Abstract
Nectins are immunoglobulin-like cell adhesion molecules (CAMs) constituting a family with four members. They exclusively localize at adherens junctions (AJs) between two neighboring cells. Nectins bind to afadin through their C-termini and are linked to the actin cytoskeleton. In addition to nectins, there are nectin-like molecules (Necls), which resemble nectins in their structures and constitute a family with five members. Nectins and Necls are involved in the formation of various kinds of cell–cell adhesion and diverse cellular functions including cell polarization, movement, proliferation, survival, and differentiation. In neuronal tissues, nectins and Necls functionally play crucial roles as CAMs at neuron–neuron and neuron–glia interactions. For example, the members of the nectin and Necl families are involved in synapse formation and remodeling in the hippocampus, a key brain region for learning and memory. Nectins also play important roles in the auditory system. Moreover, nectins and Necls are associated with human neurological diseases when mutated or upregulated. Thus, nectins and Necls are crucial for physiology and pathology in the nervous system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Cells in multicellular organisms form cell–cell junctions and contacts that play essential roles in various cellular processes, including morphogenesis, differentiation, proliferation, and migration. Cell–cell junctions and contacts can be homotypic or heterotypic; for example, the former is formed between two neighboring epithelial cells and the latter is formed between differentiating germ cells and their supporter Sertoli cells in the testis. Cell–cell junctions are mediated by cell adhesion molecules (CAMs). Cadherins, which make up a superfamily with >100 members, serve as key Ca2+-dependent CAMs in a variety of cell–cell junctions (Hirano and Takeichi 2012). The members of the immunoglobulin (Ig) superfamily also play important roles as Ca2+-independent CAMs (Brummendorf and Lemmon 2001). Nectins have emerged as Ig-like CAMs that contribute to a variety of cell–cell junctions and contacts, acting cooperatively with or independently of cadherins (Takai et al. 2008a, b). In addition, nectin-like molecules (Necls), which have domain structures similar to those of nectins, have been intensively studied (Takai et al. 2008a, b). Nectins and Necls interact in trans with each other and in cis with growth factor receptors and integrins, and regulate a variety of cell functions, including polarization, movement, proliferation, survival, and differentiation in addition to cell adhesion and contacts. On the other hand, nectins and Necls serve as viral receptors and are also associated with human diseases, such as cancer, Alzheimer’s disease (AD), and Margarita island ectodermal dysplasia (Hogle 2002; Takai et al. 2008a, b; Spear et al. 2000; Spear and Longnecker 2003). Here we first introduce the molecular and biological properties of nectins and Necls, then describe their roles in the nervous systems, and finally address neuropsychiatric diseases caused by dysfunction of nectins and Necls.
2 Nectins and Necls
2.1 Molecular Properties of Nectins
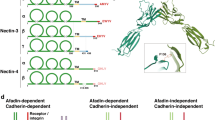
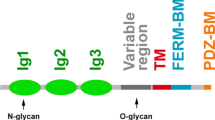
Nectins comprise a family with four members, nectin-1, nectin-2, nectin-3, and nectin-4, all of which have an extracellular region with three Ig-like loops, a single transmembrane region, and a cytoplasmic tail region (Fig. 5.1) (Takai et al. 2008a, b). Nectins have several molecular properties. (1) They show a Ca2+-independent cell–cell adhesion activity. Each nectin first forms homo-cis-dimers and then homo- or hetero-trans-dimers through the extracellular region, causing cell–cell adhesion. Heterophilic trans-interactions of nectins are stronger than their homophilic trans-interactions (Samanta et al. 2012; Satoh-Horikawa et al. 2000). By surface plasmon resonance analysis, the dissociation constants (Kds) for the interaction between nectin-1 and nectin-3 and between nectin-2 and nectin-3 are 2.3 and 360 nM, respectively (Ikeda et al. 2003), while those for homophilic binding of nectin-1, nectin-2, nectin-3, and nectin-4 are 17.5, 0.4, 228, and 153 μM, respectively (Harrison et al. 2012) (Fig. 5.2). Thus, among various combinations, the heterophilic trans-interaction between nectin-1 and nectin-3 is the strongest, followed by that between nectin-3 and nectin-2. (2) The trans-interactions of nectins induce the activation of Rap1, Cdc42, and Rac small G proteins (Takai et al. 2008a). (3) Nectins bind through their cytoplasmic tails to afadin, an actin filament (F-actin)-binding protein which connects nectins to the actin cytoskeleton (Mandai et al. 1997; Takai et al. 2008a, b) in a manner analogous to the way in which cadherins are connected to the cytoskeleton by binding through their cytoplasmic tails to the α- and β-catenin complex (Hirano and Takeichi 2012). (4) Nectins also bind through their cytoplasmic tails to partitioning defective three homologue (Par-3, also known as PARD3 in mammals), a cell polarity protein, which forms a complex with the other cell polarity proteins aPKC and Par-6 and regulates cell polarization (Ohno 2001; Takai et al. 2008a, b; Takekuni et al. 2003). (5) Nectins interact in trans heterophilically with Necls (Takai et al. 2008a, b; Ikeda et al. 2003; Shingai et al. 2003; Mueller and Wimmer 2003) and other Ig-like CAMs such as Tactile/CD96, DNAM-1/CD226, and TIGIT through their extracellular regions (Fig. 5.2) (Bottino et al. 2003; Fuchs et al. 2004; Pende et al. 2005, 2006; Stanietsky et al. 2009; Yu et al. 2009). The Kds for the heterophilic interaction between nectin-3 and Necl-5 and between nectin-3 and TIGIT are 17 and 38.9 nM, respectively (Ikeda et al. 2003; Yu et al. 2009). (6) Nectins interact with growth factor receptors. Nectin-3 interacts in cis with the platelet-derived growth factor (PDGF) receptor (Kanzaki et al. 2008), while nectin-1 interacts in cis with the fibroblast growth factor receptor (Bojesen et al. 2012). Nectin-1 and nectin-3, but not nectin-2, physically interact in cis with integrin αvβ3 at cell–cell adhesion sites (Sakamoto et al. 2006).
Molecular structures and modes of trans-interactions of nectins, Necls, and their binding proteins. Nectins and Necls share three Ig-like extracellular domains comprising an N-terminal variable region-like (V) domain and two constant region-like (C2) domains, a transmembrane region (TM), and a cytoplasmic domain. The nectin C-terminus contains interaction motifs (E/AxYV, where x represents any amino acid) that allow interaction with afadin, Par-3, PICK1, MUPP1, PATJ, and MPP3. Willin and zyxin interact with the juxtamembrane and cytoplasmic regions of nectins, respectively. The C-terminus of Necl-1 and Necl-2 contains interaction motifs (EYFI) that allow interaction with Pals2, Dlg3, and CASK. DAL-1 interacts with the juxtamembrane regions of Necl-2. Bidirectional arrows indicate the binding
Trans-interactions of nectins, Necls, and other Ig-like molecules. Homophilic (looped arrows) and heterophilic (double-headed arrows) trans-interactions are presented. Heterophilic trans-interaction between nectin-1 and nectin-3 is the strongest followed by that between nectin-3 and Necl-5 and that between nectin-2 and nectin-3. Red crossing bars indicate absence of homophilic interaction. DNAM1, DNAX accessory molecule 1; TIGIT, T cell immunoreceptor with Ig and ITIM domains; CRTAM, Class I-restricted T-cell associated molecule. Values besides arrows are Kd for hemophilic and heterophilic interactions
2.2 Molecular Properties of Necls
Necls, which are structurally similar to nectins, have five members and consist of an extracellular region with three Ig-like loops, a single transmembrane region, and a cytoplasmic region, but, unlike nectins, they do not bind afadin (Takai et al. 2008a, b). These include Necl-1/TSLL1/SynCAM3/CADM3, Necl-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1/CADM1, Necl-3/SynCAM2/CADM2, Necl-4/TSLL2/SynCAM4/CADM4, and Necl-5/poliovirus receptor (PVR)/CD155/Tage4. Necl-1 homophilically interacts in trans and heterophilically interacts in trans with nectin-1, nectin-3, Necl-2, Necl-3, and Necl-4 (Fig. 5.2) (Kakunaga et al. 2005; Niederkofler et al. 2010; Shingai et al. 2003). Necl-2 also homophilically interacts in trans and heterophilically interacts in trans with nectin-3, Necl-1, and Necl-3, as well as another Ig-like CAM CRTAM (Boles et al. 2005; Niederkofler et al. 2010; Shingai et al. 2003). Necl-3 does not homophilically interact in trans but heterophilically interacts in trans with Necl-1 and Necl-2 (Niederkofler et al. 2010; Pellissier et al. 2007). Necl-4 homophilically interacts in trans and heterophilically interacts in trans with Necl-1 (Maurel et al. 2007; Spiegel et al. 2007). Necl-5 does not homophilically interact in trans but heterophilically interacts in trans with nectin-3 and other Ig-like CAMs such as Tactile/CD96 (Fuchs et al. 2004), DNAM-1/CD226 (Bottino et al. 2003; Pende et al. 2005, 2006), and TIGIT (Stanietsky et al. 2009; Yu et al. 2009). The Kds for the interactions between Necl-5 and TIGIT, between Necl-5 and Tactile/CD96, and between Necl-5 and DNAM-1/CD226 are 3.15, 37.6, and 114 nM, respectively (Yu et al. 2009). Moreover, Necl-1 and Necl-3 interact in cis with integrin α6β4 (Mizutani et al. 2011). Necl-2 interacts in cis with the ErbB3 receptor, the ErbB4 receptor, integrin α6β4, and integrin αvβ3 (Kawano et al. 2010; Mizutani et al. 2011). Necl-5 interacts in cis with integrin αvβ3 and growth factor receptors, such as the PDGF receptor and the vascular endothelial growth factor (VEGF) receptor (Minami et al. 2010; Kinugasa et al. 2012). Necl-4 has not been reported to bind any growth factor receptors.
2.3 Proteins Associating with Nectins and Necls
2.3.1 Nectin-Binding Proteins
Some, but not all, members of the nectin family are able to bind various cytoplasmic proteins in addition to afadin, including Par-3 (Takekuni et al. 2003), protein interacting with PRKCA1 (PICK1) (Reymond et al. 2005), multiple PDZ domain protein (MUPP1, also known as MPDZ), Pals1-associated tight junction protein (PATJ) (Adachi et al. 2009), membrane palmitoylated protein 3 (MPP3) (Dudak et al. 2011), zyxin (Call et al. 2011), and willin (Ishiuchi and Takeichi 2012) (Fig. 5.1). Afadin has multiple domains: from the N-terminus to the C-terminus it has two Ras-associated domains, a forkhead-associated domain, a dilute domain, a PDZ domain, three proline-rich domains, and an F-actin-binding domain (Takai et al. 2008a, b). Afadin binds many proteins, including transmembrane proteins such as the Eph receptor tyrosine kinases, the synaptic transmembrane protein neurexins, the Notch receptor ligand jagged-1, the junction cell adhesion molecule JAM, and the gap junction protein connexin 36; peripheral membrane proteins such as the tight junction (TJ) protein ZO-1, the afadin- and α-actinin-binding protein ADIP, the actin-binding protein profilin, the vinculin-binding protein ponsin, the cadherin-binding protein α-catenin, and the Arg/Abl-interacting protein nArgBp2; and signaling molecules such as Rap1, Ras, Rit, and Rin small G proteins, Rap1 GTPase-activating protein SPA-1, Bcr and c-Src protein kinases, T-cell oncogene LIM domain only 2 (LMO2), and the tumor suppressor LIM domain only 7 (LMO7) (Kawabe et al. 1999; Begay-Muller et al. 2002; Shao et al. 1999; Takai et al. 2008a, b; Li et al. 2012). The tyrosine kinase Ryk is reported to interact with afadin, but this interaction is controversial (Trivier and Ganesan 2002). Thus, these proteins are indirectly associated with nectins through afadin. Moreover, the first Ras-associated domain of afadin mediates its self-association (Liedtke et al. 2010).
2.3.2 Necl-Binding Proteins
Necls do not directly bind afadin but binds many other proteins. Necl-1 and Necl-2 bind the membrane-associated guanylate kinase (MAGUK) family members, Pals2, Dlg3/MPP3, and calcium/calmodulin-associated Ser/Thr kinase (CASK), while Necl-2 additionally binds the tumor suppressor gene product DAL1, a band 4.1 family member protein, which connects Necl-2 to the actin cytoskeleton (Fukuhara et al. 2003; Shingai et al. 2003; Yageta et al. 2002). Pals2 is known to bind Lin-7, which is implicated in the proper localization of the Let-23 protein in Caenorhabditis elegans, the homologue of mammalian epidermal growth factor receptor (Kakunaga et al. 2004). Although PVR/CD155, a human homologue of Necl-5, was reported to bind Tctex-1, a subunit of the dynein motor complex (Mueller et al. 2002), the interaction between a mouse homologue of Necl-5 and Tctex-1 was negligible in our study (Minami et al. 2010).
3 Nectins and Necls in Non-neuronal Tissues
3.1 Nectins in a Variety of Cell–Cell Junctions of Non-neuronal Tissues
Nectins are widely expressed in many types of cells and play roles in the formation of cell–cell junctions or contacts, in some cases acting cooperatively with cadherins and in other cases independently of them. Mammalian tissues and organs are composed of two or more cell types that can adhere homotypically or heterotypically (Rikitake et al. 2012). There are homotypic and heterotypic cell–cell adhesions. Nectins and cadherins are involved in the formation of both groups of cell–cell adhesion, but cadherins are important for the formation of homotypic cell–cell adhesion, whereas nectins are important for the formation of heterotypic cell–cell adhesions, because cadherins interact in trans almost exclusively homophilically between the same members, whereas nectins interact in trans both homophilically and heterophilically between the same and different members, and, more importantly, because heterophilic trans-interactions of nectins are much stronger than their homophilic trans-interactions.
The typical homotypic cell–cell adhesion is formed between neighboring epithelial cells and endothelial cells. In these cells, there is a junctional complex comprised of TJs and adherens junctions (AJs). Nectin-1, nectin-2, and nectin-3 and possibly nectin-4 symmetrically localize at AJs and are involved in the formation of AJs, acting cooperatively with E-cadherin, a cadherin superfamily member that serves as a key CAM at AJs in epithelial cells (Gumbiner 1996; Takeichi 1991), and in the subsequent formation of TJs (Takai et al. 2008a, b). The trans-interactions of nectins induce the activation of Rap1, Cdc42, and Rac (Takai et al. 2008a, b), while those of E-cadherins induce the activation of Rac (Yap and Kovacs 2003). The activation of these molecules regulates the reorganization of the actin cytoskeleton, which is required for the formation of cell–cell adhesion (Takai et al. 2008a, b). The formation of TJs is dependent on the formation of AJs (Tsukita and Furuse 1999). The symmetric homotypic cell–cell adhesion is also formed in fibroblasts. In these cells, both N-cadherin and nectins colocalize at AJs and are involved in the formation of AJs.
The typical heterotypic cell–cell adhesion is found between many types of cells, such as between Sertoli cells and spermatids during spermatid differentiation in the testis, between commissural axons and basal processes of floor plate cells in the neural tube, between the pigment cell and non-pigment cell layers of the ciliary epithelium, between ameloblasts and stratum intermedium cells in the developing tooth, and between hair cells and supporting cells in the cochlea in the inner ear. At the Sertoli cell–spermatid junctions, nectin-2 and nectin-3 reside specifically in Sertoli cells and spermatids, respectively, and serve as essential CAMs (Ozaki-Kuroda et al. 2002). At the junctions between the apical membranes of pigment and non-pigment epithelia in the ciliary body of the eye, nectin-1 and nectin-3 localize at both sides and P-cadherin symmetrically localizes at both sides (Inagaki et al. 2005). Nectins and P-cadherin mediate the apex–apex adhesion between the pigment and non-pigment epithelia of the ciliary body. At these junctions, nectins, but not cadherins, are major CAMs. The roles of nectins in cell–cell adhesion between commissural axons and basal processes of floor plate cells in the neural tube and between hair cells and supporting cells in the cochlea in the inner ear are described below.
In addition to the stable adhesion for cell–cell junctions, such as AJs and TJs, weak and transient cell–cell adhesions are found between blood cells and between blood cells and vascular endothelial cells. Nectin-2 and nectin-3 are expressed in blood cells that lack cadherins. The exact roles of nectins in blood cells remain unknown, but they may play roles in transiently formed cell–cell contacts, such as those between macrophages and lymphocytes and between leukocytes and vascular endothelial cells during trans-endothelial migration.
3.2 Necls in Cell Adhesion, Migration, and Proliferation
Necl-2 is widely expressed in various tissues and localizes at the basolateral plasma membrane of epithelial cells, although it is not at the specialized cell–cell junctions such as AJs, TJs, and desmosomes (Shingai et al. 2003; Takai et al. 2008a, b). The ability of nectin-3 to interact in cis with Necl-2 suggests that Necl-2 is recruited to the nectin-3-based cell–cell adhesion sites during the formation of AJs (Takai et al. 2008a, b). After Necl-2 is assembled to the primordial cell–cell adhesion sites, it may be translocated from there to the extrajunctional region of the basolateral plasma membrane. The mechanism of segregation of Necl-2 from nectin-3 at the plasma membrane is currently unknown.
Necl-5 physically and functionally interacts in cis with integrin αVβ3 and the PDGF receptor and stimulates cell movement by enhancing both integrin αVβ3- and PDGF receptor-induced signalings. Necl-5 co-localizes with integrin αVβ3 and the PDGF receptor at peripheral ruffles and with integrin αVβ3 at focal complexes (Minami et al. 2010). Necl-5 facilitates the integrin αVβ3-dependent, PDGF receptor-induced activation of Rac, which regulates the formation of peripheral ruffles and focal complexes. Necl-5 is also involved in the contact inhibition of cell movement. When two moving cells collide with each other, Necl-5 on the surface of one cell interacts in trans heterophilically with nectin-3, which may be diffusely distributed along the adjacent cell surface, initiating the formation of cell–cell junctions (Ikeda et al. 2003). This trans-interaction induces the activation of Cdc42 and Rac (Sato et al. 2004), which enhances the reorganization of the actin cytoskeleton and increases the number of cell–cell adhesion sites. However, because the trans-interaction of Necl-5 with nectin-3 is transient, Necl-5 is downregulated and endocytosed from the plasma membrane in a clathrin-dependent manner (Fujito et al. 2005), which reduces cell movement. On the other hand, nectin-3 dissociated from Necl-5 is retained on the plasma membrane and subsequently interacts in trans with nectin-1, which most feasibly interacts in trans with nectin-3 (Ikeda et al. 2003). Then, cadherin is recruited to the nectin-based adhesion sites, eventually establishing AJs. Hence, the cell–cell contact-induced trans-interaction of Necl-5 with nectin-3 and the subsequent downregulation of Necl-5 are at least one of the mechanisms of the contact inhibition of cell movement (Fujito et al. 2005).
In addition, Necl-5 enhances the PDGF-induced cell proliferation by shortening the period of the G1 phase of the cell cycle (Kakunaga et al. 2004). Necl-5 enhances the PDGF-induced activation of the Ras-Raf-MEK-ERK pathway and consequently upregulates cyclins D2 and E and downregulates p27Kip1. Necl-5 regulates the VEGF-induced angiogenesis by controlling the interaction of VEGF receptor 2 with integrin αVβ3 and the VEGF receptor 2-mediated activation of downstream proangiogenic and survival signals, including Rap1, Akt, and endothelial nitric oxide synthase (Kinugasa et al. 2012).
Necl-5 also heterophilically interacts in trans with DNAM-1/CD226, which is expressed in human natural killer cells (Bottino et al. 2003). DNAM-1 has one extracellular region with two Ig-like loops, one transmembrane region, and one cytoplasmic region. Heterophilic trans-interactions of CD155/hNecl-5 with DNAM-1, poliovirus, and an anti-CD155 monoclonal antibody stimulate the phosphorylation of Necl-5 by Src kinases and recruit SH2-domain-containing tyrosine phosphatase-2 (Oda et al. 2004).
4 Nectins and Necls in Neuronal Tissues
4.1 Nectins and Necls at Neuron–Neuron Interactions
4.1.1 Nectins and Necls in Synapse Formation
Interneuronal synapses are asymmetric homotypic cell–cell adhesions. At the synapses, at least two types of intercellular junctions with different functions have been recognized: synaptic junctions (SJs) and puncta adherentia junctions (Fig. 5.3). Synaptic junctions are associated with synaptic vesicles that are docked at the presynaptic active zone where Ca2+ channels localize and with postsynaptic densities (PSDs) that are regarded as sites of specific receptors to which the neurotransmitter binds. Puncta adherentia junctions are not associated with synaptic vesicles or PSDs and appear to be similar in ultrastructure to the AJs of epithelial cells. They are regarded as mechanical adhesion sites between presynaptic axon terminals and PSDs. At the mossy fiber synapses, synapses between the mossy fiber terminals and the dendrites of pyramidal cells in the CA3 area of the hippocampus, both synaptic and puncta adherentia junctions, are highly specialized and are actively remodeled in an activity-dependent manner (Amaral and Dent 1981). N-Cadherin and αN- and β-catenins localize symmetrically at both the presynaptic and postsynaptic sides of puncta adherentia junctions, whereas nectin-1 and nectin-3 localize asymmetrically at the presynaptic and postsynaptic sides of puncta adherentia junctions, respectively (Mizoguchi et al. 2002) (Fig. 5.3). Puncta adherentia junctions have been regarded as symmetrical junctions on the basis of the morphological symmetry and symmetrical distribution of N-cadherin (Mueller and Wimmer 2003; Shingai et al. 2003), but their molecular architecture in this region is asymmetrical, at least in part, with regard to nectins (Mizoguchi et al. 2002).
Puncta adherentia junctions in neurons. Synapse between a mossy fiber terminal of a granule cell and a dendrite of a pyramidal cell in the CA3 region of the hippocampus contains two types of junctions: synaptic junctions and puncta adherentia junctions. Nectin-1 and nectin-3 asymmetrically localize at the mossy fiber terminal (presynaptic side) and at the dendrite of pyramidal cell (postsynaptic side), respectively, and form the puncta adherentia junctions in cooperation with cadherins
The molecular mechanism of synapse formation is thought to be analogous, in part, with that of the epithelial junctions in terms of the localization patterns of the junctional proteins. At the primitive synapse, synaptic and puncta adherentia junctions are not morphologically differentiated, but during their maturation membrane domain specialization is gradually formed (Amaral and Dent 1981). This neural membrane domain specialization may have some analogy with that found during the formation of the junctional complex in epithelial cells, with respect to the dynamic localization patterns of the junctional proteins. We speculate, by analogy with the formation of the junctional complex in epithelial cells, that nectins first form primordial junctions between dendrites and axons in synaptogenesis and that this event is followed by the recruitment of N-cadherin. The components of the active zones would then be recruited to the primordial junctions to form active zones at the presynaptic side. At the postsynaptic side, the components of PSDs would be assembled and membrane receptors would be transported there. The nectin and cadherin systems may serve as membrane cues for the assembly of these components. The membrane domains, comprising synaptic junctions and puncta adherentia junctions, would then gradually become segregated, followed by a maturation of synapses as AJs and TJs are segregated in epithelial cells. Thus, cell–cell adhesions in epithelia are symmetric homotypic, while synapses are asymmetric homotypic. Of the many molecules involved in synapse formation, afadin is required for synapse formation on dendritic spines in the stratum radiatum of the CA1 region of the hippocampus (Beaudoin et al. 2012). Afadin regulates spine morphology in cooperation with Rap1, which is activated by NMDA receptors (Xie et al. 2005). Afadin is recruited to the plasma membrane by activated Rap1 and induces spine neck elongation, while afadin is dissociated from the membrane by inactive Rap1 and induces spine enlargement, suggesting that afadin could be involved in activity-dependent synaptic plasticity. However, it remains unclear whether these functions of afadin are involved in the action of nectins in synapse formation.
ZO-1 associates with TJs in epithelial cells (Stevenson et al. 1986) and binds to F-actin. ZO-1 belongs to the membrane-associated guanylate kinase-like homologues (MAGUKs) family (Itoh et al. 1993; González-Mariscal et al. 2000) and plays a key role in the formation and maintenance of TJs in epithelial cells and endothelial cells (Hartsock and Nelson 2008; Wolburg and Lippoldt 2002). In neurons, ZO-1 co-localizes with nectins and cadherins at puncta adherentia junctions (Inagaki et al. 2003), which suggests that ZO-1 plays a role in the segregation of the components of synaptic junctions and puncta adherentia junctions, as is described for the role of ZO-1 in epithelial cells (Hogle 2002).
In addition to nectins, afadin, cadherins, and catenins, neuroligins and neurexins have been implicated in synapse formation (Biederer et al. 2002; Missler et al. 1998). Neuroligins and neurexins localize at the presynaptic and postsynaptic sides of SJs, respectively (Ushkaryov et al. 1992; Song et al. 1999). Neuroligins induce stable junctions with presynapse-like properties between neurons and neuroligin-expressing fibroblasts that are co-cultured with dissociated hippocampal neurons (Dean et al. 2003; Scheiffele et al. 2002). N-Cadherin and neuroligin-1 cooperate to control vesicle clustering at nascent synapses (Stan et al. 2010). They also in concert regulate the formation of glutamatergic synapses (Aiga et al. 2011). The relationship between the nectin–afadin complex and the neurexin–neuroligin complex in synaptogenesis is not known. However, there are at least afadin-dependent and/or neuroligin-dependent signaling pathways in synaptogenesis (unpublished observation).
Necls have been reported as Ig-like CAMs at synapses and named SynCAM1-3 (Biederer et al. 2002). Biederer et al. reported that SynCAM1/Necl-2 was specifically synthesized in mouse brain (Biederer et al. 2002), whereas we found that Necl-2 was ubiquitously expressed (Kakunaga et al. 2005) as reported elsewhere (Wakayama et al. 2001; Fukami et al. 2002; Shingai et al. 2003). Presumably, the reason caused this inconsistency is that the anti-SynCAM1/Necl-2 Ab used by Biederer and coworkers (Biederer et al. 2002) may recognize Necl-1 but not Necl-2. Although they reported that SynCAM1 co-localized with synaptophysin and localized at synaptic junctions (Biederer et al. 2002), we could not repeat these results and the reason for this inconsistency remains unknown. SynCAM1/Necl-2 in particular has been shown to be involved in synapse formation and remodeling. Glutamatergic synaptic transmission is reconstituted between cultured neurons and non-neuronal cells co-expressing glutamate receptors with SynCAM1/Necl-2, suggesting that a single type of SynCAM1/Necl-2 as well as the glutamate receptor is sufficient for a functional postsynaptic response (Biederer et al. 2002; Sara et al. 2005). SynCAM1/Necl-2 acts in developing neurons to shape migrating growth cones and contributes to the adhesive differentiation of their axo-dendritic contacts (Stagi et al. 2010). In addition to the involvement in the organization of synapses SynCAM1/Necl-2 may recruit both the AMPA receptors and the NMDA receptors during synapse formation (Hoy et al. 2009). Moreover, Necl-2 may be involved in neuronal migration, axon growth, path finding, and fasciculation on the axons of differentiating neurons in addition to cell adhesion in the neuroepithelium and the synapses (Fujita et al. 2005). The functions of SynCAM1/Necl-2 are modulated by polysialic acid during integration of proteoglycan NG2-positive glial cells into neural networks (Galuska et al. 2010). Overexpression of Necl-2 leads to the upregulation of CASK and increased Ca2+-independent cell adhesion (Giangreco et al. 2009). CASK is recruited to developing axon terminals by Necl-2 and neurexin/neuroligin (Kakunaga et al. 2005).
4.1.2 Nectins and Necls in Synapse Remodeling
Spines are dynamic structures that undergo rapid remodeling and experience-dependent spine remodeling provides a structural basis for learning and memory (Yuste and Bonhoeffer 2001). Synaptic activity that induces long-term potentiation, a long-lasting enhancement of synaptic strength, promotes spine enlargement and new spine formation (Matsuzaki et al. 2004). Spine structure and synaptic function are closely related (Kasai et al. 2003). The mechanisms that control the development and remodeling of spiny synapses under normal and pathological conditions need to be studied. Immature spines are often thin and elongated with filopodia; during their maturation, spine length decreases and the proportion of mushroom spines increases. The molecular details of how the filopodia are formed are still unknown, but they might be formed by the Cdc42 activated by the trans-interactions of nectins (Bottino et al. 2003; Takai et al. 2008a, b). N-Cadherin is involved in the formation of dendritic spines (Amaral and Dent 1981). It has been reported that scatter factor/hepatocyte growth factor and 12-O-tetradecanoylphorbol-13-acetate induce ectodomain shedding of nectin-1, which results in the formation of an extracellular fragment of nectin-1 (Tsukita et al. 2001; Yamada et al. 2004). In addition, nectin-1 serves as a substrate for presenilin/γ-secretase in the brain (Kim et al. 2002). The extracellular fragment of nectin-1 formed by this shedding may bind to dendritic nectin-3 and induce the formation of filopodia, which would result in changes to spine morphology. In the afadin conditionally deficient mice crossed with camk2a-Cre mice, the active zone protein, bassoon, and the postsynaptic density protein, PSD-95, are accumulated at mossy fiber-CA3 pyramidal cell synapses, while perforated PSDs tend to be more frequently observed than in control mice (Majima et al. 2009). Perforated PSDs are observed in synapses that undergo remodeling (Yuste and Bonhoeffer 2001). Thus, afadin is likely to regulate the remodeling of synapses. Whereas previous studies have advanced our understanding of molecular mechanisms of synapse formation, molecular mechanisms underlying synaptic remodeling remain largely unknown.
As the components of heterophilic trans-synaptic adhesion complexes such as a SynCAM1/Necl-2–SynCAM2/Necl-3 complex and a SynCAM3/Necl-1–SynCAM4/Necl-4 complex, Necls contribute to synapse organization and function (Fogel et al. 2007). SynCAM1/Necl-2 is also involved in synapse remodeling (Robbins et al. 2010). Necl-2 contributes to the regulation of synapse number and plasticity and impacts how neuronal networks undergo activity-dependent changes. Lateral self-assembling of SynCAM1/Necl-2 within the synaptic cleft promotes synapse induction and modulates their structure (Fogel et al. 2011). N-Glycosylation of SynCAM1/Necl-2 and SynCAM2/Necl-3 differentially affects their binding interface and implicates posttranslational modification as a mechanism to regulate trans-synaptic adhesion (Fogel et al. 2010).
4.1.3 Nectins and Necls at Contacts Between Commissural Axons and Floor Plate Cells
In the neural tube, commissural axons grow toward the ventral midline, cross the floor plate, and then abruptly change their trajectory from the circumferential to the longitudinal axis (Fig. 5.4). This axon guidance is mediated by the contacts between commissural axons and the basal processes of floor plate cells. Nectin-1 and nectin-3 asymmetrically localize at the commissural axon side and the floor plate cell side, respectively, of the plasma membranes at their contact sites and play an important role in the trajectory of the commissural axons (Okabe et al. 2004a). In addition to the nectin-1 and nectin-3 system, Necls are also involved in the trajectory of commissural axons. Necl-3 that is expressed by floor plate cells interacts with Necl-2 that is expressed by commissural axons to mediate a turning response in post-crossing commissural axons in the developing chick spinal cord in vivo (Niederkofler et al. 2010). Cadherins do not localize at the contact sites, while nectins and Necls localize there and may serve as CAMs. The weak trans-interaction between nectins and/or Necls, instead of the strong adhesion mediated by cadherins, might be advantageous when commissural axons continuously elongate while they are attached to floor plate cells.
Localization and roles of nectins and Necls at the contacts between commissural axons and floor plate cells in the neural tube. When the commissural axons make cell contacts with the dendrites of the floor plate cells, they extend across the central canal to make shift either to the rostral side or to the caudal side. Nectin-3 and Necl-3 on extending axons interact with nectin-1 and Necl-2 on dendrites of the floor plate cells, respectively. Cadherins do not localize at the contact sites
4.2 Necls at Neuron–Glia Interactions
Neurons interact not only with neurons but also with glial cells, such as astrocytes and oligodendrocytes. Interaction of neurons with glia is critical for a variety of functions in the nervous system, including neural activities and synapse transmission. Necl-1 is expressed at the contact sites among axons, their terminals, and glial cell processes that cooperatively form axon bundles, synapses, and myelinated axons (Kakunaga et al. 2005). Necl-1 is likely to serve as a CAM at the non-junctional cell–cell contact sites of the nervous tissues. In fact, Necl-1 plays an important role in the initial axon-oligodendrocyte recognition and adhesion in central nervous system myelination (Park et al. 2008). Necl-4 in Schwann cells plays an important role in initiating peripheral nervous system myelination as the glial binding partner for Necl-1 on the axon (Fig. 5.5) (Maurel et al. 2007; Spiegel et al. 2007). Necl-2-mediated glia cell adhesiveness is affected by erbB4 receptor activation (Sandau et al. 2011). Necl-3 also acts as an adhesion molecule between different cell types, interacting with other Necls in the central and peripheral nervous systems (Pellissier et al. 2007). Thus, in both the central and peripheral nervous systems, Necls are involved in myelination by mediating adhesion among different cell type such as neuron and glial cells.
Myelin sheath of the peripheral nerve. Necl-1 is specifically expressed at the contact sites among axons and glia cell processes that form the myelin sheath. Necl-4 in the Schwann cells plays an important role in initiating peripheral nervous system myelination as the glial binding partner for Necl-1 on the axon. At the Schwann cell–axon contact, Necl-1 on the axon interacts in trans with Necl-4 on the Schwann cell, while at the autotypic junctions formed between the myelin lamellae at the Schmidt–Lanterman incisure, Necl-1 interacts in trans with Necl-4
5 Nectins in the Auditory Epithelium
In the organ of Corti, sensory hair cells and supporting cells are observed. Hair cells convert sounds into electrical signals, which are transmitted to the brain. Hair cells and supporting cells are highly organized to form a checkerboard-like pattern (Kelley 2006). However, molecular mechanisms that regulate this characteristic pattern had remained unknown. In the mouse organ of Corti, hair cells and supporting cells express nectin-1 and nectin-3, respectively, but both cells possess nectin-2. The trans-interaction between nectin-1 and nectin-3 mediates the heterotypic adhesion between these two cell types, as the fine mosaic pattern is lost in nectin-1−/− mice and nectin-3−/− mice (Togashi et al. 2011). Moreover, in these mutant mice, the position of the kinocilium and the orientation of stereociliary bundles in hair cells are altered (unpublished observation). Thus, the trans-interaction between nectin-1 and nectin-3 is critical not only for checkerboard-like pattern formation, but also positioning of the kinocilium and stereociliary bundle orientation in hair cells.
6 Nectins and Necls in Diseases
6.1 Nectins and Necls as Viral Receptors
Virus interaction with cellular receptors is an essential step for recognition of the host cell and for commitment of the virus to initiate infection. Some viruses such as herpes virus and poliovirus show a tropism for neurons. Upon peripheral infection such viruses may enter the central nervous system and cause massive damage, either by direct virus-conferred effects or by immunopathology. Nectin-1 was originally isolated as one of the PVR-related proteins and named PRR-1 (Lopez et al. 1995). Nectin-2 was originally isolated as the murine homologue of human PVR, but turned out to be another PVR-related protein and was named PRR-2 (Eberlé et al. 1995). Neither PRR-1 nor PRR-2 has thus far been shown to serve as a PVR. They were later shown to serve as receptors for α-herpes viruses, facilitating their entry and intercellular spreading, and renamed HveC and HveB, respectively (Table 5.1) (Geraghty et al. 1998; Spear et al. 2000). Human nectin-1 allows entry of all α-herpes viruses tested so far, including herpes simplex virus (HSV) types 1 and 2, pseudorabies virus, and bovine herpes virus type 1 (Geraghty et al. 1998). Human nectin-2 can mediate entry of a restricted number of α-herpes viruses (Warner et al. 1998). The interaction of nectin-1 or nectin-2 with one of the HSV envelope glycoproteins recruits other viral glycoproteins to initiate fusion between the viral envelope and a cell membrane, thereby mediating the entry of the viral nucleocapsid into the cell (Spear and Longnecker 2003). The usual manifestations of HSV disease are mucocutaneous lesions. HSV establishes latent infection of neurons in sensory ganglia and causes recurrent lesions at the sites of primary infection. In HSV disease, the intercellular spreading significantly contributes to the pathogenesis. The interaction of nectin-1 with afadin increases the efficiency of intercellular spreading, but not the entry, of HSV-1. The E-cadherin–catenin system increases the efficiency of both the entry and intercellular spreading of HSV-1 (Sakisaka et al. 2001). Nectin-4 was recently identified as the epithelial cell receptor for the measles virus (Mühlebach et al. 2011; Noyce et al. 2011). Coupled with recent observations made in measles virus-infected macaques, this discovery has led to a new paradigm for how the virus accesses the respiratory tract and exits the host. Human Necl-5 (hNecl-5) was originally isolated as a receptor for poliovirus and was named hPVR (Koike et al. 1990; Mendelsohn et al. 1989). Poliovirus infects susceptible cells through hNecl-5/hPVR. It is thought that binding of hNecl-5/hPVR to poliovirus, the outer coat of which is an icosahedral protein shell, initiates conformational changes that enable the altered virion to bind to membranes and to invade cells even in the absence of the receptor (Hogle 2002). It is not clear whether the target membrane for entry is the plasma membrane or an endosomal membrane. Poliovirus is the causative agent of poliomyelitis. The usual manifestations of poliomyelitis disease are the spread and replication of virus in the central nervous system, particularly in the motor neurons. The cytoplasmic domain of hNecl-5/hPVR on the surface of endosomes that might enclose an intact poliovirion could interact with cytoplasmic dynein and the endosomes could be transported in a retrograde direction along microtubules through the axon to the neural cell body where replication of poliovirus occurs. It remains unknown whether other nectins and Necls serve as viral receptors. Thus, nectins and Necls are not only CAMs but also viral receptors and play a critical role in the pathogenesis of neurotrophic viral infections. Therefore, nectins and Necls could be therapeutic targets or probes as viral receptors. For example, nectin-4 is also a tumor cell marker that is highly expressed on the apical surface of many adenocarcinoma cell lines, making it a potential target for the oncolytic therapy by measles virus (Noyce and Richardson 2012).
6.2 Nectins in Neurological Diseases
Many lines of evidence suggest the association of nectins with pathogenesis of various neurological diseases. Mutations in the nectin-1 gene are responsible for cleft lip/palate ectodermal dysplasia, Margarita island ectodermal dysplasia and Zlotogora-Ogür syndrome, which are characterized by cleft lip/palate, syndactyly, mental retardation, and ectodermal dysplasia (Sozen et al. 2001; Suzuki et al. 2000). Impairment of the function of nectin-1 in synapse formation would explain the cause of the mental retardation. These phenotypes mainly affect the places where nectin-1 is specifically expressed (Okabe et al. 2004b). Mutations in human nectin-4 cause an ectodermal dysplasia–syndactyly syndrome that is characterized by the combination of hair and tooth abnormalities, alopecia, and cutaneous syndactyly (Brancati et al. 2010). Recent genome-wide association studies of various populations, including Japanese and African Americans, have shown a genetic association between single nucleotide polymorphisms (SNPs) in NECTIN-2 and late-onset AD (Harold et al. 2009; Logue et al. 2011; Takei et al. 2009), and mutations in NECTIN-3 are associated with human ocular disease and congenital ocular defects (Lachke et al. 2012). A SNP in the 3′UTR region of NECTIN-2 is one of the 13 genome-wide significant SNPs that map within or close to the APOE (Apolipoprotein E) locus on chromosome 19, whose polymorphic expression is widely associated with AD (Harold et al. 2009). These results suggest that together with a known association of APOE with AD, genetic variations in the NECTIN-2 gene may have implications for predisposition to this disease. At the synapses in the CA3 area of the hippocampus, the number of puncta adherentia junctions is decreased in both nectin-1−/− mice and nectin-3−/− mice (Honda et al. 2006). Furthermore, in the nectin-1-deficient mice, there is an abnormal trajectory of mossy fibers at the stratum lucidum of the hippocampus, possibly as a result of impaired puncta adherentia junctions. Both the nectin-1−/− mice and nectin-3−/− mice show microphthalmia and display a separation of the apex–apex adhesion between the pigment and non-pigment epithelia of the ciliary body (Inagaki et al. 2005).
6.3 Possible Involvement of Nectins and Necls in Other Diseases
Besides neurological diseases, nectins and Necls may be involved in the pathogenesis of various other diseases. Both nectin-2−/− mice and nectin-3−/− mice exhibit the male-specific infertility phenotype and have defects in the later steps of sperm morphogenesis, exhibiting distorted nuclei and abnormal distribution of mitochondria (Bouchard et al. 2000; Inagaki et al. 2005; Ozaki-Kuroda et al. 2002). The structure of Sertoli cell–spermatid junctions is severely impaired, and the localization of afadin and nectin-3 or nectin-2 is disorganized in the nectin-2−/− mice and nectin-3−/− mice, respectively. In all the cases, the impaired phenotypes occur at cell–cell junctions and contacts where the functions of the two nectins are not redundant. The heterophilic trans-interaction of nectins plays a particularly important role in maintaining the specialized junctions and contacts between different types of cells. Mice deficient in nectin-1, nectin-2, or nectin-3 do not apparently show impaired organization of AJs and TJs in tissues where multiple types of nectins are expressed, which might indicate that the nectins have overlapping functions in these tissues. Afadin−/− mice are embryonic lethal and show developmental defects at stages around gastrulation, including disorganization of the ectoderm, impaired migration of the mesoderm, and loss of somites and other structures that are derived from both the ectoderm and the mesoderm (Ikeda et al. 1999). In the ectoderm of the mutant mice, the organization of AJs and TJs is highly impaired. One reason why afadin−/− mice show more severe phenotypes than nectin-deficient mice is because afadin does not have family members. Necl-2 and Necl-5 are likely to contribute to tumorigenesis. Necl-2 serves as a tumor suppressor in human non-small cell lung cancer (Kuramochi et al. 2001). Rodent Necl-5 was identified as a product of a gene overexpressed in rat and mouse colon carcinoma (Chadeneau et al. 1994, 1996). Necl-5 is expressed at low levels in many cells, but its expression level is upregulated in many carcinomas (Ikeda et al. 2004; Chadeneau et al. 1994, 1996). Necl-5 is overexpressed in human colorectal carcinoma and malignant glioma (Masson et al. 2001). Upregulated Necl-5 in cancer is responsible at least partly for the enhanced motility and proliferation of cancer cells (Ikeda et al. 2003, 2004).
7 Conclusions and Perspectives
Evidence has been accumulated that nectins and Necls are important for various aspects of the nervous system physiology, such as synapse formation and remodeling, the trajectory of the commissural axons in the neural tube, myelination, and development of the auditory epithelium. However, questions about nectins and Necls still remain that include their roles in neuronal circuit formation, synaptic plasticity, neuronal cell differentiation, establishment of planar cell polarity in the auditory epithelium, and formation of heterotypic cell adhesions in the nervous system. Functional analysis combined with fine molecular and biological manipulations will answer these questions. For example, the trans-interaction between nectin-1 and nectin-3 may affect the output of the hippocampal mossy fiber circuit by changing the balance of excitatory and inhibitory synaptic transmission. Live imaging of hippocampal mossy fibers by means of a fluorescent dye will enable us to clarify the involvement of nectins and Necls in synapse formation and remodeling after induction of long-term potentiation. Further studies of the relationship between structural remodeling and change in functional parameter such as action potential firing rate and amplitude of synaptic response will provide valuable information to help answer how changes in synaptic structures contribute to changes in function of neuronal circuits. Moreover, conditional inactivation of afadin, a nectin-binding protein, also will help understand further the molecular mechanisms of synapse formation and remodeling.
On the other hand, nectins and Necls have been implicated in pathophysiology of neurological disorders. Several members of nectins and Necls have been identified as virus receptors. Mutations in the nectin genes can be the causes of hereditary neurological disorders and SNPs in the nectin genes are associated with neurodegenerative diseases. To assess the contribution of nectins and Necls in the pathogenesis of neuropsychiatric diseases, their significance should be studied at molecular, cellular, and in vivo levels. In particular, in vivo analysis is important to clarify the initiation and progression of disease processes precisely. Fortunately, nectin-1−/−, nectin-2−/−, nectin-3−/−, Necl-2/SynCAM1−/−, Necl-4−/−, Necl-5−/− mice have been already generated. Such mouse models would be powerful tools to advance our understanding of the significance of nectins and Necls in pathophysiology of neurological disorders.
References
Adachi M, Hamazaki Y, Kobayashi Y, Itoh M, Tsukita S, Furuse M, Tsukita S (2009) Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol Cell Biol 29:2372–2389
Aiga M, Levinson JN, Bamji SX (2011) N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures. J Biol Chem 286:851–858
Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195:51–86
Beaudoin GM III, Schofield CM, Nuwal T, Zang K, Ullian EM, Huang B, Reichardt LF (2012) Afadin, a Ras/Rap effector that controls cadherin function, promotes spine and excitatory synapse density in the hippocampus. J Neurosci 32:99–110
Begay-Muller V, Ansieau S, Leutz A (2002) The LIM domain protein Lmo2 binds to AF6, a translocation partner of the MLL oncogene. FEBS Lett 521:36–38
Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Südhof TC (2002) SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297:1525–1531
Bojesen KB, Clausen O, Rohde K, Christensen C, Zhang L, Li S, Kohler L, Nielbo S, Nielsen J, Gjorlund MD, Poulsen FM, Bock E, Berezin V (2012) Nectin-1 binds and signals through the fibroblast growth factor receptor. J Biol Chem 287(44):37420–37433
Boles KS, Barchet W, Diacovo T, Cella M, Colonna M (2005) The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 106:779–786
Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A (2003) Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 198:557–567
Bouchard MJ, Dong Y, McDermott BM Jr, Lam DH, Brown KR, Shelanski M, Bellve AR, Racaniello VR (2000) Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell–cell adherens junctions. Mol Cell Biol 20:2865–2873
Brancati F, Fortugno P, Bottillo I, Lopez M, Josselin E, Boudghene-Stambouli O, Agolini E, Bernardini L, Bellacchio E, Iannicelli M, Rossi A, Dib-Lachachi A, Stuppia L, Palka G, Mundlos S, Stricker S, Kornak U, Zambruno G, Dallapiccola B (2010) Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am J Hum Genet 87:265–273
Brummendorf T, Lemmon V (2001) Immunoglobulin superfamily receptors: cis-interactions, intracellular adaptors and alternative splicing regulate adhesion. Curr Opin Cell Biol 13:611–618
Call GS, Chung JY, Davis JA, Price BD, Primavera TS, Thomson NC, Wagner MV, Hansen MD (2011) Zyxin phosphorylation at serine 142 modulates the zyxin head-tail interaction to alter cell-cell adhesion. Biochem Biophys Res Commun 404:780–784
Chadeneau C, LeMoullac B, Denis MG (1994) A novel member of the immunoglobulin gene superfamily expressed in rat carcinoma cell lines. J Biol Chem 269:15601–15605
Chadeneau C, LeCabellec M, LeMoullac B, Meflah K, Denis MG (1996) Over-expression of a novel member of the immunoglobulin superfamily in Min mouse intestinal adenomas. Int J Cancer 68:817–821
Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P (2003) Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci 6:708–716
Dudak A, Kim J, Cheong B, Federoff HJ, Lim ST (2011) Membrane palmitoylated proteins regulate trafficking and processing of nectins. Eur J Cell Biol 90:365–375
Eberlé F, Dubreuil P, Mattei MG, Devilard E, Lopez M (1995) The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 159:267–272
Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T (2007) SynCAMs organize synapses through heterophilic adhesion. J Neurosci 27:12516–12530
Fogel AI, Li Y, Giza J, Wang Q, Lam TT, Modis Y, Biederer T (2010) N-glycosylation at the SynCAM (synaptic cell adhesion molecule) immunoglobulin interface modulates synaptic adhesion. J Biol Chem 285:34864–34874
Fogel AI, Stagi M, Perez de Arce K, Biederer T (2011) Lateral assembly of the immunoglobulin protein SynCAM 1 controls its adhesive function and instructs synapse formation. EMBO J 30:4728–4738
Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M (2004) Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J Immunol 172:3994–3998
Fujita E, Urase K, Soyama A, Kouroku Y, Momoi T (2005) Distribution of RA175/TSLC1/SynCAM, a member of the immunoglobulin superfamily, in the developing nervous system. Brain Res Dev Brain Res 154:199–209
Fujito T, Ikeda W, Kakunaga S, Minami Y, Kajita M, Sakamoto Y, Monden M, Takai Y (2005) Inhibition of cell movement and proliferation by cell-cell contact-induced interaction of Necl-5 with nectin-3. J Cell Biol 171:165–173
Fukami T, Satoh H, Fujita E, Maruyama T, Fukuhara H, Kuramochi M, Takamoto S, Momoi T, Murakami Y (2002) Identification of the Tslc1 gene, a mouse orthologue of the human tumor suppressor TSLC1 gene. Gene 295:7–12
Fukuhara H, Masuda M, Yageta M, Fukami T, Kuramochi M, Maruyama T, Kitamura T, Murakami Y (2003) Association of a lung tumor suppressor TSLC1 with MPP3, a human homologue of Drosophila tumor suppressor Dlg. Oncogene 22:6160–6165
Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, Geyer R, Mühlenhoff M, Geyer H (2010) Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA 107:10250–10255
Geraghty RJ, Krummenacher C, Eisenberg RJ, Cohen GH, Spear PG (1998) Entry of aherpesviruses mediated by poliovirus receptor related protein 1 and poliovirus receptor. Science 280: 1618–1620
Giangreco A, Jensen KB, Takai Y, Miyoshi J, Watt FM (2009) Necl 2 regulates epidermal adhesion and wound repair. Development 136:3505–3514
González-Mariscal L, Betanzos A, Avila-Flores A (2000) MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 11:315–324
Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–357
Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, Van Den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41:1088–1093
Harrison OJ, Vendome J, Brasch J, Jin X, Hong S, Katsamba PS, Ahlsen G, Troyanovsky RB, Troyanovsky SM, Honig B, Shapiro L (2012) Nectin ectodomain structures reveal a canonical adhesive interface. Nat Struct Mol Biol 19:906–915
Hartsock A, Nelson WJ (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669
Hirano S, Takeichi M (2012) Cadherins in brain morphogenesis and wiring. Physiol Rev 92:597–634
Hogle JM (2002) Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu Rev Microbiol 56:677–702
Honda T, Sakisaka T, Yamada T, Kumazawa N, Hoshino T, Kajita M, Kayahara T, Ishizaki H, Tanaka-Okamoto M, Mizoguchi A, Manabe T, Miyoshi J, Takai Y (2006) Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol Cell Neurosci 31:315–325
Hoy JL, Constable JR, Vicini S, Fu Z, Washbourne P (2009) SynCAM1 recruits NMDA receptors via protein 4.1B. Mol Cell Neurosci 42:466–483
Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y (1999) Afadin: a key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis. J Cell Biol 146:1117–1132
Ikeda W, Kakunaga S, Itoh S, Shingai T, Takekuni K, Satoh K, Inoue Y, Hamaguchi A, Morimoto K, Takeuchi M, Imai T, Takai Y (2003) Tage4/nectin-like molecule-5 heterophilically trans-interacts with cell adhesion molecule nectin-3 and enhances cell migration. J Biol Chem 278:28167–28172
Ikeda W, Kakunaga S, Takekuni K, Shingai T, Satoh K, Morimoto K, Takeuchi M, Imai T, Takai Y (2004) Nectin-like molecule-5/Tage4 enhances cell migration in an integrin-dependent, nectin-3-independent manner. J Biol Chem 279:18015–18025
Inagaki M, Irie K, Deguchi-Tawarada M, Ikeda W, Ohtsuka T, Takeuchi M, Takai Y (2003) Nectin-dependent localization of ZO-1 at puncta adhaerentia junctions between the mossy fiber terminals and the dendrites of the pyramidal cells in the CA3 area of adult mouse hippocampus. J Comp Neurol 460:514–524
Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Morimoto K, Inoue E, Ohtsuka T, Miyoshi J, Takai Y (2005) Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development 132:1525–1537
Ishiuchi T, Takeichi M (2012) Nectins localize Willin to cell-cell junctions. Genes Cells 17:387–397
Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S (1993) The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol 121:491–502
Kakunaga S, Ikeda W, Shingai T, Fujito T, Yamada A, Minami Y, Imai T, Takai Y (2004) Enhancement of serum- and platelet-derived growth factor-induced cell proliferation by Necl-5/Tage4/PVR/CD155 through the Ras-Raf-MEK-ERK signaling. J Biol Chem 279: 36419–36425
Kakunaga S, Ikeda W, Itoh S, Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A, Takai Y (2005) Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J Cell Sci 118:1267–1277
Kanzaki N, Ogita H, Komura H, Ozaki M, Sakamoto Y, Majima T, Ijuin T, Takenawa T, Takai Y (2008) Involvement of the nectin-afadin complex in PDGF-induced cell survival. J Cell Sci 121:2008–2017
Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H (2003) Structure-stability-function relationships of dendritic spines. Trends Neurosci 26:360–368
Kawabe H, Hata Y, Takeuchi M, Ide N, Mizoguchi A, Takai Y (1999) nArgBP2, a novel neural member of ponsin/ArgBP2/vinexin family that interacts with synapse-associated protein 90/postsynaptic density-95-associated protein (SAPAP). J Biol Chem 274:30914–30918
Kawano S, Mizutani K, Miyata M, Ikeda W, Takai Y (2010) Interaction of integrin α6β4 with ErbB3 and implication in heregulin-induced ErbB3/ErbB2-mediated DNA synthesis. Genes Cells 15:995–1001
Kelley MW (2006) Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci 7:837–849
Kim DY, Ingano LA, Kovacs DM (2002) Nectin-1alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem 277:49976–49981
Kinugasa M, Amano H, Satomi-Kobayashi S, Nakayama K, Miyata M, Kubo Y, Nagamatsu Y, Kurogane Y, Kureha F, Yamana S, Hirata K, Miyoshi J, Takai Y, Rikitake Y (2012) Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Circ Res 110:716–726
Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A (1990) The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J 9:3217–3224
Kuramochi M, Fukuhara H, Nobukuni T, Kanbe T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura T, Sekiya T, Reeves RH, Murakami Y (2001) TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat Genet 27:427–430
Lachke SA, Higgins AW, Inagaki M, Saadi I, Xi Q, Long M, Quade BJ, Talkowski ME, Gusella JF, Fujimoto A, Robinson ML, Yang Y, Duong QT, Shapira I, Motro B, Miyoshi J, Takai Y, Morton CC, Maas RL (2012) The cell adhesion gene PVRL3 is associated with congenital ocular defects. Hum Genet 131:235–250
Li X, Lynn BD, Nagy JI (2012) The effector and scaffolding proteins AF6 and MUPP1 interact with connexin36 and localize at gap junctions that form electrical synapses in rodent brain. Eur J Neurosci 35:166–181
Liedtke M, Ayton PM, Somervaille TC, Smith KS, Cleary ML (2010) Self-association mediated by the Ras association 1 domain of AF6 activates the oncogenic potential of MLL-AF6. Blood 116:63–70
Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RC, Griffith P, Obisesan TO, Shatz R, Borenstein A, Cupples LA, Lunetta KL, Fallin MD, Baldwin CT, Farrer LA, Multi-Institutional Research on Alzheimer Genetic Epidemiology (MIRAGE) Study Group (2011) A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol 68:1569–1579
Lopez M, Eberlé F, Mattei MG, Gabert J, Birg F, Bardin F, Maroc C, Dubreuil P (1995) Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene 155:261–265
Majima T, Ogita H, Yamada T, Amano H, Togashi H, Sakisaka T, Tanaka-Okamoto M, Ishizaki H, Miyoshi J, Takai Y (2009) Involvement of afadin in the formation and remodeling of synapses in the hippocampus. Biochem Biophys Res Commun 385:539–544
Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y (1997) Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol 139:517–528
Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG (2001) Overexpression of the CD155 gene in human colorectal carcinoma. Gut 49:236–240
Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766
Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL (2007) Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol 178:861–874
Mendelsohn CL, Wimmer E, Racaniello VR (1989) Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56:855–865
Minami A, Mizutani K, Waseda M, Kajita M, Miyata M, Ikeda W, Takai Y (2010) Necl-5/PVR enhances PDGF-induced attraction of growing microtubules to the plasma membrane of the leading edge of moving NIH3T3 cells. Genes Cells 15:1123–1135
Missler M, Fernandez-Chacon R, Südhof TC (1998) The making of neurexins. J Neurochem 71:1339–1347
Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, Tsutsumi T, Sonoda S, Ide C, Takai Y (2002) Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol 156:555–565
Mizutani K, Kawano S, Minami A, Waseda M, Ikeda W, Takai Y (2011) Interaction of nectin-like molecule 2 with integrin α6β4 and inhibition of disassembly of integrin α6β4 from hemidesmosomes. J Biol Chem 286:36667–36676
Mueller S, Wimmer E (2003) Recruitment of nectin-3 to cell–cell junctions through trans-heterophilic interaction with CD155, a vitronectin and poliovirus receptor that localizes to αvβ3-integrin-containing membrane microdomains. J Biol Chem 278:31251–31260
Mueller S, Cao X, Welker R, Wimmer E (2002) Interaction of the poliovirus receptor CD155 with the dynein light chain Tctex-1 and its implication for poliovirus pathogenesis. J Biol Chem 277:7897–7904
Mühlebach MD, Mateo M, Sinn PL, Prüfer S, Uhlig KM, Leonard VH, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, Ramachandran S, McCray PB Jr, Cichutek K, von Messling V, Lopez M, Cattaneo R (2011) Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480:530–533
Niederkofler V, Baeriswyl T, Ott R, Stoeckli ET (2010) Nectin-like molecules/SynCAMs are required for post-crossing commissural axon guidance. Development 137:427–435
Noyce RS, Richardson CD (2012) Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol 20:429–439
Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, Richardson CD (2011) Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog 7:e1002240
Oda T, Ohka S, Nomoto A (2004) Ligand stimulation of CD155a inhibits cell adhesion and enhances cell migration in fibroblasts. Biochem Biophys Res Commun 319:1253–1264
Ohno S (2001) Intercellular junctions and cellular polarity: the PAR–aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol 13:641–648
Okabe N, Shimizu K, Ozaki-Kuroda K, Nakanishi H, Morimoto K, Takeuchi M, Katsumaru H, Murakami F, Takai Y (2004a) Contacts between the commissural axons and the floor plate cells are mediated by nectins. Dev Biol 273:244–256
Okabe N, Ozaki-Kuroda K, Nakanishi H, Shimizu K, Takai Y (2004b) Expression patterns of nectins and afadin during epithelial remodeling in the mouse embryo. Dev Dyn 230:174–186
Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sasaki T, Wimmer E, Nishimune Y, Takai Y (2002) Nectin couples cell–cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol 12:1145–1150
Park J, Liu B, Chen T, Li H, Hu X, Gao J, Zhu Y, Zhu Q, Qiang B, Yuan J, Peng X, Qiu M (2008) Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci 28:12815–12819
Pellissier F, Gerber A, Bauer C, Ballivet M, Ossipow V (2007) The adhesion molecule Necl-3/SynCAM-2 localizes to myelinated axons, binds to oligodendrocytes and promotes cell adhesion. BMC Neurosci 8:90
Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R, Bacigalupo A, Mingari MC, Moretta A, Moretta L (2005) Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 105:2066–2073
Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini S, Rivera P, Capobianco A, Moretta L, Moretta A, Bottino C (2006) Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood 107:2030–2036
Reymond N, Garrido-Urbani S, Borg JP, Dubreuil P, Lopez M (2005) PICK-1: a scaffold protein that interacts with Nectins and JAMs at cell junctions. FEBS Lett 579:2243–2249
Rikitake Y, Mandai K, Takai Y (2012) The role of nectins in different types of cell–cell adhesion. J Cell Sci 125:3713–3722
Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, Südhof TC, Stein V, Biederer T (2010) SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron 68:894–906
Sakamoto Y, Ogita H, Hirota T, Kawakatsu T, Fukuyama T, Yasumi M, Kanzaki N, Ozaki M, Takai Y (2006) Interaction of integrin alpha(v)beta3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J Biol Chem 281:19631–19644
Sakisaka T, Taniguchi T, Nakanishi H, Takahashi K, Miyahara M, Ikeda W, Yokoyama S, Peng YF, Yamanishi K, Takai Y (2001) Requirement of interaction of nectin-1a/HveC with afadin for efficient cell–cell spread of herpes simplex virus type 1. J Virol 75:4734–4743
Samanta D, Ramagopal UA, Rubinstein R, Vigdorovich V, Nathenson SG, Almo SC (2012) Structure of Nectin-2 reveals determinants of homophilic and heterophilic interactions that control cell-cell adhesion. Proc Natl Acad Sci U S A 109:14836–14840
Sandau US, Mungenast AE, McCarthy J, Biederer T, Corfas G, Ojeda SR (2011) The synaptic cell adhesion molecule, SynCAM1, mediates astrocyte-to-astrocyte and astrocyte-to-GnRH neuron adhesiveness in the mouse hypothalamus. Endocrinology 152:2353–2363
Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Südhof TC, Kavalali ET (2005) Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci 25:260–270
Sato T, Irie K, Ooshio T, Ikeda W, Takai Y (2004) Involvement of heterophilic trans-interaction of Necl-5/Tage4/PVR/CD155 with nectin-3 in formation of nectin-and cadherin-based adherens junctions. Genes Cells 9:791–799
Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y (2000) Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem 275:10291–10299
Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2002) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101:657–669
Shao H, Kadono-Okuda K, Finlin BS, Andres DA (1999) Biochemical characterization of the Ras-related GTPases Rit and Rin. Arch Biochem Biophys 371:207–219
Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, Satoh K, Takeuchi M, Imai T, Monden M, Takai Y (2003) Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell–cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem 278:35421–35427
Song JY, Ichtchenko K, Sudhof TC, Brose N (1999) Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A 96:1100–1105
Sozen MA, Suzuki K, Tolarova MM, Bustos T, Fernandez Iglesias JE, Spritz RA (2001) Mutation of PVRL1 is associated with sporadic, non-syndromic cleft lip/palate in northern Venezuela. Nat Genet 29:141–142
Spear PG, Longnecker R (2003) Herpesvirus entry: an update. J Virol 77:10179–10185
Spear PG, Eisenberg RJ, Cohen GH (2000) Three classes of cell surface receptors for aherpesvirus entry. Virology 275:1–8
Spiegel I, Adamsky K, Eshed Y, Milo R, Sabanay H, Sarig-Nadir O, Horresh I, Scherer SS, Rasband MN, Peles E (2007) A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci 10:861–869
Stagi M, Fogel AI, Biederer T (2010) SynCAM 1 participates in axo-dendritic contact assembly and shapes neuronal growth cones. Proc Natl Acad Sci USA 107:7568–7573
Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, Lessmann V, Dresbach T, Gottmann K (2010) Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc Natl Acad Sci USA 107:11116–11121
Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O (2009) The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA 106:17858–17863
Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA (1986) Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103:755–766
Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA (2000) Mutations of PVRL1, encoding a cell–cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet 25:427–430
Takai Y, Ikeda W, Ogita H, Rikitake Y (2008a) The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342
Takai Y, Miyoshi J, Ikeda W, Ogita H (2008b) Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9:603–615
Takei N, Miyashita A, Tsukie T, Arai H, Asada T, Imagawa M, Shoji M, Higuchi S, Urakami K, Kimura H, Kakita A, Takahashi H, Tsuji S, Kanazawa I, Ihara Y, Odani S, Kuwano R (2009) Genetic association study on in and around the APOE in late-onset alzheimer disease in Japanese. Genomics 93:441–448
Takeichi M (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1455
Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, MondenM TY (2003) Direct binding of cell polarity protein PAR-3 to cell–cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem 278:5497–5500
Togashi H, Kominami K, Waseda M, Komura H, Miyoshi J, Takeichi M, Takai Y (2011) Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science 333: 1144–1147
Trivier E, Ganesan TS (2002) RYK, a catalytically inactive receptor tyrosine kinase, associates with EphB2 and EphB3 but does not interact with AF-6. J Biol Chem 277:23037–23043
Tsukita S, Furuse M (1999) Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol 9:268–273
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 4:285–293
Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC (1992) Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science 257:50–56
Wakayama T, Ohashi K, Mizuno K, Iseki S (2001) Cloning and characterization of a novel mouse immunoglobulin superfamily gene expressed in early spermatogenic cells. Mol Reprod Dev 60:158–164
Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG (1998) A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology 246:179–189
Wolburg H, Lippoldt A (2002) Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol 38:323–337
Xie Z, Huganir RL, Penzes P (2005) Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron 48:605–618
Yageta M, Kuramochi M, Masuda M, Fukami T, Fukuhara H, Maruyama T, Shibuya M, Murakami Y (2002) Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res 62:5129–5133
Yamada A, Irie K, Fukuhara A, Ooshio T, Takai Y (2004) Requirement of the actin cytoskeleton for the association of nectins and other cell adhesion molecules at adherens and tight junctions in MDCK cells. Genes Cells 9:843–855
Yap AS, Kovacs EM (2003) Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol 160:11–16
Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL (2009) The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 10:48–57
Yuste R, Bonhoeffer T (2001) Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24:1071–1089
Acknowledgments
We thank our colleagues and collaborators, for their great contributions and excellent achievements. This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Compliance with Ethics Requirements
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mori, M., Rikitake, Y., Mandai, K., Takai, Y. (2014). Roles of Nectins and Nectin-Like Molecules in the Nervous System. In: Berezin, V., Walmod, P. (eds) Cell Adhesion Molecules. Advances in Neurobiology, vol 8. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8090-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8090-7_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8089-1
Online ISBN: 978-1-4614-8090-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)