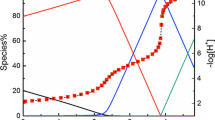
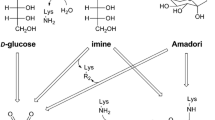
Abstract
0-Glycosyl and 0-phosphoryl groups, as well as disulfide bonds, are rapidly removed from proteins in alkaline solution primarily via β-elimination. The reaction is initiated by abstraction of the α-hydrogen of an amino acid residue by hydroxide ion. The carbanion undergoes rearrangement expelling the glyco- or phosphoryl group resulting in formation of a dehydroalanyl (from serine) or β-methyldehy-droalanyl (from threonine) residue. The unsaturated derivatives are reactive with internal protein nucleophilic groups and with external nucleophiles. These addition reactions, some leading to crosslinking, result in changed properties of the protein. Several factors may affect rates of β-elimination and addition. For these studies, we used two unique proteins: phosvitin, a well-characterized protein with 120 0-phosphoryl groups and no cystine or 0-glycosyl groups; and a glycopeptide related to the antifreeze protein from Antarctic fish, a well-characterized protein consisting of (Ala-Ala-Thr)n in which all of the threonyl residues are glycosylated. At the dilute concentrations of proteins used, rates of β-elimination and addition were independent of protein concentration but directly dependent upon the hydroxide ion concentration. With phosvitin, rates of β-elimination and addition were quite dependent on ionic strength and the rate of β-elimination was increased 20-fold in the presence of Ca2+. Activation energies for both β-elimination and addition were near 20 kcal/mole. Implications of these reactions for protein chemistry and protein processing will be discussed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Ahmed, A. I., Osuga, D. T. and Feeney, R. E. (1973). Antifreeze glycoprotein from an Antarctic fish. J. Biol. Chem., 248, 8524.
Asquith, R. S., Booth, A. K. and Skinner, J. D7–796)-7— The formation of basic amino acids on treatment of proteins with alkali. Biochim. Biophys. Acta, 181, 164.
Asquith, R. S. and Carthew, P771972). An investigation of the mechanism of alkaline degradation of cystine in intact protein. Biochim. Biophys. Acta, 278, 8.
Asquith, R. S. and Garcia-Dominguez, J. J. (1968). New amino acids in alkali-treated wool. J. Soc. Dyers Colour., 84, 155.
Asquith, R. S. and Skinner, J. D. (1970). Modification of keratin and related proteins by alkali. Textilveredlung, 5, 406.
Belitz, H. -D. (1965). Egg yolk proteins and their fractionated products. IV. Amino acid sequences in phosvitin. Lebensm.-Untersuch. Forsch., 127, 341.
Bjarnason, J. and Carpenter, K. J. (1970). Mechanisms of heat damage in proteins. 2. Chemical changes in pure proteins. Brit. J. Nutr., 24, 313.
Blackburn, S. 1968 ). “Amino Acid Determination, Method and Techniques.” Marcel Dekker, New York, N.Y.
Bohak, Z. (1964). Nc-(DL-2-Amino-2-carboxyethyl)-L-lysine, a new amino acid formed on alkaline treatment of proteins. J. Biol. Chem., 239, 2878.
Carter, C. E. and Greenstein, J. P. (1946a). A spectrophotometric method for the determination of dehydropeptidase activity. J. Biol. Chem., 165, 725.
Carter, C. E. and Greenstein, J. P. (1946b). Spectrophotometric determination of dehydropeptidase activity in normal and neo-plastic tissues. J. Nat. Cancer Inst., 7, 51.
Corfield, M. C., Wood, C., Robson, A., Williams, M. J. and Woodhouse, J. M. (1967). Formation of lysinoalanine during the treatment of wool with alkali. Biochem. J., 103, 15c.
Dakin, H. D. (1912). The racemization of proteins and their derivatives resulting from tautomeric change. J. Biol. Chem., 13, 357.
DeGroot, A. P. and Slump, P. (1969). Effects of severe alkali treatment of proteins on amino acid composition and nutritive value. J. Nutr., 98, 45.
DeGroot, A. P., Slump, P., Van Beek, L., and Feron, V. J. (1976). Severe alkali treatment of proteins. In “Proteins for Humans:
Evaluation and Factors Affecting Nutritional Value.“ C. E. Bodwell, ed., Avi, Westport, Conn.
Derevitskaya, V. A., Vafina, M. G. and Kochetkov, N. K. (1967). Synthesis and properties of some serine glycosides. Carbohyd. Res., 3, 377.
Downs, F. and Pigman, W. (1976). Determination of 0-glycosidic link- ages to L-serine and L-threonine residues of glycoproteins. Meth. Carbohydr. Chem., 7, 200.
Feeney, R. E. (1974). A biological antifreeze. Am. Scientist, 62, 712.
Feeney, R. E. (1977). Chemical modification of food proteins. In “Improvement of Food Proteins Through Chemical and Enzymatic Modifications,” R. E. Feeney and J. R. Whitaker, eds., Advances in Chemistry Series, Am. Chem. Soc., Washington, D. C.
Gallop, P. M., Blumenfeld, 0. 0. and Seifter, S. (1972). Structure and metabolism of connective tissue proteins. Ann. Rev. Biochem., 41, 617.
Geschwind, I. I. and Li, C. H. (1964). Effects of alkali-heat treatment on B-melanocyte-stimulating hormone. Arch. Biochem. Biophys., 106, 200.
Gottschalk, A. (1972). “Glycoproteins,” Elsevier Publ. Co., New York, N.Y.
Gross, E. (1977). The chemistry and biology of amino acids in food proteins: lysinoalanine. In “Improvement of Food Proteins Through Chemical and Enzymatic Modifications,” R. E. Feeney and J. R. Whitaker, eds., Advances in Chemistry Series, Am. Chem. Soc., Washington, D. C.
Horn, M. J., Jones, D. B. and Ringel, S. J. (1941). Isolation of a new sulfur-containing amino acid (lanthionine) from sodium carbonated-treated wool. J. Biol. Chem., 133, 141.
Isbell, H. S. (1944). Interpretation of some reactions in the carbohydrate field in terms of consecutive electron displacement. J. Res. Nat. Bur. Stand., Sect. A, 32, 45.
Isbell, H. S., Linek, K. and Hepner, K. E. Jr. (1971). Transformations of sugars in alkaline solutions: II. Primary rates of enolization. Carbohydr. Res., 19, 319.
Lee, H. S., Osuga, D. T., Nashef, A. S., Ahmed, A. I., Whitaker, J. R. and Feeney, R. E. Effect of alkali on glycoproteins: 13-Elimination and nucleophilic addition reactions of substituted threonyl residues. Submitted for publication (1976).
Levene, P. A. and Bass, L. W. (1928). Studies on racemization. VII. The action of alkali on casein. J. Biol. Chem., 78, 145.
Mayo, J. W. and Carlson, D. M. (1970). Effect of alkali and sodium borohydride at alkaline pH on N-acetylchondrosine: reduction vs cleavage. Carbohyd. Res., 15, 300.
Mecham, D. K. and Olcott, H. S. (1949). Phosvitin, the principal phosphoprotein of egg yolk. J. Am. Chem. Soc., 71, 3670.
Mellet, P. (1968). Influence of alkali treatment on native and denatured proteins. Text. Res. J., 38, 977.
Miro, P. and Garcia-Dominguez, J. J. Ç1973T. Action of ammonium and sodium hydroxides on keratin fibers in relation to their morphological structure. J. Soc. Dyers Colour., 89, 137.
Nashef, A. S., Osuga, D., Lee, H. S., Ahmed, A. I., Whitaker, J. R. and Feeney, R. E. Effects of alkali on proteins. Disulfides and their products. Submitted for publication (1976).
Ozeki, T. and Yosizawa, Z. (1971). Glycopeptides isolated from bovine submaxillary mucin. Arch. Biochem. Biophys., 142, 177.
Parisot, A. and Derminot, J. (197)7 Formation of amino acids in wool treated with 0.1 N sodium hydroxide at various temperatures. Bull. Inst. Text. Fr., 24, 603.
Patchornik, A. and Sokolovsky, M. (1964). Nonenzymatic cleavages of peptide chains at the cysteine and serine residues through their conversion into dehydroalanine. I. Hydrolytic and oxidative cleavage of dehydroalanine residues. J. Am. Chem. Soc., 86, 1206.
Pickering, B. T. and Li, C. H. (1964). Adrenocorticotropins XXIX. The action of sodium hydroxide on adrenocorticotropin. Arch. Biochem. Biophys., 104, 119.
Pigman, W. and Moschera, J. (1973). Uses and misuses of bases in studies of glycoproteins. In “Carbohydrates in Solution,” Advan. Chem. Ser., 117, 220.
Pisano, J. J., Finlayson, J. S., Peyton, M. P. and Nagai, Y. (1971). s-(y-Glutamyl)lysine in fibrin: Lack of crosslink formation in factor XIII deficiency. Proc. Nat. Acad. Sci. USA, 68, 770.
Plantner, J. J. and Carlson, D. M. (1975). Studies of mucin-type glycoproteins. Olefinic amino acids, products of the ß-elimination reaction. Anal. Biochem., 68, 153.
Price, V. E. and Greenstein, J. P. (1947). Dehydropeptidase activity in certain animal and plant tissues. J. Biol. Chem., 171, 477.
Provansal, M. M. P., Cuq, J. -L. A. and Cheftel, J. -C. (1975). Chemical and nutritional modifications of sunflower proteins due to alkaline processing. Formation of amino acid cross-links and isomerization of lysine residues. J. Agric. Food Chem., 23, 938.
Robson, A. and Zaidi, Z. H. (1967). The formation of lysinoalanine during the treatment of silk fibroin with alkali. J. Text. Inst. Trans., 58, 267.
Sen, L. C., Gonzalez Flores, E., Whitaker, J. R. and Feeney, R. E. Reactions of phosphoproteins in alkaline solutions. Submitted for publication (1976).
Simpson, D. L., Hranisavljevic, J. and Davidson, E. A. (1972). β-Elimination and sulfite addition as a means of localization and identification of substituted seryl and threonyl residues in proteins and proteoglycans. Biochemistry, 11, 1849.
Taborsky, G. (1974). Phosphoproteins. Adv. Protein Chem., 28, 1.
Tanaka, K., Bertolini, M. and Pigman, W.-7964). Serine and threonine glycosidic linkages in bovine submaxillary mucin. Biochem. Biophys. Res. Comm., 16, 404.
Tannenbaum, S. R., Ahern, M. and Bates, R. P. (1970). Solubilization of fish protein concentrate. Food Technol. ( Chicago ), 24, 604.
Tanzen, M. L. (1973). Cross-linking of collagen. Science, 180, 561.
Wakabayashi, K. and Pigman, W. (1974). Synthesis of some glycodipeptides containing hydroxyamino acids, and their stabilities to acids and bases. Carbohyd. Res., 35, 3.
Whiting, A. H. (1971). Isolation of lysinoalanine from the protein-polysaccharide complex of cartilage after alkali treatment. Biochim. Biophys. Acta, 243, 332.
Ziegler, K. (1964). New cross-links in alkali-treated wool. J. Biol. Chem., 239, 2713.
Ziegler, K., Melchert, I. and Lürken, C. (1967). N -(2-amino-2carboxyethyl)-ornithine, a new amino-acid fromsalkali-treated proteins. Nature (London), 214, 404.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1977 Springer Science+Business Media New York
About this chapter
Cite this chapter
Whitaker, J.R., Feeney, R.E. (1977). Behavior of O-Glycosyl and O-Phosphoryl Proteins in Alkaline Solution. In: Friedman, M. (eds) Protein Crosslinking. Advances in Experimental Medicine and Biology, vol 86. Springer, Boston, MA. https://doi.org/10.1007/978-1-4757-9113-6_10
Download citation
DOI: https://doi.org/10.1007/978-1-4757-9113-6_10
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4757-9115-0
Online ISBN: 978-1-4757-9113-6
eBook Packages: Springer Book Archive