Abstract
Coronary angiography (CA), percutaneous coronary intervention (PCI), catheter-based structural heart intervention, electrophysiological studies, and arrhythmia ablation are procedures that help cardiologists ensure better clinical diagnosis and treatment (Dawkins et al. 2005). During these procedures, catheters, guide wires, and other devices are visualized and guided by using real-time fluoroscopy. Therefore, operators are inevitably exposed to radiation (Kim and Miller 2009). Compared to other departments (radiology, urology, operating rooms, etc.), the cardiovascular or catheterization laboratory is generally considered to be an area of high radiation exposure (Raza 2011). Interventional cardiology (IC) staff is exposed more radiation per year than are radiologists by a factor of two to three (Picano et al. 2007). Invasive cardiology procedures have increased tenfold in the past decade, and growth in the field has been accompanied by concern for the safety of such staff (Picano et al. 2007).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Radiation Dose
- Percutaneous Coronary Intervention
- Personal Protective Equipment
- Catheterization Laboratory
- Interventional Cardiology
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Coronary angiography (CA), percutaneous coronary intervention (PCI), catheter-based structural heart intervention, electrophysiological studies, and arrhythmia ablation are procedures that help cardiologists ensure better clinical diagnosis and treatment (Dawkins et al. 2005). During these procedures, catheters, guide wires, and other devices are visualized and guided by using real-time fluoroscopy. Therefore, operators are inevitably exposed to radiation (Kim and Miller 2009). Compared to other departments (radiology, urology, operating rooms, etc.), the cardiovascular or catheterization laboratory is generally considered to be an area of high radiation exposure (Raza 2011). Interventional cardiology (IC) staff is exposed more radiation per year than are radiologists by a factor of two to three (Picano et al. 2007). Invasive cardiology procedures have increased tenfold in the past decade, and growth in the field has been accompanied by concern for the safety of such staff (Picano et al. 2007).
Junior cardiologists are exposed to 60% more radiation than are their seniors (Watson et al. 1997). This difference is largely a result of younger staff taking longer duration to position fluoroscopic catheters, due to their lesser skill and shorter practice (Kottou et al. 2001). High workloads, the complexity of procedures and the lack of IC specialists in hospitals are growing concerns in the health sector (ICRP 2000; Vano et al. 1998a, b). The practices employed in catheterization laboratory facilities have become routine not only in Western societies but also in the Asia Pacific region (Rotter et al. 2003; Asian Network of cardiologists 2007; Tsapaki et al. 2011).
The staff who work in IC departments employs relatively high amounts of radiation (Delichas et al. 2003), and face the risk of developing cataracts after several years of work exposure, if radiation protection tools are not properly used (Sim et al. 2010). Cumulative X-ray doses imposed on the lenses of IC staff’s eyes are often high (Vano et al. 2010a). Radiation-induced cataracts are distinct from naturally occurring ones, because they form in the posterior pole of the lens (Vano et al. 2010a). The increased incidence of lenticular changes that are occurring in IC staff, and its association with radiation doses is an important finding that underlines the need to address current concerns about the threshold dose for cataract formation (Bjelac et al. 2010).
During procedures, IC staff members are directly exposed to radiation that is reflected (scattered) from the patient (primary) and to a lesser extent from the walls of the room (secondary) (Maeder et al. 2005). The imposition of radiation dose limits for unprotected parts of the body, like eyes, hands, and the thyroid gland is crucial among IC staff, if they are to avoid the development of cataracts, cancers of the brain, skin, or thyroid (Raza 2011; Finkelstein 1998). The Ionising Radiation Regulation, introduced in 1999, reduced the maximum whole body dose for exposed personnel, but did not revise the maximum dose to the extremities (Hafez et al. 2005). The current annual dose limit is 20 mSv for the body, 150 mSv for the thyroid or eyes, and 500 mSv for the hands (International guidelines, ICRP). The recommended occupational dose of radiation for medical staff in Germany is 500 mSv for hands, 150 mSv for eyes, and 300 mSv for the thyroid [German Guidelines 2003].
In this systematic review, we address the following research questions:
-
1.
Are radiation doses for IC staff within the prescribed limits?
-
2.
Do current exposure levels produce adverse health effects for IC staff?
-
3.
Are protective measures taken against radiation exposure adequate?
2 Criteria Applied to This Systematic Review
We performed a systematic literature search by using PubMed and EMBASE and by inputting appropriate keywords into the Google search engine. The keywords used were “radiation dose,” combined with “dose,” “interventional,” “cardiologists,” “technical,” “nurses,” “hands,” “fingers,” “neck,” “thyroid,” “eyes,” “forehead,” “health,” “effects,” “cataract,” and “cancer.” The search entailed the period from January 1990 to October 2011. We also searched through reference lists of selected prominent studies for relevant publications. However, no additional eligible publication was identified by searching through these reference lists. The literature search was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The process for identifying, screening, determining eligibility, and inclusion of databases for this study is shown in a flow chart (Fig. 1). The flow chart was developed from the PRISMA flow diagram that is used for reporting databases in systematic reviews (Moher et al. 2009). The review protocol for PRISMA was based on the information given at the following website: http://www.prisma-statement.org/statement.htm.
In the present review, we included publications that addressed the types of topical information showed below.
Study design: Cohort or cross-sectional studies.
Study population: Interventional cardiology staff.
Exposure: Annual and per procedure radiation dose in the workplace for different anatomical locations (hands/fingers, eyes/forehead, neck/thyroid).
Languages: German and English.
We assessed the methodological quality of the literature and classed studies as being “moderate” or “good.” A study was deemed to be of “moderate” quality, if it did not include dosimetric measurements of the eyes, thyroid gland and hands of IC staff. A study was rated as “good,” if the radiation dose of IC staff for these anatomical locations (eyes, thyroid, and hands) was measured and/or adverse health concerns related to these findings discussed. Each of the authors of this review carried out literature screening and quality evaluation independently. Our individual findings were then compared, and in the event of disagreement, a consensus was reached by means of discussion.
In this review, we identified 42 records from the literature and 54 records from other database sources that matched the appropriate keywords (Fig. 1). After eliminating duplicates,73 records were analyzed for relevance against the topic and inclusion criteria. Twenty-four records were found to be eligible and were included in the final analysis. Twenty-eight papers that were adjudged to be of moderate quality were excluded from the study (Fig. 1).
3 Growth and Trends in Catheterization Laboratory
The number of catheterization laboratories varies according to country, with 460 labs in India, 30 in Bangladesh, 44 in Thailand, and 50 in Malaysia (Tsapaki et al. 2011). Unfortunately, no literature on dosimetric information was available from these countries (Tsapaki et al. 2011). Approximately 3,100 operations and 725 interventional cardiac catheterizations are performed annually in the UK on babies and children who are afflicted with congenital heart disease (Petersen et al. 2003). It was documented in an earlier report that the personnel of three cardiac catheterization laboratories had performed more than 15,000 cardiac procedures over a period of 5 years in Canada (Renaud 1992). The differences in X-ray systems (old film-based systems vs. digital units) and their particular settings, levels of staff training in radiation protection, frequency of use of radiation protection facilities and personal dosimeters, and workloads of specialists all affect radiation doses received by IC staff (Vano et al. 2006a). If specialists do not regularly wear their personal dosimeters, the mean values for their occupational exposure in catheterization laboratories could provide an incorrect estimate of the real radiological risk (Vano et al. 2006; Balter 1993).
Our radiation exposure assessment shows that the badges that exceed the level 1 ALARA (as low as reasonably achievable) limits (<6 mSv/year) are indeed worn by invasive cardiologists (Andreassi et al. 2005). Even if IC staff members are involved in 1,000 angiographies per year, the annual threshold exposure level of 20 mSv is unlikely to be exceeded. An operator with a comparative 1,000 procedures per year may reach the recommended occupational limits of 150 mSv for the lens of the eye and 500 mSv for the hands (Maeder et al. 2005). In this context, we presume that IC staff is at risk in the Asian Pacific region. This is because IC staff members in the Asia Pacific region may regularly conduct 1,000 procedures annually as a result of the huge demand for these procedures in treating patients, and the high workloads of these specialists.
When laboratories possess modern radioimaging equipment, use experienced operators, and adhere to standard safety precautions, coronary intervention is considered to be quite safe for both patients and operator personnel (Efstathopoulos et al. 2003). If they are to optimally protect patients and staff, operators of X-ray instruments during catheterization procedures must know the typical dose rates for each X-ray system they use (Vano et al. 2006). The dose area product (DAP) levels for CAs and PCI in six European countries was measured as 39.1 Gycm2 (CA) and 54.4 Gycm2 (PCI), respectively. Based on these data, the European Research Cardiology Group for Measures for Optimising Radiological Information and Dose in Digital Imaging and Interventional Radiology has proposed temporary reference DAP levels (viz., 45 Gycm2 for CA and 75 Gycm2 for PCI). Several authors (Maeder et al. 2005; Kuon et al. 2003, 2004) have observed that DAP levels exceed the proposed reference levels. However, finding a correlation between the Kerama Area Product (KAP) values and the eye lens doses has been difficult (Domienik et al. 2011).
Different occupational radiation doses result from using different catheter insertion sites. The most common insertion sites for PCI utilize the femoral and radial/brachial approaches. The reason for insertion-site differential dosing during cardiac procedures is that the physician’s position relative to the patient changes as the insertion site changes. The radial approach requires that the cardiologist work in closer proximity to the X-ray beam (Whitby and Martin 2005). The radial approach increased operator radiation exposure by 100% during diagnostic coronary catheterization procedures and by 50% during coronary interventions (Lange and von Boetticher 2006). No special devices or provisions were made to protect IC staff against such increased exposure. The primary reasons for the higher doses included the physician being in closer proximity to the X-ray field and longer fluoroscopy times (Kim and Miller 2009). The subclavian approach, used for implanting pacemakers and similar devices resulted in higher exposure rates than did the femoral and radial approaches, due to the operator’s proximity to the X-ray beam (Limacher et al. 1988; Lindsay et al. 1992). The operator’s external whole body dose was significantly higher when percutaneous transluminal coronary angioplasty (PTCA) was performed from the radial artery (13.5 ± 2.1 mrem/case), when compared to the femoral artery (8.8 ± 1.3 mrem/case) approach. By moving the floor shield to increase protection from the X-rays (3.3 ± 2.3 mrem/case vs. femoral), exposure from this procedure was reduced to levels less than that experienced from using the femoral artery approach. Thus, if proper procedures are followed, PTCA can be performed from the radial artery approach without producing increased operator radiation exposure (Mann et al. 1996).
Both physicians-in-training and staff physicians in cardiac catheterization laboratories are the groups who receive radiation doses that exceed the recommended limit. One other important factor that affects exposure level to radiation is the working attitude and techniques used by staff (Renaud 1992). In this regard, Watson et al. (1997) emphasized the importance of closely supervising cardiology fellows early in their training to limit radiation doses to patients and to staff personnel.
4 Radiation Doses for Interventional Cardiology Staff
No recommended limits for per procedure radiation doses were found in the literature. Therefore, exposure doses could not be analyzed against such threshold limits. In Fig. 2, we show the radiation dose incurred per procedure for hands/fingers/wrists by IC staff. These results show that, in ten cases, the doses received were >100 mSv. The annual radiation doses for hands/fingers/wrists among IC staff are well below the recommended dose (Fig. 3). Three observations for the radiation dose to hands exceeded the recommended ALARA 1 level (Fig. 3). The radiation dose received per procedure for the eyes and forehead of IC staff is presented in Fig. 4. Only four doses were >100 mSv. With one exception, the annual radiation exposure to eyes and forehead of IC staff were below the recommended dose (Fig 5). The recommended ALARA (level 1) of 6 mSv (Andreassi et al. 2005) for the radiation dose to eyes was exceeded in six observations (Fig. 5). Data were available for two cases that gave per-procedure doses (i.e., >100 mSv) for the thyroid/neck region of IC staff (Fig. 6). No literature was available for the annual dose received to the thyroid/neck region of IC staff.
Radiation dose per procedure for hands/fingers/wrists for interventional cardiology staff. Sources: Efstathopoulos et al. 2011;Tsapaki et al. 2008; Short et al. 2007; Damilakis et al. 1995; Wu et al 1991; Vano et al 1998. For each study, data points may represent combined observations from different anatomical positions (e.g., left and right wrists, fingers, and hands) and measurements with or without personal protective equipment (PPEs)
Annual radiation dose for hands/fingers/wrists for interventional cardiology staff. Sources: Pyo et al. 2008; Kim et al. 2008; Efstathopoulos et al. 2011; Whitby and Martin 2005; Domienik et al. 2011. For each study, data points may represent combined observations from different anatomical positions (e.g., left and right wrists, fingers, and hands) during procedures such as Percutaneous Transluminal Coronary Angioplasty (PTCA), femoral angioplasty, stents, embolism, angiograms
Radiation dose per procedure for eyes/forehead for interventional staff. Sources: Efstathopoulos et al. 2011; Pratt and Shaw 1993; Calkins et al. 1991; Karppinen et al. 1995; Marshall et al. 1995; Short et al. 2007; Lie et al 2008; Wu et al 1991; Vano et al. 1998. For each study, data points may represent combined observations from different anatomical positions (e.g., left and right eyes, between the eyes) and measurements with or without PPEs
Annual radiation dose for eyes/forehead for interventional cardiology staff. Sources: Vano et al. 1998, 2010; Pyo et al. 2008; Kim et al. 2008; Lie et al. 2008; Efstathopoulos et al. 2011; Domienik et al. 2011. For each study, data points may represent combined observations from different anatomical positions (e.g., left and right eyes, between the eyes) and measurements with or without PPEs
Radiation dose per procedure for thyroid/neck region for interventional cardiology staff. Sources: Steffenino et al. 1996; Williams 1997; Calkins et al. 1991; Wu et al. 1991; Vano et al. 1998. For each study, data points may represent a combination of observations made with or without the use of PPE
The exceeded limits of ALARA 1 for eyes and hands were observed only among IC surgeons. The locations from which these data were gathered included a university hospital in Athens (Attikon), 34 European hospitals, and hospitals in Spain, Norway, Bogota (Columbia), and Montevideo (Uruguay). In the present review, we show that 12.5% (3/24) of IC surgeons experienced a risk of radiation exposure for hands and 66.7% (6/9) for eyes. This risk was based on the radiation dose observed to occur between the recommended levels and ALARA 1 level (i.e., between 6 and 500 mSv for hands and 6 and 150 mSv for eyes in different studies) (Figs. 3 and 5).
4.1 Hand and Wrist Exposure
Figures 2 and 3, respectively, show single procedure (μSv) and annual radiation (mSv) doses received for hands and wrists by IC staff. When IC surgeons protect their right hands by a lead screen, the radiation dose received was 147 μSv per procedure; without such protection the right hand received 242 μSv per procedure (Vano et al. 1998). Without a lead screen the left hand received 514 μSv, compared to 235 μSv per procedure when a lead screen was used (Vano et al. 1998). The left finger and left wrist of cardiology surgeons working in IC departments received radiation doses of 7.3 and 1.3 mSv, respectively; these doses compared to 3.6 and 1.3 mSv for the left and right wrists, respectively (Domienik et al. 2011). The right and left wrists received almost the same level of radiation per procedure in nursing/assistant staff working in IC departments (26 μSv). However, fingers of the left hand received more radiation exposure (4 μSv) than did the fingers of the right hand (2 μSv) (Efstathopoulos et al. 2011). The annual radiation exposure level sustained by the nursing staff in IC departments was 1.3 m (Kim et al. 2008).
The left wrists of cardiology surgeons working in IC departments received a mean radiation dose of 493 μSv per procedure, whereas right wrists received a dose of 108 μSv (Efstathopoulos et al. 2011). In three earlier studies, the radiation doses to the hands of surgeons were 27, 482, and 710 μSv, respectively (Tsapaki et al. 2008; Short et al. 2007; Damilakis et al. 1995). The left fingers received more radiation than did the right fingers (324 vs. 88 μSv per procedure) (Efstathopoulos et al. 2011). Among 605 individuals performing coronary angiographies, the annual radiation dose for the left and right wrists of cardiology surgeons was 19.2 and 12.5 mSv, respectively (Efstathopoulos et al. 2011). Staff radiation doses varied between 34 and 235 μGy per procedure at the left wrist and 28 and 172 μGy at the right wrist (Goni et al. 2005). For different IC procedures performed by the same IC surgeons, the radiation dose to the hand nearer the procedure was different than that of the hand that was further away (Fig. 7; Whitby and Martin 2005).
Radiation dose received to the hand nearest and farthest during different IC procedures for IC surgeons (Whitby and Martin 2005)
4.2 Eye and Thyroid Gland Exposure
Figures 4 and 5 show per procedure (μSv) and annual radiation doses (mSv) for IC staff that was received to eyes and forehead, respectively. The eyes received an annual radiation dose of 1.5 mSv (Vano and Faulkner 2005), and 0.9 and 0.9 mSv (Kim et al. 2008) among IC Department nursing staff. The per-procedure radiation dose was 64 μSv (for eyes), 4 μSv (between the eyes), and 1 μSv (left eyes) (Efstathopoulos et al. 2011). A 3-year follow-up study showed the annual radiation dose received by cardiology surgeons in IC departments to be 450 mSv in 1993 and 900 mSv in 1996 (Vano et al. 1998). The per-procedure radiation dose levels for cardiology surgeons were variable: between 0.008 and 0.113 μSv (Pratt and Shaw 1993), 0.28 μSv (Calkins et al. 1991), 0.43 μSv (Karppinen et al. 1995), and 0.014 μSv (Marshall et al. 1995). The annual radiation doses recorded in various studies were as follows: 6 mSv (Vano et al. 2010), 8.2 mSv (Efstathopoulos et al. 2011), 4.1 mSv in the left eye (Domienik et al. 2011), and 3.2 mSv between the eyes (Domienik et al. 2011).
The annual radiation dose range received for 900 procedures to the eyes of cardiology surgeons was 1–11 mSv (protected eyes) and 9–210 mSv (unprotected eyes) (Lie et al. 2008). The per-procedure dose for eyes was 64 μSv between the eyes, 37 μSv in the left eye, 12 μSv between the eyes (Efstathopoulos et al. 2011), and 44 μSv for the eyes (Lie et al. 2008). For IC surgeons, the observed radiation dose for the right eye, when protected by a lead screen, was 136 μSv, whereas for the right eye, without such protection, the dose was 205 μSv; for the left eye protected by a lead screen the dose was 170 μSv, and for the unprotected left eye, the dose was 439 μSv (Vano et al. 1998). Figure 6 shows the IC staff radiation doses (μSv) received per procedure by the thyroid/neck region. The radiation dose per procedure for thyroid glands among cardiology surgeons varied across studies 0.215–0.37 μSv (Steffenino et al. 1996), 0.05–0.14 μSv (Williams 1997), 0.20 μSv (Calkins et al. 1991), 163 μSv with a lead screen (Vano et al. 1998), and 392 μSv per procedure without a lead screen (Vano et al. 1998).
5 Cataracts As an Adverse Health Effect for Interventional Cardiology Staff
A study presented at the European Society of Cardiology congress in 2009 (Duran et al. 2009) disclosed a significant difference in the frequency of lens opacity (37.9% vs. 12%, p < 0.005) between exposed IC staff and the control group. Therefore, the eyes may be regarded as a limiting organ for CA and PCI procedures (Lie et al. 2008). Bjelac et al. (2010) studied the prevalence of radiation-associated posterior lens opacity in Malaysia and reported an incidence of 52% (29/56, 95%CI 35–73) for interventional cardiologists, 45% (5/11, 95%CI 15–100) for nurses, and 9% (2/22, 95%CI 1–33) for control subjects. The risk of lens opacity was 5.7% (95%CI 1.5–22) for interventional cardiologists and 5.0% (95%CI 1.2–21) for nurses. Ophthalmological examinations of IC staff exposed to radiation revealed that 38% of surgeons and 21% of nurses had radiation-associated lens changes, in a study conducted in Bogota (Columbia) and Montevideo (Uruguay) from 2008 to 2009 (Vano et al. 2010). In 2004, a lens opacity of 37.3% and cataract case of 8% were observed among IC surgeons in North America (Junk et al. 2004). Yuan et al. (2010) observed that cardiologists who performed cardiac catheterization (CC) had more cataracts (1.2%) than doctors not performing CC (0.8%), in a study from all contracted hospitals of the Bureau of National Health insurance in Taiwan. However, this difference was not significant and there were several limitations to the study (Yuan et al. 2010).
If protection tools or procedures are not used, radiation doses to the lens of the eye may exceed the threshold for deterministic effects (lens opacity or cataracts) after several years of occupational exposure in this environment; this is true even when exposed staff perform as few as three to five procedures per day (Vano et al. 2008). The consequences to health of low dose occupational exposure of IC staff over long periods are still not clear. However, the role of personal protection equipment (PPE) is quite clear and is important in reducing radiation exposure (Vano et al. 1998; Lie et al. 2008).
Radiation-related cataracts tend to occur at an earlier age than senile cataracts do. Although radiation-induced cataracts may remain asymptomatic for several years, they still may impair visual function as lens opacity occurs and may produce severe and irreversible eye damage (Jacob et al. 2011). The O’CLOC (Occupational Cataracts and Lens Opacities in Interventional Cardiology) study provides further evidence about the potential risk of low-dose radiation-induced cataracts and has contributed to awareness of the importance of radiation protection among cardiologists (Jacob et al. 2011). In view of foregoing, there are concerns for the risk of radiation exposure to the lens of IC staff. Therefore, the current occupational guideline values of the International Commission on Radiation Protection (ICRP) for radiation exposure to eyes (150 my/year) may be considered as too high (Klein et al. 2009). The ICRP has reviewed recent epidemiological evidence for the lens of the eye and has issued a statement on sensitivity of eye lens tissue (ICRP 2011; Rehani et al. 2011). For occupational exposures that are planned, the commission now recommends an equivalent annual dose limit for the lens of 20 mSv, averaged over defined periods of 5 years, with no single annual exposure exceeding 50 mSv (ICRP 2011; Rehani et al. 2011).
Interventional radiologists need 20/20 vision in both eyes to maintain excellent stereopsis and to perform the delicate procedures demanded by their job. Treatment and surgery for cataracts is a frequent and successful surgical procedure. But, risks are associated with cataract surgery that can negatively affect outcomes and may affect visual rehabilitation prospects for interventional radiologists (Haskal 2004). The use of a mechanical injector pump for coronary arteriography has reduced the radiation exposure of cardiologists (Grant et al. 1993). This technique is safe, convenient, produces angiograms of comparable quality to hand injection and should be recommended as standard practice to reduce radiation (Grant et al. 1993). Earlier studies have confirmed that by using this pump, radiation exposure during cervical irradiation was reduced by a factor of 15; in addition, radiation exposure to the left wrist was reduced by a factor of 8 by using the protection afforded by a suspended lead screen during irradiation (Wyart et al. 1997).
The sensitivity of lens tissue to radiation damage makes eye protection essential for medical personnel (Pratt and Shaw 1993). Cardiologists often fail to routinely use protective leaded eyewear; it raises the crucial need of staff to wear radiation monitoring devices to prevent cataracts and protect the eyes (Vano and Faulkner 2005). It was observed that under high workload conditions, where inadequate protective measures were used, it is possible for the operator eye dose to exceed the recommended level (set at 3/10 of the 150 mSv dose limit) (Jeans et al. 1985). Reducing radiation-related cataract risks among interventional medical personnel can be achieved by the effective use of protective devices (Rehani et al. 2011). A systematic radiological protection training program (installing a radiation badge policy for staff) can improve compliance by 36–77% (McCormick et al. 2002). A strict policy to regularly use personal dosimeters should be part of any safety or quality program in cardiology laboratories (McCormick et al. 2002).
The relationship between the radiation eye dose received by cardiologist’s, and factors such as the dose efficiency of the X-ray equipment, effects of scattered dose rates, examination protocols, and workload are complex and may vary from center to center (Pratt and Shaw 1993). The procedure or techniques used, the catheter or catheter insertion site chosen, operator positioning and the appropriate use of personal protective devices also play an important role in the levels of radiation exposure sustained (Kim and Miller 2009). The dose rate at eye level decreases by a factor of 2 when the physician’s eye level above the floor increases from 1.6 to 1.8 m (Pratt and Shaw 1993). When a physician stands at the patient’s groin on the right-hand side and uses a femoral approach, the left anterior oblique (LAO) cranial projection results in the highest operator dose rate for scattered radiation (Pratt and Shaw 1993; Kuon et al. 2004; Camm et al. 1993). Awareness among cardiologists about the radiation risk of the LAO projection may produce a radiation dose that is two to three times lower (Pitney et al. 1994).
6 Other Adverse Health Effects
Concerns exist about the low-dose radiation (LDR) health effects among IC staff. Chronic exposure to the effects of LDR is known to increase hydrogen peroxide levels in IC staff and to alter redox balance (Russo et al. 2011). Russo et al. (2011) described two adaptive cellular responses to the effects of irradiation: (1) enhanced antioxidant defense (increases in glutathione, counteracting increased oxyradical stress) and (2) increased susceptibility to apoptotic induction, which might efficiently remove genetically damaged cells. Venneri et al. (2009) suggested that cumulative professional radiological exposure is associated with a non-negligible lifetime attributable risk of cancer for those cardiac catheterization laboratory staff that have the most exposure (Venneri et al. 2009). Such exposure was also associated with an increased micronuclei frequency for interventional cardiologists, but not for clinical cardiologists, which risk correlates with years of professional activity (Andreassi et al. 2005).
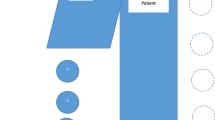
Cardiac Catheterization laboratory or cath labs and the possible radiation exposure routes for IC staff. IABP Intraaortic balloon pump. Source: Cardiac catheterization laboratory 2011
Interventional cardiologists, who have an average exposure of 4 mSv/year, show a twofold increase in certain biomarkers like circulating lymphocytes, chromosome aberrations, and/or micronuclei. The appearance of these biomarkers in IC staff are surrogates of cancer risk and could represent intermediate carcinogenesis end points (Andreassi et al. 2005; Zakeri and Assaei 2004; Maffei et al. 2004). Hence, interventional cardiology is recognized as being a practice that has high radiation risk (Finkelstein 1998; Kim et al. 2008; Vano 2003). We again underscore that monitoring for levels of occupational exposure should become an important part of any and all quality assurance (QA) programs that are established for such practices (Vano et al. 2006).
7 Need for Radiation Safety Practices in Catheterization Laboratories
As interventional procedures have increased in numbers, radiation exposure to personnel working in cardiac catheterization laboratories has increased. The current radiation precautions appear to be adequate, because the radiation dose reported for IC staff is low. However, in view of the rather high incidence of cataracts reported among IC staff, there is a need for strict implementation of radiation safety practices in the medical workplace. Programs should be implemented to initiate safety awareness and to provide for radiation protection training among IC staff (Vano 2003; Tsapaki et al. 2011). Another important undertaking is to institute the routine use of dosimeters among staff during IC procedures and to regularly perform surveillance of occupational doses for the whole body and for eyes, thyroid glands, and hands (Whitby and Martin 2005). Only instruments that comply with radiation safety standards and practices should be used during IC procedures, so that radiation exposures are optimized (Yuan et al. 2010). Of most importance in radiation safety is the regular use of personal protective equipment or shielding for staff in the workplace (Tsapaki et al. 2011; Kim et al. 2008; Pratt and Shaw 1993). Working at a safe distance from instruments, and assuring that such instruments are properly positioned can reduce the radiation dose received by IC staff (Maeder et al. 2005; Vano et al.1998; Jeans et al. 1985).
This review further underscore the importance of each catheterization laboratory undertaking routine measurement of the dose received by each IC staff member. Our review revealed that, in most of the available literature, data were given as radiation dose per procedure, and, unfortunately, no recommended dose rate has been established for individual procedures. Depending on the type of procedure and the technique used, the operator dose per procedure may range from 3 to 450 μSv at the neck, from less than 0.1 to 32 μSv at the waist or chest, and from 48 to 1,280 μSv at the hands (Miller et al. 2010). Translating such exposure values into monthly or annual worker dose limits is difficult (Miller et al. 2010). More and better monitoring of radiation doses among catheterization laboratory IC staff is needed for hands, eyes, and thyroid glands. This can be accomplished by using personal dosimeters and by developing recommended limits for IC staff per-procedure doses. Finally, we suggest that the national and international agencies (e.g., ICRP) that are responsible for medical and radiation safety do work to establish such radiation exposure limits for all relevant IC protocols.
8 Summary
To the best of our knowledge, this chapter constitutes the first systematic review of radiation exposure to eyes, thyroid, and hands for Interventional Cardiology (IC) staff. We have concluded from our review that these anatomical locations are likely to be exposed to radiation as a result of the limited use of personal protective equipment (PPE) among IC staff as shown in Fig. 8. Our review also reveals that, with the exception of three eye exposure cases, the annual radiation dose to eyes, thyroid, and hands among IC staff was within recommended levels and limits. The As Low As Reasonably Achievable (ALARA) limit was not achieved in three cases for fingers/hands and four cases for eyes. However, an increased incidence of cataracts were reported for IC staff, and this gives rise to the concern that low-dose or unnoticed exposures may increase the risk of developing cataracts among cardiology staff. Clearly, the formation of cataracts among IC staff may be an issue and should be studied in more depth.
Our review also disclosed that the two groups who receive excessive radiation doses (i.e., exceed the recommended limit) are physicians-in-training and junior staff physicians who work in cardiac catheterization laboratories. In particular, more attention should be given to assessing the effects of radiation exposure among IC staff who work in the Asia Pacific countries, because our review indicates that the number of IC procedures performed by IC staff in these countries is higher than for other continents. There is a huge demand for procedures conducted by IC staff in the Asia-Pacific area, for both treating patients and consulting with specialists.
Our review also disclosed that recommended limits for per-procedure radiation doses are needed for IC staff. We recommend that such limits be established by the appropriate national and international agencies that are responsible for occupational radiation exposure. Although our review indicates that the current precautions against LDR exposure for IC staff are adequate in most cases, we are concerned about the relatively high incidence of cataracts reported to exist among IC staff. Therefore, we believe that there is a need for a strict implementation of radiation safety practices in cardiology laboratories and associated workplaces that utilize radiation.
The action that is most important for protecting staff in the workplace against radiation exposure is the regular use of personal protective equipment or shielding. Working at a safe distance from instruments and assuring that such instruments are in the proper position are other techniques that can reduce the radiation dose received by IC staff.
References
Andreassi MG, Cioppa A, Botto N, Joksic G, Manfredi S, Federici C, Ostojic M, Rubino P, Picano E (2005) Somatic DNA damage in interventional cardiologists: a case–control study. FASEB J19(8):998–9
Asian Network of Cardiologists in Radiation Protection—under RCA/IAEA project (2007) Newsletter (Issue N1): 1–2. Available via http: 77rpop.iaea.org/RPOP/RPoP/Content/AdditionalResources/Training/2_TrainingEvents/asian-network.htm. Accessed 23 Nov 2011
Bjelac CO, Rehani MM, Sim KH, Liew HB, Vano E, Kleiman NJ (2010) Risk for radiation induced cataract for staff in interventional cardiology: Is there reason for concern? Catheter Cardiovasc Interv 76:826–834
Balter S (1993) Guidelines for personnel radiation monitoring in the cardiac catheterisation laboratory. Cathet Cardiovasc Diagn 30:277–279
Calkins H, Niklason L, Sousa J, El-Atassi R, Langberg J, Morady F (1991) Radiation exposure during radiofrequency catheter ablation of accessory atrio ventricular connections. Circulation 84:2376–82
Camm AJ, Reid J, Raphael M, Wilde P, Boyle R, Clarke M, Qureshi S, Rothman M, Shaw A (1993) Radiation hazards to the cardiologist—a report of a Subcommittee of the British Cardiac Society. Br Heart J 70:489–496
Cardiac catheterisation laboratory (2011) Available via http://cardiophile.org/common/2011/11/cardiac-catheterisation-laboratory-place-where-angiography-and-interventions-are-done. Accessed 12 Dec 2011
Dawkins KD, Gershlick T, de Belder M (2005) Percutaneous coronary intervention: Recommendations for good practice and training. Heart 91(6):1–27
Damilakis J, Koulourakis M, Hatjidakis S, Karabekios S, Gourtsoyiannis N (1995) Radiation exposure to the hands of operators during angiographic procedures. Br J Radiol 21:72–5
Domienik J, Brodecki M, Carinou E, Donadille L, Jankowski J, Koukorava C, Krim S, Nikodemova D, Ruiz Lopez N, Sans Merce M, Struelens L, Vanhavere F (2011) Extremity and eye lens doses in interventional radiology and cardiology procedures. First results of the oramed project. Radiat Prot Dosim 144(1–4):442–447
Delichas M, Psarrakos K, Molyvda-Athanassopoulou E, Giannoglou G, Sioundas A, Hatziioannou K, Papanastassiou E (2003) Radiation exposure to cardiologists performing interventional cardiology procedures. Eur J Radiol 48:268–273
Duran D, Duran G, Ramirez R, Vano E, Kleinman N, Echeverri D, Gomez G, Cabrera M (2009) Cataracts in interventional cardiology personnel. Retrospective evaluation study of lens injuries and dose (RELID Study). Eur Heart J 30:872
Efstathopoulos E, Makrygiannis SS, Kottou S, Karvouni E, Giazitzoglou E, Korovesis S, Tzanalaridon E, Rapton PD, Katritsis DG (2003) Medical personnel and patient dosimetry during coronary angiography and intervention. Phys Med Biol 48:3059–68
Efstathopoulos EP, Pantos I, Andreou M, Gkatzis A, Carinou E, Koukorava C, Kelekis NL, Brountzos E (2011) Occupational radiation doses to the extremities and the eyes in interventional radiology and cardiology procedures. Br J Radiol 84:70–77
Finkelstein MM (1998) Is brain cancer an occupational disease of cardiologists? Can J Cardiol 14:1385–1388
German Guidelines (2003) Bekanntmachung der Neufassung der Röntgenverordnung vom 30. April 2003. Bundesgesetzblatt 2003; Teil 1:604–635
Goni H, Papadopoulou D, Yakoumakis E, Stratigis N, Benos J, Siriopoulou V, Makri T, Georgiou E (2005) Investigation of occupational radiation exposure during interventional cardiac catheterisations performed via radial artery. Radiat Prot Dosim 117:107–110
Grant SCD, Faragher EB, Hufton AP, Bennett DH (1993) Use of a remotely controlled mechanical pump for coronary arteriography—a study of radiation exposure and quality implications. Br Heart J70:479–484
Hafez MA, Smith RM, Matthews SJ, Kalap G, Sherman KP (2005) Radiation exposure to the hands of orthopaedic surgeons: are we underestimating the risk? Arch Orthop Trauma Surg 125:330–335
Haskal ZJ (2004) Interventional radiology carries occupational risk for cataracts. RSNA News 14:5–6
International Commission on Radiological Protection (2000) Avoidance of radiation injuries from medical interventional procedures. ICRP Publication 85. Ann ICRP 230:7–67
International Commission on Radiological Protection (2011) Tissue reactions and other non-cancer effects of radiation. Elsevier. Ann ICRP Ref 4834-1783-0153
Junk A, Haskal Z, Worgul B (2004) Cataract in interventional radiology—An occupational hazard? Invest Ophthalmol Vis Sci 388:45
Jacob S, Bertrand A, Bernier MO (2011) Occupational cataracts and lens opacities in interventional cardiology: The O’CLOC study. Towards convergence of technical nuclear safety practice in Europe. Available via http://www.eurosafe-forum.org/userfiles/2_10_Paper_EUROSAFE_SJ.pdf. Accessed 4 Dec 2011
Jeans SP, Faulkner K, Love HG, Bardsley RA (1985) An investigation of the radiation-dose to staff during cardiac radiological studies. Br J Radiol 58:419–428
Kim KP, Miller DL (2009) Minimising radiation exposure to physicians performing fluoroscopically guided cardiac catheterisation procedures: A review. Rad Prot Dosimetry 133:227–233
Kim KP, Miller DL, Balter S, Kleinerman RA, Linet MS, Kwon D (2008) Occupational radiation doses to operators performing cardiac catheterisation procedures. Health Phys 94:211–227
Karppinen J, Parviainen T, Servomaa A, Komppa T (1995) Radiation risk and exposure of radiologists and patients during coronary angiography and PTCA. Radiat Prot Dosim 57:481–5
Klein LW, Miller DL, Blater S, Laskey W, Haines D, Norbash A, Mauro MA, Goldstein JA, Joint Inter-Society Task Force on Occupational Hazards in the Interventional Laboratory (2009) Occupational health hazards in the interventional laboratory: Time for a safe environment. J Vasc Interv Radiol 20(7 Suppl):S278–83
Kottou S, Neofotistou V, Tsapaki V, Lobotessi H, Manetou A, Molfetas MG (2001) Personnel doses in haemodynamic units in Greece. Radiat Prot Dosim 94:121–124
Kuon E, Günther M, Gefeller O, Dahm JB (2003) Standardization of occupational dose to patient DAP enables reliable assessment of radiation-protection devices in invasive cardiology. Rofo 175:1545–50
Kuon E, Empen K, Rohde D, Dahm JB (2004) Radiation exposure to patients undergoing percutaneous coronary interventions. Are the current reference values too high? Herz 29:208–17
Lie OO, Paulson GU, Wohni T (2008) Assessment of the effective dose and dose to the lens of the eye for the interventional cardiologist. Radiat prot Dosimetry 132(3):313–318
Lange HW, von Boetticher H (2006) Randomized comparison of operator radiation exposure during coronary angiography and intervention by radial or femoral approach. Catheter Cardiovasc Interv 67:12–16
Limacher MC, Douglas PS, Germano G, Laskey WK, Lindsay BD, McKetty MH, Moore ME, Park JK, Prigent FM, Walsh MN (1988) Radiation safety in the practice of cardiology. J Am Coll Cardiol 31:892–913
Lindsay BD, Eichling JO, Ambos HD, Cain ME (1992) Radiation exposure to patients and medical personnel during radiofrequency catheter ablation for supraventricular tachycardia. Am J Cardiol 70:218–223
Maffei F, Angelini S, Forti GC, Violante FS, Lodi V, Mattioli S, Hrelia P (2004) Spectrum of chromosomal aberrations in peripheral lymphocytes of hospital workers occupationally exposed to low doses of ionizing radiation. Mutat Res 547:91–9
Maeder M, Verdun FR, Stauffer JC, Ammann P, Rickli H (2005) Radiation exposure and radiation protection in interventional cardiology. Kardiovaskuläre Medizin 8:124–132
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med6:e1000097. doi:10.1371/journal.pmed.1000097
Marshall NW, Noble J, Faulkner K (1995) Patient and staff dosimetry in neuroradiological procedures. Br J Radiol 68:495–501
Miller DL, Vano E, Bartal G, Balter S, Dixon R, Padovani R, Schueler B, Cardella JF, de Baere T (2010) Occupational radiation protection in interventional radiology: A joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc Intervent Radiol 33:230–239
Mann JT, Cubeddu G, Arrowood M (1996) Operator radiation exposure in PTCA: comparison of radial and femoral approaches. J Invasive Cardiol 8:D22–D25
McCormick VA, Schultz CC, Hollingsworth-Schuler V, Campbell JM, O’Neill WW, Ramos R (2002) Reducing radiation dose in the cardiac catheterization laboratory by design alterations and staff education. Am J Cardiol 90:903–905
Picano E, Santoro G, Vano E (2007) Sustainability in the cardiac cath lab. Int J Cardiovasc Imaging 23(2):143–7
Pratt TA, Shaw AJ (1993) Factors affecting the radiation dose to the lens of the eye during cardiac catheterisation procedures. Br J Radiol 66:346–350
Petersen S, Peto V, Rayner M (2003) Congenitial heart disease statistics 2003. British Heart Foundation statistics. Available via http://www.bhf.org.uk/idoc.ashx?docid=b41e291d-0dec-41f6-b726-10b609299aa&version=−1. Accessed 13 Aug 2011
Pitney MR, Allan RM, Giles RW, Mclean D, Mccredie M, Randell T, Walsh WF (1994) Modifying fluoroscopic views reduces operator radiation exposure during coronary angioplasty. J Am Coll Cardiol 24:1660–1663
Raza SMS (2011) Radiation exposure in the cath lab-safety and precautions. Available via http://priory.com/med/radiation.htm. Accessed 20 July 2011
Rotter M, Pfiffner D, Maier W, Zeiher AM, Meier B (2003) Working Group Interventional Cardiology and Coronary Pathophysiology, European Society of Cardiology. Interventional cardiology in Europe 1999. Eur Heart J24:1164–70
Renaud L (1992) A 5-year follow-up of the radiation exposure to in-room personnel during cardiac catheterization. Health Phys 62:10–15
Rehani MM, Vano E, Bjelac OC, Kleiman NJ (2011) Radiation and cataract. Radiat Prot Dosim. doi: 10.1093/rpd/ncr299
Russo LG, Tedesco I, Russo M, Cioppa A, Andreassi MG, Picano E (2011) Cellular adaptive response to chronic radiation exposure in interventional cardiologists. Eur Heart J23. doi: 10.1093/eurheart/ehr263
Sim KH, Rehani M, Kleiman N, Bjelac OC, Vano E (2010) Radiation induced lens opacities in the eyes of cath lab staff. J Am Coll Cardiol 55(A201):E1888
Short CP, Al Hashinii H, Malone L, Lee MJ (2007) Staff radiation doses to the lower extremities in interventional radiology. Cardiovasc Intervent Radiol 30:1206–9
Steffenino G, Rossetti V, Dellavalle A, Garbarino M, Cerati R, Norbiato A, Uslenghi E (1996) Staff dose reduction during coronary angiography using low framing speed. Br J Radiol 69:860–4
Tsapaki V, Ghulam MF, Lim ST, Minh HN, Nwe N, Sharma A, Sim KH, Srimahachota S, Rehani MM (2011) Status of radiation protection in various interventional cardiology procedures in the Asia Pacific region. Heart Asia 3:16–24
Tsapaki V, Patsilinakos S, Voudris V, Magginas A, Pavlidis S, Maunis T (2008) Level of patient and operator dose in the largest cardiac centre in Greece. Radiat Prot Dosimetry 129:71–3
Vano E, Gonzalez L, Beneytez F, Moreno F (1998a) Lens injuries induced by occupational exposure in non-optimized interventional radiology laboratories. Br J Radiol 71:728–33
Vano E, KleimanNJ DA, Rehani MM, Eche D, Cabrera M (2010a) Radiation cataract risk in interventional cardiology personnel. Rad Res 174(4):490–495
Vano E, Gonzalez L, Guibelalde E, Fernandez JM, Ten JI (1998b) Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol 71:954–60
Vano E, Kleiman NJ, Duran A, Rehani MM, Echeverri D, Cabrera M (2010b) Radiation cataract risk in interventional cardiology personnel. Rad Res 174(4):490–495
Vano E (2003) Radiation exposure to cardiologists: how it could be reduced. Heart 89:1123–4
Vano E, Gonzalez L, Fernandez JM (2008) Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology 248(3):945–953
Vano E, Gonzalez L, Fernandez JM, Alfonso F, Macaya C (2006a) Occupational radiation doses in interventional cardiology: a 15 year follow up. Br J Radiol 79:383–388
Vano E, Gonzalez L, Fernandez JM, Prieto C, Guibelade E (2006b) Influence of patient thickness and operation modes on occupational and patient radiation doses in interventional cardiology. Radiat Prot Dosimetry 118(3):325–330
Vano E, Faulkner K (2005) ICRP special radiation protection issues in interventional radiology, digital and cardiac imaging. Radiat Protect Dosim 117(1–3):13–17
Venneri L, Rossi F, Botto N, Andreassi MG, Salcone N, Emad A, Lazzeri M, Gori C, Vano E, Picano E (2009) Cancer risk from professional exposure in staff working in cardiac catheterisation laboratory: Insights from the National Research Council Biological effects of ionizing radiation VII report. Am Heart J157:118–24
Watson LE, Riggs MW, Bourland PD (1997) Radiation exposure during cardiology fellowship training. Health Phys 73:690–693
Wu JR, Huang TY, Wu DK, Hsu PC, Weng PS (1991) Radiation exposure of pediatric patients and physicians during cardiac catheterisation and balloon pulmonary valvuloplasty. Am J Cardiol 68:221–225
Whitby M, Martin CJ (2005) A study of the distribution of dose across the hands of interventional radiologists and cardiologists. Br J Radiol 78:219–229
Williams JR (1997) The interdependence of staff and patient doses in interventional radiology. Br J Radiol 70:498–503
Wyart P, Dumant D, Gourdier M, Nassar F, Bouthillon JC, Chestier Y (1997) Contribution of self-surveillance of the personnel by electronic radiation dosimeters in invasive cardiology. Arch Mal Coeur Vaiss 90:233–238
Yuan MK, Chien CW, Lee SK, Hsu NW, Chang SC, Chang SC, Tang GJ (2010) Health effects of medical radiation on cardiologists who perform cardiac catheterisation. J Chin Med Assoc 73(4):199–204
Zakeri F, Assaei RG (2004) Cytogenetic monitoring of personnel working in angiocardiography laboratories in Iran hospitals. Mutat Res 562:1–9
Acknowledgments
We wish to thank Dana Wendeler, Documentation officer, Department of Occupational Health Research, Institute for Statutory Accident Insurance and Prevention in the Health and Welfare Services (BGW), Hamburg, Germany, for her support with the management of the literature. This research project was funded by the Institute for Statutory Accident Insurance and Prevention in the Health and Welfare Services (BGW), Hamburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kesavachandran, C.N., Haamann, F., Nienhaus, A. (2013). Radiation Exposure and Adverse Health Effects of Interventional Cardiology Staff. In: Whitacre, D. (eds) Reviews of Environmental Contamination and Toxicology. Reviews of Environmental Contamination and Toxicology, vol 222. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4717-7_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4717-7_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4716-0
Online ISBN: 978-1-4614-4717-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)