Abstract
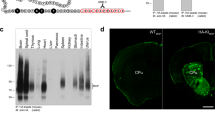
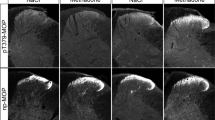
Our experience demonstrates that it is difficult to identify MOPR in rat and mouse brains by western blot, in part due to low abundance of the receptor and a wide relative molecular mass (Mr) range of the receptor associated with its heterogeneous glycosylation states. Here, we describe generation and purification of anti-μC (a rabbit polyclonal anti-MOPR antibody), characterization of its specificity in immunoblotting of HA-tagged MOPR expressed in a cell line, and ultimately, unequivocal detection of the MOPR in brain tissues by western blot with multiple rigorous controls. In particular, using brain tissues from MOPR knockout (K/O) mice as the negative controls allowed unambiguous identification of the MOPR band, since the anti-MOPR antibody, even after affinity purification, recognizes nonspecific protein bands. The MOPR was resolved as a faint, broad, and diffuse band with a wide Mr range of 58–84 kDa depending on brain regions and species. Upon deglycosylation to remove N-linked glycans by PNGase F (but not Endo H), the MOPR became a dense and sharp band with Mr of ~43 kDa, close to the theoretical Mr of its deduced amino acid sequences. Thus, MOPRs in rodent brains are differentially glycosylated by complex type of N-linked glycans in brain region- and species-specific manners. Furthermore, we characterized the MOPR in an A112G/N38D-MOPR knockin mouse model that possesses the equivalent substitution of the A118G/N40D SNP in the human MOPR gene. The substitution removes one of the four and five N-linked consensus glycosylation sites of the mouse and human MOPR, respectively. We demonstrated that the Mr of the MOPR in A112G mouse brains was lower than that in wild-type mouse brains, and that the difference was due to lower degrees of N-linked glycosylation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bodei S, Arrighi N, Spano P et al (2009) Should we be cautious on the use of commercially available antibodies to dopamine receptors? Naunyn Schmiedebergs Arch Pharmacol 379:413–441
Hamdani N, van der Velden J (2009) Lack of specificity of antibodies directed against human beta-adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 379:403–407
Jensen BC, Swigart PM, Simpson PC (2009) Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol 379: 409–412
Jositsch G, Papadakis T, Haberberger RV et al (2009) Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol 379:389–395
Lu X, Bartfai T (2009) Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol 379:417–420
Michel MC, Wieland T, Tsujimoto G (2009) How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol 379:385–388
Pradidarcheep W, Stallen J, Labruyere WT et al (2009) Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 379:397–402
Huang P, Liu-Chen LY (2009) Detection of the endogenous mu opioid receptor (mopr) in brain. Front Biosci 1:220–227
Liu-Chen L-Y, Chen C, Phillips CA (1993) Beta-[3H]funaltrexamine-labeled mu-opioid receptors: species variations in molecular mass and glycosylation by complex-type, N-linked oligosaccharides. Mol Pharmacol 44:749–756
Chen C, Xue JC, Zhu J et al (1995) Characterization of irreversible binding of beta-funaltrexamine to the cloned rat mu opioid receptor. J Biol Chem 270:17866–17870
Deng HB, Yu Y, Wang H et al (2001) Agonist-induced mu opioid receptor phosphorylation and functional desensitization in rat thalamus. Brain Res 898:204–214
Yu YK, Zhang L, Yin XX et al (1997) Mu-opioid receptor phosphorylation, desensitization, and ligand efficacy. J Biol Chem 272: 28869–28874
Carman CV, Barak LS, Chen C et al (2000) Mutational analysis of Gbeta gamma and phospholipid interaction with G Protein-coupled receptor kinase 2. J Biol Chem 275: 10443–10452
Huang P, Xu W, Yoon SI et al (2007) Agonist treatment did not affect association of mu opioid receptors with lipid rafts and cholesterol reduction had opposite effects on the receptor-mediated signaling in rat brain and CHO cells. Brain Res 1184:46–56
Huang P, Chen C, Xu W et al (2008) Brain region-specific N-glycosylation and lipid rafts association of the rat mu opioid receptor. Biochem Biophys Res Commun 365:82–88
Mague SD, Isiegas C, Huang P et al (2009) Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci U S A 106: 10847–10852
Huang P, Chen C, Mague SD et al (2012) A common single nucleotide polymorphism A118G of the mu opioid receptor alters its N-glycosylation and protein stability. Biochem J 441:379–386
Lupp A, Richter N, Dol C et al (2011) UMB-3, a novel rabbit monoclonal antibody, for assessing mu-opioid receptor expression in mouse, rat and human formalin-fixed and paraffin-embedded tissues. Regul Pept 167: 9–13
Huang P, Li J, Chen C et al (2001) Functional role of a conserved motif in TM6 of the rat mu opioid receptor: constitutively active and inactive receptors result from substitutions of Thr6.34(279) with Lys and Asp. Biochem 40: 13501–13509
Schuller AG, King MA, Zhang J et al (1999) Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2: 151–156
Mague SD, Blendy JA (2010) OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 108:172–182
Chen C, Yin J, Riel JK et al (1996) Determination of the amino acid residue involved in [3H]beta-funaltrexamine covalent binding in the cloned rat mu-opioid receptor. J Biol Chem 271:21422–21429
Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Schagger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Xu W, Yoon SI, Huang P et al (2006) Localization of the kappa opioid receptor in lipid rafts. J Pharmacol Exp Ther 317: 1295–1306
Bergen AW, Kokoszka J, Peterson R et al (1997) Mu opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry 2:490–494
Bond C, LaForge KS, Tian M et al (1998) Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A 95: 9608–9613
Acknowledgement
This work was supported by the National Institutes of Health (grant numbers R01 DA017302 and P30 DA013429).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
Huang, P., Chen, C., Liu-Chen, LY. (2015). Detection of Mu Opioid Receptor (MOPR) and Its Glycosylation in Rat and Mouse Brains by Western Blot with Anti-μC, an Affinity-Purified Polyclonal Anti-MOPR Antibody. In: Spampinato, S. (eds) Opioid Receptors. Methods in Molecular Biology, vol 1230. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1708-2_11
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1708-2_11
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1707-5
Online ISBN: 978-1-4939-1708-2
eBook Packages: Springer Protocols