Abstract
In the modern world, the attention is raised for the development of newer technologies for the transformation of biological wastes into biofuels as an alternative option of exhaustible petroleum or other sources. The organic parts of agricultural wastes, forest residues, food wastes, and municipal and industrial wastes contain an unlimited source of lignocellulosic biomass which could potentially be used for generating second-generation biofuels such as “bioethanol.” Microorganisms play an important role in all probable steps intended for lignocelluloses hydrolysis. The greener technological approach for green fuel production through application of microorganisms is a sustainable and renewable approach which is carried out in three steps such as (a) hydrolysis of lignin; (b) hydrolysis of cellulose and hemicelluloses; (c) fermentation of glucose to ethanol. The high production of ethanol is the need of the cotemporary world and therefore it becomes necessary to explore different microorganisms having a high potential for ethanol yield. Moreover, introducing metabolic engineering techniques is the current advancement for development of modified microbial cells for enhanced production of ethanol from lignocellulosic biomass. The present chapter focuses on the valorization of lignocelluloses waste through microorganisms and their mechanisms required for bioethanol synthesis from lignocellulosic biomass.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
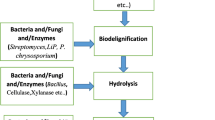
From the past few years, the global researches endeavored to find alternative sources of energy. Main reasons behind searching sustainable energy supply are (1) increase of atmospheric CO2 and concern for global climate change (Venkatramanan et al. 2020; 2021a), (2) depletion of non-renewable energy source, (3) rising energy demand, (4) energy security, (5) rural economic development, (6) rapid urbanization, (7) development of power driven technology, and (8) transportation (Baños et al. 2011; De Bhowmick et al. 2018; Prasad et al. 2019; Shah et al. 2019). It has been anticipated that in near future (approximately by 2025) around 50% increment in the energy demand will be appeared from a number of both developed and developing countries (Tong et al. 2012). Therefore, harvesting energy from plant biomass through sustainable, environmentally friendly and cost-effective approach is an important substitute of petroleum and non-renewable energy source (Prasad et al. 2021; Venkatramanan et al. 2021b). Plants and related waste materials contain cellulosic properties so they can be utilized to produce bioethanol (Prasad et al. 2019). In present world, the liquid biofuel in the form of “bioethanol” is being used having various benefits over fossil fuels. It can diminish the emission of greenhouse gases and reduce the particulate materials in the atmosphere (up to 50%) (Riccio et al. 2017; Donato et al. 2019). Various food crops such as “maize,” “sugarcane,” and “sugar beet” have become prominent source of carrying out fermentation process for bioethanol production, and such type of production is also described as “first-generation technology” which is anticipated to attain a level of approx. 100 billion liters (in 2022) (Saini et al. 2015). The maize and sugarcane are rich source of starch and sugars (sucrose) as raw materials and exhibit inadequacy to meet emergent requirement of bioethanol. Moreover, the cultivation of these crops for energy production has shown negative impact on issue of biodiversity and food chain, and considered as probable means for deforestation as huge farmland would be needed. Keeping such issues about risks associated with first-generation bioethanol, the research focus has been moved towards “second-generation technologies,” where the exploitation of non-food-based crops (with no-food parts) and wastes originated from wood or food-based industries represent most plentiful renewable organic constituents in the biosphere (Zucaro et al. 2016; Donato et al. 2019). Therefore, the second-generation bioethanol is derived from “lignocellulosic biomass” which is generated by agricultural practices, wood-based industries, municipal solid wastes, and dedicated energy crops cultivating on trivial lands (Nair et al. 2017). The biomass in form of lignocelluloses represents an economically feasible and renewable/inexhaustible reservoir for the production of eminent fuel in form of “bioethanol” (Donato et al. 2019; Prasad et al. 2019). “The lignocellulosic crop residues have huge potential to be used as feedstock for biofuel production” (Venkatramanan et al. 2021c). Although the fixing of lignocellulosic material into bioethanol production has been attributed to give numerous advantages in terms of environmental impact and sustainability, the “2G” or second-generation technology for bioethanol production is under infancy and all the concerning researches are going on all aspects (from biomass treatment to hydrolysis and fermentation). In this context, microorganisms (such as bacteria and fungi) and their enzymes have provided reasonable and cost-competitive strategy for switching the lignocellulosic biomass into bioethanol (Prasad et al. 2019). After pretreatment of raw materials (lignocellulosic biomass), the next step includes hydrolysis of biopolymers (cellulose and hemicelluloses) through hydrolytic enzymes into simpler sugars and their use in process of fermentation for bioethanol production (Fig. 3.1). Present chapter outlined the concise introduction of role of microorganisms and their enzymes in valorization of lignocellulosic materials for production of second-generation biofuel in more economically feasible and sustainable manner with considering the associated facts of less detrimental impacts on the environment.
3.2 Source of Lignocellulosic Biomass
Plant and agricultural residues (such as barley straw, corn stover, wheat, rice, husk of coconut, sugarcane bagasse, wood, sorghum stalks), forest residues, and municipal organic wastes are the key sources for the lignocellulosic biomasses (Shah and Venkatramanan 2019) (Fig. 3.2). Most of the countries produce considerable number of sources for deriving lignocellulosic material, for example corn stover is produced at high level by the USA; however, wood and large quantity of residues (agricultural and forest residues) produced by New Zealand and China (Zhu and Pan 2010). India, after China supplies approximately 0.2 billion tons of agriculture-based residues annually (De Bhowmick et al. 2018). Additionally, overall huge amount (approximately 180 million tons) of cellulosic biomass each year is derived from agricultural resources (Kurian et al. 2013; De Bhowmick et al. 2018). Lignocellulosic material in the form of either as crop or residues is chiefly produced from perennial herbaceous plants and woody plants, and such plant materials are abundantly presented on earth. Besides agricultural and forest residues, the municipal organic wastes are another main source of lignocelluloses (FitzPatrick et al. 2010; De Bhowmick et al. 2018). Structurally the “lignocellulosic biomass” is comprised of three most important biopolymers which are widely referred as cellulose, hemicellulose, and lignin. Other constituents in small quantity (such as acetyl groups, phenolic substituents, and minerals) are also present in lignocellulosic biomass (Fig. 3.2). Biopolymers involved in synthesis of lignocellulosic biomass are organized in intricate and inhomogeneous three-dimensional structures to provide varying degrees of relative composition depending on type of lignocelluloses.
3.3 Importance of Pretreatment Technologies
The three important biological materials such as cellulose, lignin, and hemicelluloses participate in the formation of lignocellulosic biomass, where cellulose and lignin as a matrix are bounded with chains of hemicelluloses. The main motto of the pretreatment process is to breakdown the lignocellulosic material which results in reduced crystallinity of cellulose and also augments the part of amorphous form of cellulose. Such cellulose form is actually exerting suitability for enzymatic activity (Sánchez and Cardona 2008). Moreover, the pretreatment is necessary to make lignocellulosic waste liable for fast hydrolysis with augmented monomeric sugars (Mosier et al. 2005), and features of pretreatment must be proficient and effective for the production of biofuel (Lu and Mosier 2008; Saxena et al. 2009; Gupta and Verma 2015). However, the important goals of pretreatment methods can be summarized in brief as (a) production of sugars through the hydrolysis, (b) avoiding the degradation of sugars, (c) avoiding the maximum formation of products having inhibitory properties, (d) to lessen the energy demand, and (e) decreasing the costs. The basic structure of plants such as “cell wall” hampers the entry of various pathogens. Number of pathogens actually produced certain hydrolytic enzymes which disrupt the internal parts of plants, but tough cell wall restricts the enzymatic activity to perform its action of degradation (Kim 2013). For the effective production of sugars required for fermentation from the cellulosic material, there is the necessity to further modify the physical and chemical characteristics of the cell wall structure of the plants. The factors involved in increment of pretreatment step are: (a) very less amount of lignocellulosic biomass (less than 20%) undergoes incomplete/partial digestion in its native state; (b) complex or mixed composition; (c) recalcitrant nature of the cellulose; (c) elevated crystallinity of cellulose fiber; and (d) enhancement in the accessibility of the enzymes (Kim 2013; De Bhowmick et al. 2018). Furthermore, it is also apparent that the preferable pretreatment processes have revealed an incredible impact on the physicochemical properties of the treated lignocellulosic biomass. Such properties influence the downstream processes including preconditioning, selection of microbes, utilization of by-products, and waste management along with the recuperation of the aimed product, concentration and purification of the product (da Costa Sousa et al. 2009). In addition, grasses and woods (both soft and hardwood) present the wide arrays of lignocellulosic material bearing different chemical and physical properties which necessitates various approaches. As a result, the suitable pretreatment processing means should be used for a particular substrate, and such aspects of interdependence between pretreatment processing and substrates make the pretreatment step as fundamental unit operational division in “lignocellulosic biorefinery” (De Bhowmick et al. 2018).
3.4 Pretreatment of Lignocellulosic Substrates
The pretreatment is an important process in valorization of lignocellulosic material and the production of second-generation biofuel, namely “bioethanol.” There are various ways of pretreatment such as physical or physio-chemical or chemical or biological or combinations of all these (Fig. 3.3). The critical step for pretreatment of biomass results the alteration of complex lignocellulosic material into amorphous and crystalline cellulose and such form of cellulose exhibits suitability for its further digestion (Saini et al. 2015; Furusato et al. 2018). Thus, it is a noteworthy step to attain elevated yield of ethanol from lignocellulosic material. One of the critical ways of pretreatment is “physical treatment” which involves certain important steps such as fragmentation, grinding, milling/shearing of the biomaterial/biomass. These all steps assist in lessening the level of polymerization and particle size, and on another side provide lignocellulosic material with increased bulk density, and surface area (Maurya et al. 2015; Amin et al. 2017). Physical treatment is considered as an ordinary step for enzymatic accessibility and effective bioconversion competence to the distorted particles (Barakat et al. 2014; Kumar and Sharma 2017). Pyrolysis, sonication, and irradiation (particularly with gamma radiation) are other methods of physical treatments (Isikgor and Becer 2015). Physio-chemical treatment is another important way of pretreatment method which involves chemical reactions for the distortion of the structure of lignocellulosic material. Physio-chemical treatment involves (a) steam explosion (also referred as hydrolysis), (b) CO2 explosion, (c) ammonia fiber explosion, (d) steam explosion with addition of sulfur dioxide (SO2), (e) liquid hot water-based pretreatment, and (f) microwave-chemical pretreatment (Brodeur et al. 2011; Isikgor and Becer 2015). Chemical treatment also played a significant role in process of pretreatment and there are number of foremost chemical treatment methods such as acidic treatment and alkaline treatment. Besides these ionic liquids (also known as green solvents), sulfite pretreatment and wet oxidation are other important methods of chemical-based pretreatment (Bensah and Mensah 2013; Amin et al. 2017). Next method of treatment is widely known as “biological treatment” process which has been illustrated as microbial mediated step to treat the biological material. As compared to other two methods of pretreatments (physical and chemical), the biological way of pretreatment is determined as an inexpensive and eco-friendly approach for the valorization of lignocelluloses (Wan and Li 2012; Maurya et al. 2015). In biological pretreatment process, the enzymes secreted by microorganisms (both bacteria and fungi) involve in degradation of the substrate. A range of bacteria such as “Actinomycetes” have been determined to produce lignocellulose degrading enzymes, and these enzymes are prominently efficient in degradation of grasses (as grasses possess huge cellulosic biomass) (Amin et al. 2017). However, biological pretreatment process of lignocelluloses is relatively economically feasible and proficient. Moreover, it is an eco-friendly source of wide arrays of enzymes for degrading complex biomass, and in industrial application enzymes hold huge potential.
3.5 Microbial Mediated Biological Pretreatment
In the modern era, the production of high-quality biofuel (such as ethanol) from least useful biomass through fermentation has given a new trend (Mohd Azhar et al. 2017). The bioethanol production is the green synthesis of renewable biofuels and may assist in reducing the need of precious fossils fuels. Moreover, it will be attributed to sustain future generation in respect of fuel-based energy. After illustrating few modern various pretreatment strategies in previous section, the greener approach in form of biological pretreatment has been assigned as most effective and eco-friendly approach causing lesser pollution. Biological approach for the pretreatment involves numerous enzymes which indirectly exhibit the role of microorganisms producing the particular enzyme. Conventional approach or the physio-chemical method for lignocelluloses degradation needs huge energy input and also determined as an important factor to cause pollution. Therefore, biological based pretreatment process of lignocelluloses could be an excellent instance of environment friendly and inexpensive strategy (Maurya et al. 2015). The conversion or transformation of the biomass/raw materials to the biofuel by using the preeminent microorganism could provide better productivity in most efficient way with less investment of money. The conversion of raw biomass might be improved by having appropriate understanding of the microorganisms participated in different steps of pretreatment. Biological pretreatment is essential because it enhances fermentation rate. This approach particularly uses the cellulose or hemicelluloses degrading microorganism for carrying out pretreatment of substrate such as lignocelluloses. Earlier studies reported the vital role of bacteria including Bacillus to degrade organic materials (Poszytek et al. 2016), and such organisms have important place in the biological pretreatments of raw materials. Bacteria are profoundly beneficial for secreting enzymes (both industrial and biotechnological important enzymes) (Singh et al. 2012). The combination of more than two microorganisms (also known as microbial consortia) aids in enhanced degradation of complex biomaterials. Microbial consortia comprising of cellulolytic bacteria (Bacillus and Streptomyces), and fungi (Candida and Aspergillus) showed wide-spectrum biodegradation (Nikiema et al. 2017). Biomolecules with complex structure such as the polysaccharides are degraded to the simpler sugars through the involvement of wide arrays of enzymes like amylase, cellobiase, cellulase, and xylanase. Moreover, protease plays a pivotal role for the degradation of protein into the amino acids and lipase breaks the lipids into two subsequent main products (such as glycerol and long-chain fatty acids) (Indrasith et al. 1988; Lass et al. 2011). However, the lignin shows extremely resistive nature against degradation, but few fungi degrade lignin too. Modification in conventional steps also required for improving the bioethanol synthesis from the biomass, and it is also reported that simultaneous “saccharification” and “fermentation” through the association of fungi can improve bioethanol productivity (Cheng et al. 2017). White-rot fungi were examined to being an effective candidate to bring out pretreatment process of most of the available lignocellulosic biomass (Kumar and Wyman 2009). Numerous white-rot fungi (Ceriporia lacerate, Cyathus stercolerus, P. chrysosporium, Pleurotus ostreatus, Phanerochaete chrysosporium, and Pycnoporus cinnabarinus) have the trait to produce lignin peroxidases (lignin-degrading enzymes) and manganese- dependent peroxidases, and these enzymes were reported to exhibit higher delignification efficacy on different lignocellulosic biomasses (Shi et al. 2008; Kumar and Wyman 2009; Maurya et al. 2015; Ummalyma et al. 2019). An effectual delignification of different biomass was reported by fungus, namely Ceriporiopsis subvermispora in the mutual action of two enzymes such as laccase and manganese peroxidase (Wan and Li 2012). Assessment of mild alkali and also the steam pretreatment of “wet-milled corn fiber” are done with using fungi, namely Gloeophyllum trabeum, P. chrysosporium, and Trichoderma reesei, which resulted into the instant hydrolysates fermentation to ethanol. This phenomenon illustrates that the yields of ethanol are 75% superior as compared to a commercially accessible cellulase enzyme utilized in instantaneous saccharification and fermentation process (Brahmachari et al. 2016). Microorganisms which had been isolated from diverse ecological niches or regions (such as soil, manure/compost, agriculture-based residues, and rumen of animals) are potential consortia having capacity for carrying out efficient degradation process of lignocelluloses (Poszytek et al. 2016). It became important to comprehend the specific microorganism involved in making a particular microbial consortium for the relevant lignocellulosic biomass to be treated, and this understanding could direct to an insightful modification in the eminent production rate of bioethanol. Consortia (mixture of pure strains of yeast and cellulolytic bacteria) screened from natural environment were also employed for successful pretreatment of lignocellulosic matter in process of biological pretreatment (Zhang et al. 2011).
3.6 Hydrolysis: A Process Involves Microbial Enzymes
Both celluloses and hemicelluloses undergo the enzymatic hydrolysis which is regulated by numerous factors such as temperature, pH, quality of substrate, incubation period, and ratio of enzyme-substrate (Achinas and Euverink 2016). Though, the use of either diluted or concentrated acid such as sulfuric acid for the acid hydrolysis is a common practice to degrade the celluloses. But, to hydrolyze the cellulosic polymers through the “acid hydrolysis” has limitations and shows unsuitability for efficient ethanol fermentation due to synthesis of toxic components such as phenols (Sun and Cheng 2002; Moe et al. 2012; Achinas and Euverink 2016). Moreover, this method of acid hydrolysis is not economically feasible as it involves high consumption of acids (Moe et al. 2012) and requires specialized reactors because of higher degree of corrosion and high toxicity rate (Wijaya et al. 2014). Therefore, it is required to use microbial based enzymes for solving the purpose of hydrolysis of celluloses and hemicelluloses in more effective manner. Plenty of researches have been performed on microbes (both bacteria and fungi) bearing cellulolytic/lignocellulolytic nature and the respective hydrolytic enzymes for efficient hydrolysis of sugars and their conversion into the ethanol (Jessen et al. 2015; Prasad et al. 2019).
Each step in the hydrolysis of polysaccharide matrix of plant cell wall is a complex phenomenon and needs a suitable treatment. The method of pretreatment of lignocellulosic material as substrate is connected with enzymatic hydrolysis, and such practices further help in enhanced porosity and enzyme accessibility to the substrate (lignocellulosic biomass) (Limayem and Ricke 2012; Prasad et al. 2019). In pretreatment process, the separation of lignin moiety from the lignocellulosic material is necessary as it interferes with the hydrolysis step through blocking the access of cellulose degrading enzyme “cellulases.” Therefore, the separation of the lignin can dramatically result into the increased hydrolysis rate of celluloses (McMillan 1994). Enzymes mediated hydrolysis have exhibited benefits over acid-based hydrolysis, as the method of enzyme hydrolysis is very mild process and potentially provides high yields with low cost. Moreover, it doesn’t have corrosion problems so it can be proposed as the preferable method for “wood-to-ethanol processes” in future (Menon and Rao 2012).
3.6.1 Cellulases
Enzymes hydrolysis coupled with activities of various kinds of hydrolytic enzymes which converts complex carbohydrate molecules into the simple monomeric sugars. In comparison with acid hydrolysis, the enzymatic hydrolysis needs less input of energy and mild conditions (Ferreira et al. 2009). Cellulase is the most significant enzyme present in various cellulolytic bacteria (Acetovibrio, Bacillus, Bacteroides, Cellulomonas, Clostridium, Erwinia, Ruminococcus, Streptomyces, Thermomonospora) and cellulolytic fungi (Fusarium, Penicillium, Phanerochaete, Schizophyllum sp., and Trichoderma). Cellulases possess the ability to convert cellulose into simplest sugars (e.g., glucose or galactose monomer) (Gupta and Verma 2015). Cellulase enzymes are comprised of a catalytic unit and a non-catalytic carbohydrate-binding unit and also associated with other accessory domains (Herve et al. 2010; Chatterjee et al. 2015). The enzyme “cellulases” belong to glycoside hydrolases family with three different classes of enzymes: (a) Endo-1,4- β-endoglucanase (cleave the glucosidic linkages randomly on the complex molecule of polysaccharide), (b) Exo-1,4- β-exoglucanase (binds to crystalline region of the cellulose and randomly cleaves the cellulose molecules), and (c) β-glucosidase or cellobiase (these enzymes specifically cleave the cellobiose molecule) (Willis et al. 2010; Chatterjee et al. 2015). Cellulose degrading microorganisms are widely known as cellulolytic microorganisms and possess the capability to degrade recalcitrant plant cell wall. The cellulolytic microorganisms, for instances thermophilic and mesophilic anaerobes, fungi, and bacteria are robustly capable to hydrolyze extremely crystalline insoluble cellulose (Shaw et al. 2008; Himmel et al. 2010). Lamed and Bayer (1988) stated that there is huge attention towards thermophiles as these microorganisms have the ability to secrete “thermo-stable cellulose” mainly under higher temperature (more than 90 °C temperature too). In case of anaerobic bacteria, the degradation of cellulose is carried out by a particular multienzyme complex, termed as “cellulosomes,” which either found in free or associated to the cell surface (Chatterjee et al. 2015). Mitchell (1998) illustrated the cellulolytic activity of Clostridium (a thermophilic anaerobe bacteria) for the degradation of cellulosic plant materials and also showed adaptable fermentable ability. For the last some years, T. reesei based cellulases have drawn attraction for the research, and are extensively employed in the laboratory and pilot-scale study for ethanol application (Gray et al. 2006). Cellulases from two prominent fungi such as Aspergillus niger and Trichoderma viride are also used for the hydrolysis of biomass (Passos et al. 2009). The majority of commercially available enzymes for hydrolysis of biomass are in fact blends of cellulases from fungi (Aspergillus or Trichoderma) supplemented with β-glucosidases. Other potent cellulases producing microorganisms are Cellulomonas sp., Clostridium sp., Thermomonospora sp., Aspergillus sp., and Trichoderma sp. (Kuhad et al. 2011).
3.6.2 Hemicellulases
“Hemicellulase” is a unique factor for plant biomass degradation and particularly acts on hemicelluloses. This enzyme is main constituent for carbon flow in nature. The main substrate of this enzyme is hemicelluloses which can be represented as an assemblage of branched and linear polysaccharides connected through hydrogen bonds to the cellulose microfibrils. Hemicellulose is comprised of a combination of glucose and sugar monomers (Ummalyma et al. 2019). Xylan is the most copious hemicelluloses which contain pentose sugars (such as xylose), and the enzyme namely “xylanase” catalyzes the hydrolysis of xylan. The catalytic or functional unit of hemicellulases can be described either as glycosidic hydrolases (which hydrolyze glycosidic bonds) or carbohydrate esterases (which catalyze the degradation of ferulic acid and acetate). Multiple xylanases with varied specificities and functions perform the action of xylan hydrolysis. There are numerous microorganisms (A. niger, Bacillus sp., Humicola insolens, and T. reesei) from which xylanases are produced on a commercial basis. The action of endoxylanases and exoxylanases commence the process of hydrolysis of hemicelluloses (Binod et al. 2011; Ummalyma et al. 2019). The redundant by-product of hemicelluloses hydrolysis is l-arabinitol which affects the diminution of d-xylose to xylitol. However, xylose reductase has the ability to reduce the l-arabinose to “l-arabinitol.” Nair and Zhao (2010) engineered a strain of Escherichia coli with a “xylose reductase mutant” which resulted into elimination of l-arabinitol production to synthesize xylitol from a combination of hemicelluloses sugars (such as l-arabinose, d-glucose, and d-xylose). Sakamoto et al. (2012) and his group designed Saccharomyces cerevisiae through genetic engineering intervention which showed the ability to degrade hemicelluloses through co-presenting the enzymes from different microorganisms such as endoxylanase (from Trichoderma reesei), β-xylosidase (from Aspergillus oryzae), β-glucosidase (from Aspergillus aculeatus), expression of xylulokinase (from S. cerevisiae) and xylose reductase and xylitol dehydrogenase (from Pichia stipitis) with the inclusion of xylose. The genetically engineered microorganisms also have the ability to produce bioethanol using rice straw, as the rice straw also provide suitable hemicelluloses (cellooligosaccharides, xylooligosaccharides, and xylan) substrate. Su et al. (2015) engineered a bacterial strain, namely E. coli W3110 to secrete xylitol to display xylose reductase from Neurospora crassa at elevated temperature without inclusion bodies. The genes of xylose isomerase (“xylA”) and xylulose kinase (“xylB”) liable for “d-xylose catabolism” were eradicated. This engineered bacterial strain can abolish catabolite repression, therefore permits the simultaneous uptaking of sugars including glucose and xylose, which is reliant on “phosphoenolpyruvate-dependent glucose phosphotransferase system (ptsG).”
3.6.3 Ligninases
Lignin is considered as second major abundant organic polymer which provides a rigidity to plant cell wall structure and also inhibits hydrolysis of hemicelluloses and celluloses. The valorization of huge biomass such as “lignocelluloses” is performed for producing green fuel “bioethanol” (Kawaguchi et al. 2016; Ragauskas et al. 2014), but degradation of lignin is prime task for efficient utilization of biomass in biorefineries. Structurally lignin is determined as a cross-linked polymer of “4-hydroxyphenylpropanoid monomers/monolignols” having various carbon(C)-carbon(C) and ether bonds. p-hydroxyphenyl, guaiacyl, and syringyl groups are the phenolic moieties of monomeric units and their proportion varied with the plant species. Generally, the most common linkages present in lignin are β-β, β-0-4, and β-5 bonds (Vanholme et al. 2010). Highly lignin selective enzyme “ligninases” is the current demand for lignin degradation. There are few fungi which produce ligninases, and among these specifically white-rot fungi synthesize some particular enzymes including MnP (Mn peroxidases), LiP (lignin peroxidases), and laccases which all arrive in category of “ligninases.” Various microorganisms produce different combinations of lignin-degrading enzymes displaying varying mechanisms of lignin degradation (Sahadevan et al. 2013). The term “enzymatic combustion” has been described in case of degradation of lignin by lignin-degrading microorganisms, where the oxidizing potential of hydrogen peroxide/molecular oxygen by two enzymes, namely “ligninolytic peroxidase” or “laccase” are subjected to oxidize aromatic units (Kirk and Farrell 1987; Bugg and Rahmanpour 2015). White-rot fungi Phanerochaete chrysosporium has been extensively studied to produce extracellular enzymes (Mn peroxidases, lignin peroxidases, and laccases) for biodegradation of lignin (Bugg and Rahmanpour 2015). Several researchers have reported MnPs production from wide range of microorganisms (bacteria, fungi, and algae) (Zhang et al. 2018; Bugg and Rahmanpour 2015). MnPs are the broadly distributed extracellular and potential peroxidases produced by fungi, especially white-rot fungi (C. subvermispora, Dichomeris squalens, P. sordida, P. chrysosporium, P. radiate, and P. rivulosu) (Hakala et al. 2006). Laccases and LiPs also show vibrant role in the course of lignin de-polymerization (Hammel and Cullen 2008; Bugg and Rahmanpour 2015). Besides aforementioned three important enzymes (cellulases, hemicellulases, and ligninases), some other enzymes including “xyloglucanase” have been employed for degradation of those secondary polysaccharides which are unable to be transformed into simple sugars through the action of “cellulases” (Stickel et al. 2014). The process of enzymatic hydrolysis carried out at elevated solid loadings is considered to be inexpensive approach due to the accumulation of higher concentration of sugar at the end phase of hydrolysis. And this plentiful amount of sugar is converted into elevated level of ethanol which exhibits low-priced approach with less energy requirement for distillation process (Modenbach and Nokes 2013). Another saccharification method is termed as simultaneous saccharification and fermentation (SSF) in which fermentative microbes are used for simultaneous SSF of hemicelluloses and celluloses (Mosier et al. 2005).
3.7 Fermentation
After hydrolysis, the next imperative step is “fermentation,” where the molecules of sugar are taken up by the enzymes synthesized by bacteria or yeasts for producing variety of organic acids and alcohols (Mussatto and Teixeira 2010; Bhagchandanii et al. 2020). The efficiency of the fermentation depends upon two main factors: (1) effective hydrolysis and (2) selection of correct microbial strains to diminish the formation of inhibitory toxic compounds to attain elevated yield of ethanol (Achinas and Euverink 2016). “SHF (Separated Hydrolysis and Fermentation)” is determined as the conventional method in which the process of hydrolysis is performed at earliest to produce monosaccharide sugar as the fermentation proceeds (Dahnum et al. 2015; Devarapalli and Atiyeh 2015; Prasad et al. 2019). One more and important method of hydrolysis and fermentation is known as “SSF (Simultaneous Saccharification and Fermentation)” where the process of cellulose hydrolysis and the process of fermentation of hexose take place in a same reactor by using yeast and enzyme together, so glucose is quickly transformed into ethanol (Cantarella et al. 2004; Dahnum et al. 2015; Prasad et al. 2019). Wyman et al. (1992) described SSF as the better process for providing high ethanol yield in comparison of SHF. Besides the better ethanol yields, the SSF process helps in elimination of end product inhibition, and eradicates the requirement for separate reactors. Saccharomyces cerevisiae is the common yeast which plentifully used in the ethanol fermentation. Moreover, Saccharomyces is also used as food additive and “generally recognized as safe (GRAS),” and as a result it became best candidate for manufacturing alcoholic beverages. Generally, S. cerevisiae has been characterized to carry out glucose fermentation to ethanol very effectively. But on contrary, the fermentation of xylose is exigent as very few conventional ethanol-producing microorganisms depict the ability to readily ferment xylose, although a lot of microorganisms consume “xylose” as a carbon (“C”) source (Lin and Tanaka 2006). Biofuel-based industries use different biomass or substrate and specific microbial strain for ethanol production, and are seeking various approaches for the modifications for huge level production of green fuel in more economical manner. In sugar-based and corn-based biofuel industries, the extensive preference has been given to Saccharomyces cerevisiae for carrying out fermentation (Achinas and Euverink 2016; Prasad et al. 2019). The role of bacteria in fermentation cannot be avoided as it is very economically feasible and easier strategy for ethanol production (Senthilkumar and Gunasekaran 2005). The common bacterial examples are Corynebacterium glutamicum and Zymomonas mobilis, which are extensively exploited in industry for ethanol production (Senthilkumar and Gunasekaran 2005; Tsuchida et al. 2007; Kang et al. 2014). Enhancement in ethanol yield is the main task for the researchers (Rai et al. 2010; Jessen et al. 2015), therefore the approach of genetic engineering accepted challenges and resulted in high ethanol production through genetically modified microorganisms. The application of first metabolic engineering surprisingly resulted into the construction of E. coli strains which selectively produce ethanol, and E. coli presents numerous benefits as a biocatalyst for the ethanol production, as well as the capacity to ferment wide ranges of sugars with no need of complex growth factors (Lin and Tanaka 2006).
3.8 Advancement in Ligocellulosic Valorization: A Biotechnological Mediated Reform
Current development in biotechnology brought a boom in excellent solubility of lignocelluloses.
Modification in genetic program depicted alterations in either microorganisms for efficient production of cellulose degrading enzymes or developing plants having nature of easy solubility of residues for improved fermentation practices. Biotechnological advances have been resulted into the development of genetically modified microorganisms for synthesizing modified cellulosome (cellulose degrading machinery). Cloned and over-expressed man5K gene in Clostridium cellulolyticum confirmed 20-fold higher activities of altered/modified form of cellulosomes on substrate “galactomannan” in comparison with control with promising cellulase activities (Perret et al. 2004). Ethanol yields and its titer can be improved by inhibition of by-products, and for accomplishing such task the three respective genes, namely lactate dehydrogenase (ldh), hygromycin phosphotransferase, and phosphotransacetylase (pta) in C. thermocellum were knocked out. Deleting only pta gene did not increase ethanol yield, but knocking out of all three genes resulted into a fourfold enhancement in production of ethanol (Argyros et al. 2011). Research on trifunctional cellulosomal complex has represented cellulosome chimera amid cellulases and hemicellulase from several microbes exhibited improved hydrolytic action on complex substrates (Fierobe et al. 2005).
3.9 Conclusion
Valorization of widely available lignocellulosic biomass and the synthesis of bioethanol is the prime need for the present world for lessening the dependency on non-renewable sources such as fossil/petroleum-based fuels. Lignocellulosic material is generated from different sources including plant materials, agricultural and forest residues, and wastes originating from wood and food-based industries. However, the practice of microbial mediated lignocellulosic waste valorization gave new trends for efficient pretreatment process for increasing the accessibility of cellulose-hemicellulose matrix. Microorganisms particularly bacteria and fungi secrete wide range of hydrolytic enzymes which assist in hydrolysis of large biopolymers such as cellulose, hemicelluloses, and lignin which eventually results in formation of fermentable sugars. Crucial step of fermentation requires activity of numerous microorganisms for utilizing various sugars and their transformation into bioethanol. The microbial mediated steps for lignocelluloses valorization is considered to be economically feasible, and provide environment friendly hub for higher yield of bioethanol. Strategies such as pretreatment and hydrolysis of lignocelluloses and subsequent fermentation step are using microorganisms and their enzymes in current era for the green production of “second-generation biofuel” at efficient level.
3.10 Future Prospects
Application of microorganisms in valorization of lignocellulosic waste is wider, but existing challenges must be addressed to further improvement in generation of second-generation biofuel. The future research is required to employ strategies for elimination of inhibitory by-products with more efficiency. Construction of genetically and metabolically engineered microbial strains should be the prime topic for research in scientific world for improving cellular machinery for many folds higher production of bioethanol with less cost. Therefore, future research needs to be intended for developing strategies through microbial strains which could reduce the duration of pretreatment period and other steps required for bioethanol production.
References
Achinas S, Euverink GJW (2016) Consolidated briefing of biochemical ethanol production from lignocellulosic biomass. Electron J Biotechnol 23:44–53. https://doi.org/10.1016/j.ejbt.2016.07.006
Amin FR, Khalid H, Zhang H, Rahman SU, Zhang R, Liu G, Chen C (2017) Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 7(1):72. https://doi.org/10.1186/s13568-017-0375-4
Argyros D, Tripathi S, Barrett T, Rogers S, Feinberg L, Olson D, Foden J, Miller B, Lynd L, Hogsett D, Caiazza N (2011) High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl Environ Microbiol 77:8288–8294. https://doi.org/10.1128/aem.00646-11
Baños R, Manzano-Agugliaro F, Montoya F, Gil C, Alcayde A, Gómez J (2011) Optimization methods applied to renewable and sustainable energy: a review. Renew Sustain Energy Rev 15(4):1753–1766. https://doi.org/10.1016/j.rser.2010.12.008
Barakat A, Mayer-Laigle C, Solhy A, Arancon RA, De Vries H, Luque R (2014) Mechanical pretreatments of lignocellulosic biomass: towards facile and environmentally sound technologies for biofuels production. RSC Adv 4(89):48109–48127. https://doi.org/10.1039/c4ra07568d
Bensah EC, Mensah M (2013) Chemical pretreatment methods for the production of cellulosic ethanol: technologies and innovations. Int J Chem Eng 2013:1–21. https://doi.org/10.1155/2013/719607
Bhagchandanii DD, Babu RP, Sonawane JM, Khanna N, Pandit S, Jadhav D, Khilari S, Prasad R (2020) A comprehensive understanding of electro-fermentation. Fermentation 6(3):92. https://doi.org/10.3390/fermentation6030092
Binod P, Janu KU, Sindhu R, Pandey A (2011) Hydrolysis of lignocellulosic biomass for bioethanol production. In: Biofuels. Elsevier, Amsterdam, pp 229–250. https://doi.org/10.1016/B978-0-12-385099-7.00010-3
Brahmachari G, Demain AL, Adrio JL (2016) Biotechnology of microbial enzymes: production, biocatalysis and industrial applications. In: Biotechnology of microbial enzymes: production, biocatalysis and industrial applications. Elsevier, Amsterdam, pp 1–608
Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S (2011) Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res 2011:787532. https://doi.org/10.4061/2011/787532
Bugg TD, Rahmanpour R (2015) Enzymatic conversion of lignin into renewable chemicals. Curr Opin Chem Biol 29:10–17. https://doi.org/10.1016/j.cbpa.2015.06.009
Cantarella M, Cantarella L, Gallifuoco A, Spera A, Alfani F (2004) Comparison of different detoxification methods for steam-exploded poplar wood as a substrate for the bioproduction of ethanol in SHF and SSF. Process Biochem 39(11):1533–1542
Chatterjee S, Sharma S, Prasad RK, Datta S, Dubey D, Meghvansi MK, Vairale MG, Veer V (2015) Cellulase enzyme based biodegradation of cellulosic materials: an overview. South Asian J Exp Biol 5(6):271–282
Cheng N, Koda K, Tamai Y, Yamamoto Y, Takasuka TE, Uraki Y (2017) Optimization of simultaneous saccharification and fermentation conditions with amphipathic lignin derivatives for concentrated bioethanol production. Bioresour Technol 232:126–132. https://doi.org/10.1016/j.biortech.2017.02.018
da Costa Sousa L, Chundawat SP, Balan V, Dale BE (2009) “Cradle-to-grave” assessment of existing lignocellulose pretreatment technologies. Curr Opin Biotechnol 20:339. https://doi.org/10.1016/j.copbio.2009.05.003
Dahnum D, Tasum SO, Triwahyuni E, Nurdin M, Abimanyu H (2015) Comparison of SHF and SSF processes using enzyme and dry yeast for optimization of bioethanol production from empty fruit bunch. Energy Procedia 68:107–116. https://doi.org/10.1016/j.egypro.2015.03.238
De Bhowmick G, Sarmah AK, Sen R (2018) Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour Technol 247:1144–1154. https://doi.org/10.1016/j.biortech.2017.09.163
Devarapalli M, Atiyeh HK (2015) A review of conversion processes for bioethanol production with a focus on syngas fermentation. Biofuel Res J 7:268–280. https://doi.org/10.18331/brj2015.2.3.5
Donato PD, Finore I, Poli A, Nicolaus B, Lama L (2019) The production of second generation bioethanol: the biotechnology potential of thermophilic bacteria. J Clean Prod 233:1410–1417. https://doi.org/10.1016/j.jclepro.2019.06.152
Ferreira S, Duarte AP, Ribeiro MHL, Queiroz JA, Domingues FC (2009) Response surface optimization of enzymatic hydrolysis of Cistus ladanifer and Cytisus striatus for bioethanol production. Biochem Eng J 45(3):192–200. https://doi.org/10.1016/j.bej.2009.03.012
Fierobe H, Mingardon F, Mechaly A, Bélaïch A, Rincon MT, Pagès S et al (2005) Action of designer cellulosomes on homogeneous versus complex substrates. J Biol Chem 280(16):16325–16334. https://doi.org/10.1074/jbc.m414449200
FitzPatrick M, Champagne P, Cunningham MF, Whitney RA (2010) A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresour Technol 101(23):8915–8922. https://doi.org/10.1016/j.biortech.2010.06.125
Furusato S, Takagaki A, Hayashi S, Miyazato A, Kikuchi R, Oyama ST (2018) Mechanochemical decomposition of crystalline cellulose in the presence of protonated layered niobium Molybdate solid acid catalyst. ChemSusChem 11(5):888–896. https://doi.org/10.1002/cssc.201702305
Gray KA, Zhao L, Emptage M (2006) Bioethanol. Curr Opin Chem Biol 10(2):141–146. https://doi.org/10.1016/j.cbpa.2006.02.035
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sustain Energy Rev 41:550–567. https://doi.org/10.1016/j.rser.2014.08.032
Hakala TK, Hildén K, Maijala P, Olsson C, Hatakka A (2006) Differential regulation of manganese peroxidases and characterization of two variable MnP encoding genes in the white-rot fungus Physisporinus rivulosus. Appl Microbiol Biotechnol 73(4):839–849. https://doi.org/10.1007/s00253-006-0541-0
Hammel KE, Cullen D (2008) Role of fungal peroxidases in biological ligninolysis. Curr Opin Plant Biol 11:349–355. https://doi.org/10.1016/j.pbi.2008.02.003
Herve C, Rogowski A, Blake AW, Marcus SE, Gilbert HJ, Knox JP (2010) Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc Natl Acad Sci 107(34):15293–15298. https://doi.org/10.1073/pnas.1005732107
Himmel ME, Xu Q, Luo Y, Ding S, Lamed R, Bayer EA (2010) Microbial enzyme systems for biomass conversion: emerging paradigms. Biofuels 1(2):323–341. https://doi.org/10.4155/bfs.09.25
Indrasith LS, Sasaki T, Yamashita O (1988) A unique protease responsible for selective degradation of a yolk protein in Bombyx mori. Purification, characterization, and cleavage profile. J Biol Chem 263(2):1045–1051
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6(25):4497–4559. https://doi.org/10.1039/c5py00263j
Jessen JE, Sveinsson T, Scully SM, Orlygsson J (2015) Ethanol production by a Paenibacillus species isolated from an Icelandic hot spring—production yields from complex biomass. Icel Agric Sci 28(1):15–24. https://doi.org/10.16886/IAS.2015.02
Kang Q, Appels L, Tan T, Dewil R (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. Sci World J 2014:298153. https://doi.org/10.1155/2014/298153
Kawaguchi H, Hasunuma T, Ogino C, Kondo A (2016) Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr Opin Biotechnol 42:30–39. https://doi.org/10.1016/j.copbio.2016.02.031
Kim TH (2013) Pretreatment of lignocellulosic biomass. In: Yang S, El-Enshasy HA, Thongchul N (eds) Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Wiley, Hoboken, NJ, pp 91–105
Kirk TK, Farrell RL (1987) Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol 41:465–501. https://doi.org/10.1146/annurev.mi.41.100187.002341
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res 2011:280696. https://doi.org/10.4061/2011/280696
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4(1):7. https://doi.org/10.1186/s40643-017-0137-9
Kumar R, Wyman CE (2009) Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol Prog 25(2):302–314. https://doi.org/10.1002/btpr.102
Kurian JK, Raveendran Nair G, Hussain A, Vijaya Raghavan G (2013) Feedstocks, logistics and pre-treatment processes for sustainable lignocellulosic biorefineries: a comprehensive review. Renew Sustain Energy Rev 25:205–219. https://doi.org/10.1016/j.rser.2013.04.019
Lamed R, Bayer EA (1988) The cellulosome of Clostridium thermocellum. Adv Appl Microbiol 33:1–46. https://doi.org/10.1016/s0065-2164(08)70203-x
Lass A, Zimmermann R, Oberer M, Zechner R (2011) Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 50(1):14–27. https://doi.org/10.1016/j.plipres.2010.10.004
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38(4):449–467. https://doi.org/10.1016/j.pecs.2012.03.002
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69(6):627–642. https://doi.org/10.1007/s00253-005-0229-x
Lu Y, Mosier NS (2008) Current technologies for fuel ethanol production from lignocellulosic plant biomass. In: Vermerris W (ed) Genetic improvement of bioenergy crops. Springer, New York
Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5(5):597–609. https://doi.org/10.1007/s13205-015-0279-4
McMillan JD (1994) Pretreatment of lignocellulosic biomass. In: Himmel ME, Baker JO, Overend RP (eds) Enzymatic conversion of biomass for fuels pro-duction. American Chemical Society, Washington, DC, pp 292–324
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38(4):522–550. https://doi.org/10.1016/j.pecs.2012.02.002
Mitchell WJ (1998) Physiology of carbohydrate to solvent conversion by clostridia. Adv Microb Physiol 39:31–130. https://doi.org/10.1016/s0065-2911(08)60015-6
Modenbach AA, Nokes SE (2013) Enzymatic hydrolysis of biomass at high-solids loadings—a review. Biomass Bioenergy 56:526–544. https://doi.org/10.1016/j.biombioe.2013.05.031
Moe ST, Janga KK, Hertzberg T, Hägg M, Øyaas K, Dyrset N (2012) Saccharification of lignocellulosic biomass for biofuel and biorefinery applications—a renaissance for the concentrated acid hydrolysis? Energy Procedia 20:50–58. https://doi.org/10.1016/j.egypro.2012.03.007
Mohd Azhar SH, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Mohd Faik AA, Rodrigues KF (2017) Yeasts in sustainable bioethanol production: a review. Biochem Biophys Rep 10:52–61. https://doi.org/10.1016/j.bbrep.2017.03.003
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686. https://doi.org/10.1016/j.biortech.2004.06.025
Mussatto S, Teixeira J (2010) Lignocellulose as raw material in fermentation processes. Appl Microbiol Microb Biotechnol 2:897–907. https://doi.org/10.1016/j.jrras.2014.02.003
Nair NU, Zhao H (2010) Selective reduction of xylose to xylitol from a mixture of hemicellulosic sugars. Metab Eng 12(5):462–468. https://doi.org/10.1016/j.ymben.2010.04.005
Nair RB, Lennartsson PR, Taherzadeh MJ (2017) Bioethanol production from agricultural and municipal wastes. In: Current Developments in biotechnology and bioengineering: solid waste management. Elsevier, Amsterdam, pp 157–190. https://doi.org/10.1016/B978-0-444-63664-5.00008-3
Nikiema M, Somda MK, Adeoti K, Traore D, Baba-Moussa F, Toukourou F, Dianou D, Traore AS (2017) Production of efficient microbial complex for organic fraction of municipal organic solid waste pretreatment upstream anaerobic digestion. Int J Environ Bioremediat Biodegrad 5(3):77–85. https://doi.org/10.12691/ijebb-5-3-1
Passos CP, Yilmaz S, Silva CM, Coimbra MA (2009) Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem 115(1):48–53. https://doi.org/10.1016/j.foodchem.2008.11.064
Perret S, Bélaich A, Fierobe HP, Bélaich JP, Tardif C (2004) Towards designer cellulosomes in clostridia: mannanase enrichment of the cellulosomes produced by Clostridium cellulolyticum. J Bacteriol 186(19):6544–6552. https://doi.org/10.1128/JB.186.19.6544-6552.2004
Poszytek K, Ciezkowska M, Sklodowska A, Drewniak L (2016) Microbial consortium with high cellulolytic activity (MCHCA) for enhanced biogas production. Front Microbiol 7:324. https://doi.org/10.3389/fmicb.2016.00324
Prasad R, Chatterjee S, Mazumder P, Gupta S, Sharma S, Vairale M, Datta S, Dwivedi S, Gupta D (2019) Bioethanol production from waste lignocelluloses: a review on microbial degradation potential. Chemosphere 231:588–606. https://doi.org/10.1016/j.chemosphere.2019.05.142
Prasad S, Venkatramanan V, Singh A (2021) Renewable energy for a low-carbon future: policy perspectives. In: Venkatramanan V, Shah S, Prasad R (eds) Sustainable bioeconomy. Springer, Singapore. https://doi.org/10.1007/978-981-15-7321-7_12
Ragauskas A, Beckham G, Biddy M, Chandra R, Chen F, Davis M, Davison B, Dixon R, Gilna P, Keller M, Langan P, Naskar A, Saddler J, Tschaplinski T, Tuskan G, Wyman C (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843–1246843. https://doi.org/10.1126/science.1246843
Rai SK, Roy JK, Mukherjee AK (2010) Characterisation of a detergent-stable alkaline protease from a novel thermophilic strain Paenibacillus tezpurensis sp. nov. AS-S24-II. Appl Microbiol Biotechnol 85(5):1437–1450. https://doi.org/10.1007/s00253-009-2145-y
Riccio A, Chianese E, Tirimberio G, Prati MV (2017) Emission factors of inorganic ions from road traffic: a case study from the city of Naples (Italy). Transp Res Pt D Transp Environ 54:239–249. https://doi.org/10.1016/j.trd.2017.05.008
Sahadevan L, Misra C, Thankamani V (2013) Ligninolytic enzymes for application in treatment of effluent from pulp and paper industries. Univ J Environ Res Technol 3(1):14–26
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5(4):337–353. https://doi.org/10.1007/s13205-014-0246-5
Sakai S, Tsuchida Y, Nakamoto H, Okino S, Ichihashi O, Kawaguchi H et al (2007) Effect of lignocellulose-derived inhibitors on growth of and ethanol production by growth-arrested Corynebacterium glutamicum R. Appl Environ Microbiol 73(7):2349–2353. https://doi.org/10.1128/AEM.02880-06
Sakamoto T, Hasunuma T, Hori Y, Yamada R, Kondo A (2012) Direct ethanol production from hemicellulosic materials of rice straw by use of an engineered yeast strain codisplaying three types of hemicellulolytic enzymes on the surface of xylose-utilizing Saccharomyces cerevisiae cells. J Biotechnol 158(4):203–210. https://doi.org/10.1016/j.jbiotec.2011.06.025
Sánchez ÓJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99:5270. https://doi.org/10.1016/j.biortech.2007.11.013
Saxena RC, Adhikari DK, Goyal HB (2009) Biomass-based energy fuel through biochemical routes: a review. Renew Sustain Energy Rev 13:167. https://doi.org/10.1016/j.rser.2007.07.011
Senthilkumar V, Gunasekaran P (2005) Bioethanol production from cellulosic substrates: engineered bacteria and process integration challenges. J Sci Ind Res 64:845–853
Shah S, Venkatramanan V (2019) Advances in microbial technology for upscaling sustainable biofuel production. In: New and future developments in microbial biotechnology and bioengineering, pp 69–76. https://doi.org/10.1016/b978-0-444-63504-4.00005-0
Shah S, Venkatramanan V, Prasad R (eds) (2019) Sustainable green technologies for environmental management. Springer Nature, Singapore. https://doi.org/10.1007/978-981-13-2772-8
Shaw A, Podkaminer K, Desai S, Bardsley J, Rogers S, Thorne P, Hogsett D, Lynd L (2008) Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. Proc Natl Acad Sci 105:13769–13774. https://doi.org/10.1073/pnas.0801266105
Shi J, Chinn MS, Sharma-Shivappa RR (2008) Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour Technol 99(14):6556–6564. https://doi.org/10.1016/j.biortech.2007.11.069
Singh AV, Sharma A, Johri BN (2012) Phylogenetic profiling of culturable bacteria associated with early phase of mushroom composting assessed by amplified rDNA restriction analysis. Ann Microbiol 62:675–682. https://doi.org/10.1007/s13213-011-0304-8
Stickel JJ, Elander RT, McMillan JD, Brunecky R (2014) Enzymatic hydrolysis of lignocellulosic biomass. In: Bioprocessing of renewable resources to commodity bioproducts, pp 77–103. https://doi.org/10.1002/9781118845394.ch4
Su B, Wu M, Zhang Z, Lin J, Yang L (2015) Efficient production of xylitol from hemicellulosic hydrolysate using engineered Escherichia coli. Metab Eng 31:112–122. https://doi.org/10.1016/j.ymben.2015.07.003
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. https://doi.org/10.1016/S0960-8524(01)00212-7
Tong Z, Pullammanappallil P, Teixeira AA (2012) How ethanol is made from cellulosic biomass 1 constituents of cellulosic biomass. UF IFAS Extension, Nov, 2–5. http://edis.ifas.ufl.edu
Ummalyma SB, Supriya RD, Sindhu R, Binod P, Nair RB, Pandey A, Gnansounou E (2019) Biological pretreatment of lignocellulosic biomass—current trends and future perspectives. In: Second and third generation of feedstocks. Elsevier, Amsterdam, pp 197–212. https://doi.org/10.1016/b978-0-12-815162-4.00007-0
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153(3):895–905. https://doi.org/10.1104/pp.110.155119
Venkatramanan V, Shah S, Prasad R (eds) (2020) Global climate change and environmental policy: agriculture perspectives. Springer Nature, Singapore. https://doi.org/10.1007/978-981-13-9570-3
Venkatramanan V, Shah S, Prasad R (eds) (2021a) Exploring synergies and trade-offs between climate change and the sustainable development goals. Springer, Singapore. https://doi.org/10.1007/978-981-15-7301-9
Venkatramanan V, Shah S, Prasad R (eds) (2021b) Sustainable bioeconomy: pathways to sustainable development goals. Springer Nature, Singapore. https://doi.org/10.1007/978-981-15-7321-7
Venkatramanan V, Shah S, Rai AK, Prasad R (2021c) Nexus between crop residue burning, bioeconomy and sustainable development goals over North-Western India. Front Energy Res 8:614212. https://doi.org/10.3389/fenrg.2020.614212
Wan C, Li Y (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30(6):1447–1457. https://doi.org/10.1016/j.biotechadv.2012.03.003
Wijaya YP, Putra RD, Widyaya VT, Ha J, Suh DJ, Kim CS (2014) Comparative study on two-step concentrated acid hydrolysis for the extraction of sugars from lignocellulosic biomass. Bioresour Technol 164:221–231. https://doi.org/10.1016/j.biortech.2014.04.084
Willis JD, Oppert C, Jurat-Fuentes JL (2010) Methods for discovery and characterization of cellulolytic enzymes from insects. Insect Sci 17(3):184–198. https://doi.org/10.1111/j.1744-7917.2010.01322.x
Wyman CE, Spindler DD, Grohmann K (1992) Simultaneous saccharification and fermentation of several lignocellulosic feedstocks to fuel ethanol. Biomass Bioenergy 3(5):301–307. https://doi.org/10.1016/0961-9534(92)90001-7
Zhang Q, He J, Tian M, Mao Z, Tang L, Zhang J, Zhang H (2011) Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour Technol 102(19):8899–8906. https://doi.org/10.1016/j.biortech.2011.06.06
Zhang H, Zhang J, Zhang X, Geng A (2018) Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching. Process Biochem 66:222–229. https://doi.org/10.1016/j.procbio.2017.12.011
Zhu J, Pan X (2010) Woody biomass pretreatment for cellulosic ethanol production: technology and energy consumption evaluation. Bioresour Technol 101(13):4992–5002. https://doi.org/10.1016/j.biortech.2009.11.007
Zucaro A, Forte A, Basosi R, Fagnano M, Fierro A (2016) Life cycle assessment of second generation bioethanol produced from low-input dedicated crops of Arundo donax L. Bioresour Technol 219:589–599. https://doi.org/10.1016/j.biortech.2016.08.022
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Upadhayay, V.K., Khan, A., Singh, J., Singh, A.V. (2021). Microbial Mediated Valorization of Lignocellulose: A Green Technology for Bioethanol Production. In: Shah, S., Venkatramanan, V., Prasad, R. (eds) Bio-valorization of Waste. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-9696-4_3
Download citation
DOI: https://doi.org/10.1007/978-981-15-9696-4_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9695-7
Online ISBN: 978-981-15-9696-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)