Abstract
Polymers are sometimes preferred for membrane filtration because they are more flexible, easier to handle, and less expensive than inorganic membranes fabricated from oxides, metals, and ceramics. The polymers are used as the membrane active layer and porous support in reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF), microfiltration (MF) processes. However, the application of polymers for filtration suffers critical drawbacks, such as the chemical attack of polymers, membrane fouling, and hydrophobicity of most polymers. In this chapter, the polymers used for membrane filtration in recent studies and their fabrication procedures are presented and discussed. The polymers used in recent applications include cellulose acetate (CA), polyamide (PA), polyvinylidene fluoride (PVDF), polysulfone (PSF), polyethersulfone (PES), polyvinyl chloride (PVC), polyimide (PI), polyacrylonitrile (PAN), polyethylene glycol (PEG), polyvinyl alcohol (PVA), poly(methacrylic acid) (PMAA), poly(arylene ether ketone) (PAEK), poly(ether imide) (PEI), and polyaniline nanoparticles (PANI). A new polymeric material named polyethersulfone amide (PESA) has also been presented recently. Most of the recent studies have focused on improving the specific energy consumption, salt rejection, water flux, chemical resistance and antifouling properties of polymeric membranes and nanocomposites through blending and surface modification techniques. These techniques involve the use of zwitterionic coatings, sulfonated poly(arylene ether sulfone) (SPAES), perfluorophenyl azide (PFPA), carbon nanotubes (CNTs) and graphene oxide (GO) as nanofillers, polyether ether ketone (PEEK), and nanoparticles such as titanium dioxide (TiO2), and mesoporous silica. The use of polymers for filtration is still gaining tremendous attention, and further improvements of polymeric characteristics for enhanced membrane performance are expected in the coming years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aryl Ether Ketone
- Polyaniline Nanoparticles (PANI)
- Arylene Ether Sulfone
- Polyether Ether Ketone (PEEK)
- Salt Rejection
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
Current water quality regulations and standards require the careful treatment of water from different sources, including seawater, brackish water, groundwater, industrial wastewater, municipal wastewater, and surface water, so that the final effluents can be useful for a wide range of applications [3, 11, 12, 14]. Desalination is a key water treatment approach, most especially in areas with inadequate natural and renewable fresh water supply. In addition, the industries in many countries are now being asked to treat their own wastewater to reduce dependence on naturally available fresh water and desalinated water [21, 59]. However, treated water from industries and municipalities require an appreciable level of treatment to prevent possible secondary or end-use problems.
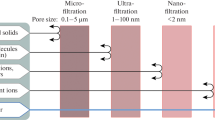
Conventionally, treatment processes such as biological treatment, distillation, evaporation, chemical coagulation, flocculation, sand filtration, and gravity sedimentation are used to remove pollutants from water [31, 35, 38]. For saline water desalination, thermal distillation processes such as multi-effect distillation, multistage flash, and thermal and mechanical vapor compression were mainly employed in parts of the world until the recent decades. Nowadays, desalination via membrane filtration processes, i.e., microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis, (RO) is gaining immense attention from desalination stakeholders [6, 88, 100]. Currently, the market share of membrane filtration processes in the desalination industry has soared and surpassed those of other approaches. MF and UF are mostly being used for pre-treatment in current desalination applications, instead of coagulation or sedimentation. In addition, there are more RO plants in the world than those that employ thermal desalination approaches currently [28]. Likewise, membrane filtration processes such as membrane bioreactors (MBRs) and osmotic membrane bioreactors (OMBRs) are being preferred for wastewater treatment than conventional approaches such as activated sludge processes (ASP), aerated lagoons, and trickling filters [17, 33].
The functional component of a membrane filtration process is the membrane. Membranes can be made from polymeric or inorganic materials [66, 104]. Most of the polymeric membranes are organic in nature, while inorganic membranes are mainly oxides, ceramics, and metals [89]. Membranes made from polymeric materials are cheaper than those fabricated from inorganic materials or ceramics [66]. Additionally, polymeric membranes can be used to achieve high water production capacity. These membranes are easy to handle during fabrication and can be arranged in different configurations such as hollow fiber and spiral wound for optimum performance [46, 66, 89]. Therefore, the objective of this chapter is to review the polymeric materials that have been used for filtration in recent times. Specifically, the recent advances in the fabrication of polymers for RO, NF, UF, and MF processes are discussed. The type of polymer used for filtration is crucial because it determines the permeate quality and the operating cost of water production. Proper selection of polymer is required for a filtration process to ensure that issues such as frequent membrane replacement and unwarranted energy consumption are avoided. The current challenges associated with recently devised polymers are also presented and discussed.
8.2 Polymers Used for Membrane Filtration
Several polymers have been used in the fabrication of MF, UF, NF, and RO membranes. Examples include cellulose acetate (CA), polyamide (PA), polyvinylidene fluoride (PVDF), polysulfone (PSF), polyethersulfone (PES), polyvinyl chloride (PVC), polyimide (PI), polyacrylonitrile (PAN), polyethylene glycol (PEG), polyvinyl alcohol (PVA), poly(methacrylic acid) (PMAA), poly(arylene ether ketone) (PAEK), poly(arylene ether sulfone) (PAES), poly(ether imide) (PEI), and polyaniline nanoparticles (PANI). A new polymeric material named polyethersulfone amide (PESA) has also been presented recently. Fabrications, characterization, and related applications of such membranes are highlighted in the subsequent sections.
8.2.1 Polymers for RO
RO technology (Fig. 8.1) has been found to be one of the most efficient and widely popular methods of desalinating water because it is suitable for the production of potable and near-to-potable water [41, 53].
RO membranes that are commercially available consist of polymeric materials such as CA and PA [13, 28]. CA is used because it is a natural polymer that is renewable, biodegradable, and eco-friendly [18, 63]. CA can be produced through the esterification of wood, cotton, recycled paper, and bagasse. CA is also a widely used polymer known for its high hydrophilicity, biocompatibility, high potential flux, etc. [26]. However, PA membranes are generally preferred among the two because of their ability to operate under a wider pH range and withstand higher temperatures [72]. Unfortunately, the practical application of PA membranes is often limited due to their continuous exposure to chlorine and other oxidizing substances [110]. The amide group that is present in the PA membranes is vulnerable to chlorine attacks during chemical cleaning [28, 92]. Hence, an additional de-chlorination step is required to reduce the concentration of chlorine to prevent the degradation of the PA membranes. Also, in order to overcome this problem, poly(arylene ether) copolymers, especially poly(arylene ether sulfone), have been used recently for RO desalination [67, 68, 92]. Since these polymers do not contain any susceptible amide linkages, it makes them to be highly resistant to chlorine attacks. It has also been established recently that thin film composite (TFC) membranes based on SPAES display high chlorine-tolerance and no significant change in water flux after 36 h of continuous exposure [110].
-
(a)
poly(arylene ether sulfone) (PAES)
Photocross-linkable disulfonated PAES copolymers have been prepared for RO applications in a recent study [64]. First, PAES oligomers with controlled degrees of sulfonation and controlled molecular weights were synthesized via nucleophilic aromatic substitution. Meta-aminophenol was used to control the molecular weight of the PAES oligomers and install telechelic amine end groups. The meta-aminophenol end-capped oligomers were reacted with acryloyl chloride to obtain novel cross-linkable PAES oligomers with acrylamide groups on both ends. The acrylamide-terminated oligomers were cross-linked using UV radiation in the presence of a multifunctional acrylate and a UV photoinitiator to obtain PAES copolymers thin films. It was shown that the cross-linked disulfonated PAES films had smooth surfaces that promoted high water passage (Fig. 8.2). The copolymer films also exhibited reduced water uptake and swelling relative to their linear counterparts.
Atomic force microscopy (AFM) images of a PA TFC membrane and b the disulfonated copolymer, showing the smooth surface of the copolymer film that ensured higher water passage [64]
-
(b)
Polyamide (PA)
Apart from the problem of chlorine attacks during chemical cleaning, PA polymeric material faces membrane fouling [19, 78, 79]. Biofouling is one of the most challenging fouling mechanisms experienced during membrane filtration [5, 39, 44]. Biofouling occurs due to the formation of a biofilm by the biological species in a membrane filtration system, resulting in the depletion of the membrane’s lifetime and selectivity. Although RO works based on the solution–diffusion principle rather than size exclusion, biofouling is a major problem in RO. This can be attributed to the thin layer of the active surface and the material of the dense and porous structures [54, 90]. Thus, zwitterionic natured coatings on membranes have been observed to be effective antibiofouling materials in recent studies [50, 80]. A desirable feature of the zwitterionic structure is that it exhibits both positively and negatively charged moieties within the same segment side chain which imparts strong hydration capacity via electrostatic interactions. These polyzwitterions are usually attached to the membrane’s surface using a grafting approach. The coatings produce densely packed polymer chains that exhibit consistent length and reduce the adhesion of cells and bacteria onto the membrane surface [27]. The surface modification of polymeric RO membrane by zwitterionic polymer can be used to achieve higher permselectivity and water flux [96]. The modification has been accomplished recently by the grafting of a commercially available membrane with N,N′dimethylaminoethyl methacrylate (DMAEMA) via redox-initiated graft polymerization reaction [96]. Then, the DMAEMA graft was modified via surface quaternization reaction with 3-bromopropionic acid (3-BPA) to obtain the zwitterionic carboxybetaine methacrylate (CBMA) polymer chains on the membrane surface. The CBMA, which has a cationic quaternary ammonium group and anionic carboxylate group on its backbone, changed the chemical structure, morphological structure, hydrophilicity, and charge of the RO membrane. The fabricated procedures are illustrated in Fig. 8.3. The modified membrane showed improved water flux (22.5% increase in water flux compared to the unmodified membrane). By using positively charged lysozyme and negatively charged bovine serum albumin as foulants, it was shown that the biofouling properties of the modified membrane were enhanced as evidenced by the higher water recovery rate after fouling test. Up to 99% mortality of Escherichia coli (E.coli) and Bacillus subtilis was achieved. Another polymer that has been used successfully for grafting is polysulfobetaine. Polysulfobetaine was grafted from the surface of commercially available TFC membranes in another recent study [27], leading to 80% reduction in microbial fouling without any adverse effect on the permeate flux.
Illustration of the fabrication procedures for the modification of RO membranes with zwitterionic polymer chains [96]
PA-RO membranes with enhanced antifouling properties have also been prepared recently by making use of the highly reactive azide group of PFPA that can form chemical bonds when activated by photoexcitation with nonreactive groups [57]. First, PEG polymers were modified with a terminal PFPA group. Then, pieces of commercially available PA were dipped into an aqueous solution containing the PEG-PFPA prepared polymers. The pieces of modified PA were dried at ambient conditions and irradiated with 254 nm UV light. Finally, the pieces of PA obtained were rinsed with water to remove unreacted azides and other by-products. The performance of the prepared membranes was evaluated through pure water permeability and sodium chloride (NaCl) rejection tests. The antibiofouling properties of the membranes were assessed by monitoring the growth of E.coli on the membranes. The prepared membranes were more hydrophilic than the commercially available PA. The membranes also exhibited lower water permeability but increased NaCl rejection. It was observed that the prepared membranes had better antibiofouling properties as evidenced by the reduced growth of E.coli bacteria on the prepared membranes.
-
(c)
Polyvinyl chloride (PVC)
Another polymeric material that has been used recently for the RO process is polyvinyl chloride (PVC) [2, 70]. This is because of its flexibility and durability along with suitable biological and chemical resistance [26]. Special selective characteristics and enhancement of separation properties have been achieved in membranes with the use of PVC/CA polymers as membrane binders. It has been observed that an increase in CA concentration in the dope solution that consists of PVC/CA polymers would result in an increase in the hydrophilic characteristics of the membrane [26]. This is because the high amount of water would be absorbed; hence, more water would pass through the membranes. An increase of CA concentration to about 10% could also improve the rejection capabilities of the fabricated membrane.
-
(d)
Chemical modifications of membrane properties using carbon nanotubes (CNTs)
Other studies have also shown that membrane properties can be improved when polymeric materials are chemically modified with other polymers of desirable properties [80, 86]. One of such additives is carbon nanotubes (CNTs) which act as nanofiller in RO desalination. Although a decrease in the membrane permeability would be observed when the CNT concentration is increased, an increase in the salt rejection and permeate flux would be achieved [77]. CA is efficient in the rejection of salts during RO desalination because of its excellent desalting properties resulting from its nanoscale characteristics [16]. Recently, raw and oxidized multiwalled CNTs (MWCNTs) in different concentrations (0.001, 0.002, 0.005, 0.01 wt%) have been incorporated into PA-RO membranes [23]. The morphology of the modified membranes was altered as a result of the MWCNTs incorporation. The membranes embedded with raw MWCNTs exhibited slightly higher contact angle compared to the pristine membrane, while membranes embedded with oxidized MWCNTs had slightly lower contact angle compared to the pristine membrane. An increase in the concentration of both raw and oxidized MWCNTs up to 0.005 wt% resulted in an increase in the water flux, after which the water flux decreased. Meanwhile, all concentrations of raw or oxidized MWCNTs resulted in better antifouling performance of the modified membranes. The modified membranes with 0.005 wt% MWCNT concentration showed the best antifouling properties.
Kim et al. [42] have also demonstrated recently that the modification of PA-RO membrane with CNT can be used to accomplish improved membrane properties [42]. The CNTs were initially functionalized by reacting them with a sulfuric acid/nitric acid mixture. Then, PA was prepared by using trimesoyl chloride (TMC) solutions in n-hexane and aqueous solutions of m-phenylenediamine (MPD) containing the functionalized CNTs. The maximum flux and salt rejection values were observed when the functionalized CNTs were prepared by the reactions of CNTs with a sulfuric acid and nitric acid mixture for 4 h at 65 °C. When shorter reaction time and lower reaction temperature were used, the CNTs were not well-dispersed in the PA active layers. Conversely, when longer reaction time and higher reaction temperature were used, the CNTs were cut down into very small pieces to form aggregated structures. Therefore, good dispersion of the functionalized CNTs in the PA layer was necessary. The membranes containing the properly modified CNTs demonstrated higher water flux than the PA membrane prepared without any CNTs. Better chemical resistance against NaCl solution compared to the pristine RO membrane was also achieved by using the modified membranes.
However, the mechanical strength and structural integrity of the nanofiller still need to be improved in future research activities so that it can be employed for large-scale commercial desalination. To achieve mechanical stability, a recent study has tested 1,2-bis(triethoxysilyl)ethane (BTESE) instead. In this study [34], a porous PSF-supported BTESE hybrid membrane was fabricated through a sol–gel spin-coating heat treatment process. A 200-nm-thick BTESE-derived silica separation layer was deposited onto the PSF support surface. The RO membrane was evaluated by using it to desalinate a NaCl aqueous solution. The membrane showed a stable and high degree of water permeability with high salt rejection reaching 96%. The membrane also showed good stability and reproducibility during the RO desalination process that was run for more than 160 h.
8.2.2 Polymers for NF
-
(a)
Polyimide (PI) and polyamide (PA)
NF membranes have gained popularity for water filtration in recent decades due to their beneficial features such as low energy consumption when compared with RO and high retention of divalent salts and neutral molecules of low molecular weight [25, 61, 112]. Nonetheless, NF membranes can only withstand aqueous solutions containing pH in the range of 2–11 due to their moderate stability. Most of the NF membranes available today consist of PI, PA, PVA, and PAN polymers in TFCs [4, 85, 87, 93, 95, 105, 108]. However, PIs are unstable when in contact with a few amines. They also exhibit very low stability and performance in polar solvents. These PIs are not preferred in aqueous solutions containing chlorinated solvents, strong amines, and strong acids/bases, but they can be modified through the process of cross-linking to obtain improved resistance against such chemicals.
-
(b)
Poly(arylene ether ketone) (PAEK)
An alternative solution that involves the use of PEEK as NF membrane material has been proposed recently [15]. It was observed that PEEK membranes have a low degree of sulfonation and are highly resistant against various solvents, acids, and bases. However, PEEK membranes exhibit low water permeability. The PEEK membranes were tested for their separation performance in tetrahydrofuran (THF) and dimethylformamide (DMF) where they exhibited a water permeance of 0.2–0.8 and 0.7–0.21 L/h m2 bar, respectively.
-
(c)
Membrane fouling in NF membranes
The challenge of membrane fouling and chemical attack is also associated with PA NF membranes. Fouling not only reduces the flux through NF membranes but also increases the energy requirement. Meanwhile, surface modification has been employed recently to impart antifouling properties to PA NF membranes [48, 52, 60, 109]. These properties have been achieved by grafting fluorinated PA onto the surface of the PA NF membranes [48]. The fluorinated PA NF membranes have lower surface energy which resulted in the minimization of the adhesion propensity membrane. The detachment of foulants from the membrane surface was achieved through the fluorination of the membrane. 98.3% permeation flux recovery was accomplished through this approach. Stability problems are also associated with PVA NF membranes. However, in a recent investigation, a novel TFC membrane has been fabricated by cross-linking PVA and 3-mercaptopropyltriethoxysilane on porous PSF support in order to enhance the ion rejection and acid/alkali stability of the membrane [109]. The introduction of a sulfonic acid group enhances the hydrophilic properties of the membrane which in turn caused an increase in the water flux across the membrane. This approach is not completely advantageous because the sulfonic acid groups also caused the swelling of the membrane, resulting in a decrease in the membrane’s rejection properties.
Meanwhile, antifouling and salt rejection features can be imparted to a TFC NF membrane by replacing the mid-layer of the TFC membrane with an electrospun nanofibrous membrane (ENM) resulting in a TFNC membrane [84]. ENMs are known for their large dirt loading capacity due to their large internal surface area. In order to achieve this, the ENM layer must be hydrophilic and heat-treated before interfacial polymerization. In addition, they are highly porous compared to conventional membranes which would ensure that the water flux across the membrane is enhanced. ENMs are produced through electrospinning and have unique properties such as high surface area to volume ratio, tailorable pore sizes, and flexibility in their surface chemistry.
8.2.3 Polymers for UF
PS and PES are widely used in UF membranes because they are polymeric materials with good mechanical properties, wide pH operation range, and strong chemical stability [20, 62, 69, 82, 83, 91, 101]. However, their application in water treatment is limited due to their hydrophobicity which ultimately leads to reduced membrane permeability. Most of the polymeric membrane materials that are widely used in UF processes exhibit hydrophobic properties. PVDF, PVC, and PMAA have also been used recently for the fabrication of UF membranes. These polymers are also naturally hydrophobic [10, 37, 55, 94 102, 111]. Membrane hydrophobicity can cause water flux decline during operation due to the accumulation of organic compounds that favor the attachment and growth of microorganisms onto the membrane surface. This usually leads to membrane fouling and subsequently membrane failure. Thus, to improve their properties and enhance their performance in water treatment applications, modifications to these polymeric materials are necessary. These modifications are carried out in such a way that the membrane hydrophilicity is increased. An increase in the membrane surface hydrophilicity would enhance the membrane’s antifouling properties for liquid water-based filtration.
-
(a)
Incorporation of TiO2 nanoparticles into polysulfone (PSF)
Blending and surface modification can be used to incorporate hydrophilic materials (nanoparticles and amphiphilic copolymers) into UF membranes to increase their hydrophilicity [49]. A hybrid PSF membrane impregnated with modified TiO2 nanoparticles for the impartation of hydrophilic property to PSF has been proposed recently [66, 107]. The membrane was prepared by grafting the hydrophilic polymer chains of (2-hydroxyethyl methacrylate) (P(HEMA)) on TiO2 nanoparticles through atom transfer radical polymerization process. PSF membranes were impregnated with the modified TiO2 nanoparticles for achieving better membrane performance, overcoming agglomeration of nanoparticles on the membrane surface and reducing the leakage of nanoparticles from the membrane during filtration. The modified TiO2 particles had better dispersibility within the polymer than unmodified TiO2. The PSF membrane modified with TiO2-HEMA exhibited improved hydrophilicity, higher water flux, and better antifouling performance than the pristine PSF membrane and unmodified TiO2 impregnated membranes.
-
(b)
Incorporation of mesoporous silica particles (MSP-1) into polysulfone (PSF)
Incorporating inorganic particles into a membrane’s casting mixture prior to phase inversion is widely studied because it is a facile approach that can be used to embed additional particle-based functionalities into membranes [16, 29, 73]. Surfactant-templated mesoporous silica particles (MSP-1) have been incorporated into PSF matrices formed with and without PEG as a molecular porogen with the aim of enhancing the properties of PSF membranes [22]. It was observed that MSP-1 additives increased the hydrophilicity of the membrane by virtue of the terminal silanol (Si–OH) groups on the pore walls and external surfaces of the particles. Both MSP-1 and PEG modified the typical morphology of the phase inversion membrane content. The mechanical properties of the PSF–MSP mesocomposite were comparable to those of their MSP-free counterparts. The addition of MSP-1 to porogen-free membranes made from casting solutions with low polymer content led to statistically significant differences in permeate flux. The addition of only 5.0 wt% MSP-1 had a detrimental effect on flux, yet a further increase to 10 wt% loading level raised the permeate flux above the value observed for MSP-free controls. However, when the PEG porogen was included in the casting mixture, no statistically significant changes either in flux or in rejection were observed. The mesocomposite membranes showed enhanced dextran rejection compared to MSP-free membranes, and fouling tests with humic acid solutions demonstrated that the mesocomposite membranes experienced lower flux decline and showed higher rejections than their MSP-free counterparts.
-
(c)
Incorporation of zinc oxide (ZnO) and silica nanoparticles into polyvinyl chloride (PVC) and poly(methacrylic acid) (PMAA)
The impact of incorporating nanoparticles into PVC for enhanced hydrophilicity of PVC UF membranes has also been studied recently [75]. The effect of incorporating zinc oxide (ZnO) nanoparticles into PVC membranes was examined. Five PVC membranes having variable ZnO percentages (0.3, 1.0, 2.0, 3.0, and 4.0 wt%) were fabricated via the phase inversion method using water as coagulant and PEG as a pore forming additive. The ZnO impregnated membranes had a higher hydrophilicity than pristine PVC membranes, with the 4.0 wt%-ZnO membrane being the most hydrophilic. An increase in ZnO concentration up to 3.0 wt% led to an increase in water flux. Further increase in ZnO concentration led to a decline in water flux due to the agglomeration of ZnO particles at the surface of the PVC membrane (Fig. 8.4). An increase in ZnO concentration up to 3.0 wt% also led to increase in membrane porosity, after which it declined. The pristine PVC membranes were only able to recover 69% of water flux after BSA permeation, whereas membranes containing 3.0 wt% ZnO were able to recover 92% of water flux after BSA permeation. Incorporation of nanoparticles into PMAA has also been examined recently for the improvement of the performance of PMAA UF membranes. Superhydrophilic silica nanoparticles have been grafted onto PMAA membranes through the process of post-fabrication tethering [49]. An increase in the wettability of the membranes was observed.
Energy dispersive X-ray (EDAX) spectroscopy showing agglomeration as more ZnO particles are included in the PVC-ZnO casting solution [75]
-
(d)
Polyethersulfone (PES)
PES has been used in most of the recent studies on UF membrane separation. The hydrophilicity of PES membrane has been improved recently by incorporating mesostructured silica particles functionalized with amine and carboxylic groups into PES [56]. The morphology, porosity, and pore size distribution of the modified membrane changed significantly as a result of the incorporation of ordered mesoporous silica particles. The hydrophilicity of the modified membrane also increased significantly. Water permeation through the membrane increased as a result of the enhanced surface porosity and hydrophilicity of the modified membrane. The antifouling property of the modified membrane was improved, especially against irreversible fouling, without negatively affecting the protein rejection potential of the membrane. It was also observed that the modified membrane exhibited a stable permeation performance during repeated stability tests. In another recent study, a new hydrophilic polymeric material that is based on PES has been proposed. This material was named polyether sulfone amide (PESA) [58]. PESA was prepared through the polycondensation reaction of diamine (4,40-diaminodiphenyl ether) with dicarboxylic acid (diphenyl sulfone 4,40-dicarboxylic acid) using triphenyl phosphite (TTP), lithium chloride (LiCl), calcium chloride (CaCl2), and pyridine (Py) as condensing agents and N-methyl-2-pyrrolidone (NMP) as a solvent. PESA was further modified by grafting it with two hydrophilic monomers, i.e., 3,5-diaminobenzoic acid (DBA) and gallic acid (GA) via interfacial polymerization. It was observed that PESA membrane was more hydrophilic than pure PES membrane. The modification of PESA membrane with DBA and GA further increased the membrane’s hydrophilicity. PESA membrane and modified PESA membrane had greater roughness compared to pure PES membranes. The pure water flux and humic acid rejection of PESA membrane were higher than those of the pristine PES membrane. PESA membrane also showed higher antifouling properties than the PES membrane. The antifouling properties of PESA membranes were further improved by surface modification with DBA and GA.
The hydrophilicity of PES membrane has also been enhanced by incorporating PANI nanoparticles into PES UF membranes [48, 76]. To do this, three different membranes—pure polyethersulfone, self-synthesized PANI impregnated into PES, and commercially available PANI impregnated into PES—were fabricated via phase inversion. The membranes were characterized via contact angle goniometry and evaluated through direct interaction with BSA, humic acid, silica nanoparticles, E.coli and Bacillus bacteria. The addition of PANI nanoparticles led to increased hydrophilicity, enhanced fouling resistance, better flux recovery, improved BSA and humic acid rejection, and reduced attack from bacteria. Interestingly, the self-synthesized PANI impregnated into PES membrane was superior to the commercially available PANI impregnated into PES membrane, in terms of membrane properties. PES UF membranes have also been modified by incorporating highly hydrophilic polyethylene glycol (PEG) and silver nanoparticles (Ag) into PES using poly(acrylonitrile-co-maleic acid) (PANCMA) as a chemical linker [71]. Hollow fiber configuration was used. Polymeric membranes with hollow fiber configuration are preferred for some separation processes because this configuration has the advantages of high surface area, self-mechanical support, excellent flexibility, and ease of handling during module fabrication. The modified membrane was shown to exhibit enhanced properties including higher hydrophilicity (75.5% decrease in contact angle), increased water flux (by 36%), and reduced bacterial growth. Another recent study has improved the hydrophilicity and antifouling property of PES UF membrane by modifying it with dextran-grafted halloysite nanotubes (HNTs) [106]. The incorporation of dextran-HNTs into PES membranes led to significant increase in hydrophilicity as evidenced by the reduction of water contact angle. In addition, the modified membranes showed higher flux and better antifouling properties than pristine PES membranes. Interestingly, the modified membranes had a slightly lower porosity, yet larger pore size than the pure PES membranes.
Meanwhile, the conventional multi-bore hollow fiber membrane consists of three or seven bore channels and an outer round-shaped geometry. However, the main drawback of this geometry is the nonuniform wall thickness. The thinner part of the membrane wall suffers as the mechanically weak point, while the thicker part generates additional mass transfer resistance. Therefore, to overcome this drawback, an attempt has been recently directed toward the fabrication of a novel tri-bore hollow fiber membrane with round-shaped bore channels but an outer triangle-shaped geometry made of Matrimids and PES materials [97]. The triangle-shaped tri-bore hollow fibers can be fabricated with a combination of a tri-bore blossom spinneret and defined spinning parameters. The new geometry, which exhibits a much more uniform wall thickness, was shown to improve the mechanical properties of both the Matrimids and PES membranes as well as their water permeation.
-
(e)
Polyvinylidene fluoride (PVDF)
PVDF is another polymeric material that can be modified for enhanced hydrophilicity. PVDF UF membrane has been recently modified by dipping the PVDF membrane into a dopamine solution such that the dopamine coats the surface of the PVDF membrane by self-polymerization [81]. The coated PVDF membrane was rinsed with water to remove unreacted polydopamine. The coated PVDF membrane obtained was then dipped into a solution containing TiO2 nanoparticles. The dopamine acted as a glue to facilitate the attachment and distribution of the TiO2 nanoparticles onto the PVDF membrane. The resulting PVDF membrane was rinsed with water to remove large TiO2 particles that are deposited onto the surface of the polydopamine-coated PVDF membrane. The TiO2 nanoparticles were homogeneously distributed on the surface of PVDF and did not agglomerate. The hydrophilicity of the modified membrane was improved as evident in the significant reduction of the water contact angle of the membrane. The pure water flux across the modified membrane also increased significantly, and the BSA rejection of the membrane was enhanced. The antifouling properties of the membrane were improved as evident in the low irreversible fouling ratio and a remarkably high flux recovery ratio (>90%) achieved for BSA separation.
-
(f)
Poly(arylene ether ketone) (PAEK) and poly(ether imide) (PEI)
Other polymeric membranes that have been tested for UF operations in recent studies are PAEK and PEI [40, 51]. Cardo PAEK membrane bearing hydrophilic carboxylic acid groups (PAEK-COOH) has been proposed as an alternative to the traditional hydrophobic PAEK membranes [51]. PAEK with pendent carboxylic acid group (PAEK-COOH) was first synthesized by the aromatic nucleophilic substitution polycondensation reaction of 2-[bis(4-hydroxyphenyl)methyl] benzoic acid (PPH-COOH) and 4,4′-bisfluorodiphenylketone in DMSO. Thermal analyses demonstrated that the synthesized PAEK-COOH polymer has a decomposition temperature of 360 °C and glass transition temperature of 220 °C, which suggests that it is well qualified for preparing membranes to deal with hot water without temperature controlling. Then, the synthesized polymer was used to prepare a tight UF membrane by the nonsolvent-induced phase inversion process. The resulting membrane had a water contact angle of 61.5°. The membrane displayed high water permeation flux and dye rejection. The antifouling performance and antidye adsorption properties of the membrane are also promising, possessing a flux recovery ratio of 91.5% for BSA, and dye adsorption rate below 5.0% for all the studied dyes (Congo red, Coomassie brilliant blue R250, Direct red 23, and Evans blue (EB). The membrane is thermally stable and suitable for high-temperature filtration applications.
PEI UF membrane has been modified recently by blending PEI with N-phthaloylated chitosan (NPHC) so as to enhance the antifouling properties of the membrane [40]. The modified membrane was more hydrophilic than the unmodified membrane. The roughness of the surface of the modified membrane was greater than that of the unmodified membrane. The surface roughness increased with increasing NPHCs content. Pure water flux increased with increasing concentration of NPHCs in the NPHCs blended membrane. Meanwhile, when the concentration of NPHCs in the NPHCs blended membrane was increased, the capacity of the fabricated membrane to reject protein became lower while permeate flux increased. However, the separation of heavy metal ions increased with increasing concentration of NPHCs in the NPHCs blended membrane. Maximum flux recovery was achieved for the PEI/NPHCs blended membrane when the NPHCs concentration was 2.0 wt%.
8.2.4 Polymers for MF
MF membranes have been mainly used in membrane distillation (MD), MBRs, and wastewater treatment processes [1, 7, 30, 32, 99]. Industrial and domestic wastewater contains harmful organic pollutants (like pharmaceutical compounds) which constitute a great threat to aquatic species and the environment in general. Advanced technologies such as advanced oxidation processes (AOPs) have proven to be very efficient in cleaning recalcitrant wastewater. One of the most important AOPs is photocatalysis via TiO2, which completely mineralizes a wide range of organic compounds. The direct incorporation of TiO2 nanoparticles onto MF polymer membranes has been proven to be a viable membrane separation technique recently [24]. Titanium tetraisopropoxide (TTIP) was used as the precursor for TiO2. TTIP hydrolysis prevented the formation of agglomerates and increased the bonding strength of the TiO2 particles formed. PES and PVDF membranes were used as the supporting structures for TiO2 nanoparticles. After the attachment of TiO2 particles, a decrease in the porosity of the membrane was observed. However, the attached TiO2 showed the ability to degrade various molecules like dyes, drugs, and pesticides.
MD is a thermally driven process by which water molecules are separated from other undesired substances through porous membranes [7, 8]. Hydrophobic membranes are required for MD applications because MD works based on the principle of vapor permeation. The vapor pressure difference across a hydrophobic membrane is the driving force in MD. Although MD is known for its easy implementation and utilization of heat, it has not yet gained industrial-scale application due to its drawbacks, among which MF membrane fouling and low flux are the most predominant and hard to tackle. Nonetheless, it has recently been reported that an increase in the flux had been observed in DCMD membranes by tetrafluoromethane (CF4) plasma surface modification [103]. Although the vapor flux through the plasma-modified membrane reached its maximum at about 15 min and then started to decrease, the overall flux of the modified membrane was still higher than that of the virgin membrane. The PVDF membranes were converted to superhydrophobic membranes through CF4 plasma treatment, which resulted in the enhancement of flux and salt rejection.
MBRs have gained popularity in wastewater treatment due to its high quality of processed water, reduction in excess sludge, controllability of solids, and minimization of required footprint [36, 65]. Although it has many positives, one of the most important drawbacks of MBR operations is also membrane fouling [9, 47]. In order to potentially overcome this, graphene oxide (GO) is currently being incorporated with MF membranes to prepare MBR membranes with antifouling properties [45]. This is due to the unique properties of GO such as hydrophilicity and large negative zeta-potential attributable to its functional groups. These properties enhance water permeation through the membrane and impede biofouling. It has been observed that the thickness of biofilm formed by the microorganism on GO-incorporated membranes decreases and the negative zeta-potential increases when the GO content within the membrane is increased [47]. The addition (up to 1.3 wt%) of GO to the membrane dope helped to prevent fouling and increase pure water flux through the membrane significantly. Above 1.3 wt% of GO would result in an increase in polymer solution viscosity, which can result in the reduction of the membrane pore size and water flux. High energy demands resulting from membrane fouling is an indirect drawback associated with MBR operations. Therefore, research on osmotic MBRs (OMBRs) has been intensified recently [43, 53, 98]. The driving force in OMBRs is the osmotic pressure difference between the feed and draw sides of the membrane, rather than hydraulic pressure difference. However, as compared to a conventional MBRs, OMBRs contain a high rejection semipermeable membrane instead of a microporous membrane. Although the fouling potential is comparatively much lower in OMBRs, membrane fouling still does occur. The elevated salinity and salt accumulation, the interactions of inorganic ions and organic foulants, and the scaling of low soluble salts under high ionic strengths might even contribute toward more complex fouling phenomena [74]. But, due to the absence of hydraulic pressure in OMBRs, the compaction of membrane foulants is milder and hence fouling could be easily curtailed by hydrodynamic shear.
8.3 Summary
The fabrication procedures, features, and performance of the polymers used in recent filtration processes are discussed in this chapter. These processes include RO, NF, UF, and MF. The polymers used recently in systems such as MBRs and MD, where membrane filtration is employed, are also discussed. The efficiency of a membrane filtration process depends on the type of polymer, the physical characteristics of a polymeric membrane, and the functional groups on the surface and interior of the polymeric membrane. A membrane with desirable properties can be fabricated through the modification of the membrane-forming polymers. This modification can be achieved through the incorporation of materials such as copolymers and nanoparticles into the membrane matrix. For RO, there is a general preference for PA TFC membranes recently, but the application of these membranes is restricted by the chemical attack of the amide group in PA due to chlorine and other oxidizing compounds. Meanwhile, surface modification by zwitterionic polymer can be used to achieve higher permselectivity and water flux. In addition, SPAES lacks amide linkages; so TFC membranes based on SPAES have high resistance to chlorine attacks. These zwitterionic coatings and reactive groups that can form chemical bonds such as PFPA also have the potential to impart antifouling properties to RO membranes. Nanofillers such as CNTs can be employed to improve the salt rejection properties of RO membranes. However, further research is needed to improve the mechanical integrity of CNT nanofillers for long-term processes. For NF, PEEK contains a low level of sulfonation and can be used with polymeric membranes to achieve resistance again solvents, acids, and bases. The grafting of fluorinated PA onto the surface of the PA NF membranes could be used to improve the resistance of PA TFC membranes to fouling. The fluorinated PA has the potential to reduce the surface energy of TFC membranes, thereby reducing the adsorption of foulants on the membranes. In addition, cross-linkers and sulfonic acid group are capable of enhancing the hydrophilicity of NF membranes.
Most of the recent works on polymers used for UF have been focused on the incorporation of inorganic particles into the polymeric casting mixture prior to phase inversion. The use of surfactant-templated mesoporous silica particles, GO, and ZnO in the fabrication of UF membranes is capable of improving the hydrophilicity of the nanocomposites formed. An improvement in membrane hydrophilicity might result in an increase in pure water flux across the membrane and membrane fouling reduction. The separation properties of the nanocomposites such as morphology, porosity, and pore size distribution can be significantly tailored through such modifications. The dipping of the PVDF membrane into a dopamine solution has also been shown as a method of imparting hydrophilicity to PVDF UF membrane in a recent investigation. Recent advancements in the modifications of the functional and structural properties of polymers for filtration are ongoing, and it is expected that further improvements in the future would ensure more efficient and less expensive filtration processes.
References and Future Readings
Abdel-Karim A, Gad-Allah TA, El-Kalliny AS, Ahmed SIA, Souaya ER, Badawy MI, Ulbricht M (2017) Fabrication of modified polyethersulfone membranes for wastewater treatment by submerged membrane bioreactor. Sep Purif Technol 175:36–46. https://doi.org/10.1016/j.seppur.2016.10.060
Ahmad A, Jamshaid F, Adrees M, Iqbal SS, Sabir A, Riaz T, Zaheer H, Islam A, Jamil T (2017) Novel polyurethane/polyvinyl chloride-co-vinyl acetate crosslinked membrane for reverse osmosis (RO). Desalination 420:136–144. https://doi.org/10.1016/j.desal.2017.07.007
Ahmad R (2017) Water worldwide—US water regulations and India’s water challenges. J Am Water Works Assoc 109:64–67. https://doi.org/10.5942/jawwa.2017.109.0041
Ahmed FE, Lalia BS, Hilal N, Hashaikeh R (2017) Electrically conducting nanofiltration membranes based on networked cellulose and carbon nanostructures. Desalination 406:60–66. https://doi.org/10.1016/j.desal.2016.09.005
Al Ashhab A, Gillor O, Herzberg M (2014) Biofouling of reverse-osmosis membranes under different shear rates during tertiary wastewater desalination: microbial community composition. Water Res 67:86–95. https://doi.org/10.1016/j.watres.2014.09.007
Amy G, Ghaffour N, Li Z, Francis L, Linares RV, Missimer T, Lattemann S (2017) Membrane-based seawater desalination: present and future prospects. Desalination 401:16–21. https://doi.org/10.1016/j.desal.2016.10.002
Ashoor BB, Mansour S, Giwa A, Dufour V, Hasan SW (2016) Principles and applications of direct contact membrane distillation (DCMD): a comprehensive review. Desalination 398:222–246. https://doi.org/10.1016/j.desal.2016.07.043
Ashoor BB, Fath H, Marquardt W, Mhamdi A (2012) Dynamic modeling of direct contact membrane distillation processes. Comput Aided Chem Eng 170–174. https://doi.org/10.1016/b978-0-444-59507-2.50026-3
Aslam M, Charfi A, Lesage G, Heran M, Kim J (2017) Membrane bioreactors for wastewater treatment: a review of mechanical cleaning by scouring agents to control membrane fouling. Chem Eng J. https://doi.org/10.1016/j.cej.2016.08.144
Behboudi A, Jafarzadeh Y, Yegani R (2017) Polyvinyl chloride/polycarbonate blend ultrafiltration membranes for water treatment. J Memb Sci 534:18–24. https://doi.org/10.1016/j.memsci.2017.04.011
Bereskie T, Haider H, Rodriguez MJ, Sadiq R (2017) Framework for continuous performance improvement in small drinking water systems. Sci Total Environ 574:1405–1414. https://doi.org/10.1016/j.scitotenv.2016.08.067
Bichai F, Ashbolt N (2017) Public health and water quality management in low-exposure stormwater schemes: a critical review of regulatory frameworks and path forward. Sustain Cities Soc 28:453–465. https://doi.org/10.1016/j.scs.2016.09.003
Buonomenna MG (2013) Nano-enhanced reverse osmosis membranes. Desalination 314:73–88. https://doi.org/10.1016/j.desal.2013.01.006
Chhipi-Shrestha G, Hewage K, Sadiq R (2017) Microbial quality of reclaimed water for urban reuses: probabilistic risk-based investigation and recommendations. Sci Total Environ 576:738–751. https://doi.org/10.1016/j.scitotenv.2016.10.105
da Silva Burgal J, Peeva LG, Kumbharkar S, Livingston A (2015) Organic solvent resistant poly(ether-ether-ketone) nanofiltration membranes. J Memb Sci 479:105–116. https://doi.org/10.1016/j.memsci.2014.12.035
Daer S, Kharraz J, Giwa A, Hasan SW (2015) Recent applications of nanomaterials in water desalination: a critical review and future opportunities. Desalination 367:37–48. https://doi.org/10.1016/j.desal.2015.03.030
Değermenci N, Cengiz İ, Yildiz E, Nuhoglu A (2016) Performance investigation of a jet loop membrane bioreactor for the treatment of an actual olive mill wastewater. J Environ Manage 184:441–447. https://doi.org/10.1016/j.jenvman.2016.10.014
Deshmukh K, Ahamed MB, Deshmukh RR, Pasha SKK, Sadasivuni KK, Polu AR, Ponnamma D, AlMaadeed MA-A, Chidambaram K (2017) Newly developed biodegradable polymer nanocomposites of cellulose acetate and Al2O3 nanoparticles with enhanced dielectric performance for embedded passive applications. J Mater Sci: Mater Electron 28:973–986. https://doi.org/10.1007/s10854-016-5616-9
Di Vincenzo M, Barboiu M, Tiraferri A, Legrand YM (2017) Polyol-functionalized thin-film composite membranes with improved transport properties and boron removal in reverse osmosis. J Memb Sci 540:71–77. https://doi.org/10.1016/j.memsci.2017.06.034
Díez B, Roldán N, Martín A, Sotto A, Perdigón-Melón JA, Arsuaga J, Rosal R (2017) Fouling and biofouling resistance of metal-doped mesostructured silica/polyethersulfone ultrafiltration membranes. J Memb Sci 526:252–263. https://doi.org/10.1016/j.memsci.2016.12.051
Drangert J-O, Sharatchandra HC (2017) Addressing urban water scarcity: reduce, treat and reuse—the third generation of management to avoid local resources boundaries. Water Policy wp2017152. https://doi.org/10.2166/wp.2017.152
Dulebohn J, Ahmadiannamini P, Wang T, Kim SS, Pinnavaia TJ, Tarabara VV (2014) Polymer mesocomposites: ultrafiltration membrane materials with enhanced permeability, selectivity and fouling resistance. J Memb Sci 453:478–488. https://doi.org/10.1016/j.memsci.2013.11.042
Farahbaksh J, Delnavaz M, Vatanpour V (2017) Investigation of raw and oxidized multiwalled carbon nanotubes in fabrication of reverse osmosis polyamide membranes for improvement in desalination and antifouling properties. Desalination 410:1–9. https://doi.org/10.1016/j.desal.2017.01.031
Fischer K, Grimm M, Meyers J, Dietrich C, Gläser R, Schulze A (2015) Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J Memb Sci 478:49–57. https://doi.org/10.1016/j.memsci.2015.01.009
Gherasim C-V, Mikulášek P (2014) Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration. Desalination 343:67–74. https://doi.org/10.1016/j.desal.2013.11.012
Gholami A, Moghadassi AR, Hosseini SM, Shabani S, Gholami F (2014) Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J Ind Eng Chem 20:1517–1522. https://doi.org/10.1016/j.jiec.2013.07.041
Ginic-Markovic M, Barclay TG, Constantopoulos KT, Markovic E, Clarke SR, Matisons JG (2015) Biofouling resistance of polysulfobetaine coated reverse osmosis membranes. Desalination 369:37–45. https://doi.org/10.1016/j.desal.2015.04.024
Giwa A, Akther N, Dufour V, Hasan SW (2016) A critical review on recent polymeric and nano-enhanced membranes for reverse osmosis. RSC Adv 6:8134–8163. https://doi.org/10.1039/C5RA17221G
Giwa A, Akther N, Housani A Al, Haris S, Hasan SW (2016) Recent advances in humidification dehumidification (HDH) desalination processes: improved designs and productivity. Renew Sustain Energy Rev 57:929–944. https://doi.org/10.1016/j.rser.2015.12.108
Giwa A, Daer S, Ahmed I, Marpu P, Hasan S (2016) Experimental investigation and artificial neural networks ANNs modeling of electrically-enhanced membrane bioreactor for wastewater treatment. J Water Process Eng 11:88–97. https://doi.org/10.1016/j.jwpe.2016.03.011
Giwa A, Dufour V, Al Marzooqi F, Al Kaabi M, Hasan SW (2017) Brine management methods: recent innovations and current status. Desalination 407:1–23. https://doi.org/10.1016/j.desal.2016.12.008
Giwa A, Hasan S (2015) Theoretical investigation of the influence of operating conditions on the treatment performance of an electrically-induced membrane bioreactor. J Water Process Eng 6:72–82. https://doi.org/10.1016/j.jwpe.2015.03.004
Gkotsis P, Banti D, Peleka E, Zouboulis A, Samaras P (2014) Fouling issues in membrane bioreactors (MBRs) for wastewater treatment: major mechanisms, prevention and control strategies. Processes 2:795–866. https://doi.org/10.3390/pr2040795
Gong G, Nagasawa H, Kanezashi M, Tsuru T (2015) Reverse osmosis performance of layered-hybrid membranes consisting of an organosilica separation layer on polymer supports. J Memb Sci 494:104–112. https://doi.org/10.1016/j.memsci.2015.07.039
Grandclément C, Seyssiecq I, Piram A, Wong-Wah-Chung P, Vanot G, Tiliacos N, Roche N, Doumenq P (2017) From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: a review. Water Res. https://doi.org/10.1016/j.watres.2017.01.005
Hasan SW, Elektorowicz M, Oleszkiewicz JA (2014) Start-up period investigation of pilot-scale submerged membrane electro-bioreactor (SMEBR) treating raw municipal wastewater. Chemosphere 97:71–77. https://doi.org/10.1016/j.chemosphere.2013.11.009
Huang YW, Wang ZM, Yan X, Chen J, Guo YJ, Lang WZ (2017) Versatile polyvinylidene fluoride hybrid ultrafiltration membranes with superior antifouling, antibacterial and self-cleaning properties for water treatment. J Colloid Interface Sci 505:38–48. https://doi.org/10.1016/j.jcis.2017.05.076
Jamaly S, Giwa A, Hasan SW (2015) Recent improvements in oily wastewater treatment: progress, challenges, and future opportunities. J Environ Sci 37:15–30. https://doi.org/10.1016/j.jes.2015.04.011
Jiang S, Li Y, Ladewig BP (2017) A review of reverse osmosis membrane fouling and control strategies. Total Environ, Sci. https://doi.org/10.1016/j.scitotenv.2017.03.235
Kanagaraj P, Nagendran A, Rana D, Matsuura T, Neelakandan S, Karthikkumar T, Muthumeenal A (2015) Influence of N-phthaloyl chitosan on poly (ether imide) ultrafiltration membranes and its application in biomolecules and toxic heavy metal ion separation and their antifouling properties. Appl Surf Sci 329:165–173. https://doi.org/10.1016/j.apsusc.2014.12.082
Katsoyiannis IA, Gkotsis P, Castellana M, Cartechini F, Zouboulis AI (2017) Production of demineralized water for use in thermal power stations by advanced treatment of secondary wastewater effluent. J Environ Manage 190:132–139. https://doi.org/10.1016/j.jenvman.2016.12.040
Kim HJ, Choi K, Baek Y, Kim D-G, Shim J, Yoon J, Lee J-C (2014) High-performance reverse osmosis CNT/polyamide nanocomposite membrane by controlled interfacial interactions. ACS Appl Mater Interfaces 6:2819–2829. https://doi.org/10.1021/am405398f
Krzeminski P, Leverette L, Malamis S, Katsou E (2017) Membrane bioreactors—a review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J Memb Sci 527:207–227. https://doi.org/10.1016/j.memsci.2016.12.010
Kwan SE, Bar-Zeev E, Elimelech M (2015) Biofouling in forward osmosis and reverse osmosis: measurements and mechanisms. J Memb Sci 493:703–708. https://doi.org/10.1016/j.memsci.2015.07.027
Le-Clech P, Chen V, Fane TAG (2006) Fouling in membrane bioreactors used in wastewater treatment. J Memb Sci 284:17–53. https://doi.org/10.1016/j.memsci.2006.08.019
Le NL, Nunes SP (2016) Materials and membrane technologies for water and energy sustainability. Sustain Mater Technol 7:1–28. https://doi.org/10.1016/j.susmat.2016.02.001
Lee J, Chae HR, Won YJ, Lee K, Lee CH, Lee HH, Kim IC, Lee JM (2013) Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J Memb Sci 448:223–230. https://doi.org/10.1016/j.memsci.2013.08.017
Li Y, Su Y, Zhao X, Zhang R, Zhao J, Fan X, Jiang Z (2014) Surface fluorination of polyamide nanofiltration membrane for enhanced antifouling property. J Memb Sci 455:15–23. https://doi.org/10.1016/j.memsci.2013.12.060
Liang S, Qi G, Xiao K, Sun J, Giannelis EP, Huang X, Elimelech M (2014) Organic fouling behavior of superhydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes functionalized with surface-tailored nanoparticles: implications for organic fouling in membrane bioreactors. J Memb Sci 463:94–101. https://doi.org/10.1016/j.memsci.2014.03.037
Liu C, Lee J, Ma J, Elimelech M (2017) Antifouling thin-film composite membranes by controlled architecture of zwitterionic polymer brush layer. Environ Sci Technol acs.est.6b05992. https://doi.org/10.1021/acs.est.6b05992
Liu C, Mao H, Zheng J, Zhang S (2017) Tight ultrafiltration membrane: preparation and characterization of thermally resistant carboxylated cardo poly (arylene ether ketone)s (PAEK-COOH) tight ultrafiltration membrane for dye removal. J Memb Sci 530:1–10. https://doi.org/10.1016/j.memsci.2017.02.005
Liu M, Chen Q, Lu K, Huang W, Lü Z, Zhou C, Yu S, Gao C (2017) High efficient removal of dyes from aqueous solution through nanofiltration using diethanolamine-modified polyamide thin-film composite membrane. Sep Purif Technol 173:135–143. https://doi.org/10.1016/j.seppur.2016.09.023
Luo W, Phan HV, Xie M, Hai FI, Price WE, Elimelech M, Nghiem LD (2017) Osmotic versus conventional membrane bioreactors integrated with reverse osmosis for water reuse: biological stability, membrane fouling, and contaminant removal. Water Res 109:122–134. https://doi.org/10.1016/j.watres.2016.11.036
Ma W, Soroush A, Van Anh Luong T, Brennan G, Rahaman MS, Asadishad B, Tufenkji N (2016) Spray- and spin-assisted layer-by-layer assembly of copper nanoparticles on thin-film composite reverse osmosis membrane for biofouling mitigation. Water Res 99:188–199. https://doi.org/10.1016/j.watres.2016.04.042
Maghsoud Z, Pakbaz M, Famili MHN, Madaeni SS (2017) New polyvinyl chloride/thermoplastic polyurethane membranes with potential application in nanofiltration. J Memb Sci 541:271–280. https://doi.org/10.1016/j.memsci.2017.07.001
Martín A, Arsuaga JM, Roldán N, de Abajo J, Martínez A, Sotto A (2015) Enhanced ultrafiltration PES membranes doped with mesostructured functionalized silica particles. Desalination 357:16–25. https://doi.org/10.1016/j.desal.2014.10.046
McVerry BT, Wong MCY, Marsh KL, Temple JAT, Marambio-Jones C, Hoek EMV, Kaner RB (2014) Scalable antifouling reverse osmosis membranes utilizing perfluorophenyl azide photochemistry. Macromol Rapid Commun 35:1528–1533. https://doi.org/10.1002/marc.201400226
Mehrparvar A, Rahimpour A (2015) Surface modification of novel polyether sulfone amide (PESA) ultrafiltration membranes by grafting hydrophilic monomers. J Ind Eng Chem 28:359–368. https://doi.org/10.1016/j.jiec.2015.03.016
Mercer KL (2017) 2017 State of the water industry: strengthening our connections. J Am Water Works Assoc 109:56–65. https://doi.org/10.5942/jawwa.2017.109.0090
Mi Y-F, Zhao F-Y, Guo Y-S, Weng X-D, Ye C-C, An Q-F (2017) Constructing zwitterionic surface of nanofiltration membrane for high flux and antifouling performance. J Memb Sci 541:29–38. https://doi.org/10.1016/j.memsci.2017.06.091
Mohammad AW, Teow YH, Ang WL, Chung YT, Oatley-Radcliffe DL, Hilal N (2015) Nanofiltration membranes review: recent advances and future prospects. Desalination. https://doi.org/10.1016/j.desal.2014.10.043
Mokhtari S, Rahimpour A, Shamsabadi AA, Habibzadeh S, Soroush M (2017) Enhancing performance and surface antifouling properties of polysulfone ultrafiltration membranes with salicylate-alumoxane nanoparticles. Appl Surf Sci 393:93–102. https://doi.org/10.1016/j.apsusc.2016.10.005
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Aristatil G, Mani N, Premalatha M, Vinoth Pandi D (2017) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47. https://doi.org/10.1016/j.carbpol.2016.09.026
Nebipasagil A, Sundell BJ, Lane OR, Mecham SJ, Riffle JS, McGrath JE (2016) Synthesis and photocrosslinking of disulfonated poly(arylene ether sulfone) copolymers for potential reverse osmosis membrane materials. Polym (United Kingdom) 93:14–22. https://doi.org/10.1016/j.polymer.2016.04.009
Neoh CH, Noor ZZ, Mutamim NSA, Lim CK (2016) Green technology in wastewater treatment technologies: integration of membrane bioreactor with various wastewater treatment systems. Chem Eng J 283:582–594. https://doi.org/10.1016/j.cej.2015.07.060
Ng LY, Mohammad AW, Leo CP, Hilal N (2013) Polymeric membranes incorporated with metal/metal oxide nanoparticles: a comprehensive review. Desalination 308:15–33. https://doi.org/10.1016/j.desal.2010.11.033
Oh HJ, McGrath JE, Paul DR (2017) Kinetics of poly(ethylene glycol) extraction into water from plasticized disulfonated poly(arylene ether sulfone) desalination membranes prepared by solvent-free melt processing. J Memb Sci 524:257–265. https://doi.org/10.1016/j.memsci.2016.11.036
Oh HJ, Park J, Inceoglu S, Villaluenga I, Thelen JL, Jiang X, McGrath JE, Paul DR (2017) Formation of disulfonated poly(arylene ether sulfone) thin film desalination membranes plasticized with poly(ethylene glycol) by solvent-free melt extrusion. Polymer (Guildf) 109:106–114. https://doi.org/10.1016/j.polymer.2016.12.035
Orooji Y, Faghih M, Razmjou A, Hou J, Moazzam P, Emami N, Aghababaie M, Nourisfa F, Chen V, Jin W (2017) Nanostructured mesoporous carbon polyethersulfone composite ultrafiltration membrane with significantly low protein adsorption and bacterial adhesion. Carbon N Y 111:689–704. https://doi.org/10.1016/j.carbon.2016.10.055
Pardeshi PM, Mungray AK, Mungray AA (2017) Polyvinyl chloride and layered double hydroxide composite as a novel substrate material for the forward osmosis membrane. Desalination. https://doi.org/10.1016/j.desal.2017.01.041
Prince JA, Bhuvana S, Boodhoo KVK, Anbharasi V, Singh G (2014) Synthesis and characterization of PEG-Ag immobilized PES hollow fiber ultrafiltration membranes with long lasting antifouling properties. J Memb Sci 454:538–548. https://doi.org/10.1016/j.memsci.2013.12.050
Pulido BA, Waldron C, Zolotukhin MG, Nunes SP (2017) Porous polymeric membranes with thermal and solvent resistance. J Memb Sci 539:187–196. https://doi.org/10.1016/j.memsci.2017.05.070
Qadir D, Mukhtar H, Keong LK (2017) Mixed matrix membranes for water purification applications. Sep Purif Rev 46:62–80. https://doi.org/10.1080/15422119.2016.1196460
Qiu G, Ting YP (2014) Short-term fouling propensity and flux behavior in an osmotic membrane bioreactor for wastewater treatment. Desalination 332:91–99. https://doi.org/10.1016/j.desal.2013.11.010
Rabiee H, Vatanpour V, Farahani MHDA, Zarrabi H (2015) Improvement in flux and antifouling properties of PVC ultrafiltration membranes by incorporation of zinc oxide (ZnO) nanoparticles. Sep Purif Technol 156:299–310. https://doi.org/10.1016/j.seppur.2015.10.015
Razali NF, Mohammad AW, Hilal N (2014) Effects of polyaniline nanoparticles in polyethersulfone ultrafiltration membranes: fouling behaviours by different types of foulant. J Ind Eng Chem 20:3134–3140. https://doi.org/10.1016/j.jiec.2013.11.056
Sabir A, Shafiq M, Islam A, Sarwar A, Dilshad MR, Shafeeq A, Zahid Butt MT, Jamil T (2015) Fabrication of tethered carbon nanotubes in cellulose acetate/polyethylene glycol-400 composite membranes for reverse osmosis. Carbohydr Polym 132:589–597. https://doi.org/10.1016/j.carbpol.2015.06.035
Safarpour M, Vatanpour V, Khataee A, Zarrabi H, Gholami P, Yekavalangi ME (2017) High flux and fouling resistant reverse osmosis membrane modified with plasma treated natural zeolite. Desalination 411:89–100. https://doi.org/10.1016/j.desal.2017.02.012
Shaffer DL, Tousley ME, Elimelech M (2017) Influence of polyamide membrane surface chemistry on gypsum scaling behavior. J Memb Sci 525:249–256. https://doi.org/10.1016/j.memsci.2016.11.003
Shafi HZ, Matin A, Akhtar S, Gleason KK, Zubair SM, Khan Z (2017) Organic fouling in surface modified reverse osmosis membranes: filtration studies and subsequent morphological and compositional characterization. J. Memb. Sci. 527:152–163. https://doi.org/10.1016/j.memsci.2017.01.017
Shao L, Wang ZX, Zhang YL, Jiang ZX, Liu YY (2014) A facile strategy to enhance PVDF ultrafiltration membrane performance via self-polymerized polydopamine followed by hydrolysis of ammonium fluotitanate. J Memb Sci 461:10–21. https://doi.org/10.1016/j.memsci.2014.03.006
Sharma N, Purkait MK (2017) Impact of synthesized amino alcohol plasticizer on the morphology and hydrophilicity of polysulfone ultrafiltration membrane. J Memb Sci 522:202–215. https://doi.org/10.1016/j.memsci.2016.08.068
Son M, Kim H, Jung J, Jo S, Choi H (2017) Influence of extreme concentrations of hydrophilic pore-former on reinforced polyethersulfone ultrafiltration membranes for reduction of humic acid fouling. Chemosphere 179:194–201. https://doi.org/10.1016/j.chemosphere.2017.03.101
Subramanian S, Seeram R (2013) New directions in nanofiltration applications—are nanofibers the right materials as membranes in desalination? Desalination 308:198–208. https://doi.org/10.1016/j.desal.2012.08.014
Tang Y-J, Xu Z-L, Xue S-M, Wei Y-M, Yang H (2017) Tailoring the polyester/polyamide backbone stiffness for the fabrication of high performance nanofiltration membrane. J Memb Sci. https://doi.org/10.1016/j.memsci.2017.07.033
Tong T, Zhao S, Boo C, Hashmi SM, Elimelech M (2017) Relating silica scaling in reverse osmosis to membrane surface properties. Environ Sci Technol 51:4396–4406. https://doi.org/10.1021/acs.est.6b06411
Tsai H-A, Wang T-Y, Huang S-H, Hu C-C, Hung W-S, Lee K-R, Lai J-Y (2017) The preparation of polyamide/polyacrylonitrile thin film composite hollow fiber membranes for dehydration of ethanol mixtures. Sep Purif Technol 187:221–232. https://doi.org/10.1016/j.seppur.2017.06.060
Turek M, Mitko K, Piotrowski K, Dydo P, Laskowska E, Jakóbik-Kolon A (2017) Prospects for high water recovery membrane desalination. Desalination 401:180–189. https://doi.org/10.1016/j.desal.2016.07.047
Ulbricht M (2006) Advanced functional polymer membranes. Polymer (Guildf) 47:2217–2262. https://doi.org/10.1016/j.polymer.2006.01.084
Vatanpour V, Safarpour M, Khataee A, Zarrabi H, Yekavalangi ME, Kavian M (2017) A thin film nanocomposite reverse osmosis membrane containing amine-functionalized carbon nanotubes. Sep Purif Technol 184:135–143. https://doi.org/10.1016/j.seppur.2017.04.038
Velu S, Arthanareeswaran G, Lade H (2017) Removal of organic and inorganic substances from industry wastewaters using modified aluminosilicate-based polyethersulfone ultrafiltration membranes. Environ Prog Sustain Energy https://doi.org/10.1002/ep.12614
Verbeke R, Gómez V, Vankelecom IFJ (2016) Chlorine-resistance of reverse osmosis (RO) polyamide membranes. Polym. Sci, Prog. https://doi.org/10.1016/j.progpolymsci.2017.05.003
Wang C, Li Z, Chen J, Li Z, Yin Y, Cao L, Zhong Y, Wu H (2017) Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J Memb Sci 523:273–281. https://doi.org/10.1016/j.memsci.2016.09.055
Wang H, Wang Z-M, Yan X, Chen J, Lang W-Z, Guo Y-J (2017) Novel organic-inorganic hybrid polyvinylidene fluoride ultrafiltration membranes with antifouling and antibacterial properties by embedding N-halamine functionalized silica nanospheres. J Ind Eng Chem 52:295–304. https://doi.org/10.1016/j.jiec.2017.03.059
Wang H, Wei M, Zhong Z, Wang Y (2017) Atomic-layer-deposition-enabled thin-film composite membranes of polyimide supported on nanoporous anodized alumina. J Memb Sci 535:56–62. https://doi.org/10.1016/j.memsci.2017.04.026
Wang JJ, Wang Z, Wang JJ, Wang S (2015) Improving the water flux and bio-fouling resistance of reverse osmosis (RO) membrane through surface modification by zwitterionic polymer. J Memb Sci 493:188–199. https://doi.org/10.1016/j.memsci.2015.06.036
Wang P, Luo L, Chung TS (2014) Tri-bore ultra-filtration hollow fiber membranes with a novel triangle-shape outer geometry. J Memb Sci 452:212–218. https://doi.org/10.1016/j.memsci.2013.10.033
Wang X, Chang VWC, Tang CY (2016) Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: advances, challenges, and prospects for the future. J Memb Sci. https://doi.org/10.1016/j.memsci.2016.01.010
Wang X, Wang C, Tang CY, Hu T, Li X, Ren Y (2017) Development of a novel anaerobic membrane bioreactor simultaneously integrating microfiltration and forward osmosis membranes for low-strength wastewater treatment. J Memb Sci 527:1–7. https://doi.org/10.1016/j.memsci.2016.12.062
Werber JR, Deshmukh A, Elimelech M (2016) The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ Sci Technol Lett 3:112–120. https://doi.org/10.1021/acs.estlett.6b00050
Wu H, Liu Y, Mao L, Jiang C, Ang J, Lu X (2017) Doping polysulfone ultrafiltration membrane with TiO2-PDA nanohybrid for simultaneous self-cleaning and self-protection. J Memb Sci 532:20–29. https://doi.org/10.1016/j.memsci.2017.03.010
Yang B, Yang X, Liu B, Chen Z, Chen C, Liang S, Chu L-Y, Crittenden J (2017) PVDF blended PVDF-g-PMAA pH-responsive membrane: effect of additives and solvents on membrane properties and performance. J Memb Sci. https://doi.org/10.1016/j.memsci.2017.07.045
Yang C, Li X-M, Gilron J, Kong D, Yin Y, Oren Y, Linder C, He T (2014) CF4 plasma-modified superhydrophobic PVDF membranes for direct contact membrane distillation. J Memb Sci 456:155–161. https://doi.org/10.1016/j.memsci.2014.01.013
Yin J, Deng B (2015) Polymer-matrix nanocomposite membranes for water treatment. J Memb Sci. https://doi.org/10.1016/j.memsci.2014.11.019
You X, Ma T, Su Y, Wu H, Wu M, Cai H, Sun G, Jiang Z (2017) Enhancing the permeation flux and antifouling performance of polyamide nanofiltration membrane by incorporation of PEG-POSS nanoparticles. J Memb Sci 540:454–463. https://doi.org/10.1016/j.memsci.2017.06.084
Yu H, Zhang Y, Sun X, Liu J, Zhang H (2014) Improving the antifouling property of polyethersulfone ultrafiltration membrane by incorporation of dextran grafted halloysite nanotubes. Chem Eng J 237:322–328. https://doi.org/10.1016/j.cej.2013.09.094
Zhang G, Lu S, Zhang L, Meng Q, Shen C, Zhang J (2013) Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J Memb Sci 436:163–173. https://doi.org/10.1016/j.memsci.2013.02.009
Zhang H, Li Bin, Pan J, Qi Y, Shen J, Gao C, Van der Bruggen B (2017) Carboxyl-functionalized graphene oxide polyamide nanofiltration membrane for desalination of dye solutions containing monovalent salt. J Memb Sci 539:128–137. https://doi.org/10.1016/j.memsci.2017.05.075
Zhang Y, Guo M, Pan G, Yan H, Xu J, Shi Y, Shi H, Liu Y (2015) Preparation and properties of novel pH-stable TFC membrane based on organic-inorganic hybrid composite materials for nanofiltration. J Memb Sci 476:500–507. https://doi.org/10.1016/j.memsci.2014.12.011
Zhang Y, Zhao C, Yan H, Pan G, Guo M, Na H, Liu Y (2014) Highly chlorine-resistant multilayer reverse osmosis membranes based on sulfonated poly(arylene ether sulfone) and poly(vinyl alcohol). Desalination 336:58–63. https://doi.org/10.1016/j.desal.2013.12.034
Zhao C, Lv J, Xu X, Zhang G, Yang Y, Yang F (2017) Highly antifouling and antibacterial performance of poly (vinylidene fluoride) ultrafiltration membranes blending with copper oxide and graphene oxide nanofillers for effective wastewater treatment. J Colloid Interface Sci 505:341–351. https://doi.org/10.1016/j.jcis.2017.05.074
Zhou D, Zhu L, Fu Y, Zhu M, Xue L (2015) Development of lower cost seawater desalination processes using nanofiltration technologies—a review. Desalination 376:109–116. https://doi.org/10.1016/j.desal.2015.08.020
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Giwa, A., Ahmed, M., Hasan, S.W. (2019). Polymers for Membrane Filtration in Water Purification. In: Das, R. (eds) Polymeric Materials for Clean Water. Springer Series on Polymer and Composite Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-00743-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-00743-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00742-3
Online ISBN: 978-3-030-00743-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)