Abstract
The primo vascular system (PVS) is a newly found organ, which is distributed throughout the entire body. The system is composed of nodes storing many small cells and thin vessels branching out from the nodes. Inside the vessel there are multiple subvessels. The PVS is found in and on most organs, including the brain, and interestingly inside some lymph and blood vessels. The PVS is normally difficult to visualize due to its semitransparent optical property and its small size, which may be the main reason why it was not discovered until recently. The diameter of primo vessels (PVs) is in the range of 20–50 μm and the size of a primo node (PN), 100–1,000 μm. The outermost layer of the PVS is more porous than that of blood or lymph capillary vessels, and the nuclei of the PVS endothelial cells are rod shaped. Important PVS properties reported are: in the fluid inside the PVS, there are cells presenting stem cell markers CD133, Oct4, and Nanog, which may imply that this system has a role in regeneration. Another very important finding is its potential relevance to cancer. According to results from an animal study using xenografts of various cancer types (lung, ovarian, skin, gastric cancer, and leukemia), as the tumor grows, the PVS is formed in a high density in the vicinity of the tumor. In addition, it was shown that PVs connect the primary and secondary tumors and that cancer cells were transported via the PVs in an active manner. In this report, we illustrated the formation of the PVS in breast cancer, and using the green fluorescent protein-expressing gastric cancer cell lines, we observed the cancer cell movement from the primary to the secondary sites during the cancer progression.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Primo Vascular System
The primo vascular system (PVS) exists in most, if not all, mammalian organs, forming an extensive network throughout the entire body. The PVS consists of primo nodes (PNs), primo vessels (PVs) that branch out of PNs, transparent fluid inside the PNs and PVs (primo-fluid, P-fluid), and the primo-microcells (P-microcells) stored mainly in PNs. The PVS is very small: the diameter of PVs is in the range of 20–50 μm and the size of a PN is in the range of 100–1,000 μm. The PVS is optically semitransparent (Fig. 39.1). It is, therefore, not easy to observe the system without special training in microsurgery and microscopy, which probably explains why this system was not discovered until the 1960s by Bonghan Kim in North Korea [1, 2]. Shortly after Kim’s reports in the 1960s, the PVS research was suddenly discontinued for unknown reasons. In the 2000s, the research was reinitiated by Kwang-Sup Soh in South Korea [3].
The PVS is distinctively different from the blood or lymphatic system. It does not express blood vessel (BV) biomarker CD31 or a lymphatic vessel (LV) biomarker LYVE-1 [4]. The external wall of the PV is porous and, therefore, when a dye (usually trypan blue) is applied to the area suspected with PVS, it gets into the PVs preferentially, allowing the observer to be able to differentiate PVs from BVs and LVs [5]. The cells of the sub-PV possess f-actin along the PV length, indicating the direction of the P-fluid [6]. The average flow speed of the P-fluid was reported to be 0.3 mm/s [6]. The nuclei of the endothelial cell in the sub-PV are rod shaped, and therefore, DAPI (dye specific to DNA or nuclei) is frequently used to confirm the PVS. Typically, the PN is packed with many small cells (P-microcell), and the cells are round and 1.0–2.5 μm in diameter [6]. The cells have small nuclei surrounded by a layer of cytoplasm and a trilaminar plasma membrane. The research team led by Soh [7, 8] confirmed that these small cells possess embryonic stem cell markers, Oct4, Nanog, and CD133. Kwon et al [9]. recently reported that the PVS also contains immune cells, enriched with granulocytes and histiocytes and chromaffin cells. Hong et al. measured the oxygen level of the P-fluid of the PVS formed in the murine melanoma [10] and in the mice embryos [11].
The PVS has been found in all organs in all animals that were studied, including intestine [12, 13], brain [14–16], heart [17], adipose tissue [18], and also within blood [19, 20] and lymphatic vessels [21, 22]. The number of animal species studied for the PVS, up to now, is 7 and the number of organs confirmed for the existence of PVS is 17 [23].
1.2 PVS and Cancer
The PVS in/on normal tissues or organs is usually not very densely populated, except for the adipose tissue. However, for four different cancer types (xenograft) in studies reported up to now, the PVS was found densely populated in proximity of the tumor.
The Soh team studied xenografts of human lung cancer NCI-H460 and human ovarian cancer SK-OV-3 [24] and observed dense PVS formed at the sites with the tumor (cancer PVS). The team then designed a unique experiment to elucidate the relationship between the cancer progression and the PVS formation. For the study, they electroporated quantum dots (QDs) into the cancer cells, and the QD-containing live cancer cells were then inoculated to nude mice. Because the QDs were inside these cells, the newly formed cells also possessed QDs and emitted fluorescence, enabling to track cancer cells by fluorescent microscopy. As the cancer progressed, secondary tumors were also formed and both LVs and PVs were generated from the primary tumor. When the fluorescence level was monitored around the area with the tumor, the fluorescence intensity from the cells passing through the PV was approximately twice that from the lymph vessel, indicating that cancer cells were transported through PVs significantly. The result from the fluorescence level emitted from PNs in the ovarian cancer showed the fluorescence at an even greater level than that from the lymph node.
In an attempt to study the origin of the PVS during the cancer progression, Heo and colleagues used cutaneous melanoma B16BL6 (nonfluorescing) into green fluorescence protein (GFP)-expressing host mice [25]. They also found denser PVS around the tumor, and the PVS formed on/in the tumor emitted green fluorescence, implying that it is initiated from the host, although it was not clear whether all PVS formed were from the host because the tumor-initiated PVS would not emit the fluorescence.
The Miller team [26] recently reported that, in a human lymphoma (U937) xenograft, PVS was formed at a higher density. The cells in the cancer PVS expressed CD68, CD45, and lysozyme and possessed the same immune phenotype of the cancer cell U937. The cells upregulated, by hundred- to thousandfold, human cancer stem cell-specific transcription factors (particularly CD68, CD45) and an upstream regulator of Nanog that maintains the pluripotent and undifferentiated state of stem cells.
Here, we report the result of PVS formation during the growth of breast cancer xenograft MDA-MB231. We also report the cancer cell tracking from the primary to the secondary tumor, utilizing the GFP-expressing MKN28 human gastric cancer cell line.
2 Methods
2.1 Observation of PVS in Normal Rat
Sprague Dawley rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.). An incision down the linea alba was made to expose the small intestine and omentum. The intestines and omentum were sprayed with a 0.2 % trypan blue solution to identify PVs. Once a PV was identified by the uptake of blue dye, it was carefully dissected with the aid of microforceps, microvessel dilators and a microscope (OP-Mi 7PH Microscope; Carl Zeiss; Germany). Images were taken with a camera (PowerShot SD940 ISl; Canon; USA) mounted on the scope.
2.2 PVS of Breast Cancer Xenograft MDA-MB231
Nude mice were inoculated with human MDA-MB231 breasts cancer cells (~107 cells) in the right flank, and tumors were allowed to grow for 1–2 months. At the time of PVS observation, mice were euthanized with carbon dioxide and, immediately, a skin incision down the flank was performed to expose the tumor. The tumor was sprayed with a 0.2 % trypan blue solution to identify PVs. The identification and imaging of the PVS were performed using the same method described above.
2.3 PVS and GFP-Expressing Gastric Cancer
MKN12 human gastric cells were transfected with GFP-expressing plasmid using Lipofectamine LTX and PLUS reagent (Invitron; Monmouth, UK), following the manufacturer’s instruction. The cells transfected with the GFP plasmid were then sorted with FACSAria (BD Biosciences; San Jose, CA, USA). Nude mice were injected with the GFP-expressing cancer cells (~107 cells) to the gastric wall, and tumors were allowed to grow for 1–2 months. At the time of the PVS observation, mice were anesthetized with Zoletil/Rompun intraperitoneal injection. Skin incision in the abdominal area was performed to expose the tumor. Fluorescence image was taken using fluorescence microscopy (MVX10; Olympus; Japan) and a monochrome CCD camera (DP30BW; Olympus; Japan) mounted on the scope.
3 Results and Discussion
3.1 Typical PVS in Normal Rat Model
Figure 39.1a shows a typical set of a PN and PVs connected to the PN, which was isolated from the surface of the omentum of a rat. PNs usually appear along the PV. The PVS on the surface of an organ can be isolated by tracking the PVs, as shown in Fig. 39.1b.
3.2 PVS of Breast Cancer Xenograft
Figure 39.2 demonstrates the PVS formed on the human breast cancer type MDA-MB231 xenograft in a nude mouse, lightly stained with trypan blue. As in the cases with the previously studied human lung, ovarian, lymphoma, and melanoma xenografts, the PVS was seen at a higher density than on the tissue without the tumor, which confirms that the PVS is densely formed in the vicinity of the cancerous tumor. This may also imply that some unknown but particular properties of cancer affect the proliferation of cancer PVS, potentially using the PVS for its growth and metastasis, although more extensive studies are required to verify this hypothesis. Assuming that the hypothesis is proven to be true, this property of the cancer PVS may be utilized for early detection of cancer.
3.3 PVS as Cancer Cell Transport Conduit
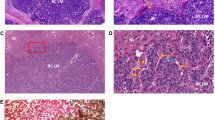
In the previous studies on the roles of the PVS in cancer cell transport, Soh’s group used QD-electroporated cancer cell lines of human lung cancer NCI-H460 and human ovarian cancer SK-OV-3, and the fluorescence generated from the QDs was traced assuming that they represented cancer cells. In this new study, to confirm the cancer cell transport via the cancer PV, GFP-expressing, human gastric cancer cell type MKN28 was xenografted in nude mice (nonfluorescing). As the primary tumor grew large, a secondary tumor was formed, as shown by optical microscopy in Fig. 39.3a. The figure describes the primary and secondary tumors and a PV that connects the two, which is indicated by the black arrow. Figure 39.3b shows an image obtained by fluorescence microscopy for the green fluorescence. Both the primary and secondary tumors were shown to be very bright and there were dots emitting bright green fluorescence from inside the cancer PV connecting the two (indicated by the white arrow), showing that the cancer cells are present inside the PV and are being transported via the PV. This cancer PV itself does not express the fluorescence, potentially indicating that the PV in the tumor is from the host, as in Heo’s [24] study result. Figure 39.3c shows the enlarged image of the PV, circled in Fig. 39.3b. The presence of the cancer cells (fluorescence) in the PV is more clearly demonstrated.
Observation of the relationship between the secondary tumor formation and the PV formed between the primary and the secondary tumors, utilizing GFP-expressing MKN28 human gastric cancer cells. (a) Optical microscopy of a PV (indicated by a black arrow) connecting the primary (P) and the secondary (S) tumors. (b) Fluorescence microscopy of the same regions is shown in (a). The primary and the secondary tumors are fluorescing. In the PV, GFP-expressing cancer cells are present, implying that the cancer cells are being transported via the PV. (c) An enlarged image of the circular region of (b). GFP-expressing cancer cells inside the PV are more clearly shown
4 Conclusions
Our study results with the breast cancer MDA-MB231 appeared to confirm that cancer (at least in the case of xenografts) induces dense PVS formation in close proximity to cancerous tumors, which suggests that a cancerous environment triggers cancer PVS formation. If it is a common signature of cancer, then it may be utilized for early detection of cancer.
The study results using GFP-expressing gastric cancer xenograft confirm that many cancer cells are present in and possibly transported via the cancer PVs, as in the previous study results by the Soh team, using QD-containing cancer cell. This result strongly implies that the cancer PVS may have a very important role in cancer metastasis. Future studies will be planned to elucidate the mechanism of the cancer PV formation, to prevent the cancer metastasis via the PVs.
The relationship between the cancer progression and the cancer PVS formation/cancer cell transportation via the cancer PV is new but very important and deserves immediate multidisciplinary efforts for more complete understanding to develop novel tools for cancer prevention, diagnostic, and treatment.
References
Kim BH (1963) On the Kyungrak system. J Acad Med Sci DPR Korea 90:1–35
Kim BH (1965) The sanal theory. J Acad Med Sci DPR Korea 108:39–62
Soh KS (2012) A brief history of the Bong-Han theory and the primo vascular system. In: Soh KS, Kang KA, Harrison DH (eds) The primo vascular system: its role in cancer and regeneration. Springer, New York, pp 3–5
Yoo JS, Ayati MH, Kim HB, Zhang W, Soh KS (2010) Characterization of the primo-vascular system in the abdominal cavity of the lung cancer mouse model and its differences from the lymphatic system. PLoS One 5(4):e9940
Ogay V, Bae KH, Kim KW, Soh KS (2009) Comparison of the characteristic features of Bonghan ducts, blood and lymphatic capillaries. J Acupunct Meridian Stud 2(2):107–117
Sung B, Kim MS, Lee BC, Yoo JS, Lee SH, Kim YJ, Kim KW, Soh KS (2008) Measurement of flow speed in the channels of novel threadlike structures on the surfaces of mammalian organs. Naturwissenschaften 95(2):117–124
Ahn SH, Lee SW, Hwang SY, Kim JH, Sohn IC (2012) Primo-microcell in a primo node as a possible origin of adult stem cells. In: Soh KS, Kang KA, Harrison DH (eds) The primo vascular system: its role in cancer and regeneration. Springer, New York, pp 163–170
Ogay V, Soh KS (2012) Identification and characterization of small stem-like cells in the primo vascular system of adult animals. In: Soh KS, Kang KA, Harrison DH (eds) The primo vascular system: its role in cancer and regeneration. Springer, New York, pp 149–156
Kwon BS, Ha CM, Yu S, Lee BC, Ro JY, Hwang S (2012) Microscopic nodes and ducts inside lymphatics and on the surface of internal organs are rich in granulocytes and secretory granules. Cytokine 60(2):587–592
Hong M, Park SS, Do H, Jhon GJ, Suh M, Lee Y (2011) Primo vascular system of murine melanoma and heterogeneity of tissue oxygenation of the melanoma. J Acupunct Meridian Stud 4(3):e159–e163
Hong M, Park SS, Do H, Jhon GJ, Suh M, Lee Y (2012) Study of the primo-vascular system and location-dependent oxygen levels for a mouse embryo. J Nanosci Nanotechnol 12(7): 5168–5172
Jiang X, Lee BC, Choi C, Baik KY, Soh KS (2004) Tubular structure of intravascular thread-like structures from rats and rabbits. J Korean Phys Soc 44:1602–1604
Shin HS, Johng HM, Lee BC, Cho SI, Soh KS, Baik KY, Yoo JS, Soh KS (2005) Feulgen reaction study of novel threadlike structures (Bonghan ducts) on the surface of mammalian organs. Anat Rec B New Anat 284(1):35–40
Dai JX, Lee BC, An P, Su Z, Qu R, Eom KH, Soh KS (2011) In vivo in situ staining of the primo vascular system in ventricles and subarachnoid space of brain by injecting trypan blue into the lateral ventricle. Neural Regen Res 6(28):2171–2175
Nam MH, Lim JK, Choi SH, Kim SC, Soh KS (2012) A primo vascular system underneath the superior sagittal sinus in the brain of a rabbit. J Acupunct Meridian Stud 5(5):210–217
Lee HS, Park WH, Je A-R, Kweon HS, Lee BC (2012) Evidence for novel structures (primo vessels and primo nodes) floating in the venous sinuses of rat brains. Neurosci Lett 522(2):98–102
Lee BC, Kim HB, Sung B, Kim KW, Sohn J, Son B, Chang B-J, Soh KS (2012) Structure of the sinus in the primo vessel inside the bovine cardiac chambers. In: Soh KS, Kang KA, Harrison DH (eds) The primo vascular system: its role in cancer and regeneration. Springer, New York, pp 57–62
Lee BC, Kim KW, Soh KS (2009) Visualizing the network of bonghan ducts in the omentum and peritoneum by using trypan blue. J Acupunct Meridian Stud 2(1):66–70
Baik KY, Lee J, Lee BC, Johng HM, Nam TJ, Sung B, Cho S, Soh KS (2005) Acupuncture meridian and intravascular bonghan duct. Key Eng Mater 277:125–129
Yoo JS, Kim MS, Ogay V, Soh KS (2008) In vivo visualization of bonghan ducts inside blood vessels of mice by using an Alcian blue staining method. Indian J Exp Biol 45(5):336–339
Lee BC, Soh KS (2008) Contrast-enhancing optical method to observe a bonghan duct floating inside a lymph vessel of a rabbit. Lymphology 41(4):178–185
Johng HM, Yoo JS, Yoon TJ, Shin HS, Lee BC, Lee C, Lee JK, Soh KS (2007) Use of magnetic nanoparticles to visualize threadlike structures inside lymphatic vessels of rats. Evid Based Complement Altern Med 4(1):77–82
Soh KS (2012) Current state of research on the primo vascular system. In: Soh KS, Kang KA, Harrison DH (eds) The primo vascular system: its role in cancer and regeneration. Springer, New York, pp 25–40
Yoo JS, Kim HB, Won N, Bang J, Kim S, Ahn S, Lee BC, Ahn S, Soh KS (2011) Evidence for an additional metastatic route: in vivo imaging of cancer cells in the primo-vascular system around tumors and organs. Mol Imaging Biol 13(3):471–480
Heo C, Hong MY, Jo A, Lee YH, Suh M (2011) Study of the primo vascular system utilizing a melanoma tumor model in a green fluorescence protein expressing mouse. J Acupunct Meridian Stud 4(3):e198–e202
Islam MA, Thomas SD, Slone S, Alatassi H, Miller DM (2013) Tumor-associated primo vascular system is derived from xenograft, not host. Exp Mol Pathol 94(1):84–90
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Kang, K.A., Maldonado, C., Perez-Aradia, G., An, P., Soh, KS. (2013). Primo Vascular System and Its Potential Role in Cancer Metastasis. In: Van Huffel, S., Naulaers, G., Caicedo, A., Bruley, D.F., Harrison, D.K. (eds) Oxygen Transport to Tissue XXXV. Advances in Experimental Medicine and Biology, vol 789. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7411-1_39
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7411-1_39
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7256-8
Online ISBN: 978-1-4614-7411-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)