Abstract
Xylanase being a hydrolytic enzyme catalyses the hydrolytic breakdown of 1,4-β-D-xylosidic linkages in xylan which is an important constituent of hemicellulose. Xylanases are hemicellulases required for depolymerization of xylans which are the second most bountiful polysaccharide occurring in nature after cellulose having plant origin. A broad range of organisms have been reported to produce xylanases that include several fungi, bacteria, protozoans, crustaceans, marine algae, insects, snails, gastropods, arthropods, several seeds and plants. Filamentous fungi have been documented to be the useful producers of xylanase because of ease of cultivation, extracellular secretion of enzymes, higher yield and industrial aspect. Fungal xylanases from Aspergillus species and Trichoderma species have been widely studied and characterized and are commercially utilized in bakery and food processing industries. Microbial xylanases have been reported to be single-chain glycoproteins having molecular masses usually 8–145 kDa and exhibit maximum activity in temperature range 40–60 °C. Thermostable xylanases are ideally suited for use in industrial applications because of numerous advantages over thermolabile xylanase such as ability to work in broad temperature range, better substrate utilization and ability to tolerate high temperature in processes as well as better shelf life. Xylanases have widespread utilization in diverse industries such as food industry, textile industry and in pulp and paper industry. Xylanases have emerged to be extremely beneficial in terms of enhancing the production of numerous fruitful products. Over the years the advancements in molecular tools and techniques have enabled the better understanding of regulatory mechanisms heading xylanase production, underlying mechanism of action of xylanases as well as more precise knowledge of xylanase gene. Such advancements have paved the way for better utilization of enzymes in a much broader sense in commercial sector. Xylanases have tremendous industrial applications in commercial sector either on their own or by associating with different enzymes in numerous processes like processing of pulp and fibres; saccharification of agricultural, industrial and municipal wastes; flour improvement for bakery products; pretreatment of forage crops and lignocellulosic biomass; as well as an alternate to treating the textile-cellulosic waste with sulphuric acid.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
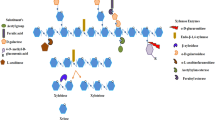
Xylanase (E.C 3.2.1.8) is an enzyme belonging to glucanase family and has a quite an expanding group of enzymes which can hydrolyse the 1,4-β-D-xylosidic linkages in xylan. Being a complex molecule, the depolymerization of xylan necessitates a cooperative action of different enzymes for its thorough disintegration. β-1,4-Endoxylanase, β-xylosidase, α-L-arabinofuranosidase, α-glucoronidase, acetyl xylan esterase and phenolic acid esterase occupy a significant place amongst the xylanases that have been reported extensively. According to Sharma and Kumar (2013), xylanase is a significant industrial enzyme that causes the random disintegration of xylan by its endo-1,4-xylanase activity and produces xylose, xylooligosaccharides and xylobiose. Amongst these xylanases, endo-1,4- xylanases (1,4-β-D-xylan xylanohydrolase, E.C.3.2.1.8) catalyse xylan depolymerization by randomly hydrolysing xylan backbone. Whistler and Masek (1955) reported xylanase for the first time. According to Bastawde (1992), the importance of xylanases is discovered over 100 years ago by Hoppe-Seyler. Xylanases were primarily termed pentosanases and were recognized by International Union of Biochemistry and Molecular Biology (IUBMB) for the first time in 1961. The systematic name of xylanases is endo-β-1,4-xylanase, but more widely accepted and universally used synonyms of the enzyme include xylanase, endo-1,4-β-D-xylanase, endoxylanase, β-1,4-D-xylan xylanohydrolase, β-xylanase and β-1,4-xylanase, respectively. A number of key factors govern the yield of xylanases in fermentation that include accessibility of substrate, rate and extent of disentangling of the xylooligosaccharides, etc. Omar et al. (2008) reported that xylanase holds significant importance due to its proficiency of degrading the plant cell wall constituents. Xylanase has attained paramount industrial importance credited to their multidimensional and multifunctional role in fermentation processes and numerous other industries.
2 Substrates for Xylanase
Hemicelluloses are the major substrates for xylanases. The word hemicelluloses points to polysaccharides occurring in plant cell wall that are confederated with cellulose and glucans. Hemicelluloses are the second most bountiful constituents in cell wall of plants behind cellulose (Nakamura 2003). Hemicelluloses consist of complex of polymeric carbohydrates that include xylan, glucomannan, xyloglucan, galactoglucomannan and arabinogalactan (Shallom and Shoham 2003). Classification of such polymeric carbohydrates relies upon the type of sugar entities present in their structure. Xylan is a major hemicellulose composed of xylose units interlinked through β-1,4-glycosidic linkage. Xylan has been reported to be the second most abundant polysaccharide behind cellulose that is loaded with a huge potential of being converted into a majority of products having paramount importance. Saha (2003) reported that xylan holds for nearly one third of entire replenishable organic carbon present on our planet. Xylan has a complex structure and heterogenic nature. Thus on account of this, xylanase plays a prodigious role in the combination of hydrolytic enzymes obligatory for the thorough disintegration of xylan (Takahashi et al. 2013). Xylan is a heterogenic polysaccharide comprising of xylose units interlinked through β-1,4-glycosidic bonds. Whistler and Richards (1970) reported that xylan major chain is built up of β-xylopyranose units. Xylan predominantly occurs in the secondary cell wall, and it constitutes the most part of the polymeric fraction of plant cell wall in association with lignin and cellulose. Xylan provides integrity to the cell wall by virtue of its association between lignin and cellulose through covalent and noncovalent bonds (Motta et al. 2013).
2.1 Structural Framework and Distribution of Xylan
Xylan being a convoluted heteropolysaccharide has an eminently branched structure that differs remarkably amidst distinctive plant species. On the grounds of common substituents occurring on the xylan backbone, xylans have been categorized into linear homoxylan, arabinoxylan, glucuronoxylan and glucuronoarabinoxylan. However as a matter of fact, there occurs microheterogeneity in each category of the xylan in relevance to the extent and characteristic of branching. The occurrence of side chains in the substituted forms of xylan determines several aspects that include physical configuration, degree of solubility, mode of the enzyme action, etc. (Motta et al. 2013). On account of its intricate structure and heterogenic nature, the thorough depolymerization of xylan necessitates a cooperative action of several enzymes (Subramaniyan and Prema 2002). Xylan has been reported to be distributed in a vast variety of tissues and cells and occurs in the major fraction of plant species. Singh et al. (2003) reported that xylan is known to exist up to significant levels in hardwoods of angiosperms (15–30%) and softwoods of gymnosperms (7–10%) and also in annual plants (<30%). Wood xylan predominantly occurs as O-acetyl-4-O-methylglucuronoxylan in hardwoods and in softwoods as arabino-4-O-methylglucuronoxylan. However xylans occurring in grasses and annual plants are generally arabinoxylans (Kulkarni et al. 1999). Xylan has a similarity to other polysaccharides having plant origin with respect to vast polydiversity and polymolecularity (Sunna and Antranikian 1997). The extent of polymerization in xylans also has significant variability, for instance, hardwood xylans generally consist of 150–200 β-xylopyranose units, whereas softwood xylans generally consist of 70–130 β-xylopyranose units (Kulkarni et al. 1999). D-Xylans hold for 20–35% of entire dry weight in hardwood and annual plants and predominantly exist as the most prevalent non-cellulosic polysaccharides (Velkova et al. 2007).
2.2 Enzymatic Disintegration of Xylan
On account of its complex structure and heterogenic nature, thorough disintegration of plant xylan necessitates the cooperative involvement of a multienzyme hydrolytic complex having broad spectrum of activities and diverse approaches of action. The main enzymes participating in multienzyme hydrolysis of xylan include β-xylosidase, endoxylanase, acetyl xylan esterase, arabinofuranosidase, glucuronidase, galactosidase and feruloyl esterase. These enzymes act in a collegial fashion to depolymerize xylan into its monomeric units (Belancic et al. 1995). Endoxylanases hold foremost place amongst all xylanases on account of their direct participation in the cleavage of glycosidic bonds as well as in liberating short xylooligosaccharides (Verma and Satyanarayana 2012).
Xylanase randomly hydrolyses xylans into xylooligosaccharides, whereas β-xylosidase dislodges xylose units from the non-reducing ends of xylooligosaccharides. Despite this, thorough disintegration needs the activity of acetyl esterase to dislodge the acetyl substituents from the D-xylose backbone (β-1,4-linked) of xylan (Coughlan and Hazlewood 1993).
3 Xylanase Sources
Xylanase sources range from microorganisms like bacteria, fungi, protozoans, actinomycetes, crustaceans, marine algae, insects, snails, gastropods, arthropods, several seeds and plants that tears the glycosidic linkages in xylans thus resulting in hemi acetyls and glycans (Motta et al. 2013). Certain invertebrate organisms such as earthworms also possess a broad spectrum of fibrolytic microbes in their gut. Thus it is quite possible that a few amongst these organisms might be the producers of novel endoxylanases (Park et al. 2007). Rumen is one of the most fascinating sources of xylanases wherein the effective hydrolysis of plant polysaccharides commences. Filamentous fungi have been documented to be the useful xylanase producers because of ease of cultivation, extracellular secretion of enzymes, higher yield and industrial aspect. Fungal xylanases from Aspergillus species and Trichoderma species have been widely and thoroughly studied and characterized and are commercially utilized in bakery and food processing industries. Certain microorganisms such as Aspergillus and Penicillium have been documented to efficiently synthesize xylanases in the presence of cheaper hemicellulosic substrates such as rice bran, bagasse corn stalk, corn cob, wheat bran and rice straw. White rot fungus is extensively utilized in manufacturing pharmaceutical products, in food industries and in the manufacturing of cosmetics (Qinnhe et al. 2004). White rot basidiomycetes conducts its depolymerization action on lignocellulosic materials through the enzymatic action of several xylanase enzymes. Several bacterial strains including Bacillus sp. have also been reported to be the efficient producers of thermostable xylanases having good stability at varying range of temperature, pH, presence of metal ions, etc. (Battan et al. 2007).
3.1 Fungal Xylanases
Fungal xylanases from genera Aspergillus, Trichoderma, Pichia and Fusarium species have been considered as the efficient and producers of novel xylanases (Absul et al. 2005). Other efficient fungal species producing xylanases include Streptomyces (Kansoh et al. 2001) Aspergillus kawachii (Ito et al. 2000) and Cunninghamella subvermispora (Ferraz et al. 2004). Haq et al. (2002) documented Aspergillus niger as the most potent xylanase producer.
3.2 Bacterial Xylanases
Bacterial species producing high activity xylanases at alkaline pH and higher temperature are Bacillus sp. (Subramaniyan et al. 2001). Main bacterial genera that are efficient producers of novel xylanases are Bacillus halodurans (Ebrahimi 2010), Saccharopolyspora pathunthaniensis (Verma and Satyanarayana 2012), Thermotoga sp. (Yoon et al. 2004), Bacillus circulans D1(Bocchini et al. 2005), Pseudomonas sp. XPB-6 (Sharma and Chand 2012), Stenotrophomonas maltophilia (Raj et al. 2013) and Bacillus genus (Kaur et al. 2015). Certain filamentous bacterial strains documented to be the efficient producers of endoxylanases, xylanase and polygalacturonase are Streptomyces sp., S. roseiscleroticus and S. cuspidosporus (Maheswari and Chandra 2000).
3.3 Thermophilic Xylanases
A good proportion of xylanase-producing thermophilic and hyperthermophilic microorganisms have been reported and successfully explored from a vast variety of sources including thermal springs, self-heating decaying organic debris, hot pools and terrestrial and marine solfataric fields (Sunna and Bergquist 2003). Family 10 xylanases have been successfully explored from a diverse range of thermophilic and hyperthermophilic organisms, including Thermoascus aurantiacus (Khasin et al. 1993) and Nonomuraea flexuosa (Hakulinen et al. 2003). Xylanases from Dictyoglomus thermophilum and Nonomuraea flexuosa have been reported amongst the most stable xylanases and possess temperature optima of 80 °C and 85 °C, respectively. Certain hyperthermophilic archaea have also been reported to be the efficient producers of novel xylanases, e.g. Pyrodictium abyssi (Andrade et al. 1999), Thermofilum strains (Andrade et al. 1999), Pyrococcus furiosus (Cady et al. 2001), Thermococcus zilligii (Cady et al. 2001), Sulfolobus solfataricus (Cannio et al. 2004) and T. leycettanus (Wang et al. 2016) (Tables 19.1, 19.2, and 19.3).
4 Classification of Xylanases
Xylanases can be categorized into two separate groups: (1) one possessing low molecular mass (<30 kDa) and basic pI and (2) another one possessing high molecular mass (>30 kDa) and acidic pI (Wong et al. 1998). Also on the basis of primary sequence homology, xylanases have been categorized into two distinct families (family F or 10 and family G or 11). Xylanase under family F has lower pI values as compared to family G. Earlier in a study, family 10 xylanases have been reported to be more complex and diverse having high molecular mass (>30 kDa) (Ducros et al. 2000). In contrast family 11 xylanases are much simpler, have consistency in their structure, have greater specificity for xylan and possess low molecular mass (>20 kDa) in comparison to family 10 xylanases. Woodward (1984) reported that three distinct types of xylanases are known for their involvement in xylan degradation.
4.1 Endo-1,4-β-xylanase (1,4-β-D-Xylan Xylanohydrolase)
Endo-1,4-β-xylanase (1,4-β-D-xylan xylanohydrolase; EC 3.2.1.8) disunites the glycosidic linkages in the xylan backbone thus leading to reduced substrate polymerization. Xylan is not degraded randomly. As a matter of fact, the xylan hydrolysis relies upon the attributes of the substrate molecule, i.e. upon the chain length, presence of substituents and extent of branching (Li et al. 2000). Xylanase categorizes themselves into two distinct types on the grounds of type of end products of the reaction: (a) non-debranching or non-arabinose liberating and (b) branching or arabinose liberating.
-
(a)
Non-debranching endoxylanases: Non-debranching or non-arabinose liberating endoxylanases are those endoxylanases that can be compartmentalized into two distinct variants, one giving off xylose and xylobiose as the end products and the other one giving off xylooligosaccharides as the end product.
-
(b)
Branching endoxylanases: Branching or arabinose liberating endoxylanases can be compartmentalized into two discrete groups: group 1 that possess the capability to hydrolyse branching points hereby giving off xylooligosaccharides and arabinose as the end products and group 2 that cleaves off the xylan and branching points thereby giving off principally xylobiose, xylose and arabinose, respectively.
4.2 Exo-1, 4-β-xylanase (β-1,4-D-Xylan Xylohydrolase)
These enzymes discharge the single xylose units from the non-reducing end of the xylan chain.
4.3 β-D-Xylosidase (1,4-β-D-Xylan Xylohydrolase)
β-D-Xylosidases (1,4-β-D-xylan xylohydrolase; EC 3.2.1.37) are exoglycosidase that actively depolymerizes short xylooligosaccharides to give off xylose. β-D-Xylosidases can be classified on the grounds of their relative fondness towards xylobiose and larger xylooligosaccharides, respectively. Octavio et al. (2006) documented that a huge fraction of bacteria and fungi efficiently produce such xylanases. They may show their existence in the culture broth surrounding the cell, in alliance with the cell, or they may also exist in both. β-D-Xylosidases play a significant role in lowering the end product inhibition of endoxylanases which is a rate-limiting factor in xylan depolymerization (Andrade et al. 2004).
5 Mechanism of Xylanase Action
Numerous stereotypes have been put forward to elucidate the action mechanism of xylanases. Subramaniyan and Prema (2002) reported that xylanase action eventually results in the hydrolysis of xylan that may cause retention or inversion of the anomeric centre of the reducing sugar monomer of the xylan thus giving an intimation of one or two chemical transition states being involved. Transfer of glycosyl eventually causes nucleophilic substitution at the saturated carbon of the anomeric centre and commences with either retention or inversion of the anomeric configuration. A great majority of hydrolytic enzymes like xylanases and cellulases that are well recognized for hydrolysing polysaccharides eventually result in the hydrolysis of their corresponding substrates with the retention of the C1 anomeric configuration. Double displacement mechanism has been reported to be directly indulged in the anomeric retention of product (Clarke et al. 1993).
6 Xylanase Production
The main driving force behind search for novel xylanases is the broader range of its tremendous industrial applications. Both solid-state fermentation (SSF) system and submerged fermentation (SMF) system can be successfully utilized. Xylanase production can be efficiently carried out using solid-state cultivation systems and submerged cultures methods. Submerged fermentation (SMF) technique has been the method of choice by most of researchers because of easier regulation of various process parameters such as pH, temperature of medium, degree of aeration as well as several environmental factors indispensable for the optimal growth of microorganisms. However as a matter of fact, solid-state fermentation has procured significant attention and acceptance from the researchers worldwide over the years and has been successfully and widely utilized for xylanase synthesis (Haltrich et al. 1996). This is credited to numerous economic and engineering advantages. Submerged fermentation (SMF) system has been preferred over solid-state fermentation (SSF) system for products involving large-scale production because the yield of enzyme is higher (about 90%) and also more cost-effective as compared to solid-state fermentation (Gouda 2000). SMF has the advantage over solid-state fermentation system in extracting a large fraction of purified enzymes. Wheat bran came out to be the best carbon source in the studies conducted on xylanase production by Stenotrophomonas maltophilia after using commercial xylans and different agro-industrial residues (Raj et al. 2013). Bacillus arseniciselenatis DSM-15340 resulted in a thermoalkalophilic cellulose-free xylanase in significant level, while it was grown in solid-state conditions by utilizing economically accessible agro-residual substrate wheat bran. Thus it could be efficiently utilized for production of xylanase on large scale by utilizing such agro-residual substrates (Kamble and Anandrao 2012). SSF conditions are exclusively favourable for the fungal growth since these organisms possess the ability to grow at rather low water activities in contrast to most of the bacteria and yeast that do not grow and proliferate efficiently in such culture environment. Mushimiyimana et al. (2015) reported that xylanolytic enzymes are efficiently produced by fungi under submerged conditions. Microbes such as Trichoderma, Aspergillus, Phanerochaete, Streptomyces, Clostridia, Ruminococcus, Chytridiomycetes, Bacillus and Fibrobacteres are loaded with huge potential to efficiently produce xylanase enzymes (Qinnhe et al. 2004).
7 Characterization and Purification
Xylanases from distinct sources vary significantly in their temperature and pH optima for the maximal activity. Concentrated and pure enzymes exhibit enhanced activity and reduced risk of inhibitory substances thus putting forward themselves as ideal materials having tremendous potential for use in industrial applications. Standard column chromatography, size exclusion chromatography and ion exchange chromatography are principally utilized techniques for purification of xylanases. Dean et al. (1991) reported that low molecular weight of xylanases has rendered their successful segregation from other proteins utilizing ultra filtration technique. Widjaja et al. (2009) purified cellulase-free xylanase from Aspergillus niger and Trichoderma reesei using ion exchange chromatography. The effective utilization of xylanase for the treatment of pulp fibres demands cellulase free xylanase. Goulart et al. (2005) successfully produced cellulase-free xylanase by utilizing Rhizopus stolonifer cultured on wheat bran. This cellulase-free xylanase exhibited optimum activity at pH 6.0 and temperature 45 °C, respectively. For maximal xylanase activity, pH 5.5 and temperature 60 °C were documented as most appropriate conditions by Huang and Penner (1991). Coelho and Carmona (2003) documented that xylanases are significantly thermostable within the pH range 4.5–10.5. Camacho and Aguilar (2003) documented a molecular weight of 22 kDa for xylanase from Aspergillus sp. Sardar et al. (2000) reported a molecular weight of 24 kDa for purified xylanase upon SDS-PAGE. Yasinok et al. (2010) reported the 186-fold purification of xylanase from Bacillus pumilus SB-M13A by hydrophobic interaction.
8 Xylanase Immobilization
Pioneer immobilization reaction was executed for introduction of reactive groups onto inert glass surface so as to increase the accessible surface area for immobilization. Therefore activation of glass beads was undertaken. Free xylanase exhibited optimum activity at pH 5.0 and 35 °C temperature. Crude enzyme was immobilized onto glass beads by physical adsorption binding. Immobilized enzyme can be reused two to three times under assay conditions. The free and immobilized xylanase activity was assayed at different pH of buffer (0.1 M) ranging from 4.0 to 8.0 and at various temperatures (35–65 °C) to determine the optimum activity under reaction conditions. The immobilized xylanase was tested for its reusability using 1 g of immobilized support repeatedly up to four times and percent relative activity determined (Kumar et al. 2014).
9 Industrial Aspects and Applications of Xylanases
Xylans and xylanases have witnessed significant rise in their biotechnological values and have shown remarkable rise in their use. The end products of xylan degradation, furfural and xylitol have gained remarkable utility in industrial applications (Parajo et al. 1998). The industrial utilization of xylanase commenced in the 1980s. Initially xylanases were used in animal feed preparation. In the successive years, they were significantly utilized in the food, textile and paper industries, respectively. Xylanases are predominantly employed in food industry to quicken the baking process of cookies, crackers, cakes as well as various other foods since they depolymerize the polysaccharides in the dough into corresponding monomeric units.
9.1 Bioprocessing of Fibres
Modern research centres on substituting the hazardous chemicals with the commercial enzymes that can precisely act upon the non-cellulosic and hemicellulosic impurities are still sustaining the quality as well as upholding the production yields of textile industries (Dhiman et al. 2008). Treatment with enzyme can apparently strengthen the water soaking characteristics of fibres by eradicating the complex impurities present in the primary cell wall. (Saha 2000) reported that utilization of pure and thermostable xylanase for pretreatment of low quality jute fibers for selectively removing xylan is entrancing. Plant fibres such as linen can be effectively processed by utlizing the xylanolytic enzyme complex. This method has an advantage that the step involving usage of strong bleaching step is bypassed as lignin won’t face oxidation which would have darkened the fibres (Csiszar et al. 2001). Xylanase precisely acts upon the hemicellulosic impurities and effectively eradicates them. Such enzymatic treatment do not create any harm to the fibre in terms of loss in fibre strength (Dhiman et al. 2008).
9.2 Biobleaching of Pulp and Paper
Treatment of the paper pulp with xylanase effectively hydrolyses the hemicellulosic chain amidst cellulose and lignin thereby eliminating the loosely held lignin from the desired cellulose. Xylanases have turned out to be a valuable as well as cost-effective asset for mills to have an edge over a vast number of bleaching benefits (Bajpai 2012). In this context, it lessens the discharge of organochlorine pollutants, for instance, dioxin, ultimately resulting in chlorineless bleaching without posing any detrimental harm to the paper’s strength (Li and Hardin 1998). The major utilization of xylanases in commercial sector is in cellulose pulp bleaching. The usage of enzymes commenced in this context ever since peroxidases were employed for degrading the lignin (Sandrim et al. 2005). Dhillon and Khanna (2000) reported chemical process is preferred over enzymatic hydrolysis method for paper production in several countries, including Brazil. The routinely used method is popularly called as the kraft process. The paper production process involves chemical pulping as the pioneer step which is characterized by the breakdown of fibres and removal of majority of lignin fraction (Hong et al. 1989). Pulp bleaching can be represented as a purification process which involves the attributes such as destruction as well as the alteration or solubilization of the coloured organic matters, lignin and other inadmissible leftovers on the fibres (Madlala et al. 2001). The efficacy as well as efficiency of microbial xylanase in context of bleaching process has been well studied for Streptomyces galbus (Kansoh and Nagieb (2004), Bacillus pumilus (Duarte et al. 2003), etc. The optimum pH of bacterial xylanases in common is somewhat uplifted compared to optimum pH of fungal xylanases (Khasin et al. 1993), which is a desirable asset in majority of paper and pulp industries.
9.3 Significant Role in Improving the Animal Feed
Xylanase is well recognized to play a significant role in uplifting the quality of animal feed. Xylanase treatment lessens the viscosity of the fodder thereby rendering the fodder readily digestible by the animal gut. It significantly elevates propagation of the pancreatic enzymes into the food thereby boosting the overall absorption of the nutrients. Gilbert and Hazlewood (1993) documented utility of xylanase in enhancing the digestibility of ruminant feeds and also in speeding up the composting process. Xylanases have been well documented for their utilization in animal feed in association with cellulases, amylases, galactosidases, glucanases, lipases, pectinases, proteases and phytases. Twomey et al. (2003) reported that these enzymes depolymerize arabinoxylans present in the constituents of the feed thereby rendering the raw material less viscous. Young fowl and swine synthesize endogenous enzymes in comparatively lesser amount as compared to adults, so that food supplements loaded with exogenous enzymes should uplift their performance as livestock. Furthermore, such diet has been found to cut down the undesirable leftovers in the excreta (nitrogen, zinc, copper and phosphorus), an effect that might play a productive role in scaling down of the environmental contamination (Polizeli et al. 2005). Addition of xylanase to feed comprising of low viscosity foods like maize and sorghum may enhance the digestion and absorption of nutrients in the foremost part of the digestive tract eventually resulting in an improved use of energy (Van Paridon et al. 1992).
9.4 Pharmaceutical and Chemical Applications of Xylanase
The end product of xylan hydrolysis, xylitol a polyalcohol, is as an artificial sweetener that is significantly used in candies, chewing gums and several other food items (Parajo et al. 1998). Xylanases are sometimes added as a cocktail (mixture) of enzymes comprising of proteases, hemicellulases and various other enzymes as a dietary supplement or as a measure to cure weak digestion. Xylitol being a noncarcinogenic sweetener is highly suited for individuals suffering with diabetes and obesity. Xylitol is also recommended in cases such as lipid metabolism disorder and respiratory infections, for the prevention of osteoporosis as well as for persons suffering with kidney and parental lesions. A vast variety of commercially available products such as candies, chewing gums and several other food products are known to have xylitol as the artificial sweetener. The advancements in xylitol production technology have facilitated a way for its utilization in a broad sense in the food, odontological and pharmaceutical (Nigam and Singh 1995). The production of ecofriendly biological fuels like bioethanol is witnessing a significant hike as the other available energy sources are depleting continuously. Moreover most of the fuels currently in use generate high levels of toxic aerosols and other polutants that pose several health hazards. The products of xylan hydrolysis can be efficiently transformed into important biological fuels such as ethanol (Sun and Cheng 2002).
9.5 Applications in Recycling of Waste Paper
Xylanases have also proved their utility in recycling of waste paper. The recycling is principally accomplished via two-staged processes, i.e. pulping and beating. Stage one essentially consists of the sundering of fibre or fibre dissemination. The entire exercise is called as hydrating process. The foremost step in enzymatic treatment primarily consists of prefatory soaking of paper followed by enzyme incubation. However stage two essentially consists of mechanical shearing of pulp, subsequent heating of pulp with a purpose of disaggregation of fibres and deactivating the enzyme. Xylanase treatment accounts for dislodging numerous reducing sugars from waste paper pulp. The release of reducing sugars is directly correlated to temperature probably on grounds that elevated temperature hydrolyses the majority of xylans held amidst the pulp fibres. Enzymatic treatment eventually promotes the swelling of pulp fibres, which aids in further processing of the pulp material as well as significantly upgrades its physical properties (Kenealy and Jeffries 2003).
9.6 Applications of Xylanases in Food, Bread and Drinks Production
The industrial applications of xylanases have witnessed a significant rise in last few decades credited to their potent efficacy in bread making process (Butt et al. 2008). The usage of starch- as well as non-starch-hydrolysing enzymes is a common practice in bread making industry for uplifting the quality and texture of bread (Javier et al. 2007). Like other hemicellulases, xylanases act in a similar manner thereby depolymerizing the hemicellulose existent in wheat flour thus assisting in uniform circulation of water thereby rendering the dough more softer as well as easy to knead. Xylanases assist in delaying the crumbing process during bread baking process thereby letting the dough to grow (Polizeli et al. 2005). Xylanase utilization in baking industry has significantly aided in a significant rise in bread volumes, better absorption of water as well as enhanced resistance to fermentation (Camacho and Aguilar 2003). Butt et al. (2008) reported that xylanases effectively transform the hemicelluloses that are water insoluble into a soluble form that actively binds to the water in dough thereby reducing the firmness in dough, enhances the volume and generates finer crumbs with increased uniformity. Various enzymes like xylanases, cellulases and proteases enhance the firmness of the gluten network thereby uplifting the worthiness of bakery products (Gray and Bemiller 2003). Xylanases are highly commended for use in biscuit industry with a purpose to make cream crackers lighter as well as to uplift the texture, uniformity and palatability of the wafers. The major desirable aspects of xylanases in food industry are endurance and ability to show optimum activity in acidic pH range. The advancements in molecular tools and techniques have paved the way for more and more uses of xylanases. Xylanases play a significant role in beer production process. They effectively depolymerize arabinoxylans to lower xylooligosaccharides thereby rendering the beer less viscous thus eliminating its muddy appearance to significant levels (Dervilly et al. 2002).
9.7 Other Important Applications of Xylanases
Xylanases along with other hydrolases can be efficiently employed for the synthesis of important biofuels like ethanol by utilizing lignocellulosic biomass (Ahring et al. 1999). Xylanase in association with pectinase, amylase and carboxymethylcellulase can be efficiently utilized for clarification of juices. Xylanases may also be employed to enhance the extraction of coffee, plant oils and starches. Xylanases may also be successfully capitalized for boosting the nutritional aspects of agricultural silage and grain feed (Malathi and Devegowda 2001). Xylanases also have significant use in rye baking wherein the addition of xylanase prompts the dough to become more soft and sloppy (Harbak and Thygesen 2002).
10 Future Prospects
The industrial importance of xylanase is well established. Amongst different hydrolytic enzymes, xylanase has attained widespread commercial importance credited to its enormous potential applications in food, in feed and in pharmaceutical industries. The surplus availability of hemicellulosic biomass especially xylan becomes a major factor in xylanase production by various microorganisms. Fungal xylanases from Aspergillus species and Trichoderma species have been widely studied and characterized and are commercially utilized in bakery and food processing industries. Xylanase production economics is governed by several key factors that include accessibility of substrate and rate and extent of disentangling of the xylooligosaccharides besides several other decisive factors including inoculum size, pH, temperature, inducers, medium additives, aeration, activators and inhibitors. Submerged fermentation (SMF) system is the most promising technique used worldwide for xylanase production. This is credited to ease of control over various key process parameters, to the higher yield of enzyme (about 90%) and because of being cost-effective as compared to solid-state fermentation. The present review focuses on the various microbial sources for novel xylanase production, the range of available substrates that can be successfully utilized to meet the industrial demands of xylanases and the widespread industrial applications of microbial xylanases.
The prospects of xylan hydrolysis by xylanase from fungal species such as Aspergillus and Trichoderma and bacterial species like Bacillus sp. look quite promising. Thus future studies to increase the xylan hydrolysis rate as well as to assure enhanced process control for increased yield of xylanase would be envisaged.
In conclusion, xylanase is an industrially important enzyme that is loaded with huge potentials for use in commercial sector in various processes such as processing of pulp and fibres; saccharification of agricultural, industrial and municipal wastes; flour improvement for bakery products; manufacturing of several food products; and enhanced bleaching of cellulose pulps that is mainly used in food and pharmaceutical industry. Since the range of applications of this enzyme is very broad, so there is always a scope for novel xylanase with better and improved characteristics, which may be utilized for various industrial applications.
References
Absul MG, Ghule JE, Shaikh H et al (2005) Enzymatic hydrolysis of delignified bagasse polysaccharides. Carbohydr Polym 62:6–10
Ahmed S, Imdad SS, Jamil A (2012) Comparative study for the kinetics of extracellular xylanases from Trichoderma harzianum and Chaetomium thermophilum. Electron J Biotechnol 15:3
Ahring BK, Licht D, Schimd AS et al (1999) Production of ethanol from wet oxidised wheat straw by Thermoanaerobacter mathranii. Bioresour Technol 68:3
Andrade CMMC, Pereira N, Antranikian G (1999) Extremely thermophilic microorganisms and their polymer-hydrolytic enzymes. Rev Microbiol 30:287–298
Andrade SV, Polizeli MLTM, Terenzi HF et al (2004) Effect of carbon source on the biochemical properties of the β-xylosidase produced by Aspergillus versicolor. Process Biochem 39:1931–1938
Azeri C, Tamer AU, Oskay M (2010) Thermoactive cellulase-free xylanase production from alkaliphilic Bacillus strains using various agro-residues and their potential in biobleaching of kraft pulp. Afr J Biotechnol 9:63–72
Bahri DO, Coskun A, Ozcan N et al (2011) Some properties of a new thermostable xylanase from alkaliphilic and thermophilic Bacillus sp. J Anim Vet Adv 10:138–143
Bajpai P (2012) Biotechnology for pulp and paper processing. Springer 3:42–45
Bastawde KB (1992) Xylan structure microbial xylanases, and their mode of action. World J Microbiol Biotechnol 8:353–368
Battan B, Sharma J, Dhiman SS et al (2007) Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzym Microbiol Technol 41:733–739
Belancic A, Scarpa J, Peirano A et al (1995) Purification and properties of two of the enzymes. J Biotechnol 41:71
Bibi Z, Ansari A, Zohra RR et al (2014) Production of xylan degrading endo-1, 4-b-xylanase from thermophilic Geobacillus stearothermophilus KIBGEIB29. J Radiat Res Appl Sci 7:478–485
Bocchini DA, Oliveira OMM, Gomes E et al (2005) Use of sugarcane bagasse and grass hydrolysates as carbon sources for xylanase production by Bacillus circulans D1 in submerged fermentation. Process Biochem 40:3653–3659
Butt MS, Nadeem MT, Ahmad Z et al (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46:22–31
Cady SG, Bauer MW, Callen W et al (2001) Beta-endoglucanase from Pyrococcus furiosus. Methods Enzymol 330:346–354
Camacho NA, Aguilar OG (2003) Production, purification and characterization of a low molecular mass xylanase from Aspergillus sp. and its application in bakery. Appl Biochem Biotechnol 104:159–172
Cannio R, Di Prizito N, Rossi M et al (2004) A xylan-degrading strain of Sulfolobus solfataricus: isolation & characterization of the xylanase activity. Extremophiles 8:117–124
Chidi SB, Godana B, Ncube I et al (2008) Production, purification and characterization of cellulase free xylanase from Aspergillus terreus UL 4209. Afr J Biotechnol 7:3939–3948
Clarke AJ, Bray MR, Strating H. (1993) Xylanases mechanism of action. In: ACS symposium American Chemical Society, vol 27
Coelho GD, Carmona EC (2003) Xylanolytic complex from Aspergillus giganteus: production and characterization. J Basic Microbiol 43:269–277
Coman G, Bahrim G (2011) Optimization of xylanase production by Streptomyces sp. P12–137 using response surface methodology and central composite design. Ann Microbiol 61:773–779
Coughlan M, Hazlewood G (1993) β-1,4-D-xylan-degrading enzyme systems: biochemistry, molecular biology and applications. Biotechnol Appl Biochem 17:259–289
Csiszar E, Urbánszki K, Szakas G (2001) Biotreatment of desized cotton fabric by commercial cellulase and xylanase enzymes. J Mol Catal 11:1065–1072
Dean JFD, Gross KC, Anderson JD (1991) Ethylene biosynthesis inducing xylanase III: product characterisation. Plant Physiol 96:571
Dervilly G, Leclercq C, Zimmerman D et al (2002) Isolation and characterization of high molecular mass water-soluble arabinoxylans from barley malt. Carbohydr Polym 47:143
Dhillon A, Khanna S (2000) Production of a thermostable alkali tolerant xylanase from Bacillus circulans AB16 grown on wheat straw. World J Microbiol Biotechnol 27:325–327
Dhiman SS, Sharma J, Battan B (2008) Pretreatment processing of fabrics by alkalothermophilic xylanase from Bacillus stearothermophilus. Enzym Microbial Technol 43:262–269
Duarte MCT, Silva DA, Gomes EC et al (2003) Xylan-hydrolyzing enzyme system from Bacillus pumilus CBMAI 0008 and its effects on Eucalyptus grandis kraft pulp for pulp bleaching improvement. Bioresour Technol 88:9–15
Ducros V, Charnock SJ, Derwenda U et al (2000) Substrate specificity in glycoside hydrolase family10 structure and kinetic analysis of S. lividans xylanase. J Biol Chem 275:230–236
Ebrahimi M (2010) Engineering thermostable xylanase enzyme mutant from Bacillus halodurans. Afr J Biotechnol 9:8110–8117
Ellis JT, Magnuson TS (2012) Thermostable and alkali stable xylanases produced by the thermophilic bacterium Anoxybacillus flavithermus TWXYL3. Int Sch Res Netw ISRN Microbiol 2012:1–8
Ellouze O, Fattouch S, Mestiri F et al (2008) Optimization of extracellular xylanase production by Sclerotinia sclerotiorum S2 using factorial design. Indian J Biochem Biophys 45:404–405
Ferraz A, De Souza-Cruz PB, Freer J, Siika-Aho M (2004) Extraction and determination of enzymes produced by Ceriporiopsis subvermispora during biopulping of Pinus taeda wood chips. Enzym Microbial Technol 34:228–234
Gabriela PMA, Mariana BO, Ronaldo APN et al (2016) Characterization and biotechnological application of recombinant xylanases from Aspergillus nidulans. Int J Biol Macromol 91:60–67
Ghoshal G, Kamble A, Shivhare US et al (2011) Optimization of culture conditions for the production of xylanase in submerge fermentation by Penicillium citrinum using response surface methodology. Int J Res Rev Appl Sci 6:132–137
Gilbert HJ, Hazelwood GP (1993) Bacterial cellulases and xylanases. J Gen Microbiol 139:187–194
Gouda MK (2000) Purification and partial characterization of cellulose free xylanase produced in solid state and submerged fermentation by Aspergillus tamarii. Adv Food Sci 22:31–37
Goulart AJ, Carmona EC, Monti R (2005) Partial purification and properties of cellulose free alkaline xylanase produced by Rhizopus stolonifer in solid state fermentation. Braz Arch Biol Technol 48:327–333
Gray JA, Bemiller (2003) Bread staling: molecular basis and control. Rev Food Sci Food Saf 2:1–21
Gupta VK, Gaur R, Yadava SK, Darmwal NS (2009) Optimization of xylanase production from free and immobilized cells of Fusarium solani F7. Bioresources 4:932945
Hakulinen N, Turunen O, Janis J et al (2003) Three-dimensional structures of thermophilic beta-1,4-xylanases from Chaetomium thermophilum and Nonomuraea flexuosa. Eur J Biochem 270:1399–1412
Haltrich D, Nidetzky B, Kulbe KD et al (1996) Production of fungal xylanases. Bioresour Technol 58:137–161
Haq I, Khan A, Butt WA et al (2002) Effect of carbon and nitrogen sources on xylanase production by mutant strain of Aspergillus niger GCBMX-45. J Biol Technol 2:143–144
Harbak L, Thygesen HV (2002) Safety evaluation of a xylanase expressed in Bacillus subtilis. Food Chem Toxicol 40(1):1–8
Herbers K, Wilke I, Sonnewald U (1995) A thermostable xylanase from Clostridium thermocellum expressed at high levels in the apoplast of transgenic tobacco has no detrimental effects and is easily purified. Nat Biotechnol 13:63–66
Hong Q, Shin NH, Chang H (1989) Effect of oxygen extraction on organic chlorine contents in bleach plant effluents. TAPPI J 72:157–161
Huang XL, Penner MH (1991) Apparent substrate inhibition of the Trichoderma reesei cellulase system. J Agri Food Chem 39:2096–2100
Ito K, Ogasawara H, Sugimoto T et al (2000) Purification and properties of acid stable xylanases from Aspergillus kawachii. Biosci Biotechnol Biochem 56:547–550
Jain KK, Dey TB, Kumar S, Kuhad RC (2014) Production of thermostable hydrolases (cellulases and xylanase) from Thermoascus aurantiacus R C K K: a potential fungus. Bioproc Biosyst Eng 38:1–10
Javier PFI, Oscar G, Sanz-Aparicio J and Diaz P (2007). Xylanases: molecular properties and applications. In: Industrial enzymes: structure, function and applications . Polaina, J., A. P. MacCabe (Eds.), Springer, Dordrecht. pp. 65– 82
Kamble RD, Anandrao RJ (2012) Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. Int J Microbiol 2:2–8
Kansoh AL, Nagieb ZA (2004) Xylanase and mannanase enzymes from Streptomyces galbus and their use in biobleaching of softwood kraft pulp. J Microbiol 85:103–114
Kansoh AL, Ali MA, El-Gammal AA (2001) Xylanolytic activities of Streptomyces sp. 1, taxonomy, production, partial purification and utilization agricultural wastes. Acta Microbiol Immunol 48:39–52
Kar S, Mandal A, Mohapatra PKD et al (2006) Production of cellulose free xylanase by Trichoderma reesei SAF3. Braz J Microbiol 37:462–464
Karlsson EN, Hachem MA, Ramchuran S et al (2004) The modular xylanase Xyn10A from Rhodothermus marinus is cell-attached, and its C-terminal domain has several putative homologues among cell-attached proteins within the phylum Bacteroidetes. FEMS Microbiol Lett 241:233–242
Kaur A, Chopra C, Joshi A et al (2015) Bioprocessing, biochemical characterisation and optimization of solid state fermentation of a new thermostable xylanase producing strain belonging to Bacillus genus. J Chem Pharm Res 7:266–276
Kenealy RW, Jeffries TW (2003) Enzyme processes for pulp and paper: a review of recent developmenst. In: Goodell B, Nicholas DD, Schultz TB (eds) Wood deterioration and preservation: Advances in our changing world, Chapter 12. ACS symposium series 845 American Chemical Society, Washington, DC, pp 210–239
Khasin A, Alchanati I, Shoham Y (1993) Purification and characterization of a thermostable xylanase from Bacillus stearothermophilus T-6. Appl Environ Microbiol 59:1725–1730
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. Fed Eur Microbiol Soc 23:411–456
Kumar PR, Eswaramoorthy S, Vithayathil PJ, Viswamitra MA (2000) The tertiary structure at 1.59 Å resolution and the proposed amino acid sequence of a family-11 xylanase from the thermophilic fungus Paecilomyces varioti Bainier. J Mol Biol 295:581–593
Kumar A, Guleria A, Kumar D et al (2014) Isolation, immobilization and characterization of xylanase from a new isolate Bacillus atrophaeus E8. Internat jour of scientific resear 3:33–35
Li Y, Hardin JR (1998) Enzymatic scouring of cotton surfactants, agitation and selection of enzyme. Textile Colour Chem 30:23–28
Li K, Azadi P, Collins R et al (2000) Relationships between activities of xylanases and xylan structures. Enzym Microbiol Biotechnol 27:89–94
Madlala A, Bissoon S, Singh S et al (2001) Xylanase-induced reduction of chlorine dioxide consumption during elemental chlorine-free bleaching of different pulp types. Biotechnol Lett 23:345–351
Maheswari U, Chandra TS (2000) Production and potential applications of xylanase from a new strain of a Streptomyces cuspidosporus. World J Microbiol Biotechnol 16:257–263
Malathi V, Devegowda G (2001) In vitro evaluation of nonstarch polysaccharide digestibility of feed ingredients by enzymes. Poult Sci 80:302
McCarthy AA, Morris DD, Bergquist PL et al (2000) Structure of XynB, a highly thermostable β-1,4-xylanase from Dictyoglomus thermophilum Rt46B.1, at 1.8 Å resolution. Acta Crystallogr 56:1367–1375
Meng DD, Ying Y, Chen XH et al (2015) Distinct roles for carbohydrate-binding modules of GH10 and GH11 xylanases from Caldicellulosiruptor sp. F32 in thermostability and catalytic efficiency. Appl Environ Microbiol 81:2006–2014
Monisha R, Uma MV, Krishna Murthy V (2009) Partial purification and characterization of Bacillus pumilus xylanase from soil source. Kathmandu Univ J Sci Eng Technol 5:137–148
Motta FL, Andrade CCP, Santana MHA (2013) A review of xylanase production by the fermentation of xylan: classification, characterization and applications. In: Chandel AK, da Silva SS (eds) Sustainable degradation of lignocellulosic biomass – techniques, applications and commercialization. INTECH, Croatia, pp 251–275
Mushimiyimana I, Padmavathi T (2015) Xylanase enzyme production by Penicillium crustosum using sugar beet peel substrate by response surface methodology. Res J Pharm, Biol Chem Sci 6:1145–1149
Nakamura S (2003) Structure and function of a multiplidomain alkaline xylanase from alkaliphilic Bacillus sp. strain 41M-1. Catal Surv Jpn 7:157164
Nigam P, Singh D (1995) Processes for fermentative production of xylitol a sugar substitute. Process Biochem 30:117–124
Norazlina I, Meenalosani N, KuHalim KH (2013) Production of xylanase by Trichoderma sp. via solid state culture using sugarcane bagasse. Int J Energy Sci 3:99–103
Octavio L, Corral, Francisco VO (2006) Xylanases overview. Adv Agric Food Biotechnol:305–322
Omar AW, Khataibeh MJ, Abu-Alruz K (2008) The use of xylanases from different microbial origin in bread making and their effects on bread quality. J Appl Sci 8:672–676
Pandey AK, Edgard G, Negi S (2016) Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew Energy 98:51–56
Parajo JC, Domingues H, Domingues JM (1998) Biotechnological production of xylitol and fundamentals of its biosynthesis. Bioresour Technol 65:191–201
Park S, Rancour DM, Bednarek SY (2007) Protein domain-domain interactions and requirements for the negative regulation of Arabidopsis CDC48/p97 by the plant ubiquitin regulatory X (UBX) domain-containing protein, PUX1. J Biol Chem 282:5217–5224
Patel K, Prajapati K (2014) Xylanase production by Cladosporium sp. from agricultural waste. Int J Curr Res Acad Rev 2:84–90
Polizeli MLT, Rizzatti M, Monti R et al (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591
Qinnhe C, Xiaoyu Y, Tiangui N et al (2004) The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Process Biochem 39:1561–1566
Raj A, Sharad K, Sudheer KS (2013) A highly thermostablexylanase from stenotrophomonas maltophilia: purification and partial characterization. Enzym Res:2–8
Ratanachomsri U, Sriprang R, Sornlek W et al (2006) Thermostable xylanase from Marasmius sp.: purification and characterization. J Biochem Mol Biol 39:105
Roy N, Habib MR (2009) Isolation and characterization of xylanase producing strain of Bacillus cereus from soil. Iran J Microbiol 1:49–53
Saha BC (2000) A-L-arabinofuranosidases biochemistry, molecular biology and application in biotechnology. Biotechnol Adv 18:403–423
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Sandrim VC, Rizzatti ACS, Terenzi HF et al (2005) Purification and biochemical characterization of two xylanases produced by Aspergillus caespitosus and their potential for kraft pulp bleaching. Process Biochem 40:1823–1828
Sanghvi GV, Koyani RD, Rajput KS (2010) Thermostable xylanase production and partial purification by solid-state fermentation using agricultural waste wheat straw. Mycology 2:106–112
Sardar M, Roy I, Gupta MN (2000) Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer. Enzym Microb Technol 27:672–679
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6:219–228
Sharma PK, Chand D (2012) Production of cellulase free thermostable xylanase from Pseudomonas sp. XPB-6. Res J Biol Sci 1:31–41
Sharma M, Kumar A (2013) Xylanases overview. British Biotechnol J 3:1–28
Shi H, Zhang Y, Li X et al (2013) A novel highly thermostable xylanase stimulated by Ca2+ from Thermotoga thermarum: cloning, expression and characterization. Biotechnol Biofuels 6:26
Shi P, Du Y, Yang H et al (2015) Molecular characterization of a new alkaline-tolerant xylanase from Humicola insolens Y1. Biomed Res Int 2015:1–7
Singh S, Madlala AM, Prior BA (2003) Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol Rev 27:3–16
Subbulakshmi S, Priya R (2014) Production and purification of enzyme xylanase by Aspergillus niger. Int J Curr Microbiol App Sci 3:664–668
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology and application. World Appl Sci J 4:277–283
Subramaniyan S, Sandhia GS, Prema P (2001) Control of xylanase production without protease activity in Bacillus sp. by the selected nitrogen source. Biotechnol Lett 23:369–371
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production. Bioresour Technol 83:1–11
Sunna A, Antranikian G (1997) Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol 17(1):39–67
Sunna A, Bergquist PL (2003) A gene encoding a novel extremely thermostable 1,4beta-xylanase isolated directly from an environmental DNA sample. Extremophiles 7(1):63–70
Takahashi Y, Kawabata H, MurakamIi S (2013) Analysis of functional xylanases in xylan degradation by Aspergillus niger E-1 and characterization of the GH family 10 xylanase XynVII. Springer Plus 2:447
Teixeira RSS, Siqueira FGA, de Souza MV et al (2010) Purification and characterization studies of a thermostable β-xylanase from Aspergillus awamori. J Ind Microbiol Biotechnol 37:1041–1051
Thomas L, Parmeswaran B, Pandey A (2016) Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew Energy 98:9–15
Twomey LN, Pluske JR, Rowe JB et al (2003) The effects of increasing levels of soluble non-starch polysaccharides and inclusion of feed enzymes in dog diets on faecal quality and digestibility. Anim Feed Sci Technol 108:71–82
Van Paridon PA, Booman JCP, Selten GCM et al (1992) Application of fungal endoxylanase in poultry diets. Elsevier:371–378
Velkova ZI, Gochev VK, Kostov G, Atev A (2007) Optimization of nutritive media composition for xylanase production by Aspergillus awamori. Bulg J Agri Sci 13:651–656
Verma D, Satyanarayana T (2012) Molecular approaches for ameliorating microbial xylanases. Bioresour Technol 17:360–367
Wang X, Huang H, Xie X et al (2016) Improvement of the catalytic performance of a hyperthermostable GH10 xylanase from Talaromyces leycettanus JCM12802. Bioresour Technol 222:277–284
Whistler R, Masek E (1955) Enzymatic hydrolysis of xylan. J Am Chem Soc 77:1241–1243
Whistler RL, Richards EL (1970) Hemicelluloses. The carbohydrates. Academic, New York, pp 447–469
Widjaja A, Lestari E, Tanjung A, Alfian W et al (2009) Optimized production of xylanase from fungal strains and its purification strategies. J Appl Sci Environ Sanitation 4:219–232
Wong KKY, Tan LUL, Saddler JN (1998) Multiplicity of β-1,4-xylanase in microorganisms. Funct Appl Microbiol Rev 52:305
Woodward J (1984) Xylanases: functions, properties and applications. Top Enzym Ferment Biotechnol 8:9–30
Yasinok AE, Biran S, Kocabas A et al (2010) Xylanase from a soil isolate, Bacillus pumilus: gene isolation, enzyme production, purification, characterization and one-step separation by aqueous-two-phase system. World J Microbiol Biotechnol 26:1641–1652
Yegin S (2016) Single-step purification and characterization of an extreme halophilic, ethanol tolerant and acidophilic xylanase from Aureobasidium pullulans NRRL Y-2311-1 with application potential in the food industry. Food Chem 221:67–75
Yin LJ, Lin HH, Chiang YI et al (2010) Bioproperties and purification of xylanase from Bacillus sp. YJ6. J Agri Food Chem 58:557–562
Yoon H, Han NS, Kim CH (2004) Expression of Thermotoga maritime endo-β 1,4-xylanase gene in E. coli and characterisation of the recombinant enzyme. Agric Chem Biotechnol 47:157–160
Zhou C, Wang Y, Liu Z et al (2011) Acidic xylanase II from Aspergillus usamii: efficient expression in Pichia pastoris and mutational analysis. Afr J Biotechnol 10:11631–11639
Zimbardi S, Cesar S, Luana P et al (2013) Optimization of β-Glucosidase, β-Xylosidase and xylanase production by Colletotrichum graminicola under solid-state fermentation and application in raw sugarcane trash saccharification. Int J Mol Sci 14:2875–2902
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dhiman, S., Mukherjee, G. (2018). Recent Advances and Industrial Applications of Microbial Xylanases: A Review. In: Gehlot, P., Singh, J. (eds) Fungi and their Role in Sustainable Development: Current Perspectives. Springer, Singapore. https://doi.org/10.1007/978-981-13-0393-7_19
Download citation
DOI: https://doi.org/10.1007/978-981-13-0393-7_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0392-0
Online ISBN: 978-981-13-0393-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)