Abstract
How cells respond to physical cues in order to meet and withstand the physical demands of their immediate surroundings has been of great interest for many years, with current efforts focused on mechanisms that transduce signals into gene expression. Pathways that mechano-regulate entry of transcription factors into the cell nucleus are emerging, and our most recent studies suggest that mechanical properties of the nucleus itself are actively controlled in response to matrix elasticity in mature, injured, and developing tissue. Here, we discuss the mechano-responsive properties of nuclei as determined by intermediate filament lamin proteins that line the inside of the nuclear envelope and that also impact transcription factor entry and broader epigenetic mechanisms. We summarize signaling pathways that regulate lamin levels and decisions of cell fate in response to matrix mechanics combined with molecular cues. We also discuss recent work that highlights the importance of nuclear mechanics in niche anchorage and cell motility in development, hematopoietic differentiation, and cancer invasion whilst also emphasizing a role in protecting chromatin from stress-induced damage.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cell mechanics
- Mechanotransduction
- Extracellular matrix
- Nucleus
- Nucleoskeleton
- Proteostasis
- Lamina
- Differentiation
- Cancer
1 Introduction
Mature tissues need to be particularly resistant to the mechanical demands of an active life. Our bones, cartilage, skeletal muscle, and heart tissues are stiff, making them robust to routine physical exertion such as walking or running when they are subjected to high-frequency shocks, stresses, and strains. With every heartbeat, the left ventricular wall experiences a 20 % radial strain (Aletras et al. 1999), and local strains of ~20 % also occur in the cartilage of knee-joints with every step (Guilak et al. 1995). Tissue-level deformations might even be amplified within cells and their nuclei (Henderson et al. 2013). Our softer tissues have less need for robustness because their function does not require them to bear load. Furthermore, some of our softest tissues, such as brain and marrow, are protected from an otherwise hard world by our bones. Nonetheless, when soft tissues are subjected to impact, such as a collision of heads in American football or rugby, occurrences of rapid straining can cause lasting damage (Viano et al. 2005).
We have recently sought to characterize the composition of cells and extracellular matrix (ECM) in tissues of increasing stiffness, and by implication, in tissues that are subjected to the greatest stress (Swift et al. 2013a, b). A close correlation between the concentration of ECM components and bulk tissue elasticity was discovered. More surprisingly, we also discovered a systematic scaling between tissue elasticity and concentration of lamins in the nucleoskeleton that was partially recapitulated in cultured cell systems. Corresponding changes in the mechanical properties of the nuclei suggest that this response may act to protect the precious chromatin cargo of the nucleus from shocks that are transmitted through the surroundings, across the cytoskeleton, and into the nucleus. An active regulation of cell or matrix composition in response to the environment implies feedback into pathways of protein turnover and remodeling, or control of the rate of new protein production. Responsive matching of mechanical properties to physical demands has classically been described as a “mechanostat” in the context of bone regulation (Frost 1987), but a recent explosion in mechanobiological studies has uncovered a host of other mechanically sensitive cellular phenomena, including contraction (Discher et al. 2005), migration (Hadjipanayi et al. 2009a, b; Winer et al. 2009), proliferation (Lo et al. 2000; Hadjipanayi et al. 2009a, b; Klein et al. 2009), differentiation (Engler et al. 2004, 2006), and apoptosis (Wang et al. 2000). Despite the recent progress, questions of how mechanical signals are transduced into specific transcriptional or regulatory pathways continue to challenge the field.
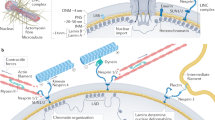
The lamina is a network structure formed from intermediate filament (IF) lamin proteins that lies inside the nuclear envelope and interacts with both chromatin and the cytoskeleton (Fig. 9.1a). In the somatic cells of humans, mice, and most vertebrates, the major forms of lamin protein are expressed from three genes: lamins -A and -C are alternative splicing products of the LMNA gene (collectively “A-type” lamins); lamins -B1 and -B2 are encoded by LMNB1 and LMNB2 genes, respectively (“B-type” lamins). The lamins share structural features, and indeed have some commonality in amino acid sequence, but differ in their posttranslational modification, with B-type lamins permanently appended by a farnesyl group that is cleaved from mature lamin-A (reviewed by Dechat et al. 2010). Like other IFs , such as keratin and vimentin, the lamins form coiled-coil parallel dimers that assemble into higher-order filamentous structures which fulfill important structural roles (Herrmann et al. 2009).
Scaling of matrix and lamin in mature tissue and during development. (a) A-type and B-type lamins form juxtaposed networks on the inside of the nuclear envelope; they are effectively located at an interface between chromatin and the cytoskeleton, to which the lamina is attached through the “LINC” (linker of nucleo- and cytoskeleton) complex. “A-type lamins,” lamins -A and -C are alternative spliceoform products of the LMNA gene; “B-type lamins,” lamins -B1 and -B2 are protein products of LMNB1 and LMNB2, respectively (adapted from ©Buxboim et al. 2010, originally published in The Journal of Cell Science). ((b)—left) The quantity of collagen-1 present in tissues scales with tissue micro-elasticity (Swift et al. 2013a, b). As collagen is one of the most prevalent proteins in the body, it is perhaps expected that it defines mechanical properties. ((b)—right) The composition of the nuclear lamina scales with tissue microelasticity. A-type lamins dominated the lamina in stiff tissue, whereas B-type lamins are prevalent in soft tissue (Swift et al. 2013a, b). ((c)—left) Observations made in adult tissue were also reflected in developing chick: the embryonic disc was initially soft, but divergent tissues either remained soft, such as brain, or became increasingly stiff, such as heart. Inset: developing chick hearts were probed by micropipette aspiration to determine micro-elasticity. ((c)—center) Tissue stiffening during development is accompanied by increased levels of collagen and A-type lamins (Lehner et al. 1987; Majkut et al. 2013). ((c)—right) Embryonic stem cells initially have negligible quantities of A-type lamins, but these levels increase as the nucleus stiffens during lineage commitment (Pajerowski et al. 2007)
Here we aim to summarize recent efforts to characterize the proteins that vary systematically with tissue stiffness. The effects of the composition of lamina on nuclear mechanical properties will be elaborated in detail, and we will consider the functions of the lamina in transducing mechanical signals from matrix and surroundings into cellular response, both in terms of an active regulation of the lamina itself and its broader role as a linkage in mechanotransduction pathways . Although we focus on a primarily protective purpose of lamin in the nucleus, there are additional regulatory consequences of such a stiff and bulky organelle, and we will summarize recent evidence that such properties limit the freedom of cells to move through tissue. The proximity of the lamina to heterochromatin within the nucleus has led it to be widely associated with epigenetic regulation (e.g., Kim et al. 2011; Meuleman et al. 2013). This review will seek to highlight the pervasive influence of the mechanical role of the lamina and hence proposes that lamin acts as both guardian and gatekeeper for chromatin .
2 Scaling of ECM and Lamina Components in Mature and Developing Tissue
Collagens and other protein constituents of the ECM are the most prevalent proteins in our bodies, largely determining the mechanical properties of tissue. Collagens are found at higher levels in stiff, mature tissues where, consistent with an expectation for proteins to behave as “biological polymers” (Gardel et al. 2004), their increased concentration is the basis of tissue elasticity (Fig. 9.1b—left). By using quantitative label-free mass spectrometry (MS) for proteomic profiling (Swift et al. 2013a, b), we have shown that collagens and other ECM-associated proteins scale with tissue elasticity (Swift et al. 2013a, b). MS was also used to quantify roughly 100 of the most abundant proteins in the cytoskeleton and nucleus, and we found the strongest correlation with bulk tissue elasticity in the composition of the nuclear lamina (Fig. 9.1b—right). Although primarily characterized by the ratio between the two main families of lamins, A-type and B-type, the compositional scaling is dominated by a 30-fold increase in the concentration of lamin-A, C from brain to bone. Although our recent observations are broadly in agreement with an extensive literature in lamin quantification (e.g., Krohne et al. 1981; Rober et al. 1990; Cance et al. 1992; Broers et al. 1997), they provide a new perspective on systematic variations across many tissues.
The relationship between tissue stiffness, ECM , and lamina during development was also determined; micropipette aspiration of embryonic chick tissue showed that the homogeneous embryonic disc is initially very soft, with proteomic profiles indicating correspondingly low levels of collagen (Fig. 9.1c—left and center). However, the properties of different tissues diverge during development with the brain remaining soft, whereas the heart stiffens as ECM proteins are deposited (Majkut et al. 2013). Cells in stiffening tissues, such as heart, are also likely to have respectively higher levels of lamin-A,C (Lehner et al. 1987). Nuclei in embryonic stem cells have indeed been shown to be very soft and to have low levels of lamin-A,C (Pajerowski et al. 2007; Eckersley-Maslin et al. 2013). As these cells commit to a lineage-specific fate, the levels of lamin-A,C increase and the nucleus becomes correspondingly stiffer (Fig. 9.1c—right).
Importantly, despite an apparent role in amplifying decisions of animal cell fate in conjunction with matrix elasticity, lamin-A,C is not essential to development as knockout mice still form all tissues (Sullivan et al. 1999). Likewise, lamin-B knockout mice survive embryogenesis (Kim et al. 2011). The most critical role of lamin may therefore be to tune the properties and regulation of maturing tissues in higher organisms, and its absence can perhaps be compensated for during development. However, the distinction here may be blurred: there is still a need to understand nuclear structure during some stages of development, such as during cell migration; and processes of trafficking and differentiation continue throughout an organism’s life span. Nonetheless, there appears to be consistency with the current notion that lamins are not expressed in yeast and plants (Dittmer and Misteli 2011), despite the latter possessing genomes that are larger and more complex than those in animals. It seems very likely that the hard cell walls of these organisms protect the chromatin in ways that are not possible for animal cells with soft cell membranes . Cell biologists could thus benefit from thinking about such physical properties that of course fit within a structure-function paradigm.
3 The Influence of Lamina Composition on the Mechanical Properties of the Nucleus
Micropipette aspiration experiments have enabled the detailed study of nuclear mechanical properties by measuring the rate of deformation under pressure (Fig. 9.2a, b; Dahl et al. 2005; Pajerowski et al. 2007). By examining nuclei with different lamina compositions, it is thus possible to approximate the characteristic contributions to nuclear mechanical properties from A-type and B-type lamins (Fig. 9.2c; Shin et al. 2013; Swift et al. 2013a, b; Harada et al. 2014). The nuclear response in deformation is a combination of elastic (spring-like) and viscous (liquid-like, flowing) properties, with lamin-B’s contributing primarily to the elastic response and lamin-A,C contributing viscosity. Thus the difference between a nucleus stoichiometrically dominated by A-type vs. B-type lamins might be akin to comparing a balloon filled with honey to one filled with water. The importance of A-type lamin in maintaining nuclear structural integrity and cell viability has been appreciated for many years (e.g., Broers et al. 2004; Lammerding et al. 2006), and its influence on nuclear viscosity has been more recently demonstrated in studies where nuclei are deformed during migration through microfluidic circuits (Rowat et al. 2013) or transwell pores (discussed later, Shin et al. 2013; Harada et al. 2014).
The mechanical role of lamin in the nucleus. (a) Deformations applied by micropipette aspiration were used to quantify nuclear compliance (effectively a measure of “softness”; the inverse of stiffness) in a range of nuclei with altered lamina compositions (for example, by overexpressing a GFP-lamin-A fusion construct). Compliance can be calculated over a range of deformation timescales as a function of the micropipette diameter, the extent of deformation (L), and the applied pressure (ΔP). (b) When a constant deforming pressure was delivered by micropipette over timescales on the order of seconds, nuclei with low LMNA were found to be more compliant than those with high LMNA. (c) The mechanical properties of the lamina can be considered as a combination of elastic (spring-like) and viscous (flowing) properties, which together define the “deformation response time,” the timescale over which the nuclear shape deforms under force. Nuclei with greater quantities of A-type lamins relative to B-type lamins were found to deform more slowly under stress (Swift et al. 2013a, b)
“Laminopathies ” are a family of diseases that are caused by mutations in lamin-A,C (reviewed, for example, by Butin-Israeli et al. 2012; Worman 2012). These disorders include muscular dystrophies (Bonne et al. 1999), cardiomyopathies (Fatkin et al. 1999), lipodystrophies (Hegele et al. 2000; Shackleton et al. 2000; Speckman et al. 2000), and premature aging (“progeria,” Merideth et al. 2008). Indeed, one of the confounding aspects of lamin-related disease is how such a widely expressed protein can cause tissue-specific symptoms. Whilst much work remains to be done to resolve this question, it is broadly consistent that laminopathies cause defects in tissues where lamin-A,C is the dominant lamin in the nucleus, i.e., bone, heart, muscle, and fat (although there are exceptions: Charcot-Marie-Tooth disorder affects the nervous system, De Sandre-Giovannoli et al. 2002). Mouse models of lamin-A,C knockout have defects in muscle and connective tissue and typically die several weeks after birth from heart failure (Sullivan et al. 1999; Kubben et al. 2011; Jahn et al. 2012). Despite the apparently constitutive expression of B-type lamins in tissue, mouse models with lamin-B1 and -B2 ablation progress through embryogenesis with eventual death due to defects in brain development (Coffinier et al. 2011; Kim et al. 2011).
4 Mechanisms of Lamin Regulation
Earlier discussion has posited that lamins are closely regulated to match the mechanical properties of the nucleus with the physical demands of tissue. In addition to being set by the epigenetic programming required to make a given tissue or organ, it is also important that protein levels vary in response to feedback from their surroundings. Even within bulk tissues, mechanical loading can cause inhomogeneous straining (for example, in human articular cartilage, meniscus, and ligaments, Chan and Neu 2012), making it beneficial to have mechanisms of lamin regulation at the local, individual cell level.
The many mechanisms by which the level of lamin-A,C can be regulated are summarized in Fig. 9.3a. We showed that the transcript and protein levels of lamin-A,C are highly correlated in tissue (Swift et al. 2013a, b), suggestive of a tight regulatory feedback. A recent study of the proteome and transcriptome in mouse fibroblasts suggested that there are around ten million copies of lamin-A,C protein per cell—accounting for about 0.7 % of cellular protein mass—and around 200 copies of the LMNA transcript (Schwanhausser et al. 2011), which seems similar to single cell measurements (Dingal et al. 2015). Half-life in the cell on rigid plastic dishes was found to be around 4 days for the protein and about 20 h for the mRNA, both slightly higher than the cellular average for all proteins and genes (Schwanhausser et al. 2011). Measurements made on proteins in a human lung cancer cell line showed the half-life of lamin-A,C to be around 12 h, roughly in the middle of the span of protein-half lives recorded in the study (Eden et al. 2011). DNA methylation is known to be an epigenetic mechanism by which gene activity can be regulated, but was discounted as the foremost means of controlling LMNA levels: no consistent changes were observed in the methylation of the LMNA promoter in a range of cell lines known to express different levels of lamin-A,C protein (Swift et al. 2013 b), or in tissues from patients with laminopathic disorders (Cortese et al. 2007). LMNA transcription has been reported to be controlled by transcription factors of the retinoic acid (RA) receptor (RAR and RXR family proteins, Olins et al. 2001; Okumura et al. 2004a, b; Shin et al. 2013; Swift et al. 2013a, b), with the resulting mRNA alternatively spliced to give the lamin-A and truncated -C forms. Soft tissue generally favors the lamin-C spliceoform (Swift et al. 2013a, b), and in brain the micro interfering-RNA MIR-9 specifically targets and deactivates the mRNA of the lamin-A spliceoform (Jung et al. 2012, 2013).
Protein regulation as a function of stress. (a) Schematic showing the factors that can regulate the levels of lamin-A,C in the cell: LMNA transcription is promoted by retinoic acid binding factors (Olins et al. 2001; Okumura et al. 2004a, b). The transcript is alternatively spliced to give rise to lamin-A and -C forms. In some tissues such as brain the -A form is suppressed through micro interfering RNA (Jung et al. 2012). Mature lamin-A (following posttranslational processing) and lamin-C assemble into the nuclear lamina, although some protein remains mobile in the nucleoplasm (Shimi et al. 2008). Phosphorylation leads to increased solubility, and may precede enzymatic protein turnover. Further stress-dependent pathways have been reported: stress on the nucleus causes unfolding of the Ig-domain of lamin-A,C and phosphorylation is suppressed under tension (Swift et al. 2013a, b). Laminopathic nuclei have been shown to have transient membrane defects that allow ingress of transcription factors (De Vos et al. 2011). (b) The nuclei of MSCs cultured on soft substrate were wrinkled, whereas those in cells on stiff substrate had a smooth, stretched appearance suggestive of greater tension. We have shown that lamin-A,C is less phosphorylated under tension (Swift et al. 2013a, b). By concentrating on one of the matrix-stiffness-regulated phosphorylation sites, we confirmed that lamin-A,C is rapidly phosphorylated with reduced cytoskeleton tension and phosphorylation leads to nuclear softening and lamin-A,C turnover (Buxboim et al. 2014)
5 Stress-Responsive Regulation of Lamin: “Use It or Lose It”
To better understand how lamin proteins are actively regulated in response to stress, mesenchymal stem cells (MSCs) were cultured on collagen-1 coated polyacrylamide hydrogels with stiffnesses that set to mimic the ECM of either brain (0.3 kPa) or pre-calcified bone (40 kPa) (Buxboim et al. 2010; Swift et al. 2013b). Images of the cultured MSCs showed that the nuclear envelopes of cells on soft matrix are wrinkled and relaxed, whereas, on stiff matrix, the nuclei are flattened by stress fibers and appear taut and smooth (Fig. 9.3b—left). Accompanying proteomic analyses revealed that, on stiff matrix, the conformation of lamin-A,C protein is maintained, the total quantity is upregulated, and the extent of phosphorylation at four sites is decreased. Phosphorylation is recognized as a key mechanism for modulating the solubility, conformation, and organization of IF proteins (Omary et al. 2006), and indeed lamins are highly phosphorylated during normal mitosis, driving disassembly of the lamina in preparation for chromosomal separation (Gerace and Blobel 1980; Heald and McKeon 1990). Thus the response we observe from matrix-induced stress is the converse of this process, with decreased phosphorylation acting to decrease lamin-A,C solubility and thereby strengthening the lamina. On soft matrix, lamin-A,C is more extensively phosphorylated, more mobile, and so, more susceptible to turnover (Buxboim et al. 2014). These observations hence point to a “use it or lose it” dynamic, whereby inessential lamin-A,C is eventually degraded. Lamin-A,C level has been reported to drive the translocation of the lamin-promoting transcription factor retinoic acid receptor gamma (RARG) to the nucleus, pointing to a feedback mechanism by which lamin protein levels promote their own transcription (see gene circuit in Swift et al. 2013a, b).
6 Mechanotransduction to the Nucleus: Downstream of Matrix and Lamin
Lamin is a key component in a system of protein linkages that allow the transmission of signals from a cell’s surroundings into the transcriptional machinery of its nucleus (Fig. 9.1a and discussed in recent reviews, e.g., Simon and Wilson 2011; Gundersen and Worman 2013; Rothballer and Kutay 2013; Sosa et al. 2013). Cell–cell interactions link to the cytoskeleton through tight and adherens junctions that tether to actin, and desmosome complexes that interact with cytoplasmic IFs such as keratin (Jamora and Fuchs 2002). Cell–matrix interactions are mediated by integrins and focal adhesion complexes that bind to cytoplasmic actin (Puklin-Faucher and Sheetz 2009; Watt and Huck 2013). The appropriately named LINC complex (“linker of nucleo- and cytoskeleton”) acts as an intermediary between cytoplasmic and nuclear structural proteins : F-actin binds to the nuclear envelope components nesprins -1 and -2, and IFs bind to the desmosome protein plectin, which in turn binds nesprin-3. Nesprins can also interact with kinesin and dynein complexes to tether to the microtubule network; Nesprins bind the SUN domain-containing family of inner nuclear membrane proteins and these in turn bind to the lamina on the inside of the nuclear envelope. Current problems for progress on understanding the roles of Nesprins are that there are few if any good antibodies to Nesprins and there are many spliceforms of Nesprins.
Lamin interactions within the nucleus are highly promiscuous (Wilson and Berk 2010; Wilson and Foisner 2010); as emphasized by Wilson and Berk in their review: “almost all characterized [inner nuclear membrane] proteins bind to A- or B-type lamins (or both) directly.” These interactions include binding to structural proteins, like actin (Simon et al. 2010), and a range of proteins that bind to the nuclear membrane, including emerin, barrier-to-autointegration factor (BAF, de Oca et al. 2009), lamina-associated polypeptide 2 (LAP2), and lamin-B receptor (LBR, Solovei et al. 2013). Of these, emerin has attracted considerable recent interest for its roles in mediating changes in the stiffness of isolated nuclei in response to tension applied to nesprin-1 (Guilluy et al. 2014), and in mechanosensing by affecting the translocation of transcription factor MKL1 (Ho et al. 2013). Furthermore, some transcription factors such as Oct-1 interact with the lamina directly (Malhas et al. 2009). Many lamin-binding proteins also interact with chromatin, particularly in its silenced heterochromatin form (Wagner and Krohne 2007), and indeed the lamins have been shown to bind DNA directly (Shoeman and Traub 1990; Luderus et al. 1992; Stierle et al. 2003). This chain of interactions thus completes a continuous physical linkage through which deformations can be transmitted from the cell exterior to chromatin (Maniotis et al. 1997). What is missing from this picture, however, is how blunt inputs—forces and perturbations acting without microscopic coherence—can be converted from mechanical to biochemical signals to activate individual genes at precise spatial locations within the nucleus. Perhaps specificity can be delivered through changes in binding, local concentration, conformation, and modification of cofactors or transcription factors.
As described above, mechanical cues from outside the cell alter protein conformations, protein modifications, and protein levels—all of which can broadly affect cell morphology and function. It is therefore of particular interest to understand the multiplicity of mechanisms that likely underlie how external factors induce stem cell lineage , with far-reaching implications for therapeutics and regenerative medicine. Populations of MSCs can be expanded in culture in a relatively naïve undifferentiated state, but they can certainly differentiate into multiple mesenchymal lineages, including fat, cartilage, muscle, and bone, dependent on external cues, such as the presence of nutrients, growth factors and cytokines, cell density, spatial constraints and mechanical forces (Pittenger et al. 1999). Cell shape influences cell fate through modulation of the activity of the small GTPase RhoA, with round cells favoring adipogenesis and well-spread cells favoring osteogenic lineage (McBeath et al. 2004). RhoA drives commitment to lineage in conjunction with its effector Rho-associated protein kinase (ROCK) through its regulation of nonmuscle myosin-II that controls cytoskeletal tension.
Although the focus of this review on the nucleus limits a deeper discussion of nonmuscle myosin-II, at least two points should be made. Knockout mice that completely lack nonmuscle myosin-IIA (MYH9) die at such an early embryonic stage that they exhibit little to no differentiation: no heart and no vasculature (Conti et al. 2004). This myosin-II isoform tends to be the dominant and early form of nonmuscle myosin-II isoforms in many tissues. Nonetheless, heterozygous mutations in human MYH9 are common and exhibit weak dominant negative effects on the wild-type protein from the normal allele (Spinler et al. 2015), which strongly suggests that even less than half of nonmuscle myosin-IIA is sufficient for near-normal differentiation in most tissues.
Cell fate can be influenced by matrix stiffness (Engler et al. 2006), and we have recently shown that this effect can be modulated by the nuclear lamina (Fig. 9.4a; Swift et al. 2013a, b). MSCs cultured on soft hydrogel substrates favor adipogenesis, but the extent of adipogenesis, as determined by oil red staining of lipid droplets, is double or more when combined with lamin-A,C knockdown. Likewise, stiff matrix induction of osteogenesis is greatly increased by lamin-A overexpression . Stiff matrix has been found to drive the nuclear translocation of the transcription factors RARG (Fig. 9.4a; Swift et al. 2013a, b) and yes-associated protein 1 (YAP1) (Dupont et al. 2011). RARG directly regulates lamin-A,C as part of differentiation and regulation of the serum response factor (SRF) pathway that amplifies levels of cytoskeletal components such as nonmuscle myosin-IIA (Fig. 9.4a; Swift et al. 2013a, b). Findings with MSCs on stiff matrix indeed are consistent with greater SRF activity in epithelial cells (Connelly et al. 2010; Ho et al. 2013). NKX2.5 is yet another transcription factor that is matrix elasticity sensitive, but it accumulates in the nucleus of MSCs on soft matrix and represses expression of at least one tension stabilizing protein, smooth muscle actin (SMA) (Dingal et al. 2015). Nuclear translocation of transcriptional regulators in response to matrix mechanics is thus an emerging theme in mechanosensing. It has also been shown to occur upon transfer of ions and changes in osmotic pressure (Finan et al. 2009; Irianto et al. 2013; Kalinowski et al. 2013). Such translocation could be driven by a change in concentration of protein-binding sites (e.g., on lamin-A,C or emerin, Ho et al. 2013; Swift et al. 2013a, b) or conceivably by protein modifications (e.g., YAP1 nuclear localization can be mediated by phosphorylation, Murphy et al. 2014). Besides conventional transport through nuclear pores via nuclear localization sequences (NLS) (Dingal et al. 2015), transient breakdown of the nuclear envelope in cells with defects in the lamina, perhaps as a consequence of a reduced robustness to mechanical stress, has been shown to affect nuclear localization of transcription factors such as RelA (De Vos et al. 2011).
Decisions of cell fate downstream of lamin-A,C regulation. (a) MSCs cultured on soft and stiff substrates take on differing phenotypes and favor alternate cell fates (Engler et al. 2006). On soft substrate, MSCs exhibit small nuclear and cellular spread areas and the nuclear lamina is thinned by a stress-sensitive phosphorylation feedback mechanism (Fig. 9.3b—left; Swift et al. 2013a, b). The transcription factors RARG and YAP1 (YAP1, Dupont et al. 2011) remain in the cytoplasm, and adipogenic cell fate is preferred. On stiff substrate, cells spread extensively with nuclei that are pinned down by well developed stress fibers. Lamin-A,C is less phosphorylated under strain, thus strengthening the lamina; RARG also translocates to the nucleus, increasing LMNA transcription. Activity of the transcription factor SRF (downstream of lamin-A,C) increases expression of cytoskeletal components (Ho et al. 2013; Swift et al. 2013b). Under these conditions, YAP1 translocates to the nucleus and cells favor osteogenesis. On both soft and stiff substrates, the effects of matrix elasticity and lamin level cooperate to enhance differentiation: lamin-A,C knockdown on soft matrix leads to more adipogenesis; lamin-A overexpression on stiff matrix leads to more osteogenesis. (b) Transcriptional activity is believed to be regulated by conserved inter-chromatin contacts that give rise to the spatial ordering of chromosomes (chromosome territories shown here in different colors, Cremer and Cremer 2001). Lamin-A,C can interact with DNA directly (“lamina-chromatin contacts”) or through protein intermediaries (Simon and Wilson 2011), but could have additional regulatory roles by mechanically determining the extent and rate that the nucleus deforms under tension, a process that could lead to the formation of altered inter-chromatin and lamina-chromatin contacts
The ability of lamins and/or its binding partners to tether to DNA has lead to interest in its role in chromatin organization and regulation (Guelen et al. 2008; Kim et al. 2011; Zullo et al. 2012; Kind et al. 2013; Lund et al. 2013; Meuleman et al. 2013). Lamina-associated domains (LADs) located at the nuclear periphery have been thought to associate with low gene expression levels whereas actively transcribed euchromatin is usually found in the nuclear interior. This might contribute to “chromosome territories ” (Cremer and Cremer 2001; Iyer et al. 2012) and to transcriptional hotspots within specific locations (Fraser and Bickmore 2007). However, it has been recently determined that nuclear lamins are not required for LAD organization in embryonic stem cells (Amendola and van Steensel 2015). Chromatin and DNA are also generally considered to make negligible contributions to overall nuclear mechanics (Pajerowski et al. 2007; Guilluy et al. 2014) unless the nucleus is condensed (Pajerowski et al. 2007), although particular cases are emerging—for example, in ESCs passing though a metastable transitional state before differentiation—where the condensation state of chromatin can become mechanically significant (Pagliara et al. 2014). It is not yet fully understood which proteins could give rise to mechanically responsive, locally defined structures and organization within the nucleus, nor how a protein as ubiquitously expressed as lamin could play a part in such specificity. Knowledge in this field will continue to improve as new experiments and models emerge to study protein-mediated changes in chromatin organization in response to perturbation (Shivashankar 2011; Talwar et al. 2013). However, based on current work, we have hypothesized that the effect of lamins on nuclear mechanics could determine the sensitivity and timescale of nuclear reorganization in response to stress (Fig. 9.4b; Swift et al. 2013a, b).
7 Cell Migration Is Slowed by the Nuclear Stiffness Needed to Protect Chromatin
As the nucleus is generally the largest and stiffest organelle, it can be a limiting factor in the migration of a cell through the 3D matrix. This means that the mechanical properties of the nucleus can have regulatory roles in processes, such as development, wound healing, hematopoiesis, cancer metastasis, and others (Fig. 9.5a—left). Studies of migration through narrow pores that mimic those in tumor tissue and require the deformation of the nucleus demonstrated a dependence on lamina composition; migration was limited when lamin-A was overexpressed, but promoted by a ~50 % knockdown of the protein (Harada et al. 2014). However, a deeper knockdown to <10 % was found to cause apoptosis in migration through small pores, underscoring the importance of lamin in providing physical protection to the nucleus (Fig. 9.5a—center). Consistent with earlier observations that lamins -A and -B, respectively, contribute primarily viscous and elastic mechanical properties to nuclei (Fig. 9.2), nuclei in which high lamin-A,C levels dominated the mechanical characteristics were observed to recover slowly following deformation, maintaining an elongated morphology after emerging from the pores (Fig. 9.5a—top right image). In contrast, nuclei with dominant levels of elastic B-type lamins rapidly returned to their more spheroid pre-migratory shapes following deformation (Fig. 9.5a—bottom right image).
The influence of the mechanical properties of the nucleus on cell migration. ((a)—left) As the largest and stiffest organelle in the cell, the nucleus can act as an “anchor” and prevents cell movement through the matrix or into surrounding vasculature. ((a)—center) As a model of migration through matrix, cells are induced to pass through 3 μm pores, a diameter sufficiently small to require deformation of the nucleus (inset). Lamin-A overexpression inhibits migration, whereas knockdown increases migration, up to a point at which significant apoptosis is observed. Thus extremely low or high lamin-A,C levels are unfavorable for cell migration, an observation with potential impact on understanding of processes such as cell migration during development and cancer metastasis. ((a)—right) Lamin-A rich nuclei (top image) showed persistence of a sausage-like morphology upon emergence from the pores (yellow arrow), while lamin-B rich nuclei (bottom image) rapidly recovered their shape (Fig. 9.5a adapted from ©Harada et al. 2014. Originally published in The Journal of Cell Biology. doi: 10.1083/jcb.201308029). (b) Effect of lamina composition on nuclear deformability during hematopoiesis. Stem cells that are retained in the marrow niche have higher lamin levels than differentiated blood lineages (Shin et al. 2013). A downregulation of nuclear cytoskeletal components in granulocytes, for example, ostensibly makes the cells better suited for passage through narrow blood vessels, but the lack of nuclear stability may contribute to their relatively short circulation times (Olins et al. 2009)
Cell migration is an important part of the development process and it is possible that the elasticity imparted by lamin-B is needed to allow nuclei to recover from the deformation (typically elongation) that occurs during migration, perhaps explaining why the brain fails to develop in lamin-B knockout mice (Coffinier et al. 2011; Kim et al. 2011; Jung et al. 2013). Neutrophilic cells also have very low levels of nucleoskeletal proteins to allow their deformation as they squeeze into confined spaces (Olins et al. 2009; Rowat et al. 2013), and indeed the composition of the nuclear lamina is continuously regulated during hematopoiesis (Fig. 9.5b; Shin et al. 2013). We hypothesize that by downregulating components of the lamina, white blood cells compromise their robustness in favor of mobility, and that this contributes to the short lifetimes of many of these cells in circulation. Cancer metastasis is an equally complex process that depends on factors including matrix remodeling and nuclear deformability (Wolf et al. 2013; Harada et al. 2014). Other work has shown that myosin-II’s ability to deform the nucleus can be a decisive factor in limiting glioma migration into brain tissue (Beadle et al. 2008; Ivkovic et al. 2012), but cancer cells in general show no universal lamina phenotype (reviewed in Foster et al. 2010). Although low levels of lamin-A,C have been correlated with increased reoccurrence of colon cancers (Belt et al. 2011), lamin-A,C was found to be upregulated in certain skin and ovarian cancers (Tilli et al. 2003; Hudson et al. 2007) and higher lamin-A,C expression was associated with better clinical outcomes in breast cancer (Wazir et al. 2013). Our own studies of tumor expansion in mouse flank have associated moderately lower levels of lamin-A,C with an increased invasiveness into the surrounding tissue, but a more complex dependence of the level of lamin-A,C with clinical prognosis might be explained by the tenuous balance between the effect of the lamina on nuclear deformability compared with that on cell survival.
8 Lamins in Cancer
Many studies have shown that lamin levels change in cancer of many organ types when compared to normal tissue (Table 9.1). Direct mechanistic links between lamins and cancer progression remain mysterious nonetheless. In cancer progression, a proliferation-competent cell acquires a cancer phenotype by either epigenetic changes (DNA methylation and histone modifications, Berdasco and Esteller 2010) or direct genomic changes (mutational, Salk et al. 2010) that lead to activation of oncogenes or inactivation of tumor suppressor genes (for review Hanahan and Weinberg 2011). Many in vitro studies have suggested a role for lamin-A in DNA damage response (Musich and Zou 2009; Mahen et al. 2013; Singh et al. 2013), but mice and humans with lamin-A deficiencies and defects are not reported to have an increased risk of cancer. Nonetheless, deep knockdown of lamin-A increases apoptosis after constrained migration through small matrix-like pores, consistent with increased DNA damage (Harada et al. 2014). The same study also showed migration—induced damage of the nuclear lamina, and nuclear ruptures have been observed in cancer cells (Vargas et al. 2012). These findings collectively suggest a protective role of lamins as an “armor” for guarding the genome . Further work is required to drill into the mechanistic link between lamins and cancer, which may lead to new treatments or at least a clearer basis for lamins as bio-marker in cancer progression.
9 Conclusions and Prospects
We have sought to outline the importance of nuclear mechanics in the context of tissue function, considering how it reflects the protective properties of the lamina, influences cell fate, and also regulates cell migration. In understanding that one of the key functions of the lamins is to ensure that the mechanical properties of the cell meets the demands of a tissue—either directly or by driving broader changes with regard to cell fate—lamins stress response factors. The response to cellular stress is classically thought in terms of how cells mitigate “heat-shock” that otherwise result in high levels of unfolded proteins (Hartl et al. 2011), but mechanical stress might also cause chromatin unfolding. Nonetheless, we are still a long way from understanding cellular protection mechanisms and how stress response pathways affect the regulation of structural features within the cell—motivating more work in nuclear biophysics.
References
Aletras AH, Ding SJ, Balaban RS, Wen H (1999) DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson 137(1):247–252
Amendola M, van Steensel B (2015) Nuclear lamins are not required for lamina-associated domain organization in mouse embryonic stem cells. EMBO Rep 16(5):610–617
Beadle C, Assanah MC, Monzo P, Vallee R, Rosenfeld SS, Canoll P (2008) The role of myosin-II in glioma invasion of the brain. Mol Biol Cell 19(8):3357–3368
Belt EJT, Fijneman RJA, van den Berg EG, Bril H, Delis-van Diemen PM, Tijssen M, van Essen HF, de lange-de Klerk ESM, Belien JAM, Stockmann HBAC, Meijer S, Meijer GA (2011) Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. Eur J Cancer 47(12):1837–1845
Berdasco M, Esteller M (2010) Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell 19(5):698–711
Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 21(3):285–288
Broers JLV, Machiels BM, Kuijpers HJH, Smedts F, van den Kieboom R, Raymond Y, Ramaekers FCS (1997) A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol 107(A1997XK99800009):505–517
Broers JLV, Peeters EAG, Kuijpers HJH, Endert J, Bouten CVC, Oomens CWJ, Baaijens FPT, Ramaekers FCS (2004) Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet 13(000224703900003):2567–2580
Butin-Israeli V, Adam SA, Goldman AE, Goldman RD (2012) Nuclear lamin functions and disease. Trends Genet 28(9):464–471
Buxboim A, Rajagopal K, Brown AEX, Discher DE (2010) How deeply cells feel: methods for thin gels. J Phys Condens Matter 22(19):194116
Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW, Discher DE (2014) Matrix elasticity regulates lamin-A, C phosphorylation and turnover with feedback to actomyosin. Curr Biol 24(16):1909–1917
Cance WG, Chaudhary N, Worman HJ, Blobel G, Cordoncardo C (1992) Expression of the nuclear lamins in normal and neoplastic human tissues. J Exp Clin Cancer Res 11(4):233–246
Chan DD, Neu CP (2012) Transient and microscale deformations and strains measured under exogenous loading by noninvasive magnetic resonance. PLoS One 7(3):8
Coffinier C, Jung HJ, Nobumori C, Chang S, Tu YP, Barnes RH, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, Fong LG, Young SG (2011) Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell 22(23):4683–4693
Connelly JT, Gautrot JE, Trappmann B, Tan DWM, Donati G, Huck WTS, Watt FM (2010) Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol 12(7):711–718
Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS (2004) Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem 279(40):41263–41266
Cortese R, Eckhardt F, Volleth M, Wehnert M, Koelsch U, Wieacker P, Brune T (2007) The retinol acid receptor B gene is hypermethylated in patients with familial partial lipodystrophy. J Mol Endocrinol 38(5–6):663–671
Cremer T, Cremer C (2001) Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2(4):292–301
Dahl KN, Engler AJ, Pajerowski JD, Discher DE (2005) Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J 89(4):2855–2864
de Oca RM, Shoemaker CJ, Gucek M, Cole RN, Wilson KL (2009) Barrier-to-autointegration factor proteome reveals chromatin-regulatory partners. PLoS One 4(9):15
De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat JM, Tazir M, Kassouri N, Szepetowski P, Hammadouche T, Vandenberghe A, Stewart CL, Grid D, Lévy N (2002) Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet 70(11799477):726–736
De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, Manders EMM, Verstraeten V, van Steensel MAM, Marcelis CLM, van den Wijngaard A, Vaux DJ, Ramaekers FCS, Broers JLV (2011) Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet 20(21):4175–4186
Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD (2010) Nuclear lamins. Cold Spring Harb Perspect Biol 2(20826548):a000547
Dingal PC, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE (2015) Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat Mater 14:951–960
Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143
Dittmer T, Misteli T (2011) The lamin protein family. Genome Biol 12(5):14
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474(7350):179–183
Eckersley-Maslin MA, Bergmann JH, Lazar Z, Spector DL (2013) Lamin A/C is expressed in pluripotent mouse embryonic stem cells. Nucleus 4(1):53–60
Eden E, Geva-Zatorsky N, Issaeva I, Cohen A, Dekel E, Danon T, Cohen L, Mayo A, Alon U (2011) Proteome half-life dynamics in living human cells. Science 331(6018):764–768
Engler AJ, Griffin MA, Sen S, Bonnetnann CG, Sweeney HL, Discher DE (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166(6):877–887
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Spudich S, De Girolami U, Seidman JG, Seidman CE, Muntoni F, Muehle G, Johnson W, McDonough B (1999) Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med 341(23):1715–1724
Finan JD, Chalut KJ, Wax A, Guilak F (2009) Nonlinear osmotic properties of the cell nucleus. Ann Biomed Eng 37(3):477–491
Foster CR, Przyborski SA, Wilson RG, Hutchison CJ (2010) Lamins as cancer biomarkers. Biochem Soc Trans 38(000274763800053):297–300
Fraser P, Bickmore W (2007) Nuclear organization of the genome and the potential for gene regulation. Nature 447(7143):413–417
Frost HM (1987) Bone mass and the mechanostat—a proposal. Anat Rec 219(1):1–9
Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA (2004) Elastic behavior of cross-linked and bundled actin networks. Science 304(5675):1301–1305
Gerace L, Blobel G (1980) Nuclear-envelope lamina is reversibly depolymerized during mitosis. Cell 19(1):277–287
Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453(7197):948–951
Guilak F, Ratcliffe A, Mow VC (1995) Chondrocyte deformation and local tissue strain in articular-cartilage—a confocal microscopy study. J Orthop Res 13(3):410–421
Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K (2014) Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol 16(4):376–381
Gundersen GG, Worman HJ (2013) Nuclear positioning. Cell 152(6):1376–1389
Hadjipanayi E, Mudera V, Brown RA (2009a) Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med 3(2):77–84
Hadjipanayi E, Mudera V, Brown RA (2009b) Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton 66(3):121–128
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Harada T, Swift J, Irianto J, Shin J-W, Spinler KR, Athirasala A, Diegmiller R, Dingal PCDP, Ivanovska IL, Discher DE (2014) Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol 204(5):669–682
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356):324–332
Heald R, McKeon F (1990) Mutations of phosphorylation sites in lamin-a that prevent nuclear lamina disassembly in mitosis. Cell 61(4):579–589
Hegele RA, Cao HN, Huff MW, Anderson CM (2000) LMNA R482Q mutation in partial lipodystrophy associated with reduced plasma leptin concentration. J Clin Endocrinol Metab 85(9):3089–3093
Henderson JT, Shannon G, Veress AI, Neu CP (2013) Direct measurement of intranuclear strain distributions and RNA synthesis in single cells embedded within native tissue. Biophys J 105(10):2252–2261
Herrmann H, Strelkov SV, Burkhard P, Aebi U (2009) Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Investig 119(7):1772–1783
Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J (2013) Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497(7450):507–511
Hudson ME, Pozdnyakova I, Haines K, Mor G, Snyder M (2007) Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc Natl Acad Sci U S A 104(44):17494–17499
Irianto J, Swift J, Martins RP, McPhail GD, Knight MM, Discher DE, Lee DA (2013) Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys J 104(4):759–769
Ivkovic S, Beadle C, Noticewala S, Massey SC, Swanson KR, Toro LN, Bresnick AR, Canoll P, Rosenfeld SS (2012) Direct inhibition of myosin II effectively blocks glioma invasion in the presence of multiple motogens. Mol Biol Cell 23(4):533–542
Iyer KV, Maharana S, Gupta S, Libchaber A, Tlusty T, Shivashankar GV (2012) Modeling and experimental methods to probe the link between global transcription and spatial organization of chromosomes. PLoS One 7(10):14
Jahn D, Schramm S, Schnolzer M, Heilmann CJ, de Koster CG, Schutz W, Benavente R, Alsheimer M (2012) A truncated lamin A in the Lmna(-/-) mouse line Implications for the understanding of laminopathies. Nucleus 3(5):463–474
Jamora C, Fuchs E (2002) Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol 4(4):E101–E108
Jung HJ, Coffinier C, Choe Y, Beigneux AP, Davies BSJ, Yang SH, Barnes RH, Hong J, Sun T, Pleasure SJ, Young SG, Fong LG (2012) Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc Natl Acad Sci U S A 109(7):E423–E431
Jung HJ, Lee JM, Yang SH, Young SG, Fong LG (2013) Nuclear lamins in the brain—new insights into function and regulation. Mol Neurobiol 47(1):290–301
Kalinowski A, Qin Z, Coffey K, Kodali R, Buehler MJ, Losche M, Dahl KN (2013) Calcium causes a conformational change in lamin A tail domain that promotes farnesyl-mediated membrane association. Biophys J 104(10):2246–2253
Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MSH, Zheng Y (2011) Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 334(6063):1706–1710
Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA, van Steensel B (2013) Single-cell dynamics of genome-nuclear lamina interactions. Cell 153(1):178–192
Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK (2009) Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 19(18):1511–1518
Krohne G, Dabauvalle MC, Franke WW (1981) Cell type-specific differences in protein-composition of nuclear-pore complex-lamina structures in oocytes and erythrocytes of xenopus-laevis. J Mol Biol 151(1):121–141
Kubben N, Voncken JW, Konings G, van Weeghel M, van den Hoogenhof MMG, Gijbels M, van Erk A, Schoonderwoerd K, van den Bosch B, Dahlmans V, Calis C, Houten SM, Misteli T, Pinto YM (2011) Post-natal myogenic and adipogenic developmental defects and metabolic impairment upon loss of A-type lamins. Nucleus 2(3):195–207
Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT (2006) Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem 281(35):25768–25780
Lehner CF, Stick R, Eppenberger HM, Nigg EA (1987) Differential expression of nuclear lamin proteins during chicken development. J Cell Biol 105(1):577–587
Lo CM, Wang HB, Dembo M, Wang YL (2000) Cell movement is guided by the rigidity of the substrate. Biophys J 79(1):144–152
Luderus MEE, Degraaf A, Mattia E, Denblaauwen JL, Grande MA, Dejong L, Vandriel R (1992) Binding of matrix attachment regions to lamin-B1. Cell 70(6):949–959
Lund E, Oldenburg AR, Delbarre E, Freberg CT, Duband-Goulet I, Eskeland R, Buendia B, Collas P (2013) Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome Res 23(10):1580–1589
Mahen R, Hattori H, Lee M, Sharma P, Jeyasekharan AD, Venkitaraman AR (2013) A-type lamins maintain the positional stability of DNA damage repair foci in mammalian nuclei. PLoS One 8(5), e61893
Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE (2013) Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol 23(23):2434–2439
Malhas AN, Lee CF, Vaux DJ (2009) Lamin B1 controls oxidative stress responses via Oct-1. J Cell Biol 184(1):45–55
Maniotis AJ, Chen CS, Ingber DE (1997) Demonstration of mechanical connections between integrins cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A 94(3):849–854
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6(4):483–495
Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith ACM, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon RO, Gahl WA, Introne WJ (2008) Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med 358(6):592–604
Meuleman W, Peric-Hupkes D, Kind J, Beaudry JB, Pagie L, Kellis M, Reinders M, Wessels L, van Steensel B (2013) Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res 23(2):270–280
Murphy AJ, Pierce J, de Caestecker C, Libes J, Neblett D, de Caestecker M, Perantoni AO, Tanigawa S, Anderson JR, Dome JS, Das A, Carroll TJ, Lovvorn HN (2014) Aberrant activation, nuclear localization, and phosphorylation of yes-associated protein-1 in the embryonic kidney and Wilms tumor. Pediatr Blood Cancer 61(2):198–205
Musich PR, Zou Y (2009) Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Aging (Albany NY) 1(1):28–37
Okumura K, Hosoe Y, Nakajima N (2004a) c-Jun and Sp1 family are critical for retinoic acid induction of the lamin A/C retinoic acid-responsive element. Biochem Biophys Res Commun 320(2):487–492
Okumura K, Hosoe Y, Nakajima N (2004b) Zic1 is a transcriptional repressor through the lamin A/C promoter and has an intrinsic repressive domain. J Health Sci 50(4):423–427
Olins AL, Herrmann H, Lichter P, Kratzmeier M, Doenecke D, Olins DE (2001) Nuclear envelope and chromatin compositional differences comparing undifferentiated and retinoic acid- and phorbol ester-treated HL-60 cells. Exp Cell Res 268(2):115–127
Olins AL, Hoang TV, Zwerger M, Herrmann H, Zentgraf H, Noegel AA, Karakesisoglou I, Hodzic D, Olins DE (2009) The LINC-less granulocyte nucleus. Eur J Cell Biol 88(19019491):203–214
Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J (2006) ‘Heads and tails’ of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci 31(7):383–394
Pagliara S, Franze K, McClain CR, Wylde GW, Fisher CL, Franklin RJM, Kabla AJ, Keyser UF, Chalut KJ (2014) Auxetic nuclei in embryonic stem cells exiting pluripotency. Nat Mater 13(6):638–644
Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE (2007) Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A 104(40):15619–15624
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Puklin-Faucher E, Sheetz MP (2009) The mechanical integrin cycle. J Cell Sci 122(2):179–186
Rober RA, Sauter H, Weber K, Osborn M (1990) Cells of the cellular immune and hematopoietic system of the mouse lack lamins A/C—distinction versus other somatic-cells. J Cell Sci 95:587–598
Rothballer A, Kutay U (2013) The diverse functional LINCs of the nuclear envelope to the cytoskeleton and chromatin. Chromosoma 122(5):415–429
Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J (2013) Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem 288(12):8610–8618
Salk JJ, Fox EJ, Loeb LA (2010) Mutational heterogeneity in human cancers: origin and consequences. Annu Rev Pathol 5:51–75
Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342
Shackleton S, Lloyd DJ, Jackson SNJ, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O’Rahilly S, Trembath RC (2000) LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 24(2):153–156
Shimi T, Pfleghaar K, Kojima S, Pack C, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, Goldman RD (2008) The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 22(000262110400006):3409–3421
Shin J-W, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE (2013) Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc Natl Acad Sci U S A 110(47):18892–18897
Shivashankar GV (2011) Mechanosignaling to the cell nucleus and gene regulation. Annu Rev Biophys 40:361–378
Shoeman RL, Traub P (1990) The in vitro DNA-binding properties of purified nuclear lamin proteins and vimentin. J Biol Chem 265(16):9055–9061
Simon DN, Wilson KL (2011) The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat Rev Mol Cell Biol 12(11):695–708
Simon DN, Zastrow MS, Wilson KL (2010) Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus 1(3):264–272
Singh M, Hunt CR, Pandita RK, Kumar R, Yang CR, Horikoshi N, Bachoo R, Serag S, Story MD, Shay JW, Powell SN, Gupta A, Jeffery J, Pandita S, Chen BP, Deckbar D, Lobrich M, Yang Q, Khanna KK, Worman HJ, Pandita TK (2013) Lamin A/C depletion enhances DNA damage-induced stalled replication fork arrest. Mol Cell Biol 33(6):1210–1222
Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, Herrmann H, Blum H, Engelkamp D, Stewart CL, Leonhardt H, Joffe B (2013) LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152(3):584–598
Sosa BA, Kutay U, Schwartz TU (2013) Structural insights into LINC complexes. Curr Opin Struct Biol 23(2):285–291
Speckman RA, Garg A, Du FH, Bennett L, Veile R, Arioglu E, Taylor SI, Lovett M, Bowcock AM (2000) Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet 66(4):1192–1198
Spinler KR, Shin JW, Lambert MP, Discher DE (2015) Myosin-II repression favors pre/proplatelets but shear activation generates platelets and fails in macrothrombocytopenia. Blood 125(3):525–533
Stierle VN, Couprie JL, Ostlund C, Krimm I, Zinn-Justin S, Hossenlopp P, Worman HJ, Courvalin JC, Duband-Goulet I (2003) The carboxyl-terminal region common to lamins A and C contains a DNA binding domain. Biochemistry 42(17):4819–4828
Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B (1999) Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 147(5):913–919
Swift J, Harada T, Buxboim A, Shin JW, Tang HY, Speicher DW, Discher DE (2013a) Label-free mass spectrometry exploits dozens of detected peptides to quantify lamins in wildtype and knockdown cells. Nucleus 4(6):450–459
Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, Rehfeldt F, Speicher DW, Discher DE (2013b) Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341(6149):1240104
Talwar S, Kumar A, Rao M, Menon GI, Shivashankar GV (2013) Correlated spatio-temporal fluctuations in chromatin compaction states characterize stem cells. Biophys J 104(3):553–564
Tilli C, Ramaekers FCS, Broers JLV, Hutchison CJ, Neumann HAM (2003) Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. Br J Dermatol 148(1):102–109
Vargas JD, Hatch EM, Anderson DJ, Hetzer MW (2012) Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 3(1):88–100
Viano DC, Casson IR, Pellman EJ, Zhang LY, King AI, Yang KH (2005) Concussion in professional football: brain responses by finite element analysis: part 9. Neurosurgery 57(5):891–915
Wagner N, Krohne G (2007) LEM-domain proteins: new insights into lamin-interacting proteins. Int Rev Cytol 261:1–46
Wang HB, Dembo M, Wang YL (2000) Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Phys Cell Phys 279(5):C1345–C1350
Watt FM, Huck WTS (2013) Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol 14(8):467–473
Wazir U, Ahmed MH, Bridger JM, Harvey A, Jiang WG, Sharma AK, Mokbel K (2013) The clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor mRNA expression in human breast cancer. Cell Mol Biol Lett 18(4):595–611
Wilson KL, Berk JM (2010) The nuclear envelope at a glance. J Cell Sci 123(12):1973–1978
Wilson KL, Foisner R (2010) Lamin-binding proteins. Cold Spring Harb Perspect Biol 2(4):17
Winer JP, Janmey PA, McCormick ME, Funaki M (2009) Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng A 15(1):147–154
Wolf K, te Lindert M, Krause M, Alexander S, te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P (2013) Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201(7):1069–1084
Worman HJ (2012) Nuclear lamins and laminopathies. J Pathol 226(2):316–325
Zullo JM, Demarco IA, Pique-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, Singh H (2012) DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 149(7):1474–1487
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 The American Physiological Society
About this chapter
Cite this chapter
Irianto, J., Ivanovska, I.L., Swift, J., Discher, D.E. (2016). The Nuclear Lamina: From Mechanosensing in Differentiation to Cancer Cell Migration. In: Chien, S., Engler, A., Wang, P. (eds) Molecular and Cellular Mechanobiology. Physiology in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-5617-3_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-5617-3_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-5615-9
Online ISBN: 978-1-4939-5617-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)