Abstract
Fungi constitute an invaluable natural resource for scientific research, owing to their diversity; they offer a promising alternative for bioprospecting, thus contributing to biotechnological advances. For a long time, extensive information has been exploited and fungal products have been tested as a source of natural compounds. In this context, enzyme production remains a field of interest, since it offers an efficient alternative to the hazardous processes of chemical transformations. Owing to their vast biodiversity and peculiar biochemical characteristics, two fungal categories, white-rot and anaerobic Neocallimastigomycota, have gathered considerable attention for biotechnological applications. These fungi are known for their ability to depolymerize complex molecular structures and are used in degradation of lignocellulosic biomass, improvement of animal feed digestibility, biogas and bioethanol production, and various other applications. However, there are only limited reports that describe proteolytic enzymes and esterases in these fungi and their synergistic action with lignocellulolytic enzymes on degradation of complex polymers. Thus, in this minireview, we focus on the importance of these organisms in enzyme technology, their bioprospecting, possibility of integration of their enzyme repertoire, and their prospects for future biotechnological innovation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi have been a prominent resource in a biotechnological study. Some of the characteristics of fungi, such as diversity, cellular secretion, degradation of complex compounds, and time- and cost-effective cultivation, make them an efficient resource for enzyme technology (Silva et al. 2013; Silva 2017).
Fungi comprise a group of widely distributed organisms in the biosphere. On the basis of their versatile lifestyles, fungi can be classified as saprophytic, pathogenic, and symbiotic with animal, plants, and algae (Mohanta and Bae 2015). Fungi are ubiquitous on earth, owing to their vast ecological diversity. They secrete a vast spectrum of enzymes, including hydrolytic enzymes, such as peptidases, esterases, and glycosidases (cellulases, amylases, xylanases, etc.), and oxidoreductases like laccases, manganese peroxidase, and lignin peroxidase, which are involved in the degradation of biopolymers from plants and animals (Graminha et al. 2008; Copete et al. 2015). This ability of enzyme secretion leads to their extensive use in industrial applications (El-Enshasy 2007).
The potential of two fungal groups—anaerobic and wood-decay fungi—for enzyme production has been explored for research and biotechnological development in the past few years. These fungi are found in a variety of ecological niches, which marks their ability for secretion of enzymes and depolymerization of complex compounds, such as lignin, cellulose, and proteins.
The phylum Neocallimastigomycota comprises unique obligate anaerobic fungi, which exist in a symbiotic relationship with herbivores. Their cellular structure is marked by the absence of mitochondria. Instead, these fungi possess hydrogenosomes, which are involved in the generation of cellular energy under anaerobic conditions (Gruninger et al. 2014). Several studies have been carried out to investigate their biological diversity and applications in biotechnology.
White-rot fungi are associated with wood-decay fungi and have a notable ability to secrete enzymes to degradation of complex chemical compounds like lignin and keratin. These processes find applications in bioremediation of toxic products. They are known to secrete a number of oxidative and hydrolytic enzymes and, thus, serve as a valuable tool for the degradation of recalcitrant substrates.
Earlier studies have demonstrated the biotechnological applications of different species of white-rot and anaerobic fungi, specifically focusing on the pretreatment for degradation of lignocellulosic biomass (Gruninger et al. 2014; Dollhofer et al. 2015; Rouches et al. 2016). Insufficient information, however, is available in the literature regarding their biotechnological prospects, and secretion of proteolytic enzymes and esterases for complementing the degradation of complex compounds.
This gap in knowledge has been focused on in the present review, to obtain a deeper insight into the importance of enzymes like peptidases, esterases, ligninases, and glycosidases in biotechnology. Based on their peculiar biochemical characteristics, the potential of white-rot and anaerobic Neocallimastigomycota fungi for enzyme secretion as well as their application prospects in enzymology and other industrial applications will be discussed.
In this review, we discussed several aspects of these two fungal groups, highlighting the biotechnological potential and the challenge of cooperative action of enzymes from these microorganisms.
Anaerobic fungi
Initially identified as protists, these organisms were characterized as obligate anaerobic fungi in the 1970s. Later, they were grouped as an order within the phylum Chytridiomycota (Griffith et al. 2010; Liggenstoffer et al. 2010). Although their role is well established in lignocellulosic biomass degradation, a little information is available about their contribution to enzyme technology. Recently, these have been classified under the phylum Neocallimastigomycota, which constitutes the order Neocallimastigales, family Neocallimastigaceae, and consists of eight genera of anaerobic fungi and symbionts in association within the gut of herbivores. The genera within Neocallimastigaceae include Neocallimastix, Piromyces, Buwchfawromyces, Ontomyces, Orpinomyces, Anaeromyces, Caecomyces, and Cyllamyces (Gruninger et al. 2014; Dollhofer et al. 2015).
Inhabiting the gastrointestinal tract of mammalian and reptilian herbivores, these anaerobic fungi are crucial for degradation of plant tissues, based on their ability to secrete of a range of cellulolytic enzymes (Gruninger et al. 2014; Samanta et al. 2008; Saxena et al. 2010). The presence of anaerobic fungi in termites has also been reported (Dollhofer et al. 2015). Plant polysaccharide degradation serves as the carbohydrate source for cellular metabolism of host herbivores.
The degradation of plant biomass by extracellular enzymes promotes the release of free oligo- and monosaccharides. Energy requirement during cellular metabolism is fulfilled by anaerobic fermentation of carbohydrates, including glucose, cellobiose, fructose, maltose, sucrose, and xylose (Nagpal et al. 2009; Dollhofer et al. 2015). The end products of fermentative pathway include acetate, lactate, ethanol, formate, succinate, CO2, and H2. These metabolites are useful in the metabolism of host herbivores, and other bacteria and archaea, inhabiting the gastrointestinal tract (Nagpal et al. 2009; Cheng et al. 2013; Gruninger et al. 2014).
Besides hydrogenosomes, these fungi possess rhizoids (filamentous or bulbous) and spores, which are self-propelled by means of flagella (zoospores) (Gruninger et al. 2014). Their capacity for mycelial dispersion by growth of rhizoids is an important parameter to improve the digestion of plant tissues and to facilitate the access of bacteria to the fermentative substrates (Dollhofer et al. 2015).
Extensive efforts have been put in to improve the handling, growth, and storage of these organisms. Besides, genic expression systems, for heterologous expression of enzymes, have also been explored (Gruninger et al. 2014).
Recently, the development of an international culture collection for cryogenic storage of fungal cultures has been proposed. It is believed that this repository would facilitate the exchange of fungal strains between different researchers worldwide (Dollhofer et al. 2015).
White-rot fungi
White-rot fungi, owing to their potential to produce lignocellulolytic enzymes, are valuable resources in enzyme technology. Several studies have revealed their role in the promotion of extensive degradation of lignin, as compared to other organisms. However, despite the mineralization of lignin to CO2 and H2O, white-rot fungi are incapable for use as a carbon and energy source. Instead, they obtain energy from metabolism of cellulose (Arora and Sharma 2010).
The phyla Ascomycota and Basidiomycota include representative species of wood-decaying fungi. Their characteristic name is originally derived from their potential to decay wood and causing rotted wood to appear white or yellow. Their ability to oxidize the recalcitrant lignin substrates signifies their importance in enzyme technology. Lignin degradation by white-rot fungi is dependent on a ligninolytic complex, comprising of manganese peroxidase, lignin peroxidase, laccase, and enzymes involved in generation of hydrogen peroxide (i.e., glucose oxidase) (Deacon 2006).
Wood decay is caused by some fungal species, based on their ability to degrade lignin. The species that hydrolyze lignin are called as white rot, due to their ability to lighten the color of rotted wood. Brown-rot fungi hydrolyze cellulose and hemicelluloses, but no lignin. Besides, some soft-rot fungi degrade lignin in angiosperm wood. They have an affinity for higher moisture and low lignin-containing biomass (Sánchez 2009).
Among the wood-decay fungi, white-rot fungi are the most well-known organisms and have been constantly explored for mycoremediation, due their capacity for degradation of complex chemical compounds. Fungi including Phanerochaete chrysosporium, Phanerochaete carnosa, Coriolus versicolor, Trametes versicolor, Pleurotus ostreatus, Irpex lacteus, Phlebiopsis gigantea, Ceriporiopsis subvermispora, and Dichomitus squalens are some representative examples of white-rot fungi, which have found applications in biotechnological research (Dashtban et al. 2009; Vaithanomsat et al. 2010; Couturier et al. 2015; Montoya et al. 2015).
Additionally, with regard to the peculiar biochemical properties, wood-decay fungi have been investigated for peptidase and esterase production. The hydrolysis of peptide and ester bond in polymers, especially in plant material, constitutes a valuable complement for wood decaying. The enzymatic arsenal and its cooperativity are crucial for the fungal growth and prospection for industrial application, including improvement of nutritional value in feed crop and biogas production, among others.
Bioprospecting and biotechnological potential
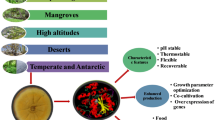
The prospecting of white-rot and anaerobic fungi is briefly discussed, highlighting the possibility of the integrated action of their enzymatic repertoire. We have demonstrated the involvement of peptidases and esterases in combination with lignocellulolytic enzymes to degrade plant biomass. To our knowledge, this is the first in-depth study on the biochemical properties of peptidases and esterases secreted by these fungi and their cooperative hydrolysis in combination with lignocellulolytic enzymes.
Lignocellulolytic enzymes and industrial prospects
Lignocellulose is an important component of plant cell wall, composed of cellulose, hemicellulose, and lignin. Although this complex is recalcitrant, some organisms, from bacteria to arthropods, have the potential to digest it (Scully et al. 2013; Wei et al. 2015).
Cellulose is a linear biopolymer consisting of glucose monomers, linked by β-1,4-glycosidic bonds. Some chains are stabilized by van der Waals forces, resulting in the formation of crystalline structures known as microfibrils. Hemicelluloses are heterogeneous polymers consisting of xylose, arabinose (pentoses), mannose, glucose, galactose (hexoses), and sugar acids. The composition of hemicelluloses depends on the plant source (Dashtban et al. 2010). Lignin is the only naturally synthesized biopolymer with an aromatic backbone. It consists of three precursor aromatic alcohols, including coniferyl, sinapyl, and p-coumaryl alcohols, which form the guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H) subunits of the lignin molecule, respectively. Upon conjugation, these chains make the structure highly recalcitrant towards degradation and are efficient enough to protect the lignocellulosic complex once linked to cellulose and hemicellulose (Dashtban et al. 2010).
There has been an emerging interest in the use of ligninolytic and cellulolytic enzymes in the biotechnology industry. Lignocellulolytic enzymes are required for a wide spectrum of applications. In particular, the role of cellulases has been established in the degradation of cellulose in fermentable sugars and its conversion to lactic acid, butanol, and ethanol as an alternative energy source to fossil fuels (Montoya et al. 2015).
Ligninolytic enzymes can be used in a number of processes, such as improvement of access to cellulose and hemicellulose in plant biomass, manufacturing of paper pulp (biobleaching), improvement of nutritional value and feed digestibility for ruminants, and waste effluent treatment, among others (Arora and Sharma 2010).
Cellulose is completely hydrolyzed by the activity of endoglucanases (EGs), cellobiohydrolases (CBHs), and β-glucosidases (BGLs) (Cantarel et al. 2009). EGs catalyze the hydrolysis of cellulose chains, releasing cellobiose or bigger polymers. The pH and temperature for maximum EG activity range between 4 and 6 and between 45 and 70 °C, respectively. There are, however, certain exceptions to these conditions (Manavalan et al. 2015). CBHs are monomeric proteins, with molecular weights ranging from 36 to 75 kDa. CBHs catalyze the hydrolysis of cellulose to generate cellobiose. Maximum CGH activity is obtained in the pH range 4–5 at 37–60 °C (Momeni et al. 2013; Rytioja et al. 2014; Manavalan et al. 2015). BGLs, in general, have molecular weights ranging between 35 and 640 kDa and can also exist in trimeric forms. They catalyze the hydrolysis of cellobiose, releasing glucose monomers. The optimum pH for BGL activity ranges between pH 3.5 and 5.5, and the temperatures for maximum activity range from 45 to 75 °C (Manavalan et al. 2015).
Enzymes acting in hemicellulose degradation consist of xylan-degrading endoxylanases, alpha-glucuronidases, beta-xylosidases, acetyl xylan esterases, ferulic acid esterases, and alpha-l-arabinofuranosidase, and glucomannan-degrading beta-mannosidases and beta-mannanases (Van den Brink and de Vries 2011).
A number of enzymes, including lignin peroxidase (LiP), manganese peroxidase (MnP), and laccases, are involved in complete decomposition of lignin. The white-rot fungi are usually differentiated from other fungal classes by their ability to efficiently degrade lignin.
LiPs are proteins having molecular weights between 30 and 50 kDa. The optimum pH and temperatures for maximum LiP activity range between pH 2 and 5 and between 35 and 55 °C, respectively. Iron ions (Fe3+) act as co-factors for the enzyme and act by mediating the oxidation of veratryl alcohol using H2O2, in the active site of LiPs (Manavalan et al. 2015).
MnPs are glycoproteins with molecular weights ranging from 32 to 62.5 kDa and act at an optimum pH of 4–7 and an optimum temperature from 40 to 60 °C. The mechanism of action of MnP is similar to that of LiP, the only difference being the presence of Mn2+ as the proton donor in MnP (Manavalan et al. 2015; Kellner et al. 2014).
Laccases are oxidases with molecular weight between 38 and 383 kDa. This enzyme has a tricopper site and is able to catalyze a ring cleavage of aromatic compounds. In general, laccases are N-glycosylated and act in a wide variation of pH and temperature conditions. The expression of laccases in Trametes velutina was enhanced by the addition of Cu2+ and Fe2+ ions as well as some aromatic compounds like tannic acid, syringic acid, cinnamic acid, gallic acid, and guaiacol (Yang et al. 2013).
The production of peptidases, esterases, and lignocellulolytic enzymes by white-rot and anaerobic fungi is summarized in Table 1. The synergism of white-rot and anaerobic fungi has a great potential in plant biomass degradation and is an efficient alternative for enzyme technology.
White-rot and anaerobic fungi in lignocellulose degradation
Both anaerobic and wood-decay fungi have an extensive ability to degrade plant tissue. Thus, they serve as promising candidates in investigation of secretion of cellulolytic and ligninolytic enzymes, for obtaining renewable fuel, like bioethanol from cellulose-containing biomass.
In anaerobic fungi, the ability to secrete cellulolytic enzymes has received extensive attention. It was identified that some of these enzymes are acquired by fungi through horizontal gene transfer from bacteria (Griffith et al. 2010). In addition, these enzymes can either be found in a free state within the fungal cell or as a part of cellulosomes, which is a multi-enzyme complex associated with cell wall.
Several studies have revealed the presence of cellulolytic enzymes in the genome of anaerobic fungi (Nicholson et al. 2005; Dollhofer et al. 2015). Transcriptome analysis of Orpinomyces sp. strain C1A growing in the presence of lignocellulosic biomass (alfalfa, energy cane, corn stover, and sorghum) revealed the crucial role of cellulosome and free glycoside hydrolases in degradation of plant biomass (Couger et al. 2015).
However, the forage degradation by anaerobic fungi is commonly limited, owing to the presence of recalcitrant lignin. It is estimated that 40–60% of organic carbon remains unused, owing to decreased accessibility of anaerobic fungi to cellulose and hemicellulose embedded within lignin biomass. Several pretreatment strategies, like mechanical, thermal, oxidative, chemical, and ultrasonic technologies, to improve the degradation of plant biomass are reported in the literature (Graminha et al. 2008; Dollhofer et al. 2015).
An additional strategy for lignocellulose degradation is the integration of hydrolytic and ligninolytic enzymes from white-rot fungi. Therefore, the cumulative action of anaerobic and wood-decay fungi results in higher potential for degradation of plant biomass (Arora and Sharma 2010; Gruninger et al. 2014; Dollhofer et al. 2015).
Biofuel production research has recently focused on the potential of white- and brown-rot fungal species to efficiently perform the saccharification of alkaline-pretreated sugarcane bagasse. The enzymes secreted by these fungi were proposed to enhance the efficiency of production of bioethanol from lignocellulosic substrates (Valadares et al. 2016).
Secretomic analysis of Schizophyllum commune revealed a unique wood biodegradation system, with higher diversity of carbohydrate-degrading enzymes, as compared to other white-rot fungi P. chrysosporium, C. subvermispora, and Gloeophyllum trabeum. The higher wood-decay activity was contributed by the differential expression of hemicellulases, pectinases, and accessory proteins involved in the generation of hydroxyl radicals (Zhu et al. 2016).
Biogas production
Based on the information from a rumen environment, it has been suggested that the biogas production under reactor conditions is dependent on the microbial interactions and activities to degrade organic waste. The applications of white-rot fungi are also investigated to enhance its ligninolytic activity and production performance (Gruninger et al. 2014).
In rumen, unlike axenic cultures, the interactions between symbiotic organisms, such as bacteria, archaea, and anaerobic fungi, are fundamental to increase the cellulolytic and xylanolytic activity and, ultimately, the biogas production. The anaerobic digestion provides the basic substrate to methanogens during biogas production. The metabolic products, especially acetate and formate, derived from anaerobic fungi are the preferred by methanogens, which carry out interspecies hydrogen transfer via methanogenesis (Gruninger et al. 2014; Dollhofer et al. 2015).
Marvin-Sikkema et al. (1990) reported an increase in the cellulolytic activity of anaerobic fungi growing in association with methanogens, which was marked by a 5–10% enhancement in cellulose fermentation with several anaerobic fungi, such as Piromonas communis P, Neocallimastix patriciarum C, Sphaeromonas communis FG10, and Neocallimastix frontalis RE1, and up to 15–25% with Neocallimastix sp. strain L2. Other studies also co-related the stimulatory effect of co-culture of anaerobic fungi and methanogens in methane production by degradation of plant biomass (Jin et al. 2011; Cheng et al. 2013).
Although the use of anaerobic fungi in biogas production has been established, its exploitation at the industrial level has not been achieved as yet, possibly due to concerns like decreased enzymatic activity and compromised fungal growth (Dollhofer et al. 2015).
Kazda et al. (2014) reported about the importance of anaerobic fungi in biogas reactors. Molecular techniques by analyzing internal transcribed spacer 1 (ITS1) sequences enabled to detect anaerobic fungi in full-scale biogas plants and in laboratory reactors. However, the compromised fungal growth has been an obstacle for anaerobic cultivation and their visualization in microscopic surveys. Further, the difficulty for maintaining the culture flow contributes to the low level of enzyme activity.
Inhabiting the gastrointestinal tract, anaerobic fungal populations remain in equilibrium due to rumen conditions, such as salivary and digesta flow, and the absorption of metabolites across the rumen epithelium which contributes to maintain the rumen microbial ecosystem (Zhu et al. 1996). In the laboratory, the major difficulty is to simulate these rumen conditions (Zhu et al. 1996; Gruninger et al. 2014). Currently, investigations have focused on the fungal growth in closed batch cultures in which the culture medium is composed of lower substrate concentration than that found in the rumen. Additionally, for maintaining the fungal population, a subculture into a fresh medium can be performed.
Additionally, to address these problems, extensive efforts have been put in to improve the industrial application of these fungi. Heterologous expression offers an alternative for application of lignocellulolytic enzymes in reactors under ideal reaction conditions (Gruninger et al. 2014).
There has been an emerging interest in the use of symbiotic anaerobic fungi in biotechnological applications. To achieve this, some reports have described the heterologous expression of glycosidases, such as xylanases from Orpinomyces sp. (Li et al. 2007; Madhavan et al. 2009) and N. frontalis (Tsai and Huang 2008). Li et al. (1997) also exhibited the heterologous expression of cellulase and xylanase from anaerobic fungus Orpinomyces sp. strain PC-2 using Escherichia coli as an expression system, encoding a polypeptide of 471 amino acids for cellulase complementary DNA (cDNA) and a polypeptide of 362 amino acids for xylanase cDNA. In this work, Orpinomyces sp. strain grown on Avicel and the glycosidases demonstrated an identity of 80 to 85% to the corresponding enzymes from N. patriciarum.
The key role of fungi and methanogen symbionts in biowaste treatment is demonstrated, for example, by the conversion of organic waste to alternative fuel. Household and industrial activities generate a large amount of organic residues annually. Anaerobic digestion of biowaste is an alternative to generation of methane, since it is a biogas sustainable source in comparison to processes like incineration or landfill (Kazda et al. 2014).
In this context, once again, the white-rot fungi show them as valuable candidates for pretreatment of lignocellulosic biomass, integrating lignocellulolytic enzymes, esterases, and peptidases in cumulative action with strict anaerobic fungi.
Esterases and proteolytic enzymes
Different to the reported in other studies (Gruninger et al. 2014; Dollhofer et al. 2015; Rouches et al. 2016), in this minireview, we highlight the biotechnological attention devoted to esterases and peptidases secreted by anaerobic and white-rot fungi and, in particular, to the enzymatic synergisms with lignocellulolytic enzymes on degradation of forage biomass.
In general, peptidases and esterases are involved in fundamental biological processes, including germination, sporulation, and diet of fungi. Their physiological importance and the alternative use in biotechnology fields reinforce the extensive attention devoted to these hydrolytic enzymes (Lawrence 1967; Inácio et al. 2015).
Esterases are hydrolases capable to catalyze the cleavage and formation of ester bonds, and peptidases are enzymes that catalyze the cleavage of peptide bonds into proteins and peptides. The specificity study in proteolytic enzymes is a crucial parameter for a higher understanding of its catalytic preference, in order to provide important information about the degradation of protein substrate (Silva et al. 2014; Silva 2017).
Peptidases are important enzymes involved in the metabolism of all organisms and are also significant in a number of industrial processes. Currently, seven types of proteolytic enzymes are known, including aspartic, cysteine, serine, metallo, glutamic, and threonine peptidases, and non-hydrolytic asparagine peptide lyase (Rawlings et al. 2011; Silva 2017). Fungi are known to secrete a range of proteolytic enzymes, which find applications in a number of processes, such as cheese manufacturing, peptide synthesis, animal waste treatment, pharmaceutical, detergent, and food industries, as well as other aspects of basic research (Silva et al. 2013, 2014, 2016, 2017; Biaggio et al. 2016; Silva 2017).
Fungi have been widely explored for peptidase production (Silva 2017). Recent researches have evaluated the biochemical properties and potential for application of fungal peptidases. For example, Graminho et al. (2013) and Silva et al. (2014) reported the secretion of non-specific serine peptidases, by which they can be prospected for bioactive peptide synthesis and detergent formulation.
On cheese production, peptidases are investigated to promote the cleavage of the peptide bond between phenylalanine (Phe105) and methionine (Met106) on k-casein substrate (Silva 2017). The milk clotting is a crucial step for cheese manufacturing. Silva et al. (2016) exhibited an aspartic peptidase with potential to be an alternative enzyme in milk clotting during the preparation of cheese.
Ida et al. (2016) also documented the use of peptidases in detergent formulation. In this study, the washing performance in commercial detergent compatibility of two collagenolytic serine peptidases was evaluated, by which a successful assay for removing an egg protein stain was demonstrated.
In plant degradation, wood nitrogen is especially found in proteins into cell wall, by which wood-decay fungi secrete proteolytic enzymes in order to promote protein hydrolysis and its use as a nitrogen source (Inácio et al. 2015). The nitrogen limitation is known to enhance the secretion of enzymes by white-rot fungi (Dorado et al. 2001). Dass et al. (1995) and Dorado et al. (2001) reported an increase on production of peptidase and ligninolytic enzymes when subjected to nitrogen limitation.
Wood decay fungi, owing to their distinctive biochemical properties, have been explored in detail for peptidase production and regulation of activity of lignocellulolytic enzymes. For instance, production of subtilisin-like peptidase from the white-rot fungal strain P. ostreatus has been described (Palmieri et al. 2001). The authors exhibited a monomeric glycoprotein with a molecular mass estimated at 75 kDa. They pointed out the importance of this enzyme on regulation of the laccase activity.
Additionally, secretion of different subclasses of peptidases, including aspartic, cysteine, metallo, and serine peptidases, with varying molecular weights, and different pH and temperature optima for enzymatic activities, has been reported in many species of the genus Pleurotus (Inácio et al. 2015). Recently, production of peptidases has also been reported from Cerrena unicolor, Phlebia lindtneri, and Pycnoporus sanguineus (Janusz et al. 2016).
Rahul et al. (2013) also described the production of two alkaline peptidases from P. chrysosporium. In this screening, the peptidases exhibited an optimum caseinolytic activity at pH 7.5 and molecular mass estimated at 33 and 40 kDa.
Several investigations have revealed the effect of peptidases on the regulation of lignocellulolytic activity (Silva 2017). A study performed in white-rot T. versicolor demonstrated an improvement in laccase and peroxidase activities after inhibition of peptidase activity by phenylmethylsulfonyl fluoride (PMSF) (Staszczak et al. 2000). In this work, the authors suggested an influence of peptidases on the degradation of ligninases.
Peptidases provide amino acids from protein diet in the digestive tract of ruminants. Metallopeptidase from anaerobic N. frontalis leads to protein breakdown in rumen. The symbiotic association of Neocallimastigomycota fungi within herbivores has indicated the possibility of its use in animal diet (Wallace 1996). Improvement of in vitro digestibility of feed by mixing microflora in buffalo rumen was attained by the proteolytic activity of Piromyces sp., Anaeromyces sp., and Orpinomyces sp. (Paul et al. 2004).
White-rot and anaerobic fungi are known to produce another class of valuable hydrolytic enzymes—the esterases—which are involved in degradation of plant biomass. Studies about esterase activity involved in hydrolysis of the ester bond in plant tissue have been reported as an additional/cooperative enzymatic action in order to complete degradation of forage fiber. Some esterases, like acetyl xylan esterase, have been characterized from anaerobic fungus Orpinomyces sp. strain PC-2, which has a molecular mass of 34,845 Da and shows a 56% amino acid homology with the acetyl xylan esterase from N. patriciarum (Blum et al. 1999). In the rumen of Hereford bulls fed on an alfalfa-based diet, the presence of several glycosidases, acetyl esterases, and peptidases, as an enzymatic complex involved in feed degradation, has been reported (Lee et al. 2002).
In biochemical evaluation, investigators detected the esterase activity of two feruloyl esterases secreted by Neocallimastix strain MC-2, by which they were purified and partially characterized. The enzymes were classified as ferulic acid esterase (FAE) I and FAE II and exhibited a molecular mass at 69 and 24 kDa, respectively (Borneman et al. 1992). Paul et al. (2004) also reported the production of esterases from Piromyces sp., Anaeromyces sp., and Orpinomyces sp. in a study demonstrating the effect of anaerobic fungi on feed degradation. All these documented researches reported that esterases are essential actors on cooperative hydrolysis of ester bonds in complex crop fiber.
In white-rot fungus, MacDonald et al. (2011) related the transcriptomic responses of P. carnosa in growth on coniferous and deciduous wood, the transcript sequences encoded to acetyl xylan esterase and glucuronoyl esterase.
In another study with P. carnosa, glucuronoyl esterase was reported to be involved in wood degradation. This enzyme mediated the hydrolysis of glucuronoxylan linked to lignin via ester bonds (Gandla et al. 2015). The production of glucuronoyl esterase is also reported in S. commune (Spánikova and Biely 2006).
Peptidases and esterases have been implicated in feed processing. The enzymatic repertoire of white-rot and anaerobic fungi has shown a great potential for improvement of feed digestibility. The integration of esterases and proteolytic enzymes contributes to the degradation of poorly digested feed. The role during degradation of plant materials is shown in Table 2.
Enzyme cooperativity and improvement of feed digestibility in ruminants
The increasing demand for meat consumption has generated an intensive interest in the improvement of animal nutrition. In such cases, anaerobic and white-rot fungi have been explored as possible alternatives to enhance the digestibility of poorly processed plant fibers during digestion in ruminants (Nagpal et al. 2009).
The complex of hydrolytic enzymes secreted from anaerobic fungi is crucial to digestion of plant tissue in the gastrointestinal tract of ruminants. Apart from glycosidases, peptidases and esterases are important for degradation of grasses. Esterases hydrolyze the ester bonds between lignin and hemicellulose to favor the access to cellulose and hemicellulose, and peptidases catalyze the cleavage of peptide bonds in proteins from plant material, providing organic nitrogen and facilitating the penetration of rhizoids of anaerobic fungi (Nagpal et al. 2009; Silva 2017).
Akin et al. (1993) reported the use of P. chrysosporium to improve the digestibility of grass cell walls and the biodegradation by rumen microorganisms. In this report, the esterase activity is noticed as a crucial tool for lignocellulosic biodegradation. It has been proposed that the phenolic acid esterases gave ruminal fungi an important advantage over ruminal bacteria.
The extensive interest in anaerobic symbiotic fungi within ruminants is potentially due to the possibility of its application in feed supplementation, especially to improve the nutritional content of cattle feed and to promote the animal growth (Gruninger et al. 2014; Lee et al. 2000; Dey et al. 2004; Paul et al. 2004).
It is estimated that the anaerobic fungi account for about 8–12% of the microbial biomass in rumen. Symbiotic fungi in ruminant can be used as probiotic additives, which serve as a supplement to the animal, owing to their potential to improve the microbial activity in the digestive tract and through promotion of degradation of plant fiber (Nagpal et al. 2009).
Using the anaerobic fungus Piromyces sp. in addition to mixed rumen buffalo microflora, Paul et al. (2004) reported an improvement on in vitro feed digestibility of lignocellulosic material (wheat straw and wheat bran, 80:20 w/w). The experiments indicated a feed digestibility for about 43.64 ± 1.73% using the anaerobic fungus as a feed additive, and a 35.37 ± 1.65% for the control group (without feed additive). Additionally, for a period of 24 h of plant biomass treatment, the fungal inoculum increased the production of carboxymethyl cellulase, xylanase, acetyl esterase, and β-glucosidase. The final conclusion of this study suggests the potential to use Piromyces sp. as a feed additive for plant fiber degradation.
In another study, anaerobic fungi were manipulated to improve the utilization of poor-quality feed in sheep (Gordon et al. 2001). The oral dosing with the anaerobic fungus Piromyces sp. CS15 was effective in increasing feed intake (straw-based diet), thus contributing to increase the live weight by penned sheep.
An additional tool to improve the degradation of forage material and nutrient value addition to animal diet is the production of ligninolytic and proteolytic enzymes by white-rot fungi. The use of these microorganisms can improve the nutritional yield of plant biomass, for enhancement of the crop fiber degradation and to facilitate the access to cellulose and hemicellulose substrates by the activity of glycoside and peptide hydrolases from anaerobic and wood-decay fungi.
Several studies have revealed an improvement in the digestibility of plant residues in ruminants due to the activity of white-rot fungi. An improvement in the nutritional value of wheat straw, accompanied with an increase of protein content was observed after treatment with Pleurotus sp. (Fazaeli et al. 2004). In another study, treatment with the fungus P. ostreatus using corn straw as a substrate resulted in an increase in crude protein and soluble carbohydrates after 15 days (Ramirez-Bribiesca et al. 2010). Raghuwanshi et al. (2014) also related the use of the fungus Ganoderma spp. to improve the nutritional content of wheat straw residue.
The combination of enzymatic complexes from these fungi is complementarily functional with plant fibers and is involved in reinforcement of the digestion of lignocellulosic material and adding on to the nutritional value in cattle. The enzyme cooperativity of peptidases, esterases, glycosidases, and ligninases is crucial for plant fiber degradation and optimum nutrient assimilation. The synergism of white-rot and anaerobic fungi has a great potential in feed processing and is an efficient alternative for livestock farming.
Future prospects
In the past few years, there has been an emerging interest in white-rot and anaerobic fungi, particularly owing their peculiar biochemical characteristics and ability to secrete enzymes involved in the degradation of complex polymers. These fungi are prominent candidates for applications involving generation of sustainable energy like bioethanol and biogas, and improvement of feed digestibility. In the future, technological advances are expected to improve their handling, storage, and biodiversity prospecting, providing new information on the development of new biotechnological products to contribute to the scientific innovation and development.
References
Akin DE, Sethuraman A, Morrison WH III, Martin SA, Eriksson KEL (1993) Microbial delignification with white rot fungi improves forage digestibility. Appl Environ Microbiol 59:4274–4282
Alberts JF, Gelderblom WCA, Botha A, van Zyl WH (2009) Degradation of aflatoxin B1 by fungal laccase enzymes. Int J Food Microbiol 135:47–52
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160:1760–1788
Bending GD, Friloux M, Walker A (2002) Degradation of contrasting pesticides by white rot fungi and its relationship with ligninolytic potential. FEMS Microbiol Lett 121:59–63
Biaggio RT, Silva RR, da Rosa NG, Leite RSR, Arantes EC, Cabral TPF, Juliano MA, Juliano L, Cabral H (2016) Purification and biochemical characterization of an extracellular serine peptidase from Aspergillus terreus. Prep Biochem Biotech 46:298–304
Blum DL, Li X-L, Chen H, Ljungdahl LG (1999) Characterization of an acetyl xylan esterase from the anaerobic fungus Orpinomyces sp. strain PC-2. Appl Environ Microbiol 65:3990–3995
Borneman WS, Ljungdahl LG, Hartley RD, Akin DE (1992) Purification and partial characterization of two feruloyl esterases from the anaerobic fungus Neocallimastix strain MC-2. Appl Environ Microbiol 58:3762–3766
Cabaleiro DR, Rodríguez-Couto S, Sanromán A, Longo MA (2002) Comparison between the protease production ability of ligninolytic fungi cultivated in solid state media. Process Biochem 37:1017–1023
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Cheng YF, Jin W, Mao SY, Zhu W-Y (2013) Production of citrate by anaerobic fungi in the presence of co-culture methanogens as revealed by 1H NMR spectrometry. Asian-Aust J Anim Sci 26:1416–1423
Copete LS, Chanagá X, Barriuso J, López-Lucendo MF, Martínez MJ, Camarero S (2015) Identification and characterization of laccase-type multicopper oxidases involved in dye-decolorization by the fungus Leptosphaerulina sp. BMC Biotechnol 15:74
Couger MB, Youssef NH, Struchtemeyer CG, Liggenstoffer AS, Elshahed MS (2015) Transcriptomic analysis of lignocellulosic biomass degradation by the anaerobic fungal isolate Orpinomyces sp. strain C1A. Biotechnol Biofuels 8:1–17
Couturier M, Navarro D, Chevret D, Henrissat B, Piumi F, Ruiz-Dueñas FJ, Martinez AT, Grigoriev IV, Riley R, Lipzen A, Berrin J-G, Master ER, Rosso M-N (2015) Enhanced degradation of softwood versus hardwood by the white-rot fungus Pycnoporus coccineus. Biotechnol Biofuels 8:1–16
Dashtban M, Schraft H, Qin W (2009) Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int J Biol Sci 5:578–595
Dashtban M, Schraft H, Syed TA, Qin W (2010) Fungal biodegradation and enzymatic modification of lignin. Int J Biochem Mol Biol 1:36–50
Dass SB, Dosoretz CG, Reddy CA, Grethlein HE (1995) Extracellular proteases produced by the wood-degrading fungus Phanerochaete chrysosporium under ligninolytic and non-ligninolytic conditions. Arch Microbiol 163:254–258
Deacon JW (2006) Fungal ecology: saprotrophs. In: Deacon JW (ed) Fungal ecology: saprotrophs. Blackwell, Oxford, pp 213–236
Dey A, Sehgal JP, Puniya AK, Singh K (2004) Influence of an anaerobic fungal culture (Orpinomyces sp.) administration on growth rate, ruminal fermentation and nutrient digestion in calves. Asian Aus J Anim 17:820–824
Doi RH, Kosugi A, Murashima K, Tamaru Y, Han SO (2003) Cellulosomes from mesophilic bacteria. J Bacteriol 185:5907–5914
Dollhofer V, Podmirseg SM, Callaghan TM, Griffith GW, Fliegerová K (2015) Anaerobic fungi and their potential for biogas production. Adv Biochem Eng Biotechnol 151:41–61. doi:10.1007/978-3-319-21993-6_2
Dorado J, Field JA, Almendros G, Sierra-Alvarez R (2001) Nitrogen-removal with protease as a method to improve the selective delignification of hemp stemwood by the white-rot fungus Bjerkandera sp. strain BOS55. Appl Microbiol Biotechnol 57:205–211
El-Enshasy HA (2007) Filamentous fungal cultures—process characteristics, products, and applications. In: Shang-Tian Y (ed) Bioprocessing for value-added products from renewable resources. Elsevier, Amsterdam, pp 225–262
Fazaeli H, Mahmodzadeh H, Azizi A, Jelan ZA, Liang JB, Rouzbehan Y, Osman A (2004) Nutritive value of wheat straw treated with Pleurotus fungi. Asian-Aust J Anim Sci 17:1681–1688
Fliegerová K, Pazoutová S, Mrázek J, Kopecny J (2002) Special properties of polycentric anaerobic fungus Anaeromyces mucronatus. Acta Vet Brno 71:441–444
Gandla ML, Derba-Maceluch M, Liu X, Gerber L, Master ER, Mellerowicz EJ, Jonsson LJ (2015) Expression of a fungal glucuronoyl esterase in Populus: effects on wood properties and saccharification efficiency. Phytochemistry 112:210–220
Gondim-Tomaz MA, Franco TT, Durrant LR (2005) Biodegradation of diuron and pyruthiobac-sodium by white-rot and soil fungi. In: Calabrese EJ, Kostecki PT, Dragun J (eds) Contaminated soils, sediments and water. Springer, US, pp 21–32
Gordon GLR, Phillips MW, White SW, Rintoul AR, Mitchell PA (2001) Can anaerobic fungi be manipulated in the sheep rumen to improve the utilisation of poor-quality feed? Recent Advances in Animal Nutrition in Australia 13:43–48
Graminha EBN, Gonçalves AZL, Pirota RDPB, Balsalobre MAA, Da Silva R, Gomes E (2008) Enzyme production by solid-state fermentation: application to animal nutrition. Anim Feed Sci Tech 144:1–22
Graminho ER, Silva RR, Cabral TPF, Arantes EC, Da Rosa NG, Juliano L, Okamoto DN, Oliveira LCG, Kondo MY, Juliano MA, Cabral H (2013) Purification, characterization, and specificity determination of a new serine protease secreted by Penicillium waksmanii. Appl Biochem Biotechnol 169:201–214
Griffith GW, Baker S, Fliegerova K, Liggenstoffer A, van der Giezen M, Voigt K, Beakes G (2010) Anaerobic fungi: Neocallimastigomycota. IMA Fungus 1:181–185
Gruninger RJ, Puniya AK, Callaghan TM, Edwards JE, Youssef N, Dagar SS, Fliegerova K, Griffith GW, Forster R, Tsang A, McAllister T, Elshahed MS (2014) Anaerobic fungi (phylum Neocallimastigomycota): advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol Ecol 90:1–17
Hori C, Gaskell J, Igarashi K, Samejima M, Hibbett D, Henrissat B, Cullen D (2013) Genomewide analysis of polysaccharides degrading enzymes in 11 white- and brown-rot Polyporales provides insight into mechanisms of wood decay. Mycologia 105:1412–1427
Howard RL, Masoko P, Abotsi E (2003) Enzyme activity of a Phanerochaete chrysosporium cellobiohydrolase (CBHI.1) expressed as a heterologous protein from Escherichia coli. Afr J Biotechnol 2:296–230
Ida EL, Silva RR, de Oliveira TB, Souto TB, Leite JA, Rodrigues A, Cabral H (2016) Biochemical properties and evaluation of washing performance in commercial 1 detergent compatibility of two collagenolytic serine peptidases secreted by Aspergillus fischeri and Penicillium citrinum. Prep Biochem Biotech
Inácio FD, Ferreira RO, Vaz de Araujo CA, Brugnari T, Castoldi R, Peralta RM, de Souza, CGM (2015) Proteases of wood rot fungi with emphasis on the Genus Pleurotus. BioMed Res Int 1–10. doi.org/10.1155/2015/290161
Janusz G, Sulej J, Jaszek M, Osińska-Jaroszuk M (2016) Effect of different wavelengths of light on laccase, cellobiose dehydrogenase, and proteases produced by Cerrena unicolor, Pycnoporus sanguineus and Phlebia lindtneri. Acta Biochim Pol 63:1–6
Jin W, Cheng YF, Mao SY, Zhu WY (2011) Isolation of natural cultures of anaerobic fungi and indigenously associated methanogens from herbivores and their bioconversion of lignocellulosic materials to methane. Bioresour Technol 102:7925-7931
Kanaly R, Hur H (2006) Growth of Phanerochaete chrysosporium on diesel fuel hydrocarbons at neutral pH. Chemosphere 63:202–211
Kazda M, Langer S, Bengelsdorf FR (2014) Fungi open new possibilities for anaerobic fermentation of organic residues. Energy, Sustain Soc 4:1–9
Kellner H, Luis P, Pecyna MJ, Barbi F, Kapturska D, Krüger D, Zac DR, Marmeisse R, Vandenbol M, Hofrichter M (2014) Widespread occurrence of expressed fungal secretory peroxidases in forest soils. PLoS One 9:e95557
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Research. doi:10.4061/2011/280696
Lawrence RC (1967) The metabolism of triglycerides by spores of Penicillium roquefort. J Gen Microbiol 46:65–76
Lee SS, Ha JK, Cheng KJ (2000) Influence of an anaerobic fungal culture administration on in vivo ruminal fermentation and nutrient digestion. Anim Feed Sci Technol 88:201–217
Lee SS, Kim CH, Ha JK, Moon YH, Choi NJ, Cheng KJ (2002) Distribution and activities of hydrolytic enzymes in the rumen compartments of Hereford bulls fed alfalfa based diet. Asian-Aust J Anim Sci 15:1725–1731
Li X-L, Chen H, Ljungdahl LG (1997) Monocentric and polycentric anaerobic fungi produce structurally related cellulases and xylanases. Appl Environ Microbiol 63:628–635
Li XL, Skory CD, Ximenes EA, Jordan DB, Dien BS, Hughes SR, Cotta MA (2007) Expression of an AT-rich xylanase gene from the anaerobic fungus Orpinomyces sp. strain PC-2 in and secretion of the heterologous enzyme by Hypocrea jecorina. Appl Microbiol Biotechnol 74:1264–1275. doi:10.1007/s00253-006-0787-6 72
Liggenstoffer AS, Youssef NH, Couger MB, Elshahed MS (2010) Phylogenetic diversity and community structure of anaerobic gut fungi (phylum Neocallimastigomycota) in ruminant and non-ruminant herbivores. The ISME Journal 4:1225–1235
MacDonald J, Doering M, Canam T, Gong Y, Guttman DS, Campbell MM, Master EM (2011) Transcriptomic responses of the softwood-degrading white-rot fungus Phanerochaete carnosa during growth on coniferous and deciduous wood. Appl Environ Microbiol 77:3211–3218
Madhavan A, Tamalampudi S, Ushida K, Kanai D, Katahira S, Srivastava A, Fukuda H, Bisaria VS, Kondo A (2009) Xylose isomerase from polycentric fungus Orpinomyces: gene sequencing, cloning, and expression in Saccharomyces cerevisiae for bioconversion of xylose to ethanol. Appl Microbiol Biotechnol 82:1067–1078. doi:10.1007/s00253-0081794-6
Manavalan T, Manavalan A, Heese K (2015) Characterization of lignocellulolytic enzymes from white-rot fungi. Curr Microbiol 70:485–498. doi:10.1007/s00284-014-0743-0
Marvin-Sikkema FD, Richardson AJ, Stewart CS, Gottschal JC, Prins RA (1990) Influence of hydrogen-consuming bacteria on cellulose degradation by anaerobic fungi. Appl Environ Microbiol 56:3793–3797
Mohanta TK, Bae H (2015) The diversity of fungal genome. Biol Proced Online 17:1–9. doi:10.1186/s12575-015-0020
Momeni MH, Payne CM, Hansson H, Mikkelsen NE, Svedberg J, Åke E, Sandgren M, Beckhan GT, Ståhlberg J (2013) Structural, biochemical, and computational characterization of the glycoside hydrolase family 7 cellobiohydrolase of the tree-killing fungus Heterobasidion irregulare. J Biol Chem 288:5861–5872
Montoya S, Sánchez OJ, Levin L (2015) Production of lignocellulolytic enzymes from three white-rot fungi by solid-state fermentation and mathematical modeling. Afr J Biotechnol 14:1304–1317. doi:10.5897/AJB2014.14331
Nagpal R, Puniya AK, Griffith GW, Goel G, Puniya M, Sehgal JP, Singh K (2009) Anaerobic rumen fungi: potential and applications. In: Khachatourians GG, Arora DK, Rajendran TP, Srivastava AK (eds) Agriculturally important microorganisms, vol. I. Academic World International, pp. 375–393
Nicholson MJ, Theodorou MK, Brookman JL (2005) Molecular analysis of the anaerobic rumen fungus Orpinomyces—insights into an AT-rich genome. Microbiology 151:121–133
Palmieri G, Bianco C, Cennamo G, Giardina P, Marino G, Monti M, Sannia G (2001) Purification, characterization, and functional role of a novel extracellular protease from Pleurotus ostreatus. Appl Environ Microbiol 67:2754–2759
Paul S, Kamra D, Sastry VRB, Sahu N, Agarwal N (2004) Effect of anaerobic fungi on in vitro feed digestion by mixed rumen microflora of buffalo. Reprod Nutr Dev 44:313–319
Raghuwanshi S, Misra S, Saxena RK (2014) Treatment of wheat straw using tannase and white-rot fungus to improve feed utilization by ruminants. J Anim Sci Biotechnol 5:1–8
Rahul G, Rao DS, Rajyalakshmi A, Kiran SR (2013) Screening of alkaline proteases in Phanerochaete chrysosporium grown in non-lignocellulose médium. Afr J Microbiol Res 7:4297–4305
Ramirez-Bribiesca JE, Soto-Sanchiez A, Hernandez-calva LM, Salinas Chavira J, Galaviz-Rodriguez JR, Cruz-Monterrosa RG, Vargas Lopez S (2010) Influence of Pleurotus ostreatus spent corn straw on performance and carcass characteristics of feedlot Pelibuey lambs. Indian J Anim Sci 80:754–757
Rawlings ND, Barret AJ, Bateman A (2011) Asparagine peptide lyases. J Biol Chem 286:38321–38328
Rouches E, Herpoel-Gimber I, Steyer JP, Carrere H (2016) Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: a review. Renew Sust Energ Rev 59:179–198
Rytioja J, Hildén K, Yuzon J, Hatakka A, de Vries RP, Mäkelä MR (2014) Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiology and Molecular Biology Reviews: MMBR 78:614–649
Samanta AK, Singh KK, Das MM, Pailan GH (2008) Effect of direct fed anaerobic fungal culture on rumen fermentation, nutrient utilization and live weight gain in crossbred heifers. Indian J Anim Sci 78:1134–1137
Sánchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194
Saxena S, Sehgal JP, Puniya AK, Singh K (2010) Effect of administration of rumen fungi on production performance of lactating buffaloes. Benef Microbes 1:183–188
Scully ED, Hoover K, Carlson JE, Tien M, Geib SM (2013) Midgut transcriptome profiling of Anoplophora glabripennis, a lignocellulose degrading cerambycid beetle. BMC Genomics 14:850
Silva RR (2017) Bacterial and fungal proteolytic enzymes: production, catalysis and potential applications. Appl Biochem Biotechnol. doi:10.1007/s12010-017-2427-2
Silva RR, Cabral TPF, Rodrigues A, Cabral H (2013) Production and partial characterization of serine and metallo peptidases secreted by Aspergillus fumigatus Fresenius in submerged and solid state fermentation. Braz J Microbiol 44:235–243
Silva RR, Caetano RC, Okamoto DN, de Oliveira LCG, Bertolin TC, Juliano MA, Juliano L, Oliveira AHC, Rosa JC, Cabral H (2014) The identification and biochemical properties of the catalytic specificity of a serine peptidase secreted by Aspergillus fumigatus Fresenius. Protein & Pept Lett 21:663–671
Silva RR, Souto TB, Oliveira TB, Oliveira LCG, Karcher D, Juliano MA, Juliano L, Oliveira AHC, Rodrigues A, Rosa JC, Cabral H (2016) Evaluation of the catalytic specificity, biochemical properties, and milk clotting abilities of an aspartic peptidase from Rhizomucor miehei. J Ind Microbiol Biotechnol. doi:10.1007/s10295-016-1780-4
Silva RR, Oliveira LCG, Juliano MA, Juliano J, Rosa JC, Cabral H (2017) Activity of a peptidase secreted by Phanerochaete chrysosporium depends on lysine to subsite S’1. Int J Biol Macromolec 94:474–483
Spániková S, Biely P (2006) Glucuronyl esterase-novel carbohydrate esterase produced by Schizophyllum commune. FEBS Lett 580:4597–4601
Staszczak M, Zdunek E, Leonowicz A (2000) Studies on the role of proteases in the white-rot fungus Trametes versicolor: effect of PMSF and chloroquine on ligninolytic enzymes activity. J Basic Microbiol 40:51–63
Tekere M, Ncube I, Read JS, Zvauya R (2002) Biodegradation of the organochlorine pesticide, lindane by a sub-tropical white-rot fungus in batch and packed bed bioreactor systems. Environ Technol 23:199–206
Tsai CT, Huang C-T (2008) Overexpression of the Neocallimastix frontalis xylanase gene in the methylotrophic yeasts Pichia pastoris and Pichia methanolica. Enzym Microb Technol 42(6):459–465. doi:10.1016/j.enzmictec.2008.01.018
Vaithanomsat P, Apiwatanapiwat W, Petchoy O, Chedchant J (2010) Production of ligninolytic enzymes by white-rot fungus Datronia sp. KAPI0039 and their application for reactive dye removal. Int J Chem Eng 1–6. doi:10.1155/2010/162504
Valadares F, Gonçalves TA, Gonçalves DSPO, Segato F, Romanel E, Milagres AMF, Squina FM, Ferraz A (2016) Exploring glycoside hydrolases and accessory proteins from wood decay fungi to enhance sugarcane bagasse saccharification. Biotechnol Biofuels 9:110. doi:10.1186/s13068-016-0525-y
Van den Brink J, de Vries RP (2011) Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 91(6):1477–1492
Vodovnik M, Logar RM (2010) Cellulosomes—promising supramolecular machines of anaerobic cellulolytic microorganisms. Acta Chim Slov 57:767–774
Wallace RJ (1996) The proteolytic systems of ruminal microorganisms. Ann Zootech 45:301–308
Wei Y, Zhou H, Zhang J, Zhang L, Geng A, Liu F, Zhao G, Wang S, Zhou Z, Yan X (2015) Insight into dominant cellulolytic bacteria from two biogas digesters and their glycoside hydrolase genes. PLoS One 10(6)
Wilson DB (2011) Microbial diversity of cellulose hydrolysis. Curr Opin Microbiol 14:1–5
Wilson DB, Kostylev M (2012) Cellulase processivity. Methods Mol Biol 908:93–99
Yang Y, Wei F, Zhuo R, Fan F, Liu H, Zhang C, Zhang X (2013) Enhancing the laccase production and laccase gene expression in the white-rot fungus Trametes velutina 5930 with great potential for biotechnological applications by different metal ions and aromatic compounds. PLoS One 8(11)
Zhu W-Y, Theodorou MK, Longland AC, Nielsen BB, Dijkstra J, Trinci APJ (1996) Growth and survival of anaerobic fungi in batch and continuous-flow cultures. Anaerobe 2:29–37
Zhu N, Liu J, Yang J, Lin Y, Yang Y, Ji L, Li M, Yuan H (2016) Comparative analysis of the secretomes of Schizophyllum commune and other wood-decay basidiomycetes during solid-state fermentation reveals its unique lignocellulose-degrading enzyme system. Biotechnol Biofuels 9:42
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
In this article, we did not perform any studies with human participants or animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
da Silva, R.R., Pedezzi, R. & Souto, T.B. Exploring the bioprospecting and biotechnological potential of white-rot and anaerobic Neocallimastigomycota fungi: peptidases, esterases, and lignocellulolytic enzymes. Appl Microbiol Biotechnol 101, 3089–3101 (2017). https://doi.org/10.1007/s00253-017-8225-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8225-5