Abstract
Malignant catatonia (MC) represents a life-threatening neuropsychiatric disorder that was widely reported both in the United States and abroad long before the introduction of antipsychotic drugs. Lack of recognition probably accounts for the relative paucity of contemporary North American reports on MC. Furthermore, MC is a syndrome rather than a specific disease entity that may occur as an outgrowth of diverse neuromedical illnesses as well as with the major psychoses. From this perspective, neuroleptic malignant syndrome (NMS), a potentially deadly complication of antipsychotic drug treatment, may be conceptualized as a drug-induced form of MC. The hypothesis that MC and NMS share a common pathophysiology, involving reduced dopamine functioning in the frontal-subcortical circuits, provides additional support for a view of NMS as a subtype of MC. Electroconvulsive therapy is the preferred treatment for MC stemming from a major psychotic disorder, and appears also effective in cases caused by neuromedical illnesses. Antipsychotic drugs should be withheld whenever MC is suspected.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Medial Prefrontal Cortex
- Neuroleptic Malignant Syndrome
- Imitation Behavior
- Antipsychotic Drug Treatment
- Akinetic Mutism
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Clinical Vignettes
Patient 1
A 27-year-old female with a personal and family history of bipolar disorder has taken no psychiatric medications for the past 6 months. One week prior to admission, she develops elevated mood, pressured speech, and flight of ideas. Over the ensuing days, she grows markedly agitated and unable to sleep, talks constantly, paces relentlessly, and refuses to eat or drink. On admission to the psychiatric unit, she requires four-point restraints. She is confused and intensely hyperactive with periods of incoherent chatter alternating with hostile verbal outbursts. She frequently thrashes from side to side, is delusional, and appears to be responding to both auditory and visual hallucinations. She exhibits muscular rigidity, posturing, echolalia, and echopraxia. Temperature is 39 °C with tachycardia, tachypnea, profuse diaphoresis, and a blood pressure of 170/120 mm Hg. Laboratory abnormalities include leukocytosis, elevation in creatinine phosphokinase (CPK) (2,800 IU), and serum transaminases and a serum iron of 38 μg/dl (75–175 μg/dl). Lumbar puncture, EEG, and CT scan of the head are normal.
During the next 24 hours, she lapses into stupor with increased rigidity and a temperature of 40.2 °C. The diagnosis of malignant catatonia associated with a manic episode is made and electroconvulsive therapy (ECT) initiated. Body temperature and other vital signs return to normal after the first bilateral ECT treatment. She receives one bilateral ECT treatment daily for the next 5 days with three more over the next week. She responds with a marked decrease in agitation and progressive improvement in confusion, hallucinations, delusions, and catatonic features. She starts divalproex sodium and olanzapine with good response and is discharged 2 weeks later.
Patient 2
A 46-year-old male schizophrenic patient has taken no psychiatric medications for the past 2 years. He is admitted to the Intensive Care Unit with a 1-week history of progressive mutism, immobility, negativism, and staring. On exam, he exhibits marked muscular rigidity. Temperature is 40.1 °C with tachycardia, tachypnea, diaphoresis, and a blood pressure of 190/110 mm Hg. Laboratory evaluation reveals elevated CPK and leukocytosis. All other studies are noncontributory. Malignant catatonia is diagnosed. Intravenous lorazepam 2 mg, administered four times daily for 2 days, is without benefit. He is referred for bilateral ECT and responds promptly.
Introduction
Catatonia is a syndrome of striking motor and behavioral abnormalities that may occur in association with diverse neuromedical, drug-induced, and psychiatric illnesses. Furthermore, catatonia may be conceptualized as a continuum, with milder forms at one end (termed simple or benign) and more severe forms, involving hyperthermia and autonomic dysfunction (termed malignant), at the other [1]. In 1934, Stauder [2] described lethal catatonia, characterized by extreme motor excitement followed by stuporous exhaustion, coma, cardiovascular collapse, and death. The entire course involved progressive hyperthermia, autonomic dysfunction, clouding of consciousness, and prominent catatonic features. In those cases ending in death, the paucity of findings was puzzling and in sharp contrast to the catastrophic clinical manifestations. In fact, this disorder had been discussed previously by Calmeil (1832) [3] and Bell (1849) [4] and was the subject of numerous North American and foreign publications during the pre-antipsychotic drug era. Other names used to describe this same disorder included Bell’s mania, acute delirious mania, delirium acutum, delire aigu, psychotic exhaustion syndrome, and Scheid’s cyanotic syndrome, among others [5–10]. More recently, stressing that not all cases are fatal, Philbrick and Rummans [1] have promulgated the term malignant catatonia (MC).
Although the incidence of MC has likely declined worldwide following the introduction of modern psychopharmacologic agents, it has remained widely reported in Europe and Asia. In contrast, contemporary North American publications on MC have now become more limited with an almost complete lack of reference to the current foreign work or the large North American literature from the pre-antipsychotic drug era. In this chapter we review the historical and modern world literature on MC. On the basis of this review, we conclude that MC continues to occur and represents an uncommon but potentially fatal neuropsychiatric disorder. Lack of recognition appears to account for the scarcity of recent North American reports on MC.
Furthermore, our data indicate that MC, like simple catatonia, represents a syndrome rather than a specific disease. Although most often presenting as an outgrowth of the major psychoses, MC may also occur in association with diverse neurologic, infectious, and toxic-metabolic conditions. From this perspective, neuroleptic malignant syndrome (NMS), a life-threatening complication of antipsychotic drug treatment [8, 11], may be viewed as a drug-induced form of MC. In addition, findings from our review indicate that MC and NMS share a common pathophysiology involving reduced dopaminergic neurotransmission within the basal ganglia-thalamocortical circuits. Recognition of the clinical features of MC and an appreciation of its diverse etiologies are essential for the effective management of patients who develop this catastrophic reaction.
Clinical Presentation: Pre-antipsychotic Drug Era
Despite the diversity of nomenclature, there is considerable consistency to early accounts of MC [5–10]. A prodromal phase was observed in most, but not all, cases. It lasted an average of 2 weeks and involved insomnia, anorexia, and labile mood. In roughly 90 % of cases, the disease proper began with a phase of intense motor excitement that then continued almost without interruption (as exemplified by Patient 1). Features of this excited phase included refusal of foods and fluids, clouding of consciousness, tachycardia, tachypnea, cyanosis, labile or elevated blood pressure, and profuse perspiration. Acrocyanosis and spontaneous hematomas of the skin were frequently noted. At times, excitement might be interrupted by periods of catatonic stupor and rigidity. Other catatonic signs, such as mutism, catalepsy, posturing, echolalia, and echopraxia were often present. Thought processes became increasingly disorganized and speech grew progressively incoherent. Auditory and visual hallucinations accompanied by bizarre delusions were frequently prominent.
In this “classic” excited phase of MC, excitement was always associated with hyperthermia that could attain levels approaching 43.3 °C prior to the final stuporous phase of MC. This presentation differs phenomenologically from NMS in that although NMS is often preceded by a period of hyperactivity, hyperthermia first emerges concomitantly with, or shortly after, the onset of stupor and rigidity. The excited phase of MC was noted to vary in duration but lasted an average of 8 days [12].
In the final phase of MC, excitement gave way to stuporous exhaustion and extreme hyperthermia, often followed by coma, cardiovascular collapse, and death [5]. In all of Stauder’s 27 cases [2], rigidity of the skeletal muscles was described during this terminal stupor, similar to that seen in NMS. Although other accounts of MC echoed the findings of Stauder, some reports described flaccid muscles in contrast to NMS [12]. About 10 % of cases reported during the pre-antipsychotic drug era involved hyperthermia and a primarily stuporous course unassociated with a preceding hyperactive phase (Patient 2).
During the pre-antipsychotic drug era, MC was reported fatal in 75–100 % of cases [5]. It was observed to occur predominantly in young adults between the ages of 18 and 35 and involved women roughly seven times more often than men. During this period, MC was estimated to account for 0.25–3.5 % of admissions to psychiatric hospitals and occurred with equal frequency throughout the seasons [5]. Stauder [2], and others, reported findings consistent with a familial pattern of occurrence.
Kraepelin [13], who called this disorder delirium acutum, considered it a nonspecific syndrome that could occur as an outgrowth of neuromedical illness as well as the major psychoses. In contrast, most early French authors viewed MC as an unusual but deadly form of encephalitis preferentially involving the hypothalamus [14]. Subsequent to Stauder’s [2] publication, however, MC was increasingly seen as confined to the major psychoses, although Stauder himself never fully dismissed the possibility that some or all of his patients may have had encephalitis. Most German and American authors emphasized lack of autopsy findings that could account for death, with the CNS abnormalities reported by the French either unconfirmed or deemed trivial. Bronchopneumonia and other infections were considered “opportunistic,” occurring in an already exhausted and compromised host.
Contemporary Presentation
In 1986, we identified a series of 292 MC cases reported between 1960 and 1985 [5]. Two hundred and sixty-five cases came from 20 reports representative of more than 50 publications from Europe and Asia. The remaining 27 cases came from just 12 articles found in an exhaustive search of the North American literature. Most patients had received antipsychotic drug treatment. Since then, we have identified 107 additional cases reported in the world literature between 1986 and 2010, thus extending our series to 399 total cases [8–10, 15–32]. Although MC remains more frequently mentioned in the foreign literature, the disparity in these more recent 107 cases appears somewhat reduced, suggesting improved recognition of this disorder in North America.
Among 341 cases in which sex was specified, 218 (64 %) were female. The mean age of occurrence was 33, compared with 25 during the pre-antipsychotic drug era. Of considerable interest, mortality, which exceeded 75 % during the pre-antipsychotic drug era, remained at 60 % between 1960 and 1985 [5] and has fallen to 10 % in the 107 cases reported since 1986 [8–10, 15–32]. This recent decline is striking and presumably reflects enhanced awareness of MC, early diagnosis, and rapid institution of appropriate treatment. Nevertheless, MC continues to represent a potentially lethal disorder. Among cases reported since 1960, MC was estimated to occur in 0.07 % of psychiatric admissions [33] or annually in 0.0004 % of community adults [34].
Table 5.1 summarizes the clinical features of MC. Along with catatonic stupor and hyperactivity, they remain to be hyperthermia, altered consciousness, and autonomic instability manifested by diaphoresis, tachycardia, labile or elevated blood pressure, and varying degrees of cyanosis. Catatonic signs aside from stupor and excitement continue to be noted. One large series [35] identified 62 patients with psychogenic MC and reported that each exhibited at least three catatonic features. In our 107 most recent cases, muscle rigidity was present in 41 of 48 (85 %) cases in which muscle tone was characterized.
Among the 107 recent MC cases, CPK was elevated in 45 of 48 patients (94 %) in whom it was tested. Leukocytosis was reported in 24 of 35 patients (66 %) and serum transaminases were elevated in 13 of 29 patients (45 %). Serum iron levels were obtained in only eight patients, but were decreased in all eight. Less consistent findings among the 107 recent cases included non-focal generalized slowing on electroencephalography, elevated erythrocyte sedimentation rates, mild hyperglycemia, elevated serum creatinine, hyponatremia, hypernatremia, and dehydration. Philbrick and Rummans [1] found that three of five MC cases treated at their facility had evidence of frontal atrophy on CT scans of the head. Furthermore, one patient with a normal head CT had decreased frontal perfusion on posttreatment SPECT imaging.
In 60 (15 %) of the 399 contemporary cases, a preexisting neuromedical illness was believed to have initiated the full syndromal picture of MC. Reports of infectious causes predominated, including 25 cases of acute or post-infectious viral encephalitis [8–10, 16, 20, 21, 28, 31]; single cases of Borrelia encephalitis, general paresis, bacterial meningoencephalitis, and viral hepatitis; and bacterial septicemia that evolved from five cases of endometritis, and from single cases each of pyelonephritis, tuberculosis of the large intestine, aortitis, cholangitis, endocarditis, and gingival abscess [5–10]. In two cases of septic origin, the original focus of infection was not indicated [5]. Cerebrovascular thrombosis [5] and paraneoplastic limbic encephalitis accounted for two cases each [17, 22], and MC developed in the context of normal-pressure hydrocephalus and multiple sclerosis in single cases [9, 26]. Two cases occurred secondary to hyperthyroidism, and single cases were attributed to uremia, systemic lupus erythematosus, and cerebral anoxia [5, 9]. Reports of toxic causes included single cases due to tetraethyl lead poisoning, barbiturate withdrawal, clonazepam withdrawal, renal transplantation, toxic epidermal necrolysis, therapeutic ingestion or overdose of cyclobenzaprine, and intrathecal administration of Ziconotide [5, 9, 15, 27].
Three hundred and thirty-nine of the 399 cases (85 %) were considered as the outgrowth of a major psychotic disorder, diagnosed as schizophrenia in 127 cases, mania in 22 cases, major depression in 31 cases, psychotic disorder not otherwise specified in 22 cases, and “periodic catatonia” in 10 cases. Among these 339 MC cases arising from the major psychoses, 167 (49 %) ended in death and 104 went to autopsy. Seventy-nine of the 104 proved autopsy negative. In the remaining 20 cases, however, death could be attributed to specific consequences of catatonic immobility, such as deep venous thrombosis with pulmonary embolism. These cases of simple (benign) catatonia rendered fatal by severe intercurrent medical complications were differentiated from “genuine” psychogenic MC.
The Malignant Catatonia Syndrome
Our review of the modern world literature supports Kraepelin’s [14] conceptualization of MC as a nonspecific syndrome that may occur in association with diverse neurologic, medical, drug-induced, and psychiatric illnesses. Table 5.2 summarizes known causes of the MC syndrome.
Consistent with this view, it is appropriate to consider the relationship between MC and NMS. Among the 399 contemporary MC cases, the “classic” excited form (Patient 1) involving extreme hyperactivity and progressive hyperthermia prior to the onset of stupor has continued to predominate with 66 % of cases presenting in this fashion. However, 34 % of patients exhibited a primarily stuporous course. This represents a change from the pre-antipsychotic drug era when only about 10 % of patients presented as primarily stuporous [9]. Furthermore, a selective analysis of the 107 cases reported since 1986 indicates that this trend has continued, with only 56 % exhibiting excitement and 44 % presenting as stuporous (Patient 2).
In many of these cases involving a stuporous course, stupor and hyperthermia developed only following the initiation of antipsychotic drug treatment, giving rise to questions concerning the differentiation of MC from NMS. Furthermore, the clinical features of the presentation of classic excited MC, once stupor emerges, appear equally difficult to distinguish from those of NMS. Viewing MC as a syndrome, we have suggested that NMS represents an antipsychotic drug-induced toxic or iatrogenic form of MC. Accordingly, the emergence of NMS as a subtype of MC could help explain the increased percentage of primarily stuporous MC cases reported in the contemporary literature.
The recognition that MC is a well-defined neuropsychiatric syndrome that occurs in association with both neuromedical and psychiatric disorders has significant clinical implications. The worldwide prevalence of MC has probably declined in recent years; the effects of modern psychopharmacologic agents and other advances in medical care have likely altered the course of underlying disorders associated with the syndrome, thereby reducing the frequency with which these disorders progress into MC. However, it appears likely that lack of familiarity with MC due to barriers of time, language, culture, and diagnostic systems has contributed to its relatively rare mention in the contemporary North American literature. MC involves a dramatic admixture of medical and behavioral manifestations, and unless clinicians are armed with an appreciation of MC as a syndrome with diverse etiologies, patients are likely to be labeled “psychiatric” or “medical” largely on the basis of the treating physician’s orientation.
Clearly, it is difficult for clinicians to accept that high fever and confusion may occur as a direct outgrowth of a psychiatric condition. Hafner and Kafner [34] concluded that even in Germany, where MC appears better recognized, neurologists and internists rather than psychiatrists now more commonly care for patients who previously would have been diagnosed with MC. These patients are likely to receive diagnoses such as “nonspecific organic encephalopathy with fever.” Conversely, reports resembling those on viral encephalitis “imitating” catatonic schizophrenia indicate that failure to recognize MC may result in a narrow focusing on behavioral manifestations, with neglect of ominous physical signs [36]. Once developed, MC, independent of etiology, assumes an autonomous and frequently fatal course. Only with prompt recognition of this distinctive syndrome can the proper diagnostic evaluation and treatment be initiated.
Pathophysiology
A consideration of the pathogenesis of MC with a particular focus on the dopamine system further supports a view of NMS as a subtype of this disorder. A number of authors have posited a key role for dopamine hypoactivity in triggering MC [5, 8, 9, 37, 38]. Furthermore, there is compelling clinical evidence implicating antipsychotic drug-induced dopamine receptor blockade in the pathogenesis of NMS [8, 39]. Fricchione [37, 38] along with our group [8–10] proposed that the onset of MC coincides with a reduction in dopaminergic activity within the frontal subcortical circuits. As elucidated by Alexander [40, 41], these circuits represent one of the brain’s principal organizational networks underlying brain–behavior relationships. Five circuits connecting the basal ganglia with their associated areas in the cortex and thalamus have been identified and are named according to their cortical site of origin (see Fig. 5.1). They include the “motor circuit,” the “oculomotor circuit,” the “dorsolateral prefrontal circuit,” the “lateral orbitofrontal circuit,” and the “anterior cingulate-medial orbitofrontal circuit.” Each circuit involves the same member structures, including an origin in a specific area of the frontal cortex; projections to the striatum (putamen, caudate, and ventral striatum); connections to the globus pallidus interna and the substantia nigra pars reticulata; which, in turn, project to specific thalamic nuclei; and a final link back to the frontal area from which they originated, thus creating a feedback loop.
Proposed basal ganglia-thalamocortical circuits. Parallel organization of the five basal ganglia-thalamocortical circuits. Each circuit engages specific regions of the cerebral cortex, stiatum, pallidum, substantia nigra, and thalamus (adapted from [40] with permission)
Dopamine is in a key position to influence activity in each of the circuits. Mesocortical dopamine pathways project directly to circuit areas of origin in the supplementary motor area, frontal eye fields, and the three prefrontal cortical areas. Additionally, dopamine modulates each circuit through its projections to the striatum [42]. The motor, the anterior cingulate-medial orbitofrontal circuit, and the lateral orbitofrontal circuits represent the most likely candidates for involvement in the pathogenesis of MC.
Specifically, the onset of hypodopaminergia in the motor circuit may underlie muscular rigidity [8, 9, 39]. In addition, hypodopaminergia developing in the anterior cingulate-medial orbitofrontal circuit could participate in causing diminished responsiveness, akinesia, and mutism and contribute to hyperthermia and autonomic dysfunction. Bilateral lesions of this circuit have been associated with akinetic mutism, which involves severe hyomotility, diminished arousal, and mutism and has been mistaken for simple catatonia [42]. Furthermore, certain cases of akinetic mutism have presented with hyperthermia and autonomic dysfunction, making them difficult to distinguish from MC [8, 9, 39]. In this regard, it is of considerable interest that the anterior cingulate-medial orbitofrontal circuit contains a spur from the ventral pallidum to the lateral hypothalamus [43]. This suggests that reduced dopamine activity could cause hyperthermia and autonomic dysfunction in MC by disrupting anterior cingulate-medial orbitofrontal circuit transmission to the lateral hypothalamus.
Lastly, hypodopaminergia involving the lateral orbitofrontal subcortical circuit may mediate selected catatonic features observed in MC. Dysfunction in the lateral orbitofrontal circuit has been associated with utilization and imitation behaviors [44]. These behaviors involve automatic imitation of the gestures and actions of others or inappropriate use of objects such as tools or utensils. Utilization and imitation behaviors reflect enslavement to environmental cues [44] and share striking clinical similarities with catatonic features such as echopraxia, echolalia, gegenhalten, all of which are viewed as stimulus bound or motor perseverative phenomena consistent with frontal lobe dysfunction [44]. Utilization and imitation behaviors may also occur in association with dorsolateral prefrontal circuit dysfunction.
We have proposed that in addition to dopamine-2 receptor blockade, NMS is the product of preexisting central dopamine hypoactivity that represents a trait vulnerability marker for this disorder, coupled with state-related downward adjustments in the dopamine system occurring in response to acute or repeated exposure to stress [8, 9, 39]. Here, we suggest that such state- and trait-related factors are also critical in causing hypodopaminergia in the frontal subcortical circuits in MC. A number of lines of evidence indicate that certain individuals may exhibit baseline hypodopaminergia, including reduced homovanillic acid (HVA) levels in post-NMS patients; reduced striatal HVA levels or lack of elevated HVA-to-dopamine ratios in patients who died from MC or NMS; lower cerebrospinal fluid HVA levels and more severe baseline parkinsonian symptoms in patients with Parkinson’s disease following recovery from NMS; and reports of abnormalities in the dopamine-2 receptor gene in NMS [8–10, 39].
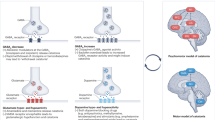
Furthermore, the enhanced responsiveness of the dopamine system to stress may be implicated as a state-related cofactor predisposing to MC. In particular, the dopaminergic innervation of the medial prefrontal cortex in the rat is unique in that it is activated by very mild stressors such as limited footshock or conditioned fear [45]. In addition, there is considerable data indicating a functional interdependence of dopamine systems innervating the medial prefrontal cortex and subcortical dopamine systems; changes in the medial prefrontal cortex dopamine system appear to have an inverse relationship with dopamine turnover in the dorsal and ventral striatum [46]. Consistent with this, lesions of the mesocortical dopamine pathway to the medial prefrontal cortex in the rat result in increased indexes of subcortical dopamine functioning [46] (see Fig. 5.2).
Interdependence of Medial Prefrontal and Subcortical Dopamine System: Normal State (right) and after lesioning of the dopamine input to the Medial Prefrontal Cortex (left) (adapted from [46]. Copyright 1987, American Medical Association, All rights reserved
Conversely, increased mesocortical dopaminergic neurotransmission to the medial prefrontal cortex has been associated with decreased indexes of subcortical dopamine functioning [46, 47]. Accordingly, if stress activates the stress-sensitive mesocortical dopaminergic pathway to the medial prefrontal cortex, it could have feedback effects in both the dorsal and ventral striatum, rendering these areas hypodopaminergic and predisposing to MC and NMS in individuals with preexisting central dopaminergic hypoactivity (see Fig. 5.3).
Interdependence of Medial Prefrontal and Subcortical Dopamine System: Normal State (right) after stress-inducted activation of dopamine input to the Medial Prefrontal Cortex (left) (adapted from [46]. Copyright 1987, American Medical Association, All rights reserved
Evaluation and Treatment
Familiarity with the distinctive clinical features and varied etiologies of MC is essential for effective management of this potentially fatal condition. In both clinical vignettes, it was critical to exclude neuromedical or drug-induced causes of MC before assigning a psychiatric etiology. The potential for severe autonomic symptoms and high rates of medical complications dictate early institution of intensive medical care focusing on fluid replacement, reduction of temperature, and support of cardiac, respiratory, and renal functions. Careful monitoring for complications, particularly aspiration pneumonia, thromboembolism, and renal failure, is essential. Many clinicians, not recognizing the syndrome they are witnessing, are apt to treat the patient’s unusual symptoms with antipsychotic drugs. However, the bulk of evidence indicates that the dopamine receptor blocking effects of antipsychotics are likely to aggravate MC episodes, as in NMS, where continuation of antipsychotic drug treatment clearly increases the likelihood of death. Antipsychotics should be withheld whenever MC is suspected.
Benzodiazepines have been highly effective in the treatment of simple (benign) catatonia, including antipsychotic drug-induced catatonia [37, 38]. Philbrick and Rummans [1] observed that the benefits of benzodiazepines in MC appeared less uniform than in simple catatonia but were nonetheless impressive at times. They asserted that even a partial response might be beneficial and retard the progression of MC until more definitive treatment can be instituted. Fricchione [37, 38] suggested that if simple catatonia proves unresponsive to benzodiazepines after 5 days of treatment, ECT should be considered as a definitive measure. In MC, however, these researchers argued against a 5-day wait and urged that ECT be started if benzodiazepines do not briskly reverse the MC process. Such was the case in Patient 2 where 2 days of intravenous lorazepam therapy was without benefit. Lack of response led to early initiation of ECT followed by dramatic resolution of MC.
Indeed, ECT has been viewed as a safe and effective treatment for MC when it occurs as an outgrowth of a major psychotic disorder [5–10]. Although controlled studies are lacking, case reports as well as series of consecutive cases indicate excellent results with its use. Among 50 patients reported in four large series [5], 40 of 41 patients treated with ECT survived. In contrast, only five of nine who received only antipsychotics and supportive care recovered. Similarly, in Philbrick and Rummans [1] review of 18 MC cases, 11 of 13 treated with ECT survived, compared to only 1 of 5 who did not receive ECT.
However, ECT appears effective only if initiated before severe progression of MC symptoms. Sedvic [48] reported that the onset of coma or a temperature in excess of 41 °C predicts a poor response even to ECT. Arnold and Stepan [12] found that in 19 patients starting ECT within 5 days of the onset of hyperthermia, 16 survived, whereas in 14 patients who began treatment beyond this 5-day point, ECT had no effect in preventing a fatal outcome. Although earlier protocols called for particularly intensive treatment [12], recent trials have indicated that ECT can be efficacious when given once or twice daily or every other day for a total of 5–15 treatments (usually bilateral) [5–10]. Substantial improvement often becomes evident after one to four treatments. There can be little doubt that prompt initiation of ECT represented a life-saving intervention in both of our clinical vignettes.
Other data, also anecdotal, suggests that MC due to the major psychoses can be effectively treated with adrenocorticotropic hormone (ACTH) and corticosteroids [5–10]. However, since severely ill patients have tolerated ECT without incident, and since the utility of hormonal therapy is less well documented, ECT appears to be the preferred treatment. ACTH and corticosteroids may be used if ECT proves ineffective.
Several investigators have suggested that ECT in combination with dantrolene, a drug that inhibits contraction and heat production in muscle, represents the optimal treatment for MC [5–10]. Additional cases have involved successful treatment with dantrolene alone; bromocriptine, dantrolene, and ECT; bromocriptine and benzodiazepines; and dantrolene and bromocriptine; as well as artificial hibernation [8–10].
In MC occurring as an outgrowth of a neuromedical illness, treatment must obviously be directed at the underlying disorder. Nevertheless, anecdotal reports have described ECT as dramatically effective in suppressing the symptoms of MC-like states complicating a diversity of neuromedical conditions [5–10]. In such cases, the efficacy of ECT appears largely independent of the underlying illness, and improvement is likely to be transient if the neuromedical condition persists. If, however, the underlying disorder either remits or is corrected, permanent recovery may be possible. Along these lines, ECT has been used effectively in the treatment of NMS.
Conclusions
MC represents a life-threatening neuropsychiatric disorder described long before the introduction of antipsychotic drugs. A review of the world literature on MC indicates that although the incidence of the condition may have declined since the pre-antipsychotic drug era, it continues to occur and is now reported more frequently in foreign publications. Lack of recognition probably accounts for the relative paucity of contemporary North American reports on this disorder. Failure to recognize MC has significant clinical implications since, once developed, this condition assumes an autonomous and potentially fatal course.
Furthermore, MC represents a nonspecific syndrome that develops as an outgrowth of neuromedical illness as well as the major psychoses. From this perspective, NMS may be conceptualized as an antipsychotic drug-induced form of MC. The hypothesis that MC and NMS share a common pathophysiology involving reduced dopamine functioning with the frontal-subcortical circuits provides additional support for a view of these disorders as manifestations of a unitary diagnostic entity. ECT appears to be the preferred treatment for MC stemming from a major psychotic disorder and it may also be effective in cases caused by neuromedical illness. Antipsychotic drugs should be withheld whenever MC is suspected.
References
Philbrick KL, Rummans TA. Malignant catatonia. J Neuropsychiatry Clini Neurosci. 1994;6:1–13.
Stauder KH. Die todliche Katatonie. Arch Psychiatr Nervenkr. 1934;102:614–34.
Calmeil LF. Dictionnaire de Medecine ou Repertoire General des Sciences. Medicales sous le Rapport Theorique et Practique. 2nd ed. Paris: Bechet; 1832.
Bell LV. On a form of disease resembling some advanced stages of mania and fever. Am J Insan. 1849;6:97–127.
Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143:1374–81.
Mann SC, Caroff SN, Bleier HR, et al. Electroconvulsive therapy of the lethal catatonia syndrome: case report and review. Convuls Ther. 1990;6:239–47.
Mann SC, Auriacombe M, Macfadden W, et al. Lethal catatonia: clinical aspects and therapeutic intervention; a review of the literature. Encéphale. 2001;27:213–6 (in French).
Mann SC, Caroff SN, Keck Jr PE, et al. The neuroleptic malignant syndrome and related conditions. 2nd ed. Arlington, VA: American Psychiatric Publishing Inc; 2003.
Mann SC, Caroff SN, Fricchione G, et al. Malignant catatonia. In: Caroff SN, Mann SC, Francis A, Fricchione G, editors. Catatonia: from psychopathology to neurobiology. Arlington, VA: American Psychiatric Publishing; 2004. p. 105–19.
Mann SC, Caroff SN, Campbell EC, et al. Malignant catatonia. In: Frucht SJ, Fahn S, editors. Movement disorder emergencies: diagnosis and treatment. Totowa, NJ: Humana Press, Inc.; 2005. p. 53–67.
Caroff SN. The neuroleptic malignant syndrome. J Clin Psychiatry. 1980;41:79–83.
Arnold OH, Stepan H. Untersuchungen zur Frage der akuten todlichen Katotonie. Wien Z Nervenheilkd Grenzgeb. 1952;4:235–58.
Kraepelin E (1905) Lectures on Clinical Psychiatry (2nd ed.), Edited by Johnstone T. New York: William Wood
Ladame C. Psychose aigue idiopathique ou foudroyante. Schweizer Archiv fur Neurologic und Psychiatrie. 1919;5:3–28.
Levine T, Petrides G, Weiner J, et al. Intractable delirium associated with ziconotide successfully treated with electroconvulsive therapy. Psychosomatics. 2002;43:10–7.
Slooter JC, Braun KPJ, Balk FJE, et al. Electroconvulsive therapy for catatonia in childhood. Pediatr Neurol. 2005;32:190–2.
Lee A, Glick DB, Dinwiddie SH. Electroconvulsive therapy in a pediatric patient with malignant catatonia paraneoplastic limbic encephalitis. J ECT. 2006;22:267–70.
Oyama Y, Suzuki K, Honda T, et al. Respiratory distress due to elderly catatonia resolved with ECT.Clinical. Psychiatry. 2006;48:51–6.
Ter Haar IM, Rutgers RJ, Egbers PH. A young woman with a labile mood, hyperactivity, hyperthermia, and exhaustion: symptoms of lethal catatonia. Ned Tijdschr Geneeskd. 2006;150:1753–5 (in Dutch).
Dale RC, Webster W, Gill D. Contemporary encephalitis lethargica presenting with agitated catatonia, stereotypy, and dystonia-parkinsonism. Mov Disord. 2007;15:2281–4.
Ono Y, Manabe Y, Hanakawa Y, et al. Steroid responsive encephalitis lethargica with malignant catatonia. Intern Med. 2007;46:307–10.
Kaestner F, Mostert C, Behnken I, et al. Therapeutic strategies for catatonia in paraneoplastic encephalitis. World J Biol Psychiatry. 2008;9:236–40.
Dhossche DM, Shashidhar SM, Kumar T, et al. Electroconvulsive therapy for malignant catatonia in adolescence. South Med J. 2009;102:1170–2.
Nisijima K. Malignant catatonia accompanied by high urinary catecholamine levels mimicking the presentation of pheochromocytoma. Psychiatry Clin Neurosci. 2009;63:428–9.
Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia; a report of 3 cases and review. Psychitr Q. 2009;80:23–40.
Shafti SS, Nicknam Z, Fallah P, et al. Early psychiatric manifestations in a patient with primary progressive multiple sclerosis. Arch Iran Med. 2009;12:595–8.
Brown M, Freeman F. Clonazepam withdrawal-induced catatonia. Psychosomatics. 2009;50:289–92.
Suzuki H, Fukushima T, Makino K, et al. Patient with encephalitis lethargica presenting with olanzapine-responsive malignant catatonia. Rinsho Shinkeigaku. 2010;50:329–31 (in Japanese).
Moltke K, Lublin H. Malignant catatonia, a neuropsychiatric syndrome. Ugeskr Laeger. 2010;172:1305–6 (in Dutch).
Wachtel LE, Crawford TO, Dhossche DM, et al. Electroconvulsive therapy for pediatric malignant catatonia with cerebellar dysgenesis. Pediatr Neurol. 2010;43:427–30.
Rasmussen KG, Hart DA, Lineberry TW. ECT in patients with psychopathology related to acute neurologic illness. Psychosomatics. 2010;49:67–72.
Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical care and treatment. Gen Hosp Psychiatry. 2010;32:631–5.
Koziel-Schminda E. “Ostra Smierteina Katatonia” Typu Staudera O Przebiegu Letalny(Analiza Materialow Kliniczynch I Sekcyjnch Szpitala W Kochborowie Z Lat (1950–1970). Psychiatr Pol. 1973;7:563–7.
Hafner H, Kasper S. Akute lebensbedrohliche Katatonie: Epidemiologische und Klinische Befunde. Nervenzart. 1982;53:385–94.
Singerman S, Raheja R. Malignant catatonia-a continuing reality. Ann Clin Psychiatry. 1994;6:259–66.
Wilson LG. Viral encephalopathy mimicking functional psychosis. Am J Psychiatry. 1976;133:165–70.
Fricchione G, Bush G, Fozdar M, et al. Recognition and treatment of the catatonic syndrome. J Intensive Care Med. 1997;12:135–47.
Fricchione G, Mann SC, Caroff SN. Catatonia, lethal catatonia, and neuroleptic malignant syndrome. Psychiatr Ann. 2000;3:347–55.
Mann SC, Caroff SN, Fricchione G, et al. Central dopamine hypoactivity and the pathogenesis of neuroleptic malignant syndrome. Psychiatr Ann. 2000;30:363–74.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–81.
Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–46.
Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80.
Deutch AY, Bourdelais AJ, Zahm DS. The nucleus accumbens core and shell: accumbal compartments and their functional attributes. In: Kalivas PW, Barnes CD, editors. Limbic Motor Circuits and neuropsychiatry. Boca Raton FL: CRC Press; 1993. p. 163–75.
Taylor MA. Catatonia: a review of a behavioral neurologic syndrome. Neuropsychiatry Neuropsychol Behav Neurol. 1990;3:48–72.
Thierry AM, Tassin JP, Blanc G, et al. Selective activation of the mesocortical dopamine system by stress. Nature. 1976;263:242–4.
Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9.
Pycock CL, Kerwin RW, Carter CJ. Effects of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–6.
Sedivic V. Psychoses endangering life. Cesk Psychiatr. 1981;77:38–41 (In Czech).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mann, S.C., Caroff, S.N., Bleier, H.R., Campbell, E.C. (2013). Malignant Catatonia. In: Frucht, S. (eds) Movement Disorder Emergencies. Current Clinical Neurology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60761-835-5_5
Download citation
DOI: https://doi.org/10.1007/978-1-60761-835-5_5
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60761-834-8
Online ISBN: 978-1-60761-835-5
eBook Packages: MedicineMedicine (R0)