Abstract
This chapter describes a method for GMP-compliant expansion of human mesenchymal stromal/stem cells (hMSC) from bone marrow aspirates, using the Quantum® Cell Expansion System from Terumo BCT. The Quantum system is a functionally closed, automated hollow fiber bioreactor system designed to reproducibly grow cells in either GMP or research laboratory environments. The chapter includes protocols for preparation of media, setup of the Quantum system, coating of the hollow fiber bioreactor, as well as loading, feeding, and harvesting of cells. We suggest a panel of quality controls for the starting material, the interim product, as well as the final product.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Bioreactor
- Functionally closed
- Hollow fiber
- Glucose consumption
- Lactate generation
- Mesenchymal stromal cells
- Mesenchymal stem cells
- MSC
- Platelet lysate
- GMP
- Clinical application
1 Introduction
Human MSC have emerged as attractive candidates for cell therapy, tissue repair, and tissue engineering. According to a position paper of the International Society for Cell Therapy (ISCT) [1], both mesenchymal stromal cells and mesenchymal stem cells are denominated as MSC. Human MSC (hMSC) are multipotent adult progenitor cells of perivascular origin [2] present in many tissues. hMSC can be isolated from a wide range of tissues, including the bone marrow (BM), adipose tissue, and cord blood , from each of which they are readily expandable. When maintained in vitro, hMSC are defined by plastic adherence, expression of a specific set of surface markers, and the ability to differentiate into osteoblasts, adipocytes, and chondroblasts under differentiation-inducing cell culture conditions [3]. Significant ex vivo expansion is strictly necessary due to the low frequency of primary MSC in human tissues and the relatively large size of therapeutic cell doses. Isolation and expansion of hMSC is typically performed using classic cell culture systems, e.g., CellSTACKs (Corning) or Cell Factory Systems (Nunc). While the large surfaces offered by these systems are advantageous for the growth of adherent hMSC, difficulty in handling rapidly increases with the increase in surface area. Accordingly, various bioreactor systems have emerged in the last years, including microcarrier-based systems [4, 5], cell-stack-based systems [6], and hollow-fiber-based systems [7–10]. This chapter describes a method for GMP-compliant expansion hMSC in one of these bioreactor systems, namely, the Quantum Cell Expansion System (Terumo BCT, Lakewood, USA).
1.1 Quantum ® Cell Expansion System
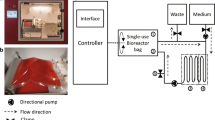
The Quantum system (Fig. 1a, b) is a functionally closed and automated bioreactor system, integrating incubation, fluid handling, and access to a gas supply, in addition to a touch screen interface for operation. A sterile, single-use disposable unit called the Quantum Cell Expansion Set (Fig. 2) is loaded onto the Quantum system to incorporate the hollow fiber bioreactor and fluid circuits, which will provide continuous exchange and circulation of media, as well as gas exchange. The bioreactor itself is comprised of ~11,500 hollow fibers with a total intracapillary (IC) surface area of 2.1 m2. Typical culture manipulations (e.g., cell seeding, media exchanges, trypsinization, cell harvest, etc.) are managed by the computer-controlled system, which synchronizes pumps and valves to achieve the designated task. All media and reagents are added to the system using sterile welding technology, thereby maintaining the functionally closed nature of the system. Moreover, the premixed gas supply enters the system through a sterile barrier filter, flows continually over circulating media within a hollow fiber gas transfer module, and leaves the system again via a sterile barrier filter (see Note 1 ). An external gas mixer may be used to supply individual gas mixtures, or gas may be supplied from a user-provided premixed gas tank which allows the user to expand cells at their optimal gas composition.
1.2 Quantum Cell Expansion System Hydraulics
The Quantum system fluid circuit is composed of two loops: the intracapillary (IC) loop and the extracapillary (EC) loop. The IC loop, which has a volume of 189 mL, is the fluid circuit including the inside of the hollow fibers. The EC loop, which has a volume of 305 mL, is the fluid circuit including the outside of the hollow fibers. A semipermeable membrane forms the hollow fibers, thus separating the IC and EC loops. Small molecules, such as glucose and dissolved gases, easily cross the membrane between the two loops. Large molecules, however, are sequestered on the side of the membrane in which they are added (or produced by the cells) (see Notes 2 and 3 ). For this reason, it is important to ensure that media and reagents supplying large molecules critical for the culture process (e.g., cytokines , growth factors , trypsin) are provided to the IC loop.
Five inlet lines allow for the connection of bags to the Quantum system for cell loading, media provision, and reagent addition. Though these inlet lines are named for ease of use (e.g., Cell, IC Media, Wash), the content of a bag connected to any inlet line is at the discretion of the operator. The operator can also choose to provide fluid from each of these lines to the IC loop, the EC loop, or both loops simultaneously. The fluid entering the system will necessarily displace the fluid present in the constant-volume system. This displaced fluid will exit through one of two outlets: the harvest line or the outlet line. Typically, the outlet line is used to collect process waste in a waste bag, while the harvest line is only used at the conclusion of the process to collect the harvested product.
1.3 Operation of the Quantum System
The Quantum process is analogous in many ways to a traditional cell culture process. The media (e.g., Alpha MEM plus 10 % PL), saline solution (e.g., PBS), and release agent (e.g., trypsin) can be the same (but GMP grade). Moreover, most of the process steps are logically consistent, e.g., seeding, feeding, and harvesting. However, routine operation of the Quantum system is significantly different than the repetitive flask-based or CellSTACK-based process. Instead, operation involves the following standard procedures:
-
1.
Operating ....................................................................................................3the touch screen interface
-
2.
Managing the cell expansion set
-
(a)
Loading the cell expansion set
-
(b)
Unloading the cell expansion set
-
(a)
-
3.
Filling bags:
-
(a)
Media bags
-
(b)
Cell inlet bags
-
(a)
-
4.
Connecting and disconnecting bags:
-
(a)
Use of a sterile welding device
-
(b)
Use of an RF (radio frequency) sealer
-
(a)
-
5.
Taking samples:
-
(a)
From the sample port
-
(b)
From the sampling coil
-
(a)
Each of these procedures is discussed in Subheading 3 and placed in the context of the overall protocol.
The Quantum system is operated via a touch screen located on the front of the system. Via this screen, operators can configure system settings, run predefined or custom tasks, and observe the live status of the system. The live status, located on the home screen, indicates all current settings (i.e., inlet sources, outlet line, pump rates, bioreactor motion, and stop condition), temperature of the incubator, and fluid pressures. Reports including this information, the time of all events, and the operator performing each event are also recorded for each run, enabling easier documentation and troubleshooting.
A “Task” button on the right of the touch screen provides access to a library of typical processes or tasks. Settings may be modified for all tasks, in order to meet the needs of a particular protocol. Additionally, custom tasks can be prepared to meet process needs that are not presently available in the predefined task library. Upon defining the task settings that are necessary for a particular protocol, the “Configuration” button, also on the right of the touch screen, provides the ability to save all settings.
2 Materials
Reagents and disposables should be GMP suitable, implying provision of certificates of analysis, certificates of release, certificates of origin, certification by qualified person, and/or certificates of compliance for each batch from the manufacturer, where applicable. The manufacturers should have a production license from the corresponding competent authority, an approval according to the Council Directive of the EC 93/42/EEC Annex II and/or Annex V, Article 3 concerning medical devices, and/or be certified or have an accreditation according to at least one of the following standards or directives by the corresponding competent authorities: good manufacturing practice (GMP), EN ISO 13485: 2003/AC, ISO 9001, DIN EN ISO 15189, DIN EN ISO/IEC 17025, and ISO 18001.
The materials listed in Subheadings 2.1–2.4 should be appropriate for GMP-grade isolation and expansion of hMSC as advanced therapy medicinal product (ATMP) for clinical trials.
2.1 Equipment
-
1.
Quantum system (Quantum® Cell Expansion System), Terumo BCT, Lakewood, USA.
-
2.
Pump or pump with pump head.
-
3.
Sealer.
-
4.
Sterile tubing welder.
-
5.
Scales.
-
6.
Lactate monitoring device.
-
7.
Mixed gas tanks.
-
8.
Plasma separator.
2.2 Reagents
-
1.
DMSO.
-
2.
Basic media as preferred by the user, e.g., Alpha MEM Eagle w/UGln1 and nucleosides or DMEM with 1 g/l glucose.
-
3.
DPBS 0.0095 M(PO4) w/o Ca and Mg.
-
4.
Platelet lysate (PL).
-
5.
TrypZean/EDTA solution.
-
6.
Fibronectin, 5 mg/5 mL, plasma derived, GMP quality, contract manufacturing.
-
7.
NaCl 0.9 % injectable solution.
-
8.
20 % HSA NaCl solution.
-
9.
Sodium heparin.
-
10.
Lactate test strips.
2.3 Disposables
-
1.
Syringes (3, 10, 20 mL).
-
2.
Sampling site coupler with needle injection site.
-
3.
CryoMACS Freezing Bag 50 mL.
-
4.
Transfer bag 600 mL.
-
5.
Cell inlet bag (CIB) 0.5 L, accessory set.
-
6.
Media bag 4 L, accessory set.
-
7.
Sampling coil, accessory set.
-
8.
Waste bag 4 L, accessory set.
-
9.
In-line filter 200 μm, accessory set.
-
10.
Cell expansion set (CES).
2.4 MSC Culture Media
Composition of complete media (see Notes 4 – 7 ): 90 % basic media supplemented 10 % of HPL and 1–2 IU/mL of heparin. Heparin should be added to the Alpha MEM or DMEM media first, preceding addition of the HPL.
3 Methods
3.1 Overview on the Expansion Process
The manufacture of MSC from BM is comprised of multiple expansion steps within the Quantum system. The first step includes both the isolation of hMSC from the whole bone marrow via adherence and the expansion (P0) of these primary hMSC (see Note 9 ). After harvest of the expanded hMSC, a portion of the pre-cultured hMSC is further expanded (P1) in a second bioreactor to generate a larger and more pure hMSC population.
Due to the functionally closed nature of the Quantum system, the system could potentially be used in lower-grade clean rooms (class C or D) than traditional cell culture processes, as per the discretion of local regulatory authorities. However, depending on the particular process design, preparation of cells, reagents, etc. may remain as open procedures, thereby requiring environments consistent with traditional cell culture procedures (e.g., a class A laminar air flow cabinet within a class B clean room environment).
Here we describe a protocol that is suitable for many autologous or small-scale allogeneic applications, from which other cell production applications can be easily derived. A few steps (see Note 10 ) remain as “open procedures” and are indicated accordingly.
Figures 3, 4, and 5 provide a visual summary of the overall Quantum protocol. At the top of the figure, all bags (i.e., both media bags and cell inlet bags) are illustrated, indicating their contents. The rows within the figure indicate the sequential steps of the process, highlighting the following information in the provided columns:
-
The name of the task, in addition to the buttons used to locate the task. For example, “Task >> Feed and Add” indicates that the Task button should be followed by the Feed and Add button, after which the Feed Cells task can be found.
-
A brief description of the task.
-
The approximate duration of the task.
-
An illustration of which bags are connected to each of the five inlet lines.
-
A list of notes pertinent to the task.
The remainder of this section explores the details underlying the protocol.
3.2 Tasks
Via the Quantum system touch screen (Fig. 1a, b), the user can select and modify specific tasks. A task can be considered as a programmed unit consisting of one or more consecutive steps, which are cumulatively intended to achieve a specific result (e.g., loading cells from the cell inlet bag into the bioreactor and distributing them). Each step is defined by the following settings: IC Inlet (i.e., which inlet line feeds the IC inlet pump), IC Inlet Rate, IC Circulation Rate, EC Inlet, EC Inlet Rate, EC Circulation Rate, Outlet, Rocker (i.e., stationary or in motion), and Stop Condition. Tasks are divided into seven categories: Set Management, System Management, Washout, Load and Attach, Feed and Add, Release and Harvest, and Custom.
The first six categories include tasks that are predefined as a part of the Quantum software, though the details of the tasks are flexible within the intended design of each task. The Custom category provides further flexibility when the designs of the predefined tasks do not suffice. Each of eight Custom tasks allows both maximum flexibility within each setting and the ability to add up to 99 steps.
3.3 Loading the Cell Expansion Set (CES)
The “Load Cell Expansion Set” task opens all valves and turns off all alarms to allow the operator to load the CES onto the device, integrating it with the pumps, valves, gas supply, and fluid sensors. The primary steps are as follows:
-
1.
Start task “Load Cell Expansion Set” which can be found in Set Management.
-
2.
Open the incubator door.
-
3.
Open the four pumps and the five external mounting clips.
-
4.
Remove the CES from the packaging, and align to place the tubing organizer on the mounting plate by aligning the hole of the center of the tubing organizer with the rocker arm on the mounting plate. Rest the bioreactor on the spill tray.
-
5.
Ensure that all five external mounting clips lock over the edges of the tubing organizer.
-
6.
Rotate the base of the mounting clips a quarter turn counterclockwise to secure the tubing organizer in the mounting plate.
-
7.
Ensure all tubes are pulled over the center of the corresponding pump rotors and attached behind the notch.
-
8.
Ensure that the tubing is centered over the pump rotor on all four pumps, and close the rotor cover and lock rotor latch on each of the four pumps.
-
9.
Load the EC inlet line into the EC fluid detector.
-
10.
Push the rocker assembly into the rocker arm completely so the bioreactor will be in the home position.
-
11.
Hang the waste and harvest bags on the bag pole.
-
12.
Insert the tubing guide onto the pegs.
-
13.
Remove the two blue and the one red caps from the CES.
-
14.
Connect the gas inlet line to the gas quick disconnect. You should hear a click sound when you connect it. Be sure the gas inlet line is behind the bioreactor and does not hinder the rocker motion.
-
15.
Ensure all lines are clear of the incubator door. Close the door.
-
16.
Touch finish to complete the task.
3.4 Prime Cell Expansion Set
After loading the CES, this task is used to fill the system with PBS, removing all air. To prime the CES, the following steps need to be performed (see Notes 6 and 8 ):
-
1.
Fill a media bag with PBS (see Subheading 3.5).
-
2.
Turn on the external gas supply.
-
3.
Attach the media bag containing the desired electrolyte solution to the cell line (see Subheading 3.7).
-
4.
Start task “Prime Cell Expansion Set” which is located in Set Management.
-
5.
Touch “Prime Cell Expansion Set,” and on the following Setup Screen, “Prime Cell Expansion Set” touch Start.
-
6.
Take a sample from the sample port to prime the filter (see Subheading 3.16).
-
7.
After you have finished sampling, touch “Finish” and afterward “Yes.”
3.5 Filling Media Bags
Media bags are used for large volume solutions up to 4 L, such as culture media or an electrolyte solution (see Notes 6 and 7 ) (e.g., phosphate-buffered saline or physiologic NaCl solution). Though the media bags are filled via a connected sterile barrier filter, this procedure is typically handled as an “open procedure” (see Notes 10 and 11 ). Complete the steps in this section to fill media bags:
-
1.
Open the pouch that contains the media bag and take it out.
-
2.
Remove the protective cover from the end of the tubing.
-
3.
Connect the end of the tubing to the fluid source; for example, place the tubing into the fluid container. Use an appropriate method to exclude contamination of the fluid.
-
4.
Load the white tube into the tubing pump.
-
5.
Ensure that the cap on the pressure relief valve on the filter is tight.
-
6.
Set the tubing pump to a low flow rate, such as 150 mL/min, to prime the filter. When priming the filter, hold it upright. After the filter cartridge is full and the fluid begins to flow into the bag, the flow rate may be increased up to a maximum of 500 mL/min.
-
7.
When the last of the fluid has been pumped from the fluid source:
-
(a)
Set the tubing pump to a low flow rate.
-
(b)
Slowly pump the fluid from the white tubing into the inlet of the filter.
-
(c)
Stop the pump.
-
(a)
-
8.
Use a tubing sealer to double seal the clear tubing. To ensure that you will have enough tubing to attach the bag to the CES using a sterile tubing welder, leave as much of the clear tubing as possible connected to the bag.
-
9.
Separate the media bag from the filter at the double seal.
-
10.
If the media bag will be used the same or the next day, leave it at room temperature. Store media bags in the dark.
3.6 Filling Cell Inlet Bags (CIBs)
CIBs are used for any small-volume solution less than 500 mL (e.g., BM, MSC suspension, or other reagents, such as TrypZean). Filling a CIB is an “open procedure” (see Note 10 ).
Complete the following steps to fill a CIB with the desired solution:
-
1.
Open the pouch that contains the CIB and take it out.
-
2.
Fill a syringe with as much culture media/vehicle solution as necessary to reach, together with the subsequently added liquid of interest, a final volume of 80–100 mL.
This step does not apply for TrypZean.
-
3.
Remove the blue cap from the luer of the CIB. Via luer, pre-fill the CIB with the culture media/vehicle solution from step 2.
-
4.
Transfer the liquid of interest via luer from the syringe(s) into the CIB. Remove the syringe.
-
5.
Via luer, push approx. 50 mL of air from a syringe into the CIB.
3.7 Loading Media Bags
Media bags are used to load cell culture media or electrolyte solution (PBS, NaCl solution) onto the Quantum bioreactor, e.g., to feed the cells or flush the system (see Notes 6 and 7 ).
-
1.
Hang the media bag on the bag pole.
-
2.
Locate the desired inlet line (typically IC inlet line or Wash line).
-
3.
Connect the media bag tube to the inlet line via welding.
The CIB’s content is usually loaded onto the Quantum bioreactor at the beginning or during a specific task (e.g., “IC EC Washout,” “Condition Media”).
3.8 Loading Cell Inlet Bags (CIBs)
Cell inlet bags are intended to hold any small-volume solution (i.e., <500 mL), such as cells or reagents.
-
1.
Hang the CIB on the bag pole.
-
2.
Locate the desired inlet line (typically cell inlet line or reagent line).
-
3.
Connect the CIB’s tube to the desired inlet line via welding.
-
4.
The CIB’s content is loaded onto the Quantum bioreactor by running an appropriate task (e.g., “Load Cells Without Circulation,” “Coat Bioreactor”).
-
5.
Wash the cell line or reagent line, respectively (see Subheading 3.10).
3.9 Coating of the Bioreactor
Once the cell expansion set has been primed, the IC side of the bioreactor needs to be coated with fibronectin to promote cell adhesion (see Note 6 ). To coat the Quantum bioreactor, complete the following steps:
-
1.
Aspirate the fibronectin solution into a syringe.
-
2.
Transfer the fibronectin into a cell inlet bag (CIB; see Subheading 3.6). As a vehicle solution, we recommend phosphate-buffered saline (PBS) to increase the total volume to 100 mL.
-
3.
Load the fibronectin onto the Quantum system (see Subheading 3.8). Connect the bag to the reagent line.
-
4.
Coat the bioreactor by running the “Coat Bioreactor” task for 12–18 h. Remove the fibronectin bag using the RF Sealer.
-
5.
Wash the reagent line (see Subheading 3.10).
3.10 Inlet Line Washout
This task is used to wash a line and remove possible residuals to leave a clean line for future use (see Notes 10 and 12 ). For example, this is helpful after a BM load or a coating procedure.
-
1.
Choose the “Inlet Line Washout” task. In the Setup screen, choose the Inlet Source and Inlet Destination.
For an Inlet Line Washout following a “Load Cells…” task, use the following settings: Inlet Source: Wash; Inlet Destination: Cell.
For an Inlet Line Washout following the “Coat Bioreactor” task, use the following settings: Inlet Source: Wash; Inlet Destination: Reagent.
-
2.
Lower the destination bag so that it hangs below the source bag.
-
3.
After touching “Start” the liquid flow from the Inlet Source to the Inlet Destination. When the desired volume has reached the destination bag, touch the “Finish” button.
-
4.
Touch “Yes” to confirm, and the home screen appears showing the system in an idle state.
3.11 IC EC Washout
After coating, the PBS and excess fibronectin should be flushed out of the bioreactor with the media (see Notes 6 and 7 ).
-
1.
Attach the appropriate media onto the IC media line (see Subheading 3.7).
-
2.
Run the “IC EC Washout” task. Modified setting: EC Inlet = IC media.
3.12 Condition Media
In preparation for the cells, media within the system should be allowed to reach equilibrium with the attached gas mixture, as well as the temperature of the system (see Notes 6 and 7 ).
Accordingly, run the “Condition Media” task. Modified setting: EC inlet = IC media.
3.13 Expansion of MSC from Bone Marrow (P0): Loading of BM, Removal of Non-adherent Cells, and Feeding
- 1.
-
2.
Use the “Attach Cells” task for 48 h. Modified settings: EC inlet = IC media, rocker at 180 °C.
-
3.
Upon completion of the 48 h attachment period, use the “High Density Washout” task to remove non-adherent cells (e.g., red blood cells ). Modified settings: EC inlet = IC media.
-
4.
Use the “Feed Cells” task to continuously feed the cells with the default inlet rate until predefined lactate concentration is observed (see Fig. 4). Upon reaching the next defined threshold concentration, the operator should progressively double the inlet rate as indicated in the figures.
-
5.
When the lactate concentration reaches 6 mM at an inlet rate of 0.4, proceed to Subheading 3.18.
3.14 Expansion of MSC from Pre-cultured MSC (P1): Loading of MSC, Removal of Non-adherent Cells, and Feeding
-
1.
Loading pre-cultured MSC (see Note 9 ).
-
2.
Use the “Attach Cells” task for 24 h. Modified settings: EC inlet = IC media, rocker at 180 °C.
-
3.
Use the “Feed Cells” task to continuously feed the cells with the default inlet rate until predefined lactate concentration is observed (see Fig. 4). Upon reaching the next defined threshold concentration, the operator should progressively double the inlet rate as indicated in the figures.
-
4.
When the lactate concentration reaches 7 mM at an inlet rate of 0.8 mL/min, proceed to Subheading 3.18.
3.15 Taking Samples from the Sample Coil
Samples from the IC circulation loop can be taken from the sampling coil (see Notes 2 and 16 ).
-
1.
If running a task, touch “Pause.”
-
2.
Open the incubator door and remove sample coil from the rocker.
-
3.
Unravel the sample coil from the rocker and remove sample coil strain relief from sample coil line.
-
4.
Use the sterile connection device to remove a piece of the sampling coil.
-
5.
Open the sterile weld on the sample coil.
-
6.
Connect sample coil relief to the sample coil, recoil it around the rocker assembly, and connect the coil strain relief to the rocker.
-
7.
Close the incubator door.
3.16 Taking Samples from the Sample Port
The sample port gives access to the media within the EC loop which can be used for analysis of lactate and glucose levels (see Note 3 ).
-
1.
Open the incubator door.
-
2.
Use a cleansing wipe that contains an appropriate laboratory disinfectant to clean the sample port surface that will interface with the syringe. Note that this sterilization is secondary to the presence of a sterile barrier between the port and the EC loop, through which the sample is drawn.
-
3.
Connect the syringe to the sample port.
-
4.
Draw 3 mL of media through the filter and the sample port, and then discard the media.
-
5.
Repeat step 2 to clean the sample port.
-
6.
Draw the volume of sample needed.
-
7.
Repeat step 2 to clean the sample port.
-
8.
Close the incubator door.
3.17 Measuring Glucose and Lactate Concentrations
Glucose and lactate concentrations may be measured using samples taken from the either the sample port or the sampling coil (see Subheadings 3.15 and 3.16, Notes 2 , 3 and 16 ). Concentrations can be measured by handhold devices according to the manufacturer’s protocol.
3.18 Harvest of MSC
This section describes the release of MSC from the Quantum bioreactor.
-
1.
Fill a CIB with 180 mL of TrypZean (see Subheading 3.6; do not dilute TrypZean). Place bag at 37 °C.
-
2.
Fill a media bag with NaCl solution containing 5 % of HSA (see Note 17 , Subheading 3.5). Weld the NaCl-HSA solution to the EC media line (see Subheading 3.7). Do not start a task yet.
-
3.
For P0, run the “Rapid IC Washout” task to remove any cells remaining in suspension. Modified settings: IC inlet = wash, EC Inlet = wash. (When harvesting P1 MSC, you can directly proceed to step 4).
-
4.
Weld the TrypZean to the reagent line (see Subheading 3.8).
-
5.
Run the task “Release Adherent Cells and Harvest” (see Note 18 ). Modified settings for step 5: IC inlet = EC media.
3.19 Unload the Cell Expansion Set
The Unload Cell Expansion Set task opens all valves and turns off all alarms, allowing the cell expansion set to be easily removed.
-
1.
Seal all inlet and outlet lines connected to bags.
-
2.
Under the Task menu, choose “Set Management,” followed by “Unload Cell Expansion Set.” On the appearing screen, touch “Start.”
-
3.
Open the incubator door and unlock all rotor latches, open the rotor covers on the pumps, and remove the tubing from the pumps.
-
4.
Disconnect the gas inlet line from the gas quick connect by pressing down the silver button on top of it, and turn off the external gas supply afterward.
-
5.
Remove the bioreactor from the rocker arm.
-
6.
Remove the tubing line guide from the pegs.
-
7.
Rotate the base of the mounting clips a quarter turn clockwise to loosen the tubing organizer from the mounting plate.
-
8.
Unlock the five external mounting clips and pull the tubing organizer from the mounting plate.
-
9.
Close the rotor covers and lock the latches on all pumps.
-
10.
Touch “Finish” and afterwards “Yes.” The home screen appears showing the system status as “Idle.”
3.20 Post-Processing Procedures
Subheadings 3.21–3.23 describe the procedures which are usually conducted after hMSC harvest. In summary, the quality of the cells should be confirmed, TrypZean should be washed from the suspension, and the hMSC should either be cryopreserved or prepared for immediate administration to the patient.
3.21 Taking hMSC Samples for Quality Controls
After harvest of both P0 and P1 cells, it is desirable to obtain a sample of hMSC for quality controls (see Tables 1 and 2). Sampling via the method described below is considered an open procedure (see Note 10 ).
-
1.
Pierce a sampling site coupler with needle injection site through the membrane of the harvest bag.
-
2.
Remove the cap from the sampling site coupler. Connect a syringe via luer and aspirate sufficient cell suspension to conduct all required quality controls. Do not remove the sampling site coupler as it will be helpful again in later steps (and will leave an open bag).
3.22 Cryopreser-vation of P0 and P1 hMSC
The following protocol describes the cryopreservation of the complete harvested product into one freezing bag. It comprises the removal of TrypZean and resuspension of the MSC in a freezing solution consisting of NaCl with 10 % DMSO and 5 % of human serum albumin (HSA). The protocol may be easily adapted for freezing in several bags. Generally, it is recommendable not to freeze more than 1 × 107 MSC/mL. The total number of hMSC per bag depends on the number of P0-MSC to be loaded for P1 expansion (e.g., 1 × 107–2 × 107 cells) or the number of MSC to be administered to a patient in one dose after P1 (e.g., 1 × 106–5 × 106 MSC per kg body weight). It should also be noted that this protocol is one of many potential washing methods. Other options could include more automated processes that have recently become available (e.g., the Sepax from Biosafe).
-
1.
Prepare 10 mL of a cryopreservation stock solution (CSS) by aspirating 5 mL of each solution into a syringe to produce a 50 % DMSO and 50 % NaCl solution. Place the syringe between cool packs, precooled at −20 °C.
-
2.
Prepare a second syringe with 40 mL of a NaCl solution containing 8 % of HSA (see Note 19 ). Place the syringe between cool packs.
-
3.
Weld an empty transfer bag to the harvest bag and transfer the cell suspension by gravity (see Notes 20 and 21 ).
-
4.
Centrifuge the transfer bag (300 × g, 5 min, weak break).
-
5.
Reconnect the transfer bag with the harvest bag by welding.
-
6.
Immediately put the transfer bag into a plasma extractor.
-
7.
Press the supernatant into harvest bag and disconnect the two bags by sealing. Discard the harvest bag with the supernatant.
-
8.
Immediately resuspend the hMSC in the residual volume via manual manipulation. After resuspension, there should be no visible aggregates.
-
9.
Tare the scale with empty transfer bag.
-
10.
Place the transfer bag on the scale to determine the volume of the suspension by weight.
-
11.
Remove the cap from the sampling site coupler with needle injection site (has been attached after harvest, see Subheading 3.21). Connect a syringe to the sampling site coupler and remove a sample from the hMSC suspension. Determine the cell number (counting chamber) to calculate the total amount of cells.
-
12.
Via luer, add sufficient NaCl-8%HSA (step 2) solution to the cell suspension, to reach a final volume of 40 mL.
-
13.
Recap the luer and agitate the bag carefully.
-
14.
Remove the cap from the sampling site coupler with needle injection site. Connect a syringe via luer and aspirate the hMSC suspension.
-
15.
Connect the syringe via luer to the freezing bag and inject the hMSC suspension.
-
16.
Connect the syringe with the CSS (see step 1) to the freezing bag.
-
17.
Place the freezing bag between cool packs, precooled at −20 °C.
-
18.
Inject the CSS into the freezing bag while gently and constantly agitating the bag.
-
19.
Connect a syringe via luer to the freezing bag. Aspirate several mL of hMSC suspension for testing (e.g., microbial testing) and retain several samples.
-
20.
Immediately freeze bags and retain samples under controlled conditions.
3.23 Preparation of P1 hMSC for Direct Administration to the Patient
The following protocol describes the preparation of the harvested suspension for administration to the patient. It comprises the removal of TrypZean and resuspension of the hMSC in NaCl-5 % HSA. The protocol could be easily adapted for the preparation of multiple doses from a single harvested product.
-
1.
Prepare a transfer bag with 50 mL of physiological NaCl solution containing 5 % of HSA.
-
(a)
Place the transfer bag on a scale. Tare the scale.
-
(b)
Pierce the spike of the transfer bag through the lid of a squeezable PE plastic bottle containing NaCl solution.
-
(c)
Squeeze the NaCl bottle to force 37.5 g of NaCl solution into the transfer bag.
-
(d)
Add 12.5 mL of 20 % HSA solution.
-
(a)
-
2.
Conduct steps 3–11 of Subheading 3.22.
-
3.
Weld the transfer bag containing the NaCl-5 % HSA solution to the transfer bag with the hMSC suspension.
-
4.
By gravity, add as much NaCl-5 % HSA solution as necessary to reach a final volume of approximately 50 mL.
-
5.
Disconnect the two bags from each other using the sealer.
-
6.
Pierce a sampling site coupler with needle injection site through the sample port of the transfer bag. Remove the cap from the sampling site coupler.
-
7.
Connect a syringe to the sampling site coupler with needle injection site.
-
8.
Aspirate several mL of hMSC suspension for, e.g., microbial testing and as retention samples for cryopreservation.
-
9.
Use the remaining suspension in the syringe for determination of cell number (counting chamber).
-
10.
The hMSC suspension is ready for release.
3.24 Quality Controls
Tables 1 and 2 summarize proposed quality controls that could be utilized for the isolation and expansion process. The release criteria and test methods for the ATMP must be defined in detail in both the clinical protocol and the Investigational Medicinal Product Dossier (IMPD), according to the specific requirements of the clinical trial, requests of the pertinent authorities, and the guidance of the country-specific pharmacopeia.
3.25 Release Criteria
Table 3 proposes release criteria pertaining to either the hMSC (e.g., identity) or the media in which they were cultured (e.g., sterility). Release criteria should be performed for each expansion, according to the requirements of the responsible authorities. Specifications may differ according to the therapeutic application, the method of delivery, and the nature of the final cell product preparation (e.g., cryopreserved vs. freshly harvested). Although genetic stability of (Quantum-derived) hMSC has been shown and the risk of tumorigenicity for hMSC is considered low [8, 10, 14], it might be advantageous to additionally analyze these parameters. Regarding the transplant itself, the endotoxin threshold is 5 EU/kg body weight per hour for intravenous administration and consider the number of transplanted leukocytes/CD45+ cells.
4 Notes
-
1.
Daily routine should ensure that the Quantum system is still supplied with gas.
-
2.
Samples for endotoxin, mycoplasma, and microbial testing of the culture media should be taken from the IC loop via the sampling coil. The sample port on the EC loop contains a sterile filter which limits its utility for such purposes. After having disconnected a portion of the sampling coil using the sterile connection device, culture media can be withdrawn from the coil in a sterile and safe manner by welding the filter end of a sampling coil accessory set to one end of the sample and a luer (e.g., that of an in-line filter accessory set) to the opposite end, thus allowing aspiration of the media into a syringe.
-
3.
Samples taken from the EC loop sample port with a luer-lock syringe may be used to analyze metabolite concentrations (e.g., glucose and lactate) as an indicator of cell proliferation.
-
4.
Any frozen solution (e.g., hMSC suspension, PL and TrypZean) should be thawed under controlled conditions, e.g., using a CE-marked plasmatherm device.
-
5.
Before adding PL to the basic media, add 1–2 IU/mL heparin to the basic media and mix thoroughly, in order to avoid gelation.
-
6.
All inlet solutions to the Quantum system should be at or above room temperature, in order to minimize bubble formation within the system.
-
7.
If the media is light sensitive, it is important to protect the media bags from light, e.g., by wrapping them in aluminum foil.
-
8.
Make sure that there is >2 L of electrolyte solution in the bag when priming the CES. Do not stop the “Prime Cell Expansion Set” task. If necessary, use Pause.
-
9.
The first harvest of MSC, originating from the BM aspirate, generates Passage 0 (P0) MSC; accordingly, expanding these cells in a second step yields Passage 1 (P1) cells.
-
10.
While the Quantum system is a functionally closed system and, therefore, may not require installation in a class A-C clean room, a few steps (e.g., filling CIBs and media bags) may remain open, where “open” is defined as allowing contact between the fluid and ambient air. Accordingly, these open steps should be performed in a class A clean room (e.g., class A laminar air flow in class B environment).
-
12.
Upon arrangement, some companies can prepare their GMP-grade culture media in bags with polyurethane tubing that is compatible with the Quantum system (e.g., Biochrom, Berlin, Germany). These prefilled bags simplify handling and decrease the risk of contamination by obviating the open filling step.
-
13.
The Inlet Line Washout task can also be used to transfer liquid from one bag to another in a sterile manner, which could be beneficial for the development of an individualized expansion protocol.
-
14.
For BM aspiration, a variety of protocols may be used. However, care should be taken to add sufficient sodium heparin to the aspiration set.
-
15.
It is advisable to determine cell number and viability before loading the BM onto the Quantum bioreactor, e.g., by using an automated system like Sysmex KX21-N analyzer (Sysmex Deutschland GmbH, Norderstedt, Germany).
-
16.
It is advisable to load unmanipulated BM within 24 h after its aspiration. If intermediate storage is necessary, use a temperature range of 21 °C ± 3 °C.
-
17.
Approximately 12.7 cm of the coil contains 1 mL of media. The total accessible length of the original sampling coil on the cell expansion set is approximately 225 cm.
-
18.
The HSA is present to inactivate TrypZean.
-
19.
If a noncritical alarm occurs during the “Release Adherent Cells and Harvest” task, mute it. Only press “Continue” after the task is complete, thereby avoiding repetitive alarms.
-
20.
Final HSA concentration of the cryopreservant should be approximately 5 %.
-
21.
The tubing connected directly to the harvest bag is made of a common polyvinyl chloride (PVC) and can, therefore, be sterile-connected to another PVC line with equal dimensions: inner diameter, 2.95 mm; outer diameter, 4.06 mm; and wall thickness, 0.56 mm.
-
22.
The harvest bag that comes pre-attached to the cell expansion set is not suitable for centrifugation.
References
Horwitz EM, Le Blanc K, Dominici M et al (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7:393–395
Crisan M, Yap S, Casteilla L et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
dos Santos F, Andrade P, Eibes G et al (2011) Ex vivo expansion of human mesenchymal stem cells on microcarriers. In: Chase LG, Rao MS, Vemuri M (eds) Mesenchymal stem cell assays and applications. Humana Press, New York, pp 189–198
Schop D, Janssen FW, Borgart E et al (2008) Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: growth and metabolism. J Tissue Eng Regen Med 2:126–135
Gottipamula S, Muttigi MS, Chaansa S et al (2014) Large-scale expansion of pre-isolated bone marrow mesenchymal stromal cells in serum-free conditions. J Tissue Eng Regen Med 2014:n/a–n/a
Hanley PJ, Mei Z, Durett AG et al (2014) Efficient manufacturing of therapeutic mesenchymal stromal cells with the use of the Quantum Cell Expansion System. Cytotherapy 16:1048–1058
Jones M, Varella-Garcia M, Skokan M et al (2013) Genetic stability of bone marrow-derived human mesenchymal stromal cells in the Quantum System. Cytotherapy 15:1323–1339
Nold P, Brendel C, Neubauer A et al (2013) Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochem Biophys Res Commun 430:325–330
Rojewski MT, Fekete N, Baila S et al (2013) GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device Quantum Cell Expansion System. Cell Transplant 22:1981–2000
Garg A, Houlihan DD, Aldridge V et al (2014) Non-enzymatic dissociation of human mesenchymal stromal cells improves chemokine-dependent migration and maintains immunosuppressive function. Cytotherapy 16:545–559
Krampera M, Galipeau J, Shi Y et al (2013) Immunological characterization of multipotent mesenchymal stromal cells – The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 15:1054–1061
Menard C, Pacelli L, Bassi G et al (2013) Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: standardization of immune quality controls. Stem Cells Dev 22:1789–1801
Barkholt L, Flory E, Jekerle V et al (2013) Risk of tumorigenicity in mesenchymal stromal cell-based therapies – bridging scientific observations and regulatory viewpoints. Cytotherapy 15:753–759
Acknowledgments
We appreciate the excellent technical assistance of G. Baur, T. Becker, D. Erz, S. Chester, C. Späth, C. Loechelt, and A. Wachtel. This work was supported by grants from the 7th Framework Programme of the European Commission: CASCADE (Cultivated Adult Stem Cells as Alternative for Damaged Tissue) (HEALTH-F5-2009-223236), REBORNE (Regenerating Bone Defects using New biomedical Engineering approaches) (HEALTH-2009-1.4.2-241879) to Luc Sensebé and Hubert Schrezenmeier, the von Behring-Röntgen Foundation (Cellular Interaction of Nanoparticle-Labeled MSC with Tumor Cells), and the UKGM-Kooperationsvertrag Grant (GMP-conform manufacturing of MSC) to Cornelia Brendel and Holger Hackstein.
Financial Disclosure Statement
MTR and HS work for nonprofit organizations producing platelet lysate and clinical-grade MSC. BR and SB work for Terumo BCT.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Barckhausen, C. et al. (2016). GMP-Compliant Expansion of Clinical-Grade Human Mesenchymal Stromal/Stem Cells Using a Closed Hollow Fiber Bioreactor. In: Gnecchi, M. (eds) Mesenchymal Stem Cells. Methods in Molecular Biology, vol 1416. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3584-0_23
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3584-0_23
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3582-6
Online ISBN: 978-1-4939-3584-0
eBook Packages: Springer Protocols