Abstract
Proteases are enzymes that hydrolyse proteins and polypeptides into smaller constituents. Holding two-thirds of the global enzyme market, proteases are used to execute unique functions in industries of food, textile, detergent, therapeutics and environmental remediation. Fungi have emerged as the most dominant source of proteases due to several cultivational advantages over other sources. Expeditious industrial growth and emerging environment problems necessitate search for novel enzymes from more efficient fungal sources. Endophytic fungi form an uncharted ecological group of fungi with assorted synthesizing potential. Their efficacy has been proven within a short amount of time, as distinguished bioactive secondary metabolites have already been obtained from them. Commercial hydrolases are customarily isolated from soil-borne genera of fungi, but their endophytic counterparts offer a potential alternative, owing to recent findings that endophytes too display the same array of enzymes as do the soil fungi. Prospecting of endophytic fungi for protease production is not only promising, but there also exists the possibility of isolating and characterizing novel proteases that might be suitable alternatives for specialized industries. Several researchers have endorsed this postulate as they have discovered endophytic fungi with optimum protease producing capabilities and novel proteases with projected industrial applications prima facie.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Proteases are enzymes that catalyse the cleavage of peptide bonds of proteins and polypeptides resulting in the formation of oligopeptides or free amino acids. They are also known as proteinases and polypeptidases. Proteases are ubiquitously present in all biological systems for carrying out proteolysis—a process vital for all forms of life. Proteolysis is necessary for both structural and functional aspects of a living being. Proteases are produced by every living cell either for intracellular operations or are secreted to the surroundings for nutritional and defensive functions. Besides being physiologically vital, they have also found over the years several critical roles to play in industries pertaining to health, food, textile and medicine (Correa et al. 2014). Their significance on the industrial level can be estimated by the fact that they claim up to 60% of the global enzyme market and constitute one of three major groups of industrial enzymes (Saran et al. 2007; Ningthoujam et al. 2009).
The industrial processes involving catalysis of proteins can be executed either enzymatically or chemically. Chemical degradation of proteins often leads to hydrolysates with undesirably modified amino acids and uncontrollable reactions. Chemical modification has been preferably substituted with highly specific biocatalysis. Proteases represent a reusable, sustainable environmental friendly alternative. Products formed from proteases are gaining popularity and preference among commoners as people are inclining towards natural products rather than chemically synthesized ones (Sumantha et al. 2006; Tavano 2013; Saxena et al. 2014, 2015; Suman et al. 2016; Verma et al. 2017).
Earlier, proteases were classified on the basis of some practical and functional facets, such as their catalytic actions (endoprotease and exoprotease), source (animal, plant or microbial), pH optima (alkaline, neutral or acidic), substrate specificity, etc. A more rational system was contrived by the Enzyme Commission (EC) under which all enzymes had been grouped into six classes. Proteases fall under class three, which comprises of hydrolases, and subgroup four—which characterizes enzymes with the ability to hydrolyse peptide bonds (EC 3.4). This class is further divided into families; six such families have been recognized hitherto—serine carboxy proteases (EC 3.4.16), metallo carboxy proteases (EC 3.4.17), serine proteases (EC 3.4.21), cysteine proteases (EC 3.4.22), aspartic proteases (EC 3.4.23) and metalloproteases (EC 3.4.24) (Whitaker 1994).
Classification of proteases on the basis of their functional pH range is one of the most feasible and workable basis as it can readily identify the type of industrial sector the protease can be applied to. Acidic proteases function optimally in the range of pH 0–6.0. They act on the breakdown of bonds involving aromatic amino acids bulky side chains at both sides of the cleaving bond. Acid proteases find use mainly in food industries. Neutral proteases are active in the pH range of 5.0–8.0. They have a characteristic high affinity for hydrophobic amino acids in the polypeptide chain and have low degree of hydrolysis. Their low thermal tolerance provides a mechanism of reaction control and facilitates achieving hydrolysates with limited hydrolysis. The proteases that show maximum activity in the neutral-alkaline (7.0–14) pH range are called alkaline proteases. They either have a serine centre or are of metallo-type. They are perhaps the most extensively studied among the three groups of proteases. This is due to their potentially huge marketability as they are useful in a variety of industries like detergent, food, pharmaceutical and leather industries (Sharma et al. 2017; dos Santos Aguilar and Sato 2018).
10.2 Applications of Enzymes Proteases in Industries
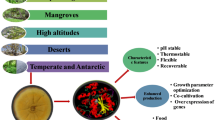
Proteases have served in the industries since time immemorial, the earliest application of these being in the food industry. The reaction involving degradation of proteins into smaller constituents has found disparate uses in various fields. Proteases have emerged as a significant enzyme group holding two-thirds of the global enzyme market. In addition to their conventional uses in dairy, bakery, leather and detergent industry, modern times are seeing proteases being exploited for some unconventional purposes, ranging from bioremediation to treatment of diabetes, cancer and AIDS (Ladenburger et al. 1997; Rao et al. 1998; Abdennabi et al. 2017; Yadav et al. 2016, 2017a, b). Their utility in a specific industry is generally a reflection of their chemical nature and optimum working conditions (Fig. 10.1). Some commercial applications of proteases are described below.
10.2.1 Therapeutics
Proteases are digestive enzymes that are required for breakdown of proteinaceous food, providing essential amino acids to the body. Many pathophysiological conditions can cause obstruction in this normal catalytic process. Administration of protease formulations to such patients improves the digestion process (Craik et al. 2011; Rajput et al. 2016). Zenpep® (Eurand) is one such protease preparation available in the market for treating malabsorption of nitrogen in patients with cystic fibrosis. Patients of cystic fibrosis suffer from a decreased production and release of pancreatic enzymes which compromises their ability to absorb nitrogen from food sources. Zenpep® is a porcine-derived preparation of proteolytic and lipolytic enzymes that have tremendously helped people as a digestive aid. However, such replacement enzymes of porcine origin have caused allergies in humans. Trizytek™ (Eli Lilly) is another digestive aid currently in development that has shown promising results. The formulation comprises a lipase, amylase and an alkaline elastolytic protease from the fungus Aspergillus melleus. The oral delivery of the preparation has been shown to improve protein digestion in cancer and cystic fibrosis patients with pancreatic insufficiency (Littlewood et al. 2006; Wooldridge et al. 2009).
Cosmetic reconstruction and wound healing are medical techniques where proteases have been employed extensively. Proteases have been used in skin ointments for removal of necrotic tissue in skin ulcers, debridement of wounds, removal of keratin in acne or psoriasis, degradation of keratinized skin and elimination of human callus (Gupta et al. 2002a; Shubha and Srinivas 2017). Keratinolytic proteases are a potential resource for accelerating healing process by scar removal and renewal of epithelia. Cosmetic preparations of plant proteases papain and bromelain have been in use for regenerating skin through peeling and smoothing. They act by eliminating collagenous and keratinous debris, thereby removing dead cells from the epithelia and renewing the same. Proteases have been an additive in vaccine therapies for dermatophytosis. Collagenolytic proteases have been employed in treatment of sciatica in herniated intervertebral discs, in treatment of retained placenta and as a pretreatment for enhancing adenovirus-mediated cancer gene therapy. Penzyme is a commercial concoction of trypsin and chymotrypsin that can be helpful in treatment of psoriasis by digesting outer damaged layers of skin (Sim et al. 2000; Vignardet et al. 2001; Brandelli et al. 2010).
Antibody fragments are gaining importance in diagnostics and drug designing since the past few years. Generation of antibody fragments involves proteolysis of entire immunoglobulin molecules. Specific digestion of IgG molecules by papain, pepsin and ficin has been traditionally done to dissect the antibody molecule into fragments. Fragments of monoclonal antibodies provide advantage over whole antibody molecules due to their small size, faster diffusion and lower immunogenicity while maintaining specificity. These properties have led to their application in diagnostics, therapeutics and biopharmaceutical research (Holliger and Hudson 2005; Mótyán et al. 2013).
Proteases execute diverse functions ranging from the cellular to the organism level. They are involved in regulation of cascades of haemostasis and inflammation in the vertebrate system. Apart from being a crucial element in the normal physiology of cells, proteases also play an important regulatory part in pathophysiological conditions of an organism. The necessary role they have in completion of life cycle of pathogens and anomalous cells has led to the development of new drugs that target them for treating terminal diseases such as cancer and AIDS. Microbial proteases are used as immunostimulatory agents and in combinatorial treatment with antibiotics (Okumura et al. 1997). Studies done by Ladenburger and his team (Ladenburger et al. 1997) show that protease administration successfully delayed the onset of insulin-dependent diabetes mellitus in mice with autoimmune diabetes.
10.2.2 Food
The most notable purpose served by proteases has been in the food industry, particularly in cheese-making and bakery. The biochemical process of proteolysis is accountable for the modification of milk to cheese, and it has been traditionally performed using animal rennet (chymosin). Chymosin is the most suitable and preferable enzyme for cheese-making since it has high specificity for casein and its low thermal tolerance ensures that the enzymatic activity ceases upon cooking, which can otherwise cause bittering of cheese. But due to inadequate supply of this enzyme of animal origin, focus has shifted to microbial milk coagulants. An increasing number of cheese manufacturing industries have employed proteases of microorganisms that have been deemed GRAS (Generally Recognized as Safe) by the US Food and Drug Administration, like Mucor miehei, Bacillus subtilis and Endothia parasitica.
Proteases have also assisted in bakeries through their action of limited proteolysis on gluten. Gluten is an insoluble protein present in wheat, and it determines the viscoelastic and mass expansion properties and flavour of bakery dough. Pretreatment of dough using protease results in reduction of mixing time and improvement of loaf volume. Protease treatment is in general used to manipulate gluten strength to achieve multifarious bakery products each with unique flavour and properties. Endo- and exoproteases from Aspergillus oryzae (BakeZyme B500BG) and a neutral metalloprotease from Bacillus amyloliquefaciens (Neutrase®) have been utilized for degrading proteins in flour dough for preparing biscuits, cakes, crackers and cookies (Sawant and Nagendran 2014).
Proteases are important to the brewing industry too, as addition of protease can increase the growth of yeast in fermentation media, resulting in better and faster yield of alcohol. It also aids in extraction of nutritional proteins from malt and barley. Clarification of protein drinks, alcoholic beverages and fruit juices also requires protease. Proteases are also used in clarification of xanthan gum. Kojizyme and Flavourzyme are commercial fungal proteases that are used in fermentation of soy sauce and seasoning and limited hydrolysis of proteinaceous food materials like meat, fish, casein, gelatin, etc. The protein hydrolysates formed from partial hydrolysis are valued as nutritional supplements (Ward et al. 2009).
10.2.3 Leather and Textile
Leather processing involves steps of soaking, dehairing, bating and tanning of the animal hide. Dehairing process could be carried out either chemically or enzymatically. Chemical processing utilizes strong alkali for soaking, followed by hydrogen sulphide application for dissolving hair roots. The extreme alkaline conditions and dangerous chemicals like hydrogen sulphide render the chemical method extremely hazardous for the workers. Moreover, the huge amount of chemical wastewater contributes significantly to environmental pollution. Processing by proteases is a much safer option both for industrial workmen as well as the environment. Protease treatment reduces soaking time by accelerating water absorption. This reduces the requirement of water and minimizes effluent release. Protease operates by removing non-collagenous materials of leather and dissolving non-fibrillar proteins like globulins and albumins. Physical conditions of leather processing happen to be optimal for alkaline proteases. Application of alkaline proteases with sodium chloride and hydrated lime is efficient in dehairing animal skin. Various combinations of proteases from Bacillus and Aspergillus along with trypsins have been used in leather processing. Enzyme use has been shown to improve leather texture and quality (Ward et al. 2009; dos Santos Aguilar and Sato 2018).
Proteases are a valuable resource for the silk industry. Raw silk consists of two protein components – sericin (22%) and fibroin (76%). Fibroin is the major component that forms the finished product. Sericin is a water-soluble protein that forms a protective and adhesive layer over the fibroin. Removal of sericin, known as degumming or silk scouring, is necessary to achieve strength, texture, lustre and colour in the finished fabric. It is accomplished through digestion of raw silk by proteases. This method is superior to soap treatment as protease action can be controlled to get desired strength of silk fabric and it is environmentally friendly too (Gulrajani and Gupta 1996).
10.2.4 Detergents
The idea of incorporating proteases to detergent was pioneered by German chemist Otto Röhm. He obtained a patent in 1913 for using tryptic enzymes of animal origin with laundry detergent and formulated the first enzyme detergent named Burnus® with his associate Otto Haas. Unfortunately, the formulation did not gain popularity due to inefficiency. This was later attributed to the inactivation of the tryptic enzymes in the alkaline conditions produced by the detergent. The first preparation of detergents with proteolytic enzymes that gained popularity and acceptance among the common mass was introduced by Novo Industri in the year 1961. It comprised of an alkaline protease from Bacillus licheniformis that was stable at high pH range of 8–10. Since then, proteases have become the most sought-after enzymes in the detergent industry. Mostly serine alkaline proteases are used for this purpose. Addition of protease provides several perks such as easy removal of proteinaceous dirt (blood, body secretions, milk, fish and meat stains, etc.) and reduction in amount of water required for washing, minimizing the physical effort and time that go into doing laundry. Enzymatic detergents have made cold washing more effective, thus saving energy. Various proteases capable of functioning in a variety of pH and temperature ranges are added to detergents, and this has given way to a new class of detergents that have minimum impact on the environment (Samal et al. 1990; Ward et al. 2009; Valls et al. 2011; Sahay et al. 2017; Saxena et al. 2016; Suman et al. 2015).
10.2.5 Organic Synthesis
Radically, proteases have been used to disintegrate polypeptides. Proteolysis involves an equilibrium reaction between synthesis and disintegration, the equilibrium being driven and controlled by the amount of water in the reaction solution. By manipulating the moisture content and through use of appropriate solvent, the reverse reaction can be coerced. This is termed Protease-Mediated Peptide Synthesis (PMPS). A remarkable use of protease in organic synthesis is the industrial manufacture of aspartame, an artificial sweetener used as a sugar substitute. A heat-stable extracellular Zn2+ metalloprotease from Bacillus thermoproteolyticus is employed for reverse hydrolysis that yields the dipeptide aspartame (Kühn et al. 2002; Ward et al. 2009; Birrane et al. 2014).
10.2.6 Research
Many techniques in biological research avail proteases. Proteinase K is a well-known protease that is exploited in laboratory-scale biochemical processes. It was first described by Ebeling and his team in 1974 from the fungus Tritirachium album. It was found to possess a superlative proteolytic, specifically keratin-hydrolysing, property. It is applied in nucleic acid isolation procedure for removing unwanted protein components of the cells and also to inactivate nucleases that might attack the nucleic acids, thereby increasing extent of purification and yield of the isolated DNA/RNA (Ebeling et al. 1974; Mótyán et al. 2013).
Tissue culture techniques also require protease application for dissociation of intact tissue and liberation and isolation of detached viable cells. Separation of cells involves digestion of junctions connecting the cells and dissolution of the extracellular matrix. Proteases form an important component of the array of enzymes utilized in this process. During flask culture of tissues also proteases are used to separate the cell layer from the surface of flask by dissolving the protein bridges. Trypsin is generally used for this purpose (Canavan et al. 2005; Huang et al. 2012).
Proteases are nowadays proving to be tremendously helpful in management of industrial and household wastes, for accelerating the process of degrading waste material, in wastewater management and other bioremediation processes. Proteases are used in cleaning solutions for contact lenses for removing proteinaceous debris. They also find use in photography and biomedical industries for dissolving gelatin off scrap films of photos and X-rays that allow recovery of silver from the films. Proteases perform dynamic functions, and interests are ever growing in finding proteases with unique and novel biological properties (Anwar and Saleemuddin 1998; Nielsen and Oxenboll 1998; Kumar and Takagi 1999; Gupta et al. 2002b; Harrison and Bonning 2010; Hasan et al. 2013; Alberto et al. 2016; Rajput et al. 2016). A synopsis of commercial proteases currently in use, with their respective sources of origin, is provided in Table 10.1.
10.3 Proteases from Fungi: How They Are Prevalent and What Advantages They Have
A few decades ago, animals used to be the sole source of industrial enzymes. Pigs and cows were slaughtered, and proteases recovered from them were utilized in the industries. A few proteases were described from plants later on, and they served some particular functions in commercial sectors. Remarkable expansion of the enzyme trade occurred after realization that microbial sources surpass animal and plant sources in terms of enzyme production. Microbial production of enzymes is more profitable due to several reasons. Microorganisms (bacteria and fungi) have limited space requirement (Najafi et al. 2005). The enzymes they produce are extracellular in nature, i.e. they are secreted into the culture media, and this makes the downstream processing easier. They can grow in simple media composition with a rapid growth rate and faster rate of enzyme production. They are also amenable to genetic manipulation and recombination (Anbu et al. 2013). The proteases obtained from microbes also have an edge over animal and plant proteases as they have been found to have greater thermal stability, allowing longer shelf life (Maria et al. 2005; Sharma et al. 2014). They have minimum loss of function even over adverse storage conditions. Microorganisms provide a reliable and consistent yield with constant composition along predictable and controllable fermentation conditions.
Over the past few years, fungi have outperformed other enzyme sources, and currently about 60% of industrial enzymes are of fungal origin (Fig. 10.2). Fungi have become more acceptable as industrial enzyme synthesizers than their bacterial counterparts due to greater efficiency of the fungal mycelia, easy separation of mycelia from the culture media and greater biochemical diversity of proteases found in fungi (Ningthoujam et al. 2009; Sharma et al. 2017). Serine, threonine, metallo-, aspartic and cysteine protease and other uncharacterized proteases have been produced from fungi, and they have been employed in manufacturing food, beverages, leather and textile and in simplifying the processing of raw materials (Rawlings et al. 2004; Maria et al. 2005). The excellent enzyme-synthesizing abilities of fungi are accredited to their saprotrophic and parasitic mode of nutrition. This lifestyle demands exhibit of a gamut of degrading enzymes that dissolve host tissues or disintegrate dead tissues so that the released nutrients can be absorbed by the fungi. Extracellular proteases released by fungi hydrolyse peptides and facilitate nitrogen uptake (Schulz et al. 2002; Suryanarayanan et al. 2012).
10.4 Need to Look for New Sources
Insatiable demand for industrial enzymes has led to extensive research efforts into optimizing and maximizing production from existing protease sources (Kirk et al. 2002). Industrial growth has caught up a breakneck speed, and to meet demands, research is ever-going for finding novel and more proficient protease sources as well as organisms that produce unprecedented kinds of proteolytic enzymes. Novel proteases are providing solutions to many previously unresolved challenges in the biomedical and biotechnological sectors. When it comes to enhancement of enzyme synthesis, natural selection has always been preferable to combinatorial chemistry (Schulz et al. 2002).
Increasing health consciousness of the society has led to rejection of many synthetic products. Demand for products from natural sources has increased, and focus has shifted to screening microorganisms for finding bioactive compounds (Strobel and Daisy 2003). Although fungi have been the dominant enzyme producers in industries, it is perplexing that only a handful of fungal species are exploited on the industrial scale. Species of Aspergillus (particularly A. niger and A. oryzae), Humicola, Penicillium, Rhizopus and Trichoderma have been prepotent in commercial bioproduct synthesis (Østergaard and Olsen 2010; Correa et al. 2014). It is safe to presume that out of an estimated 1.5 million members, many undocumented and uninvestigated fungal species are promising and potent sources of bioactive products (Hawksworth 1991; Peterson et al. 2011).
Bioactive product discovery relies heavily on the strength of culture collection for screening and requires exploration of atypical environments (Hyde 2001). It is a general notion that organisms from unusual habitats possess unusual characters corresponding to the habit requirements. The terrestrial environment has been thoroughly scanned, and resources in this front have been exhausted, so there is an urgent need to scrutinize other ecological groups of fungi (Correa et al. 2014). Studies on endophytic fungal flora have been initiated recently after it was comprehended that plant tissues are not sterile but are indeed inhabited by a large number of fungi and bacteria. Endophytic fungi have been explored in terms of their species diversity and bioactive metabolite production. Within a short amount of time and with a handful of studies, endophytes have proved to be an unexplored repository of natural products and have already offered distinguished bioactive metabolites. Fascinatingly, most studies on screening endophytic fungi are for secondary metabolites. Prospecting of endophytic fungi for protease production is not only promising, but there also exists the possibility of isolating and characterizing novel proteases that might be suitable alternatives for specialized industries (Alberto et al. 2016).
10.5 Endophytic Fungi as Enzyme Synthesizers
10.5.1 What Are Endophytic Fungi?
Endophytic fungi are a highly diverse ecological group of fungi circumscribed by their habitat- internal tissues of plants. They live inside plant tissues without making their presence apparent. They are a polyphyletic group of taxonomically and metabolically diverse fungi that make up a significant component of microbial diversity in the environment (Wilson et al. 1991; Arnold 2007; Rana et al. 2016a, b, 2017; Suman et al. 2016). In the initial years following their discovery, endophytes were thought to have a neutral relation with their host plant. But later investigations, beginning from 1970, revealed that they in fact form a mutual partnership with their host. This was corroborated by the finding that clavicipitaceous members residing inter- and intracellularly in grasses deter herbivore feeding. They compensate for the inability of grasses to produce toxic secondary metabolites. The repugnant toxic alkaloids produced by the endophytes thwart consumption of the plant by herbivores. Subsequent studies established that endophytic fungi aid in survival and health of their hosts through production of heterogeneous bioactive metabolites. The relation is mutualistic in the sense that the fungi help the plant through biotic and abiotic stresses and, in turn, derive nutritional carbon source from the host. Consequently, they are predominantly found in the sink tissues of plants, regions of sucrose unloading such as leaf sheaths and pith (Hinton and Bacon 1985). They are transmitted either vertically, through seeds or vegetative propagation of host plant, or horizontally, invading the plant tissues via natural (stomata, lenticels) or artificial (mechanical injury) openings (Carroll 1988). Their invasion and ramification inside the host tissues require display of a battery of degrading enzymes, and survival inside the host involves active production of primary and secondary metabolites.
10.5.2 Their Synthesizing Abilities
Endophytes exist in symbiotic partnership with their hosts. They actively synthesize diverse compounds in their habitat that facilitate better nutrient uptake by their host, enhancing host health and fitness. They prevent pathogen invasion and upregulate plant defense system. In the ecological niche they occupy, they constantly engage in biological warfare with other microbial species, many of which are phytopathogens, for space and nutritional requirements. This necessitates production of antagonistic substances that endow the endophytes with better chance of survival. Due to their ability to live inside plant tissues facing defense chemicals of the host plant, their capacity to detoxify and transform bioactive molecules can be rightly predicted to be employed on an industrial scale (Suryanarayanan et al. 2012). Endophytic fungi isolated from many angiosperms and gymnosperms have been bioprospected and found to be producing unique structures, including alkaloids, benzopyranones, chinones, flavonoids, phenols, phenolic acids, quinines, isocoumarin derivatives, steroids, peptides, terpenoids, tetralones and xanthones (Tan and Zou 2001; Strobel et al. 2004).
There have been many instances where endophyte of a particular plant was observed to produce phytochemicals specific to the plant. Tan and Zou (2001) postulated that millions of years of co-evolution has led to genetic recombination between such endophytes and their hosts, and fungi show a greater affinity towards accepting foreign genetic material through horizontal gene transfer. Genes for novel product synthesis might have been shared in this way between the two symbiotic partners through evolutionary time. Many of the secondary metabolites produced by endophytes have diverse applications in agrochemicals, medical therapy, as antiparasites, immune-modulatory agents, antioxidants, cytotoxic agents, etc. Endophytic fungi have been found to produce such chemicals in independent cultures—a feature that renders them suitable as bioactive product sources at the industries. Several studies have substantiated their ability to produce distinctive substances that possess bioactivity, such as novel antibiotics; antimycotics; immunosuppressants; anticancer, antiviral and volatile organic compounds including volatile antimicrobials; insecticides; and antidiabetic compounds (Strobel and Daisy 2003). Many fungi isolated from plants have been screened for enzyme synthesis potential, but majority remain unexplored, providing huge scope for finding alternative sources of enzymes (Alberto et al. 2016).
10.5.3 Their Promising Candidature as Protease Sources
Commercial hydrolases are customarily isolated from soilborne genera of fungi like Aspergillus, Penicillium and Rhizopus (Lee et al. 2014). But their endophytic counterparts offer a potential alternative as it has been established that endophytes too display the same array of enzymes as do the soil fungi (Promputtha et al. 2007). Enzyme synthesis is an integral part of endophytic life cycle as enzymes have several important functions:
-
Hydrolytic enzymes dissolve the lignocellulosic material of plant cells to enable fungal mycelia to penetrate the host surface and ramify inside the host plant tissues.
-
Enzymes disintegrate sources of nitrogen, phosphorus, calcium, etc. external to the plant roots and enable the plant to absorb these otherwise inaccessible sources.
-
Enzymes are necessary for the absorptive mode of nutrition of fungi. Enzymes breakdown complex food materials, such as starch and sucrose obtained from the host tissues, into simpler units that get absorbed by the fungal mycelia.
-
Enzymes prevent pathogen invasion and expansion by targeting substrates on the surface and interior of the pathogen’s anatomy.
Role of enzymes extends after senescence of host tissue, as fungi’s lifestyle shifts to saprotrophic mode. From that point onwards, the enzymes perform degradation of the dead organic matter, and the decaying tissues supply nourishment to the fungi (Maria et al. 2005). There have been a number of studies on enzymatic profiling of endophytic fungi. Endophytic fungal isolates have been scrutinized for general or specific hydrolytic enzymes such as amylase, protease, lipase, cellulase, tannase, laccase, etc. and have given promising results (Sunitha et al. 2013). Caldwell et al. (2000) reported that Phialophora finlandia and P. fortinii, endophytic fungi isolated from alpine plant communities, were able to breakdown complex forms of phosphorus, nitrogen and carbon found in plants. Choi et al. (2005) screened the endophytic fungi for their ability to produce lignocellulases, amylase, cellulase, ligninase, pectinase and xylanase. Maria et al. (2005) performed similar studies on fungi isolated from mangrove fern Acrostichum aureum L. and mangrove angiosperm Acanthus ilicifolius L. Screening tests have revealed that all endophytic fungal strains do not share the property of enzyme synthesis, and this specialization arises due to their specific adaptation to the environment in which their host plants are found (Sunitha et al. 2013).
Adequate literature is present in bioprospection of fungi for secondary metabolite production, but it is scarce in case of enzyme profiling of endophytic fungi. Their huge potential and promising results in preliminary tests rationalize their candidature as potent enzyme producers. Sunitha et al. (2013) postulated that the possibility of endophytic fungi actually being weak parasites or latent pathogens warrants their protease producing capacity. Since nitrogen is an important macronutrient that endophytes derive from plants, protease can be found as an integral and crucial component of the assemblage of enzymes made by endophytes. There is additional likelihood of endophytes acquiring novel protease genes from its host plants over evolutionary time (Priest 1984; Vasundhara et al. 2016). Pavithra et al. (2012) conjecture that since extracts of Basil (Ocimum sp.) containing proteases are effective in control of diabetes, protease enzymes from the endophytic fungi residing in this plant will have similar properties. Table 10.2 depicts some proteases described from fungi of endophytic origin.
10.5.4 Advantages They Might Have Over Present Fungal Sources
Fungal enzymes predominant in the industries are all from soil fungi. These enzymes are also produced by endophytic fungi, their distinguishing character being that they are biochemically adapted to the endophyte’s natural environment (Borges et al. 2009). Aspergillus niger, a soil fungus regarded as GRAS, used widely for obtaining various enzymes, has been recently found as an endophyte of several plants (Ward et al. 2005; Meijer et al. 2011). The industries not only seek sources with better production but also novel proteases and newly discovered functions of existing proteases. Attaining novel structures from fungal cultures is always lucrative and sought after. A comparative study of structure determination between soil fungi and endophytic fungi to determine the percentage of novel structures in their metabolome revealed that endophytic fungi have a higher proportion of unknown structures (51%) in their culture extracts than soil fungi (38%) (Fig. 10.3).
Since isolation, characterization and structure determination of a new compound is a tedious process, it is intelligent to screen endophytic fungi, for they offer better probability of finding novel products (Schulz et al. 2002). Zaferanloo and her team (Zaferanloo et al. 2013) showed through their work how relatively easy it is to rapidly screen endophytic fungi using low-cost substrates and identify industry-ready isolates with excellent synthesizing capacity. Enzyme biosynthesis pathways and products in endophytic fungi are adapted to a particular ecological condition and attuned to perform discrete functions. Such enzymes are often more stable and harmless than those obtained from other sources (Raju et al. 2015). Production of enzymes by endophytic fungi is more eco-friendly and sustainable and provides better quality control (Tenguria et al. 2011). Li and her associates (Li et al. 2012a) suggest through their work that endophytes isolated from plants of Baima Snow Mountain are adapted to cold climate and their enzymes might be cold attuned. Isolation of those enzymes can be helpful in biotransformation of heat-labile substances. Enzymatic profiling proves helpful in screening capable fungi, and further characterization can identify proteases that can cater to various industrial demands (Sunitha et al. 2013; Alberto et al. 2016).
10.6 Screening of Endophytic Fungi for Protease Production
10.6.1 Isolation from Appropriate Niches
The foremost step in searching potent protease-producing endophytic fungi is the selection of host plant. Two aspects have to be kept under consideration while choosing a plant specimen- ecological habitat of the plant and its established and potential phytochemistry. Among all geographical regions of the world, the tropical rainforests are speculated to harbour more than 60% of the world’s biodiversity. Plants of this region have inevitably a richer diversity of endophytic fungi than plants of other terrains. They have a larger number of representatives that are potential candidates for biosynthesis. Endophytic fungi from other phytogeographic regions might be less diverse, but there could be some unique strains that one might not find in the hot and humid equatorial regions. Choice of the plant itself is also equally important and depends on the phytochemical profile of the plant. For this reason, plants with proven medicinal properties or ethnomedicinal applications are favoured for endophyte isolation (Zaferanloo et al. 2013). Here the concept of shared properties between host and endophyte provides rationale for selection. Environmental screening programmes are set up to evaluate and select appropriate samples from a region’s vegetation. These programmes are beneficial for discovering novel enzyme synthesizers with distinct properties, and the ecological habitats of microorganisms help in anticipation of properties of the enzymes.
After selection and collection of plant sample, it must be quickly processed for isolating endophytes. Isolation is done using various culture media. Composition of the media is adjusted and modified to support growth of desired organism and suppress the growth of other undesirable organisms that might be present in the incubated tissue or could be contaminants coming from aseptic conditions. Different workers have used different isolation media, such as water-agar medium (WA), Sabouraud dextrose agar (SDA) media, malt extract agar, Czapek dox agar and potato dextrose agar (PDA) media (Patil et al. 2015; Meshram et al. 2016; Abdennabi et al. 2017; Fareed et al. 2017), for isolating fungi from plant tissues. The plant part plated for incubation is also an important element. Some parts are found to have more fungal endophytes than others, and again there is marked difference in the biosynthetic abilities of fungi based on their location within the plant body. Shubha and Srinivas (2017) performed screening tests on endophytes of Cymbidium aloifolium and found that root endophytes were most efficient in protease production, followed by endophytes of leaves and flowers. After successful isolation, the isolates are screened for protease production qualitatively and/or quantitatively. Further selection is always based on quantitative assessment of enzyme production by the isolates (dos Santos Aguilar and Sato 2018).
10.6.2 Screening Methods
Screening methods involve testing whether the endophytic fungal candidate in question is capable of producing protease. The initial screening can be performed to simply assess the presence of protease synthesizing ability, whereas further investigation ascertains if the fungus is able to synthesize in quantities useful at the industrial level. The screening procedures have to be kept constant for all the isolates. Results of screening methods provide potential producers, often more capable than commercially used strains. Endophytic fungi isolated from different plants have been screened for protease production, and many workers have obtained positive results in screening (Table 10.3). Alberto et al. (2016) found an endophytic strain of Diaporthe sp. that had proteolytic activity comparable to the commercially used Aspergillus oryzae. Screening techniques also reveal the competence in protease production of endophytic isolates procured from the same plant. Patil et al. (2015) observed that among the endophytic assemblage comprising of species of Aspergillus, Biosporus and Rhizoctonia, Biosporus sp. showed maximum protease activity. Various strains of endophytic Aspergillus, such as Aspergillus sp. from Alpinia calcarata and Aspergillus japonicus from Cymbidium aloifolium, have been reported by many workers to have remarkable proteolytic ability (Sunitha et al. 2013; Shubha and Srinivas 2017). Some rare fungal endophytes like Isaria sp. isolated from Calophyllum inophyllum and Stemphylium sp. of Eremophila longifolia have demonstrated exceptional proteolytic activity (Sunitha et al. 2013; Zaferanloo et al. 2013). Likewise, common genera of fungal endophytes have also exhibited similar properties, for example, Colletotrichum gloeosporioides and Trichoderma spp.—endophytes of Cymbidium aloifolium— and Phoma herbarum, Phoma sp. and Alternaria alternata isolated from Eremophila longifolia (Zaferanloo et al. 2013; Shubha and Srinivas 2017). Many workers have reported strains of sterile fungi to have extraordinary potential in protease production. Screening methods can also reveal if the enzyme sources are resilient to temperature and pH fluctuations, indicating potential use in industrial applications.
10.6.2.1 Solid Plate Methods
Agar plate methods are the most popular among screening methods. They are based on the fungi’s capability to utilize a polymeric nitrogen source by secreting proteolytic enzymes into the surrounding medium. It involves selection of appropriate medium composition along with a protein substrate. The fungal isolate is either directly grown on the medium or its culture broth is applied to see if it can digest the proteinaceous substrate. The digested protein gives a clear halo that is visible unaided, or it can be enhanced by flooding the plate with various chemical solutions. The solid plate screening methods are the most simple and cheap techniques that do not require much skill. But these are only qualitative in nature and tell little or nothing about the endophytes’ quantitative abilities to produce protease. A number of studies have utilized glucose yeast peptone agar (GYP-agar) medium amended with 0.4% gelatin (Maria et al. 2005; Sunitha et al. 2013; Fareed et al. 2017). Fungal blocks were incubated on the plate, and after the period of incubation, saturated ammonium sulphate solution was poured on the plates that precipitated the undigested gelatin and gave visible clear zones. Fouda and his team (2015) used the same protein substrate but used yeast-malt agar as the basal media and mercuric chloride as the indicator. Katoch and her team (2014) inoculated endophytic fungi on casein starch agar plates with 1% skimmed milk. In one study, fungi were allowed to grow on skim milk-agar plates, and the clear zones were enhanced with 10% tannic acid solution (Zaferanloo et al. 2013). Budiarto and his co-workers (2015) used PDA modified with 0.1% gelatin to grow fungi directly on this media. Trichloroacetic acid (TCA) has also been used as an indicator for precipitating unused protein substrate. Alberto et al. (2016) used Manachini solution with 0.5% gelatin as inducer for growing fungi and separated the culture broth by filtration. The broth was then placed in wells on media prepared with 10% gelatin, 10% skim milk and 2% agar-agar in citrate-phosphate buffer (pH 5.0). Commercial protease of Aspergillus oryzae was used as reference. After proper incubation, enzymatic activity was evaluated by measuring the size of the clear halo around the wells. Shubha and Srinivas (2017) devised a method for partially quantifying the proteolytic activity shown by fungi on solid plates. By measuring the zone of clearance and the diameter of fungal colony and by calculating the difference between these two, they obtained the enzyme index of respective fungal isolates, which is helpful for comparing their enzyme-synthesizing ability.
10.6.2.2 Liquid Culture Method
Spectroscopic studies involving growing the fungi in liquid media and then studying extracellular enzyme properties are more time-consuming than simple solid-media screening but carry several advantages. This quantitative method can detect infinitesimal proteolytic activity that might go undetected in agar plate assays, since spectroscopy is a sophisticated technique that is sensitive to even slight changes in optical density. Screening through liquid culture was performed by Patil et al. (2015). Filtered liquid culture broths of fungi were added to 1% casein solutions. Digestion of protein by enzyme was allowed for 1 h, followed by addition of 0.5 M trichloroacetic acid to stop the catalysis. The reaction mixtures were then centrifuged to remove precipitate, and absorption was read at 275 nm. The quantity of enzyme which liberated 1 μg of tyrosine under assay conditions was termed as one enzymatic unit. This procedure is suitable for carrying out screening and enzyme assay simultaneously.
10.6.2.3 Gel Dot Blot Method
Thirunavukkarasu et al. (2017) developed this method that allows rapid screening of a huge number of fungi for extracellular protease production as well as partial characterization of the protease. The method involves preparation of wells created onto the plate and acrylamide gel with gelatin as substrate. A composite gel is made with gel strips of varying pH—pH 5.0, pH 7.0 and pH 9.0. The lyophilized culture filtrates of fungi are spotted onto the gel and incubated for 8–10 h, followed by staining of the gel using 0.025% Coomassie Brilliant Blue. Commercially available alkaline and acidic proteases were used as controls by the authors. Enzymatic action is visualized as clear zones on the deep blue gel. This method uses Coomassie Brilliant Blue which is very sensitive to low protein concentrations, as low as 30 ng. This method is superior to usual agar plate and spectroscopic assays as:
-
It is more accurate in detecting enzyme activity and low concentrations of protein.
-
A large number of samples can be screened in a short time.
-
Their range of optimum pH can be speculated simultaneously.
10.6.2.4 Molecular/Genomic screening
Bryant and her team (2009) approached molecular techniques for identifying protease synthesis genes, particularly subtilisin-like proteases (SLPs) in the fungus Epichloë festucae, an obligate endophyte found in many grass species. Using a combination of polymerase chain reaction (PCR), transcriptome and whole genome analysis, they predicted 15 different kinds of SLPs in the genome of the endophyte. Degenerate primers for sequence amplification were designed based on the conserved SLP sequences in the evolution clade. The predicted subtilisin-like protease genes were identified in a genomic library from Neotyphodium lolii.
10.6.3 Understanding More About Protease Function and Synthesis Efficiency
10.6.3.1 Scale-Up Culture in Liquid Media
Selection of an appropriate media to produce elevated amounts of enzyme is an important task and might require several steps of trial and error. The media composition ought to be favourable for both fungal growth and enzyme secretion. In laboratory context, generally submerged cultivation is preferred over solid-state cultivation (Li et al. 2012b). Media that are typically used for luxurious growth of all kinds of filamentous fungi are applied and might be accompanied by some inducers for better production of extracellular enzyme. For harvesting secreted protease from filamentous fungi, most scientists have utilized potato dextrose broth (PDB) in scale-up cultures (Budiarto et al. 2015).
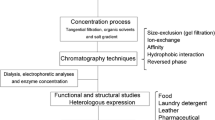
10.6.3.2 Extraction and Purification of the Protein
Purification process of enzyme can be single-step or multistep, depending upon the extent of purification demanded by its potential use. Therapeutic applications require highly purified enzymes in small amounts, whereas others like the detergent and food industry need crude enzymes in huge quantities. Methods of extraction and purification of the protease exploit some chemical and physical characteristics of the protein molecule, viz. its solubility, size, polarity, binding affinity, charge, etc.
The most elaborate method of protein extraction and purification from an endophytic fungus has been described by Budiarto and his co-workers (2015). Following large-scale culture in PDB, they implemented a three-step purification process for achieving maximum purification of the protease. The culture broth was centrifuged, and the filtered supernatant was saturated with 90% ammonium sulphate. The resulting precipitate of extracellular protein was resuspended in a buffer of 25 mM Tris-HCl (pH 7.4). The solution was then dialysed for 24 h at 4 °C in dialysis tubing and then applied on DEAE-Sepharose with the same buffer used previously. The column was eluted using gradient concentration of NaCl. Active fractions were applied onto a Sephadex SG-75 column with the same buffer. Finally, obtained active fractions were freeze-dried and resuspended in the tris-HCl buffer for further experiments. Estimation of protein content of the active fractions was done by Bicinchoninic Acid Protein Assay method. Activity of fractions at each purification step through plate assay was assessed by placing 0.5% agarose medium containing 0.2% of gelatine in Tris-HCl buffer on a Petri dish. Fractions from column chromatography were loaded into wells created onto the plate and incubated for 24 h. The development of clear zone around the wells was detected by applying Coomassie Blue dissolved in a mixture of methanol, acetic acid and water, followed by destaining step to remove staining solution using destain solution made from methanol, acetic acid and water until the clear zone could be seen visually. This three-step purification process, involving first step of ammonium sulphate precipitation followed by two steps of ion-exchange chromatography and gel filtration, was also enacted by Meshram and his associates (2016).
10.6.3.3 Characterization of the Enzymes
Ascertaining the physicochemical properties of protease is essential to identify industrial sectors it might find use in. In order to biochemically characterize the enzyme, one must determine enzyme activity and study enzyme kinetics. Most authors have followed the method described by Kunitz (1947) with varied modifications to determine protease activity. It is based on monitoring spectrophotometrically the amount of tyrosine liberated through casein hydrolysis. In work done by Maria et al. (2005) and Budiarto et al. (2015), 1 ml enzyme filtrate was added to 1 ml 2% casein suspended in 100 mM phosphate buffer (pH 7.6). After 20 min of incubation at 35 ± 1 °C, the reaction was terminated by adding 3 ml of ice cold 0.306 M TCA. One millimetre of the clear TCA soluble extract was mixed with 5 ml 0.4 M Na2CO3 and 0.5 ml 1 N Folin-Ciocalteu reagent. The absorbance was measured at 660 nm/540 nm. One unit of protease activity was defined as 1 μmol of tyrosine released during catalysis per ml of reaction mixture per minute under the experimental conditions. Mayerhofer et al. (2015) quantified protease activity of culture broths of endophytic fungi using fluorescently labelled casein as substrate. Incubation was done in citrate-citric acid buffer ranging from pH 2.0 to 6.0. Proteolysis was studied by reading the excitation at 590 nm and emission of 645 nm. Fluorescence of each sample was calculated by subtracting uninoculated control values from the obtained sample values.
Determination of optimum conditions for protease activity and enzyme kinetics study are based on varying incubation parameters such as temperature, pH and concentrations of substrate. Budiarto et al. (2015) following the protocol of Zhang et al. (2010) created a temperature gradient of 20 to 90 °C and pH gradient using different buffer systems (citrate buffer for pH 4–6, Tris-HCl for pH 7–9, glycine-NaOH for pH 10–11). The value of Km and Vmax was determined based on the Lineweaver-Burk plot created by plotting the reciprocal of substrate concentration on the X-axis and reciprocal of the enzyme reaction velocity on the Y-axis by mixing crude enzyme with different concentration of casein ranging from 0.2% to 0.02%. They found protease-specific activity to be 0.091 IU/mg, optimum temperature as 60 °C and optimum pH 7 and Km and Vmax values of 0.183 mg/ml and 7.01 μg/min, respectively. Meshram et al. (2016) through similar experiments determined specific activity of their isolated protease from endophytic Xylaria curta to be 36.67 U/mg with an optimum activity at pH 8 and temperature 35 °C. They obtained Km and Vmax of 246 μM and 1.22 U/ml towards fibrin, 282 μM and 0.13 U/ml for plasmin, 298.2 μM and 0.15 U/ml for streptokinase and 240.0 μM and 1.10 U/ml for fibrinogen, respectively.
Determination of molecular mass of the protein is either done through traditional technique of gelatin zymography or contemporary methods of mass spectrometry. Budiarto and his co-workers (2015) performed gelatin zymography for molecular mass determination by running the sample on 0.2% gelatin-containing gel electrophoresis. After complete run and separation of individual components, denatured protein was reactivated by incubation in 2.5% Triton X-100 for 40 min at 37 °C. Then the gel was stained with 0.05% Coomassie Blue and kept for 2 h. Removal of excess stain using destaining solution until clear band appeared on gel indicated protease activity. Interpolation deduced from linear logarithmic plot of relative molecular mass against Rf value of the protein band appearing on the gel gives the molecular mass of the protein. Through this method, they arrived at a molecular mass of 43 kDa on gelatin zymogram. Meshram and his team (2016) employed the sophisticated technique of MALDI-TOF (matrix-assisted laser desorption/ionization-time of flight) mass spectrometry for determining the molecular mass of their protease and found it to be a 33.76 kDa protein. Mass spectrometry assisted by MALDI-TOF is the most suitable modern method for molecular mass determination of protein that gives accurate results in a short span of time without much hassles of carrying out physical experiments.
A simple and fast way to determine the family or kind of protease is to investigate the effect of chemical inhibitors on protease activity. A number of inhibitors are used for this purpose, each one specifically inhibiting a particular class of protease as depicted below:
-
Serine protease inhibitors—phenylmethylsulfonyl fluoride (PMSF) and Leupeptin
-
Metalloprotease inhibitors—ethylenediaminetetraacetic acid (EDTA) and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA)
-
Cysteine protease inhibitors—tosyl phenylalanyl chloromethyl ketone (TPCK), iodoacetate, E-64 and Leupeptin
-
Aspartic protease inhibitor—pepstatin A
-
Threonine inhibitor—Leupeptin
By studying residual activity upon inhibition, Meshram et al. (2016) characterized their enzyme as a metalloprotease which was inhibited by EDTA and EGTA. Budiarto et al. (2015) identified theirs to be a serine protease as it was inhibited by PMSF. Characterization of protease reasserts their suitability for use in industrial settings where robustness and resilience are required over uncontrollable physical conditions that may lead to hostile temperatures, pH, presence of inhibitors and oxidizing agents. Proteases with broad range of activity and versatile applications are always coveted.
10.7 Industry Scale Production
10.7.1 Optimization
Under natural conditions without forcing any kind of manipulation, proteases produced by endophytic fungi are quite moderate in amount. To upscale their production to levels that are industrially sustainable, optimization of the fermentation process needs to be done. Protease production by endophytic fungi is affected by several intrinsic as well as extrinsic parameters. Intrinsic factors include the morphology and metabolic state of the fungal culture, while extrinsic factors consist of external media composition, temperature, pH, aeration, presence of inhibitors and inducers, competing species, etc. Process optimization has recently come under focus with regard to fungal endophytes, as they are increasingly finding use in industrial processes as manufacturers of bioactive products. Optimization studies shed light on the interactions between various factors affecting production, so that negative interactions can be avoided and positive interactions can be promoted. Finding the optimum fermentation conditions begins with fixing on the type of fermentation to be approached—solid or submerged. Parameters common to both types and exclusive to each type need to be assessed for establishing the most appropriate conditions for protease production with regard to a particular strain of endophytic fungi.
In the past few years, several researchers have performed optimization of protease production by endophytic fungal isolates. Maria et al. (2005) optimized the period of incubation best suited for Pestalotiopsis sp. in static culture using wheat bran seawater medium, observing fluctuations over 3, 6, 9, 12 and 15 days of culture. Zaferanloo and her co-workers (2013) assessed protease production by endophytes over a range of pH and incubation temperature and concluded that enzyme secretion was dominant at low pH and low temperatures. Comprehensive optimization of protease production by endophytic strain Alternaria alternata EL17 was accomplished by Zaferanloo and her team (2014). Regulation of fermentation process needs study of impact of changes in one parameter while keeping other parameters constant. Culture conditions that are economically viable need to be ascertained. The discrete parameters affecting enzyme production are briefly described below.
10.7.1.1 Incubation Period
Age of the culture affects enzyme production significantly since production of each metabolite is characteristic of a definite phase in the growth curve of a fungus. Optimum enzyme production can occur anytime between 24 h incubation to a week depending on the culture conditions and metabolic state of the fungus. Budiarto et al. (2015) noted that the early phase produced maximum harvest of protease from Xylaria psidii. Endophytic Alternaria citrimacularis and Curvularia australiensis both showed maximum enzyme secretion at 7th and 11th day which then remained constant up to day 20 (Mani et al. 2018). Maria and her team (2005) made the observation that protease production reached peak on 6th day irrespective of pH of the culture solution. Though incubation period acts as a critical element, in many cases, it has been found that enzyme production by an organism is not growth associated (Sharma et al. 2017).
10.7.1.2 pH
pH of the culture medium is pivotal in determining the growth and morphology of the organism since cells are sensitive to the hydrogen ion concentration of the surrounding media (Rajput et al. 2016). pH of the media regulates all enzymatic reactions and transports across membrane, affecting chemiosmosis by proton motive force. Under optimum pH levels, the metabolic efficiency of a cell is highest, and consequently, its enzyme synthesis is also at its highest at this pH (Sharma et al. 2017). Optimum pH of culture conditions may or may not reflect the optimum pH of the protease. Mani et al. (2018) observed optimum pH for fermentation of endophytic Alternaria citrimacularis and Curvularia australiensis to be pH 7. Similar observation was made by Zaferanloo and team (2013) in case of Phoma moricola, Nigrospora sp., Cladosporium sp. and Alternaria spp. and in another work by Zaferanloo et al. (2014) in fermentation of Alternaria alternata. Zaferanloo and her co-workers (2013) reported Stemphylium sp. and Phoma herbarum to have maximum protease activity at the alkaline pH of 9, while Phoma minima had an acidic pH optimum of 5.5. Rajput et al. (2016) studied relative effect of different pH ranging from acidic to alkaline and found neutral pH range to be most suitable, followed by alkaline range and drop in activity at acidic pH range.
10.7.1.3 Temperature
Temperature is one of the most vital parameters that need to be controlled and kept in optimum range for maximum cell growth and enzyme synthesis. Fluctuations in incubation temperature can throw the organism into stress conditions and lead to the synthesis of obnoxious toxic metabolites, reducing the output of protease. Optimum temperature required by the fungus corresponds to its habit, whether it is psychrophilic, mesophilic or thermophilic. Most endophytic fungi produce maximum protease at the thermophilic range (37 °C) like Leptosphaerulina sp., Phoma minima and Alternaria alternata (Zaferanloo et al. 2013). Zaferanloo et al. (2014) also found their strain of endophytic Alternaria alternata to be most active at 37 °C. They discovered best production of protease in Phoma herbarum at 50 °C and in another species of Phoma at 9 °C.
10.7.1.4 Metal Ions
Various chemicals and metal ions have been reported to have modulatory effects on enzyme synthesis pathways. Some act as inducers, while others have inhibitory effects. Calcium ions are, in general, known to be inducers and help in stabilizing many enzymes by preventing conformational changes. This was confirmed by Meshram et al. (2016) as Ca2+ increased xylarinase activity. Proteolysis by this enzyme was found to decrease in presence of Cu2+ and Mn2+ and was completely inhibited by Zn2+ and Fe2+.
10.7.1.5 Substrate
Selection of a substrate is perhaps the most important factor in making the enzyme production process commercially feasible. The culture media claims up to 30% of the cost of enzyme manufacture. Hence, it is important to use ingredients that maximize protease production while cost-cutting at the same time. Submerged fermentation allows amalgamation of different ingredients, each having positive upregulating effect on the process and giving the liberty of exclusively selecting each macro- and micronutrient. Solid-state fermentation allows the use of low-cost substrates from industrial and agricultural wastes. Solid wastes like wheat straw or barley, sugar cane bagasse, coffee pulp, grape wastes, copra paste, inert materials like resins of ionic exchange, acrolite or polyurethane foam have been applied for use as solid substrates for protease production. Gabres et al. (2016) investigated the proteolytic activity of endophytic fungi of bamboo leaves on the bamboo leaf litter through solid-state fermentation. They utilized bamboo leaves as a substrate with distilled water making moisture levels of 60–65%. Sometimes, two or more substrates are used in combination to elevate yield of protease. Substrate selection based on optimization must be verified for cost-effectiveness and regular supply and availability of the raw materials.
10.7.1.6 Carbon Source
Carbon is the element most abundantly required by any organism. Besides being a nutritional requirement, the carbon source also affects protease synthesis by having upregulating or downregulating activity. Various organic and inorganic sources of carbon have been investigated for their effect on proteolytic activity of endophytic fungi. Rajput et al. (2016) concluded that glucose was most effective in optimizing protease secretion, followed by maltose, sucrose, galactose and lactose. Zaferanloo et al. (2014) found the complex carbohydrates of soybean to be most effective, among starch, glucose, sucrose and maltose when used as a carbon source. Interestingly, some carbon sources inhibit protease synthesis through catabolic repression mechanism. In their absence, the protease has to play an additional role of providing carbon from amino acids. Conversely, protease activity declines when the energy status of the cell is high and the cell has overabundance of carbon source. It is now known that the catabolite control protein (CcpA) is responsible for such regulation and acts as a signal for the repression in protease synthesis (Tehran et al. 2016). Hence, it is important to identify such sources of carbon to either avoid them in media composition or to adjust their concentrations to achieve desirable results.
10.7.1.7 Nitrogen Source
As important variable needed for growth and sustenance, nitrogen is preferable in diverse forms by each living being. Since nitrogen is requisite for amino acid and hence protein synthesis, adequate amounts of the form feasibly processable by the fungus need to be provided in the culture medium. Researchers have utilized a number of organic and inorganic, simple and complex forms of nitrogen to find the one that provides highest yield of protease. Zaferanloo et al. (2014) investigated impact of tryptone, yeast extract, casein, peptone and l-asparagine on protease production and found tryptone to give the most desirable results. Rajput and co-workers (2016) report yeast extract to be most suitable, followed by beef extract, peptone, ammonium nitrate, ammonium carbonate and urea.
10.7.1.8 Moisture Content
In case of solid-state fermentation, where the water availability is limited, it is necessary to provide the adequate amount of moisture for optimal growth and produce. Increased moisture content decreases porosity of the substrate, thereby reducing oxygen availability to the growing mycelia. As reduced porosity decreases gas exchange, temperature of the solid substrate rises, disturbing the ideal conditions of incubation. Low moisture content retards growth, decreases nutrient solubility and lowers the degree of swelling of substrate, all contributing to poor yield of enzyme. Optimized water levels in the substrate are necessary both for proper growth and fermentation as well as ease of product recovery (Sharma et al. 2017).
10.7.1.9 Particle Size of Substrate
Surface area available for growth of fungal mycelia in solid culture is important in determining enzyme synthesis rate. Smaller particles provide greater surface area for fungal hyphae to grow and attach to, facilitating nutrient exchange. But it may also lead to agglutination of the particles, resulting in decrease in aeration and diffusion. Larger particle size enhances diffusion but limit the surface area for growth. Hence, for optimum production of enzyme, a compromised particle size needs to be provided (Sharma et al. 2017).
10.7.1.10 Agitation and Aeration
In submerged fermentation, agitation and aeration of the liquid culture media are required for two reasons—for dissolving oxygen needed by the growing hyphae and for homogenization of mycelial mass, nutrients and products within the culture broth (dos Santos Aguilar and Sato 2018).
10.7.2 Systems of Fermentation
Fermentations performed at the laboratory scale are mostly submerged fermentation (Smf). Industries employ both submerged and solid-state fermentation (Ssf) for elevated levels of product formation. The difference between the two fermentation techniques lies in the availability of free water to the growing fungal filaments. Both systems have their own benefits and drawbacks, and choosing the appropriate technique is crucial for optimum product recovery.
10.7.2.1 Submerged Fermentation
This fermentation technique involves growing the fungal culture in liquid substratum with predefined composition. Submerged culturing allows greater control over incubation parameters, such as temperature, aeration and pH. Individual ingredients of the media can be adjusted according to demand of fermentation process. It has added advantage of ease of sterilization of media. Despite being cost-intensive, due to the benefits this process provides, submerged cultivation is preferred for protease synthesis where consistent production is required.
10.7.2.2 Solid-State Fermentation
Solid-state fermentation is the cultivation of filamentous fungi over solid material in the absence of any free liquid. Ssf is a promising technology that allows the use of agro-industrial wastes, carrying out both enzyme synthesis and degradation of waste material discarded by industries. Through ssf, many industrial residues have been put to good use, like cassava bagasse, sugarcane bagasse, sugar beet pulp/husk, orange bagasse, oil cakes, apple pomace, grape juice, grape seed, coffee husk, wheat bran, coir pith, etc., and have been used as raw materials for growing protease producing fungi (Bhargav et al. 2008). Ssf provides many advantages over smf and is considered more instinctive for fungi, since in nature filamentous fungi are found growing on solid substrates. Due to minimal amounts of available water, ssf results in formation of highly concentrated products that make the downstream processing quite effortless. Solid culture also deters bacterial contamination. Ssf is emerging as an economically and ecologically viable option that requires low capital investment, simpler machinery, low-energy input, use of cheap substrate and reduced catabolite repression and yields superior productivity and low wastewater output (Sharma et al. 2017).
10.7.2.3 Downstream Process
After completion of fermentation, the protease must be separated, concentrated and purified. This final step in commercial enzyme production is known as downstream processing or bioseparation. It can constitute up to 60% of the total production costs, excluding the charge incurred in purchase of raw materials. The downstream processing includes methods such as extraction, concentration, purification and stabilization and requires various chemical solvents, highly efficient machinery and skilled labour. The general scheme representing downstream processing for enzyme isolation is depicted in Fig. 10.4.
10.8 Attempts at Characterizing Proteases with Specific Industrial Use
Most studies involving proteolytic abilities of endophytic fungi have been confined to qualitative screening for enzyme production. Despite promising display of proteolytic activity by many endophytic strains, it would be superfluous to expect endophytic fungi and their proteases to be superior to the existing sources and enzymes. In this chapter, a few representative studies have been described where the researchers have went beyond conventional approaches and established their discovered proteases as appealing solutions to lingering problems.
10.8.1 Detergent Industry
Among all commercial protease applications, detergent industries are the most dominant protease demanders. For this particular application, proteases need to have certain characteristics, like optimum activity at high and low temperatures and high pH, stability in presence of chelating and oxidizing agents, etc. Sources that secrete protease in huge amounts in cultivation media are sought after. Zaferanloo and her team (2013) studied physicochemical properties of protease produced by Phoma herbarum isolated from Eremophila longifolia and characterized this protease to be active at low temperatures and high pH. They suggest potential use of this protease in detergents for cold washing. Suggested disposition is through wax-coated granules to prevent inhalation of protease dust.
10.8.2 Food Industry
Rajput and his co-workers (2016) identified the optimum conditions for the protease production of endophytic isolate of Alternaria alternata, and their findings show that the endophyte possesses the ability to produce protease in a wide range of pH (3–12) and temperature (25–50 °C). The optimum temperature and pH for fermentation were noted to be 27 °C and pH 7, respectively. Deducing from these preliminary data, the authors suggest that the protease from A. alternata EL-17 can be applied to cheese-making and in milk clotting where the fermentation conditions are suitable for the activation of protease and its limited thermal tolerance ensures deactivation upon cooking. Zaferanloo et al. (2013) characterized protease of Phoma sp. as being most active at low pH and low temperature, making it a suitable candidate for use in food and confectionary.
10.8.3 Biomedical Sectors
A few workers have screened endophytes with the distinctive purpose of investigating their fibrinolytic activity and potential use in thrombolytic therapy. Naturally produced fibrinolytic agents can play a pivotal role in thrombolytic therapy which might be able to cure many heart-related diseases that are caused by accumulation of fibrin, the primary protein component of blood clot, in blood vessels forming a haemostatic plug or clot. Diseases such as myocardial infarction, high blood pressure, valvular heart disease and ischaemic heart diseases call for such thrombolytic therapy that is cost-effective with fewer side effects (Meshram et al. 2016). Significant contribution in this sector was made by Wu et al. (2009) who described a novel fibrinolytic enzyme from the endophyte Fusarium sp. CPCC 480097. The endophyte was isolated from chrysanthemum stems, and protease produced by it showed excellent fibrinolytic activity. The protease was studied to be a 28 kDa protein, with an isoelectric point of 8.1 and maximum fibrinolysis at 45 °C and pH 8.5.
Li et al. (2007) tested endophytic isolates of Clonostachys sp., Cladosporium sp., Fusarium sp. BLB and Verticillium sp. from Trachelospermum jasminoides for in vitro thrombolytic, fibrinolytic, fibrinogenolytic and anticoagulant activity and found positive results. Another endophyte isolated from Hibiscus leaves was screened for fibrinolytic activity after observing its capability of utilizing skim-milk agar. Ahmad et al. (2014) tested the fibrinolytic activity of two endophytic fungi, identified as Penicillium citrinum and Fusarium sp. through fibrin plate screening and found both of these to possess positive fibrinolytic protease activity.
The most remarkable work was done by Meshram and his team (2016) in their description of a bifunctional metalloprotease produced by Xylaria curta that they have named xylarinase. The protease possesses superlative plasmin-like ability of hydrolysing fibrin, independent of plasminogen. Xylarinase can hydrolyse both fibrin and its precursor. Dose-dependent dissolution of thrombus revealed minimum amount of 50 μl of protease required. This suggested better efficacy than plasmin. N-terminal sequencing of the protein revealed it to be a novel protease. Its molecular mass was determined at 33.76 kDa. The mechanism of its action is postulated to involve blocking the activation of blood clotting cascade by suppressing the thrombin pathway. It has been shown to have no hemorrhagic effect in vitro. As stated by the authors, Xylarinase stands out as a prospective candidate in producing therapeutic agents, as evidenced in preclinical studies in thrombolytic therapy.
10.8.4 Agri-industries
10.8.4.1 Litter Degradation
The role of endophytes in litter degradation of their associated senescent host tissues had been postulated for many years. Two unrelated groups of researchers confirmed this hypothesis through their work. Kumaresan and Suryanarayanan (2002) studied the role of foliar endophytes in mangrove litter degradation. The endophytic assemblage of intact as well as senescent leaves in both wet and dry fallen conditions was investigated, and the enzyme activity of the isolated endophytes was tested. Glomerella sp. MG108 was found to be an active producer of protease and many other hydrolytic enzymes that degrade the plant litter. Endophytic community, in a whole, possesses the complete enzyme array to degrade leaf litter, and protease is an important part of that conglomeration of hydrolytic enzymes. The authors suggest that future studies involving the role of endophytes in agri-industry waste degradation is worthwhile and holds promising results.
Sun et al. (2011) also concluded through similar findings that the degrading enzymes of endophytes of Acer truncatum had a significant role to play in litter degradation. Gabres et al. (2016) studied the digestion of bamboo leaf litter by native endophytic fungi. The fungi were able to lower the protein content of the litter through proteolysis. The workers noticed an increased amount of fibre in the fermented litter that could have been formed due to increased tannin-protein complex production and suggest the use of such leaves as fodder for horses, as increased fibre imparts greater stamina through improved digestion and peristalsis. The authors foresee that these endophytes have the potential for use in industries as sources of protease. Orlandelli et al. (2015) vouch for the use of agro-industrial wastes and other such waste products as substrates for production of proteases by endophytic fungi. They found that endophytic fungi from Piper hispidum could efficiently produce protease in media consisting of rice flour and soy flour, both by-products of agro-industries. This can provide the industries with cost-effective raw materials that are both cheap and abundantly available.
10.8.4.2 Protease in Bio-control Tool Designing
While insecticides have been the primary dependence to protect commercial crops, it has not gone unnoticed that they cause severe deterioration of the environment and are also detrimental to all forms of life. Keeping in mind these facts and the increasing instances of insecticide resistance in previously susceptible pests, scientists have been working on devising safer options of biological control (Kour et al. 2017). In this front, Bensaci et al. (2015) observed that the endophytic fungus Cladosporium oxysporum isolated from Euphorbia bupleuroides subsp. luteola can be effectively used to control the black bean aphid (Aphis fabae) through formulations that contain protease from the endophyte. These genera include some species that are natural entomopathogens. Finding endophytic forms of this natural entomopathogen increases the chances of obtaining biocontrol formulations that are fast-effective and stable. The authors created invert emulsions of conidial suspensions of the fungus, characterized by a discontinuous aqueous phase within a continuous oily phase. Spray treatment of the aphids by the invert emulsions resulted in more than 90% mortality within half an hour. The fungus could effectively germinate and invade the cuticle of the aphid. Proteolytic activity of the fungus is a predominant factor in this pathogenesis. The enzyme is responsible for degradation of the cuticle of insect and invasion of the fungal hyphae; its production and activity were observed to be consistent with the aphicidal activity. Authors note that the fungus and its proteolytic formulations can be successfully exploited in biological control programmes for several aphids in semiarid and arid agricultural ecosystems. Other studies involving aphicidal activity of endophytic counterparts of natural entomopathogenic fungi have demonstrated that endophytic fungi are more efficient in producing proteases that increase chances of successful colonization inside the host insect.
In a similar vein, Potshangbam and her co-workers (2017) tested endophytic fungal isolates for enzyme production and inhibition of pathogens in the quest to devise a bio-control agent. They also studied their growth parameters in different environmental conditions that could reflect their dynamic living conditions inside host plants. Their results conclude that protease is a key enzyme that decides their suitability as a biocontrol agent as it provides protection against insect pests. The study found that the endophytic fungal isolates that produced highest amounts of protease were able to inhibit pathogens and also their colonization following artificial inoculation was successful. The authors suggest that such endophytic fungi are promising bio-resource agents.
10.8.5 Bioremediation
A rather prodigious work concerning practical application of endophytic protease was done by Russell and his associates (2011). Endophytic fungi were isolated from rainforest trees and screened for their ability to degrade the polyester polyurethane (PUR). PUR is a widely used polymer that has responded to a few attempts of biodegradation. Screening for positive-degrading ability was done by inoculating the endophytic fungal isolates in media containing PUR as the sole carbon source. Two strains of Pestalotiopsis microspora were found to possess excellent ability to degrade PUR even under anaerobic conditions. The enzyme responsible for degradation was identified as a diffusible secreted protein obtained by filtration through a 0.22 μm membrane that was denatured when the temperature was raised to 98 °C. The enzyme was further characterized to be a serine protease when it was observed that serine hydrolase-specific inhibitor PMSF inhibited its activity. The culture filtrates containing the enzyme were capable of clearing the polymer to a huge extent within a short period of time. Authors conjecture that endophytes are a useful bioresource and these enzymes from endophytes can be effectively utilized in bioremediation programs. They also suggest screening of endophytes for finding out degraders of other recalcitrant polymers that have been polluting the ecosystem .
10.9 Conclusion and Future Prospects
Growth of civilization has always depended on searching for newer and better resources. Focus is now on sustainable growth that aims at overall well-being of the planet. For adopting a carbon-neutral mode of development, enhancement of existing products and processes for increased efficiency and rational systems of waste management are required. Endophytic fungi as protease producers are advocates of sustainable system. With a limited number of studies, their modus operandi has been substantiated to be more efficacious than other traditional sources of proteases. Novel proteases have been extracted from them that have the potential to function in challenging areas and provide solution to lingering problems ranging from the field of therapeutics to bioremediation. These primary metabolites are promising substitutes to harmful inorganic chemicals and secondary metabolites in industrial processes. This emerging field of research welcomes more, in-depth studies that will enable us to delve into the arsenal of metabolites produced by endophytic fungi and their applications on a broad scale in the practical world.
References
Abdennabi R, Triki MA, Salah RB, Gharsallah N (2017) Antifungal activity of Endophytic fungi isolated from date palm sap (Phoenix dactylifera L.). EC Microbiol 13(4):123–131
Ahmad MS, Noor ZM, Ariffin ZZ (2014) Isolation and identification fibrinolytic protease endophytic fungi from Hibiscus leaves in Shah Alam. Int J Agri Biosyst Eng 8(10):1104–1107
Alberto RN, Costa AT, Polonio JC, Santos MS, Rhoden SA, Azevedo JL, Pamphile JA (2016) Extracellular enzymatic profiles and taxonomic identification of endophytic fungi isolated from four plant species. Genet Mol Res. https://doi.org/10.4238/gmr15049016
Amirita A, Sindhu P, Swetha J, Vasanthi NS, Kannan KP (2012) Enumeration of endophytic fungi from medicinal plants and screening of extracellular enzymes. World J Sci Technol 2:13–19
Anbu P, Gopinath SCB, Cihan AC, Chaulagain BP (2013) Microbial enzymes and their applications in industries and medicine. Biomed Res Int 204014. https://doi.org/10.1155/2013/204014
Anwar A, Saleemuddin M (1998) Alkaline proteases: a review. Bioresour Technol 64:175–183
Arnold AE (2007) Understanding the diversity of foliar endophytic fungi: progress, challenges and practise. Fungal Biol Rev 21:51–56
Bensaci OA, Daoud H, Lombarkia N, Rouabah K (2015) Formulation of the endophytic fungus Cladosporium oxysporum Berk. & M.A. Curtis, isolated from Euphorbia bupleuroides subsp. luteola, as a new biocontrol tool against the black bean aphid (Aphis fabae Scop.). J Plant Prot Res 55(1):80–87
Bezerra JD, Santos MG, Svedese VM, Lima DM, Fernandes MJ, Paiva LM, Souza-Motta CM (2012) Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J Microbiol Biotechnol 28:1989–1995
Bhagobaty RK, Joshi SR (2012) Enzymatic activity of fungi endophytic on five medicinal plant species of the pristine sacred forests of Meghalaya, India. Biotechnol Bioprocess Eng 17:33–40
Bhargav S, Panda BP, Ali M, Javed S (2008) Solid-state fermentation: an overview. Chem Biochem Eng Q 22:49–70
Birrane G, Bhyravbhatla B, Navia MA (2014) Synthesis of aspartame by thermolysin: an X-ray structural study. ACS Med Chem Lett 5:706–710
Borges WS, Borges KB, Bonato PS, Said S, Pupo MT (2009) Endophytic fungi: natural products, enzymes and biotransformation reactions. Curr Org Chem 13:1137–1163
Brandelli A, Daroit DJ, Riffel A (2010) Biochemical features of microbial keratinases and their production and applications. Appl Microbiol Biotechnol 85:1735–1750
Bryant MK, Schardl CL, Hesse U, Scott B (2009) Evolution of subtilisin–like protease gene family in the grass endophytic fungus Epichloë festucae. BMC Evol Biol 9:168
Budiarto BR, Mustopa AZ, Tarman K (2015) Isolation, purification and characterization of extracellular protease produced by marine-derived endophytic fungus Xylaria psidii KT30. J Coast Life Med 3(1):56–63
Caldwell BA, Jumpponen A, Trappe JM (2000) Utilization of major detrital substrates by dark-septate, root endophytes. Mycologia 92:230–232
Canavan HE, Cheng X, Graham DJ, Ratner BD, Castner DG (2005) Cell sheet detachment affects the extracellular matrix: a surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J Biomed Mater Res A 75(1):1–13
Carroll G (1988) Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology 69(1):2–9
Chathurdevi G, Gowrie SU (2016) Endophytic fungi isolated from medicinal plant – a promising source of potential bioactive metabolites. Int J Curr Pharm Res 8(1):50–56
Choi YW, Hodgkiss IJ, Hyde KD (2005) Enzyme production by endophytes of Brucea javanica. J Agric Technol 1:55–56
Correa RCG, Rhoden SA, Mota TR, Azevedo JL, Pamphile JA, de Souza CGM, Polizeli MLTM, Bracht A, Peralta RM (2014) Endophytic fungi: expanding arsenal of industrial enzyme producers. J Ind Microbiol Biotechnol 41:1467–1478
Craik CS, Page MJ, Madison EL (2011) Proteases as therapeutics. Biochem J 435(1):1–16
dos Santos Aguilar JG, Sato HH (2018) Microbial proteases: production and application in obtaining protein hydrolysates. Food Res Int 103:253–262
Ebeling W, Hennrich N, Klockow M, Metz H, Orth HD, Lang H (1974) Proteinase K from Tritirachium album limber. Eur J Biochem 47:91–97
Fareed S, Jadoon UN, Ullah I, Jadoon MA, Rehman MU, Bibi Z, Waqas M (2017) Isolation and biological evaluation of endophytic fungus from Ziziphus nummularia. J Entomol Zool Stud 5(3):32–38
Fouda AH, Hassan SED, Eid AH, Ewais EED (2015) Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann Agric Sci 60(1):95–104
Gabres CA, Undan JR, Valentino MJG (2016) Proteolytic enzyme like activity of fungal endophytes and their effects in the proximate composition of dried bamboo leaves. Int J Biol Pharm Allied Sci 5(6):1298–1306
Gulrajani ML, Gupta SV (1996) Degumming of silk with different protease enzymes. Indian J Fibre Text Res 21:270–275
Gupta R, Beg QK, Chauhan B (2002a) An overview on fermentation, downstream processing and properties of microbial proteases. Applied Microbiol Biotechnol 60:381–395
Gupta R, Beg QK, Larenz P (2002b) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Harrison RL, Bonning BC (2010) Proteases as insecticidal agents. Toxins 2:935–953
Hasan S, Ahmad A, Purwar A, Khan N, Kundan R, Gupta G (2013) Production of extracellular enzymes in the entomopathogenic fungus Verticillium lecanii. Bioinformation 9:238–242
Hawksworth DL (1991) The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res 95:641–655
Hinton DM, Bacon CW (1985) The distribution and ultrastructure of the endophyte of toxic tall fescue. Can J Bot 63:36–42
Holliger P, Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nature Biotechnol 23:1126–1136
Huang HL, Hsing HW, Lai TC, Chen YW, Lee TR, Chan HT, Lyu PC, Wu CL, Lu YC, Lin ST, Lin CW, Lai CH, Chang HT, Chou HC, Chan HL (2012) Trypsin-induced proteome alteration during cell subculture in mammalian cells. J Biomed Sci 17(1):36
Hyde KD (2001) Increasing the likelihood of novel compound discovery from filamentous fungi. In: Pointing SB, Hyde KD (eds) Bio-Exploitation of filamentous fungi, Fungal Diversity Research Series 6, Fungal Diversity Press, Hong Kong, pp 77–91
Katoch M, Salgotra A, Singh G (2014) Endophytic fungi found in association with Bacopa monnieri as potential producers of industrial enzyme and antimicrobial bioactive compounds. Braz Arch Biol Technol 57(5):714–722
Katoch M, Singh A, Singh G, Wazir P, Kumar R (2017) Phylogeny, antimicrobial, antioxidant and enzyme-producing potential of fungal endophytes found in Viola odorata. Ann Microbiol 67:529–540
Kirk O, Borchert TV, Fuglsan CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13:345–351
Kour D, Rana KL, Verma P, Yadav AN, Kumar V, Singh DH (2017) Biofertilizers: eco-friendly technologies and bioresources for sustainable agriculture. In: Proceeding of International Conference on Innovative Research in Engineering Science and Technology IREST/PP/014
Kühn D, Dürrschmidt P, Mansfeld J, Ulbrich-Hofmann R (2002) Biolysin and thermolysin in dipeptide synthesis: a comparative study. Biotechnol Appl Biochem 36:71–76
Kumar CG, Takagi H (1999) Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv 17:561–594
Kumaresan V, Suryanarayanan TS (2002) Endophyte assemblages in young, mature and senescent leaves of Rhizophora apiculata: evidence for the role endophytes in mangrove litter degradation. Fungal Divers 9:81–91
Kunitz MJ (1947) Crystalline soybean trypsins inhibitor II. General properties. J Gen Physiol 30:291–310
Ladenburger WU, Richter W, Moeller P, Boehm BO (1997) Protease treatment delays diabetes onset in diabetes-prone nonobese diabetic (NOD) mice. Int J Immunother 13(3/4):75–78
Lee JM, Tan WS, Ting ASY (2014) Revealing the antimicrobial and enzymatic potentials of culturable fungal endophytes from tropical pitcher plants (Nepenthes sp.). Mycosphere 5(2):364–377
Li Y, Shuang JL, Yuam WW, Huang WY, Tan RX (2007) Verticase: a fibrinolytic enzyme produced by Verticillium sp. Tj33, an endophyte of Trachelospermum jasminoides. J Integr Plant Biol 49:1548–1554
Li HY, Shen M, Zhou ZP, Li T, Wei Y-I, Lin L-B (2012a) Diversity and cold adaptation of endophytic fungi from five dominant plant species collected from the Baima Snow Mountain, Southwest China. Fungal Divers 53:1–8
Li S, Yang X, Yang S, Zhu M, Wang X (2012b) Technology prospecting on enzymes: application, marketing and engineering. Comput Struct Biotechnol J 2:1–11
Lindstrom JT, Belanger FC (1994) Purification and characterization of an endophytic fungal proteinase that is abundantly expressed in the infected host grass. Plant Physiol 106:7–16
Littlewood JM, Wolfe SP, Conway SP (2006) Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol 41:35–49
Mani VM, Soundari AJPG, Preethi K (2018) Enzymatic and phytochemical analysis of endophytic fungi on Aegle marmelos from Western Ghats of Tamil Nadu, India. Int J Life Sci Pharma Res 8(1):1–8
Maria GL, Sridhar KR, Raviraja NS (2005) Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. J Agr Tech 1:67–80
Mayerhofer MS, Fraser E, Kernaghan G (2015) Acid protease production in fungal root endophytes. Mycologia 107(1):1–11
Meijer M, Houbraken JAMP, Dalhuijsen S, Samson RA, Vries RO (2011) Growth and hydrolase profiles can be used as characteristics to distinguish Aspergillus niger and other black aspergilla. Stud Mycol 69:19–30
Meshram V, Saxena S, Paul K (2016) Xylarinase: a novel clot busting enzyme from an endophytic fungus Xylaria curta. J Enzyme Inhib Med Chem 31(6):1502–1511
Mótyán JA, Tóth F, Tőzsér J (2013) Research applications of proteolytic enzymes in molecular biology. Biomol Ther 3:923–942
Najafi MF, Deobagkar D, Deobagkar D (2005) Potential application of protease isolated from Pseudomonas aeruginosa PD100. Electron J Biotechnol 8:197–203
Ng’ang’a MP, Kahangi EM, Onguso JM, Losenge T, Mwaura P (2011) Analyses of extra-cellular enzymes production by endophytic fungi isolated from bananas in Kenya. Afr J Hortic Sci 5:1–8
Nielsen RI, Oxenboll K (1998) Enzymes from fungi: their technology and uses. Mycologist 12:69–71
Ningthoujam DS, Kshetri P, Sanasam S, Nimaichand S (2009) Screening, identification of best producers and optimization of extracellular proteases from moderately halophilic alkali thermotolerant indigenous actinomycetes. World Appl Sci J 7:907–916
Okumura H, Watanabe R, Kotoura Y, Nakane Y, Tangiku O (1997) Effects of a proteolytic enzyme preparations used concomitantly with an antibiotic in Osteroarticular infections. Jpn J Antibiot 30(3):223–227
Orlandelli RC, de Almeida TT, Alberto RN, Polonio JC, Azevedo JL, Pamphile JA (2015) Antifungal and proteolytic activities of endophytic fungi isolated from Piper hispidum Sw. Braz J Microbiol 46(2):359–366
Østergaard LH, Olsen HS (2010) Industrial applications of fungal enzymes. In: Hofrichter XM (ed) The mycota. Springer, Berlin, pp 269–290
Patel C, Yadav S, Rahi S, Dave A (2013) Studies on biodiversity of fungal endophytes of indigenous monocotaceous and dicotaceous plants and evaluation of their enzymatic potentialities. Int J Sci Res Publ 3(7):1–4
Patil MG, Pagare J, Patil SN, Sidhu AK (2015) Extracellular enzymatic activities of endophytic fungi isolated from various medicinal plants. Int J Curr Microbiol App Sci 4(3):1035–1042
Pavithra N, Sathish L, Ananda K (2012) Antimicrobial and enzyme activity of endophytic fungi isolated from Tulsi. J Pharm Biomed Sci 16(12):1–5
Peterson R, Grinyer J, Nevalainen H (2011) Extracellular hydrolase profiles of fungi isolated from koala faeces invite biotechnological interest. Mycol Progr 10:207–218
Potshangbam M, Indira Devi S, Sahoo D, Strobel GA (2017) Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front Microbiol 8:325
Prathyusha P, Rajitha Sri AB, Satya Prasad K (2015) Diversity and enzymatic activity of foliar endophytic fungi isolated from medicinal plants of Indian dry deciduous forest. Pharm Lett 7(8):244–251
Priest FG (1984) Extracellular enzymes. In: Aspects of microbiology, Volume 9. Van Nostrand Reinhold, Wokingham, UK
Promputtha I, Lumyong S, Dhanasekaran V, McKenzie EHC, Hyde KD, Jeewon R (2007) A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb Ecol 53:579–590
Rajput K, Chanyal S, Agrawal PK (2016) Optimization of protease production by endophytic fungus, Alternaria alternata isolated from gymnosperm tree- Cupressus torulosa D.Don. World J Pharm Pharm Sci 5(7):1034–1054
Raju DC, Thomas SM, Thomas SE (2015) Screening for extracellular enzyme production in endophytic fungi isolation from Calophyllum inophyllum L. leaves. J Chem Pharm Res 7:900–904
Rana KL, Kour D, Yadav AN, Kumar V, Dhaliwal HS (2016a) Biotechnological applications of endophytic microbes associated with barley (Hordeum vulgare L.) growing in Indian Himalayan regions. In: Proceeding of 86th Annual Session of NASI & Symposium on “Science, Technology and Entrepreneurship for Human Welfare in The Himalayan Region”, p 80
Rana KL, Kour D, Yadav AN, Kumar V, Dhaliwal HS (2016b) Endophytic microbes from wheat: diversity and biotechnological applications for sustainable agriculture. In: Proceeding of 57th Association of Microbiologist of India & International symposium on “Microbes and Biosphere: What’s New What’s Next”, p 453
Rana KL, Kour D, Verma P, Yadav AN, Kumar V, Singh DH (2017) Diversity and biotechnological applications of endophytic microbes associated with maize (Zea mays L.) growing in Indian Himalayan regions. In: Proceeding of National Conference on Advances in Food Science and Technology, pp 41
Rao MB, Tanskale AM, Ghatger MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62(3):597–635
Rawlings ND, Tolle DP, Barrett AJ (2004) MEROPS: the peptidase database. Nucleic Acids Res 32:D160–D164
Russell JR, Huang J, Anand P, Kucera K, Sandoval AG, Dantzler KW, Hickman DS, Jee J, Kimovec FM, Koppstein D, Marks DH, Mittermiller PA, Núñez SJ, Santiago M, Townes MA, Vishnevetsky M, Williams NE, Vargas MPN, Boulanger L-A, Bascom-Slack C, Strobel SA (2011) Biodegradation of polyester polyurethane by endophytic fungi. J Appl Environ Microbiol 77(17):6076–6084
Sahay H, Yadav AN, Singh AK, Singh S, Kaushik R, Saxena AK (2017) Hot springs of Indian Himalayas: potential sources of microbial diversity and thermostable hydrolytic enzymes. 3 Biotech 7:1–11
Samal BB, Karan B, Stabinsky Y (1990) Stability of two novel serine proteinases in commercial laundry detergent formulations. Biotechnol Bioeng 35:650–652
Saran S, Isar J, Saxena RK (2007) A modified method for the detection of microbial proteases on agar plates using tannic acid. J Biochem Bioph Meth 70:697–699
Sawant R, Nagendran S (2014) Protease: an enzyme with multiple industrial applications. World J Pharm Pharm Sci 3(6):568–579
Saxena AK, Yadav AN, Kaushik R, Tyagi S, Kumar M, Prasanna R, Shukla L (2014) Use of microbes from extreme environments for the benefits of agriculture. In: Afro-Asian Congress on microbes for human & environmental health. https://doi.org/10.13140/RG.2.1.3479.1841
Saxena AK, Yadav AN, Kaushik R, Tyagi SP, Shukla L (2015) Biotechnological applications of microbes isolated from cold environments in agriculture and allied sectors. In: International conference on “low temperature science and biotechnological advances”, society of low temperature biology, p 104. https://doi.org/10.13140/RG.2.1.2853.5202
Saxena AK, Yadav AN, Rajawat M, Kaushik R, Kumar R, Kumar M, Prasanna R, Shukla L (2016) Microbial diversity of extreme regions: an unseen heritage and wealth. Indian J Plant Genet Resour 29:246–248
Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106(9):996–1004
Sharma KM, Kumar R, Vats S, Gupta A (2014) Production, partial purification and characterization of alkaline protease from Bacillus aryabhattai K3. Int J Adv Pharm Biol Chem 3(2):290–298
Sharma KM, Kumar R, Panwar S, Kumar A (2017) Microbial alkaline proteases: optimization of production parameters and their properties. J Genet Eng Biotechnol 15:115–126
Shubha J, Srinivas C (2017) Diversity and extracellular enzymes of endophytic fungi associated with Cymbidium aloifolium L. Afr J Biotechnol 16(48):2248–2258
Sim YC, Lee SG, Lee DC, Kang BY, Park KM, Lee JY, Kim MS, Chang IS, Rhee JS (2000) Stabilization of papain and lysozyme for application to cosmetic products. Biotechnol Lett 22:137–140
Strobel GA, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67:491–502
Strobel GA, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Suman A, Verma P, Yadav AN, Saxena AK (2015) Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Res J Biotechnol 10:33–42
Suman A, Yadav AN, Verma P (2016) Endophytic microbes in crops: diversity and beneficial impact for sustainable agriculture. In: Singh D, Abhilash P, Prabha R (eds) Microbial inoculants in sustainable agricultural productivity, research perspectives. Springer, India, pp 117–143. https://doi.org/10.1007/978-81-322-2647-5_7
Sumantha A, Larroche C, Pandey A (2006) Microbiology and industrial biotechnology of food-grade proteases: a perspective. Food Technol Biotechnol 44(2):211–220
Sun X, Guo L, Hyde KD (2011) Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers 47:85–95
Sunitha VH, Nirmala Devi D, Srinivas C (2013) Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J Agric Sci 9:01–09
Suryanarayanan TS, Thirunavukkarasu N, Govindarajulu MB, Gopalan V (2012) Fungal endophytes: an untapped source of biocatalysts. Fungal Divers 54:19–30
Tan RX, Zou WX (2001) Endophytes: a rich source of natural metabolites. Nat Prod Rep 8:448–459
Tavano OL (2013) Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzym 90:1–11
Tehran MM, Shahnavaz B, Birjandi RG, Mashreghi M, Fooladi J (2016) Optimization of protease production by psychrotrophic Rheinheimera sp. with response surface methodology. Appl Food Biotechnol 3(4):236–245
Tenguria RK, Khan FN, Quereshi S (2011) Endophytes- mines of pharmacological therapeutics. World J Sci Technol 1(5):127–149
Thirunavukkarasu N, Suryanarayanan TS, Rajamani T, Paranetharan MS (2017) A rapid and simple method for screening fungi for extracellular protease enzymes. Mycosphere 8(1):131–136
Valls C, Pujadas G, Garcia-Vallve S, Mulero M (2011) Characterization of the protease activity of detergents laboratory practicals for studying the protease profile and activity of various commercial detergents. Biochem Mol Biol Educ 39(4):280–290
Vasundhara M, Kumar A, Reddy MS (2016) Molecular approaches to screen bioactive compounds from endophytic fungi. Front Microbiol 7:1774
Verma P, Yadav AN, Kumar V, Singh DP, Saxena AK (2017) Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crops improvement. In: Singh DP, Singh HB, Prabha R (eds) Plant-microbe interactions in agro-ecological perspectives. Springer Nature, Singapore, pp 543–580. https://doi.org/10.1007/978-981-10-6593-4_22
Vignardet C, Guillaume YC, Michel L, Friedrich J, Millet J (2001) Comparison of two hard keratinous substrates submitted to the action of a keratinase using an experimental design. Int J Pharm 224:115–122
Ward OP, Qin WM, Dhanjoon J, Ye J, Singh A (2005) Physiology and biotechnology of Aspergillus. Adv Appl Microbiol 58:1–75
Ward OP, Rao MB, Kulkarni A (2009) Proteases, production. In: Schaechter M (ed) Encyclopaedia of microbiology, 3rd edn. Elsevier, New York, pp 495–511
Whitaker JR (1994) Principles of enzymology for the food science, 2nd edn, Marcel Dekker, New York, pp 469–497
Wilson AD, Clement SL, Kiaser WJ (1991) Survey and detection of endophytic fungi in Lolium germplasm by direct staining and aphid assays. Plant Dis 75:169–173
Wooldridge JL, Heubi JE, Amaro-Galvez R, Boas SR, Blake KV, Nasr SZ, Chatfield B, McColley SA, Woo MS, Hardy KA, Kravitz RM, Straforini C, Anelli M, Lee C (2009) EUR-1008 pancreatic enzyme replacement is safe and effective in patients with cystic fibrosis and pancreatic insufficiency. J Cystic Fibrosis 8:405–417
Wu B, Wu L, Chen D, Yang Z, Luo M (2009) Purification and characterization of a novel fibrinolytic protease from Fusarium sp. CPCC 480097. J Ind Microb Biotechnol 36:451–459
Yadav AN, Sachan SG, Verma P, Kaushik R, Saxena AK (2016) Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. J Basic Microbiol 56:294–307
Yadav AN, Kumar R, Kumar S, Kumar V, Sugitha T, Singh B, Chauhan VS, Dhaliwal HS, Saxena AK (2017a) Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J App Biol Biotech 5:1–13
Yadav AN, Verma P, Kumar R, Kumar V, Kumar K (2017b) Current applications and future prospects of eco-friendly microbes. EU Voice 3:1–3
Zaferanloo B, Virkar A, Mahon PJ, Palombo EA (2013) Endophytes from an Australian native plant are a promising source of industrially useful enzymes. World J Microbiol Biotechnol 29:335–345
Zaferanloo B, Quang TD, Daumoo S, Ghorbani MM, Mahon PJ, Palombo EA (2014) Optimization of protease production by endophytic fungus, Alternaria alternata, isolated from Australian native plant. World J Microbiol Biotechnol 30:1755–1762
Zhang XQ, Liu QH, Zhang GQ, Wang HX, Ng TB (2010) Purification and molecular cloning of a serine protease from mushroom Hypsizigus marmoreus. Process Biochem 45:724–730
Acknowledgments
Authors are thankful to the Council of Scientific and Industrial Research (CSIR), Government of India, for financial support in the form of Junior Research Fellowship to SM and Department of Biotechnology (DBT), New Delhi for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mandal, S., Banerjee, D. (2019). Proteases from Endophytic Fungi with Potential Industrial Applications. In: Yadav, A., Mishra, S., Singh, S., Gupta, A. (eds) Recent Advancement in White Biotechnology Through Fungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-10480-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-10480-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10479-5
Online ISBN: 978-3-030-10480-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)