Abstract
Antibodies are important tools for protein and peptide research, including for the kappa opioid receptor (KOR) and dynorphins (Dyns). Well-characterized antibodies are essential for rigorous and reproducible research. However, lack of validation of antibody specificity has been thought to contribute significantly to the reproducibility crisis in biomedical research. Since 2003, many scientific journals have required documentation of validation of antibody specificity and use of knockout mouse tissues as a negative control is strongly recommended. Lack of specificity of antibodies against many G protein-coupled receptors (GPCRs) after extensive testing has been well-documented, but antibodies generated against partial sequences of the KOR have not been similarly investigated. For the dynorphins, differential processing has been described in distinct brain areas, resulting in controversial findings in immunohistochemistry (IHC) when different antibodies were used. In this chapter, we summarized accepted approaches for validation of antibody specificity. We discussed two KOR antibodies most commonly used in IHC and described generation and characterization of KOR antibodies and phospho-KOR specific antibodies in western blotting or immunoblotting (IB). In addition, applying antibodies targeting prodynorphin or mature dynorphin A illustrates the diversity of results obtained regarding the distribution of dynorphins in distinct brain areas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Antibodies are widely used in biomedical research. They are employed to label specific antigens (most commonly proteins and peptides) via techniques such as western blotting or immunoblotting (IB), immunohistochemistry (IHC), immunocytochemistry (ICC), enzyme-linked immunosorbant assay (ELISA), immunoprecipitation (IP) and fluorescence-activated cell sorting (FACS). For any antibody to be useful, its specificity for the antigen in the intended application is of utmost importance. Well-characterized antibodies that consistently perform as expected are essential for rigorous and reproducible research. However, problems with validation of antibody specificity or lack of validation have been cited as one of the important factors for the “reproducibility crisis” in biomedical research (Freedman et al. 2015). It was estimated that 36.1% of irreproducible research was attributed to biological reagents and reference materials (Freedman et al. 2015), of which antibodies constitute a large portion. For the Human Protein Atlas (HPA), Berglund et al. (2008) examined 6,120 antibodies for 5,067 proteins in the human genome and showed that only 7% and 15% of antibodies achieved high and medium validation scores for IHC of proteins, respectively. Many journal editors and researchers have raised concerns about the lack of validation of antibody specificity in research (Baker 2015a, b; Bordeaux et al. 2010; Gautron 2019; Pillai-Kastoori et al. 2020; Rhodes and Trimmer 2006; Saper 2005; Saper and Sawchenko 2003; Uhlen et al. 2016). In 2003, Journal of Comparative Neurology was the first journal to introduce the requirements of validation of antibody specificity for publishing research work in the journal. Many journals followed, including Nature, Endocrinology, British Journal of Pharmacology, Journal of Histochemistry and Cytochemistry, Molecular Endocrinology.
The research community launched several initiatives in attempts to enhance quality and standardization of antibodies used in research, such as antibody evaluation, protocols and documentation. Bourbeillon et al. (2010) developed guidelines called the Minimum Information about A Protein Affinity Reagent (MIAPAR), as an important first step in formalizing standards in reporting the production and properties of protein binding reagents, such as antibodies. It constructed a checklist of required information, including production/purification process, experimental evidence of specificity, updated protocols, and other relevant details. Subsequently, the International Working Group for Antibody Validation (IWGAV) was formed in 2016 and recommended guidelines for raising standards for antibody validation (Uhlen et al. 2016). In 2017 the National Institutes of Health (NIH) issued a notice (NOT-OD-17-068), which required research grant applicants to authenticate key biological reagents, including antibodies.
In addition to improving the standardization of antibodies, antibody performance is another common source of variability, which may vary considerably between suppliers and even batches.
2 General Considerations for Validation of Specificity of Antibodies
2.1 Unique Issues Associated with Antibodies Against G Protein-Coupled Receptors (GPCRs)
GPCR antibodies are typically generated against synthetic peptides corresponding to partial sequences of the N- or C-terminal domains of GPCRs because these two regions have the most divergent sequences and they are accessible in the extracellular and intracellular space, respectively. The peptides are usually coupled to a carrier protein, such as keyhole limpet haemocyanin or thyroglobulin, for use as antigens. Most GPCR antibodies are polyclonal antibodies generated largely in rabbits using conventional methods. GPCR antibodies present unique challenges in that GPCRs are generally present in very low levels in tissues, including the brain. It is thus necessary to have antibodies that have very high affinity for the antigen to allow use of very low concentrations so as to minimize nonspecific interactions. However, such high affinity antibodies are not commonly available for GPCRs. Thus, one drawback of GPCR antibodies frequently encountered is low signal-to-noise ratios, that is low specificity. In IB, it may be necessary to partially enrich the GPCRs by, for example, IP if appropriate antibodies are available, or lectin affinity chromatography taking advantage of the glycoprotein nature of many GPCRs. Most GPCRs are glycosylated, largely in the N-terminal domain; therefore, they appear as broad and diffuse band(s) in IB with relative molecular weights (Mr’s) higher than molecular weights predicted from amino acid sequences [for example, (Huang et al. 2015, 2008; Li et al. 2007; Petaja-Repo et al. 2000, 2002)]. Because of different degrees of glycosylation, Mr’s of the same GPCR may have species, tissue and brain region differences (Huang et al. 2008, 2015; Liu-Chen et al. 1993). Many GPCRs appear as two bands: one band represents the fully glycosylated form present on plasma membranes and the other partially glycosylated in the Golgi and endoplasmic reticulum (Huang et al. 2008, 2015; Li et al. 2007; Petaja-Repo et al. 2000, 2002). Because of these issues, it is not possible to use Mr in IB as predicted from amino acid sequences as the first-line characterization criterion of antibodies against a GPCR.
Studies published in 2009 examined several commercially available antibodies against partial sequence of each of D1, D4 and D5 dopamine receptors, β1-, β2- and β3-adrenergic receptors, α1A-, α1B- α1D- and α2B-adrenergic receptors, M1, M2, M3, M4 and M5 muscarinic receptors and GalR1 and GalR2 galanin receptors. The results revealed that none of the commercially available antibodies against these GPCRs showed specificity when tested in IHC, IB or both [(Michel et al. 2009) and other articles in the same issue]. Subsequently similar studies on other GPCRs were published.
2.2 Validation of Specificity of Antibodies
Michel et al. (2009) proposed that specificity of antibodies against GPCRs (or any other proteins) should be validated with at least one of the following methods: (1) Staining should be absent in tissues from knockout mice. (2) Intensity of staining should be reduced following siRNA knockdown of the target protein in cells or in vivo. (3) Closely related receptors expressed in the same cell lines should yield no staining. (4) Antibodies generated against at least two distinct epitopes of the same receptor should yield the same staining. Many labs used haemagglutinin (HA) or FLAG epitope tagged GPCRs and checked the specificity of GPCR antibodies by use of FLAG or HA antibodies as the positive controls. We would suggest that at least two approaches should be taken to more stringently define antibody specificity. The approaches outlined here for validation of antibody specificity are applicable for any immunological techniques. Antibodies suitable for IHC may not be appropriate for IB and vice versa, therefore validation for IHC and IB should be performed separately. This is because in each application samples are treated differently, which may affect the epitopes exposed on the target protein and this, in turn, may profoundly influence binding of antibodies to the target protein. Previously, antibodies pre-absorbed with an excessive amount of the antigen were commonly used as the controls to validate specificity of antibodies, which is now proven to be inadequate because it eliminated staining of not only the target protein, but also many other proteins which may have similar epitopes.
For GPCRs for which highly selective radiolabeled ligands are available, it is generally accepted that receptor autoradiography results yield the most reliable neuroanatomical distribution of the receptor, albeit with low resolution. Two highly selective KOR agonists, [3H]U69,593 and [3H]CI977, have been used as the radioligands for autoradiography (Mansour et al. 1994; Slowe et al. 1999; Unterwald et al. 1991). IHC staining should produce similar distribution as receptor autoradiography. Yet, it has to be kept in mind that receptor autoradiography only detects receptors with binding activity. Internalized or inactive receptors, which may be targeted by IHC, are not visualized.
3 Antibodies for IHC of the KOR
IHC of the KOR has been carried out with antibodies raised against synthetic peptides corresponding to partial sequences of N- and C-terminal domains the KOR [for example, (Appleyard et al. 1997; Arvidsson et al. 1995; Drake et al. 1996; Mansour et al. 1996)]. A synthetic peptide was conjugated to a carrier protein and polyclonal antibodies were generated with conventional methods. KT2 and KOR1 antibodies, both from rabbits, are discussed here because they are more widely used in IHC. KT2 and KOR1 antibodies were raised against 371–380 and 366–380 peptides in the C-terminal domain of the rat KOR, respectively.
3.1 Characterization of KT2 Antibody and KOR1 Antibodies for IHC
Because the two antibodies were used mostly for IHC, their characterizations for IHC are described. Drake et al. (1996) reported that in ICC, KT2 antibodies label the outer membranes of Xenopus oocytes transfected with the rat KOR, but not the untransfected ones. IHC of rodent brain sections with KT2 showed staining in central grey and spinal cord, which was abolished when KT2 antibodies were pre-absorbed with the antigen.
Arvidsson et al. (1995) observed that in ICC, KOR1 antiserum stained Cos-7 cells transfected with HA-conjugated rat KOR (HA-rat KOR) in a similar manner as HA antibodies, but did not stain cells transfected with HA-MOR or HA-DOR. The antigen peptide [KOR(366–380)] blocked the staining with KOR1 antibodies in brain sections and in transfected cells, but shorter peptides [KOR 366–373, 369–376 and 374–380] did not.
At the time of publication, neither antibody was tested in KOR knockout mice in IHC.
3.2 IHC of the KOR in the Brain
IHC of the KOR in the brain was performed on guinea pig brain sections with KOR1 or KT2 antibodies (Arvidsson et al. 1995; Drake et al. 1996) because of higher levels of KOR in this species (Mansour et al. 1988). As discussed by Drake et al. (1996), the KOR1 and KT2 antibodies labeled several brain regions found to have KOR binding by receptor autoradiography, including the substantia nigra, nucleus accumbens, basal forebrain, and endopiriform nucleus. However, neither KT2 nor KOR1 labeled the claustrum, which has the highest level of binding of [3H]U69,593 in the guinea pig brain (Unterwald et al. 1991; Wang et al. 2011), or the cerebellum, which expresses a moderate level of KOR in this species (Unterwald et al. 1991). Distributions of KOR1 and KT2 immunoreactivities (KOR1-IR, KT2-IR, respectively) showed significant differences. KOR1-IR, but not KT2-IR, was present in the diagonal band, suprachiasmatic nucleus, supraoptic nucleus, and VTA. Conversely, KT2-IR, but not KOR1-IR, was observed in the central grey and lateral septum. At the electron microscopy level, KOR1-IR appeared predominantly postsynaptic since it was localized to cell bodies and dendrites, whereas KT2-IR was found mostly in processes with varicosities, which had the appearance of axons (Arvidsson et al. 1995; Drake et al. 1996).
The reasons for the discrepancies in staining patterns of KOR1 and KT2 antibodies are not clear. The findings suggest that antibodies, even raised against similar antigens, may recognize different epitopes, which underscores the difficulties associated with raising specific antibodies against GPCRs.
3.3 Generation of a Mouse Line Expressing a Fusion Protein of the KOR Conjugated with tdTomato (KOR-tdT)
To circumvent the issues associated with KOR antibodies, Chen et al. (2020) generated a mouse line expressing the KOR conjugated in-frame with tdTomato 5′ to the stop codon (KOR-tdT) to facilitate identification of KOR-containing neurons. Clearing of whole brains with CLARITY revealed 3-dimensional (3-D) images of distribution of KOR for the first time. It was also the first 3-D image of a GPCR distribution in the rodent brain. 3-D brain images of KtdT and IHC on brain sections with antibodies against tdTomato show similar distribution to that of autoradiography of [3H]U69,593 binding to KOR in wildtype mice. KOR was visualized at the cellular level, such as co-localization with tyrosine hydroxylase (TH) and agonist-induced KOR translocation into intracellular space in some ventral tegmental area (VTA) neurons. These mice thus represent a powerful and heretofore unparalleled tool for neuroanatomy of KOR at both the 3-D and cellular levels.
4 KOR Antibodies for IB
In this section, antibodies for IB of KOR generated by Chen et al. (2016) are described. They generated antibodies against the rat/mouse KOR peptide (368–380) in rabbits (PA847) and in guinea pigs (5698) and purified each with antigen affinity chromatography (PA847p and 5698p, respectively). The antibodies were fully characterized for IB only.
4.1 Detection of KOR Expressed in Cells
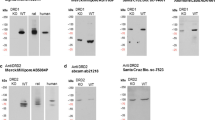
In IB of CHO cells stably transfected with FLAG-human KOR (FLAG-hKOR), rabbit KOR antibodies PA847p recognized a broad and diffuse protein band of Mr. 52 kDa and a less diffuse band of 42 kDa, which were absent in untransfected CHO cells (Fig. 1a). When IB was performed with anti-FLAG antibodies [Purified rabbit anti-FLAG antibody (F7425), Sigma Aldrich], two protein bands with the same Mr’s were detected (Fig. 1a). The Mr 52-kDa band is a full glycosylated form of the KOR, whereas the Mr 42 kDa band represents immature forms of the KOR (Li et al. 2007). The broad and diffuse nature of the 52-kDa band is due to heterogeneity of glycosylation (Li et al. 2007).
(a) Antigen affinity chromatography-purified rabbit antibodies against mouse KOR(371–380) peptide (PA847p) recognize human KOR expressed in CHO cells in immunoblotting with high specificity. CHO-FLAG-hKOR cells and CHO cells were solubilized with Laemmli buffer and subject to SDS-PAGE. Immunoblotting with PA847p revealed two protein bands (indicated by arrow heads) of Mr 52 kDa and Mr 42 kDa in CHO-FLAG-hKOR cells, which were not present in CHO cells. Anti-FLAG antibodies and PA847 recognized the same two protein bands of Mr 52 kDa and Mr 42 kDa in CHO-FLAG-hKOR cells. (c) Immunoblotting of KOR in mouse brains with rabbit antibodies against mouse KOR(371–380) peptide (PA847p) revealed two bands (indicated by arrow heads) of Mr 55 kDa and Mr 45 kDa in wildtype (WT) brains, but not in KOR−/− (KO) brains. Mouse brains (with cerebella removed) were solubilized and KOR was immunoprecipitated with guinea pig antibodies against mouse KOR(371–380) (5698p) followed by SDS-PAGE and immunoblotting with rabbit antibodies PA847p. Each experiment was performed twice with similar results
4.2 Detection of the KOR in Mouse Brains
Detection of the KOR in mouse brains by IB is much more challenging than in cells because of the very low KOR expression level as well as the great complexity of protein constituents in brains. When KOR contents in brain membranes are calculated based on the Bmax value of [3H]U69,593 binding reported by Kitchen et al. (1990) (7.3 fmol/mg protein) and molecular weight of the protein backbone of ~42 kDa (KOR has 380 amino acids in length), the KOR constitutes only 0.000031% of total brain membrane proteins, making its detection difficult. Liu et al. (2020) thus partially purified KOR from solubilized brain membranes by IP with purified guinea pig anti-KOR (5698p) followed by IB with PA847p rabbit anti-KOR. Compared with brains of KOR knockout mice, wildtype mouse brains had one broad band of high intensity of ~55 kDa and a sharper band of light intensity of ~45 kDa (Fig. 1b), which most likely represent fully glycosylated and immature forms of the KOR, respectively. The differences in Mr’s between CHO cells and brains are likely due to variations in the extent of glycosylation, similar to what was observed for the MOR (Huang et al. 2008; Huang and Liu-Chen 2009; Liu-Chen et al. 1993). The much higher level of the immature form of the KOR in CHO cells may be due to the stronger CMV promoter in the KOR plasmid transfected into CHO cells. It is noteworthy that even in the KOR knockout, there are immunoreactive bands, demonstrating that it is crucial to have tissues from KOR knockout mice as a control to discern the truly positive bands.
5 Antibodies for IB of Phosphorylated KOR
Following agonist activation, GPCRs are phosphorylated by G protein-coupled receptor kinases (GRKs) and second messenger-activated protein kinases, such as protein kinase A and protein kinase C. Antibodies specifically recognizing phosphorylated form of GPCRs are useful research tools. Importantly, phospho-specific antibodies should recognize phosphorylated GPCRs, but not unphosphorylated GPCRs. Specificity of phospho-specific antibodies should be validated by at least two of the following experiments: 1) staining of the phospho-specific antibodies should be increased by a prototypic agonist that is known to induce receptor desensitization and internalization. 2) staining of the phospho-specific antibodies is abolished by treatment of samples or IB transfer membranes with phosphatase. 3) staining of the phospho-specific antibodies is abolished by mutation of the phosphorylation site to Ala. 4) in IB, phospho-specific GPCR antibodies should recognize protein bands of similar apparent Mr as GPCR antibodies.
5.1 Detection of Phosphorylated KOR in Cells
Chen et al. (2016) determined the sites of U50,488H-promoted mouse KOR (mKOR) phosphorylation to be S356, T357, T363 and S369 in the C-terminal domain. Antibodies were generated against three phosphopeptides (pS356/pT357, pT363 and pS369) and purified first with phospho-peptide affinity chromatography followed by adsorption with unphosphorylated peptide affinity beads to enhance specificity against the phosphorylated peptide. The antibodies were fully characterized for IB only.
Using mouse neuro2a neuroblastoma (N2A) cells stably transfected with FLAG-tagged mouse KOR conjugated with 6 x His (N2A-FmK6H cells), Chen et al. (2016) demonstrated that following U50,488H treatment and IP of KOR with FLAG antibodies, IB with each of rabbit anti-pS356/pT357, anti-pT363 and ant-pS369 revealed a high-intensity broad and diffuse band of ∼52 kDa in U50,488H-treated samples (Fig. 2a, phosphorylated mKOR). In saline-treated samples, there was no staining with anti-pT363 and ant-pS369; however, there was a faint staining with anti-pS356/pT357, suggesting basal phosphorylation (Fig. 2a). Blots were then stripped and re-blotted with rabbit anti-FLAG antibodies to stain total KOR. mKOR was revealed as a diffuse band of 52 kDa (Fig. 2a, total mKOR) and the amounts of total KOR were not different between the saline- and U50,488H-treated groups. In N2A-FmK6H cells, the intensity of the immature mKOR form was low, like in mouse brains.
(a) Phospho-peptide antibodies have high specificity for phosphorylated KOR. left: U50,488H greatly enhanced anti-pS356/pT357, anti-pT363 and anti-pS369 immunoblotting intensity of the mKOR. Murine neuro2A neuroblastoma cells stably transfected with FLAG-tagged mouse KOR conjugated with 6 x His (N2A-FmK6H) were treated with vehicle or 10 μM U50,488H for 30 min. Cells were solubilized and the receptors were partially purified with a Ni-NTA agarose column. Eluates were subject to SDS-PAGE and immunoblotting with indicated antibodies. Membranes were stripped and re-blotted with anti-FLAG antibodies for total KOR. The experiments were performed three times with similar results. Untransfected N2A cells were subjected to similar treatment and immunoprecipitation procedures, and none of anti-pS356/pT357, anti-pT363 and anti-S369 detected a 52-kDa band in immunoblotting (data not shown). right: Immunoblotting intensity was greatly reduced by dephosphorylation. Experiments were performed as described above, except that PVDF membranes with transferred proteins were incubated with lambda phosphatase and then subject to immunoblotting with indicated antibodies. Membranes were stripped and re-blotted with anti-FLAG antibodies for total KOR. Phosphatase treatment reduced staining of U50,488H-treated samples, indicating phospho-specificity of the antibody. In addition, phosphatase reduced pS356/pT357 staining in the control, demonstrating constitutive phosphorylation of the sites. These experiments were performed four times with similar results. (b) Effects of mutations in the mKOR on U50,488H-promoted receptor phosphorylation detected with phospho-KOR antibodies. The cDNA construct of the wildtype, S356A, S357A, S356A/S357A, T363A or S369A mutant of the mKOR was transfected into N2A cells and stable mixed clonal cells were established. Cells were treated with vehicle or U50,488H (10 μM) for 30 min, harvested and receptor proteins were purified and resolved with SDS-PAGE. Immunoblotting was performed with the indicated antibodies. The amount of total mKOR was determined with another gel loaded with the same aliquots. S356A, S357A or S356A/S357A substitutions abolished basal and U50,488H-promoted mKOR phosphorylation detected by anti-pS356/pT357. T363A and S369A mutations eliminated mKOR phosphorylation detected by anti-pT363 and anti-pS369 staining, respectively. The experiments were performed two times with similar results (from Chen et al. 2016)
When transfer membranes were treated with lambda protein phosphatase, which dephosphorylates phosphoserine, phosphothreonine and phosphotyrosine, U50,488H-promoted staining in the 52-kDa protein band by anti-pS356/pT357, anti-pT363 and anti-pS369 was eliminated (Fig. 2a, dephosphorylated mKOR). These results indicate that the immunoreactivity of the 52-kDa band is due to phospho-KOR.
S356A, T357A or S356A/T357A substitution abrogated anti-pSr356/pT357 staining in control and U50,488H-treated mKOR (Fig. 2b). T363A and S369A mutations of the FmK6H eliminated U50,488H-induced staining by anti-pT363 and anti-pS369, respectively (Fig. 2b).
Thus, anti-pS356/pThr357, anti-pT363 and anti-pS369 react with mKOR phosphorylated at S356/T357, T363 and S369, respectively. These results validate further the specificity of antibodies for phosphorylated KOR.
5.2 Detection of Phosphorylated KOR in Mouse Brains
In mouse brains, phosphorylated KOR has to be enriched by IP before IB because of the low level of KOR. KOR in solubilized membranes was immunoprecipitated with guinea pig antibodies against KOR(368–380) (5698p) and IB was performed with the rabbit anti-pT363 or anti-pS369 for detection of phosphorylated KOR or rabbit antibodies against KOR(368–380) for total KOR (PA847p). Guinea pig antibodies were used for IP, whereas rabbit antibodies were used for IB to avoid cross reactivities. Wildtype or KOR knockout C57BL/6J mice were treated with saline or U50,488H (10 mg/kg, s.c.) and killed 30 min later and brains were dissected and frozen on dry ice immediately. Solubilization of brains, IP of KOR and IB of phosphorylated KOR were performed as described (Liu et al. 2020) (see Fig. 3 legend). In wildtype C57BL/6J mice, U50,488H treatment greatly increased the intensity of anti-pT363 and anti-pS369 staining in brains (Fig. 3a). In contrast, in the brains of KOR knockout mice, there is no staining by either antibody following saline or U50,488H pretreatment, indicating specificity for the phosphorylated KOR. Staining with anti-pS356/pT357 was not performed because T363 and S369 are the two primary phosphorylation sites and are phosphorylated before S356/T357 (Chen et al. 2016). Without enrichment with IP, it was not possible to detect phosphorylated KOR with IB.
Detection of U50,488H-promoted KOR phosphorylation in mouse brains by immunoblotting. (a) In immunoblotting, anti-pT363 and anti-pS369 antibodies are specific for phospho-KOR in mouse brains. Wildtype (WT) and KOR−/− (KO) C57BL/6 adult mice were injected with saline or U50,488H (10 mg/kg, s.c.) and euthanized 30 min later and brains were immediately removed and frozen on dry ice. Four brains were pooled as one sample because of the low expression level of KOR. Brains were solubilized with 2% dodecyl-β-D-maltoside (DDM) and centrifuged and the supernatant was incubated with Pansorbin to remove proteins interacting with protein A and centrifuged again. KOR in the supernatant was immunoprecipitated with guinea pig antibodies against the KOR(371–380) peptide (custom-generated, Ab5699) (2 μg/ml, overnight, 4°C) followed by goat anti-guinea pig IgG conjugated to agarose. Pelleted agarose beads were washed extensively and KOR was dissociated from agarose with Laemmli buffer. The mixture was resolved with SDS-PAGE followed by IB with rabbit anti-pT363 and anti-pS369 antibodies. In the wildtype, the p-KOR band appeared as a broad diffuse band with a median Mr of 60 ~ kDa and U50,488H treatment greatly enhanced the staining. KOR−/− mice did not show any staining in saline- or U50,488H-treated group. The blot was then stripped and re-blotted for total KOR with purified rabbit antibodies against the KOR(371–380) peptide (PA847p). Thus, anti-pT363 and anti-pS369 are specific for the phosphorylated KOR in the mouse brain. The experiment was performed twice with similar results. S: saline; U: U50,488H; WT, wildtype; KO, KOR−/−. (b), U50,488 dose-dependently promoted KOR phosphorylation at T363 and S369 in mouse brains as detected by immunoblotting with phospho-specific antibodies. Male CD-1 mice were injected (s.c.) with saline or an indicated dose of U50,488H (1, 2, 4, 6, or 10 mg/kg) and euthanized 30 min later. Brains were immediately removed and frozen. KOR was partially purified by immunoprecipitation, resolved with SDS-PAGE and IB was performed with rabbit anti-pT363 and anti-pS369 antibodies. The blot was then stripped and re-blotted for total KOR with purified rabbit antibodies against the KOR(371–380) peptide (PA847p). Experiments were performed twice with similar results. (c) Quantification of (b). Intensity of protein bands were quantified with ImageGauge software. p-KOR staining intensity was normalized against that of the total KOR in the same lane. The resulting data were then normalized against those of 10 μM U50,488H. Data are the mean of two samples, each from 4 mouse brains
5.3 U50,488H Promoted KOR Phosphorylation at T363 and S369 in Mouse Brains in a Dose-Dependent Manner
Male CD-1 mice were treated with saline or 1, 2, 4, 6 or 10 mg/kg U50,488H (s.c.) and killed 30 min later and brains were removed and frozen on dry ice immediately. KOR phosphorylation following IP of KOR was detected with IB as described above. As shown in Fig. 3b, U50,488H promoted KOR phosphorylation at T363 and S369 in a dose-dependent manner. The staining intensities of phosphorylated KOR were normalized against that of the total KOR in respective lanes, which were then normalized against that at 10 mg/kg U50,488H (Fig. 3c). The EC50 values of U50,488H-induced KOR phosphorylation were estimated to be ~1.5 mg/kg for T363 and ~ 3 mg/kg for S369 (Fig. 3c).
6 KOR Antibodies from Commercial Sources
Many antibodies generated against KOR peptides are available from commercial sources with varying levels of validation, but there have not been systematic studies as for other GPCRs [for example, [(Michel et al. 2009) and other articles in the same issue]. The burden of specificity validation is on researchers, who have to perform experiments to check antibody specificity in their own particular application(s). Anecdotal evidence indicates that researchers often found that the antibodies were not specific after spending money and devoting time and efforts.
7 Antibodies against Dynorphins: Some Considerations
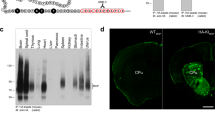
Dyn peptides, like other neuropeptides, are synthesized as large precursors (Watson et al. 1983) and sorted into large dense core vesicles. There they are processed by proteolytic cleavage and subsequent modifications like amidation to yield mature functional peptides [for a review, see (Schwarzer 2009)]. The precursor prodynorphin (pDyn) is processed to produce dynorphin A, dynorphin B, α-neoendorphin, and β-neoendorphin. For IHC, this provides high antigen density within these vesicles. In contrast to the membrane bound GPCRs, peptides are well protected from a direct influence of fixation of tissue. Still, target retrieval may enhance the permeability of membranes (cells and vesicle) and enhance signal intensity (Fig. 4). Interestingly, different antibodies yield partially contradictive results in IHC, irrespective of the fixation protocol applied. The processing of the precursor peptides depends on the presence of enzymes needed for maturation within the vesicle. This results in differently sized intermediate and mature peptides along the axon or between different neuronal populations. One example was reported from the pituitary gland. In the posterior lobe, processing to mature peptides appeared almost complete. In contrast, predominantly larger precursor fragments were isolated from the anterior lobe (Day and Akil 1989; Seizinger et al. 1984). Coexistence of pDyn and dynorphins in the same axon, even the same vesicle, was also reported from brain (Yakovleva et al. 2006). Available antibodies target different regions of the precursor and may be affected by the processing in opposite directions. Endo- and exoproteolytic processing may destroy the antigen, resulting in loss of signal. By contrast, some antibodies detect only free ends of peptides, thereby depending on the processing to generate the antigen (Fig. 4). Likewise in the hippocampus, pDyn is highly expressed in granule cells (Hurd 1996), but hard to detect with antibodies against pDyn. By contrast antibodies against mature DynA nicely label the axons of granule cells (Fig. 4). Processing of the propeptide also is reflected in the appearance of differently sized fragments in IB. The specificity of antibodies targeting mature dynorphins can hardly be controlled in IB. Therefore, KO animals are an essential control. Antibodies targeting mature DynA (Peninsula) or DynB (Peninsula and ABD serotec) and those targeting pDyn (Avivasysbio, Neuromics) or the middle segment of pDyn (Acris) yielded similar results, yet with some discrepancies (see Fig. 4b) and clear batch to batch variability.
Immunofluorescence images comparing antibodies against pDyn and Dyn A in central amygdala (a) and dentate gyrus (b) of WT and Dyn KO mice. For the optional antigen retrieval step, free-floating PFA-fixed 40 μm coronal brain sections were incubated in a 10 mM sodium citrate solution (pH 8.7) in an 80°C water bath for 20 min. After blocking, the sections were incubated with primary antibodies against pDyn 1:1,000 (Neuromics, host guinea pig, GP 10110, lot 100,031) or Dyn A 1:2,000 (Peninsula, host rabbit, T-4268, lot 06613) overnight at room temperature. Following washes, Alexa Fluor488 goat anti-guinea pig 1:1,000 (A11073, lot 1,637,243) was applied as secondary antibody for pDyn and Alexa Fluor488 donkey-anti-rabbit 1:1,000 (A21206, lot 2,156,521) for Dyn A for 2.5 h at room temperature. Images were acquired using a ZEISS Axio Imager M1 wide-field fluorescence microscope with a 20x objective. WT wildtype, KO pDyn knockout mice, TR target retrieval
8 Conclusion
When antibodies against the KOR, Dyn peptides or proDyn are used, it is critical to validate specificity of the antibodies in the intended application using exactly the same conditions for the experiments. Experimental conditions (most importantly antibody dilution) need to be optimized to minimize the background and maximize the signal and, in the process, optimal conditions may be achieved to have no staining in knockout mice. Proper controls have to be performed, including knockout mice, siRNA knockdown, transfected vs. untransfected cells, transfected cells expressing the target vs. closely related molecules. Knockout mouse samples are considered the most important controls for experiments involving animal tissues.
References
Appleyard SM, Patterson TA, Jin WZ, Chavkin C (1997) Agonist-induced phosphorylation of the kappa-opioid receptor. J Neurochem 69:2405–2412
Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law P-Y, Wessendorf MW, Elde R (1995) The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci U S A 92:5062–5066
Baker M (2015a) Antibody anarchy: a call to order. Nature 527:545–551. https://doi.org/10.1038/527545a
Baker M (2015b) Reproducibility crisis: blame it on the antibodies. Nature 521:274–276. https://doi.org/10.1038/521274a
Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, Persson A, Ottosson J, Wernérus H, Nilsson P, Lundberg E, Sivertsson A, Navani S, Wester K, Kampf C, Hober S, Pontén F, Uhlén M (2008) A genecentric human protein atlas for expression profiles based on antibodies. Mol Cell Proteomics 7:2019–2027. https://doi.org/10.1074/mcp.R800013-MCP200
Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, Anagnostou V, Rimm D (2010) Antibody validation. BioTechniques 48:197–209. https://doi.org/10.2144/000113382
Bourbeillon J, Orchard S, Benhar I, Borrebaeck C, de Daruvar A, Dübel S, Frank R, Gibson F, Gloriam D, Haslam N, Hiltker T, Humphrey-Smith I, Hust M, Juncker D, Koegl M, Konthur Z, Korn B, Krobitsch S, Muyldermans S, Nygren PA, Palcy S, Polic B, Rodriguez H, Sawyer A, Schlapshy M, Snyder M, Stoevesandt O, Taussig MJ, Templin M, Uhlen M, van der Maarel S, Wingren C, Hermjakob H, Sherman D (2010) Minimum information about a protein affinity reagent (MIAPAR). Nat Biotechnol 28:650–653. https://doi.org/10.1038/nbt0710-650
Chen C, Chiu YT, Wu W, Huang P, Mann A, Schulz S, Liu-Chen LY (2016) Determination of sites of U50,488H-promoted phosphorylation of the mouse kappa opioid receptor (KOPR): disconnect between KOPR phosphorylation and internalization. Biochem J 473:497–508. https://doi.org/10.1042/BJ20141471
Chen C, Willhouse AH, Huang P, Ko N, Wang Y, Xu B, Huang LHM, Kieffer B, Barbe MF, Liu-Chen LY (2020) Characterization of a knock-in mouse line expressing a fusion protein of kappa opioid receptor conjugated with tdTomato: 3-dimensional brain imaging via CLARITY. eNeuro 7. https://doi.org/10.1523/ENEURO.0028-20.2020
Day R, Akil H (1989) The posttranslational processing of prodynorphin in the rat anterior pituitary. Endocrinology 124:2392–2405
Drake CT, Patterson TA, Simmons ML, Chavkin C, Milner TA (1996) Kappa opioid receptor-like immunoreactivity in Guinea pig brain: ultrastructural localization in presynaptic terminals in hippocampal formation. J Comp Neurol 370:377–395
Freedman LP, Cockburn IM, Simcoe TS (2015) The economics of reproducibility in preclinical research. PLoS Biol 13:e1002165. https://doi.org/10.1371/journal.pbio.1002165
Gautron L (2019) On the necessity of validating antibodies in the immunohistochemistry literature. Front Neuroanat 13:46. https://doi.org/10.3389/fnana.2019.00046
Huang P, Liu-Chen LY (2009) Detecting the mu opioid receptor in brain following SDS-PAGE with multiple approaches. Front Biosci (Elite Ed) 1:220–227
Huang P, Chen C, Xu W, Yoon SI, Unterwald EM, Pintar JE, Wang Y, Chong PL, Liu-Chen LY (2008) Brain region-specific N-glycosylation and lipid rafts association of the rat mu opioid receptor. Biochem Biophys Res Commun 365:82–88
Huang P, Chen C, Liu-Chen LY (2015) Detection of mu opioid receptor (MOPR) and its glycosylation in rat and mouse brains by western blot with anti-muC, an affinity-purified polyclonal anti-MOPR antibody methods. Mol Biol 1230:141–154. https://doi.org/10.1007/978-1-4939-1708-2_11
Hurd YL (1996) Differential messenger RNA expression of prodynorphin and proenkephalin in the human brain. Neuroscience 72:767–783. https://doi.org/10.1016/0306-4522(96)00002-4
Kitchen I, Kelly M, Viveros MP (1990) Ontogenesis of kappa-opioid receptors in rat brain using [3H]U-69593 as a binding ligand. Eur J Pharmacol 175:93–96
Li JG, Chen C, Liu-Chen LY (2007) N-glycosylation of the human kappa opioid receptor enhances its stability but slows its trafficking along the biosynthesis pathway. Biochemistry 46:10960–10970
Liu JJ, Chiu YT, Chen C, Huang P, Mann M, Liu-Chen LY (2020) Pharmacological and phosphoproteomic approaches to roles of protein kinase C in kappa opioid receptor-mediated effects in mice. Neuropharmacology 181:108324. https://doi.org/10.1016/j.neuropharm.2020.108324
Liu-Chen L-Y, Chen C, Phillips CA (1993) Beta-[3H]funaltrexamine-labeled mu-opioid receptors: species variations in molecular mass and glycosylation by complex-type, N-linked oligosaccharides. Mol Pharmacol 44:749–756
Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1988) Anatomy of CNS opioid receptors. Trends Neurosci 11:308–314
Mansour A, Fox CA, Meng F, Akil H, Watson SJ (1994) Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci 5:124–144
Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ (1996) Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience 71:671–690
Michel MC, Wieland T, Tsujimoto G (2009) How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedeberg's Arch Pharmacol 379:385–388
Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M (2000) Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem 275:13727–13736
Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M (2002) Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J 21:1628–1637
Pillai-Kastoori L, Heaton S, Shiflett SD, Roberts AC, Solache A, Schutz-Geschwender AR (2020) Antibody validation for Western blot: by the user, for the user. J Biol Chem 295:926–939. https://doi.org/10.1074/jbc.RA119.010472
Rhodes KJ, Trimmer JS (2006) Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci 26:8017–8020. https://doi.org/10.1523/JNEUROSCI.2728-06.2006
Saper CB (2005) An open letter to our readers on the use of antibodies. J Comp Neurol 493:477–478. https://doi.org/10.1002/cne.20839
Saper CB, Sawchenko PE (2003) Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol 465:161–163. https://doi.org/10.1002/cne.10858
Schwarzer C (2009) 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 123:353–370. https://doi.org/10.1016/j.pharmthera.2009.05.006
Seizinger BR, Höllt V, Herz A (1984) Proenkephalin B (prodynorphin)-derived opioid peptides: evidence for a differential processing in lobes of the pituitary. Endocrinology 115:662–671. https://doi.org/10.1210/endo-115-2-662
Slowe SJ, Simonin F, Kieffer B, Kitchen I (1999) Quantitative autoradiography of mu-,delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res 818:335–345
Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, Rimm DL, Rodriguez H, Hiltke T, Snyder M, Yamamoto T (2016) A proposal for validation of antibodies. Nat Methods 13:823–827. https://doi.org/10.1038/nmeth.3995
Unterwald EM, Knapp C, Zukin RS (1991) Neuroanatomical localization of kappa 1 and kappa 2 opioid receptors in rat and Guinea pig brain. Brain Res 562:57–65
Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY (2011) Sex difference in ê-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5'-O-(3-[35S]thiotriphosphate) binding in the Guinea pig. J Pharmacol Exp Ther 339:438–450
Watson SJ, Khachaturian H, Taylor L, Fischli W, Goldstein A, Akil H (1983) Pro-dynorphin peptides are found in the same neurons throughout rat brain: immunocytochemical study. Proc Natl Acad Sci U S A 80:891–894. https://doi.org/10.1073/pnas.80.3.891
Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekström TJ, Hauser KF, Pickel VM, Bakalkin G (2006) Prodynorphin storage and processing in axon terminals and dendrites. FASEB J 20:2124–2126. https://doi.org/10.1096/fj.06-6174fje
Acknowledgements
The writing of this manuscript was supported by the NIH grants R01DA041359, R21DA045274 and P30DA013429 (LYLC) and by the FWF grant W1206-B05 (SC). We thank Dr. Stefan Schulz of Jena University Hospital, Friedrich-Schiller-Universität, Jena, Germany for pS356/pT357 antibodies.
Conflict of Interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chen, C., Widmann, M., Schwarzer, C., Liu-Chen, LY. (2021). Considerations on Using Antibodies for Studying the Dynorphins/Kappa Opioid Receptor System. In: Liu-Chen, LY., Inan, S. (eds) The Kappa Opioid Receptor. Handbook of Experimental Pharmacology, vol 271. Springer, Cham. https://doi.org/10.1007/164_2021_467
Download citation
DOI: https://doi.org/10.1007/164_2021_467
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-89073-5
Online ISBN: 978-3-030-89074-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)