Abstract
The cytoskeleton, mainly consisting of microtubules, intermediate filaments and microfilaments, along with cytoskeleton associated and interconnecting proteins as well as the centrosome, plays enormously important roles in all stages of embryogenesis and undergoes significant changes to accommodate a diversity of cellular functions during gametogenesis, oocyte maturation, fertilization and pre-implantation embryo development. The varied functions of the cytoskeleton can be accomplished on many different levels, among which are a diversity of different posttranslational modifications (PTMs), chemical modifications that regulate activity, localization and interactions with other cellular molecules. PTMs of the cytoskeleton, including phosphorylation, glycosylation, ubiquitination, detyrosination/tyrosination, (poly)glutamylation and (poly)glycylation, acetylation, sumoylation, and palmitoylation, will be addressed in this chapter. Focus will be on (1) Microtubules, microtubule organizing centers (centrosomes), intermediate filaments, microfilaments and their PTMs; (2) Cytoskeletal functions and cytoskeletal PTMs during gametogenesis and oocyte maturation; and (3) Cytoskeletal functions and cytoskeletal PTMs during fertilization and pre-implantation embryo development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Posttranslational modifications
- Cytoskeleton
- Microtubules
- Centrosomes
- Meiosis
- Sperm
- Fertilization
- Mitosis
- Cell division
- Embryo development
Introduction
The cytoskeleton plays enormously important roles in all stages of embryogenesis and it undergoes significant changes to accommodate a diversity of cellular functions during gametogenesis, oocyte maturation, fertilization and pre-implantation embryo development. Three major components comprise the cytoskeleton that are discussed in more detail below and include microtubules, intermediate filaments and microfilaments. The present chapter will also include the centrosome, a major microtubule organizing center that plays an essential role in the organization of the interconnected cytoskeleton and significantly impacts structural and metabolic functions through its microtubule organizing capabilities. The varied functions of the cytoskeleton can be accomplished on many different levels, among which are a diversity of different posttranslational modifications (PTMs), chemical modifications that regulate activity, localization and interactions of cytoskeletal proteins with other cellular molecules. PTM modifications of the cytoskeleton include phosphorylation, glycosylation, ubiquitination, detyrosonation/tyrosination, (poly)glutamylation and (poly)glycylation, acetylation, sumoylation, and palmitoylation, among others, as will be discussed in more detail below. These modifications are important for regulated cellular functions and they also allow dynamic changes in response to different stimuli.
Cytoskeletal reorganization and remodeling occurs throughout embryo development and allows for the establishment of the germ cell lineage and the migration of primordial germ cells (PGCs) from the hindgut region to the genital ridges in the post-implantation embryo. Oocyte maturation depends on cytoskeletal functions for the formation of the meiotic spindle at the oocyte center and its migration to the oocyte periphery followed by meiosis I and meiosis II, to separate chromosomes and achieve haploidy in preparation for fertilization. The process of fertilization triggers a cascade of cytoskeletal reorganizations to form the sperm aster, zygote aster, mitotic apparatus and subsequent symmetric and asymmetric cell divisions, and cellular polarization in the developing pre-implantation embryos. These processes will be addressed in more detail in the specific sections below, with focus on (1) Microtubules, microtubule organizing centers (centrosomes), intermediate filaments, microfilaments and their PTMs; (2) Cytoskeletal functions and cytoskeletal PTMs during gametogenesis and oocyte maturation; and (3) Cytoskeletal functions and cytoskeletal PTMs during fertilization and pre-implantation embryo development.
Microtubules, Microtubule Organizing Centers (Centrosomes), Intermediate Filaments, Microfilaments and Their Posttranslational Modifications
As mentioned above, the cytoskeleton consists of a complex network of fibers primarily composed of three families of protein molecules that are assembled to form three main types of filaments: microtubules, intermediate filaments and microfilaments. Hundreds of accessory proteins link these filaments to each other as well as to different cellular components that allow intra- and intercellular communications and signal transduction to fulfill specific cellular functions.
Microtubules are composed of α/β subunit heterodimers that typically are linked into 13 protofilaments to compose one single complete microtubule. The lateral association of protofilaments forms a cylindrical microtubule with an outer diameter of 25 nm. The number of protofilaments to form one microtubule may differ in different systems and the regulation of protofilament number may be linked to tubulin acetylation [1]. Various and different α and β-tubulin isotypes are expressed within one cell to form microtubules with clearly differentiated and specific cellular functions, although the isoforms may to some extent be functionally interchangeable.
Microtubules are polarized structures with a dynamic plus end and a minus end that can be stabilized by attachment to cellular structures such as microtubule organizing centers (MTOC; centrosome). Individual microtubules undergo highly dynamic changes within a cell which is referred to as ‘dynamic instability’, allowing for phases of growth and rapid depolymerization. In addition to α- and β-tubulin subunits, four more tubulins have been discovered in more recent years which are delta (Δ)-, epsilon (ε)-, zeta (ζ)- and eta (η)-tubulins [2], whose functions are linked to eukaryotic centrioles and/or basal bodies. The γ-tubulin is a member of the tubulin family whose functions include microtubule nucleation and organization; it is primarily found at MTOCs (centrosomes) but also can be localized to other cellular compartments such as the plasma membrane where microtubule nucleation can take place.
Microtubules are heterogeneous in length and fulfill a variety of different functions in cells either alone or by interacting with different cellular components. The plus-end directed microtubule motor protein kinesin and minus-end directed microtubule motor protein dynein are important for transport of cargo along microtubules to their functional destinations and therefore also play a role in cellular and intercellular signal transductions. The regulation of microtubule dynamics and stability includes participation of a heterogeneous group of numerous non-motor microtubule-associated proteins (MAPs) and microtubule-interacting molecules that provide additional functional microtubule diversity.
Microtubules are important for maintenance of cell shape, cellular transport of membrane vesicles, macromolecules and organelles such as mitochondria, for cell motility, meiosis, mitosis and cell division, and for the formation of centrioles, immotile primary cilia, and motile cilia and flagella.
Drugs that interfere with microtubule polymerization include colcemid, colchicine, nocodazole, podophyllotoxin, and griseofulvin, among others; the best-known drug that promotes tubulin polymerization and interferes with tubulin depolymerization is taxol [3–5], a drug that is used for cancer treatment, as it prevents depolymerization of mitotic microtubules, therefore preventing cancer cells from rapid cell division.
Biological structures that are distinctly enriched in microtubules include cilia, primary cilia, centrioles, the meiotic apparatus, and the mitotic apparatus that are briefly described as follows.
Cilia: Cilia or flagella are microtubule-based structures with a cytoskeletal core referred to as the axoneme that is important for sperm tail motility, for functions of cells in the Fallopian tubes to move the egg from the ovary to the uterus, and for a diversity of ciliated cell functions throughout development. Motile cilia are found in all stages of embryo development and they are distinguished from non-motile primary cilia by their microtubule organizations and by the presence of dynein. Motile cilia are known for their 9 + 2 microtubule arrangement which refers to their composition of nine outer doublet microtubules and a central pair of single microtubules. Each outer microtubule doublet contains a complete A tubule and an incomplete B tubule that is fused with the complete A tubule.
Primary cilia: The primary cilium is a specialized non-motile cilium that protrudes as one single cilium from almost all cells in our body [6]. It originates from a basal body that develops from the mother (elder) centriole of the cell’s centrosome complex and becomes coordinated with cell cycle regulation. Primary cilia contain 9 outer microtubule doublets but no central microtubule pair (9 + 0), and no dynein. These sensory cellular antennas with a receptor-rich membrane coordinate a large number of signaling pathways that are coupled with nuclear activation and cell division. A close relationship exists between primary cilia and the centrosome complex, as primary cilia undergo cell cycle-specific assembly and disassembly and share mother centriole components during this process. Typically, in the G1 stage, primary cilia are assembled when the distal end of the centrosome’s mother centriole becomes reorganized to form the basal body for the primary cilium’s axoneme that directly assembles onto the microtubules of the modified mother centriole. Primary cilia are disassembled at the entry into mitosis when fully matured centrioles become located at the mitotic poles; they are reassembled during exit from mitosis (reviewed in [7–10]). Primary cilia play important roles in embryo development and are critical for signal transduction pathways [11, 12]. Primary cilia dysfunction has been implicated in a variety of diseases and disorders (reviewed in [13]).
Centrioles: Centrioles are cylindrical structures composed of nine outer triplet microtubules, each consisting of a complete A, an incomplete B, and an incomplete C microtubule. Centrioles do not contain central microtubule pairs. In mitotic cells, a typical centriolar duplex consists of two centrioles representing mother and daughter centriole that are organized perpendicular to one another. The mother (older) centriole is structurally and functionally distinguished from the daughter (younger) centriole by characteristic distal and subdistal appendages. Both centrioles are connected through interconnecting fibers.
Meiotic apparatus: The meiotic apparatus (meiotic spindle) consists of acentriolar centrosome material, chromosomes, and two classes of microtubules that connect both meiotic spindle poles (pole-to-pole or interpolar microtubules) and those that connect one pole with the kinetochores of chromosomes (kinetochore microtubules). The meiotic apparatus is formed at the oocyte center during oocyte maturation, following germinal vesicle breakdown (GVBD). The fully formed spindle moves to the oocyte periphery to become the MI spindle followed by formation of the MII spindle. Two asymmetric cell divisions following meiosis I and meiosis II take place, resulting in two polar bodies and a haploid oocyte.
Mitotic apparatus: The mitotic apparatus is formed to separate chromosomes equally into two new daughter cells. A typical mitotic apparatus consists of centrosomes containing a pair of perpendicularly oriented centrioles, chromosomes, and two classes of microtubules (pole-to-pole or interpolar microtubules and kinetochore microtubules) that along with numerous accessory proteins provide the complete machinery to separate chromosomes into the dividing daughter cells. In the mouse preimplantation embryo, no centrioles have been observed during mitotic cell divisions up to the blastula stage, while in non-rodent mammalian systems, the sperm-derived centrioles are present during mitosis that participate in symmetric and asymmetric cell divisions throughout pre-implantation embryo development (reviewed in [7, 9]).
Microtubule Organizing Centers (MTOCs; Centrosomes): In addition to the above-mentioned microtubule-enriched structures, the microtubule organizing centers (MTOCs; centrosomes) are important for cytoskeletal coordination and functions (reviewed in more detail in [8, 9]). The functional units of MTOCs can have different molecular compositions that organize various microtubule formations and include spindles of the meiotic and mitotic spindle apparatus as well as cytoplasmic asters (reviewed in [7–9]). MTOCs may or may not contain centrioles. Most scientists in the field define MTOCs as structural units that contain γ-tubulin and numerous other proteins that are important for cell cycle-specific nucleation and organization of microtubules (reviewed in [14]). The best-studied MTOCs are the centriole-containing centrosomes in somatic cells that are composed of a large number of centrosomal proteins, with at least 60 of them being directly associated with the interphase centrosome structure, and numerous others that are associated with centrosomes to perform cell cycle-specific functions (reviewed in [14]). Details on centrosome structure and functions are not included in the present chapter but have been reviewed previously [7–9, 15–17].
Intermediate Filaments are ropelike fibers with a diameter of ~10 nm. These fibers are important for cellular structure and organization, serving as mechanical scaffolds in many capacities. They are composed of intermediate filament proteins that comprise a large and heterogeneous family of over 50 different proteins being subcategorized into six different types or classes in vertebrates. One type of intermediate filaments is organized into the nuclear lamina, a meshwork of filaments underlining the inner nuclear envelope. Other types play a role in intra- and intercellular communications and include vimentin and cytokeratin, among others (reviewed in [18]). Intermediate filaments are more stable to experimental treatments compared to microtubules and microfilaments.
Microfilaments (actin filaments) are composed of actin subunits that form two-stranded helical polymers resulting in filaments with a typical diameter of ~7–8 nm. Microfilaments are important for a great variety of cellular functions including cellular motility, membrane trafficking and shape changes, among numerous others. Actin filaments can be highly dynamic or they can be anchored, such as in muscle tissue. Cellular microfilaments can be organized in linear bundles, two-dimensional networks, and three-dimensional gels. Similar to microtubules, different actin isoforms allow different actin filament functions.
A large number of microfilament-associated and microfilament-interacting molecules have been identified that assure varied microfilament functions. Of the actin-based cell motility proteins, several have been well studied while others are still being investigated in numerous laboratories. The Arp2/3 (actin-related protein 2/3) complex is a well-known actin nucleation complex that is important for the formation of new actin filaments off the sides of existing microfilaments (reviewed by [19]).
Posttranslational Modifications of Cytoskeletal Proteins with a Focus on Tubulins
PTMs allow for significant diversity, complexity, and heterogeneity of gene products on qualitative and quantitative levels and for spatio-temporal control of protein activities and dynamics in biological processes. As mentioned above, PTMs modulate molecular interactions, protein localization, and protein stability which are important for cellular functions, while dysfunctions have been implicated in various diseases including cancers, diabetes, dysmetabolic syndrome, neurological disorders and others for which onset may be traced back to PTM disorders in early embryo development.
PTMs are chemical alterations to protein structure that frequently involve substrate-specific enzymes and may include more than 300 different types of modifications. Gene products can be modified in various combinations resulting in a large heterogeneity of the protein population. Several methods have been employed to determine PTMs including mass spectrometry, two-dimensional gel electrophoresis (2DE), immunological probes, and others (reviewed in [20]). The following will provide an overview of PTMs and will highlight PTMs of the cytoskeletal proteins including tubulins, intermediate filament proteins, actin, and centrosomes.
Of the PTMs referred to in the introduction, the most generally studied are phosphorylation, ubiquitination, and sumoylation [21–23], for which functions have been established, although a number of functions associated with these PTM changes for tubulin are not yet fully understood. The best understood PTMs for tubulin (microtubules) include detyrosination and the related Δ2 modification, (poly)glutamylation, (poly)glycylation, and acetylation [20, 21, 23, 24], which will be addressed below. Most of the PTMs occur on formed microtubules and provide specific characteristics for specific functions. In general, PTM takes place at amino acid side chains or peptide linkages with the majority of them being enzyme-mediated. PTMs can alter the functional capabilities, dynamics and biophysical properties of microtubules and their interacting molecules.
Protein phosphorylation most frequently occurs on serine, threonine or tyrosine residues and plays significant roles in the fine-regulation of cell cycle stages and in a number of different signal transduction pathways.
Ubiquitination is important for the degradation of proteins and plays a role in numerous specific cell cycle regulation events, as will be discussed below. Ubiquitination and acetylation are the best characterized lysine modifications of cytoskeletal proteins.
Glycosylation affects protein folding, distribution, stability, and activity.
Glutamylation and glycation refer to the addition of glutamate (glutamylation) or glycine (glycylation) residues onto glutamate residues in the C-terminal tails (CTTs) of both α-and β-tubulin [21, 24]. Glycylation is mainly associated with tubulin incorporated into axonemes (cilia and flagella) while glutamylation is seen in neuronal cells, centrioles, axonemes, and the mitotic spindle.
Glycylation and glutamylation can take place on either α-tubulin or β-tubulin, forming long or short side chains. These PTM modifications can occur on a single microtubule or on different microtubules, but the selectivity for different modification sites on tubulin tails or their spatial and temporal regulation is not yet known. Both PTMs can overlap and compete with each other for modification sites [25, 26].
Tubulin polyglutamylation and polyglycylation: Polyglutamylation refers to progressive addition of Glu residues onto the γ-carboxyl group of one or more Glu residues near the C-terminus of polymerized tubulin [27–29]. Hydrolysis of linear Glu chains removes polyglutamylation from tubulin and it also generates Δ2-tubulin from detyrosinated tubulin, as addressed below. Enzymes of the TTLL (tubulin Tyr ligase-like) family carry out the polyglycylation PTM of tubulin which plays a role in the dynamics and stability of axonemes. The TTLL enzyme family comprises glutamylating [30–32] and glycylating [25, 26, 33] enzymes.
Sumoylation refers to the reversible addition and removal of SUMO (small ubiquitin-related modifier) polypeptides on lysine residues that play a role in cell cycle regulation as well as in other cellular processes and in the DNA damage response (DDR). Sumoylation is associated with changes in stability and function of various cytoskeletal structures.
Palmitoylation: Tubulin has been shown to incorporate radioactively labeled [3H]palmitate, predominantly on the α-subunit at cysteine 376. Palmitoylation has been studied in mutants of the budding yeast Saccharomyces cerevisiae in which mitosis occurred but aster microtubules showed defects and may have affected microtubule interactions with the cortex (reviewed in [21]).
Detyrosonation/tyrosination: Detyrosination of microtubule polymers is based on the removal of the gene-encoded C-terminal tyrosine of α-tubulin by a yet to be identified carboxypeptidase [21, 24, 34, 35]. The removal of the C-terminal Glu residue on detyrosinated tubulin results in Δ2-tubulin [36]. The Δ2-tubulin PTM is irreversible and catalyzed by deglutamylase enzymes of the CCP family [37]. Historically, detyrosinated tubulin was referred to as Glu-tubulin, and it was renamed to avoid confusion when glutamylated tubulin was discovered [27]. Tyrosination on the other hand involves the addition of a tyrosine residue to the C-terminal glutamate residue of α-tubulin within the soluble tubulin heterodimers that is catalyzed by tubulin tyrosine ligase (TTL) [21, 24]. Tyrosination refers to the enzymatic addition of Tyr to α-tubulin; the reversible tyrosination–detyrosination cycle is initiated by the removal of a Tyr functional group (detyrosination), whereas re-addition of Tyr (tyrosination) returns tubulin to its nascent state. The detyrosination/tyrosination PTM cycle plays a role in the recruitment of two types of microtubule-binding proteins, molecular motors and plus-end tracking proteins (+TIPs). For example, the microtubule motor protein kinesin-1 binds preferentially to detyrosinated microtubules in neuronal cells [38] while kinesin selectively binds to detyrosinated tubulin, therefore allowing selective interactions with other cellular components such as vimentin ([39]; reviewed in [20]).
Acetylation is a reversible PTM that plays a major role in tubulin modifications and is also an important modification for cross-talk with other PTMs including phosphorylation, ubiquitination and methylation, all of which can modify the biological function of acetylated proteins [40].
Acetylation of Lys-40 on α-tubulin takes place on the luminal face of the microtubule polymer [41]; newer studies identified acetylation on Lys252 of β-tubulin that preferentially takes place on non-polymerized tubulin [42]. While the enzyme for acetylation has not yet been identified, two tubulin deacetylating enzymes are known: the histone deacetylase 6 (HDAC6) [43, 44] to reverse acetylation of Lys40, and sirtuin 2 (SIRT2) [45] which shows preferential activity towards a tubulin peptide substrate as compared to a histone peptide substrate.
So far, PTMs have mainly been described for α- and β-tubulins. Most α-tubulins are acetylated on the ε-amino group of a conserved lysine residue at position 40 in the N-terminus. Acetylation is frequently associated with stable microtubules as seen in axonemes, and it takes place after microtubule assembly; however, acetylation does not necessarily cause stabilization, which has been shown in several studies and includes the finding that the HDAC6 protein, itself, can inhibit microtubule growth [46]. PTMs on the other hand, can protect microtubules from severing enzyme activities such as that of Katanin and Spastin [47]; on the other hand, tubulin modification by detyrosination allows the microtubule-depolymerizing kinesins from the kinesin-13 family (mitotic centromere-associated kinesin (MCAK; also known as KIF2C) and KIF2A) to preferentially depolymerize the now tyrosinated microtubules [48]. The studies by Peris et al. [48] were the first to demonstrate a mechanism explaining how detyrosination can stabilize microtubules, as it was shown that tyrosinated microtubules are better substrates of depolymerizing kinesins.
It is also important that microtubule motor activity can be regulated by PTMs which may serve as an effective mechanism to spatially and temporally segregate a range of subcellular transport activities. For example, tubulin detyrosination regulates the binding and motor activity of the ubiquitous Kinesin-1 (the ‘conventional’ kinesin, KIF5) to microtubules [39, 49–51]. Polyglutamylation and polyglycylation may further play a role in the fine-tuning of microtubule motor activities (reviewed in [23]).
The above-mentioned studies and others show that PTMs create marks on microtubules that allow targeting of cytoskeletal structures for their destination during specific cellular functions.
The intermediate filament proteins vimentin and cytokeratin are acetylated on lysine residues which has been reported to destabilize the polymer [52, 53], thereby destabilizing intermediate filament functions, which differs from microtubule and microfilament acetylation.
In regard to microfilaments, six of the seven ARP2/3 complex subunits are acetylated on lysine residues [54]. A regulator of the ARP2/3 complex, cortactin, is important for invadopodia functions [55]; it is acetylated along with the actin/ARP2/3-interacting proteins cofilin and coronin [54, 56]. Cortactin can be acetylated on nine different lysine residues, which results in decreased actin-binding capacity and decreased translocation to the periphery (reviewed in [57].
The three major isoforms of actin, α, β, and γ-actin all can be acetylated [54, 58]. Acetylation of γ-actin has been implicated in stabilization of stress fibers. Formins participate in the microfilament-mediated formation of the contractile ring at the end of mitosis which may involve ubiquitin-mediated degradation of formin to complete cell division.
Posttranslational Modifications of Centrosomes, Basal Bodies, Cilia and Flagella
As mentioned above, posttranslational modifications can define the location, interactions, and function of proteins which also holds true for centrosomal proteins and centrosome protein complexes.
While relatively little is known about PTMs in centrosomes, various data are available on some aspects that in part are related to the entire centriole-centrosome complex; a non-catalytic subunit of the TTLL1 complex, polyglutamylase complex subunit 1 (PGS1) [59], was specifically localized to the centrosome when overexpressed in cultured cells [60], perhaps implying a role for polyglutamylation in centrosome functions.
Several other TTLL enzymes have been associated with the centrosome complex and basal bodies in overexpression studies [32]. However, as mentioned above, centrosomes are communication centers for various cellular processes and it is not clear which enzymes are responsible for the modification of centrioles and which enzymes accumulate on the centrosome or basal body to assume other functions for related cellular activities such as the modification of the mitotic spindle or for cilia-related functions.
Perhaps the best-understood posttranslational modifications of centrosomes are phosphorylations that modulate the functions of centrosome proteins. Recent proteomic screens and computational analysis identified a variety of substrates for centrosome-associated kinases [61–63]. Phosphorylation-dependent functions of specific centrosome proteins have been described and include CPAP, the human homologue of Sas-4 involved in centriole duplication, which has been identified as a PLK2 substrate. The PLK2-phosphorylated CPAP localizes to procentrioles and plays a role in procentriole elongation [64]. Other posttranslational modifications include Sumoylation and ubiquitination that play important roles in regulating centrosome functions. Ubiquitination of the centrosomal protein CP110 during the G2 phase of cell cycle, and its subsequent degradation is required for centrosome and spindle integrity [65–72]. Several investigators have shown that ubiquitin-dependent proteolysis is important for centrosome duplication, procentriole formation and control of daughter centriole length [69, 70, 73].
The centriolar protein SAS6 is required in human cells for procentriole nucleation and formation which is regulated through the ubiquitin ligase APCCdh1 that targets SAS-6 for degradation [74]. The E3 ubiquitin ligase complex SCF-FBXW5 ubiquitinates SAS6 and is negatively regulated by the Polo kinase PLK4 [75]. Autophosphorylation of PLK4 results in ubiquitin and proteasome-dependent degradation of PLK4 which then causes a block of centriolar reduplication by releasing the activity of the SCF-FBXW5 complex. These molecular cascades use cycles of phosphorylation and proteolytic processes for centriolar replication [75].
In addition to phosphorylation and ubiquitination, Sumoylation of centrosomal proteins is important for regulation of the nuclear localization of centrin-2, which otherwise resides in mammalian centrosomes as core centrosomal protein [76]. While these data provide some insights into PTMs of centrosomes, this aspect of centrosome biology is only partially understood and more studies are required to better understand mechanisms, regulation, and functions of PTMs of centrosomal proteins.
Proteins of the NEK family are important for centrosome functions as well as for basal body, motile cilia, and primary cilia functions [77, 78]. NEK49, a NIMA (never in mitosis gene A)-related kinase localizes to the basal body and shows a decreasing gradient along the flagellum which correlates with the glutamylation profile of the axoneme. The human genome encodes at least 11 NEKS [79] and disruption of the Nek1 gene in mice causes pleiotropic effects, including male sterility and polycystic kidney disease [80], which is caused by primary cilia dysfunction and potentially consistent with a role for NEK1 in tubulin modification.
Detyronisation is unique in motile cilia in that the central pairs of microtubules are mostly detyrosinated, while the B-tubule of the outer microtubule doublets is more heavily tyrosinated compared to the A-tubule which has been studied in Chlamydomonas flagella (reviewed in [23]). In axonemes, it has been shown that Δ2-tubulin is enriched on the B-tubule [81] which may indicate irreversible detyrosination to provide stability.
Acetylation is significantly enriched in axonemal microtubules [82, 83] but it is not clear whether or not it affects the assembly and function of cilia; this relates to other findings in mice lacking HDAC6 which did not affect axonemal functions such as male fertility. Nevertheless, developmental defects were seen in HDAC6-knockout mice which included accelerated bone growth that may be the result of dysfunctional signal transductions in primary cilia with increased acetylation. Functional studies [84, 85] revealed that αTAT1 (enzyme MEC-17) that carries out acetylation of Lys40 on α-tubulin decreased acetylation and slowed ciliary assembly, affecting primary cilia functions. Other strong enrichment of PTMs in cilia and flagella include polyglutamylation and polyglycylation [86].
PTMs may be developmentally regulated. For example, in Drosophila melanogaster testes, the assembly of sperm axonemes was not affected by depletion of a key glycylase while it was affected in later stages, in which loss of glycylation resulted in complete disassembly of sperm axonemes and total sterility of male flies [25].
While there are evolutionary differences in cilia, in most animal species polyglutamylation is required for ciliary assembly and functions and it may be involved in intraflagellar transport (IFT). Beating asymmetry in cilia was prevented in Ttll1- knockout mice in which the levels of polyglutamylation on both α- and β-tubulin were reduced. Airway cilia and sperm flagella were most affected in these animals [87, 88].
Glutamylation and glycylation may allow different functions in axonemes and they may regulate each other. Glycylation allows structural stabilization while glutamylation is important for the regulation of beating behavior. Nearly complete loss of cilia was shown in zebrafish with double knockdown of the glycylase Ttll3 and the polyglutamase Ttll6.
Centrioles and basal bodies are enriched in PTMs with the highest enrichment of detyrosinated [89], acetylated [90], and polyglutamylated tubulin and Δ2-tubulin [81]. Polyglutamylation is distinguished by long Glu side chains [89, 91]. In HeLa cells, injection of the glutamylation-specific antibody GT335 [92] resulted in disassembly of centrosomes [93], possibly as a result of centriole destabilization during the G2/M stage of cell cycle [94].
Posttranslational Modifications in Cycling Cells
In cultured cells, detyrosination [95], acetylation [90], and polyglutamination [91] are enriched during cell division in the central mitotic spindle and in the midbody, but not in astral mitotic microtubules. The PTMs shown in the central mitotic spindle may play a role in stabilizing kinetochore microtubules. Stabilization of microtubules by tyrosination may regulate the activity of the depolymerizing kinesin MCAK [48], which becomes important for chromosome segregation during anaphase [96] and may serve as regulator for chromosome segregation. Also, polyglutamylation induces enzymatic microtubule severing [97], thereby controlling the length of the mitotic spindle via katanin-mediated microtubule severing [98, 99]. Further, by controlling spastin-dependent severing [100], polyglutamylation may ensure timely abscission during cytokinesis.
Temporal and spatial control of tubulin PTMs may allow for specific functions of multiple microtubule-interacting proteins during cell division.
Cytoskeletal Functions and Cytoskeletal PTMs During Gametogenesis and Oocyte Maturation
The stages of male and female gametogenesis and the role of the cytoskeleton in these processes have been described in detail in previous papers and reviews [101–107], as well as in several chapters of this book, and are not specifically addressed in this section. Briefly, gametogenesis refers to a complex developmental process resulting in the production of male of female germ cells. During spermatogenesis, mature spermatozoa are produced in a well-organized sequence of events in which cell proliferation, meiosis and differentiation take place. To accomplish this process, the proliferating primordial germ cells (PGCs) migrate from their site of origin to the future gonad position to associate with somatic gonadal precursor cells for gonad formation. PGCs then differentiate in a sex-specific manner while undergoing a distinct program of proliferation and quiescence. In the male genital ridge, the PGCs become enclosed by precursor somatic Sertoli cells followed by seminiferous cord formation during which PGCs and Sertoli cells form solid strands of cells which later become seminiferous tubules when the cords form a lumen. PGCs enclosed in seminiferous cords undergo morphological changes resulting in gonocytes that proliferate for several days before being arrested at the G0/G1 stage of cell cycle. In rats and mice, gonocytes resume proliferation within a few days after birth to give rise to spermatogonial stem cells (SSCs) which initiate the first round of spermatogenesis to produce spermatozoa at the onset of reproductive age [108].
Centrioles and centrosomes undergo important developmental processes during gametogenesis. As reviewed in detail by Manandhar et al. [109] and Sun and Schatten [110], when spermatids transform into mature spermatozoa, most of the centrosomal material is lost. However, the proximal centriole is completely retained and becomes localized close to the nucleus to perform critically important functions after fertilization, as will be discussed in section “Cytoskeletal Functions and Cytoskeletal PTMs During Fertilization and Pre-implantation Embryo Development”. The distal centriole becomes partially reduced, and it becomes associated with the sperm axoneme in the midpiece and tail after being restructured to lose the triplet microtubule organization while forming a central pair of microtubule doublets, as is characteristic for the axoneme.
Of all the microtubule-containing structures that play a role in spermiogenesis, we mainly have data on the transient caudal manchette and on the stable axoneme (reviewed in [111]). Both structures contain PTMs but only the PTMs of the axoneme have been studied in detail.
Oocyte development begins during fetal growth and is completed in the adult. During oogenesis, centrioles that are present in oogonia until the pachytene stage of oogenesis [112] (reviewed in [109]) become lost, and the mature oocyte is devoid of centrioles in most species. However, reduced amounts of centrosomal components are present in the cytoplasm (reviewed in [109]) that can be visualized in parthenogenetically activated oocytes [15, 16, 113] and become associated with the sperm centriole after fertilization.
Mammalian oocytes are arrested in diakinesis of meiotic prophase I at birth and are located within the primordial follicle pool. A large nucleus (germinal vesicle; GV) forms during follicle growth. Stimulation by gonadotrophins induces GV-breakdown (GVBD) and meiotic resumption followed by the first meiotic cell cycle stages of prometaphase I, metaphase I, anaphase I, and telophase I to accomplish chromosome separation and extrusion of the first polar body (PB1). The second meiotic cell cycle continues up to the metaphase II stage (MII), at which the oocyte becomes arrested until fertilization or parthenogenetic activation takes place. The MII oocyte is the end result of a complex process of oocyte maturation during which the oocyte becomes fertilization-competent and achieves developmental potential. In most species except in the mouse, the MII spindle is localized perpendicular to the cell surface, and it displays a barrel-shaped to pointed spindle morphology (it is parallel to the egg surface in the mouse which represents one of the features that are different in mouse oocytes compared to other non-rodent mammalian oocytes). The MII spindle takes a special place in the reproductive cell cycle as this is the stage at which fertilization occurs in most mammalian species. Although it appears static in immunofluorescence and transmission electron microscopy (TEM) images, the MII spindle is a highly dynamic structure whose integrity is actively maintained by a complex set of regulatory kinases and other regulatory proteins. Loss of spindle integrity includes alterations in regulatory kinases as has been shown in aging oocytes (reviewed in [114, 115]). Failure in MII spindle function can lead to aneuploidy and subsequent cell and developmental abnormalities resulting in abortion, disease, or developmental defects (reviewed in [114–116]).
Posttranscriptional regulation of pre-existing maternal mRNA and posttranslational modification of proteins is critical for meiotic progression from the primordial stage to the zygote and several key proteins such as CDK1/cyclin B are posttranslationally modified to precisely control meiotic progression. Others include the kinases PKA, AKT, MAPK, Aurora A, CaMKII, the phosphatases CDC5, CDK14s and others that participate in the meiotic process. The posttranscriptional and posttranslational modifications of proteins other than cytoskeletal proteins have recently been reviewed for mouse oocytes by Kang and Han [117] and are not addressed in this chapter that is focused on cytoskeletal proteins.
Compared to our knowledge of PTMs in somatic cells, the information for cytoskeletal PTMs in germ cells is still sparse despite the fact that microtubule organization and centrosome functions are among the most important processes for oocyte maturation with consequences for successful fertilization and embryo development. In this section, cytoskeletal functions will be correlated to their known PTMs keeping in mind that the information on PTM-related cytoskeletal functions is incomplete, as only sporadic data are available for selected aspects. While the best data for PTMs come from studies of mature sperm tails, only scarce data are available for maturing oocytes from the GV stage to the formation of the central, MI, and MII meiotic spindles.
The mechanisms for spindle formation and migration in different systems have previously been reviewed in detail [8, 116, 118, 119] and are not specifically addressed here. Significant reorganization of the microtubule network takes place during oocyte maturation which is shown in Fig. 4.1, highlighting microtubule organization and centrosome formation at the meiotic spindle poles that are critical for accurate chromosome segregation. Two asymmetric divisions take place in oocytes after first and second meiosis, when the first and second polar bodies are extruded, respectively, to remove half of the chromosome complement and excess centrosomal material.
Schematic diagram of oocyte maturation stages. (a): Germinal vesicle (GV) stage; (b) germinal vesicle breakdown (GVBD); (c) first meiotic spindle in metaphase; (d) first meiotic spindle in anaphase and first polar body extrusion; (e) meiosis II (MII) metaphase II spindle; (f ) anaphase II spindle with extruded second polar body. Blue = microtubules; green = chromatin/chromosomes; red = centrosomal components
The acentriolar centrosome formation at the two meiotic spindle poles includes participation of the centrosomal proteins γ-tubulin, pericentrin, centrin, and the nuclear mitotic apparatus protein, NuMA. The specific participation of these proteins may differ in different systems on both qualitative and quantitative levels. The acentriolar centrosomes play important roles in microtubule stabilization and in maintenance of functional meiotic spindles. As mentioned above, spindle integrity is lost in aging oocytes which includes centrosome and microtubule instabilities, with consequences for chromosomal mis-segregation resulting in female infertility and developmental abnormalities. It is not known whether PTMs are altered in microtubules of aging oocytes.
While we do not yet have a complete understanding of PTMs in MII oocytes, some data have emerged in recent years that have mainly been generated in the mouse system. In the unfertilized mouse oocyte, it was shown that the acetylated form of α-tubulin is predominantly localized at the poles of the arrested MII spindle, as detected with a monoclonal antibody to acetylated α-tubulin and analyzed with immunofluorescence and immuno high-voltage electron microscopy (immuno HVEM) using colloidal gold [120]. Microtubules in the cytoplasmic cytasters that are present in the unfertilized mouse oocyte [121, 122] were not acetylated. The meiotic spindle became labeled with acetylated α-tubulin at meiotic anaphase; by telophase and during second polar body formation, the acetylated α-tubulin was only detected at the meiotic midbody. After perturbing microtubule dynamics with cold, colcemid, or griseofulvin treatment, the remaining stable meiotic spindle microtubules showed positive staining for acetylated α-tubulin; however, taxol stabilization of microtubules did not alter tubulin acetylation patterns. These results show that acetylated microtubules are present during meiosis and display a cell-cycle-specific pattern of acetylation, with acetylated microtubules associated with microtubules at the centrosomal area in meiotic metaphase, an increase in spindle microtubule acetylation at anaphase, and selective deacetylation at telophase with acetylated microtubules in the midbody during the asymmetric meiotic cell division prior to polar body extrusion.
Cytoskeletal Functions and Cytoskeletal PTMs During Fertilization and Pre-implantation Embryo Development
Preimplantation embryo development includes all stages from fertilization to implantation and it is a well-orchestrated program that includes symmetric and asymmetric cell divisions, morula and blastocyst formation. Preimplantation embryo development is regulated genetically, epigenetically and posttranslationally.
In mammals, exit from MII arrest and meiotic resumption is typically achieved by the fertilizing spermatozoon, which evokes in the oocyte a cascade of calcium signaling followed by significant cytoskeletal reorganization and remodeling. During non-rodent mammalian (including human) fertilization, the spermatozoon contributes the proximal centriole as major microtubule nucleating and organizing center that is important for the aggregation of oocyte centrosomal components and the enlargement of the sperm aster into the zygote aster and mitotic apparatus. The components involved in sperm centrosomal functions have previously been reviewed in detail [7, 15, 16, 109] and will not be discussed in the present chapter. For successful fertilization and sperm aster organization, the sperm-derived centriole must first disengage from the sperm tail connecting piece; release of the proximal centriole is facilitated by sperm (and oocyte) proteasomes [123], which then allows rapid nucleation and accurate formation of microtubules into sperm aster, zygote aster and mitotic apparatus; failure in accurate centrosome, and microtubule organization can lead to infertility or to disorders that are manifested later in life (reviewed in [15, 16]). Centriole duplication occurs during the pronuclear stage (Fig. 4.2) but our knowledge of PTMs during these fertilization stages is sparse. We also still do not fully understand how the centriole-centrosome complex becomes duplicated during the pronuclear stage, although we understand from centriole duplication in some model organisms and in somatic cells that duplication of this complex is under cytoplasmic control and driven by cyclin-dependent kinase 2 (CDK2) complexed with cyclin E or cyclin A (reviewed by [124]).
(a) Schematic diagram representing spermatozoa in non-rodent mammalian species displaying nucleus, proximal and distal centrioles, and sperm tail; (b) while the distal centriole deteriorates, the proximal centriole serves as microtubule organizing center (MTOC) to form the sperm aster during the pronuclear stage after fertilization; (c) formation of the procentrioles from the older centrioles that form the mitotic poles and organize the mitotic apparatus
The sperm centrioles duplicate during the pronuclear stage (in subsequent cell cycles during the G1/S phases) and starts with procentriole growth from the existing centriole, a process termed semiconservative centriole duplication. The procentriole grows into the daughter centriole that is oriented perpendicular to the older (mother) centriole, resulting in two pairs of centrioles that also indicate duplication of centrosomal material. The duplicated centrioles separate and migrate around the zygote nucleus to form the opposite poles of the first mitotic spindle.
Remodeling of the centrosome complex is critically important for proper development. While the centriole complex itself does not change its structure during development this complex has the capability to attract centrosomal material that surrounds the centriole complex and allows centrosomal plasticity throughout development for embryo-specific functions. Phosphorylation and other posttranslational modifications are important aspects in centrosome regulation required for cell cycle-specific changes (reviewed in [125, 126]).
In most systems, the non-membrane bound centrosome organelle of ~1 μm in size (Fig. 4.3) consists of a large number of centrosomal proteins embedded in a centrosomal matrix that typically surround a pair of perpendicularly oriented cylindrical centrioles; however, it is important to note that centrioles are not present in the centrosome complex during mitosis and cell divisions in mouse embryos up to the blastula stage. Details on mouse and non-rodent mammalian centrosomes have been presented in several recent review papers [7–9, 14–17] and are not included in the present chapter.
Schematic diagram of a typical somatic cell centrosome composed of two centrioles, termed mother and daughter centrioles, that are connected to each other by interconnecting fibers and surrounded by centrosomal material (also termed pericentriolar material; PCM). The mother centriole is distinguished from the daughter centriole by distal (shown) and subdistal (not shown) appendages. Microtubules are nucleated by the gamma-tubulin ring complex (γ-TuRC) and accessory proteins, and anchored at their minus ends by the microtubule anchoring complex within the centrosomal core structure. Microtubule growth is regulated by distal plus-end addition of tubulin subunits
Centrosome functions play major roles in cell cycle regulation (reviewed in [14]), symmetric and asymmetric cell divisions, in cellular differentiation, in stem cell maintenance and stem cell differentiation, and in embryo development. Centrosomal composition varies in different cell cycle stages, in order to perform cell cycle-specific functions including controlling the length and amount of microtubule organization. The centrosome complex plays a major role during preimplantation embryo development. Through its microtubule organizing capabilities, the centrosome facilitates many cellular activities including cell motility, polarity, maintenance of cell shape, cell division, transport of vesicles, distribution of cell fate determinants, and targeting of a variety of signaling molecules. Differential accumulation of cellular components is critical for proper development and assures targeted distribution of cellular components during subsequent cell divisions. It includes cytoplasmic factors such as transcripts of developmental genes, which allows for cell type-specific gene activity.
In the developing embryo, centrosome duplication occurs during the S phase and is tightly synchronized with DNA replication. After duplication during each embryonic cell cycle, centrosomes separate toward the opposite poles, establishing the bipolar mitotic apparatus that contributes to cellular differentiation. During each cell cycle, the maturation of interphase centrosomes into mitotic centrosomes includes acquisition of mitosis-specific centrosome proteins such as the Nuclear Mitotic Apparatus protein (NUMA) that moves out of the nucleus during the cell’s exit from interphase and associates with the centrosomal core structure during mitosis. At this stage, centrosomal material can separate symmetrically or asymmetrically. Molecular centrosome asymmetry will have consequences for microtubule organization and transport of cargo, generating unequal inheritance of cell fate altering molecules in different cells during subsequent development.
Most of our knowledge about centrosomes comes from somatic cells and other studies derived from the Drosophila or C. elegans model systems, both of which may provide information applicable to some extent to mitotic cells during pre-implantation embryo development in mammals, which has not been studied extensively.
Several studies have focused on specific aspects of PTM by acetylation and tyrosination during preimplantation embryo development. It was shown that α-tubulin in microtubules of mouse embryos is acetylated in a specific spatial and temporal sequence during preimplantation embryo development [120]. Furthermore, the sperm axoneme retains its acetylation after incorporation while interphase, oocyte-derived microtubules are not detected with an antibody to the acetylated form of α-tubulin. Similar to meiosis, results for mitosis show the presence of acetylated mitotic microtubules and demonstrate a cell-cycle-specific pattern of tubulin acetylation, with acetylated microtubules found at the centrosomes at metaphase, an increase in spindle labeling at anaphase, and the selective deacetylation of all but midbody microtubules at telophase. First mitosis follows a pattern similar to that observed during second meiosis, as described in section “Cytoskeletal Functions and Cytoskeletal PTMs During Gametogenesis and Oocyte Maturation”; only the mitotic midbodies are acetylated and no other acetylated microtubules are detectable in the interphase daughter cells. As described for meiosis in section “Cytoskeletal Functions and Cytoskeletal PTMs During Gametogenesis and Oocyte Maturation”, after treatment with cold, colcemid, or griseofulvin, the remaining stable microtubules showed staining for acetylated microtubules (Fig. 4.4).
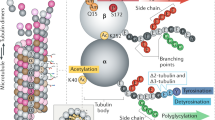
Localization of microtubule structures and PTMs in typical mammalian somatic cells. (a) In interphase cells, dynamic (more labile; orange) and stable microtubules (blue) are present. While dynamic microtubules lack PTMs, stable microtubules accumulate PTMs in a cell cycle-specific progression pattern as described in the text. Microtubules in motile cilia contain PTMs displaying high accumulation on the B tubule of the outer microtubule doublets. While motile cilia shown here contain the 9 + 2 arrangement of nine doublet microtubules with dynein arms surrounding a central pair, primary cilia (not shown) lack the central pair of microtubules (9 + 0) and such primary non-motile cilia do not contain dynein. PTMs are also accumulated in centriolar/basal body triplet microtubules. (b) In mitotic cells, spindle microtubules (kinetochore and/or interpolar) show a high amount of PTMs, while astral microtubules are mostly unmodified. The centrioles within the mitotic centrosomes at the spindle poles display PTMs as described in the text and are distinguished by high levels of polyglutamylation. Differentiated microtubule structures, such as centrioles, cilia and flagella are highly polyglutamylated, acetylated and detyrosinated, and show specific accumulation of Δ2-tubulin. Glycylation is highly specific for cilia and flagella, and can appear as monoglycylation and polyglycylation. Combinations of different PTMs play a role in the control of stability and movement of cilia and flagella. Modified from [20]
The first embryonic cell cycle is completed when the zygote chromosomes become separated during first mitosis. Centrosomes organize microtubules equally in both spindle halves during mitosis and up until recently it was also thought that they receive equal amounts of centrosome material. However, newer studies show that cellular asymmetry can already occur when centrosomal material becomes distributed unequally or when it becomes differentially modified [127]. Differences in centrosome quantity and quality in the two dividing daughter cells may set up the pattern for differentiation and polarization of the preimplantation embryo.
Stages of preimplantation development have been explored in great detail in mouse embryos, relative to gene expression patterns and specific phases of development with phase I spanning fertilization to the 2-cell stage; phase II spanning the 4-cell to the 8-cell stage; and phase III spanning the 8-cell embryo to the blastocyst stage.
As mentioned above, the mouse preimplantation embryo does not contain centrioles and the formation of the preimplantation mitotic and division stages is acentriolar (reviewed in [7–9, 116]), which is an important difference compared to other mammalian systems including humans.
Preimplantation development has also been studied in vivo and in vitro in several suitable animal models that do have sperm-derived centrioles in all stages of embryo development, including sheep, bovine and porcine embryos, and human embryos that had been discarded because of poor embryo quality but provided valuable research material.
In all mammalian species studied so far, the first preimplantation development stages are characterized by a synchronous doubling of cell numbers until the 8-cell stage as shown in the representative diagram in Fig. 4.5. Asynchronous cell divisions take place after morula compaction. At the 8- to 16-cell stage, the embryo starts developing into a blastocyst, at which time the first events of cellular differentiation are observed. At the blastocyst stage, the embryo hatches from the zona pellucida and implants in the uterus.
Schematic diagram of early preimplantation development from fertilization to the 8 cell stage. Spermatozoon enters the MII-stage oocyte (a), followed by pronuclear apposition (b), metaphase (c), early anaphase (d), late anaphase (e), early cell division (f ), divided, 2 cell stage (g), 4-cell stage (h), and 8-cell stage (i). PTMs take place in different stages and include different types of modifications in different subcellular microtubule organizations. Subcellular distribution of tubulin PTMs takes place in the MII spindle and in mitotic spindles as described in the text for developing embryo cells during the first cell cycles. Subsequent preimplantation PTMs are likely to follow those described for somatic cells although specific analysis for embryo cells is not yet available except for limited data for TE and ICM cells. We know from somatic cells that in undifferentiated cells, PTM modification levels are low in interphase as depicted in Fig. 4.3; detyrosination (Δ1), acetylation on Lys40 (Ac) and glutamylation are increased at the inner mitotic spindle and on the midbody that also shows a slight increase in polyglutamylation. The high levels of polyglutamylation of centrioles have been linked to centrosomal stability
Several changes are associated with the development of the preimplantation embryo and include changes in protein synthesis and changes in energy requirements that correlate with morphological changes [128, 129], resulting in compaction that clearly indicates cellular differentiation.
Species-specific differences are observed during these stages in the mouse and other mammalian species. In the mouse, development from the 1-cell stage to the blastocyst stage containing 32 or more cells takes about three and a half days, with the first cleavage from one into two cells taking 16–20 h and the second cleavage from 2 to 4-cell stage taking 18–22 h. Embryonic genome activation occurs during a lengthened cell cycle from the 2- to 4-cell stage in the mouse. During the earliest developmental changes, mouse embryos are under post-transcriptional maternal control, relying on changes in the translation of mRNAs synthesized during oocyte growth, and/or post-translational protein modifications. New transcription takes place during the late 4- and 8-cell stage to prepare the embryo for compaction. During the morula-blastocyst transition stage, an increase in the rate of protein synthesis is observed leading to the formation of the inner cell mass (ICM) and trophectoderm (TE) at the blastocyst stage.
As indicated above, compaction indicates the first morphological changes in the preimplantation embryo when cellular differentiation takes place. During this stage, two distinct cell populations are produced. The blastomeres remaining in contact with the outside of the preimplantation embryo will differentiate into the trophectodermal (TE) lineage while the blastomeres differentiating inside the embryo will form the inner cell mass (ICM). Cellular polarization takes place during the 8-, 16- and 32-cell stages, with specific cells changing their morphological and functional phenotype to a polarized phenotype. Specifically, divisions of the 8-cell embryo will result in an average of 9 cells located on the outside and 7 cells located on the inside of the developing embryo. The outer cells become polarized and larger while the inner cells remain apolar. The polarization process of the outer cells is apparent by the basal migration of the nucleus and the apical accumulation of actin, clathrin, endosomes and microvilli.
While we do not have complete information on PTMs during these stages, we have some information obtained on mouse preimplantation embryos and we do have information on the shift in PTMs during polarization in somatic cells.
As has been reported by Houliston and Maro [130], posttranslational modification of distinct microtubule subpopulations takes place during cell polarization and differentiation in the mouse preimplantation embryo. These studies showed that during the process of cellular differentiation (the time of compaction) into trophectoderm cells and inner cell mass, tyrosinated α-tubulin was detected by immunofluorescence microscopy in subsets of microtubules within and between cells. All microtubules contained tyrosinated α-tubulin but acetylated α-tubulin was only detected in a subpopulation located predominantly at the cell cortices. It was shown that during development one population of cytoplasmic microtubules containing tyrosinated α-tubulin redistributed toward the apex of cells during the 8-cell stage, while at the same time a population of cortical microtubules displaying acetylated α-tubulin accumulated near the intercellular contact zone at the basal part of the cell. This finding indicates differential microtubule dynamics between apical and basal regions during this stage of preimplantation development. Such a pattern of microtubule PTM was also seen during the 16-cell stage. Cortical microtubules were preferentially acetylated in the cortex which correlates well with the PTM pattern described for polarized epithelial cells [131], discussed below. The inside cells of the developing embryos contained more acetylated microtubules than the outside cells. Most acetylated microtubules could be depolymerized by nocodazole but some remained in the outside cells confirming that acetylation does not necessarily relate to microtubule stability.
As mentioned above, while the trophectodermal cells are polar, the ICM cells are adhesive and compact. During blastocyst formation, the trophectodermal cells acquire characteristics of epithelial cells and may display PTMs as described below for polarization of tissue culture cells. However, we do not yet have detailed information on the sequential acquisition of PTMs that play a role in the polarization process during preimplantation embryo development.
Most of our knowledge regarding preimplantation development comes from the mouse and many of the events that occur during mouse preimplantation development have also been observed in other mammalian embryos. Nevertheless, it is important to point out that significant species-specific differences exist, with major differences in the duration of cell cycle stages and timing of specific events. Common to most mammalian species are the synchronous cell divisions for the first few cell cycles followed by asynchronous divisions, typically after the 8-cell stage. Significant differences exist during the blastocyst stage when various embryos undergo long periods of blastocyst expansion which is especially characteristic for farm animals, including the sheep and pig in which increases in cell number and size take place [128, 129]. Differences are also significant in the timing of embryonic genome activation.
In porcine embryos, pre-implantation embryonic development has been described in detail [128, 132] including stages of blastula formation and cellular differentiation into trophectoderm and inner cell mass. In porcine embryos ICM, formation takes place on day 5 and continues with differentiation into epiblast and hypoblast at around the time when the embryo hatches from the zona pellucida [133, 134]. ICM and TE cell lineages are vital and essential for embryonic and fetal survival. TE cells and ICM-derived extra-embryonic membranes form the fetal placenta during later development.
The preimplantation stages and cell cycle timing has been reviewed by Niakan et al. [129] and time-lapse imaging studies have provided excellent data on dynamic behavior of human embryos during the first week of in vitro development [135]. These studies showed that human embryos undergo cytokinesis within 14.3 ± 6.0 min and complete second division within 11.1 ± 2.2 h after completion of first cytokinesis. In humans, the implantation occurs at ca. day 7 of development.
PTMs during cellular polarization: Using immunocytochemistry and immunoblotting techniques in somatic cells, Quinones et al. [131] reported that PTM of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. By using Madin-Darby Canine Kidney (MDCK) epithelial cells, the authors showed that the composition and distribution of modified microtubules change as the cells undergo morphogenesis associated with polarization. They showed that two-dimensionally spreading cells contain more detyrosinated microtubules with orientations toward the leading edge. In contrast, three-dimensionally polarized cells contain more acetylated microtubules that are oriented toward the apical domain, which correlates well with the data reported for polarizing cells in the developing mouse embryo [130]. While the functional aspects of these modifications await further clarification, it may be assumed that such modifications allow directional transport of cargo along microtubules. Data by these authors also revealed that microtubules are not necessarily acetylated along their entire length but that only short segments of microtubules may be acetylated rather than the entire microtubule. Polyglutamylation was seen stochastically on tubulin of most microtubules but further studies are needed to characterize functional aspects correlated to this observation. Interestingly, when the authors used nocodazole to depolymerize microtubules, they found that in subconfluent cells, detyrosinated microtubules initially appeared more stable compared to acetylated microtubules; after treatment for one hour, few acetylated microtubules remained while detyrosinated microtubules were still present. Based on these results, the authors propose that there are different classes of stable microtubules that are affected differently by drug treatment. Corresponding results were also obtained for confluent cells in which a small number of acetylated microtubules persisted after treatment with nocodazole for one hour while no polyglutamylated microtubules were resistant to drug treatment. In polarized cells, more microtubules were resistant to drug treatment, with most of them being acetylated. Detyrosinated and polyglutamylated tubulin was mostly detected in primary cilia. Taken together, the authors were able to demonstrate that there are different degrees of microtubule stability which may not directly correlate to PTMs, but PTMs are important for functional diversity in polarized cells. Further studies by Zink et al. [136] using depletion and overexpression approaches revealed that tubulin detyrosination promotes monolayer formation and apical trafficking in epithelial cells. These studies also identified alternating stretches of detyrosinated and tyrosinated tubulin. In detyrosination-depleted cells, premature polarization of cells could be induced. The authors propose that the detyrosinated tubulin-enriched microtubules may serve as cytoskeletal tracks to guide membrane cargo in polarized MDCK cells. These recent findings are most informative as to the possible functions of PTMs in cellular polarization and may be extended to preimplantation embryo in which cellular polarization is crucial for successful development. It may also be useful for studies on embryonic stem cells and their differentiation into various tissues.
Conclusions and Perspectives
The present chapter has provided an overview of our current knowledge on cytoskeletal PTMs during gametogenesis, oocyte maturation, fertilization and pre-embryo development with focus on the microtubule cytoskeleton. The importance of PTMs in cytoskeletal functions has been well recognized, and significant new information has been gained by studying PTMs in neuronal cells and in a variety of somatic cell systems. However, data on reproductive system cells are still incomplete and further investigations are needed, particularly since the cytoskeleton plays critically important roles in fertilization, symmetric and asymmetric cell divisions, stem cell maintenance and differentiation, cellular polarization, cilia and primary cilia formation, and various other aspects that are important for maintaining and reorganizing cell and tissue architecture to accommodate a diversity of cellular functions during embryo development. Investigations of specific cytoskeletal PTMs during fertilization and all subsequent stages of embryo development are important to address the role of cytoskeletal PTMs in allowing functional differences of this complex network of interconnected fibers. PTM abnormalities may be among the underlying reasons for cytoskeletal dysfunctions with consequences for infertility and developmental abnormalities.
While not yet included in the present chapter it should be noted that septins have been called a fourth novel unconventional component of the cytoskeleton [137]. Septins are a family of proteins that can form non-polar filaments or rings and can interact with the actin and microtubule cytoskeleton. Septins play a role in cytokinesis by recruiting different proteins to the contractile ring; by doing so they are important for cell division and for budding in yeast as is known for Saccharomyces cerevisiae in which septins had originally been discovered. In mouse oocytes, it has been shown that Septin2 is posttranslationally modified by SUMOylation and required for chromosome congression [138]. Septin 1 is required for spindle assembly and chromosome congression [139] and Septin 7 is required for orderly meiosis [140]. These studies have opened up an important new area for further investigations into septin functions and PTMs during fertilization and cell divisions throughout embryo development when septin may play a role in cellular polarization.
References
Cueva JG, Hsin J, Huang KC, Goodman MB. Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22(12):1066–74.
McKean PG, Vaughan S, Gull K. The extended tubulin superfamily. J Cell Sci. 2001;114: 2723–33.
Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7.
De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci U S A. 1981;78:5608–12.
Schatten G, Schatten H, Bestor T, Balczon R. Taxol inhibits the nuclear movements during fertilization and induces asters in unfertilized sea urchin eggs. J Cell Biol. 1982;94:455–65.
Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81.
Schatten H, Sun Q-Y. The role of centrosomes in fertilization, cell division and establishment of asymmetry during embryo development. Semin Cell Dev Biol. 2010;21:174–84.
Schatten H, Sun QY. The significant role of centrosomes in stem cell division and differentiation. Microsc Microanal. 2011;17(4):506–12. Epub 2011 Jul 11.
Schatten H, Sun QY. New insights into the role of centrosomes in mammalian fertilisation and implications for ART. Reproduction. 2011;142:793–801.
Schatten H, Sun QY. Centrosome dynamics during meiotic spindle formation in oocyte maturation. Mol Reprod Dev. 2011;78:757–68.
Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–13.
Lancaster MA, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol. 2011;13:702–9.
Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205.
Schatten H. The mammalian centrosome and its functional significance. Histochem Cell Biol. 2008;129:667–86.
Schatten H, Sun Q-Y. The role of centrosomes in mammalian fertilization and its significance for ICSI. Mol Hum Reprod. 2009;15(9):531–8.
Schatten H, Sun Q-Y. The functional significance of centrosomes in mammalian meiosis, fertilization, development, nuclear transfer, and stem cell differentiation. Environ Mol Mutagen. 2009;50(8):620–36.
Schatten H, Sun Q-Y. Nuclear-centrosome relationships during fertilization, cell division, embryo development, and in somatic cell nuclear transfer (SCNT) embryos. In: Schatten H, editor. The centrosome. LLC: Springer Science and Business Media; 2012.
Goldman RD, Grin B, Mendez MG, Kuczmarski ER. Intermediate filaments: versatile building blocks of cell structure. Curr Opin Cell Biol. 2008;20(1):28–34. doi:10.1016/j.ceb.2007.11.003.
Sun SC, Kim NH. Molecular mechanisms of asymmetric division in oocytes. Microsc Microanal. 2013;19:883–97.
Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol. 2008;20:71–6.
Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4:938–47. PubMed: 14685172.
Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–55.
Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86.
Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–60. PubMed: 17786050.
Rogowski K, Juge F, van Dijk J, Wloga D, Strub JM, Levilliers N, Thomas D, Bré MH, Van Dorsselaer A, Gaertig J, Janke C. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell. 2009;137:1076–87.
Wloga D, Webster DM, Rogowski K, Bré MH, Levilliers N, Jerka-Dziadosz M, Janke C, Dougan ST, Gaertig J. TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell. 2009;16:867–76.
Eddé B, Rossier J, LeCaer JP, Desbruyères E, Gros F, Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990;247:83–5.
Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, MacDonald TL, Sundberg RJ, Rebhun LI, Frankfurter A. Characterization of posttranslational modifications in neuron-specific class III β-tubulin by mass spectrometry. Proc Natl Acad Sci U S A. 1991;88:4685–9.
Rόdiger M, Plessman U, Kloppel KD, Wehland J, Weber K. Class II tubulin, the major brain β tubulin isotype is polyglutamylated on glutamic acid residue 435. FEBS Lett. 1992;308:101–5.
Ikegami K, Mukai M, Tsuchida JI, Heier RL, MacGregor GR, Setou M. TTLL7 is a mammalian β-tubulin polyglutamylase required for growth of MAP2-positive neurites. J Biol Chem. 2006;281:30707–16.
Ikegami K, Horigome D, Mukai M, Livnat I, MacGregor GR, Setou M. TTLL10 is a protein polyglycylase that can modify nucleosome assembly protein 1. FEBS Lett. 2008;582: 1129–34.
van Dijk J, Rogowski K, Miro J, Lacroix B, Eddé B, Janke C. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell. 2007;26:437–48.
Ikegami K, Setou M. TTLL10 can perform tubulin glycylation when co-expressed with TTLL8. FEBS Lett. 2009;583:1957–63.
Kalinina E, Biswas R, Berezniuk I, Hermoso A, Aviles FX, Fricker LD. A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 2007;21:836–50. PubMed: 17244818.
Rodriguez de la Vega M, Sevilla RG, Hermoso A, Lorenzo J, Tanco S, Diez A, Fricker LD, Bautista JM, Aviles FX. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 2007;21:851–65 [PubMed: 17244817].
Paturle-Lafanechère L, Eddé B, Denoulet P, Van Dorsselaer A, Mazarguil H, Le Caer JP, Wehland J, Job D. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry. 1991;30:10523–8.
Rogowski K, van Dijk J, Magiera MM, Bosc C, Deloulme JC, Bosson A, Peris L, Gold ND, Lacroix B, Grau MB, Bec N, Larroque C, Desagher S, Holzer M, Andrieux A, Moutin MJ, Janke C. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell. 2010;143:564–78.
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–72. PubMed: 17084703.
Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem. 1998;273:9797–803.
Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–61.
L’Hernault SW, Rosenbaum JL. Chlamydomonas α-tubulin is posttranslationally modified by acetylation on the ε-amino group of a lysine. Biochemistry. 1985;24:473–8.
Chu CW, Hou F, Zhang J, Phu L, Loktev AV, Kirkpatrick DS, Jackson PK, Zhao Y, Zou H. A novel acetylation of β-tubulin by San modulates microtubule polymerization via down-regulating tubulin incorporation. Mol Biol Cell. 2011;22:448–56.
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–31. PubMed: 12486003.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. PubMed: 12024216.
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+− dependent tubulin deacetylase. Mol Cell. 2003;11:437–44. PubMed: 12620231.
Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A. Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci. 2009;122:3531–41.
Sharma N, Bryant J, Wloga D, Donaldson R, Davis RC, Jerka-Dziadosz M, Gaertig J. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J Cell Biol. 2007;178:1065–79.
Peris L, Wagenbach M, Lafanechère L, Brocard J, Moore AT, Kozielski F, Job D, Wordeman L, Andrieux A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–66.
Kreitzer G, Liao G, Gundersen GG. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol Biol Cell. 1999;10:1105–18.
Dunn S, Morrison EE, Liverpool TB, Molina-París C, Cross RA, Alonso MC, Peckham M. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci. 2008;121:1085–95.
Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–67.
Drake PJ, Griffiths GJ, Shaw L, Benson RP, Corfe BM. Application of high-content analysis to the study of post-translational modifications of the cytoskeleton. J Proteome Res. 2009;8:28–34.
Leech SH, Evans CA, Shaw L, Wong CH, Connolly J, Griffiths JR, Whetton AD, Corfe BM. Proteomic analyses of intermediate filaments reveals cytokeratin 8 is highly acetylated: implications for colorectal epithelial homeostasis. Proteomics. 2008;8:279–88.
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–40.
Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. Eur J Cell Biol. 2008;87:581–90.
Samant SA, Courson DS, Sundaresan NR, Pillai VB, Tan M, Zhao Y, Shroff SG, Rock RS, Gupta MP. HDAC3-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem. 2011;286:5567–77.
Zenckeck WD, Xiao H, Weiss LM. Lysine post-translational modifications and the cytoskeleton. Essays Biochem. 2012;52:135–45.
Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–18.
Janke C, Rogowski K, Wloga D, Regnard C, Kajava AV, Strub JM, Temurak N, van Dijk J, Boucher D, van Dorsselaer A, Suryavanshi S, Gaertig J, Eddé B. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science. 2005;308:1758–62.
Regnard C, Fesquet D, Janke C, Boucher D, Desbruyères E, Koulakoff A, Insina C, Travo P, Eddé B. Characterisation of PGs1, a subunit of a protein complex co-purifying with tubulin polyglutamylase. J Cell Sci. 2003;116:4181–90.
Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 2007;26:2262–73.
Vaz Meirelles G, Ferreira Lanza DC, da Silva JC, Santana Bernachi J, Paes Leme AF, Kobarg J. Characterization of hNek6 interactome reveals an important role for its short N-terminal domain and colocalization with proteins at the centrosome. J Proteome Res. 2010;9: 6298–316.
Sardon T, Pache RA, Stein A, Molina H, Vernos I, Aloy P. Uncovering new substrates for Aurora A kinase. EMBO Rep. 2010;11:977–84.
Chang J, Cizmecioglu O, Hoffmann I, Rhee K. PLK2 phosphorylation is critical for CPAP function in procentriole formation during the centrosome cycle. EMBO J. 2010;29: 2395–406.
D’Angiolella V, Donato V, Vijayakumar S, Saraf A, Florens L, Washburn MP, Dynlacht B, Pagano M. SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature. 2010;466:138–42.
Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–8.
Freed E, Lacey KR, Huie P, Lyapina SA, Deshaies RJ, Stearns T, Jackson PK. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 1999;13:2242–57.
Gstaiger M, Marti A, Krek W. Association of human SCF(SKP2) subunit p19(SKP1) with interphase centrosomes and mitotic spindle poles. Exp Cell Res. 1999;247:554–62.
Fisk HA. Many pathways to destruction: the centrosome and its control by and role in regulated proteolysis. Chapter 8. In: Schatten H, editor. The centrosome. LLC: Springer Science and Business Media; 2012.
Prosser SL, Fry AM. Regulation of the centrosome cycle by protein degradation. Chapter 9. In: Schatten H, editor. The centrosome. LLC: Springer Science and Business Media; 2012.
Fukasawa K. Molecular links between centrosome duplication and other cell cycle associated events. Chapter 10. In: Schatten H, editor. The centrosome. LLC: Springer Science and Business Media; 2012.
Kais Z, Parvin JD. Centrosome regulation and breast cancer. Chapter 14. In: Schatten H, editor. The centrosome. LLC: Springer Science and Business Media; 2012.
Korzeniewski N, Cuevas R, Duensing A, Duensing S. Daughter centriole elongation is controlled by proteolysis. Mol Biol Cell. 2010;21:3942–51.
Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gonczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell. 2007;13:203–13.
Puklowski A, Homsi Y, Keller D, May M, Chauhan S, Kossatz U, Grunwald V, Kubicka S, Pich A, Manns MP, et al. The SCF-FBXW5 E3-ubiquitin ligase is regulated by PLK4 and targets HsSAS-6 to control centrosome duplication. Nat Cell Biol. 2011;13:1004–9.
Klein UR, Nigg EA. SUMO-dependent regulation of centrin-2. J Cell Sci. 2009;122: 3312–21.
Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–46.
Meraldi P, Nigg EA. Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J Cell Sci. 2001;114:3749–57.
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34.
Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci U S A. 2000;97:217–21.
Paturle-Lafanechère L, Manier M, Trigault N, Pirollet F, Mazarguil H, Didier JD. Accumulation of δ 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J Cell Sci. 1994;107:1529–43.
L’Hernault SW, Rosenbaum JL. Chlamydomonas α-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983;97:258–63.
Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101:2085–94.
Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major α-tubulin K40 acetyltransferase αTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A. 2010;107:21517–22.
Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–22. doi:10.1038/nature09324.
Bré MH, de Nechaud B, Wolff A, Fleury A. Glutamylated tubulin probed in ciliates with the monoclonal antibody GT335. Cell Motil Cytoskeleton. 1994;27:337–49.
Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc Natl Acad Sci U S A. 2010;107:10490–5.
Vogel P, Hansen G, Fontenot G, Read R. Tubulin tyrosine ligase-like 1 deficiency results in chronic rhinosinusitis and abnormal development of spermatid flagella in mice. Vet Pathol. 2010;47:703–12.
Geimer S, Teltenkotter A, Plessmann U, Weber K, Lechtreck KF. Purification and characterization of basal apparatuses from a flagellate green alga. Cell Motil Cytoskeleton. 1997;37:72–85.
Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated α-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302.
Bobinnec Y, Moudjou M, Fouquet JP, Desbruyères E, Eddé B, Bornens M. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil Cytoskeleton. 1998;39:223–32.
Wolff A, de Nechaud B, Chillet D, Mazarguil H, Desbruyeres E, Audebert S, Edde B, Gros F, Denoulet P. Distribution of glutamylated α and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol. 1992;59:425–32.
Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Eddé B, Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. 1998;143:1575–89.
Abal M, Keryer G, Bornens M. Centrioles resist forces applied on centrosomes during G2/M transition. Biol Cell. 2005;97:425–34.
Gundersen GG, Bulinski JC. Distribution of tyrosinated and nontyrosinated α-tubulin during mitosis. J Cell Biol. 1986;102:1118–26.
Maney T, Hunter AW, Wagenbach M, Wordeman L. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J Cell Biol. 1998;142:787–801.
Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, Gerlich DW, Janke C. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol. 2010;189:945–54.
McNally K, Audhya A, Oegema K, McNally FJ. Katanin controls mitotic and meiotic spindle length. J Cell Biol. 2006;175:881–91.
Sonbuchner TM, Rath U, Sharp DJ. KL1 is a novel microtubule severing enzyme that regulates mitotic spindle architecture. Cell Cycle. 2010;9:2403–11.
Connell JW, Lindon C, Luzio JP, Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10:42–56.
Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Mammalian spermatogenesis. In: Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED, editors. Histological and histopathological evaluation of the testis. 1st ed. Clearwater: Cache River Press; 1990. p. 1–40.
Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microsc Res Tech. 2003;61:88–102.
Sutovsky P, Manandhar G, Wu A, Oko R. Interactions of the sperm perinuclear theca with the oocyte: Implications for oocyte activation, anti-polyspermy defense and assisted reproduction. Microsc Res Tech. 2003;61:362–78.
Sutovsky P. Visualization of sperm accessory structures in the mammalian spermatids, spermatozoa and zygotes. In: Schatten H, editor. Methods in molecular biology, vol 253: germ cell protocols: vol. 1 sperm and oocyte analysis. Totowa: Human Press; 2004. p. 59–77.
Baska KM, Sutovsky P. Protein modification by ubiquitination and is consequences for spermatogenesis, sperm maturation, fertilization and pre-implantation embryonic development. In: Tokumoto T, editor. New impact on protein modifications in the regulation of reproductive system. Kerala: Research Signpost; 2005. p. 83–114.
Sutovsky P, Manandhar G. Mammalian spermatogenesis and sperm structure: anatomical and compartmental analysis. In: DeJonge C, Barrat C, editors. The sperm cell: production, maturation, fertilization, regeneration. Cambridge: Cambridge University Press; 2006. p. 1–30.
Manandhar G, Sutovsky P. Comparative histology and subcellular structure of mammalian spermatogenesis and spermatozoa. In: Schatten H, editor. Comparative reproductive biology. Malden: Iowa State Press. Ames, Iowa: A Blackwell Publishing Company; 2007. p. 81–98.
Nayernia K, Li M, Engel W. Spermatogonial stem cells. In: Schatten H, Totowa NJ, editors. Methods in molecular biology, vol. 253: germ cell protocols: vol. 1 sperm and oocyte analysis. New Jersey, Totowa: Humana Press Inc.; 2004.
Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13.
Sun QY, Schatten H. Centrosome inheritance after fertilization and nuclear transfer in mammals. In: Sutovsky P, editor. Somatic cell nuclear transfer. Vol. 591. Landes bioscience. Adv Exp Med Biol. 2007. pp. 58–71
Kieszenbaum AL. Sperm axoneme: a tale of tubulin posttranslational diversity. Mol Reprod Dev. 2002;62:1–3.
Szollosi D, Calarco P, Donahue RP. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972;11:521–41.
Schatten H, Walter M, Biessmann H, Schatten G. Activation of maternal centrosomes in unfertilized sea urchin eggs. Cell Motil Cytoskeleton. 1992;23:61–70.
Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15(5):573–85.
Miao YL, Sun QY, Zhang X, Zhao JG, Zhao MT, Spate L, Prather RS, Schatten H. Centrosome abnormalities during porcine oocyte aging. Environ Mol Mutagen. 2009;50(8):666–71.
Schatten H, Sun QY. Chromosome behaviour and spindle formation in mammalian oocytes. In: Trounson A, Gosden R, Eichenlaub-Ritter U, editors. Biology and pathology of the oocyte: role in fertility, medicine and nuclear reprogramming. New York: Cambridge University Press; 2013.
Kang MK, Han SJ. Post-transcriptional and post-translational regulation during mouse oocyte maturation. BMB Rep. 2011;44(3):147–57.
Ai J-S, Wang Q, Li M, Shi LH, Ola SI, Xiong B, Yin S, Chen DY, Sun QY. Roles of microtubules and microfilaments in spindle movements during rat oocyte meiosis. J Reprod Dev. 2008;54:391–6.
Ai J-S, Wang Q, Yin S, Shi L-H, Xiong B, Ouyang Y-C, Hou Y, Chen D-Y, Schatten H, Sun Q-Y. Regulation of peripheral spindle movement and spindle rotation during mouse oocyte meiosis: new perspectives. Microsc Microanal. 2008;14:349–56.
Schatten G, Simerly C, Asai DJ, Szöke E, Cooke P, Schatten H. Acetylated α-tubulin in microtubules during mouse fertilization and early development. Dev Biol. 1988;130:74–86.
Schatten G, Simerly C, Schatten H. Microtubule configurations during fertilization, mitosis and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci U S A. 1985;82:4152–6.
Maro B, Howlett SK, Webb M. Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol. 1985;101:1665–72.
Rawe VY, Díaz ES, Abdelmassih R, Wójcik C, Morales P, Sutovsky P, Chemes HE. The role of sperm proteasomes during sperm aster formation and early zygote development: Implications for fertilization failure in humans. Hum Reprod. 2008;23(3):573–80.
Sluder G. Centrosome duplication and its regulation in the higher animal cell. In: Nigg E, editor. Centrosomes in development and disease. Weinheim: Wiley-VCA Verlag GmbH & CoKGaG; 2004. p. 167–89.
Wilkinson CJ, Andersen JS, Mann M, Nigg EA. A proteomic approach to the inventory of the human centrosome. In: Nigg E, editor. Centrosomes in development and disease. Weinheim: Wiley-VCA Verlag GmbH & CoKGaG; 2004. p. 125–42.
Wojcik C, DeMartino GN. Intracellular localization of proteasomes. Int J Biochem Cell Biol. 2004;35:579–89.
Fuentealba LC, Eivers E, Geissert D, Taelman V, DeRobertis EM. Asymmetric mitosis: unequal segregation of proteins destined for degradation. Proc Natl Acad Sci U S A. 2008;105:7732–7.
Martin L, Besch-Williford C, Lai L, Cheong HT, Im GS, Park KW, Murphy C, Hao Y, Ellersieck MR, Keisler DH, Schatten H, Green JA, Prather RS. Morphologic and histologic comparisons between in vivo and nuclear transfer derived porcine embryos. Mol Reprod Dev. 2007;74:952–60.
Niakan KK, Han J, Pedersen RA, Simon C, Reijo Pera RA. Human pre-implantation embryo development. Development. 2012;139(5):829–41. doi:10.1242/dev.060426.
Houliston E, Maro B. Posttranslational modification of distinct microtubule subpopulations during cell polarization and differentiation in the mouse preimplantation embryo. J Cell Biol. 1989;108:543–51.
Quinones GB, Danowski BA, Devaraj A, Singh V, Ligon LA. The posttranslational modification of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. Mol Biol Cell. 2011;22:1045–57.
Hall VJ, Jacobsen JV, Rasmussen MA, Hyttel P. Ultrastructural and molecular distinctions between the porcine inner cell mass and epiblast reveal unique pluripotent cell states. Dev Dyn. 2010;239:2911–20.
Hyttel P, Niemann H. Ultrastructure of porcine embryos following development in vitro versus in vivo. Mol Reprod Dev. 1990;27:136–44.
Oestrup O, Hall V, Petkov SG, Wolf XA, Hyldig S, Hyttel P. From zygote to implantation: morphological and molecular dynamics during embryo development in the pig. Reprod Domest Anim. 2009;44 Suppl 3:39–49.
Wong C, Loewke K, Bossert N, Behr B, DeJonge C, Baer T, Reijo Pera RR. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–21. PubMed: 20890283.
Zink S, Grosse L, Freikamp A, Bänfer S, Müksch F, Jacob R. Tubulin detyrosination promotes monolayer formation and apical trafficking in epithelial cells. J Cell Sci. 2012;125(Pt 24):5998–6008. doi:10.1242/jcs.109470.
Mostowy S, Pascale CP. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–94.
Zhu JL, Lin SL, Li M, Ouyang YC, Hou Y, Schatten H, Sun QY. Septin2 is modified by SUMOylation and required for chromosome congression in mouse oocytes. Cell Cycle. 2010;9(8):1607–16.
Zhu J, Qi ST, Wang YP, Wang ZB, Ouyang YC, Hou Y, Schatten H, Sun QY. Septin1 is required for spindle assembly and chromosome congression in mouse oocytes. Dev Dyn. 2011;240(10):2281–9. doi:10.1002/dvdy.22725. PMID: 21932310.
Li S, Ou XH, Wei L, Wang ZB, Zhang QH, Ouyang YC, Hou Y, Schatten H, Sun QY. Septin 7 is required for orderly meiosis in mouse oocytes. Cell Cycle. 2012;11(17):3211–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Schatten, H., Sun, QY. (2014). Posttranslationally Modified Tubulins and Other Cytoskeletal Proteins: Their Role in Gametogenesis, Oocyte Maturation, Fertilization and Pre-implantation Embryo Development. In: Sutovsky, P. (eds) Posttranslational Protein Modifications in the Reproductive System. Advances in Experimental Medicine and Biology, vol 759. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0817-2_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0817-2_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0816-5
Online ISBN: 978-1-4939-0817-2
eBook Packages: MedicineMedicine (R0)