Abstract
Artemisinin, a potent antimalarial sesquiterpene lactone, is produced in low quantities by the plant Artemisia annua L. We used inhibitors of both the mevalonate and nonmevalonate terpenoid pathways to study in both seedlings and hairy root cultures the source of isopentenyl diphosphate (IPP), the channeling of carbon from sterols to sesquiterpenes, and the role that sugars may play in controlling artemisinin biosynthesis. Together, our results indicated that artemisinin is likely biosynthesized from IPP pools originating in both the plastid and the cytosol and that channeling of carbon can be directed away from competing sterol pathways and toward sesquiterpenes. Although glucose stimulated artemisinin production, the response is very complex with ratios of glucose to fructose involved; artemisinin levels increased proportionate to increasing amounts of glucose. Disaccharides mainly inhibited artemisinin production, but the response was less definitive. Glucose also increased expression of some of the genes in the artemisinin biosynthetic pathway, thereby suggesting that this sugar is acting not only as a carbon source but also as a signal. As we develop a better understanding of the regulation of the artemisinin biosynthetic pathway, results suggest that many factors can possibly be harnessed to increase artemisinin production in A. annua.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Artemisinin (Fig. 8.1) and its derivatives are the most important drugs for treatment of malaria and, as part of a multidrug cocktail, artemisinin combination therapy (ACT), minimize the development of resistant forms of the infecting parasite. Besides malaria, artemisinin also has been shown to be effective against other infectious agents including human cytomegaloviruses, herpes simplex, hepatitis B and C (Efferth et al. 2002; Romero et al. 2005), Toxoplasma gondii (Jones-Brando et al. 2006; D’Angelo et al. 2009), Schistosoma (Utzinger et al. 2001), and Pneumocystis carinii (Merali and Meshnick 1991). Artemisinin derivatives have also shown efficacy against numerous cancers (Singh and Lai 2004; Nam et al. 2007; de Vries and Dien 1996). Thus, there is an urgent need for considerable quantities of the drug. Unfortunately, there is currently not even enough artemisinin produced to treat malaria, let alone any of these other diseases.
Field-grown plants of Artemisia annua L. still remain the main source of the drug. After 4–6 months of growth, the plant produces ≤1.8% artemisinin of its total shoot dry weight, and this amount varies depending on environmental conditions. Much is still not known about the control of its biosynthesis in planta, and not all of the biosynthetic steps are confirmed. Here, we describe some of our recent efforts to enhance our general understanding of artemisinin biosynthesis and its regulation.
8.2 Where Does Artemisinin IPP Originate?
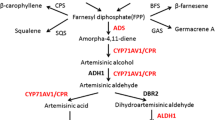
Terpenes in plants are produced by the successive condensation of multiple subunits of isopentenyl diphosphate (IPP), which can originate from one of two sources: either from the cytosolic mevalonic acid pathway (MVA) or from the plastidic nonmevalonic acid independent pathway (MEP) (Fig. 8.2a). IPP can shuttle between these two cellular compartments depending on the plant species and the product being synthesized (Laule et al. 2003; Hampel et al. 2005; Dudareva et al. 2005). For example, in monocots (Maier et al. 1998) and bryophytes (Adam et al. 1998), all the IPP for sesquiterpenes derives from the MEP pathway. In contrast, dicots from the Compositae, plants closely related to A. annua, derive their IPP in sesquiterpenes from both the MEP and the MVA pathways (Adam and Zapp 1998; Steliopoulos et al. 2002). Until recently (Towler and Weathers 2007), the source of IPP leading to artemisinin biosynthesis in A. annua was not known.
Terpene and artemisinin metabolism showing (a) IPP compartmentalization and points of inhibition for metabolic studies. (b) Artemisinin biosynthesis post FPP (latter adapted from a graphic courtesy of KH Teoh). HMG-CoA hydroxymethylglutaryl CoA, MEV mevinolin, HMGR HMG-CoA reductase, MVA mevalonic acid, DMAPP dimethylallyl diphosphate, IPP isopentenyl diphosphate, FPP farnesyl diphosphate, GGPP geranylgeranyl diphosphate, MIC miconazole, 14DM 14α-demethylase, G3P glyceraldehyde 3-phosphate, DXP deoxyxylulose 5-phosphate, DXR DXP reductoisomerase, FOS fosmidomycin, DXS DXP synthase, MEP methylerythritol 4-phosphate, GPP geranyl diphosphate, ABA abscisic acid, ADS amorphadiene synthase, CYP71AV1 p450 catalyst specific to artemisinin biosynthesis
We used inhibitors specific to either the MVA or the MEP pathway to determine the effect of each on the routing of IPP for artemisinin production in 14-day axenic seedlings of A. annua (YU strain) (Towler and Weathers 2007). Fosmidomycin (FOS) inhibits the first enzyme, DXR (1-deoxy-d-xylulose 5-phosphate reductoisomerase), in the MEP pathway (Rodríguez-Concepción et al. 2004), while mevinolin (MEV) inhibits the enzyme HMG-CoA reductase in the MVA pathway (Bach and Lichtenthaler 1983). After 14 days of incubation and growth in the inhibitors, shoots were harvested, weighed, and extracted for artemisinin analysis by HPLC. Growth was inhibited from 30% to 40% when seedlings were grown in either FOS or MEV, but when both inhibitors were added, growth inhibition was effectively doubled, indicating an additive effect (Table 8.1). Inhibition of growth was about the same for roots and shoots; however, in FOS shoots also became chlorotic, probably because of the inhibition of chlorophyll synthesis (Towler and Weathers 2007). Each inhibitor also significantly inhibited artemisinin production about 70–80%; in the presence of both FOS and MEV, no artemisinin was detected in the seedlings (Table 8.1). Together, these data show that artemisinin is likely synthesized using IPP originating from both the MEP and the MVA pathways.
8.3 Channeling Carbon Toward Artemisinin Biosynthesis
The complex terpenoid pathway offers several points whereby carbon can be rerouted, and for artemisinin, one especially attractive point is at farnesyl diphosphate (FDP) where sesquiterpenes and sterols diverge into separate branches (Fig. 8.2a). Shunting carbon from sterol biosynthesis to sesquiterpenes has been demonstrated in both yeast and plants through either genetic engineering or inhibition of specific enzymes (Asadollahi et al. 2008; Ro et al. 2006). These two pathways have shown evidence of coordinate control; conditions that stimulate sesquiterpene production also cause a decrease in sterols (Vögeli and Chappell 1988). Using similar reasoning, miconazole (MIC), an inhibitor of sterol 14α-demethylases (Zarn et al. 2003), was fed to A. annua shoot cultures (Woerdenbag et al. 1993; Kudakasseril et al. 1987) to inhibit sterol biosynthesis and to shunt carbon instead toward sesquiterpenes, and artemisinin increased. We further showed in A. annua seedlings that in the presence of MIC, artemisinin concentrations rose significantly (Table 8.2) compared to uninhibited controls, demonstrating that indeed carbon flux was likely rerouted from sterol biosynthesis toward sesquiterpenes and artemisinin production (Towler and Weathers 2007). Together, these results show that inducible shunting of carbon is a reasonable strategy for engineering increased levels of artemisinin in planta.
8.4 Elicitation Effects on Artemisinin Production
More traditional approaches for increasing artemisinin production in culture have focused on use of nutrients (Wang and Tan 2002) and other cues including phytohormones (Weathers et al. 2005; Woerdenbag et al. 1993), chitosan (Putalun et al. 2007), light (Wang et al. 2001; Souret et al. 2003), or other abiotic elicitors (Ferreira et al. 1995; Baldi and Dixit 2008). Success, however, has been limited. For example, although Putalun et al. (2007) showed that chitosan increased artemisinin yields in A. annua hairy roots sixfold to a final concentration of about 1.8 mg/g DW, we could not confirm their work and also did not see any significant stimulation of artemisinin production in the presence of abiotic elicitors or methyl jasmonate in hairy roots (Ryan 1996) or seedlings (Fig. 8.3). Early experiments appeared to show that methyl jasmonate had some effect (Fig. 8.3). That experiment, however, required dissolving methyl jasmonate in DMSO, and when compared to the DMSO-only controls, the artemisinin production in the presence of methyl jasmonate was not statistically significant (Fig. 8.3).
Although none of the standard elicitors seemed to have increased production of artemisinin, we observed on numerous occasions that DMSO did. Indeed, both our studies with miconazole (Table 8.2; Towler and Weathers 2007) and with methyl jasmonate (Fig. 8.3) showed that the DMSO controls produced significant increases in artemisinin beyond the untreated control. This stimulatory effect on artemisinin or any other secondary metabolite production has not to our knowledge been previously reported. In A. annua, DMSO is detected only by the roots, not the shoots, and was shown to increase peroxide levels in the plant resulting in enhanced dihydroartemisinic acid and artemisinin production in the leaves (Mannan et al. 2010).
8.5 Sugars Affect Artemisinin Production
A number of years ago, we serendipitously observed that when filter-sterilized medium was used to culture hairy roots of A. annua, the yield of artemisinin was more consistent. During autoclaving, sucrose is partially hydrolyzed to glucose and fructose (Schenk et al. 1991) as well as to small amounts of toxic products including furfurals (Rédei 1974), so autoclaving of sucrose produces an undefined medium with differing ratios of glucose, fructose, and sucrose (Weathers et al. 2004). We observed that both growth and artemisinin levels of A. annua hairy roots were further affected by the type of sugar provided in the culture medium. Glucose inhibited root growth, while sucrose and fructose stimulated it (Weathers et al. 2004). Production of artemisinin, however, was increased 300% and 200% by glucose and fructose, respectively, when compared to sucrose (Weathers et al. 2004). Sugars have been known for some time to play key regulatory roles in plant growth and development (Jang and Sheen 1994; Rolland et al. 2006), but our results suggested they may also be affecting the production of the secondary metabolite, artemisinin.
Others had reported that sugars had some effects on secondary metabolites, including in Vitis vinifera, where Larronde et al. (1998) showed that sucrose dramatically stimulated the production of anthocyanins. In an effort to provide clues to the mechanism of this sugar effect, they also used several glucose analogs. Mannose, which can be transported into plants and phosphorylated by hexokinase, mimicked the effect of sucrose in the production of anthocyanins, while another glucose analog, 3-O-methylglucose (3OMG), which can be taken up into plant cells but only slowly phosphorylated by hexokinase (HXK), did not. Mannoheptulose is a specific inhibitor of HXK, and when it was added, anthocyanin production by sucrose was inhibited. It was later suggested that HXK appeared to be involved in a sugar signal transduction pathway related to anthocyanin production (Vitrac et al. 2000).
8.5.1 Monosaccharide Effects on A. annua and Artemisinin
Considering these results, we subsequently used sugars alone, in combination, or with their respective analogs to investigate their role in the production of artemisinin in A. annua seedlings. When compared to sucrose, glucose increased artemisinin in seedling shoots by >200%; in contrast, fructose decreased artemisinin production by about 50% (Wang and Weathers 2007). Because it appeared in our earlier work that the plant was sensitive to varying ratios of glucose, fructose, and sucrose, we fed seedlings differing ratios of glucose to sucrose, but saw no significant differences in artemisinin production. When fed different ratios of glucose to fructose, however, specific artemisinin levels increased in direct proportion to increases in the relative glucose concentration (Fig. 8.4; Wang and Weathers 2007). These results suggested that artemisinin production was indeed responding to changing amounts of glucose in the seedling culture media. This stimulation by glucose was also observed in A. annua hairy root cultures, but the response was not as dramatic (Weathers et al. 2004).
We then used three glucose analogs, 3-O-methylglucose (3OMG), mannose, and mannitol, to obtain some clues on the possible mechanism of glucose control of artemisinin production. Although the 3OMG analog can be transported into the cell and phosphorylated to 3-O-methyl glucose-6-phosphate by HXK, the rate of phosphorylation is about 5 orders of magnitude slower than for mannose or glucose (Cortès et al. 2003), and thus this analog is useful to determine if HXK is involved in glucose sensing. Mannose can also be transported into the cell and phosphorylated to mannose-6-phosphate, but is thereafter very slowly metabolized (Pego et al. 1999; Baskin et al. 2001). Mannitol alters osmotic pressure; it does not enter and is not metabolized by most plant cells (Gibson 2000). Inhibition of a specific process by 3OMG would suggest involvement of HXK (Gonzali et al. 2002; Cortès et al. 2003), while inhibition by mannose might suggest that a step downstream of HXK is involved (Pego et al. 1999; Baskin et al. 2001). Any effect by mannitol suggests an osmotic effect or possible involvement of a monosaccharide transporter (Gibson 2000).
Each analog at 3 g/L along with glucose at 27 g/L was fed to seedlings for 14 days. Shoots were then harvested, weighed, and extracted for assay of artemisinin by HPLC. The analog 3OMG decreased artemisinin production >90%. Mannitol, on the other hand, seemed to stimulate artemisinin production by 50%, while mannose showed no significant effect on artemisinin yield compared to glucose (Fig. 8.5; Wang and Weathers 2007). Mannitol and 3OMG both decreased overall growth by about 30% (Fig. 8.5). Although the stimulation of artemisinin production by mannitol is interesting, there is no apparent explanation for this response and, thus, requires more study. Together, these results suggest that glucose plays some key role in regulating artemisinin production possibly through one of the glucose signaling pathways involving HXK because of the strong inhibition by 3OMG.
We also used tagatose, a stereoisomer of D-fructose, and found that when added in small amounts to fructose, it inhibited both growth and artemisinin production ∼50% compared to fructose. Tagatose is not metabolized by plant cells, and its mechanism of inhibition is not yet understood; however, it has been hypothesized that if the analog were transported into cells, it would be phosphorylated by HXK to yield tagatose-6-phosphate (Kim 2004). Together, the results using tagatose support the results showing that increasing amounts of fructose relative to glucose are inhibitory to artemisinin production and that fructose clearly plays a role in regulating this sesquiterpene.
8.5.2 Disaccharide Effects on A. annua and Artemisinin
Disaccharides also seemed to interfere with artemisinin biosynthesis. We added palatinose, trehalose, maltose, cellobiose, or lactose as 10% of total sugars with the remaining 90% as sucrose. Compared to 100% sucrose, seedlings fed either palatinose or trehalose in conjunction with sucrose showed decreased biomass (Fig. 8.6). Palatinose and cellobiose, however, significantly inhibited artemisinin production up to 75% compared to plantlets grown in 100% sucrose. Palatinose is a sucrose analog that cannot be transported into cells through the sucrose transporter and is not made by plants. It is used to identify signals that may be detected external to the plant or by the sucrose transporter. As an example, extracellular invertases are induced by palatinose (Sinha et al. 2002). Although the role of cellobiose, the smallest subunit of cellulose, is not well understood, it is likely produced during cell wall degradation by pathogenic fungi and may, thus, play a role in pathogen defense. For example, cellobiose inhibits cellulase (Mandels and Reese 1965) and when added in conjunction with a fungal extract, stimulates lignin production in peach bark wounds (Biggs 1990). In A. annua, however, cellobiose inhibition of artemisinin production does not seem connected to pathogen defense.
8.5.3 Sugars Affect Artemisinin Gene Regulation
To determine if glucose, fructose, and sucrose affect mRNA transcription of the genes in the artemisinin biosynthetic pathway, young seedlings of A. annua were fed one of those three sugars for 14 days and then harvested. RNA was extracted from the shoots and real-time PCR was used to measure mRNA transcription using probes generated from the highly conserved regions of genes encoding enzymes that are associated with artemisinin biosynthesis including HMGR, FPS, DXS, DXR, SQS, ADS, and CYP71AV1. Results showed that compared to sucrose, glucose significantly upregulated and fructose downregulated transcription of both ADS and CYP71AV1 in young seedlings (Fig. 8.7), providing further evidence that sugars, in particular glucose and fructose, are acting as signals to regulate artemisinin production (Arsenault et al. 2010a).
Overall, these results suggest that both monosaccharide and disaccharide sugars may play a role well beyond that of a carbon source in downstream regulation of secondary metabolites like artemisinin and that the mechanism of these responses is complex.
8.6 Conclusions and Future Directions
In our efforts to provide a better understating of how artemisinin production is regulated in A. annua plants, we were the first to have shown that this sesquiterpene lactone is produced from IPP produced both in the cytosol via the MVA pathway and also from IPP stemming from the MEP pathway in the plastid. Additional inhibitor studies confirm earlier work with shoot cultures that indicated carbon can be rerouted from sterol production toward sesquiterpenes resulting in increased artemisinin. Although we have not observed any significant increase in artemisinin using typical elicitation molecules like methyl jasmonate or chitosan, we were the first to show that DMSO increases artemisinin production significantly, probably by increasing the ROS peroxide. Furthermore, compared to sucrose, artemisinin production is enhanced by glucose, inhibited by fructose, and particularly sensitive to the ratio of these two monosaccharides. Disaccharides also affect artemisinin production, but the response is not well understood. The increase in artemisinin production by glucose appears to be the result of the perception by the plant of glucose as a sugar signal, and this is further supported by results showing that at least two of the enzymes, ADS and CYP71AV1, in the artemisinin biosynthetic pathway are upregulated after seedlings are grown 14 days in glucose.
The mechanisms of action of both the DMSO and sugar stimuli have been further studied to show modulation of gene expression and metabolite levels during reproductive development of A. annua (Arsenault et al. 2010b), including at the level of leaf trichome formation. Although some progress has been made, as summarized in the review by Nguyen et al. (2011), the mechanisms of overall regulation of artemisinin synthesis require more study, as holds true with optimizing and upscaling the culture conditions of artemisinin-producing hairy root cultures (Sivakumar et al. 2010).
References
Adam K, Zapp J (1998) Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochemistry 48:953–959
Adam K, Thiel R, Zapp J, Becker H (1998) Involvement of the mevalonic acid pathway and the glyceraldehyde-pyruvate pathway in terpenoid biosynthesis of the liverworts Ricciocarpos natans and Conocephalum conicum. Arch Biochem Biophys 354:181–187
Arsenault PR, Vail D, Wobbe KK, Weathers PJ (2010a) Effect of sugars on artemisinin production in Artemisia annua L.: transcription and metabolite measurements. Molecules 15:2302–2318
Arsenault PR, Vail D, Wobbe KK, Erickson K, Weathers PJ (2010b) Reproductive development modulates gene expression and metabolite levels with possible feedback inhibition of artemisinin in Artemisia annua. Plant Physiol 154:958–968
Asadollahi MA, Maury J, Møller K, Nielsen KF, Schalk M, Clark A, Nielsen J (2008) Production of plant sesquiterpenes in Saccharomyces cerevisiae: effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol Bioeng 99:666–677
Bach TJ, Lichtenthaler HK (1983) Inhibition by mevinolin of plant growth, sterol formation and pigment accumulation. Physiol Plant 59:50–60
Baldi A, Dixit VK (2008) Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour Technol 99:4609–4614
Baskin TI, Remillong EL, Wilson JE (2001) The impact of mannose and other carbon sources on the elongation and diameter of the primary root of Arabidopsis thaliana. Aust J Plant Physiol 28:481–488
Biggs AR (1990) Managing wound-associated disease by understanding wound healing in the bark of woody plants. J Arboric 16:108–112
Cortès S, Gromova M, Evrard A, Roby C, Heyraud A, Rolin DB, Raymond P, Brouquisse RM (2003) In plants, 3-O-methylglucose is phosphorylated by hexokinase but not perceived as a sugar. Plant Physiol 131:824–837
D’Angelo JG, Bordón C, Posner GH, Yolken R, Jones-Brando L (2009) Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. J Antimicrob Chemother 63:146–150
de Vries PJ, Dien TK (1996) Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52:818–836
Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102:933–938
Efferth T, Marschall M, Wang X, Huong SM, Hauber I, Olbrich A, Kronschnabl M, Stamminger T, Huang ES (2002) Activity of artesunate towards wild-type, recombinant GFP-expressing sensitive and ganciclovir-resistant human cytomegaloviruses. J Mol Med 80:233–242
Ferreira JF, Simon JE, Janick J (1995) Relationship of artemisinin content of tissue-cultured, greenhouse-grown, and field-grown plants of Artemisia annua. Planta Med 61:351–355
Gibson SI (2000) Plant sugar-response pathways: part of a complex regulatory web. Plant Physiol 124:1532–1539
Gonzali S, Alpi A, Blando F, Bellis LD (2002) Arabiodopsis (HXK1 and HXK2) and yeast (HXK2) hexokinases over expressed in transgenic lines are characterized by different catalytic properties. Plant Sci 163:943–954
Hampel D, Mosandl A, Wust M (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66:305–311
Jang JC, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6:1665–1679
Jones-Brando L, D’Angelo J, Posner GH, Yolken R (2006) In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob Agents Chemother 50:4206–4208
Kim P (2004) Current studies on biological tagatose production using L-arabinose isomerase: a review and future perspective. Appl Microbiol Biotechnol 65:243–249
Kudakasseril GJ, Lam L, Staba EJ (1987) Effect of sterol inhibitors on the incorporation of 14C-isopentenyl pyrophosphate into artemisinin by a cell-free system from Artemisia annua tissue cultures and plants. Planta Med 53:280–284
Larronde F, Krisa S, Decendit A, Chèze C, Deffieux G, Mérillon JM (1998) Regulation of polyphenol production in Vitis vinifera cell suspension cultures by sugars. Plant Cell Rep 17:946–950
Laule O, Fürholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100:6866–6871
Maier W, Schneider B, Strack D (1998) Biosynthesis of sesquiterpenoid cyclohexenone derivatives in mycorrhizal barley roots proceeds via the glyceraldehyde 3-phosphate/pyruvate pathway. Tetrahedron Lett 39:521–524
Mandels M, Reese ET (1965) Inhibition of cellulases. Annu Rev Phytopathol 3:85–102
Mannan A, Liu CZ, Arsenault PR, Towler MJ, Vail DR, Lorence A, Weathers PJ (2010) DMSO triggers the generation of ROS leading to an increase in artemisinin and dihydroartemisinic acid in Artemisia annua shoot cultures. Plant Cell Rep 29:143–152
Merali S, Meshnick SR (1991) Susceptibility of Pneumocystis carinii to artemisinin in vitro. Antimicrob Agents Chemother 35:1225–1227
Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim HJ, Cha IH (2007) Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck 29:335–340
Nguyen KT, Arsenault PR, Weathers PJ (2011) Trichomes + roots + ROS = artemisinin: regulating artemisinin biosynthesis in Artemisia annua L. In Vitro Cell Dev Biol Plant 47:329–338
Pego JV, Weisbeek PJ, Smeekens SCM (1999) Mannose inhibits Arabidopsis germination via a hexokinase-mediated step. Plant Physiol 119:1017–1023
Putalun W, Luealon W, De-Eknamkul W, Tanaka H, Shoyama Y (2007) Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol Lett 29:1143–1146
Rédei GP (1974) ‘Fructose effect’ in higher plants. Ann Bot 38:287–297
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Rodríguez-Concepción M, Forés O, Martínez-García JF, González V, Phillips MA, Ferrer A, Boronat A (2004) Distinct light-mediated pathways regulate the biosynthesis and exchange of isoprenoid precursors during Arabidopsis seedling development. Plant Cell 16:144–156
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Romero MR, Efferth T, Serrano MA, Castaño B, Macias RIR, Briz O, Marin JJG (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res 68:75–83
Ryan JF (1996) The examination of culture conditions, biomass optimization, and terpene elicitation with chitosan, heavy metals, and light exposure of transformed roots cultures of Artemisia annua. M.S. thesis (Biotechnology), Worcester Polytechnic Institute, Worcester
Schenk N, Hsiao K-C, Bornman CH (1991) Avoidance of precipitation and carbohydrate breakdown in autoclaved plant tissue culture medium. Plant Cell Rep 10:115–119
Singh NP, Lai HC (2004) Artemisinin induces apoptosis in human cancer cells. Anticancer Res 24:2277–2280
Sinha AK, Hofmann MG, Römer U, Köckenberger W, Elling L, Roitsch T (2002) Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol 128:1480–1489
Sivakumar G, Liu C, Towler MJ, Weathers PJ (2010) Biomass production of hairy roots of Artemisia annua and Arachis hypogaea in a scaled-up mist bioreactor. Biotechnol Bioeng 107:802–813
Souret FF, Kim Y, Wyslouzil BE, Wobbe KK, Weathers PJ (2003) Scale-up of Artemisia annua L. hairy root cultures produces complex patterns of terpenoid gene expression. Biotechnol Bioeng 83:653–667
Steliopoulos P, Wüst M, Adam K-P, Mosandl A (2002) Biosynthesis of the sesquiterpene germacrene D in Solidago canadensis: 13C and 2H labeling studies. Phytochemistry 60:13–20
Towler MJ, Weathers PJ (2007) Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep 26:2129–2136
Utzinger J, Xiao S, N’Goran EK, Bergquist R, Tanner M (2001) The potential of artemether for the control of schistosomiasis. Int J Parasitol 31:1549–1562
Vitrac X, Larronde F, Krisa S, Decendit A, Deffieux G, Mérillon JM (2000) Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry 53:659–665
Vögeli U, Chappell J (1988) Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol 88:1291–1296
Wang JW, Tan RX (2002) Artemisinin production in Artemisia annua hairy root cultures with improved growth by altering the nitrogen source in the medium. Biotechnol Lett 24:1153–1156
Wang Y, Weathers PJ (2007) Sugars proportionately affect artemisinin production. Plant Cell Rep 26:1073–1081
Wang Y, Zhang H, Zhao B, Yuan X (2001) Improved growth of Artemisia annua L. roots and artemisinin production under red light conditions. Biotechnol Lett 23:1971–1973
Weathers PJ, DeJesus-Gonzalez L, Kim YJ, Souret FF, Towler MJ (2004) Alteration of biomass and artemisinin production in Artemisia annua hairy roots by media sterilization method and sugars. Plant Cell Rep 23:414–418
Weathers PJ, Bunk G, McCoy MC (2005) The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell Dev Biol Plant 41:47–53
Woerdenbag HJ, Lüers JFJ, Uden W, Pras N, Malingré T, Alfermann AW (1993) Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia annua L. Plant Cell Tissue Cult 32:247–257
Zarn JA, Brüschweiler BJ, Schlatter JR (2003) Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14α-demethylase and aromatase. Environ Health Perspect 111:255–261
Acknowledgments
The authors are indebted to Worcester Polytechnic Institute, the Arkansas Bioscience Institute, and NIH 2R15GM069562-03 for partial financial support of this work. Special thanks to Dan Vail (WPI) for Fig. 8.7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this chapter
Cite this chapter
Weathers, P., Towler, M., Wang, Y., Wobbe, K.K. (2012). Artemisinin: Controlling Its Production in Artemisia annua . In: Bach, T., Rohmer, M. (eds) Isoprenoid Synthesis in Plants and Microorganisms. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4063-5_8
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4063-5_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4062-8
Online ISBN: 978-1-4614-4063-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)