Abstract
Malignant catatonia (MC) is a life-threatening neuropsychiatric disorder that was reported long before the introduction of modern antipsychotic drugs. A review of the world literature on MC indicates that although the incidence of the condition may have declined since 1960 when antipsychotics were introduced, it continues to occur and is likely under-recognized. MC is a syndrome rather than a specific disease, and it may develop as an outgrowth of diverse neuromedical illnesses as well as psychiatric disorders. Current data suggest that a substantial proportion of MC cases previously attributed to schizophrenia were more likely due to autoimmune encephalitis, particularly anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. Neuroleptic malignant syndrome (NMS), a potentially deadly complication of antipsychotic drug treatment, may be conceptualized as a drug-induced form of MC. Our review also supports the idea of catatonia as a continuum, with milder forms at one end (termed simple or benign) and more severe forms, involving hyperthermia and autonomic dysfunction (termed malignant) at the other. The hypothesis that simple catatonia, MC, and NMS share a common pathophysiology involving reduced dopaminergic neurotransmission within the basal ganglia-thalamocortical circuits underscores their relationship as variants of a larger unitary catatonic syndrome. Electroconvulsive therapy is the preferred treatment for MC stemming from a psychiatric disorder and also appears effective in cases caused by neuromedical illness. Antipsychotic drugs should be withheld whenever MC is suspected.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Malignant catatonia
- Catatonia
- Neuroleptic malignant syndrome
- Bell’s mania
- Lethal catatonia
- Delirious mania
- Excited delirium
- Electroconvulsive therapy
- Autoimmune encephalitis
- Anti-NMDA receptor encephalitis
- Benzodiazepines
Patient Vignette
A 27-year-old female with bipolar disorder had been off psychiatric medications for 6 months. One week prior to admission, she developed elevated mood, pressured speech, and flight of ideas. She grew markedly agitated, talked constantly, paced relentlessly, and refused food or drink. On admission, she was confused and intensely hyperactive with periods of hostile verbal outbursts and responded to auditory hallucinations. She exhibited muscular rigidity, posturing, echolalia, and echopraxia. Temperature was 39 °C with tachycardia, tachypnea, profuse diaphoresis, and blood pressure of 170/120 mm Hg. Laboratory abnormalities showed leukocytosis and elevation in creatinine phosphokinase (CK). Lumbar puncture, EEG, and CT scan of the head were normal. Over the next 24 h, she lapsed into stupor with increased rigidity and a fever of 40.2 °C. The diagnosis of malignant catatonia associated with a manic episode was made and electroconvulsive therapy (ECT) initiated. Body temperature and other vital signs returned to normal after the first bilateral ECT treatment. She received one bilateral ECT treatment daily for the next 5 days with three more over the next week with marked improvement in all symptoms.
Introduction
Catatonia is a syndrome of striking motor and behavioral abnormalities that may occur in association with diverse neuromedical, drug-induced, and psychiatric illnesses. Furthermore, catatonia may be conceptualized as a continuum, with milder forms at one end (termed simple or benign) and more severe forms, involving hyperthermia and autonomic dysfunction (termed malignant), at the other [1]. In 1934, Stauder [2] described lethal catatonia characterized by extreme motor excitement followed by stuporous exhaustion, coma, cardiovascular collapse, and death. The entire course involved progressive hyperthermia, autonomic dysfunction, clouding of consciousness, and prominent catatonic features. In cases ending in death, the paucity of findings was puzzling and in sharp contrast to the catastrophic clinical manifestations. In fact, this disorder had been discussed previously by Calmeil (1832) [3] and Bell (1849) [4] and was the subject of numerous North American and foreign publications during the pre-antipsychotic drug era. Other names used to describe this same disorder included Bell’s mania , acute delirious mania , delirium acutum , delire aigu , psychotic exhaustion syndrome , and Scheid’s cyanotic syndrome , among others [5,6,7,8]. More recently, stressing that not all cases are fatal, Philbrick and Rummans [1] have promulgated the term malignant catatonia (MC).
In this chapter, we review the historical and modern world literature on MC. On the basis of this review, we conclude that MC represents a currently under-recognized but potentially fatal neuropsychiatric disorder. Our data indicate that MC, like benign catatonia, represents a syndrome rather than a specific disease that may occur in association with diverse neuromedical illnesses as well as with psychiatric disorders. Current data suggest that a substantial proportion of MC cases previously attributed to schizophrenia were more likely linked to autoimmune encephalitis, particularly anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis [9]. Neuroleptic malignant syndrome (NMS), a life-threatening complication of antipsychotic drug treatment [6, 10], may be viewed as an antipsychotic drug-induced toxic or iatrogenic subtype of MC. The hypothesis that simple catatonia, MC, and NMS share a common pathophysiology involving reduced dopaminergic neurotransmission within the basal ganglia-thalamocortical circuits underscores their identity as variants of a larger unitary catatonic syndrome. Recognition of the clinical features of MC and an appreciation of its diverse etiologies are essential for the effective management of patients who develop this catastrophic reaction.
Clinical Presentation: Pre-antipsychotic Drug Era
Despite the diversity of nomenclature, there is considerable consistency to early accounts of MC [5,6,7,8]. A prodromal phase was observed in most but not all cases, lasting an average of 2 weeks, and involved insomnia, anorexia, and labile mood. In roughly 90% of cases, the disease proper began with a phase of intense motor excitement that then continued almost without interruption (as exemplified by the Patient Vignette). Features of this excited phase included refusal of foods and fluids, clouding of consciousness, tachycardia, tachypnea, cyanosis, labile or elevated blood pressure, and profuse perspiration. Acrocyanosis and spontaneous hematomas of the skin were frequently noted. At times, excitement might be interrupted by periods of catatonic stupor and rigidity. Other catatonic signs, such as mutism, catalepsy, posturing, echolalia, and echopraxia were often present. Thought processes became increasingly disorganized and speech grew progressively incoherent. Auditory and visual hallucinations accompanied by bizarre delusions were frequently prominent.
In this “classic” excited phase of MC, excitement was always associated with hyperthermia that could attain levels approaching 43.3 °C prior to the final stuporous phase of MC. This presentation differs phenomenologically from NMS in that although NMS is often preceded by a period of hyperactivity, hyperthermia first emerges concomitantly with, or shortly after, the onset of stupor and rigidity. The excited phase of MC was noted to vary in duration but lasted an average of 8 days [11].
In the final phase of MC, excitement gave way to stuporous exhaustion and extreme hyperthermia, often followed by coma, cardiovascular collapse, and death [5]. In all of Stauder’s 27 cases [2], rigidity of the skeletal muscles was described during this terminal stupor, similar to that seen in NMS. Although other accounts of MC echoed the findings of Stauder, some reports described flaccid muscles in contrast to NMS [6]. About 10% of cases reported during the pre-antipsychotic drug era involved hyperthermia and a primarily stuporous course unassociated with a preceding hyperactive phase. During the pre-antipsychotic drug era, MC was reported fatal in 75–100% of cases [5]. It was observed to occur predominantly in young adults between the ages of 18 and 35 and involved women roughly seven times more often than men. During this period, MC was estimated to account for 0.25–3.5% of admissions to psychiatric hospitals and occurred with equal frequency throughout the seasons [5]. Stauder [2] and others reported findings consistent with a familial pattern of occurrence.
Kraepelin [12], who called this disorder delirium acutum, considered it a nonspecific syndrome that could occur as an outgrowth of neuromedical illness as well as the major psychoses. In contrast, most early French authors viewed MC as an unusual but deadly form of encephalitis preferentially involving the hypothalamus [13]. Subsequent to Stauder’s [2] publication, however, MC was increasingly seen as confined to the major psychoses, although Stauder himself never fully dismissed the possibility that some or all of his patients may have had encephalitis. Most German and American authors emphasized lack of autopsy findings that could account for death, with the CNS abnormalities reported by the French either unconfirmed or deemed trivial. Bronchopneumonia and other infections were considered “opportunistic,” occurring in an already exhausted and compromised host.
Contemporary Presentation
In 1986, we conducted a computerized search of the PubMed database and identified 292 MC cases reported between 1960 and 1985 [5]. In 2013 [8], we added 107 cases found in the literature between 1986 and 2010 and reported on the total 399 cases identified over a 50-year period. Since then, we have reviewed 105 cases published between 2011 and 2020 [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113], thus extending our series to 504 MC cases. Most patients had received antipsychotic drugs at some point during their treatment. All cases involved hyperthermia, since its presence is required for a diagnosis of MC.
Gender was specified in 450 of the full series of 504 MC cases; in 280 (62%) of the cases, the patient was a female. This indicates that women continue to be disproportionately affected, although the trend now appears somewhat moderated compared to the pre-antipsychotic drug era. Mean age of occurrence was 32, compared with 25 during the pre-antipsychotic drug era. Mortality, which had exceeded 75% during the pre-antipsychotic drug era and was still at 60% between 1960 and 1985, has fallen to 10% in cases reported between 1986 and 2020. This decline in mortality is striking and presumably reflects enhanced awareness of MC, early diagnosis, and rapid institution of appropriate treatment. Nevertheless, MC continues to represent a potentially lethal disorder. MC has been estimated to occur in 0.07% of psychiatric admissions or annually in 0.0004% of community adults [5].
Table 7.1 summarizes the clinical features of MC. Along with catatonic stupor and hyperactivity, they have hyperthermia, altered consciousness, and autonomic instability manifested by diaphoresis, tachycardia, labile or elevated blood pressure, and varying degrees of cyanosis. Catatonic signs aside from stupor and excitement continue to be noted. One large series [114] identified 62 patients with psychogenic MC and reported that each exhibited at least three catatonic features. In our most recent 105 MC cases, muscle rigidity was present in 64 of the 79 (81%) cases in which muscle tone was characterized. Among these recent cases, CK was elevated in 41 of the 48 patients (85%) and leukocytosis was present in 19 of 35 (54%). A reduction in serum iron, which has been associated with both MC and NMS [115], was reported in the single case in which serum iron level was measured.
In 60 (15%) of the 399 MC cases reported during the 50-year period between 1960 and 2010 [8], a neuromedical illness was believed to have initiated the full syndromic picture of MC. Infectious causes predominated, in particular, acute and postinfectious viral encephalitis and bacterial septicemia with cerebrovascular disorders, normal pressure hydrocephalus, and various metabolic and toxic disorders accounting for additional cases. Two cases were associated with anti-NMDAR encephalitis and two with paraneoplastic limbic encephalitis. This 15% figure for organic MC was similar to that found in our previous reports [5,6,7,8], as well as in surveys of simple or benign organic catatonia in general hospital settings [116]. During the 50-year period between 1960 and 2010, 339 (85%) of the 399 MC cases reported were considered the outgrowth of a psychiatric disorder, diagnosed as schizophrenia in 127 cases, mania in 22 cases, major depression in 31 cases, psychotic disorder not otherwise specified in 22 cases, and “periodic catatonia” in 10 cases.
However, our most recent 105 MC cases reported during the nine years between 2011 and 2020 differ markedly in this regard. Eighty of 105 (76%) are now attributed to neuromedical illness whereas only 25 (24%) are associated with a psychiatric disorder. Table 7.2 lists the causes associated with these 105 MC cases. Symptom profiles and neurodiagnostic findings in these most recent cases contrast distinctly with those of our earlier reports. For example, seizures, observed in only 14 of the 399 cases (3%) prior to 2010, were reported in 38 of the most recent 105 MC cases (36%). Findings rarely seen in earlier cases included cerebrospinal fluid lymphocytic pleocytosis (27%), abnormalities on electroencephalography (slowing, epileptic activity, and delta brush) (73%), and abnormalities on magnetic resonance imaging (hyperintensities in cortical or subcortical brain regions) (18%).
Two principle factors appear to account for this recent inversion in the ratio of neuromedical to psychiatric MC cases: a decline in cases attributed to schizophrenia and a dramatic increase in cases due to autoimmune encephalitis, particularly NMDAR encephalitis. A critical observation regarding MC cases seen as an outgrowth of schizophrenia is that 117 of the 127 cases reported between 1960 and 2010 appeared in the literature prior to 1985 [6]. Previously, we had cautioned that the frequent association of MC with schizophrenia may be spurious, resulting from a continued misconception that catatonic signs imply catatonic schizophrenia, a view more prominent at that time [5]. Furthermore, although methodologic limitations preclude a definitive answer, studies using consistent diagnostic criteria over time support a true decrease in the incidence of benign or simple catatonic schizophrenia [117, 118].
Anti-NMDAR encephalitis , first described in 2007, is a common and severe form of autoimmune encephalitis [9, 119]. It primarily involves young patients with a median age of 21 but can be seen at any age [120]. Like MC, it more commonly affects women (80%), a percentage of whom will have an ovarian teratoma [121]. Early in its course, anti-NMDAR encephalitis possesses a remarkable capacity to mimic psychiatric disorders [122]. Dalmau et al. [123] reported that 77% of anti-NMDAR encephalitis cases were seen initially by a psychiatrist. Prompt recognition is critical as the condition is treatable and can be diagnosed serologically. Gurrera [122] retrospectively identified 230 adult cases of anti-NMDAR encephalitis associated with prominent psychiatric symptoms and scored them for the presence of clinical features. Regarding the manifestations of MC, catatonic features were present in 45.7%, autonomic dysfunction in 45.7%, fever in 28.3%, and reduced arousal in 30%. This symptom profile clearly predicts that a certain percentage of anti-NMDAR cases with prominent psychiatric symptoms should meet criteria for MC. Frequently observed neurologic features included seizures (60.4%), orofacial dyskinesias (39.1%), dyskinesias involving other body parts (36.1%), memory disturbance (34.8%), and impaired language/aphasia (25.7%).
Other recent studies have explored the frequency of catatonia in anti-NMDAR encephalitis . Serra-Mestres and associates [121] retrospectively applied the Bush-Frances Catatonia Screening Instrument (BFCSI) to 189 anti-NMDAR encephalitis patients identified in a systematic literature search; catatonia was present in 60%. Additionally, autonomic dysfunction was found in 58% of patients who were catatonic compared to 38% who were not. Furthermore, the first prospective study has now been conducted [124]. It assessed the presence of catatonia in 58 anti-NMDAR encephalitis patients entering an inpatient research unit using both the BFCSI and the Braunig Catatonia Rating Scales and identified catatonia in 70.6% of cases.
 Anti-NMDA receptor-mediated encephalitis
is a frequent cause of malignant catatonia.
Anti-NMDA receptor-mediated encephalitis
is a frequent cause of malignant catatonia.
In a paper exploring evidence for immune dysregulation in catatonia, Rogers and associates [125] conducted a systematic literature search for autoimmune disorders causing catatonia. Similar to our observations regarding MC, they found that 72% (249/346) of all cases of autoimmune catatonia reported were due to anti-NMDAR encephalitis. They observed that catatonia described in anti-NMDAR encephalitis cases is often of the MC variety and tends to co-occur in association with psychosis or mania. This led them to propose that MC represents an entity that could be largely accounted for by autoimmune disorders such as anti-NMDAR encephalitis . We would contend that the causes of MC are multiple and diverse. Nevertheless, our findings appear consistent with the impression that autoimmune encephalitis, particularly anti-NMDAR encephalitis, may account for many MC cases previously attributed to psychosis or schizophrenia, MC cases viewed as an outgrowth of viral encephalitis where no causative pathogen is identified, and, as Rogers and associates suggest, cases previously ascribed to encephalitis lethargica.
The Malignant Catatonia Syndrome
Our review of the modern world literature supports Kraepelin’s [12] conceptualization of MC as a nonspecific syndrome that may occur in association with diverse neuromedical, drug-induced, and psychiatric disorders. Table 7.3 summarizes known causes of the MC syndrome. Consistent with this view of MC as a nonspecific syndrome, it is appropriate to consider the relationship between MC and NMS. Among the total 504 contemporary MC cases, the “classic” excited form (clinical vignette) involving extreme hyperactivity and progressive hyperthermia prior to the onset of stupor has continued to predominate with 61% of cases presenting in this fashion. However, 39% of patients exhibited a primarily stuporous course. This represents a change from the pre-antipsychotic drug era when only about 10% of MC cases were presented in this fashion. Furthermore, a selective analysis of the 105 cases reported since 2011 indicates a reversal in this trend with only 43% presenting as excited and 57% exhibiting a primarily stuporous course.
In many of these cases involving a stuporous course, stupor and hyperthermia developed only following the initiation of antipsychotic drug treatment, giving rise to questions concerning the differentiation of MC from NMS. Furthermore, the clinical features of the classic excited form of MC, once stupor emerges, appear equally difficult to distinguish from those of NMS. Viewing MC as a syndrome, we have suggested that NMS represents an antipsychotic drug-induced toxic or iatrogenic subtype of MC. Accordingly, the emergence of NMS as a subtype of MC could help explain the increased percentage of primarily stuporous MC cases reported in the contemporary literature. Furthermore, anti-NMDAR encephalitis, by far the single most frequent cause of MC in cases reported since 2011, appears to entail an extraordinarily high risk for NMS that could prove critical in explaining the current dominance of the primarily stuporous presentation in our most recent cases. In one study [126], NMS was diagnosed or suspected in 22% of anti-NMDAR encephalitis patients exposed to antipsychotic drugs. Another report suggested that as many as 18 of 33 (55%) patients with anti-NMDAR encephalitis developed suspected NMS upon exposure to antipsychotic drugs [127]. These rates dramatically exceed recent estimates of NMS as occurring in about 0.02 percent of psychiatric patients treated with antipsychotics.
A number of authors have concurred with our conceptualization of NMS as a drug-induced subtype of MC [1, 128]. Fricchione [129] suggested a close relationship between catatonic states triggered by antipsychotics and those that are not, and proposed that antipsychotic drug-induced catatonia is to simple catatonia what NMS is to MC. Koch and associates [130] provided further evidence for overlap among catatonia, MC, and NMS. Among 16 patients with NMS, 15 met clinical and research criteria for catatonia. Furthermore, in each of their cases, there was a strong positive correlation between the severity of NMS and the number of catatonic signs reported, strengthening the argument for a relationship between the disorders and consistent with a view of NMS as a severe variant of catatonia.
In contrast, other authors have argued that simple catatonia, MC, and NMS are distinct clinical entities. Both Castillo and associates [131] and Fleischhacker and associates [132] proposed that excited or agitated behavior points to a diagnosis of MC. However, agitation is commonly a feature of the psychosis preceding NMS for which antipsychotic drugs were originally used. Castillo and associates [132] maintained that prominent muscle rigidity might be a distinguishing feature. However, patients with agitated catatonia usually receive medications early in treatment. Accordingly, if rigidity is present, it may be difficult to know whether this represents NMS or drug-related extrapyramidal symptoms superimposed on MC. Based on our review, we concur with Fricchione and colleagues’ [129, 133] conclusion that aside from a few differences in presentation, catatonia, MC, and NMS appear to be variants of a larger unitary catatonic syndrome.
Pathogenesis
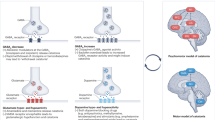
There is evidence for involvement of several neurotransmitter systems in the pathogenesis of catatonia including dopamine, glutamate, gamma-aminobutyric acid, and serotonin. On biochemical grounds, motor symptoms in catatonia appear modulated by dopamine [134]. A consideration of the role of dopamine further supports a view of catatonia, MC, and NMS as subtypes of the same disorder. A number of authors have posited a key role for dopaminergic hypoactivity in triggering both simple catatonia and MC [6, 129, 133]. Furthermore, there is compelling clinical evidence implicating antipsychotic drug-induced dopamine receptor blockade in the pathogenesis of NMS [135]. Our group [6, 135], along with Fricchione and colleagues [129, 133], have proposed that the onset of simple catatonia, MC, and NMS coincides with a reduction in dopaminergic activity within the basal ganglia-thalamocortical circuits. As elucidated by Alexander and associates [136, 137], these circuits represent one of the brain’s principal organizational networks underlying brain–behavior relationships. Five circuits connecting the basal ganglia with their associated areas in the cortex and thalamus have been identified and are named according to their cortical site of origin (Fig. 7.1). They include the “motor circuit,” the “oculomotor circuit,” the “dorsolateral prefrontal circuit,” the “lateral orbitofrontal circuit,” and the “anterior cingulate-medial orbitofrontal circuit.” Each circuit involves the same member structures, including an origin in a specific area of the frontal cortex; projections to the striatum (putamen, caudate, and ventral striatum); connections to the globus pallidus interna and the substantia nigra pars reticulata, which, in turn, project to specific thalamic nuclei; and a final link back to the frontal area from which they originated, thus creating a feedback loop.
Proposed basal ganglia-thalamocortical circuits . Parallel organization of the five basal ganglia-thalamocortical circuits. Each circuit engages specific regions of the cerebral cortex, striatum, pallidum, substantia nigra, and thalamus. (Adapted with permission from Alexander et al. [136])
Dopamine is in a key position to influence activity in each of the circuits. Mesocortical dopamine pathways project directly to circuit areas of origin in the supplementary motor area, frontal eye fields, and the three prefrontal cortical areas. Additionally, dopamine modulates each circuit through its projections to the striatum [138]. The motor, the anterior cingulate-medial orbitofrontal circuit, and the lateral orbitofrontal circuits represent the most likely candidates for involvement in the pathogenesis of simple catatonia, MC, and NMS.
Specifically, the onset of hypodopaminergia in the motor circuit may underly muscular rigidity [6,7,8, 135]. In addition, hypodopaminergia developing in the anterior cingulate-medial orbitofrontal circuit could participate in causing diminished responsiveness, akinesia and mutism, and contribute to hyperthermia and autonomic dysfunction. Bilateral lesions of this circuit have been associated with akinetic mutism, which involves severe hypomotility, diminished arousal and mutism, and has been mistaken for simple catatonia [138]. Furthermore, certain cases of akinetic mutism have presented with hyperthermia and autonomic dysfunction, making them difficult to distinguish from MC and NMS [6, 8, 135]. In this regard, it is of considerable interest that the anterior cingulate-medial orbitofrontal circuit contains a spur from the ventral pallidum to the lateral hypothalamus [139]. This suggests that reduced dopamine activity could cause hyperthermia and autonomic dysfunction in MC and NMS by disrupting anterior cingulate-medial orbitofrontal circuit transmission to the lateral hypothalamus.
Finally, hypodopaminergia involving the lateral orbitofrontal subcortical circuit may mediate selected catatonic features observed in simple catatonia, MC, and NMS. Dysfunction in the lateral orbitofrontal region has been associated with utilization and imitation behaviors [140]. These behaviors involve automatic imitation of the gestures and actions of others or inappropriate use of objects such as tools or utensils. Utilization and imitation behaviors reflect enslavement to environmental cues [140] and share striking clinical similarities with catatonic features such as echopraxia, echolalia, and geigenhalten, all of which are viewed as stimulus bound or motor preservative phenomena consistent with frontal lobe dysfunction [140]. Utilization and imitation behaviors may also occur in association with dorsolateral prefrontal circuit dysfunction.
We have proposed that in addition to dopamine-2 receptor blockade, NMS is the product of pre-existing central dopamine hypoactivity that represents a trait vulnerability marker for this disorder, coupled with state-related downward adjustments in the dopamine system occurring in response to acute or repeated exposure to stress [6, 8, 135]. Here, we suggest that such state- and trait-related factors are also critical in causing hypodopaminergia in the frontal subcortical circuits in simple catatonia, MC, and NMS. A number of lines of evidence indicate that certain individuals may exhibit baseline hypodopaminergia, including reduced homovanillic acid (HVA) levels in post-NMS patients; reduced striatal HVA levels or lack of elevated HVA to dopamine ratios in patients who died from MC or NMS; lower cerebrospinal fluid HVA levels and more severe baseline parkinsonian symptoms in patients with Parkinson’s disease following recovery from NMS; and reports of abnormalities in the dopamine-2 receptor gene in NMS [6, 8, 135].
Furthermore, the enhanced responsiveness of the dopamine system to stress may be implicated as a state-related co-factor predisposing to simple catatonia, MC, and NMS. In particular, the dopaminergic innervation of the medial prefrontal cortex in the rat is unique in that it is activated by very mild stressors such as limited foot shock or conditioned fear [141]. In addition, there are considerable data indicating a functional interdependence of dopamine systems innervating the medial prefrontal cortex and subcortical dopamine systems; changes in the medial prefrontal cortex dopamine system appear to have an inverse relationship with dopamine turnover in the dorsal and ventral striatum [142]. Consistent with this, lesions of the mesocortical dopamine pathway to the medial prefrontal cortex in the rat result in increased indexes of subcortical dopamine functioning [142].
Conversely, increased mesocortical dopaminergic neurotransmission to the medial prefrontal cortex has been associated with decreased indexes of subcortical dopamine functioning [142, 143]. Accordingly, if stress activates the stress-sensitive mesocortical dopaminergic pathway to the medial prefrontal cortex, it could have feedback effects in both the dorsal and ventral striata, rendering these areas hypodopaminergic and predisposing to MC and NMS in individuals with pre-existing central dopaminergic hypoactivity.
Management
Effective management hinges on early recognition of this disorder. Excluding neuromedical or drug-induced causes of MC is critical before assigning a psychiatric etiology. The potential for severe autonomic symptoms and the high rate of medical complications dictate early institution of intensive medical care focusing on fluid replacement, temperature reduction, and support of cardiac, respiratory, and renal functions. Careful monitoring to prevent complications, particularly aspiration pneumonia, thromboembolism, and renal failure is essential. Many clinicians, not recognizing the syndrome they are witnessing, are apt to treat MC patients with antipsychotic drugs. However, the bulk of evidence indicates that the dopamine receptor blocking effects of both first- and second-generation antipsychotics are likely to aggravate MC episodes, as in NMS where continuation of antipsychotic drug treatment clearly increases the likelihood of death. Antipsychotics should be withheld whenever MC is suspected. A treatment algorithm is shown in Fig. 7.2.
Proposed treatment algorithm for malignant catatonia. (Adapted with permission from Fricchione et al. [133]. Copyright 1997, Sage Publications Inc)
Benzodiazepines have been highly effective in the treatment of simple catatonia, including antipsychotic drug-induced catatonia [133]. Philbrick and Rummans [1] observed that the benefits of benzodiazepines in MC appeared less uniform than in simple catatonia but were nonetheless impressive at times. They asserted that even a partial response might be beneficial to retard the progression of MC until more definitive treatment can be instituted. Van Den Eede and associates [144] suggested that lorazepam be started at 2–4 mg per day (PO, IM, IV) and increased to 8–16 mg per day if simple catatonia does not resolve in 2 days. For MC, however, they proposed that lorazepam be initiated at 8–16 mg per day. Fricchione and associates [133] suggested that if simple catatonia proves unresponsive to lorazepam after 5 days of treatment, ECT should become a consideration. In MC, however, these researchers argued against a 5-day wait and urged that ECT be started expeditiously if lorazepam does not briskly reverse the MC process.
Indeed, ECT is a safe and effective treatment for MC occurring as an outgrowth of a psychiatric disorder [1, 145]. Although controlled studies are lacking, case reports and series of consecutive cases indicate excellent results with its use. Among 50 patients reported in four large series [1, 5, 145], 40 of 41 patients treated with ECT survived. In contrast, only five of nine who received antipsychotics and supportive care survived. However, ECT appears effective only if initiated before severe progression of MC symptoms. Sedvic [146] stressed that the onset of coma or a temperature exceeding 41 °C predicts a poor response even to ECT. Arnold and Stepan [11] found that of 19 patients starting ECT within 5 days of the onset of hyperthermia, 16 survived, whereas in 14 patients starting treatment beyond this point, ECT had no effect in preventing a fatal outcome. Although earlier protocols called for particularly intensive treatment [11], recent trials have indicated that ECT can be efficacious when given once or twice daily or every other day for a total of 5–20 treatments (usually bilateral). Substantial improvement often becomes evident after 1–4 treatments [145].
 Lorazepam and ECT are safe and effective treatments for MC.
Lorazepam and ECT are safe and effective treatments for MC.
Several investigators have suggested that ECT in combination with dantrolene, a drug that inhibits contraction and heat production in muscle, represents the optimal treatment for MC [5,6,7,8]. Other cases have reported successful treatment with dantrolene alone; the dopamine agonist bromocriptine combined with dantrolene and ECT; bromocriptine and benzodiazepines; dantrolene and bromocriptine [5,6,7,8]; the NMDA antagonist and dopamine agonist amantadine [26, 147]; and the NMDA antagonist memantine combined with lorazepam [74]. Successful treatment with transcranial magnetic stimulation (rTMS) has been observed in one case [90]. Lastly, intravenous propofol has been reported effective as an interim measure until ECT can be instituted [148], considered a superior anesthetic for MC during ECT [150], and proposed as capable of “lysing” MC on its own [28, 149, 150]. In MC occurring as an outgrowth of a neuromedical illness, specific treatment must be directed at the underlying disorder. Nevertheless, in our full series of 504 MC cases, ECT has been reported as highly effective in treating MC complicating a diversity of neuromedical conditions [1, 5,6,7,8, 145]. In such cases, the efficacy of ECT appears largely independent of the underlying condition and improvement is likely to be transient if the neuromedical condition persists. If, however, the underlying disorder either remits or is corrected, permanent recovery may be possible. Along these lines, ECT has been used effectively in stabilizing MC (and NMS) in anti-NMDA receptor encephalitis, permitting definitive measures such as tumor removal (if indicated) and sequential immunotherapies to take effect [49, 151].
Conclusion
MC represents a life-threatening neuropsychiatric disorder described long before the introduction of modern antipsychotic drugs. A review of the world literature on MC indicates that although the incidence of the condition may have declined since the pre-antipsychotic drug era, it continues to occur but is under-recognized. MC represents a syndrome rather than a specific disease and may develop as an outgrowth of diverse neuromedical illnesses as well as with psychiatric disorders. Among 399 MC cases reported during the 50-year period between 1960 and 2010, 339 (85%) were attributed to a psychiatric condition and only 60 (15%) to a neuromedical condition. In contrast, among our most recent 105 MC cases reported during the nine years between 2011 and 2020, 80 (76%) are now attributed to neuromedical illness and only 25 (24%) are associated with a psychiatric disorder. These findings are striking but appear consistent with the impression that anti-NMDAR encephalitis may now account for many MC cases previously attributed to psychosis or schizophrenia, MC cases viewed as an outgrowth of viral encephalitis where no pathogen was identified, and MC cases previously ascribed to encephalitis lethargica.
Also, from a perspective of MC as a syndrome, NMS may be conceptualized as an antipsychotic drug-induced toxic or iatrogenic subtype of MC. The hypothesis that simple catatonia, MC, and NMS share a common pathophysiology involving reduced dopaminergic neurotransmission within the basal ganglia-thalamocortical circuits underscores their identity as variants of a larger unitary catatonic syndrome. ECT appears to be the preferred treatment for MC. Antipsychotic drugs should be withheld whenever MC is suspected. Recognition of the clinical features of MC and an appreciation of its diverse etiologies are essential for the effective management of patients who develop this catastrophic reaction.
References
Philbrick KL, Rummans TA. Malignant catatonia. J Neuropsychiatry Clin Neurosci. 1994;6(1):1–13.
Stauder KH. Die todliche Katatonie. Arch Psychiatr Nervenkr. 1934;102:614–34.
Calmeil LF. Dictionnaire de Medecine ou Repertoire General des Sciences. Medicales sous le Rapport Theorique et Practique. 2nd ed. Paris: Bechet; 1832.
Bell LV. On a form of insanity resembling some advanced stages of mania and fever. Am J Insan. 1849;6:97–127.
Mann SC, Caroff SN, Bleier HR, Welz WK, Kling MA, Hayashida M. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374–81.
Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck Jr PE, Lazarus A, editors. The neuroleptic malignant syndrome and related conditions. 2nd ed. Washington, DC: American Psychiatric Publishing Inc.; 2003. p. 121–43.
Mann SC, Caroff SN, Campbell EC, Bleier HR, Greenstein RA. Malignant catatonia. In: Frucht SJ, Fahn S, editors. Movement disorder emergencies: diagnosis and treatment. Totowa: Humana Press; 2005. p. 53–67.
Mann SC, Caroff SN, Bleier HR, Campbell EC. Malignant catatonia. In: Frucht SJ, editor. Movement disorder emergencies: diagnosis and treatment. 2nd ed. New York: Humana Press; 2013. p. 59–74.
Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378(9):840–51.
Caroff SN. The neuroleptic malignant syndrome. J Clin Psychiatry. 1980;41(3):79–83.
Arnold OH, Stepan H. Untersuchungen zur Frage der akuten todlichen Katatonie. Wien Z Nervenheilkd Grenzgeb. 1952;4:235–58.
Kraepelin E. In: Johnstone T, editor. Lectures on clinical psychiatry. 2nd ed. New York: William Wood; 1905.
Ladame C. Psychose aigue idiopathique ou foudroyante. Schweizer Archiv fur Neurologic und Psychiatre. 1919;5:3–28.
Karacetin G, Bayer R, Demir T. Successful treatment of benzodiazepine-resistant malignant catatonia with electroconvulsive therapy. J Neuropsychiatry Clin Neurosci. 2012;24(1):E48.
Wong S, Hughes B, Pudek M, Dailin L. Malignant catatonia mimicking pheochromocytoma. Case Rep Endocrinol. 2013;2013:815821. https://doi.org/10.1155/2013/815821.
Nisijima K. Increased biogenic catecholamine and metabolite levels in two patients with malignant catatonia. Neuropsychiatr Dis Treat. 2013;9:1171–4.
Hobo M, Uezato A, Nishiyama M, Suzuki M, Kurata J, Makita K, et al. A case of malignant catatonia with idiopathic pulmonary arterial hypertension treated by electroconvulsive therapy. BMC Psychiatry. 2016;16:130. https://doi.org/10.1186/s12888-016-0835-4.
Duncan MD, Vazirani SS. An unusual rapid response call: malignant catatonia. Am J Med. 2016;129(7):678–80.
Park J, Tan J, Krzeminski S, Hazeghazam M, Bandiamuri M, Carlson RW. Malignant catatonia warrants early psychiatric-critical care collaborative management: two cases and literature review. Case Rep Crit Care. 2017;2017:1951965. https://doi.org/10.1155/2017/1951965.
Ohi K, Kuwata A, Shimada T, Yasuyama T, Nitta Y, Uehara T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia. Medicine (Baltimore). 2017;96(16):e6566. https://doi.org/10.1097/MD.0000000000006566.
Ghaziuddin N, Hendricks M, Patel P, Wachtel LE, Dhossche DM. Neuroleptic malignant syndrome/malignant catatonia in child psychiatry: literature review and a case series. J Child Adolesc Psychopharmacol. 2017;27(4):359–65.
Kurose S, Koreki A, Funayama M, Takahashi E, Kaji M, Ogyu K, et al. Resting-state hyperperfusion in whole brain: a case of malignant catatonia that improved with electric convulsion therapy. Schizophr Res. 2019;210:287–8.
Ozan E, Aydin EF. Challenges in diagnosing and treating malignant catatonia and its fatal consequences. J Neuropsychiatry Clin Neurosci. 2014;26(1):E52.
Wachtel L, Commins E, Park M, Rolider N, Stephens R, Reti I. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319–20.
Dessens F, van Passen J, van Westerloo DJ, van der Wee N, van Vliet IM, Van Noorden MS. Electroconvulsive therapy in the intensive care unit for the treatment of catatonia: a case series and review of the literature. Gen Hosp Psychiatry. 2016;38:37–41.
Maki M, Kato O, Kunimatsu J, Sato T, Fujie S. Significant response to amantadine in a patient with malignant catatonia. Eur Psychiatry. 2016;33(S1):S336.
Onofrei C, Singh R, Sears C. 1821: diagnostic challenges and treatment of malignant catatonia with electroconvulsive therapy. Crit Care Med. 2016;44(12):530.
Bellani M, Zanette G, Zovetti N, Barillari M, Del Piccolo L, Brambilla P. Adult mild encephalitis with reversible splenial lesion associated with delirious mania: a case report. Front Psych. 2020;11:79. https://doi.org/10.3389/fpsyt.2020.00079.
Adams G, Brown A, Burnside R, Tandy D, Lowe K, Malhotra A, et al. A undiagnosed stupor in the acute medical unit: a case of malignant catatonia. Q J Med. 2015;108(4):335–6.
Matias DFM, de Mello AS, Riera R, Teixeira de Gois A. Malignant catatonia responsive to low doses of lorazepam: case report. Sao Paulo Med J. 2016;134(2):176–9.
Shenai N, White CD, Azzam PN, Gopalan P, Solai LK. Practical and legal challenges to a electroconvulsive therapy in malignant catatonia. Harv Rev Psychiatry. 2016;24(3):238–41.
Hirayama I, Inokuchi R, Hiruma T, Doi K, Morimura N. Malignant catatonia mimics tetanus. Clin Pract Cases Emerg Med. 2018;2(4):369–70.
Buvanaswari P. Behaviour changes with autonomic disturbances-malignant catatonia? Eur Psychiatry. 2015;30(Supplement 1):1264.
Averna R, Battaglia C, Labonia M, Riccioni A, Vicari S. Catatonia in adolescence: first onset psychosis or anti-NMDAR encephalitis? Clin Neuropharmacol. 2019;42(4):136–8.
Hefter D, Topor CE, Gass P, Hirjak D. Two sides of the same coin: a case report of first-episode catatonic syndrome in a high-functioning autism patient. Front Psych. 2019;10:224. https://doi.org/10.3389/fpsyt.2019.00224.
Consoli A, Ronen K, An-Gourfinkel I, Barbeau M, Marra D, Costedoat-Chalumeau N, et al. Malignant catatonia due to anti-MNDA-receptor encephalitis in a 17-year old girl: case report. Child Adolesc Psychiatry Ment Health. 2011;5(1):15. https://doi.org/10.1186/1753-2000-5-15.
Wingfield T, McHugh C, Vas A, Richardson A, Wilkens E, Boninton A, et al. Autoimmune encephalitis: a case series and comprehensive review of the literature. Q J Med. 2011;104(11):921–31.
Achour NB, Youssef-Turki IB, Messelmani M, Kraoua I, Yaacoubi J, Klaa H, et al. Anti-NMDA receptor encephalitis mimicking a primary psychiatric disorder in a 13-year-old girl. Turk J Psychiatry. 2012;24(2):145–7.
Finke C, Kopp UA, Pruss H, Dalmau J, Wandinger K-P, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2012;83(2):195–8.
Haththotuwa HR, Malhas L, Jagadeeswaran A. Anti-NMDA receptor encephalitis an intensive care perspective. J Intensive Care Soc. 2012;13(2):147–50.
McCarthy A, Dineen J, McKenna P, Keogan M, Sheehan J, Lynch T, et al. Anti NMDA receptor encephalitis with associated catatonia during pregnancy. J Neurol. 2012;259(12):2632–5.
Obligar P, Ortiz M, Lee L. Rapid response of methylprednisone in a 14 year old male with proven anti-NMDA receptor encephalitis. Philipp J Neurol. 2012;16(1):54–5.
Di Capua D, Garcia-Ptacek S, Garcia-Garcia ME, Abarrategui B, Porta-Etessam J, Garcia-Morales I. Extreme delta brush in a patient with anti-NMDAR encephalitis. Epileptic Disord. 2013;15:461–4.
Wilson JE, Shuster J, Fuchs C. Anti-NMDA receptor encephalitis in a 14-year-old female presenting as malignant catatonia-medical and psychiatry approach to treatment. Psychosomatics. 2013;54(6):585–9.
Young PJ, Baker S, Cavazzoni E, Erickson SJ, Krishnan A, Kruger PS, et al. A case series of critically ill patients with anti-NMDA receptor encephalitis. Crit Care Resusc. 2013;15(1):8–14.
Serban-Pereteanu AS, Trasca D, Stefanescu VC, Bustan M, Zurac S, Cojocaru IM. Anti-NMDA receptor encephalitis in a young woman: a diagnostic challenge. Rom J Neurol. 2014;8(4):200–11.
Acien P, Ruiz-Macia E, Acien M, Martin-Estefania C. Mature ovarian teratoma-associated limbic encephalitis. J Obstet Gynaecol. 2015;35(3):317–9.
Hur J. Fever of unknown origin: an unusual presentation of anti-NMDA receptor encephalitis. Infect Chemother. 2015;47(2):129–32.
Jones KC, Schwartz AC, Hermida AP, Kahn DA. A case of anti-NMDA receptor encephalitis treated with ECT. J Psychiatr Pract. 2015;21(5):374–80.
Kiani R, Lawden M, Eames P, Critchley P, Bhaumik S, Odedra S, et al. Anti-NMDA-receptor encephalitis presenting with catatonia and neuroleptic syndrome in patients with intellectual disability and autism. BJPsych Bull. 2015;39(1):32–5.
Koksal A, Baybas S, Mutluay B, Altunkaynak Y, Keskek A. A case of NMDAR encephalitis missed diagnosed as post partum psychosis and neuroleptic malignant syndrome. Neurol Sci. 2015;36(7):1257–8.
Simabukuro MM, de Andrade Freitas CH, Castro LHM. A patient with a long history of relapsing psychosis and mania presenting with anti-NMDA receptor encephalitis 10 years after first episode. Dement Neuropsychol. 2015;9(3):311–4.
Afanasiev V, Brechemier M-L, Boisseau W, Ducoudray R, Mayer M-E, Meyronet D, et al. Anti-NMDA receptor antibody encephalitis and neuroendocrine pancreatic tumor: causal link? Neurology. 2016;87(1):112–3.
Aulicka S, Horak O, Mrazova L, Mikolasek P, Strba J, Krbkova L, et al. Malignant catatonia due to anti-NMDA-receptor encephalitis in a 15-year-old girl: case report and summary of current knowledge. Neuropsychiatry (London). 2016;6(4):136–41.
Halbert RK. Anti-N-Methyl-D-Aspartate receptor encephalitis: a case study. J Neurosci Nurs. 2016;48(5):270–3.
Milovac Z, Santini M, Pisk SV, Caratan S, Gorsic V, Filipcic E. Acute psychosis-anti-NMDA receptor encephalitis phase. Psychiatr Danub. 2016;28(3):301–3.
Rozier M, Morita D, King M. Anti-N-Methyl-D-Aspartate receptor encephalitis: a potential mimic of neuroleptic malignant syndrome. Pediatr Neurol. 2016;63:71–2.
Splendiani A, Felli V, Di Sibio A, Gennarelli A, Patriarca L, Stratta P, et al. Magnetic resonance imagining and magnetic resonance spectroscopy in a young male patient with anti-N-methyl-D-aspartate receptor encephalitis and uncommon cerebellar involvement: a case report with review of the literature. Neuroradiol J. 2016;29(1):30–5.
Vargas RJ, Farid H, Goldenson RP, Fairchild AH, Dorton BJ, Bromley BS. Ovarian teratomas and Anti-N-Methyl-D-Aspartate receptor encephalitis: why sonography first? J Ultrasound Med. 2016;35(4):852–4.
Ziplow J, Chadha T, Wen A. 1834: psychosis seizures and autonomic instability in a teenage girl with an ovarian mass. Crit Care Med. 2016;44(12):534.
Bota RG, Groysman L, Momii A. Catatonia as a syndrome characterized by GABAergic interneuronal dysfunction mediated by NMDA receptors. Br J Med Med Res. 2017;19(11):1–6.
Doden T, Sekijima Y, Ikeda J, Ozawa K, Ohashi N, Kodaira M, et al. Postpartum anti-N-Methyl-D-aspartate receptor encephalitis: a case report and literature review. Intern Med (Tokyo). 2017;56(3):357–62.
Hermans T, Santens P, Matton C, Oostra K, Heylens G, Herremans S, et al. Anti-NMDA receptor encephalitis: still unknown and underdiagnosed by physicians and especially psychiatrists? Acta Clin Belg. 2017;73(5):364–7.
Liang Z, Yang S, Sun X, Li B, Li W, Liu Z, et al. Teratoma-associated anti-NMDAR encephalitis. Two cases report and literature review. Medicine (Baltimore). 2017;96:e9177.
Sivarooban V, Yogitagavari Y, Che CK, Lee CW. Organic disorder with neuropsychiatric symptoms-case report of anti-NMDA receptor encephalitis with neuropsychiatric manifestations. Malaysian J Psychiatry Ejournal. 2017;26(1):37–42.
Tsutsui K, Takaki M, Omori Y, Imai Y, Nishino S, Tanaka K, et al. N-Methy-D-aspartate receptor antibody could be the cause of catatonic symptoms in psychiatric patients: case reports and methods for detection. Neuropsychiatr Dis Treat. 2017;13:339–45.
Voice J, Ponterio JM, Lakhi N. Psychosis secondary to an incidental teratoma: a “heads-up” for psychiatrists and gynecologists. Arch Womens Ment Health. 2017;20(5):703–7.
Vasenina EE, Levin OS, Gan’kina OA, Chimagomedova AS, Levikov DI. Auto immune anti-NMDA receptor encephalitis. Neurosci Behav Phys. 2018;48(6):650–6.
Amugoda C, Foroush NC, Akhlaghi H. Anti-NMDAR encephalitis: higher suspicious needed for earlier diagnosis (Case report, literature review and diagnostic criteria). Neurol Med. 2019:7476254. https://doi.org/10.1155/2019/7476254.
Ford B, McDonald A, Srinivasan S. Anti-NMDA receptor encephalitis: a case study and illness overview. Drugs Context. 2019;8:212589. https://doi.org/10.7573/dic.212589.
Moussa T, Afzal K, Cooper J, Rosenberger R, Gerstle K, Wagner-Weiner L. Pediatric anti-NMDA receptor encephalitis with catatonia: treatment with electroconvulsive therapy. Pediatr Rheumatol. 2019;17:8. https://doi.org/10.1186/s12969-019-0310-0.
Schermann H, Ponomareva IV, Gennadievich V, Yakushev KB, Sherman MA. Clinical variants of limbic encephalitis. SAGE Open Med Case Rep. 2019;7:1–10. https://doi.org/10.1177/2050313X19846042.
AlShimemeri S, Alsaeed M, Lai J, Uy C. Delayed N-methyl-D-aspartate receptor encephalitis relapse. Can J Neurol Sci. 2020;47(2):264–6. https://doi.org/10.1017/cjn.2019.332.
Ramirez-Bermudez J, Restrepo-Martinez M, Diaz-Victoria AR, Espinola-Nadurille ME. Memantine as an adjunctive therapy in a patient with anti-NMDA receptor encephalitis. J Clin Psychopharmacol. 2019;40(1):92–3. https://doi.org/10.1097/JCP.0000000000001145.
Sokhi DS, Bhogal OS. Autoimmune encephalitis is recognized as an important differential diagnosis in a Kenyan tertiary referral center. BMJ Mil Health. 2020; https://doi.org/10.1136/jramc-2019-001338.
Nikolaus M, Knierim E, Meisel C, Kreye J, Pruss H, Schnabel D, et al. Severe GABA A receptor encephalitis without seizures: a paediatric case successfully treated with early immunomodulation. Eur J Paediatr Neurol. 2018;22(3):558–62.
Samra K, Rogers J, Mahdi-Rogers M, Stanton B. Catatonia with GABA A receptor antibodies. Pract Neurol. 2020;20:139–43.
Carneiro S, Fernades I, Abuowda Y, Oliveria AA, Santos C, Palos A, et al. Anti-vgkc antibody-associated limbic encephalitis presenting with recurrent catatonia. Eur Psychiatry. 2015;30(Supp 1):812.
Xu Z, Prasad K, Yeo T. Progressive encephalomyelitis with rigidity and myoclonus in an intellectually disabled patient mimicking neuroleptic malignant syndrome. J Mov Disord. 2017;10(2):99–101.
Mon T, L’Ecuyer S, Farber NB, White AJ, Baszis KW, Hearn JK, et al. The use of electroconvulsive in a patient with juvenile systemic lupus erythematosus and catatonia. Lupus. 2012;21(14):1575–81.
Ali A, Taj A. Misbah-uz-Zehra. Lupus catatonia in a young girl who presented with fever and altered sensorium. Pak J Med Sci. 2014;30(2):446–8.
Strohmayer K. D47 case vignette in critical care: a case of malignant catatonia due to central nervous system involvement of systemic lupus erythematosus (sle) necessitating treatment in the intensive care unit (icu). Am J Respir Crit Care Med. 2014;189:A6128.
Jones M, Gausche E, Reed E. A case of neuropsychiatric lupus with severe malignant catatonia that improves with daily electroconvulsive therapy. J Neuropsychiatry Clin Neurosci. 2016;28(1):e19–20. https://doi.org/10.1176/appi.neuropsych.15080211.
Bharadwaj B, Sugaparaneetharan A, Rajkumar RP. Graves’ disease presenting with catatonia: a probable cause of encephalopathy associated with autoimmune thyroid disease. Acta Neuropsychiatr. 2012;24(6):374–9.
Saito T, Saito R, Suwa H, Yakushiji F, Takezawa K, Nakamura M. Differences in the treatment response to antithyroid drugs versus electroconvulsive therapy in a case of recurrent catatonia due to Graves’ disease. Case Rep Psychiatry. 2012:868490. https://doi.org/10.1155/2012/868490.
Rosado SN, Silveira V, Reis AI, Gordinho A, Noronha C. Catatonia and psychosis as manifestations of primary Sjogren’s syndrome. Eur J Case Rep Intern Med. 2018;5(6):000855. https://doi.org/10.12890/2018_000855.
Mischel NA, Mooneyham GLC, Lau C, Van Mater H, Weiner RD. Non-N-methyl-D-aspartate autoimmune encephalopathy and catatonia treatment with electroconvulsive therapy: a pediatric case series and treatment guidelines. Psychosomatics. 2020; https://doi.org/10.1016/j.psym.2019.12.005.
Aggarwal A, Kumar P, Faridi MMA. Neurological manifestation as presenting feature of dengue infection. J Pediatr Neurosci. 2015;10:76–7.
Halder A, Biswas A. Multifactorial organic etiological agents causing catatonia: a learning experience. Int J Educ Psychol Res. 2015;1:301–3.
Kate MP, Raju D, Vishwanathan V, Khan FR, et al. Successful treatment of refractory organic catatonic disorder with repetitive transcranial magnetic stimulation (rTMS) therapy. J Neuropsychiatry Clin Neurosci. 2011;22(3):E2–3.
Shukla L, Narayanaswamy JC, Gopinath S, Math SB. Electroconvulsive therapy for the treatment of organic catatonia due to viral encephalitis. J ECT. 2012;28(3):E-27–E28. https://doi.org/10.1097/YCT.0b013e31824e9228.
Saini SM, Eu CL, Yahya WNN, Rahman AHA. Malignant catatonia secondary to viral menigoencephalitis in a young man with bipolar disorder. Asia Pac Psychiatry. 2013;5(Supp 1):55–8.
Vadala SF, Pellegrini D, Silva ED, Minarro D, Finn BC, Bruetman JE, et al. Lethargic encephalitis. Report of one case. Rev Med Chile. 2013;141(4):531–4.
Bigman DY, Bobrin BD. Von Economo’s disease and post-encephalitic parkinsonism responsive to carbidopa and levodopa. Neuropsychiatr Dis Treat. 2018;14:927–31.
Becker MA, Cannon J, Certa KA. A case of mycoplasma pneumonia encephalopathy presenting as mania. Psychosomatics. 2020; https://doi.org/10.1016/j.psym.2020.02.004.
Aghamollaii V, Ahmadinejad Z, Mohammadian F, Mirsepassi Z. Catatonic state as a rare presentation of neurobrucellosis: a case report. Iran J Psychiatry Behav Sci. 2019;13(3):e95824. https://doi.org/10.5812/ijpbs.95824.
Hocker SE, Wijdicks EFM. Neurological complications of sepsis. Continuum (Minneapolis, MN). 2014;20(3):598–613.
Kanagasundram S, Chengappa KNR. Meningoencephalitis or clozapine withdrawal catatonia or both in a patient with schizophrenia. Acta Neuropsychiatr. 2011;23(2):85–7.
Koch A, Reich K, Wielopolski J, Clepce M, Fischer M, Cornhuber J, et al. Catatonic dilemma in a 33-year-old woman: a discussion. Case Rep Psychiatry. 2013:542303.
Bilbily J, McCollum B, de Leon J. Catatonia secondary to sudden clozapine withdraw: a case with three repeated episodes and a literature review. Case Rep Psychiatry. 2017:2402731. https://doi.org/10.1155/2017/2402731.
Fryml LD, Williams KR, Pelic CG, Fox J, Sahlem G, Robert S, et al. The role of amantadine withdrawal in 3 cases of treatment-refractory altered mental status. J Psychiatr Pract. 2017;23(3):191–9.
Amos JJ. Lorazepam withdrawal-induced catatonia. Ann Clin Psychiatry. 2012;24(2):170–1.
Dada MU, Oluwole L, Obadeji A, Ajayi OA. Dexamethasone induced psychosis presenting with catatonic features. Afr J Psychiatry. 2011;14:316–8.
Clough Z, Henry R, Ekelund A. Delirium associated with therapeutic levels of lithium in bipolar disorder. Prog Neurol Psychiatry. 2014;18(2):10–2.
van Esch AMJ, Fest A, Hoffland BS, Janzing JGE, Steens SCA, Esselink RAJ, et al. Toxic leukoencephalopathy presenting as lethal catatonia. J Addict Med. 2019;13(3):241–4.
Keary CJ, Nejad SH, Rasimas JJ, Stern TA. Intoxications associated with agitation, tachycardia, hypertension, and fever: differential diagnosis, evaluation, and management. Prim Care Companion CNS Disord. 2013;15(3):PCC.12f01459. https://doi.org/10.4088/PCC.12f01459.
Kumar KK, Bondade S, Sattar FA, Singh N. Malignant catatonia and neuroleptic malignant syndrome in relation to disulfiram overdose. Indian J Psychol Med. 2016;38(4):344–7.
Johansson A, Lindstedt D, Roman M, Thelander G, Nielson EI, Lennborn U, et al. A non-fatal intoxication and seven deaths involving the dissociative drug 3-MeO-PCP. Forensic Sci Int. 2017;275(6):76–82.
Bulbena-Cabre A, DiGenova P, Sigel P, Dunn NR, Swift RG. Synthetic cannabinoid intoxication presenting as malignant catatonia: a case report. Int J Ment Health Addict. 2020;18(3):582–6.
Heekin RD, Bradshaw K, Calarge CA. First known case of catatonia due to cyclosporine A-related neurotoxicity in a pediatric patient with steroid-resistant nephrotic syndrome. BMC Psychiatry. 2019;19:123. https://doi.org/10.1186/s12888-019-2107-6.
Rojas PG, Morton L. An unusual case of fever and altered mental status. JAMDA. 2020;21(3):B4. https://doi.org/10.1016/j.jamda.2020.01.019.
Lee S, Jun GW, Jeon SB, Kim CJ, Kim JH. Paroxysmal sympathetic hyperactivity in brain-compressing huge benign tumors: clinical experience and literature review. Springerplus. 2016;5:340. https://doi.org/10.1186/s40064-016-1898-x.
Rengers E, Pop-Purceleanu M, Rietveld L, van der Weyer RW, Frenzael T. Recognize malignant catatonia early: it is well treatable! A case report and review of the literature. Neth J Crit Care. 2017;25(2):67–70.
Singerman S, Raheja R. Malignant catatonia-a continuing reality. Ann Clin Psychiatry. 1994;6(4):259–66.
Lee JW. Serum iron in catatonia and neuroleptic syndrome. Biol Psychiatry. 1998;44(6):499–507.
Oldham MA. The probability that catatonia in the hospital has a medical cause the relative proportions of its causes: a systematic review. Psychosomatics. 2018;59(4):333–40.
Caroff SN, Mann SC, Campbell EC, Sullivan KA. Epidemiology. In: Caroff SN, Mann SC, Francis A, Fricchione G, editors. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Press; 2004. p. 15–31.
Stompe T, Ortwein-Swoboda G, Ritter K, Schanda H, Friedman A. Are we witnessing the disappearance of catatonic schizophrenia? Compr Psychiatry. 2002;43:167–74.
Dalmau J, Tuzun E, Wu H-Y, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36.
Tanguturi YC, Cundiff AW, Fuchs C. Anti-N Methyl d-aspartate receptor encephalitis and electroconvulsive therapy. Literature review and future directions. Child Adolesc Psychiatric Clin N Am. 2019;28:79–89.
Serra-Mestres J, Villagrasa-Blasco B, Thacker V, Jaimes-Albornoz W, Sharma P, Isetta M. Catatonia in N-methyl-d-aspartate receptor antibody encephalitis: phenomenological characteristics from a systematic review of case reports. Gen Hosp Psychiatry. 2020;64:9–16.
Gurrera RJ. Frequency and temporal sequence of clinical features in adults with anti-NMDA receptor encephalitis presenting with psychiatric symptoms. Psychol Med. 2019;49:2709–16.
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of effects of antibodies. Lancet Neurol. 2008;7(12):1091–8.
Espinola-Nadurille M, Flores-Rivera J, Rivas-Alonso V, Vargas-Canas S, Fricchione GL, Bayliss L, et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci. 2019;73(9):574–80.
Rogers JP, Pollak TA, Blackman G, David AS. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6:620–30.
Sarkis RA, Coffey MJ, Cooper JJ, Hassan I, Lennox B. Anti-N-Methyl-D-Aspartate receptor encephalitis: a review of psychiatric phenotypes and management considerations: a report of the American Neuropsychiatric Committee on Research. J Neuropsychiatry Clin Neurosci. 2019;31(2):137–42.
Lejuste F, Thomas L, Picard G, Desestret V, Ducray F, Rogemond V, et al. Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e280.
Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge: Cambridge University Press; 2003.
Fricchione GL. Neuroleptic catatonia and its relationship to psychogenic catatonia. Biol Psychiatry. 1985;20(3):304–13.
Koch M, Chandragiri S, Rizvi S, Petrides G, Francis A. Catatonic signs in neuroleptic malignant syndrome. Compr Psychiatry. 2000;41(1):73–5.
Castillo E, Rubin RT, Holsboer-Trachsler E. Clinical differentiation of lethal catatonia and neuroleptic malignant syndrome. Am J Psychiatry. 1989;146(3):324–8.
Fleischhacker WW, Unterweger B, Kane JM, Hinterhuber H. The neuroleptic malignant syndrome and its differentiation from lethal catatonia. Acta Psychiatr Scand. 1990;81(1):3–5.
Fricchione G, Bush G, Fozdar M, Frances A, Fink M. Recognition and treatment of the catatonic syndrome. J Intensive Care Med. 1997;12(3):135–47.
Hirjak D, Kubera KM, Wolf RC, Northoff G. Going back to Kahlbaum’s psychomotor (and GABAergic) origins. Is catatonia more than just a motor and dopaminergic syndrome? Schizophr Bull. 2020;46(2):272–85.
Mann SC, Caroff SN, Fricchione G, Campbell EC. Central dopamine hypoactivity and the pathogenesis of neuroleptic malignant syndrome. Psychiatric Ann. 2000;30(5):363–74.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–81.
Alexander GE, Curtcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1991;85:119–46.
Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50(8):873–80.
Deutch AY, Bourdelais AJ, Zahm DS. The nucleus accumbens core and shell: accumbal compartments and their functional attributes. In: Kalivas PW, Barnes CD, editors. Limbic motor circuits and neuropsychiatry. Boca Raton: CRC Press; 1993. p. 163–75.
Taylor MA. Catatonia: a review of a behavioral neurologic syndrome. Neuropsychiatry Neuropsychol Behav Neurol. 1990;3(1):48–72.
Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of the mesocortical dopamine system by stress. Nature. 1976;263:242–4.
Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–9.
Pycock CL, Kerwin RW, Carter CJ. Effects of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–6.
Van Den Eede F, Van Hecke J, Van Dalfsen A, Van den Bossche B, Cosyns P, Sabbe BGC. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry. 2005;20(5–6):422–9.
Mann SC, Caroff SN, Bleier HR, Antelo RE, Un H. Electroconvulsive therapy of the lethal catatonia syndrome. Convuls Ther. 1990;6(3):239–47.
Sedvic V. Psychoses endangering life. Cesk Psychiatr. 1981;77:38–41. (In Czech)
Northoff G, Lins H, Boker H, Danos P, Bogerts B. Therapeutic efficacy of N-methyl-D-aspartate antagonist amantadine in febrile catatonia. J Clin Psychopharmacol. 1999;19(5):484–6.
Schonfeldt-Lecuona C, Cronemeyer M, Hiesener L, Connemann BJ, Gahr M, Sartorius A, et al. Comparison of international guidelines with regard to the treatment of malignant catatonia. Pharmacopsychiatry. 2020;53(1):14–20.
Alfson ED, Awosika OO, Singhal T, Fricchione GL. Lysis of catatonic withdrawal by propofol in a bone-marrow transplant recipient with adenovirus limbic encephalitis. Psychosomatics. 2013;54(2):192–5.
Fox FL, Bostwick JM. Propofol sedation of refractory delirious mania. Psychosomatics. 1997;38(3):288–90.
Coffey MJ, Cooper JJ. Electroconvulsive therapy in anti-N-methyl-D-aspartate receptor encephalitis. J ECT. 2016;32(4):225–9.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mann, S.C., Caroff, S.N., Campbell, E.C. (2022). Malignant Catatonia. In: Frucht, S.J. (eds) Movement Disorder Emergencies. Current Clinical Neurology. Humana, Cham. https://doi.org/10.1007/978-3-030-75898-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-75898-1_7
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-75897-4
Online ISBN: 978-3-030-75898-1
eBook Packages: MedicineMedicine (R0)