Abstract
In adult skeletal muscles, satellite cells are the primary myogenic stem cells involved in myogenesis. Normally, they remain in a quiescent state until activated by a stimulus, after which they proliferate, differentiate, and fuse into an existing myofiber or form a de novo myofiber. To study satellite cell dynamics in adult murine models, most studies utilize regeneration models in which the muscle is severely damaged and requires the participation from satellite cells in order to repair. Here, we describe a model to study satellite cell behavior in muscle hypertrophy that is independent of muscle regeneration.
Synergist ablation surgery involves the surgical removal of the gastrocnemius and soleus muscles resulting in functional overload of the remaining plantaris muscle. This functional overload results in myofiber hypertrophy, as well as the activation, proliferation, and fusion of satellite cells into the myofibers. Within 2 weeks of functional overload, satellite cell content increases approximately 275 %, an increase that is accompanied with a ~60 % increase in the number of myonuclei. Therefore, this can be used as an alternative model to study satellite cell behavior in adulthood that is different from regeneration, and capable of revealing new satellite cell functions in regulating muscle adaptation.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Adult skeletal muscles have the remarkable ability to fully regenerate following extensive injury through the participation of resident stem cells , making skeletal muscles an ideal model system to study stem cell behavior in an adult tissue. Of the multiple stem cells in skeletal muscle, satellite cells are the primary myogenic stem cell that gives rise to mature myofibers. In order to study satellite cell dynamics in adult animals, the majority of studies employ a regenerative model, typically induced by injection of a myotoxin or through a damaging injury, such as a crush or freeze [1]. While these models clearly result in the activation, proliferation and differentiation of satellite cells, they also produce extensive damage to many cellular structures, in addition to the myofibers. Thus, the relevance to human muscle adaptation, for example in response to exercise training, may be limited.
Here, we present a surgical model which results in the induction of the same myogenic program in satellite cells, without requiring extensive damage to the myofibers [2, 3]. Synergist ablation involves the surgical removal of synergist muscles thereby placing functional overload on the remaining plantaris muscle. Using this model, we see rapid expansion of the satellite cell pool within 7 days of functional overload, which further increases by 14 days and starts to decline by 56 days (Fig. 1). Furthermore, this model induces a 60 % increase in myonuclear number within 14 days of overload, which appears to be solely the result of satellite cells differentiating and then fusing to existing myofibers [3]. Therefore, this model can be used as an alternative method to study satellite cell behavior in adulthood that is distinct from regeneration , and thus, may involve different signaling events and/or molecular regulation.
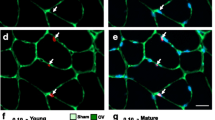
Synergist ablation results in a rapid expansion of the satellite cell pool. (a) Satellite cells were identified immunohistochemically on mouse skeletal muscle cross sections using an antibody against the satellite cell-specific transcription factor, paired box 7 (Pax7). White/red arrows indicate Pax7 + cells. Myofibers can be visualized using an antibody against laminin. Scale bar—100 μm. (b) Satellite cells proliferate within 7 days of functional overload , further increasing in number by 14 days of overload, and then gradually returning to baseline by 56 days of overload
2 Materials
2.1 Surgical Suite Setup (Fig. 2)
Example of a surgery suite for performing rodent surgeries. (a) Heated Plexiglas surgical table; (b) Nose cone attached to isoflurane via tubing; (c) Closed induction chamber attached to isoflurane; (d) Light source; (e) Digital temperature gauge with probe located on surgical table; (f) Isoflurane vaporizer; (g) Oxygen tank with associated regulator
-
1.
Fume hood.
-
2.
Oxygen take.
-
3.
Isoflurane.
-
4.
Isoflurane vaporizer machine.
-
5.
Heated Plexiglas surgery pad.
-
6.
Dissection microscope (optional).
-
7.
Light source.
-
8.
Tubing and plastic nose cone.
-
9.
Directional control valves.
-
10.
Digital temperature gauge with probe.
2.2 Animal Anesthetization, Analgesic and Restraint
-
1.
Artificial tears.
-
2.
70 % ethanol.
-
3.
Carprofen.
-
4.
27 G insulin syringe.
-
5.
Labeling tape.
2.3 Surgical Site Preparation
-
1.
Betadine surgical scrub.
-
2.
#3 scalpel handle.
-
3.
#11 scalpel blades.
-
4.
70 % ethanol.
2.4 Incision and Fascia Separation
-
1.
Fine curved forceps.
-
2.
Coarse forceps.
-
3.
Fine scissors.
-
4.
#3 scalpel handle.
-
5.
#11 scalpel blades.
-
6.
Surgical probe.
2.5 Separating and Cutting Away the Gastrocnemius/Soleus from the Plantaris
-
1.
Fine curved forceps.
-
2.
Surgical probe.
-
3.
Fine scissors.
-
4.
Cotton tip swabs.
-
5.
5-0 polypropylene surgical suture.
2.6 Contralateral Leg and Analgesic Administration
-
1.
Carprofen.
-
2.
27 G insulin syringe.
2.7 Tissue Collection and Processing
-
1.
Piece of scratch-resistant glass (10 in. by 10 in.).
-
2.
RNAse removal solution (optional).
3 Methods
3.1 Surgical Suite Setup
-
1.
Attach temperature probe to the top of the Plexiglas surgical table.
-
2.
Turn on heating pad located on the underside of the Plexiglas table. We found that pads designed for animal aquarium use work well, since they are designed to be mounted underneath a glass surface. Temperature should be between 25 and 27 °C at the time of surgery.
-
3.
Fill isoflurane vaporizer machine with isoflurane.
-
4.
Turn oxygen tank on and set to a flow rate of 0.5–2 L of O2 per minute.
-
5.
Set flow to the closed chamber. Turn on isoflurane vaporizer machine to deliver 3–4 % isoflurane at a flow rate of 0.8–1.0 L/min.
3.2 Animal Anesthetization, Analgesic and Restraint
-
1.
Using an insulin syringe, administer Carprofen (10 mg/kg) through an intraperitoneal (IP) injection 30 min prior to surgery.
-
2.
Immediately prior to surgery, wipe down surgical table with 70 % ethanol.
-
3.
Place animal in closed chamber connected to the isoflurane vaporizer machine.
-
4.
Once animal is sufficiently under anesthetic, quickly remove from box, apply artificial tears to the animals eyes to prevent their eyes from drying out, and immediately place face inside the nose cone.
-
5.
Reduce isoflurane to 1–3 % and set flow to the nose cone.
-
6.
Gently apply tape to the forepaws to avoid the animals head from dislodging from the nose cone during surgery (Fig. 3).
3.3 Surgical Site Preparation
-
1.
Apply 70 % ethanol to the posterior side of both hindlimbs.
-
2.
Using a #11 scalpel blade, gently shave hair away from the posterior side of both hindlimbs.
-
3.
Secure the first hindlimb by placing tape across the underside of the hindpaw, placing the leg in full plantar flexion.
-
4.
Apply Betadine in a circular motion from the inside to the outside of the site of incision followed by alcohol in a similar fashion. This should be repeated two times.
3.4 Incision and Fascia Separation
-
1.
Place the scalpel just medial to the great saphenous vein at the mid-belly of the gastrocnemius. Incision should run in between the medial and lateral tendons of the gastrocnemius (Fig. 4) (see Note 1 ).
-
2.
With constant pressure, make an incision down the length of the hindlimb stopping just proximal to the heel (see Notes 2 and 3 ).
-
3.
Use forceps to grab the skin and fascia on the medial side, while simultaneously using the surgical probe to gently separate the skin and fascia from the gastrocnemius.
-
4.
For the lateral side, place the probe on the lateral tendon of the gastrocnemius and gently position the probe between the lateral head of the gastrocnemius and the lateral hamstring (see Note 4 ).
3.5 Separating and Cutting Away the Gastrocnemius/Soleus from the Plantaris
-
1.
Use forceps to grab the gastrocnemius tendon and gently pull upwards.
-
2.
Pulling the gastrocnemius upwards should reveal the bifurcation between the gastrocnemius tendon and the plantaris tendon (Fig. 5a).
Fig. 5 Isolating the plantaris tendon and removing the gastrocnemius and soleus. (a) Gently pull up on the gastrocnemius tendons to identify the bifurcation from the plantaris tendon; (b) Place surgical probe in between plantaris and gastrocnemius/soleus tendon (note medial gastrocnemius tendon has been cut to expose plantaris tendon located underneath); (c) Surgical area after gastrocnemius and soleus have been removed; (d) Sutures closing the incision site
-
3.
From the medial side, insert the surgical probe between this bifurcation and gently run it up and down the distal 1/2 of the muscle to fully separate the gastrocnemius/soleus from the plantaris (Fig. 5b) (see Note 5 ).
-
4.
Once the probe is between the two sets of tendons, leave the probe in place and use a fine pair of scissors to cut the gastrocnemius/soleus tendon which is sitting above the probe.
-
5.
Take toothed-forceps and grab the severed gastrocnemius/soleus tendon and slowly and gently lift up and away from the plantaris.
-
6.
Cut the gastrocnemius/soleus complex at approximately mid-belly and remove from the hindlimb (Fig. 5c) (see Note 6 ).
-
7.
Bleeding should be minimal, however use cotton swabs to absorb any blood that may accumulate at the surgical site
-
8.
Depending on the amount of satellite cell activation and myofiber hypertrophy desired, additional variations to the surgical procedure can be employed (see Note 7 ).
-
9.
Use standard suturing procedures to place 3–4 sutures and close the incision (Fig. 5d) (see Note 8 ).
3.6 Contralateral Leg and Analgesic Administration
-
1.
Repeat steps of Subheadings 3.2–3.5 on the contralateral leg (see Note 9 for control surgery).
-
2.
As a postoperative analgesic Carprofen (10 mg/kg IP) or Meloxicam (2–5 mg/kg IP) should be given at 24 h and 48 h post-surgery.
3.7 Tissue Collection and Processing
-
1.
Following the desired length of functional overload , euthanize the animal according to the guidelines approved by the institution.
-
2.
Use 70 % ethanol to wet the fur and remove all fur and skin from the hindlimb.
-
3.
Dissect away both medial and lateral hamstrings from the plantaris and remaining proximal portion of the gastrocnemius.
-
4.
Remove as much of the residual gastrocnemius as possible while the plantaris is still attached to the hindlimb.
-
5.
Dissect away the plantaris at the furthest proximal origin and distal insertion points.
-
6.
Place plantaris on glass and use a scalpel to remove any remaining gastrocnemius muscle (see Notes 10 and 11 ).
-
7.
Finish processing tissue in accordance with downstream analyses.
4 Notes
-
1.
A dissection scope may be used, particularly during the learning phase, in order to aid in identification of anatomical landmarks. Modifications will be needed to be made to the surgical suite setup in order to accommodate the microscope, while still maintaining proper care of the animal.
-
2.
The larger the incision, the easier it will be to locate, cut, and remove the gastrocnemius and soleus. However, this will inevitability result in a greater healing response. As the surgeon becomes more proficient, a smaller incision can be made in order to minimize trauma and recovery.
-
3.
Careful consideration should be taken to make sure the incision reaches at least mid-way down the Achilles tendon. This will make identification and separation of the gastrocnemius/soleus tendon from the plantaris tendon much easier.
-
4.
If the incision was done correctly, the saphenous vein should be just lateral to the incision. It is important to make sure these vessels stay intact during the surgery. Using the probe, it is important to separate the lower hamstring which has the saphenous vein and nerves from the gastrocnemius muscle.
-
5.
The more distal along the tendon bifurcation that you insert your probe, the greater the likelihood that you will successfully remove the soleus in addition to the gastrocnemius. This is because the soleus tendon attaches into the gastrocnemius tendon more proximal on the Achilles tendon. Make sure to not probe underneath the saphenous vein, which should have been adequately separated away in the previous step.
-
6.
After removing the complex, closely examine the surgical site to make sure that no part of the soleus remains. Despite being careful, sometimes the probe will not get all the way under the soleus tendon when probing between the gastrocnemius/soleus tendon and plantaris tendon. If this occurs, you may not end up removing all of the soleus muscle. However, the presence of the remaining soleus should be easy to identify based on the dark red color of the soleus relative to the plantaris. To remove the rest of the soleus, gently guide the probe between the plantaris and soleus and cut the soleus away. At this point, removal of the soleus may be challenging; however, as long as it has been severed it should not affect the functional overload of the plantaris.
-
7.
Based on empirical evidence, the more gastrocnemius that is removed, the greater the mechanical load placed on the plantaris. Removing a large portion (i.e., >3/4) of the gastrocnemius may result in a regenerative response, in addition to hypertrophy of existing myofibers, characterized by the appearance of small, centrally nucleated myofibers. Removing only the lower 1/3 of the gastrocnemius should minimize the regenerative response. It is recognized that this surgical model results in a robust growth response, which may be undesirable for some studies. As an alternative, researchers may wish to leave the soleus intact resulting in a dual soleus/plantaris overload.
-
8.
Since the plantaris is now exposed, careful consideration should be taken to not accidentally damage it while suturing. The distal aspect of the incision can be the most challenging, since there is little excess skin to suture through. In this case, the surgeon should be careful to not accidentally suture through the plantaris tendon.
-
9.
We believe that in order to get maximal functional overload of the plantaris, surgical removal of the gastrocnemius/soleus should be performed on both legs. If one leg is left as a contralateral control, the animal with undoubtedly favor this leg and not put as much weight on the leg with which the surgery was performed. To address this, we always delegate animals to act as “sham” operated controls. For these animals, perform all of the procedures up until Subheading 3.4, step 4 (i.e., cutting the tendon) and then skip to Subheading 3.5 (suturing).
-
10.
Typically if the plantaris is overloaded for longer than 14 days, the small amount of gastrocnemius that remains will fuse to the proximal portion of the plantaris. It is important to remove this excess tissue as best as possible, particularly if the muscle will be homogenized for RNA or protein analysis. Since this excess gastrocnemius has not been subjected to mechanical overload, it will undoubtedly possess a different molecular signature from that of the plantaris.
-
11.
If tissue is to be used for RNA analyses, apply RNAse removal solution liberally to the glass surface to minimize potential RNA degradation.
References
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA (2014) Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28:1654–1665
McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA (2011) Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138:3657–3666
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Kirby, T.J., McCarthy, J.J., Peterson, C.A., Fry, C.S. (2016). Synergist Ablation as a Rodent Model to Study Satellite Cell Dynamics in Adult Skeletal Muscle. In: Kyba, M. (eds) Skeletal Muscle Regeneration in the Mouse. Methods in Molecular Biology, vol 1460. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3810-0_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3810-0_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3808-7
Online ISBN: 978-1-4939-3810-0
eBook Packages: Springer Protocols