Abstract
Xylanase is a class of hydrolytic enzymes which cleaves the linear polysaccharide, the major constituent of hemicellulose beta-1,4-xylan into xylose. The structure of xylanase is complex, repeated linear polymers of xylopyranosyl groups at numerous carbon positions with different acidic compounds or sugars. It plays a critical physiological role in plant tissue like seed germination, plant defense system, and softening of fruits. Among microbial sources, actinomycetes, fungi, bacteria, and yeast are the principal sources of xylanases. The chief xylanase producers from fungal genera include Aspergillus, Coriolus versicolor, Fusarium, Phanerochaete chrysosporium, Trichoderma, and Pichia. The commercialization of xylanase into the industry has increased significantly due to wide number of applications. They are used in paper industries, bio-bleaching of wood pulp, bioprocessing of textiles, food additives to poultry, improvement in the nutritional properties of grain feed and silage, extraction of plant oils, starch, and coffee, etc. Solid-state fermentation is an effective method for xylanase synthesis, predominantly by fungal culture due to the advantages like high productivity at low cost as it produces xylanase by consuming cheap substrate, which serve as the carbon source as a resultant total cost of the process decreases. Advancement in recombinant DNA technology led to the selection of xylanase-producing microorganisms which are more likely suitable for industrial applications. The advancement in the genetic engineering can help us to amend the fungal expression system for hyper-expression of the heterologous xylanase for production as well as industrial use. Using improved technical advancement systems, development of recombinant fungal expression systems by genetic approach will help in hyper-expression of xylanases and xylanase families for their production management at the industrial level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
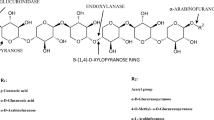
Xylanase (EC 3.2.1.8, beta-xylanase, beta-1,4-xylan xylanohydrolase, xylanohydrolase, beta-D-xylanase, 1,4-beta-xylan, endo-1,4-beta-D-xylanase, beta-1,4-xylanase, endo-1,4-beta-xylanase, endo-1,4-xylanase, endo-(1->4)-beta-xylan 4-xylanohydrolase) is a class of hydrolytic enzymes which cleaves the linear polysaccharide which is the major constituent of hemicellulose beta-1,4-xylan into xylose (Talamantes et al. 2016; Vogel 2018). It plays a critical physiological role in plant tissue like seed germination, plant defense system, and softening of fruits (Saleem et al. 2008). It is second most abundant natural polysaccharide consisting mainly of D-xylose as its monomeric unit commonly present in the middle lamellae and cell wall of plant cells (Saulnier et al. 2007; Caffall and Mohnen 2009). The major chain of xylan is composed of β-xylopyranose residues which covers different groups of noncellulosic polysaccharides of small monosaccharide units such as L-arabinose, D-galactose, D-glucuronic acid, D-galacturonic acid, D-glucose, D-mannose, D-xylose, etc. (de Vries and Visser 2001; Menon et al. 2010; Segato et al. 2014). Because of the complex chemical structure and heterogeneity of plant xylan, the complete degradation requires different hydrolytic enzymes having diverse mode of action and specificities. Thus, it explains the reason for arsenal production of polymer-degrading proteins (Motta et al. 2013).
The xylanolytic enzyme system which hydrolyzes the xylan comprises different hydrolytic enzymes like α-arabinofuranosidase (α-L-arabinofuranosidase, E.C.3.2.1.55), acetylxylan esterase (E.C.3.1.1.72), α-glucuronidase (α-glucosiduronase, E.C.3.2.1.139), β-xylosidase (xylan-1,4-β-xylosidase, E.C.3.2.1.37), and endoxylanase (endo-1,4-β-xylanase, E.C.3.2.1.8) (Rahman et al. 2003; Selvarajan and Veena 2017). These diverse enzymes act in cooperation for the conversion of xylan to constituent sugar molecules (Hu et al. 2011; Su et al. 2013). Out of all xylanases, endoxylanases are considered to be of extreme importance as they are directly involved in the cleaving of glycosidic bonds and liberation of small stretches of xylooligosaccharides (Dey and Roy 2018). Reliable with their side group substitutions and structural chemistry, xylanase seems to be intertwined, covalently linked, and interspersed at many points with the superimposing sheath of lignin by hydrogen bonding (Zhang 2008; Youssefian and Rahbar 2015). Xylanases are not restricted to plants; they also can be found in majority of the species of crustaceans, snails, insects, protozoans, marine algae, etc. (Kumar et al. 2016a, b; Chakdar et al. 2016). Among microbial sources, actinomycetes, fungi, bacteria, and yeast are the principal sources of xylanases (Juturu and Wu 2012).
The characteristics of various xylanase-producing bacteria and fungi are mentioned in Table 12.1. From the past few decades, the commercialization of xylanase into the industry has increased significantly due to wide number of applications. They are used in paper industries, bio-bleaching of wood pulp, bioprocessing of textiles, food additives to poultry, improvement in the nutritional properties of grain feed and silage, extraction of plant oils, starch, and coffee, etc. (Yadav 2015; Motta et al. 2013; Goswami and Rawat 2015). Apart from these wider applications, xylanases also have potential for application in bakery processes and fruit juice processing units (Butt et al. 2008; Harris and Ramalingam 2010). The production of xylanase levels in filamentous fungi is very much higher than those found in actinomycetes, bacteria, and yeasts as they secrete xylanase directly into the medium without any processes by eliminating the need for cell disruption (Sepahy et al. 2011). Filamentous fungi also produce auxiliary enzymes which are essential for the degradation/debranching of substituted xylans (Nair and Shashidhar 2008; Brink and Vries 2011). The objective of this chapter is to discuss the various types and sources of xylanases, their industrial applications, and factors affecting the production of xylanases.
12.2 Types of Xylanases
Xylanases have been broadly classified in at least three ways: the crystal structure (Jeffries 1996), product profile or the substrate specificity and kinetic properties (Motta et al. 2013), and based on the isoelectric point and molecular weight (Wong et al. 1988). The acceptable system for the classification of xylanases is simply based on the comparison of the catalytic domains and its primary structure. According to the CAZy database (http://www.cazy.org), xylanases (EC3.2.1.8) are linked to glycoside hydrolase (GH) families 5, 7, 8, 9, 10, 11, 12, 16, 26, 30, 43, 44, 51, and 62. Out of these, xylanases GH 10 and 11 are the two families which were extensively studied. GH family 10 comprises endo-1,3-β-xylanases and endo-1,4-β-xylanases (Motta et al. 2013). These members of the family possess the ability to hydrolyze the aryl β-glycosides at the aglyconc bond within xylobiose and xylotriose (Heo et al. 2004; Qing and Wyman 2011). On the basis of amino acid similarity index, xylanases are classified under glycoside hydrolases into families 10 and 11. It has been documented that GH10 xylanases have low pI and molecular weight ≥30 kDa, whereas GH11 xylanases have high pI and molecular weight 20 kDa approximately. Moreover, enhanced activity of these enzymes is observed on small stretches of xylooligosaccharides, thus indicating the presence of small substrate-binding site (Henrissat 1991; Gallardo et al. 2004; Murphy et al. 2011; Mathur et al. 2015). Family 11 is made up of xylanases and stated to be “true xylanases” as they are highly active on substrate having d-xylose (Liu and Kokare 2017). Among all xylanases, endoxylanases are considered to be of extreme importance as they are directly involved in hydrolyzing of glycosidic bond and liberating small stretches of xylooligosaccharides (Collins et al. 2005a). Bacillus species have been reported to secrete large amount of extracellular xylanase (Beg et al. 2001), along with filamentous fungi like Aspergillus , Penicillium , and Trichoderma which also secretes large amount of extracellular xylanases accompanied by cellulolytic enzymes (Kohli et al. 2001; Polizeli et al. 2005; Wong and Saddler 1992).
12.3 Xylanase Structure
Xylanases are ubiquitous in nature; they are reported from rumen bacteria, terrestrial bacteria, crustaceans, snails, marine algae, insects, germinating seeds, rumen bacteria, protozoa, and fungi (Walia et al. 2015). The structure of xylanases is assumed to be 8 TIM-barrel fold of 8 parallel α strands of 32.5 kDa polypeptide chain forming cylinder-like structure followed by eight main α helices (Natesh et al. 1999). The structure of xylanase is complex, repeated linear polymers of xylopyranosyl groups at numerous carbon positions with different acidic compounds or sugars. The efficient and complete hydrolysis of the polymer needs an array of different enzymes with diverse mode of action and specificity (Segato et al. 2014). Endo-1,4-b D-xylanase (E.C. 3.2.1.8) haphazardly cleaves the xylan backbone, and xylosidases degrade the monomers of the xylose. α-L-arabinofuranosidases play an important role in the removal of the side groups, and the phenolic and acetyl side branches were removed by acetylxylan esterases, and they act on complex polymer (Drzewiecki et al. 2010; Takahashi et al. 2013). The conversion of xylan into its constituent sugar is supported by all these enzymes, and such kind of multifunctional system is commonly found in actinomycetes (Walia et al. 2015), bacteria (Azeri et al. 2010), and fungal species (Driss et al. 2011) (Fig. 12.1).
12.4 Fungal Xylanases
Advancement in research on fungus that utilizes xylan, and on its substituted enzyme systems involved, is becoming more and more relevant in economic and ecological terms. Xylanases are synthesized by both thermophiles and mesophiles (Smith et al. 1991). The chief xylanase producers from fungal genera includes Aspergillus, Penicillium, Fusarium, Trichoderma, and Pichia (Yadav et al. 2018; Kavya and Padmavathi 2009; Sakthiselvan et al. 2014). White-rot fungi have been reported to synthesize extracellular xylanase which can act on broad range of hemicellulose materials such as the following: Coriolus versicolor synthesize mixture of xylanolytic enzyme and Phanerochaete chrysosporium synthesize α-glucuronidase in large amount (Castanares et al. 1995; El-Nasser et al. 1997). Among the mesophilic fungi, Trichoderma and Aspergillus are the two genera which are preeminent in xylanase production (Shah and Madamwar 2005; Alvarez-Zúñiga et al. 2017). In the past few decades, lots of steps and effort have been put to isolate extremophilic and thermophilic xylanase-producing bacteria of high stability (Monti et al. 2003; Bruins et al. 2001; Rizzatti et al. 2001; Maheshwari et al. 2000; Puchart et al. 1999; Niehaus et al. 1999; Andrade et al. 1999; Kalogeris et al. 1998). Various species of thermophilic fungi have been reported which include Thermoascus aurantiacus, Thermomyces lanuginosus, Talaromyces emersonii, Talaromyces byssochlamydoides, Paecilomyces variotii, Melanocarpus albomyces, Humicola grisea, Humicola lanuginosa, Humicola insolens, and Chaetomium thermophile (Ishihara et al. 1997; Polizeli et al. 2005; Li et al. 2011; Saxena et al. 2016).
All these species of xylanase-producing fungus retain temperature between 60 °C and 80 °C and are highly stable (Amir et al. 2013). Even the enzyme produced by archaea and eubacteria is stable at high temperature, but the amount of enzyme is comparatively low in comparison to fungi (Nigam 2013). Generally, the xylanase is more in fungal culture to that of bacteria and yeast. These are mostly glycoproteins and highly active at pH (4.5 to 6.5). They have molecular weight ranging from 6 to 38 kDa and exist in multiple forms (Chakdar et al. 2016). Although it has been also reported that the degree of structural homology is similar in endoxylanases of thermophiles and mesophiles (Collins et al. 2005b; Meruelo et al. 2012). Various authors put forth the reason behind the high stability of xylanases in thermophiles is mainly due to the presence of N-terminal proline which changes reduction in conformational freedom, extra disulfide bridges, salt bridges, and presence of hydrophobic sides (Wang et al. 2014; Panja et al. 2015). Later on, Hakulinen et al. (2003) studied that the thermal stability of xylanases is strictly based on the higher Thr/Ser ratio and the number of charged residues which results in enhance polar interactions.
From fungal kingdom, the genus Aspergillus is considered to be the potent producer of both β-D-xylosidase and xylanase enzyme, and moreover it has been well-characterized (Knob et al. 2010; Chakdar et al. 2016). These filamentous fungi are of industrial importance as synthesized xylanases are extracellular in nature. Additionally, fungal species have high yield in contrast to bacteria and yeast (Motta et al. 2013; Patel and Savanth 2015). On exploring xylan-degrading enzyme, many new enzymes with unique characteristics for microbes were discovered which attained the attention of industries for various applications (Nigam 2013; Anbu et al. 2017). Thermophilic fungi, unique microbes which are able to survive at high temperature, are generally associated with heaps of agricultural and forestry products. The colonization and distribution of thermostable fungal population present in the compost largely depend on a variety of degrading enzymes as fungal strains perform the enhanced function in lignocellulose waste on xylan present in it (Maheshwari et al. 2000; Singh et al. 2016a). Each enzyme has its specialized function as well as biological importance (Ali et al. 2017). Xylanases produced by thermophilic fungi which are active at alkaline pH have found their application in paper and pulp industry during bleaching process and eliminating the need of chlorine; as a result, the process is becoming eco-friendly (Raghukumar et al. 2004; Medeiros et al. 2007; Harris and Ramalingam 2010; Gangwar et al. 2014; Kumar et al. 2016a, b).
12.5 Xylanase Production
Two methods, i.e., solid-state and submerged fermentation, are most commonly used for the production of xylanases. It has been observed that production of enzyme is relatively high in solid-state fermentation (SSF) in comparison with submerged fermentation (Suman et al. 2015; Alberton et al. 2009; Ling Ho and Heng 2015). Therefore, in recent years, SSF has gained more attention by researcher because of commercial and engineering advantages (Subramaniyam and Vimala 2012). SSF can be executed on various lignocellulosic wastes like corncob, ragi bran, rice bran, soya bran, and wheat bran and have been found effective substrate for xylanase production (Kavya and Padmavathi 2009; Soccol et al. 2017). Thus, SSF is an effective method for xylanase synthesis, predominantly by fungal culture due to the advantages like high productivity at low cost as it produces xylanase by consuming cheap substrate, which serves as the carbon source as a resultant total cost of the process decreases (Harris and Ramalingam 2010; Walia et al. 2017). Therefore, in order to reduce the cost of xylanase synthesis, lignocellulosic waste can be used as substrate instead of pure xylans (Goyal et al. 2008; Motta et al. 2013).
12.6 Application of Xylanases
From the past few decades, the biotechnological and commercial use of xylanase enzymes has increased remarkably. The major applications of xylanases are in food industries, paper industries, feed industries, biofuel production, and pharmaceutical industries (Singh et al. 2016b; Yadav et al. 2015a, b; Pedersen et al. 2015; Ahlawat et al. 2007). Xylanases are also commercially produced in developed countries such as the USA, Canada, Denmark, the Republic of Ireland, Germany, Finland, and Japan (Bajpai 2014). The commonly used microorganisms used for this purpose include Humicola insolens, Aspergillus niger , and Trichoderma spp. (Polizeli et al. 2005; Harris and Ramalingam 2010). In the future, it might be used for the biodegradation of organic (Shukla et al. 2016; Kumar et al. 2017; Singh et al. 2017a, b, Kaur et al. 2017) and inorganic contaminants (Kumar et al. 2015a; Mishra et al. 2016; Singh et al. 2016b; Kumar et al. 2016a, Kumar et al. 2016b) such as pesticides (Kumar et al. 2013, 2014b) heavy metal, etc. (Kumar et al. 2014c. Kumar et al. 2015b). However, no study is reported till date. Before 1980, it was used in the preparation of the feeds for animals. Nowadays, xylanase along with cellulose and pectinase accounts for more than 20% of enzyme market worldwide (Choct 2006; M’hamdi et al. 2014; Sahay et al. 2017). Presently, some industries have put forth their interest in the development of various efficient enzymatic processes which could replace acid hydrolysis treatment of hemicellulose-containing material (Hu et al. 2011). The major application of xylanases in industries and their uses were described in Table 12.2.
Due to biotechnological potential of xylanase, it has aroused the great interest in the industrial sector like ethanol and xylitol synthesis in paper and cellulose industry and liquid fuel, cellular protein, and chemical production in food industry (Yadav et al. 2017a, b, c, d; Kulkarni et al. 1999; Guimaraes et al. 2013). Most of the agricultural waste comprises of cellulose and hemicellulose which needs to be converted in constituent sugar (Anwar et al. 2014; Saini et al. 2015). Waste synthesized by agro-industry and food industry is available in staggered amount all over the world and is becoming the health hazard (Kanimozhi and Nagalakshmi 2014). In order to utilize the waste, we require strategic planning and chemicals for hydrolyzing the constituent (Paritosh et al. 2017). Due to xylan being the major polymer in the plant structure, xylanases and microbes producing xylanase enzyme can be adapted for processing of food, paper pulp, sugar, ethanol, and agro-industries (Sridevi et al. 2016; Walia et al. 2017).
For the production of ethanol, first delignification of the lignocellulose biomass is required, followed by the hydrolysis of cellulose and hemicellulose polymer to monosaccharide sugar (Lee et al. 2014; Kumar and Sharma 2017). Hydrolysis can be conducted either by acid treatment at elevated temperature or action of enzyme. If the acid hydrolysis procedure is assessed in context to cost, it becomes expensive because of energy consumption and equipment (Woiciechowski et al. 2002; Timung et al. 2016; Amin et al. 2017). The lignocellulosic biomass comprises complex constituent that requires action of various enzymes like β-glucosidases, β-xylosidases, endoglucanases, and xylanases in synergistic manner for proper hydrolysis (Yeoman et al. 2010; Hu et al. 2011). Xylanase also has the application in paper and pulp industry for bleaching of kraft pulp (Azeri et al. 2010). Generally, xylanase documented till date is found to be effective at neutral pH 6 and temperature 50 °C (Chakdar et al. 2016). In enzyme associated with pulp bleaching process, the temperature and pH of incoming pulp are high, thus making the thermostable alkaline xylanase the enzyme of interest (Kumar et al. 2014a; Cunha et al. 2018a). Moreover, usage of xylanase in paper industry during bleaching processes decreased the usage of chemicals and gives enhanced brightness to paper (Sharma et al. 2017).
For various processes like juice clarification, extraction of coffee, plant oils, and starch requires the amalgam of pectinase, xylanase, and other enzymes (Goswami and Rawat 2015; Tallapragada and Venkatesh 2017). Xylanases have various potentials in various industries like paper, animal, food, and biofuel industries (Beg et al. 2001; Polizeli et al. 2005; Harris and Ramalingam 2010). During the formulation of feed, xylanase along with amylase, glucanase, and pectinase decreases the feed viscosity and elevates the nutrient adsorption. Generally, the nutrients are liberated by hydrolyzing the nondegradable fibers by enzyme, or they liberate the enzyme arrested by fibers (Mathlouthi et al. 2002).
In the last few decades, xylanolytic enzymes have also attained their importance in bread-making industry (Butt et al. 2008), in which non-starch and starch hydrolyzing enzyme is predominantly used for improving the bread quality. Xylanases have been reported to enhance tolerance of dough to diverse flour quality parameters as well as the amendment in processing methods (Ahmad et al. 2014; Cunha et al. 2018b). They make the dough softer, decrease the work supplies, and increase the quantity of leavened pan bread (Jaekel et al. 2012). These xylanolytic complexes have their role in textile industries for plant fiber processing in case of linen and hessian (Polizeli et al. 2005). Thus, the overall scenario favors and depicts that fungal xylanases have great potential and industrial advantages and in association with other enzymes can aid in gaining profit for industries (Walia et al. 2017; Kumar et al. 2018).
12.7 Cloning of Fungal Xylanase Genes
Advancement in recombinant DNA technology led to the selection of xylanase-producing bacteria which are more likely suitable for industrial applications (Singh et al. 2016b). The key challenge for this technology includes the production of xylanolytic systems and upgrading of fermentation characteristic of bacterial and fungal species by inserting genes for xylosidase and xylanase (Knob et al. 2014; Kapilan and Arasaratnam 2017). Filamentous fungi come in the category of xylanase producers which show both homologous and heterologous gene expression. Their promoter region expresses the enzymes with high yields. It’s not possible to attain particular enzyme in its pure form (Ahmed et al. 2009; Mustafa et al. 2016). Therefore, such technology can be applied to achieve such purposes. The genes coded for xylanases have been cloned in heterologous and homologous hosts with the intention to overproduce the enzyme and change its property to be best suited for industrial applications (Lambertz et al. 2014; Walia et al. 2017). Various genes have been cloned and expressed to enhance the production of enzymes, their specificity, substrate utilization, and other industrial applications. E. coli has been selected worldwide for heterologous or homologous expression of recombinant proteins and gene cloning in xylanase-producing organisms (Adrio and Demain 2014; Chakdar et al. 2016). This is due to its widespread cloning vectors, ease of DNA cloning, secretion of homologous proteins, and overproduction of recombinant proteins directly into the natural hosts. They are used since long times for production of recombinant enzymes either extracellularly or intracellularly (Walia et al. 2017). The major drawback of using E.coli as expression vector is that some of the proteins are not secreted efficiently (Rosano and Ceccarelli 2014).
However, E.coli has been found as virtuous host for recombinant protein for cloning xylanase genes and can be further used to carry out its gene structure (Reeves et al. 2000). Other microbes such as S. cerevisiae and P. pastoris are also used to secrete high amount of xylanase production in batch mode medium at low cost (Damaso et al. 2003; Shang et al. 2017). Due to high-expression characteristics, they both emerge as excellent host under its own promoters. One of the major drawbacks of both the species is its use in large-scale production and health hazards of methanol (Motta et al. 2013; Walia et al. 2017).
Usage of xylanases for various roles largely depends on the kinetics, pH stability, and optimum temperature (Liao et al. 2015). The recombinant xylanases synthesized by fungal and yeast strains have been reported to show equivalent or enhanced properties than the native enzymes. Thermostable enzymes are employed in the various processes in the industry, but propagation of thermostable microbes is found to be ineffective at large scale because of extreme fermentation conditions (Damaso et al. 2003; Kumar et al. 2016a, b). It has been reported that T. reesei and P. pastoris express the thermostable xylanase at a high level (Mellitzer et al. 2012; Huang et al. 2012). In the same way, anaerobic microbes also show the expression of xylanase and thus can be used in the fermentation industry. There are chances for unraveling the new strains of fungi which can produce recombinant xylanases (Motta et al. 2013; Nigam 2013).
Moreover, the advancement in the genetic engineering can help us to amend the fungal expression system for hyper-expression of the heterologous xylanase for production as well as industrial use. Sometimes, overexpression of recombinant proteins led to site-direct mutagenesis using recombinant technology (Kim et al. 2012; Lambertz et al. 2014). Lists of various fungal species along with their cloning vectors and hosts are depicted in Table 12.3.
12.8 Conclusions and Future Prospects
Xylanases have extensive range of application in various industries such as paper, pulp, animal feed, pharmaceutical, and pulp industries. Due to its varying properties of hydrolysis and low toxicity, they are also used in food industry. It also reduces load of chemical additives and emulsifiers in food industry. The current review shows that production of xylanases in large-scale production is still a challenging task. New approaches, such as consensus polymerase chain reaction screening of genome sequencing, functional approaches, and study of extremophilic enzymes, will further add new prospects to understand the other applications of the xylanase. There is also possibility of isolating new fungal species for producing recombinant xylanases. Using improved technical advancement systems, development of recombinant fungal expression systems by genetic approach will help in hyper-expression of xylanases and xylanase families for their production management at the industrial level.
References
Adrio JL, Demain AL (2014) Microbial enzymes: tools for biotechnological processes. Biomol Ther 4:117–139
Ahlawat S, Battan B, Dhiman SS, Sharma J, Mandhan RP (2007) Production of thermostable pectinase and xylanase for their potential application in bleaching of kraft pulp. J Ind Microbiol Biotechnol 34:763–770
Ahmad Z, Butt MS, Riaz M (2013) Partial purification and characterization of Xylanase produced from Aspergillus niger using wheat bran. Pak J Agric Sci 50:433–437
Ahmad Z, Butt MS, Ahmed A, Riaz M, Sabir SM, Farooq U, Rehman FU (2014) Effect of Aspergillus niger xylanase on dough characteristics and bread quality attributes. J Food Sci Technol 51:2445–2453
Ahmed S, Riaz S, Jamil A (2009) Molecular cloning of fungal xylanases:an overview. Appl Microbiol Biotechnol 84:19–35
Alberton LR, Vandenberghe LPDS, Assmann R, Fendrich RC, Rodriguéz-León J, Soccol CR (2009) Xylanase production by Streptomyces viridosporus T7A in submerged and solid-state fermentation using agro-industrial residues. Braz Arch Biol Technol 52:171–180
Ali SS, Wu J, Xie R, Zhou F, Sun J, Huang M (2017) Screening and characterizing of xylanolytic and xylose-fermenting yeasts isolated from the wood-feeding termite, Reticulitermes chinensis. PLoS One 12:e0181141
Alvarez-Zúñiga MT, Santiago-Hernández A, Rodríguez-Mendoza J, Campos JE, Pavón-Orozco P, Trejo-Estrada S, Hidalgo-Lara ME (2017) Taxonomic identification of the thermotolerant and fast-growing fungus Lichtheimia ramosa H71D and biochemical characterization of the thermophilic xylanase LrXynA. AMB Express 7:194
Amin FR, Khalid H, Zhang H, Rahman S, Zhang R, Liu G, Chen C (2017) Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 7:72
Amir A, Arif M, Pande V (2013) Purification and characterization of xylanase from Aspergillus fumigatus isolated from soil. Afr J Biotechnol 12(20):3049–3057
Andrade CM, Pereira N Jr, Antranikian G (1999) Extremely thermophilic microorganisms and their polymer-hidrolytic enzymes. Rev Microbiol 30:287–298
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy:a brief review. J Radiat Res Appl Sci 7:163–173
Apel PC, Panaccione DG, Holden FR, Walton JD (1993) Cloning and targeted gene disruption of XYL1, a beta 1, 4-xylanase gene from the maize pathogen Cochliobolus carbonum. Mol Plant-Microbe Interact 6:467–473
Apel-Birkhold PC, Walton JD (1996) Cloning, disruption, and expression of two endo-beta 1, 4-xylanase genes, XYL2 and XYL3, from Cochliobolus carbonum. Appl Environ Microbiol 62:4129–4135
Anbu P, Gopinath SC, Chaulagain BP, Lakshmipriya T (2017) Microbial enzymes and their applications in industries and medicine 2016. Biomed Res Int 2017:1–3
Azeri C, Tamer UA, Oskay M (2010) Thermoactive cellulase-free xylanase production from alkaliphilic Bacillus strains using various agro-residues and their potential in biobleaching of kraft pulp. Afr J Biotechnol 9(1):063–072
Bajpai P (2014) Xylanolytic enzymes. Academic Press, Amsterdam
Baraznenok VA, Becker EG, Ankudimova NV, Okunev NN (1999) Characterization of neutral xylanases from Chaetomium cellulolyticum and their biobleaching effect on eucalyptus pulp. Enzym Microb Technol 25:651–659
Bataillon M, Cardinali APN, Castillon N, Duchiron F (2000) Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain SPS-0. Enzym Microb Technol 26:187–192
Battan B, Sharma J, Dhiman SS, Kuhad RC (2007) Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzym Microb Technol 41:733–739
Beg QKM, Kapoor L, Mahajan G, Hoondal S (2001) Microbial xylanase from the newly isolated Bacillus sp. Strain BP-23. Can J Microbiol 39:1162–1166
Bibi Z, Ansari A, Zohra RR, Aman A, Qader SAU (2014) Production of xylan degrading endo-1, 4-β-xylanase from thermophilic Geobacillus stearothermophilus KIBGE-IB29. J Radiat Res Appl Sci 7:478–485
Brink J, Vries RP (2011) Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 6:1477–1492
Bruins ME, Janssen AE, Boom RM (2001) Thermozymes and their applications. Appl Biochem Biotechnol 90:155
Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46:22–31
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344:1879–1900
Carmona EC, Brochettobraga MR, Pizzirani kleiner AA, Jorge JA (1998) Purification and biochemical characterization of an endoxylanase from Aspergillus versicolor. FEMS Microbiol Lett 166:311–315
Castanares A, Hay AJ, Gordon AH, McCrae SI, Wood TM (1995) d-Xylan-degrading enzyme system from the fungus Phanerochaete chrysosporium. isolation and partial characterisation of an α-(4-O-methyl)-d-glucuronidase. J Biotechnol 43:183–194
Cesar T, Mrša V (1996) Purification and properties of the xylanase produced by Thermomyces lanuginosus. Enzym Microb Technol 19:289–296
Chadha BS, Ajay BK, Mellon F, Bhat MK (2004) Two endoxylanases active and stable at alkaline pH from the newly isolated thermophilic fungus, Myceliophthora sp. IMI 387099. J Biotechnol 109:227–237
Chakdar H, Kumar M, Pandiyan K, Singh A, Nanjappan K, Kashyap PL, Srivastava AK (2016) Bacterial xylanases: biology to biotechnology. 3 Biotech 6:150
Choct M (2006) Enzymes for the feed industry: past, present and future. Worlds Poult Sci J 62:5–16
Christakopoulos P, Nerinckx W, Kekos D, Macris B, Claeyssens M (1996) Purification and characterization of two low molecular mass alkaline xylanases from Fusarium oxysporum F3. J Biotechnol 51:181–189
Collins T, De Vos D, Hoyoux A, Savvides SN, Gerday C, Van Beeumen J, Feller G (2005a) Study of the active site residues of a glycoside hydrolase family 8 xylanase. J Mol Biol 354:425–435
Collins T, Gerday C, Feller G (2005b) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Cunha L, Martarello R, Souza PMD, Freitas MMD, Barros KVG, Ferreira Filho EX, Homem-de-Mello M, Magalhães PO (2018a) Optimization of xylanase production from Aspergillus foetidus in soybean residue. Enzyme Res 2018
Cunha CCDQB, Gama AR, Cintra LC, Bataus LAM, Ulhoa CJ (2018b) Improvement of bread making quality by supplementation with a recombinant xylanase produced by Pichia pastoris. PLoS One 13:e0192996
de Vries RP, Visser J (2001) Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65:497–522
Damaso MCT, Almeida MS, Kurtenbach E, Martins OB, Pereira N, Andrade CM, Albano RM (2003) Optimized expression of a thermostable xylanase from Thermomyces lanuginosus in Pichia pastoris. Appl Environ Microbiol 69:6064–6072
De Faria FP, Te'o VSJ, Bergquist PL, Azevedo MO, Nevalainen KMH (2002) Expression and processing of a major xylanase (XYN2) from the thermophilic fungus Humicola grisea var. thermoidea in Trichoderma reesei. Lett Appl Microbiol 34:119–123
Decelle B, Tsang A, Storms RK (2004) Cloning, functional expression and characterization of three Phanerochaete chrysosporium endo-1, 4-β-xylanases. Curr Genet 46:166–175
Dey P, Roy A (2018) Molecular structure and catalytic mechanism of fungal family G acidophilic xylanases. 3 Biotech 8:78
Dhillon A, Gupta JK, Jauhari BM, Khanna S (2000) A cellulase-poor, thermostable, alkalitolerant xylanase produced by Bacillus circulans AB 16 grown on rice straw and its application in biobleaching of eucalyptus pulp. Bioresour Technol 73:273–277
Driss D, Bhiri F, Elleuch L, Bouly N, Stals I, Miled N, Chaabouni SE (2011) Purification and properties of an extracellular acidophilic endo-1, 4-β-xylanase, naturally deleted in the “thumb”, from Penicillium occitanis Pol6. Process Biochem 46:1299–1306
Drzewiecki K, Angelov A, Ballschmiter M, Tiefenbach KJ, Sterner R, Liebl W (2010) Hyperthermostable acetyl xylan esterase. Microb Biotechnol 3:84–92
Dwivedi P, Vivekanand V, Ganguly R, Singh RP (2009) Parthenium sp. as a plant biomass for the production of alkalitolerant xylanase from mutant Penicillium oxalicum SAUE-3.510 in submerged fermentation. Biomass Bioenergy 33:581–588
El-Nasser NHA, Helmy SM, El-Gammal AA (1997) Formation of enzymes by biodegradation of agricultural wastes with white rot fungi. Polym Degrad Stab 55:249–255
Fernandez-Espinar M, Pinaga F, De Graaff L, Visser J, Ramón D, Vallés S (1994) Purification, characterization and regulation of the synthesis of an Aspergillus nidulans acidic xylanase. Appl Microbiol Biotechnol 42:555–562
Fujimoto H, Ooi T, Wang SL, Takizawa T, Hidaka H, Murao S, Arai M (1995) Purification and properties of three xylanases from Aspergillus aculeatus. Biosci Biotechnol Biochem 59:538–540
Gallardo O, Diaz P, Pastor FJ (2004) Cloning and characterization of xylanase A from the strain Bacillus sp. BP-7: comparison with alkaline pI-low molecular weight xylanases of family 11. Curr Microbiol 48:276–279
Gangwar AK, Prakash NT, Prakash R (2014) Applicability of microbial xylanases in paper pulp bleaching: a review. Bioresources 9:3733–3754
Georis J, Giannotta F, De Buyl E, Granier B, Frère JM (2000) Purification and properties of three endo-β-1, 4-xylanases produced by Streptomyces sp. strain S38 which differ in their ability to enhance the bleaching of kraft pulps. Enzym Microb Technol 26:178–186
Ghanem NB, Yusef HH, Mahrouse HK (2000) Production of Aspergillus terreus xylanase in solid-state cultures: application of the Plackett–Burman experimental design to evaluate nutritional requirements. Bioresour Technol 73:113–121
Ghareib M, El Dein MMN (1992) Purification and general properties of xylanase from Aspergillus terreus. Zentralbl Mikrobiol 147:569–576
Ghosh M, Nanda G (1994) Physiological studies on xylose induction and glucose repression of xylanolytic enzymes in Aspergillus sydowii MG49. FEMS Microbiol Lett 117:151–156
Goswami GK, Rawat S (2015) Microbial Xylanase and their applications-A review. Int J Curr Res Acad Rev 3:436–450
Goyal M, Kalra KL, Sareen VK, Soni G (2008) Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of Trichoderma viride. Braz J Microbiol 39:535–541
Guimaraes NCA, Sorgatto M, Peixoto-Nogueira SC, Betini JHA, Zanoelo FF, Marques MR, Moraes MDLT, Giannesi GC (2013) Bioprocess and biotechnology: effect of xylanase from Aspergillus niger and Aspergillus flavus on pulp biobleaching and enzyme production using agroindustrial residues as substrate. Springerplus 2:380
Hakulinen N, Turunen O, Jänis J, Leisola M, Rouvinen J (2003) Three-dimensional structures of thermophilic β-1, 4xylanases from Chaetomium thermophilum and Nonomuraea flexuosa: comparison of twelve xylanases in relation to their thermal stability. Eur J Biochem 270:1399–1412
Harris AD, Ramalingam C (2010) Xylanases and its application in food industry: a review. J Exp Sci 1:1–11
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Heo SY, Kim JK, Kim YM, Nam SW (2004) Xylan hydrolysis by treatment with endoxylanase and $\beta $-xylosidase expressed in yeast. J Microbiol Biotechnol 14:171–177
Hessing JG, Van Rotterdam CO, Verbakel JM, Roza M, Maat J, van Gorcom RF, van den Hondel CA (1994) Isolation and characterization of a 1, 4-β-endoxylanase gene of A. awamori. Curr Genet 26:228–232
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels 4:36
Huang Y, Chen Y, Mo D, Cong P, He ZY (2012) Attenuated secretion of the thermostable xylanase xynB from Pichia pastoris using synthesized sequences optimized from the preferred codon usage in yeast. J Microbiol Biotechnol 22:316–325
Iefuji H, Chino M, Kato M, Iimura Y (1996) Acid xylanase from yeast Cryptococcus sp. S-2: purification, characterization, cloning, and sequencing. Biosci Biotechnol Biochem 60:1331–1338
Inagaki K, Nakahira K, Mukai K, Tamura T, Tanaka H (1998) Gene cloning and characterization of an acidic xylanase from Acidobacterium capsulatum. Biosci Biotechnol Biochem 62:1061–1067
Ishihara M, Tawata S, Toyama S (1997) Purification and some properties of a thermostable xylanase from thermophilic fungus strain HG-1. J Ferment Bioeng 83:478–480
Ito K, Iwashita K, Iwano K (1992) Cloning and sequencing of the xynC gene encoding acid xylanase of Aspergillus kawachii. Biosci Biotechnol Biochem 56:1338–1340
Jaekel LZ, Silva CBD, Steel CJ, Chang YK (2012) Influence of xylanase addition on the characteristics of loaf bread prepared with white flour or whole grain wheat flour. Food Sci Technol 32:844–849
Jeffries TW (1996) Biochemistry and genetics of microbial xylanases Thomas W Jeffries. Curr Opin Biotechnol 7:337–342
Jørgensen H, Eriksson T, Börjesson J, Tjerneld F, Olsson L (2003) Purification and characterization of five cellulases and one xylanase from Penicillium brasilianum IBT 20888. Enzym Microb Technol 32:851–861
Juturu V, Wu JC (2012) Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30:1219–1227
Kalogeris E, Christakopoulos P, Kekos D, Macris BJ (1998) Studies on the solid-state production of thermostable endoxylanases from Thermoascus aurantiacus: characterization of two isozymes. J Biotechnol 60:155–163
Kanimozhi K, Nagalakshmi PK (2014) Xylanase production from Aspergillus niger by solid state fermentation using agricultural waste as substrate. Int J Curr Microbiol App Sci 3:437–446
Kapilan R, Arasaratnam V (2017) Industrial applications of bacterial xylanases: a review. Middle-East J Sci Res 25:79–89
Kaur P, Singh S, Kumar V, Singh N, Singh J (2017) Effect of rhizobacteria on arsenic uptake by macrophyte Eichhornia crassipes (Mart.) Solms. Int J Phytoremediation 20:114–120
Kaushik P, Mishra A, Malik A (2014) Dual application of agricultural residues for xylanase production and dye removal through solid state fermentation. Int Biodeterior Biodegrad 96:1–8
Kavya V, Padmavathi T (2009) Optimization of growth conditions for xylanase production by Aspergillus niger in solid state fermentation. Pol J Microbiol 58:125–130
Khanna P, Sundari SS, Kumar NJ (1995) Production, isolation and partial purification of xylanases from and Aspergillus sp. World J Microbiol Biotechnol 11:242–243
Kim IK, Roldão A, Siewers V, Nielsen J (2012) A systems-level approach for metabolic engineering of yeast cell factories. FEMS Yeast Res 12:228–248
Kimura I, Sasahara H, Tajima S (1995) Purification and characterization of two xylanases and an arabinofuranosidase from Aspergillus sojae. J Ferment Bioeng 80:334–339
Kimura T, Kitamoto N, Kito Y, Karita S, Sakka K, Ohmiya K (1998) Molecular cloning of xylanase gene xynG1 from Aspergillus oryzae KBN 616, a shoyu koji mold, and analysis of its expression. J Ferment Bioeng 85:10–16
Kimura T, Ito J, Kawano A, Makino T, Kondo H, Karita S, Sakka K, Ohmiya K (2000) Purification, characterization, and molecular cloning of acidophilic xylanase from Penicillium sp. 40. Biosci Biotechnol Biochem 64:1230–1237
Kimura T, Suzuki H, Furuhashi H, Aburatani T, Morimoto K, Sakka K, Ohmiya K (2002) Molecular cloning, characterization, and expression analysis of the xynF3 gene from Aspergillus oryzae. Biosci Biotechnol Biochem 66:285–292
Kitamoto N, Yoshino S, Ito M, Kimura T, Ohmiya K, Tsukagoshi N (1998) Repression of the expression of genes encoding xylanolytic enzymes in Aspergillus oryzae by introduction of multiple copies of the xynF1 promoter. Appl Microbiol Biotechnol 50:558–563
Kitamoto N, Yoshino S, Ohmiya K, Tsukagoshi N (1999) Purification and characterization of the overexpressed Aspergillus oryzae xylanase, XynF1. Biosci Biotechnol Biochem 63:1791–1794
Knob A, Terrasan CRF, Carmona EC (2010) β-Xylosidases from filamentous fungi: an overview. World J Microbiol Biotechnol 26:389–407
Knob A, Fortkamp D, Prolo T, Izidoro SC, Almeida JM (2014) Agro-residues as alternative for xylanase production by filamentous fungi. Bioresources 9:5738–5773
Kohli U, Nigam P, Singh D, Chaudhary K (2001) Thermostable, alkalophilic and cellulase free xylanase production by Thermoactinomyces thalophilus subgroup C. Enzym Microb Technol 28:606–610
Kolenová K, Vršanská M, Biely P (2005) Purification and characterization of two minor endo-β-1, 4-xylanases of Schizophyllum commune. Enzym Microb Technol 36:903–910
Kormelink FJM, Searle-Van Leeuwen MJF, Wood TM, Voragen AGJ (1993) Purification and characterization of three endo-(1, 4)-β-xylanases and one β-xylosidase from Aspergillus awamori. J Biotechnol 27:249–265
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4:7
Kumar M, Joshi A, Kashyap R, Khanna S (2011) Production of xylanase by Promicromonospora sp MARS with rice straw under non sterile conditions. Process Biochem 46:1614–1618
Kumar V, Upadhyay N, Singh S, Singh J, Kaur P (2013) Thin-layer chromatography: comparative estimation of soil’s atrazine. Curr World Environ 8(3):469–472
Kumar L, Kumar D, Nagar S, Gupta R, Garg N, Kuhad RC, Gupta VK (2014a) Modulation of xylanase production from alkaliphilic Bacillus pumilus VLK-1 through process optimization and temperature shift operation. 3 Biotech 4:345–356
Kumar V, Upadhyay N, Kumar V, Kaur S, Singh J, Singh S, Datta S (2014b) Environmental exposure and health risks of the insecticide monocrotophos—a review. J Biodivers Environ Sci 5:111–120
Kumar V, Singh S, Manhas A, Singh J, Singla S, Kaur P (2014c) Bioremediation of petroleum hydrocarbon by using Pseudomonas species isolated from petroleum contaminated soil. Orient J Chem 30:1771–1776
Kumar V, Singh S, Kashyap N, Singla S, Bhadrecha P, Kaur P (2015a) Bioremediation of heavy metals by employing resistant microbial isolates from agricultural soil irrigated with industrial waste water. Orient J Chem 31:357–361
Kumar V, Singh S, Singh J, Upadhyay N (2015b) Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull Environ Contam Toxicol 94:807–815
Kumar V, Kaur S, Singh S, Upadhyay N (2016a) Unexpected formation of N′-phenyl-thiophosphorohydrazidic acid O, S-dimethyl ester from acephate: chemical, biotechnical and computational study. 3 Biotech 6:1
Kumar V, Marín-Navarro J, Shukla P (2016b) Thermostable microbial xylanases for pulp and paper industries: trends, applications and further perspectives. World J Microbiol Biotechnol 32:34
Kumar V, Singh S, Singh R, Upadhyay N, Singh J (2017) Design, synthesis, and characterization of 2, 2-bis (2, 4-dinitrophenyl)-2-(phosphonatomethylamino) acetate as a herbicidal and biological active agent. J Chem Biol 10:179–190
Kumar V, Dangi AK, Shukla P (2018) Engineering thermostable microbial xylanases toward its industrial applications. Mol Biotechnol:1–10
Lambertz C, Garvey M, Klinger J, Heesel D, Klose H, Fischer R, Commandeur U (2014) Challenges and advances in the heterologous expression of cellulolytic enzymes: a review. Biotechnol Biofuels 7:135
Lee HV, Hamid SBA, Zain SK (2014) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014:1–20
Levasseur A, Asther M, Record E (2005) Overproduction and characterization of xylanase B from Aspergillus niger. Can J Microbiol 51:177–183
Li XL, Zhang ZQ, Dean JF, Eriksson KE, Ljungdahl LG (1993) Purification and characterization of a new xylanase (APX-II) from the fungus Aureobasidium pullulans Y-2311-1. Appl Environ Microbiol 59:3212–3218
Li XL, Skory CD, Ximenes EA, Jordan DB, Dien BS, Hughes SR, Cotta MA (2007) Expression of an AT-rich xylanase gene from the anaerobic fungus Orpinomyces sp. strain PC-2 in and secretion of the heterologous enzyme by Hypocrea jecorina. Appl Microbiol Biotechnol 74:1264–1275
Li DC, Li AN, Papageorgiou AC (2011) Cellulases from thermophilic fungi: recent insights and biotechnological potential. Enzyme Res 2011:1–9
Liao H, Zheng H, Li S, Wei Z, Mei X, Ma H, Shen Q, Xu Y (2015) Functional diversity and properties of multiple xylanases from Penicillium oxalicum GZ-2. Sci Rep 5:12631
Ling Ho H, Heng KL (2015) Xylanase production by Bacillus subtilis in cost-effective medium using soybean hull as part of medium composition under submerged fermentation (SmF) and solid-state fermentation (SsF). J Biodivers Biopros Dev 2:143
Liu X, Kokare C (2017) Microbial enzymes of use in industry. In: Biotechnology of microbial enzymes, Academic Press, Amsterdam, pp 267–298
Liu W, Zhu W, Lu Y, Kong J, Ma G (1998) Production, partial purification and characterization of xylanase from Trichosporon cutaneum SL409. Process Biochem 33:331–336
Liu N, Qin M, Gao Y, Li Z, Fu Y, Xu Q (2012) Pulp properties and fiber characteristics of xylanase-treated aspen APMP. Bioresources 7:3367–3377
M’hamdi N, Darej C, Jebali J, Bouraoui R, Metahni S, Frouja I, Singh DG, Jarboui I, Brar SK (2014) Different enzymes and their production. In: Enzymes in value-addition of wastes. Nova Science Publishers, Inc., New York, pp 109–132
Maheshwari R, Bharadwaj G, Bhat MK (2000) Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev 64:461–488
Mäntylä A, Paloheimo M, Hakola S, Lindberg E, Leskinen S, Kallio J, Suominen P (2007) Production in Trichoderma reesei of three xylanases from Chaetomium thermophilum: a recombinant thermoxylanase for biobleaching of kraft pulp. Appl Microbiol Biotechnol 76:377–386
Martínez-Anaya MA, Jiménez T (1998) Physical properties of enzyme-supplemented doughs and relationship with bread quality parameters. Z Lebensm Unters Forsch 206:134–142
Mathlouthi N, Saulnier L, Quemener B, Larbier M (2002) Xylanase, β-glucanase, and other side enzymatic activities have greater effects on the viscosity of several feedstuffs than xylanase and β-glucanase used alone or in combination. J Agric Food Chem 50:5121–5127
Mathur N, Goswami GK, Pathak AN (2015) In silico study of Bacillus brevis xylanase—structure prediction and comparative analysis with other bacterial and fungal xylanase. Int J Biomed Data Min 4:112
Medeiros RG, Silva FGD Jr, Báo SN, Hanada R, Ferreira Filho EX (2007) Application of xylanases from Amazon Forest fungal species in bleaching of eucalyptus kraft pulps. Braz Arch Biol Technol 50:231–238
Mellitzer A, Weis R, Glieder A, Flicker K (2012) Expression of lignocellulolytic enzymes in Pichia pastoris. Microb Cell Factories 11:61
Menon V, Prakash G, Rao M (2010) Value added products from hemicellulose: biotechnological perspective. Global J Biochem 1:36–67
Meruelo AD, Han SK, Kim S, Bowie JU (2012) Structural differences between thermophilic and mesophilic membrane proteins. Protein Sci 21:1746–1753
Mishra V, Gupta A, Kaur P, Singh S, Singh N, Gehlot P, Singh J (2016) Synergistic effects of Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in bioremediation of iron contaminated soils. Int J Phytoremediation 18:697–703
Monti R, Terenzi HF, Jorge JA (1991) Purification and properties of an extracellular xylanase from the thermophilic fungus Humicola grisea var. thermoidea. Can J Microbiol 37:675–681
Monti R, Cardello L, Custódio MF, Goulart AJ, Sayama AH, Contiero J (2003) Production and purification of an Endo–1, 4-beta-Xylanase from Humicola grisea var. thermoidea by electroelution. Braz J Microbiol 34:124–128
Moreira LRS, Campos MC, Siqueira PHVM, Silva LP, Ricart CAO, Martins PA, Queiroz RML, Filho EXF (2013) Two β-xylanases from Aspergillus terreus: characterization and influence of phenolic compounds on xylanase activity. Fungal Genet Biol 60:46–52
Morosoli R, Durand S, Letendre ED (1987) Induction of xylanase by β-methylxyloside in Cryptococcus albidus. FEMS Microbiol Lett 48:261–266
Motta FL, Andrade CCP, Santana MHA (2013) A review of xylanase production by the fermentation of xylan: classification, characterization and applications. In: Sustainable degradation of lignocellulosic biomass-techniques, applications and commercialization, Intech, London, United Kingdom
Murphy C, Powlowski J, Wu M, Butler G, Tsang A (2011, 2011) Curation of characterized glycoside hydrolases of fungal origin. Database:bar020
Mustafa G, Kousar S, Rajoka MI, Jamil A (2016) Molecular cloning and comparative sequence analysis of fungal β-Xylosidases. AMB Express 6:30
Nair SG, Shashidhar S (2008) Fungal xylanase production under solid state and submerged fermentation conditions. Afr J Microbiol Res 2:82–86
Nair SG, Sindhu R, Shashidhar S (2008) Purification and biochemical characterization of two xylanases from Aspergillus sydowii SBS 45. Appl Biochem Biotechnol 149:229–243
Natesh R, Bhanumoorthy P, Vithayathil PJ, Sekar K, Ramakumar S, Viswamitra MA (1999) Crystal structure at 1.8 Å resolution and proposed amino acid sequence of a thermostable xylanase from Thermoascus aurantiacus1. J Mol Biol 288:999–1012
Niehaus F, Bertoldo C, Kähler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 51:711–729
Nigam PS (2013) Microbial enzymes with special characteristics for biotechnological applications. Biomol Ther 3:597–611
Panja AS, Bandopadhyay B, Maiti S (2015) Protein thermostability is owing to their preferences to non-polar smaller volume amino acids, variations in residual physico-chemical properties and more salt-bridges. PLoS One 10:e0131495
Paritosh K, Kushwaha SK, Yadav M, Pareek N, Chawade A, Vivekanand V (2017) Food waste to energy: an overview of sustainable approaches for food waste management and nutrient recycling. Biomed Res Int 2017:2370927
Patel SJ, Savanth VD (2015) Review on fungal xylanases and their applications. Int J 3:311–315
Pedersen MB, Dalsgaard S, Arent S, Lorentsen R, Knudsen KEB, Yu S, Lærke HN (2015) Xylanase and protease increase solubilization of non-starch polysaccharides and nutrient release of corn-and wheat distillers dried grains with solubles. Biochem Eng J 98:99–106
Pérez-González JA, van Peij NN, Bezoen A, Maccabe AP, Ramón D, de Graaff LH (1998) Molecular cloning and transcriptional regulation of the Aspergillus nidulans xlnD gene encoding a β-xylosidase. Appl Environ Microbiol 64:1412–1419
Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591
Puchart V, Katapodis P, Biely P, Kremnický L, Christakopoulos P, Vršanská M, Kekos D, Macris BJ, Bhat MK (1999) Production of xylanases, mannanases, and pectinases by the thermophilic fungus Thermomyces lanuginosus. Enzym Microb Technol 24:355–361
Puls J, Sousa MVD, Ferreira Filho EX (1999) Purification and characterization of a low molecular weight xylanase from solid-state cultures of Aspergillus fumigatus Fresenius. Rev Microbiol 30:114–119
Qing Q, Wyman CE (2011) Hydrolysis of different chain length xylooliogmers by cellulase and hemicellulase. Bioresour Technol 102:1359–1366
Raghukumar C, Muraleedharan U, Gaud VR, Mishra R (2004) Xylanases of marine fungi of potential use for biobleaching of paper pulp. J Ind Microbiol Biotechnol 31:433–441
Rahman AS, Sugitani N, Hatsu M, Takamizawa K (2003) A role of xylanase, α-L-arabinofuranosidase, and xylosidase in xylan degradation. Can J Microbiol 49:58–64
Raj KC, Chandra TS (1996) Purification and characterization of xylanase from alkali-tolerant Aspergillus fischeri Fxn1. FEMS Microbiol Lett 145:457–461
Reeves RA, Gibbs MD, Morris DD, Griffiths KR, Saul DJ, Bergquist PL (2000) Sequencing and expression of additional xylanase genes from the hyperthermophile Thermotoga maritima FjSS3B. 1. Appl Environ Microbiol 66:1532–1537
Rizzatti ACS, Jorge JA, Terenzi HF, Rechia CGV, Polizeli MLTM (2001) Purification and properties of a thermostable extracellular β-D-xylosidase produced by a thermotolerant Aspergillus phoenicis. J Ind Microbiol Biotechnol 26:156–160
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5:172
Ryan SE, Nolan K, Thompson R, Gubitz GM, Savage AV, Tuohy MG (2003) Purification and characterization of a new low molecular weight endoxylanase from Penicillium capsulatum. Enzym Microb Technol 33:775–785
Saarelainen R, Paloheimo M, Fagerström R, Suominen PL, Nevalainen KH (1993) Cloning, sequencing and enhanced expression of the Trichoderma reesei endoxylanase II (pI 9) gene xln2. Mol Gen Genet 241:497–503
Sahay H, Yadav AN, Singh AK, Singh S, Kaushik R, Saxena AK (2017) Hot springs of Indian Himalayas: potential sources of microbial diversity and thermostable hydrolytic enzymes. 3 Biotech 7:1–11
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5:337–353
Sakthiselvan P, Naveena B, Partha N (2014) Molecular characterization of a Xylanase-producing fungus isolated from fouled soil. Braz J Microbiol 45:1293–1302
Saleem F, Ahmed S, Jamil AMER (2008) Isolation of a xylan degrading gene from genomic DNA library of a thermophilic fungus Chaetomium thermophile ATCC 28076. Pak J Bot 40:1225–1230
Salles BC, Cunha RB, Fontes W, Sousa MV (2000) Purification and characterization of a new xylanase from Acrophialophora nainiana. J Biotechnol 81:199–204
Salles BC, Te’o VS, Gibbs MD, Bergquist PL, Edivaldo Filho XF, Ximenes EA, Nevalainen KH (2007) Identification of two novel xylanase-encoding genes (xyn5 and xyn6) from Acrophialophora nainiana and heterologous expression of xyn6 in Trichoderma reesei. Biotechnol Lett 29:1195–1201
Saulnier L, Guillon F, Sado PE, Chateigner-Boutin AL, Rouau X (2007) Plant cell wall polysaccharides in storage organs: xylans (food applications) (2007):653–689
Saxena AK, Yadav AN, Rajawat M, Kaushik R, Kumar R, Kumar M, Prasanna R, Shukla L (2016) Microbial diversity of extreme regions: an unseen heritage and wealth. Indian J Plant Genet Resour 29:246–248
Segato F, Damásio AR, de Lucas RC, Squina FM, Prade RA (2014) Genomics review of holocellulose deconstruction by Aspergilli. Microbiol Mol Biol Rev 78:588–613
Selvarajan E, Veena R (2017) Recent advances and future perspectives of thermostable xylanase. Biomed Pharmacol J 10:261–279
Sepahy AA, Ghazi S, Sepahy MA (2011) Cost-effective production and optimization of alkaline xylanase by indigenous Bacillus mojavensis AG137 fermented on agricultural waste. Enzyme Res 2011:593624
Shah AR, Madamwar D (2005) Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochem 40:1763–1771
Shang T, Si D, Zhang D, Liu X, Zhao L, Hu C, Fu Y, Zhang R (2017) Enhancement of thermoalkaliphilic xylanase production by Pichia pastoris through novel fed-batch strategy in high cell-density fermentation. BMC Biotechnol 17:55
Sharma D, Agrawal S, Yadav RD, Mahajan R (2017) Improved efficacy of ultrafiltered xylanase–pectinase concoction in biobleaching of plywood waste soda pulp. 3 Biotech 7:2
Shukla L, Suman A, Yadav AN, Verma P, Saxena AK (2016) Syntrophic microbial system for ex-situ degradation of paddy straw at low temperature under controlled and natural environment. J Appl Biol Biotech 4:30–37
Singh S, Reddy P, Haarhoff J, Biely P, Janse B, Pillay B, Pillay D, Prior BA (2000) Relatedness of Thermomyces lanuginosus strains producing a thermostable xylanase. J Biotechnol 81:119–128
Singh RN, Gaba S, Yadav AN, Gaur P, Gulati S, Kaushik R, Saxena AK (2016a) First, high quality draft genome sequence of a plant growth promoting and cold active enzymes producing psychrotrophic Arthrobacter agilis strain L77. Stand Genomic Sci 11:54. https://doi.org/10.1186/s40793-016-0176-4
Singh S, Singh N, Kumar V, Datta S, Wani AB, Singh D, Singh J (2016b) Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ Chem Lett 14:317–329
Singh S, Kumar V, Upadhyay N, Singh J, Singla S, Datta S (2017a) Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and Fe(III)], and humic acid. 3 Biotech 7:262
Singh S, Kumar V, Chauhan A, Datta S, Wani AB, Singh N, Singh J (2017b) Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 16(1):211–237
Smith DC, Bhat KM, Wood TM (1991) Xylan-hydrolysing enzymes from thermophilic and mesophilic fungi. World J Microbiol Biotechnol 7:475–484
Soccol CR, da Costa ESF, Letti LAJ, Karp SG, Woiciechowski AL, de Souza Vandenberghe LP (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Innov 1:52–71
Song HY, Lim HK, Lee KI, Hwang IT (2014) A new bi-modular endo-β-1, 4-xylanase KRICT PX-3 from whole genome sequence of Paenibacillus terrae HPL-003. Enzym Microb Technol 54:1–7
Sridevi A, Sandhya A, Ramanjaneyulu G, Narasimha G, Devi PS (2016) Biocatalytic activity of Aspergillus niger xylanase in paper pulp biobleaching. 3 Biotech 6:165
Su X, Han Y, Dodd D, Moon YH, Yoshida S, Mackie RI, Cann IK (2013) Reconstitution of a thermostable xylan-degrading enzyme mixture from the bacterium Caldicellulosiruptor bescii. Appl Environ Microbiol 79:1481–1490
Subramaniyam R, Vimala R (2012) Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int J Sci Nat 3:480–486
Suman A, Verma P, Yadav AN, Saxena AK (2015) Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Res J Biotechnol 10:33–42
Szendefy J, Szakacs G, Christopher L (2006) Potential of solid-state fermentation enzymes of Aspergillus oryzae in biobleaching of paper pulp. Enzym Microb Technol 39:1354–1360
Takahashi Y, Kawabata H, Murakami S (2013) Analysis of functional xylanases in xylan degradation by Aspergillus niger E-1 and characterization of the GH family 10 xylanase XynVII. Springerplus 2:447
Talamantes D, Biabini N, Dang H, Abdoun K, Berlemont R (2016) Natural diversity of cellulases, xylanases, and chitinases in bacteria. Biotechnol Biofuels 9:133
Tallapragada P, Venkatesh K (2017) Isolation, identification and optimization of xylanase enzyme produced by Aspergillus niger under submerged fermentation. J Microbiol Biotechnol Res 1:137–147
Tan LUL, Wong KKY, Yu EKC, Saddler JN (1985) Purification and characterization of two D-xylanases from Trichoderma harzianum. Enzym Microb Technol 7:425–430
Taneja K, Gupta S, Kuhad RC (2002) Properties and application of a partially purified alkaline xylanase from an alkalophilic fungus Aspergillus nidulans KK-99. Bioresour Technol 85:39–42
Timung R, Naik Deshavath N, Goud VV, Dasu VV (2016) Effect of subsequent dilute acid and enzymatic hydrolysis on reducing sugar production from sugarcane bagasse and spent citronella biomass. J Energy 2016:1–12
Vogel K (2018) Analytics of enzymes. In: Enzymes in human and animal nutrition, Academic Press, Amsterdam, pp 441–455
Walia A, Mehta P, Guleria S, Chauhan A, Shirkot CK (2015) Molecular cloning and sequencing of Alkalophilic Cellulosimicrobium cellulans CKMX1 Xylanase gene isolated from mushroom compost and characterization of the gene product. Braz Arch Biol Technol 58:913–922
Walia A, Guleria S, Mehta P, Chauhan A, Parkash J (2017) Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech 7:11
Wang K, Luo H, Tian J, Turunen O, Huang H, Shi P, Hua H, Wang C, Wang S, Yao B (2014) Thermostability improvement of a Streptomyces xylanase by introducing proline and glutamic acid residues. Appl Environ Microbiol 80(7):2158–2165. https://doi.org/10.1128/AEM.03458-13
Winterhalter C, Liebl W (1995) Two extremely thermostable xylanases of the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl Environ Microbiol 61:1810–1815
Woiciechowski AL, Nitsche S, Pandey A, Soccol CR (2002) Acid and enzymatic hydrolysis to recover reducing sugars from cassava bagasse:an economic study. Braz Arch Biol Technol 45:393–400
Wong KK, Saddler JN (1992) Trichoderma xylanases, their properties and application. Crit Rev Biotechnol 12:413–435
Wong KK, Tan LUL, Saddler JN (1988) Multiplicity of beta-1, 4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305
Ximenes FA, Sousa MV, Puls J, Silva FG Jr, Filho EXF (1999) Purification and characterization of a low-molecular-weight xylanase produced by Acrophialophora nainiana. Curr Microbiol 38:18–21
Yadav AN (2015) Bacterial diversity of cold deserts and mining of genes for low temperature tolerance. Ph.D. Thesis, IARI, New Delhi/BIT, Ranchi pp. 234, doi: https://doi.org/10.13140/RG.2.1.2948.1283/2
Yadav AN, Sachan SG, Verma P, Saxena AK (2015a) Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J Biosci Bioeng 119:683–693
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015b) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31:95–108
Yadav AN, Kumar R, Kumar S, Kumar V, Sugitha T, Singh B, Chauhan VS, Dhaliwal HS, Saxena AK (2017a) Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J Appl Biomater Biomech 5:1–13
Yadav AN, Verma P, Kumar R, Kumar V, Kumar K (2017b) Current applications and future prospects of eco-friendly microbes. EU Voice 3(1):1–3
Yadav AN, Verma P, Kumar V, Sachan SG, Saxena AK (2017c) Extreme cold environments: a suitable niche for selection of novel psychrotrophic microbes for biotechnological applications. Adv Biotechnol Microbiol 2:1–4
Yadav AN, Verma P, Sachan SG, Saxena AK (2017d) Biodiversity and biotechnological applications of psychrotrophic microbes isolated from Indian Himalayan regions. EC Microbiol ECO 01:48–54
Yadav AN, Verma P, Kumar V, Sangwan P, Mishra S, Panjiar N, Gupta VK, Saxena AK (2018) Biodiversity of the genus Penicillium in different habitats. In: Gupta VK, Rodriguez-Couto S (eds) New and future developments in microbial biotechnology and bioengineering, Penicillium system properties and applications. Elsevier, Amsterdam, pp 3–18. https://doi.org/10.1016/B978-0-444-63501-3.00001-6
Yeoman CJ, Han Y, Dodd D, Schroeder CM, Mackie RI, Cann IK (2010) Thermostable enzymes as biocatalysts in the biofuel industry. In: Advances in applied microbiology, vol 70. Academic Press, Amsterdam/Boston, pp 1–55
Yoshino S, Oishi M, Moriyama R, Kato M, Tsukagoshi N (1995) Two family G xylanase genes from Chaetomium gracile and their expression in Aspergillus nidulans. Curr Genet 29:73–80
Youssefian S, Rahbar N (2015) Molecular origin of strength and stiffness in bamboo fibrils. Sci Rep 5:11116
Zhang YHP (2008) Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J Ind Microbiol Biotechnol 35:367–375
Ziaie-Shirkolaee Y, Talebizadeh A, Soltanali S (2008) Comparative study on application of T. lanuginosus SSBP xylanase and commercial xylanase on biobleaching of non wood pulps. Bioresour Technol 99:7433–7437
Acknowledgments
The authors are highly grateful to the Head of School, School of Bioengineering and Biosciences, Lovely Professional University, for providing necessary research facilities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, S., Sidhu, G.K., Kumar, V., Dhanjal, D.S., Datta, S., Singh, J. (2019). Fungal Xylanases: Sources, Types, and Biotechnological Applications. In: Yadav, A., Mishra, S., Singh, S., Gupta, A. (eds) Recent Advancement in White Biotechnology Through Fungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-10480-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-10480-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10479-5
Online ISBN: 978-3-030-10480-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)