Abstract
The increased demand for biodiesel and the difficulties in obtaining enough quantities of raw materials for its production are stimulating the search for alternative feedstocks. Among the various possibilities, the utilization of residual fatty materials, in particular waste frying oils and animal fat residues from the meat and fish processing industries, are increasingly seen as viable options for biodiesel production. This work reviews the state of the art regarding the utilization of waste oils and animal fats as feedstocks for biodiesel production, which are characterized by the presence of high levels of impurities such as high acidity and moisture content. The relative advantages and disadvantages of the different routes for biodiesel production are presented and discussed in this chapter, focusing on their chemical and technological aspects. Also discussed are the questions related to the viability and potential economic advantages of using this type of feedstocks in biodiesel production for road transportation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
As energy demands increase and the fossil fuel reserves are limited or are becoming harder and harder to explore, research is being directed towards the development of renewable fuels. This aspect is particularly relevant in the transportation sector, where the dependence on fossil fuels is even more evident and any possible alternative (e.g. fuel cells and hydrogen) is harder to develop and implement in practice. In the short term, especially in Europe, biodiesel (mono-alkyl esters of long-chain fatty acids) derived from renewable biological sources such as vegetable oils or animal fats are attracting a lot of attention. Among its main key features one can point out its renewability, biodegradability, improved viscosity, better quality of exhaust gases, and also the possibility of being used, as a petroleum diesel substitute or combined with diesel fuels, in conventional combustion ignition engines without significant modifications.
Biodiesel promises to supplement and even replace at a local/regional level fossil diesel while contributing to rural development and reducing the dependence on fossil fuels. However, under current production technology, its use in transportation even blended with diesel has some pros and cons. First, biodiesel production costs are higher than those of petroleum diesel, mainly due to its production from expensive edible vegetable oils that account for 88% of the total estimated cost for biodiesel production [90]. This is one of the major hurdles in biodiesel commercialization, making it difficult to compete in price with fossil diesel and requiring in many cases subsidies or fixed prices policies to be competitive with current fossil fuels or to fulfil specific national or international targets for the incorporation of bio-based fuels. Second, the continued development, market growth, and market share of biodiesel, with the corresponding need of raw materials for its production, has risks of their own and is causing more harm than good. For example, some of the most relevant feedstocks, such as soybean oil and palm oil, are placing additional pressure on food supplies during a period of great demand increase in developing countries and diverting valuable resources away from food production. Until new technologies and/or feedstocks unconnected with the human food supply chain are developed, the use of edible vegetable oils to produce biodiesel might further strain the already tight supplies of arable land and water all over the world, potentially pushing food prices up even further. Furthermore, biodiesel feedstocks are impacted by previous and current land use practices, and cultures are adapted to specific climate and soil conditions available in restricted regions of the world. Thus, moving a culture from one region of the world to another will surely influence the crop yield potential. For example, requiring the utilization of more fertilizers, having an impact on the local biodiversity as some of the species can be invasive and displace native species, or bringing pests with them, with potential direct consequences to local ecosystems. Also, a more intense agriculture normally increases the soil erosion due to carbon loss and nitrate and phosphorous loss [82].
To circumvent the problems referred above, new feedstocks are needed what is currently an extensive area of research. An example includes microalgae that have the ability to grow under harsher conditions, in areas unsuitable for agricultural purposes, and with reduced needs for nutrients. This way, the competition with other crops for arable soil, in particular for human consumption, is greatly reduced. Also, microalgae are easy to cultivate and can grow at low cost with little attention, using water unsuitable for human consumption. However, very high energy requirement for drying the algal biomass is a barrier to its commercialization at present [66]. Another example is Jatropha curcas L., currently at a very early stage of development for biodiesel production. Since the markets of the different products from this plant have not yet been properly explored or quantified, the optimum economic benefit of its production has not been achieved [57].
From the currently available alternative feedstocks for biodiesel production, some attention is being given to residual oil and fat, such as waste frying oils from restaurants or food industry, and animal fats resulting from the meat or fish processing industries, which otherwise need to be disposed off with care and represents an operational cost. Even though the residual oil or fat are of lesser quality than virgin vegetable oils and more difficult to process due to the presence of impurities or to their high acidity, they may be a good option for biodiesel production, allowing one to use a waste and treating it appropriately in the production of a product (biodiesel) with value that can be used internally by the company or sold out. Moreover, these fatty materials are available at a lower cost (in many cases even for free) and can be used as feedstock for biodiesel production. Araujo et al. [8] evaluated the biodiesel production from waste frying oil concluding that it can be economically feasible provided that logistics are well configured.
The most common animal fats that can be processed into biodiesel are beef tallow, pork lard, and poultry fat. Fish oils are also possible to be converted into biodiesel, although research in this area is not so advanced as for the animal fats. In most of these cases, the oil or fat are not readily available for use in biodiesel production, but need to be firstly extracted from the fatty residues. It is estimated that about 38% of the bovine, 20% of the pork, and 9% of the poultry are fatty material for rendering (e.g. bones, fat, head, other non edible materials, etc.) from which can be obtained about 12–15% of tallow, lard, or poultry fat that can be used for biodiesel production [25, 36].
The lipid content in fish varies a lot depending on the type of fish and by-product. For example, Gunasekera et al. [45] reported the lipid content of 17% in carp offal, 13% in carp roe, 57% in trout offal, 31% in fish frames, and 13% in “surimi” processing waste and fish meal. Oliveira and Bechtel [74] reported 11.5% of lipids in pink salmon heads and 4% in salmon viscera. Kotzamanis et al. [55] reported 12% of lipids in trout heads.
In the European Union, about one million tonnes of tallow is rendered each year [69]. The United States generates in average about 4 kg/person of yellow grease per year, and based on this statistic, Canada should produce about 120,000 tonne/year of waste fats of various origins [100]. Brazil generates about 1,382,472 tonne/year of beef tallow and 194,876 tonne/year of lard from slaughterhouses, which is normally used for producing meal and oil for animal feed [25]. The world fish capture and aquaculture production was in 2004 about 140 million tonnes of fish, from which about 25% was for non-food uses, in particular for the manufacture of fish oil and animal meal [34]. The amount of waste frying oil generated annually in several countries is also huge, accounting for more than 15 million tonnes, varying according to the amount of edible oil consumed. For example, the United States generates around 10 million tonnes of waste frying oils, followed by China with 4.5 million tonnes and by the European Union with a potential amount ranging from 0.7 to 1.0 million tonnes [44]. However, the worldwide amount of waste oils generated should be much larger than that and it is expected to increase in the near future.
Some studies available in the open literature show some potential for these feedstocks. For example, Chua et al. [30] performed a LCA to study the environmental performance of biodiesel derived from waste frying oils in comparison with low sulphur diesel and concluded that biodiesel is superior in terms of global warming potential, life cycle energy efficiency, and fossil energy ratio. Godiganur et al. [41] tested biodiesel from fish oil in compression ignition engines, showing overall good combustion properties and environmental benefits. In particular, there are no major deviations in diesel engine’s combustion and no significant changes in the engine performance. Moreover, there is a reduction of the main noxious emissions in comparison with fossil diesel, with the exception on the nitrogen oxide (NOx) emissions. Wyatt et al. [97] produced biodiesel from lard, beef tallow, and chicken fat by alkali-catalyzed transesterification. The biofuel obtained from these animal fats were tested and the NOx emissions determined and compared with soybean biodiesel as 20% volume blends (B20) in petroleum diesel. Results show that the three animal fat-based B20 fuels have lower NOx emission levels (3.2–6.2%) than the soy-based B20 fuel.
Animal fats and vegetable oils differ on their physical and chemical properties. While vegetable oils have a large amount of unsaturated fatty acids, animal fats have in their composition a large amount of saturated fatty acids [20]. Animal fats such as tallow or lard are solid at room temperature. An exception is the poultry fat which is liquid at room temperature and has in its composition a low percentage of saturated triglycerides, comparable to soybean oil. Fish oils contain a wide range of fatty acids, some of them with more than 18 atoms in their carbon chain and even some with an odd number of carbons [37]. Chiou et al. [28] analysed and compared the methyl esters derived from salmon oil extracted from fish processing by-products with methyl esters derived from corn oil, concluding that, although there are some differences in the fatty acid composition, salmon and corn oil methyl esters have similar physical properties.
The physical and chemical properties of waste frying oil and the corresponding fresh edible oil are almost identical, but differ from source to source depending on the oil source. Waste oils have normally higher moisture and free fatty acids (FFA) contents than fresh edible oil, particles of different composition, and also polymerized triglycerides are formed during frying due to the thermolytic, oxidative, and hydrolytic reactions that may occur [44]. Additionally, during frying, the oil is heated at temperatures of 160–200°C in the presence of air and light for a relatively long period of time, what contributes to increase its viscosity, specific heat, and darkens its colour.
For processing these fatty waste materials and to improve the quality of biodiesel produced, different solutions can be employed. For example, Guru et al. [46] studied biodiesel production from waste animal fats in a two-step catalytic process and adding organic-based nickel and magnesium compounds as additives in order to achieve a reduction in the biodiesel pour point. Canoira et al. [22] evaluated biodiesel obtained from different mixtures of animal fat and soybean oil using a process simulation software (Aspen Plus™), concluding that a mix of 50% (v/v) of both raw materials is the most suitable to obtain a final product with a quality according to the standards and with the minimum costs. This is relevant to optimize the production processes and ensure that the costs of disposal should be higher than the costs of making biodiesel corrected by the potential economical gains, for example reducing the consumption of fossil fuels.
As most of the biodiesel feedstocks have similar characteristics, any improvement in the way how the pre-processing, reaction, and final processing are done, in particular related to the reaction time and final product quality, will have a profound impact in the production capacity and in the overall process. As two phases are formed and the diverse reactants are presented in different phases, the effects of mixing are significant to the process. The interfacial area between phases increases with high mixing intensity, facilitating the mass transfer between phases and naturally increasing the reaction rates [10]. Noureddini and Zhu [73] confirm these conclusions and have shown that, depending on the reaction stage, both the mass transport and the reaction kinetics are dominant aspects controlling the process performance.
In this work, the various steps for biodiesel production are described, depending on the characteristics of the waste oil or animal fat, having in mind the process improvement.
2 Biodiesel Production Processes
Processes for producing biodiesel from fatty waste materials should be similar to those from vegetable oils, the dominant feedstock according to the European point of view. Nevertheless, the special characteristics of these feedstocks, in particular their high content in FFA, moisture, and other contaminants, such as dirt and other chemicals that appear during processing and/or utilization of these fatty materials, requires that additional processing steps are employed, such as pre-treatment operations.
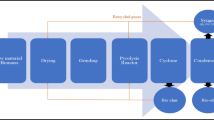
Currently, the process most widely used industrially for biodiesel production is the alkali-catalyzed transesterification of triglycerides, with low molecular weight alcohols and operated in batch mode. This process is more efficient and less corrosive than the acid-catalyzed transesterification, the reaction is faster, and requires lower amount of catalyst to carry out the reaction, presenting only problems in the glycerol separation. Also, the alkaline catalysts (NaOH, KOH, NaOCH3, etc.) are the most commonly preferred and are cheaper than the ones employed in the acid-catalyzed process (H2SO4, HCl, etc.). However, the alkali-catalyzed process has several drawbacks, in particular it is very sensitive to the lipidic feedstock purity, mainly operating in batch mode and needing large reaction times to obtain a complete conversion of oil, and has complex biodiesel purification steps after the reaction. For waste fats, these factors have to be considered explicitly to ensure a proper conversion of the fats to biodiesel, meaning that in most cases pre-treatment steps are required. Figure 1 shows a simplified process flowsheet for biodiesel production from waste oil or animal fat with high acidity by alkali-catalyzed transesterification preceded by a pre-treatment by esterification.
2.1 Extraction of Oil or Fat from Fatty Waste Materials
Depending on the characteristics of the fatty waste materials, normally three main steps are performed:
-
Extraction of the oil or fat from the fatty feedstock.
-
Filtration and removal of contaminants.
-
Neutralization or esterification of the FFA.
The extraction process is relevant in the meat and fish processing industry, as most types of fatty waste materials are normally associated with other materials, for example meat residues and bones or fish’s heads and viscera. Thus, it is necessary to separate the oil or fat from the remaining materials. Depending on the fatty residues to separate, the process varies, involving for example heating or solvent extraction.
At the laboratory scale, the waste animal fats (e.g. tallow, lard, or poultry fat) collected from slaughterhouses or food processing companies can be melted and filtered in order to obtain the fat and remove gums, protein residues, and suspended particles [67]. For extracting the fish oil from the fish’s residues, firstly fish’s viscera and heads can be cooked thoroughly in boiling water. The supernatant oil is taken from the top of the boiling vessel and placed in a separatory funnel, where the oil is washed (with distilled water at about 60°C) and separated from the water and solid residues. The fish soapstock is squeezed and as a result the crude fish oil containing some solid impurities is separated from the cake of fish dregs. The resulting crude oil is centrifuged and placed in a separatory funnel where it is washed. Finally, the oil is vacuum-filtered to remove any remaining impurities.
Industrially, the bulk of the material to be rendered consists of the leftover parts of a slaughtered animal (fats, bones, and other parts). The first step in the rendering process is the milling and grinding of a mixture of materials to generate a mass that is screw-conveyed to a batch digester where it remains for 4–5 h to be cooked with saturated vapour at about 110°C, until it loses about 70% of its moisture content. Then the digester is opened and its contents are discharged into a percolator tank, heated by steam, where the liquid fat separates from solids by percolation and sieving. After percolation, the fat is centrifuged and/or filtered and sent to a decanter tank for storage and eventual final separation from the aqueous phase present. The solid material removed from the fat in this operation is added to the solid material from percolation. The solid material is hot-pressed generating more fat that is added to the one percolated for purification. The pressed material is milled in a hammer mill, and then sent for screening to obtain the particle size of flour. The material retained in screening returns to the mill. Passing through the screening the meat/bone meal is bagged and stored for shipping and using in pet food [25].
The industrial process for extracting oil from fish by-products (e.g. heads, viscera, fish bones, and skin) operates in a continuous mode. Thus, after milling and grinding the fish, by-products are screw-conveyed to a continuous steam cooker with a residence time of about 15 min. After cooking, the coagulated mass is pre-strained in a strainer conveyor before entering a screw press that separates the press cake from the press liquor. The press cake is disintegrated in a tearing machine (a wet mill) and dried in an indirect steam dryer with internal rotating blades. The meal passes through a vibrating screen furnished with a magnet to remove extraneous matter-like pieces of wood and metal (e.g. fish hooks) before entering the hammer mill. The ground meal is automatically weighed out in bags that are closed and stored. The press liquor then passes through a buffer tank before separation into oil, “stick” water, and fine sludge in a centrifuge. The oil passes through a buffer tank before water and sludge impurities are removed (polishing) in the oil separator. After polishing, the oil often passes through an inspection tank before storage in the oil tank [35].
Another possibility for extracting lipids from fatty waste materials is by using an organic solvent, such as n-hexane. For example, Nebel and Mittelbach [72] tested nine solvents for extracting fat from meat and bone meal, obtaining about 15% fat with all solvents, but n-hexane was found to be the most suitable solvent to perform the extraction, because it is relatively cheap and has a low boiling point. The fat was then converted to methyl esters via a two-step process, whose quality was according to the European specification for biodiesel (EN 14214) except the cold-temperature behaviour and the oxidation stability. Oliveira and Bechtel [74] described a solvent extraction procedure using a 2:3 solution of isopropyl alcohol/hexane (99.9% purity) for extracting lipids from salmon’s by-products including heads, viscera, frames, and skin.
2.2 Pre-treatment of Waste Oils and Fats
When dealing with high acidity feedstocks, in particular waste frying oils or animal fats from the meat or fish processing industry, one needs to perform a pre-treatment to guarantee that the transesterification reaction is performed in an efficient way and that the quality of the biodiesel obtained follows all the applicable norms such as the EN 14214. Also, it is important to know their characteristics and the presence of contaminants that reduce the efficiency and effectiveness of the alkali-catalyzed transesterification.
For the removal of contaminants, of special concern is the presence of moisture that has a strong negative influence in the transesterification reaction. Water content of waste oils and animal fats may vary considerably depending on the origin. Rice et al. [81] reported a range of 1–5% (w/w) of water contents in waste frying oils. The presence of water inhibits the esterification and transesterification reactions, favours the hydrolysis of triglycerides and FFA, lowers the esters yield, and renders the ester and glycerol separation difficult [7, 18]. If the water concentration is greater than 0.5%, the ester conversion rate may drop below 90% [19]. Water also promotes soap formation in the presence of the alkali catalysts, increasing catalyst consumption and diminishing its efficiency. The water content in the feedstock should be lower than 0.06% (w/w) [64, 81]. Heating the waste frying oil or tallow over 100°C, to about 120°C, can boil off any excess water present in the feedstock. For other contaminants, other strategies should be employed in a case-by-case scenario.
The waste frying oils may have other impurities such as solid particles resulting from the food frying and sodium chloride that is added to the fried food. Depending on the feedstock characteristics, the separation of these solid particles may be accomplished by filtration, pressing, or centrifugation. The presence of chlorides may cause corrosion problems in the process equipment and piping system.
The acid value of oil is another important parameter to be determined, since it allows one to evaluate which is the most adequate method to produce biodiesel. For example, depending on the oil acidity, one- or two-step process can be used, where in a first step, the level of FFA is reduced to below 3% by acid-catalyzed esterification with methanol as reagent and sulphuric acid as catalyst and, in a second step, triglycerides in product from the first step are transesterified with methanol by using an alkaline catalyst to produce methyl esters and glycerol [94].
The FFA content of waste frying oil and animal fats vary widely. Waste oils typically contain 2–7% (w/w) of FFA [95], while animal fats may contain 15% FFA but can be as high as 40% [18, 93, 94]. In order to maximize the methyl esters yield, Freedman et al. [39] proposed to use vegetable oils with a FFA content lower than 0.5% (w/w) in order to not affect the yield of transesterification reaction. Rice et al. [81] reported that a reduction of FFA from 3.6 to 0.5% increased yields from 73 to 87%. Canakci and Van Gerpen [19] referred that a FFA level above 5% can lower the ester conversion rate below 90%. A study from the Sustainable Community Enterprises [85] concluded that due to its high acidity, salmon oil requires an esterification pre-treatment to be possible to perform the transesterification
In the presence of FFA and moisture, saponification reactions occur because the fatty acids react with the catalyst to produce soaps, decreasing the methyl esters yield, or even inhibiting the transesterification reaction. Even in small amounts, these contaminants can reduce the reaction rate by orders of magnitude [18]. Moreover, the formation of soap consumes catalyst and causes emulsions to be formed, which limits the mass transfer between phases, significantly reducing the chemical reaction rate and the selectivity to biodiesel. This further complicates the separation of phases after the reaction completion and makes it difficult to recover and purify biodiesel [7].
The equations (1) and (2) represent, respectively, the saponification of FFA and esters.
Aryee et al. [9] used FTIR and titrimetric analytical methods for FFA determination in fish oils extracted from salmon skin, concluding that the FFA content of Atlantic salmon skin lipids increased linearly from 0.6 to 4.5% within the 120 days it was stored at 20°C, as a result of auto-oxidation. Wu and Bechtel [96] also found that the FFA level in salmon heads and viscera increases with the storage time and temperature. From a practical point of view, this results show that at least the fish oils should be used immediately after their extraction, limiting somehow the utilization at a local scale or when the logistical networks are efficient.
Refined vegetable oils normally do not need a pre-treatment in order to produce biodiesel. However, the waste frying oils and the animal fats with high acidity (more than 2.5% w/w of FFA) need a pre-treatment to reduce their FFA content. This is normally done by acid-catalyzed esterification, using H2SO4 as catalyst and methanol as reagent in the proportions of 2.25 g of methanol and 0.05 g of sulphuric acid per each gram of FFA in oil. From the several approaches proposed in literature such as esterification and distillation refining method [99], Bianchi et al. [14] concluded that esterification is the most attractive to lower the FFA content of waste animal fat to 0.5% (from a typical range of 10 to 25%) using a solid acid ion-exchange resin as catalyst.
During esterification, the FFA are converted to methyl esters, but the triglycerides remain essentially unconverted to esters for low methanol to oil molar ratios and short reactor residence times [7, 29, 51, 60]. The esterification reaction can be represented as follows
Since water is formed as a by-product during esterification, it needs to be removed or the reaction will be quenched prematurely. One possible approach is to remove water while the reaction occurs, for example, using a membrane reactor. Another approach is to perform the reaction in two rounds with the removal of methanol, sulphuric acid, and water phase in between, followed by the addition of more fresh reactant to perform a second-round reaction driving it closer to completion [19–21, 94]. Zhang et al. [100] suggested the addition of glycerine after the second-round reaction to remove all the water from the oil stream, having the advantage of removing the acid catalyst which may cause neutralization of the alkali-catalyst during the transesterification reaction.
2.3 Biodiesel Production Processes
2.3.1 Alkali-Catalyzed Process
The alkali-catalyzed transesterification of triglycerides (1) is the process normally used for low amounts of FFA present in triglycerides (less than 2.5% w/w) since this reaction is very sensitive to the oil or fat purity, requiring in many cases pre-treatment steps. The pre-treated oil can then be transesterified with an alkali-catalyst to convert triglycerides into methyl esters.
As shown in (4), transesterification is a multiple reaction including three reversible steps in series, where triglycerides are converted to diglycerides, then diglycerides are converted to monoglycerides, and monoglycerides are converted to fatty acid alkyl monoester (biodiesel) and glycerol (by-product). Although several alcohols can be used in this reaction, such as ethanol, methanol, or butanol to obtain respectively, methyl, ethyl, or butyl esters, it is methanol that is most commonly used due to its low cost by comparison with the other alcohols. An excess of alcohol needs to be used (normally an alcohol to oil molar ratio of 6:1) at a reaction temperature of about 60°C, if methanol is used, or 70°C for ethanol [39]. The amount of catalyst used in the mixture is in the range of 0.5–1.0% (w/w), a higher amount may have as consequences gel formation and difficulty in separating glycerol. Generally, the transesterification reaction is affected by operating conditions such as alcohol/oil molar ratio, kind of alcohol (e.g. methanol, ethanol, propanol, or butanol), type and amount of catalysts, reaction time and temperature, and purity of reactants.

(4)
After the chemicals are mixed for the transesterification, two essentially immiscible phases are formed: one non-polar containing triglycerides and esters, and the other polar containing glycerol and alcohol. As two immiscible phases are formed, the reactor vessels are intensely stirred to promote mass transfer [10]. Some emulsification also occurs due to saponification reaction since the alkalis catalyst is used [11]. For temperatures of 60 or 70°C, the conversion of the oil is complete in few hours [1].
Tashtoush et al. [91] performed experiments to determine the optimum conditions for converting animal fats into ethyl and methyl esters, concluding that absolute ethanol performs better than absolute methanol and that 50°C is the optimum temperature to perform the transesterification reaction, during 2 h maximum. Other authors have also devised different ways of using residual oils or fats with high FFA and moisture contents, for biodiesel production. For example, Alcantara et al. [3] calculated the cetane index of waste frying oil and concluded that it is similar to that of fossil diesel fuel. Ma et al. [64] studied the effect of catalyst, FFA, and water in the transesterification of beef tallow, concluding that the presence of water has the most negative effect on the reaction conversion and should be kept beyond 0.06% (w/w), while FFA should be kept beyond 0.5% (w/w).
Piu [77] studied the production of biodiesel from waste animal fats, including the oil pre-treatment, biodiesel production, purification, and its final combustion in a diesel-powered generated for emissions determination, concluding that using 20/80 (v/v) of biodiesel/diesel blends has the better results in terms of NOx, CO, HC, and smoke emissions.
Alptekin and Canakci [5] studied the production of biodiesel from chicken fat extracted from chicken wastes (feathers, blood, offal, and trims) after the rendering process. These authors investigated the variables affecting the FFA level of chicken fats such as alcohol molar ratio, amount of acid catalyst, and reaction time by using a chicken fat with 13.45% FFA. The optimum esterification condition was found to be 20% (wt/wt) sulphuric acid and 40:1 of methanol:oil molar ratio, for a reaction time of 80 min at 60°C of temperature. For these conditions, the methyl ester yield was 87.4% after transesterification.
The alkali-catalyzed process suffers from some significant drawbacks, in particular the pronounced adverse effects of water, high acidity, and long reaction time in batch mode. Many options are being suggested and some are under development and even implementations to improve the process, in particular to be able to operate in continuous mode with reduced reaction time, and trying to reduce the operating costs, especially associated with the feedstocks consumption and with the biodiesel purification steps [11]. Some examples are listed bellow and some applications to waste fats are discussed in the next sub-sections
-
Acid-catalyzed transesterification [56].
-
Non-catalytic supercritical methanol or ethanol for the transesterification reaction [26, 58, 59, 65].
-
Heterogeneous or Biological catalyzed process (inorganic chemical, enzymes, and living organisms) to avoid the need for the removal and recycling of the catalyst [16, 31, 54, 79].
-
Transesterification with co-solvents to enhance the solubility of reactants, by diminishing the mixture polarity and increasing the reaction rate [15, 43].
-
In situ transesterification [47].
-
Microwave-assisted transesterification [61].
-
Catalytic cracking [50].
2.3.2 Acid-Catalyzed Process
The usage of a strong acid instead of a strong base is better suited for high acidity feedstocks, a situation normally found in waste oils and fats, making it possible to avoid the oil pre-treatment operation and providing high conversion rates with no soap formation [19, 49]. Nevertheless, it is seldom used due to its longer reaction times and higher temperatures required, when compared to the alkali-catalyzed process, and it is more corrosive to the process equipment [1, 19]. For example, Kulkarni and Dalai [56] report 88 and 95% conversion obtained, respectively, for 48 and 96 h reaction time.
Also, a higher methanol to oil molar ratio is needed to promote high equilibrium conversions of triglycerides to esters, which generally increases the production costs, due to an increase in the volume needed for the reactor and the separation of glycerol that becomes more difficult. Kulkarni and Dalai [56] report that 98% conversion is obtained for a methanol:oil molar ratio of 30:1 by comparison with a 87% conversion for a molar ratio of 6:1.
Among the several acid catalysts (e.g. sulphuric, sulfonic, phosphoric, or hydrochloric acid) that can be used, sulphuric acid is the most common. Zhang et al. [101] evaluated economically both the alkali-catalyzed and the acid-catalyzed process, concluding that though the first one, using virgin vegetable oil, has the lowest fixed capital cost, the second one, using waste frying oil, is more economically feasible overall.
Kulkarni and Dalai [56] present the effect of various parameters on the acid-catalyzed transesterification, showing that the FFA and moisture content of oils are the parameters that most affect the reaction conversion. For instance, with less than 0.5% water the conversion is above 90%, and for 3 or 5% of moisture the conversion is, respectively, 32 and 5%. The FFA effect is not so accentuated allowing one to obtain 90, 80, and 60% conversion for 5, 15, and 33% of FFA content, respectively.
Bhatti et al. [13] studied the effect of various parameters in the production of biodiesel from animal fats, concluding that the optimum conditions for 5 g of chicken and mutton tallow are, respectively, a temperature of 50 and 60°C, 1.25 and 2.5 g of H2SO4, and an oil:methanol molar ratio of 1:30 and 1:30, yielding 99.01 ± 0.71% and 93.21 ± 5.07% of methyl esters, after 24 h, in the presence of acid. Gas chromatographic analysis showed a total of 98.29 and 97.25% fatty acids in chicken and mutton fats, respectively.
2.3.3 Non-catalytic Supercritical Processes
Other interesting option for producing biodiesel from feedstocks with high concentration of impurities such as water and FFA is the transesterification of triglycerides with supercritical methanol, which is receiving a lot of attention [17, 58, 59]. This process is catalyst-free and it is able to obtain full conversion of the triglycerides in a matter of minutes [58], with the possibility of continuous operation mode [26].
The operation is also simpler, as the transesterification of triglycerides and methyl esterification of fatty acids occurs simultaneously without using any catalyst. Because no catalyst is used and has to be recovered, the downstream processing is much simpler, and soap-free glycerol can be obtained [89]. Other advantage this process presents is the insensitivity to the presence of impurities in the vegetable oil, such as water and FFA [59]. The presence of moisture is not only negligible, but it can also be advantageous in this process [6].
The reaction is carried out in supercritical methanol (or ethanol), in which feedstocks react with the alcohol under conditions of high pressure (above 100 atm) and high temperature (more than 276°C). At these conditions, the alcohol is in a supercritical gaseous state and the triglycerides are somewhat dissolved in a single phase. The reasons for this behaviour are not yet fully understood, but are certainly related to the high solubility of triglycerides in supercritical alcohol and solvent effects [65]. Also, some authors have observed a dependence on the type of alcohol and triglyceride used [4].
Notwithstanding its clear advantages over other processes, significant hurdles remain for the full scale implementation of supercritical production units. First of all, high temperatures and pressures are necessary to ensure that the alcohol is in supercritical state, requiring the utilization of special equipment designed to support these conditions. This will lead to high equipment and operational costs, making the process economics not so attractive when compared to other options. Also, the excess of methanol used in the reaction is much larger when compared to the conventional process; normally an alcohol/oil molar ratio of 42:1 is used, which needs to be recovered and recycled back to the reactor this way complicating the process design [58].
Alternatively, Cao et al. [23] proposed the supercritical methanol process, using propane as co-solvent, which decreases the reaction temperature and pressure, as well as the alcohol to oil molar ratio. This is because propane decreases the critical point of methanol allowing the supercritical reaction to be carried out under milder conditions than those of 424 atm and 350°C reported by Kusdiana and Saka [58]. In this case, the optimal reaction conditions are a temperature of 280°C, a pressure of 126 atm, an alcohol to oil molar ratio of 24:1, and propane to oil molar ratio of 0.05:1. At these conditions, 98% of oils are converted to biodiesel for a reaction time of 10 min. Kasteren and Nisworo [53] performed an economic analysis of this process, considering the industrial production of biodiesel from waste frying oil and concluded that it can compete with the existing alkali and acid-catalyzed processes.
2.3.4 Heterogeneous Catalyzed Process
A natural evolution in process development is the replacement of the homogeneous catalysis with a solid base catalyst, change that simplifies extensively the post-processing process. In particular, it makes it easier to operate in continuous mode, eases the catalyst separation and recycling after reaction, avoids the saponification problem, and yields a cleaner biodiesel product, a purer glycerol that can be more easily marketed.
Various types of heterogeneous catalysts are being considered and studied for biodiesel production, including titanium silicates, ion-exchange resins, and zeolite metal-supported catalysts, among others. Extensive reviews of the current status on the use of solid base catalysts can be found in Liu et al. [63] and Di Serio et al. [33]. Peng et al. [75] prepared, characterized, and studied a solid acid catalyst for its activity in the production of biodiesel from several feedstocks with a high FFA content, showing that heterogeneous catalysis is a worthy option to process those types of raw materials.
Heterogeneous catalysts such as zeolites and metals may also allow for the use of feedstocks with a high FFA content [16, 31, 54]. However, some scientific and technical barriers persist relatively to their application at industrial-scale. For instance, Albuquerque et al. [2] concluded that the catalyst activity strongly depends on the metal composition of the oxides used, and new materials with well-defined structures, high surface area, and adequate basic or acid properties have yet to be developed. Although many of the solid catalysts proposed in literature for biodiesel production have good catalytic performances, they require high temperatures and pressure to work properly. Also, it is necessary to address the questions of deactivation, reusability, and regeneration of the catalysts in practical conditions to assess their real potential for using in commercial applications. As heterogeneous catalyzed processes have advantages over homogeneous catalyzed and are easier to operate, more commercial applications will certainly be introduced in the near future with important impact on the biodiesel production.
2.3.5 Biological Catalyzed Process
Biological catalysts for biodiesel production, including both enzymes and living organisms, are considered to be one of the most promising alternatives for future use in biodiesel production [38, 79]. They can be implemented either in solution or supported (e.g. in biological films or in packed beds). An advantage of enzymes is that they do not require the utilization of nutrients. An advantage of living organisms is that they can be genetically engineered to improve their performance, resilience, and capacity to operate in harsher conditions.
Lipases obtained from different biological sources are examples of enzymes that can be used to perform the transesterification reaction and that have shown a good tolerance to the oil FFA content [95]. Kaieda et al. [52] show that different enzymes have different capacities and report that certain lipases can be used for biodiesel production even if the oil has high water and methanol contents.
Although extensive research has been devoted to this area, the use of bio-catalysts for biodiesel production is still at the laboratory stage [1, 79, 86, 87]. They can be more efficient, selective, require a lower reaction temperature, and produce less side products or wastes, when compared with other types of catalyzed processes, but the reaction rates are much lower than for the conventional process, normally taking several hours (8–12 h) for similar conversions.
Some of the main problems include the difficulty in determining:
-
What are the best enzymes or microorganisms to perform the reaction, depending on the feedstocks characteristics and on the impurities that may exist?
-
What are the optimum reaction conditions, in particular what are the optimal molar ratio of reactants, solvents to be used, temperature, and water content?
-
How the enzymes will be used, if supported or in solution?
-
How to recover and reuse the enzymes?
-
How to avoid the enzymes’ deactivation, or the living organisms’ death?
Some studies can be found in literature addressing some of the problems listed above. For example, Shimada et al. [87] concluded that the best way to avoid the inhibition or deactivation of enzymes and maintain the enzyme activity for longer periods of time is their stepwise addition to the reaction mixture, in order to maintain the oil/methanol ratio at certain optimal levels. Although the addition of co-solvents appears in some cases to have a positive effect on the enzyme stability [1], there is still some work to be done in order to identify the most adequate solvents and how they influence the ongoing reaction.
2.3.6 Transesterification Reaction Using Co-solvents
The use of co-solvents such as dimethyl ether (DME), diethyl ether (DEE), methyl tert-butyl ether (MTBE) and tetra-hydro-furan (THF) has attracted much attention since they allow one to increase the reaction rate, under milder conditions, by diminishing the mixture polarity [15, 43].
For example, the use of co-solvents and high mixing intensity for the reaction reduces the need to use higher temperatures to enhance the solubility among reactants and the mass transfer between both phases [11]. Moreover, transesterification in supercritical methanol, employing propane and CO2 as co-solvents, was also developed [23, 48, 84, 98]. Nevertheless, the selection of the appropriate co-solvent and the mixing intensity are critical factors contributing to the correct operation and performance of the reaction system.
Sabudak and Yildiz [83] studied biodiesel production from waste frying oils by applying three different processes: a one-step alkali-catalyzed transesterification, a two-step alkali-catalyzed transesterification, and a two-step acid-catalyzed transesterification followed by alkali-catalyzed transesterification. For each reaction, these authors added THF as a co-solvent concluding that the effect of THF on reaction yield is not significant, and instead of using the co-solvent, it is more economical to improve the mixing ability of the reactor.
2.3.7 In Situ Transesterification
In situ transesterification is another possibility that simplifies or even eliminates the need to perform the pre-processing of feedstocks, in particular the oil extraction and refining steps. The transesterification reaction is directly performed in the macerated oil seeds, such as soybeans flakes or animal fats containing the lipidic material [24, 40, 70, 88, 102].
Although the in situ transesterification was proposed some years ago, it has not yet been used extensively. Reasons for this may be the large molar ratios between oil and alcohol that are necessary to obtain full oil conversion and the dependence on the seeds characteristics and on its oil content [88]. This process may seem simple, but it is still not fully worked out for practical applications and it is not economically efficient.
Haas et al. [47] investigated biodiesel production by in situ transesterification using as feedstocks corn dried grains (a by-product of ethanol production) and meat and bone meal (a by-product of animal rendering). As a result, these authors achieved almost the maximum theoretical transesterification conversion (91.1%) at ambient pressure and 35°C of temperature. For a higher temperature of 55°C, no significant increase in the conversion was achieved. Partial drying of the corn grains contributed to reduce the methanol requirements to achieve a high degree of transesterification. For meat and bone meal, drying was not required to achieve a high degree (93.3%) of transesterification.
2.3.8 Microwave-Assisted Transesterification
Microwave-assisted transesterification is another possibility for biodiesel production from lipidic feedstocks with high acidity [61]. Refaat et al. [80] compared both microwave-assisted and the conventional process for producing biodiesel from high acidity feedstocks, concluding that reaction time is reduced by about 97% and separation time by about 94% using microwave irradiation.
Perin et al. [76] compared the acid-catalyzed and alkali-catalyzed transesterification assisted by microwave irradiation concluding that the best results are obtained under basic conditions, i.e. the reaction takes place in 5 min, and 95% conversion is obtained. Azcan and Danisman [12] have considered microwave heating to perform the transesterification of rapeseed oil, showing that increased yields and reduced reaction times are possible.
2.3.9 Catalytic Cracking
A variant of thermal cracking is the catalytic cracking, extensively used in the petrochemical industry to produce a significant percentage of the fossil-derived fuel currently used. This possibility has also been pursued for the production of biofuels from a wide variety of feedstocks, especially from low-value triglyceride-based biomass. The reaction takes place in fluid catalytic cracking (FCC) units where triglyceride molecules are transformed into water, CO2, CO, and a mixture of hydrocarbons, some of the aromatic type [68]. The employment of a catalyst permits the utilization of milder conditions of temperature and pressure, with a better control of the final products [27, 92].
Hua et al. [50] studied the catalytic cracking transformation of vegetable oils and animal fats in the laboratory. The results show that they can be used as FCC feed singly or co-feeding with vacuum gas oil, which can give high yield (by mass) of liquefied petroleum gas (LPG), C2–C4 olefins, for example, 45% LPG, 47% C2–C4 olefins, and 77.6% total liquid yield produced with palm oil cracking. Co-feeding with vacuum gas oil gives a high yield of LPG (39.1%) and propylene (18.1%).
Different combinations of reactors and catalysts can be used, as for example pillared clays, alumina metal-supported catalysts, zeolites, among others. Also, the huge experience gathered in the petrochemical industry can be relevant in the development and implementation of cracking processes for biodiesel production.
2.4 Post-processing
After the transesterification reaction, the post-processing steps needed to purify biodiesel according to the existing regulations and norms are the same as those involved in the biodiesel production from currently edible vegetable oils. Although the final product composition in terms of esters may be different depending on the feedstocks used, their physical properties are similar and no significant differences are expected between the two variants.
After the reaction is finished, the mixture is allowed to separate into an upper layer of methyl esters and a lower layer of glycerol diluted with methanol. Glycerol is removed by allowing the two phases to form and settle. Then, any unreacted alcohol is air-stripped or vacuum-distilled away from the esters phase and recycled back to the reactor.
Depending on the process, water can be used to wash catalyst residues and sodium soaps from the methyl esters. Moreover, small amounts of concentrated phosphoric acid (H3PO4) can be added to the raw methyl esters to break down catalyst residues and sodium soaps. Predojevic [78] studied different purification steps of biodiesel obtained from waste frying oils, by a two-step alkali-catalyzed transesterification reaction, concluding that the best results are obtained when using silica gel and phosphoric acid treatments (with a yield of 92%) and the lowest yields (89%) are obtained using hot water. Also, Sabudak and Yildiz [83] applied three different purification methods to biodiesel produced from waste frying oils (water washing with distilled water, dry wash with addition of magnesol, and an ion-exchange resin) concluding that the most effective one is the ion-exchange resin.
The same situation occurs for the storage of biodiesel, where potential problems of decomposition may occur. For example, Lin and Lee [62] studied the oxidative stability of marine fish-oil biodiesel showing that the addition of antioxidant significantly retards the fuel deterioration over time, although it increases the kinematic viscosity and carbon residue at the beginning of the storage period. These authors also concluded that the operating temperature is a dominant factor in the deterioration of the fuel characteristics.
3 Economic and Environmental Considerations
As stated above, the conversion to biodiesel of waste oils and fats from the meat and fish processing industries represents an opportunity to valorize a residue and obtain a higher value product (biodiesel). In many situations, the adequate disposal of residual oils and fats represents an operational cost, as they cannot be burnt directly in a boiler without special equipment. Thus, from this point of view, there is an economic incentive to valorize those residues.
However, depending on the total quantity of residual oils and fats generated, different approaches have to be considered. If the total amount is small, as it is the case of the waste frying oils generated in restaurants, it will be easier to make the selective collection of those materials to be processed in a centralized production facility. If a good logistic system is developed and properly implemented, and incentives are available for the residue producers, this is proved to be a good option [8], applicable even for the small- and medium-sized companies of the meat and fish processing industries. This situation may change if small and compact units for the production of small quantities of biodiesel from a wide variety of feedstocks become available, although the costs of energy and raw materials, and the hazards involved in the manipulation of dangerous chemicals may render this possibility impracticable.
However, if the quantity of residues generated is large, the option of having an in-house facility for the production of biodiesel may be viable from an economic point of view. In any case, the reduction in the consumption of fuel by the company, either by the utilization of biodiesel or by burning of the glycerol produced in the process, must be compared with the investment in equipment and operational costs due to the consumption of materials and energy necessary to produce biodiesel. With the increase in price of fossil fuels, this option is expected to become more and more attractive. It is also relevant for companies operating close by or even interconnected, that generate large quantities of residual fats, and that may be interested on a common processing plant to take care of all the fat residues generated in their activities.
From an environmental point of view, the valorization of residual oils and fats to biodiesel production makes sense. This corresponds to the reutilization of a waste material originated from a renewable source, thus reducing the consumption of non-renewable fossil fuels. Although at a first glance there is a reduction in greenhouse gas emissions, in particular of carbon dioxide [71], the actual reduction depends on how the residues are collected and transported to the production site. To minimize those emissions, the logistical network should be properly optimized, for example, by giving the residue generators special containers for storing the waste fats and defining the more adequate collection routes. Although for in-house biodiesel production facilities this problem does not occur, additional savings may be accomplished through an adequate process optimization and integration.
Moreover, with the advent of more stringent limits for greenhouse gas emissions and the development of trading schemes for carbon emissions, the production of biodiesel from residual oils and fats can be a good form to combine the environmental and the economical aspects to one’s advantage. However, the current legislation and regulations still need to be improved or even created to be able to have a clear vision of the trade-offs involved on these decisions.
4 Conclusions
This article presents and discusses the main questions regarding the utilization of residual oils and fats for biodiesel production. Some key aspects are identified and strategies to deal with them are presented. Among them, the high content of FFA and moisture in waste frying oils and animal fats, as compared to fresh edible oils, makes the alkaline-catalyzed transesterification reaction to be less efficient for biodiesel production. A pre-treatment method suitable to handle this type of feedstocks is presented. Also, more efficient and robust production processes are presented that are able to use feedstocks with the characteristics normally encountered in waste fats.
As the global demand for biodiesel increases and the pressure to be more environmental friendly, yet maintaining market competitiveness, increases, more and more waste residues will be seen as valuable raw materials. Besides helping companies to fulfil their goals, policy targets defined at governmental and regional levels may be easier to reach.
References
Akoh CC, Chang SW, Lee GC, Shaw JF (2007) Enzymatic approach to biodiesel production. J Agric Food Chem 55:8995–9005
Albuquerque MCG, Santamaría-González J, Mérida-Robles JM, Moreno-Tost R, Rodríguez-Castellón E, Jiménez-López A, Azevedo DCS, Cavalcante CL, Maireles-Torres P (2008) MgM (M=Al and Ca) oxides as basic catalysts in transesterification processes. Appl Catal Gen 347(2):162–168
Alcantara R, Amores J, Canoira L, Fidalgo E, Franco MJ, Navarro A (2000) Catalytic production of biodiesel from soy-bean oil, used frying oil and tallow. Biomass Bioenergy 18:515–527
Alonzo DEL (2007) Heterogeneous catalysis and biodiesel forming reactions. PhD Thesis in Chemical Engineering, Graduate School of Clemson University
Alptekin E, Canakci M (2010) Optimization of pretreatment reaction for methyl ester production from chicken fat. Fuel 89:4035–4039
Al-Zuhair S (2007) Production of biodiesel: possibilities and challenges. Biofuels Bioprod Bior 1(1):57–66
Aranda DAG, Santos RTP, Tapanes NCO, Ramos ALD, Antunes OAC (2008) Acid-catalyzed homogeneous esterification reaction for biodiesel production from palm fatty acids. Catal Lett 122:20–25
Araujo VKWS, Hamacher S, Scavarda LF (2010) Economic assessment of biodiesel production from waste frying oils. Bioresour Technol 101:4415–4422
Aryee ANA, van de Voort FR, Simpson BK (2009) FTIR determination of free fatty acids in fish oils intended for biodiesel production. Process Biochem 44:401–405
Ataya F (2008) Mass transfer limitations in the transesterification of canola oil to fatty acid methyl ester. PhD Thesis in Chemical Engineering, University of Ottawa
Ataya F, Dube MA, Ternan M (2008) Transesterification of canola oil to fatty acid methyl ester (FAME) in a continuous flow liquid-liquid packed bed reactor. Energy Fuel 22:3551–3556
Azcan N, Danisman A (2008) Microwave assisted transesterification of rapeseed oil. Fuel 87:1781–1788
Bhatti HN, Hanif MA, Qasim M, Ata-ur-Rehman (2008) Biodiesel production from waste tallow. Fuel 87(13–14):2961–2966
Bianchi CL, Boffito DC, Pirola C, Ragaini V (2010) Low temperature de-acidification process of animal fat as a pre-step to biodiesel production. Catal Lett 134:179–183
Boocock DGB, Konar SK, Mao V, Sidi H (1996) Fast one-phase oil-rich processes for the preparation of vegetable oil methyl esters. Biomass Bioenergy 11(1):43–50
Brito A, Borges ME, Arvelo R, Garcia F, Diaz MC, Otero N (2007) Reuse of fried oil to obtain biodiesel: Zeolites Y as a catalyst. Int J Chem React Eng 5:Article A104
Bunyakiat K, Makmee S, Sawangkeaw R, Ngamprasertsith S (2006) Continuous production of biodiesel via transesterification from vegetable oils in supercritical methanol. Energy Fuel 20:812–817
Canakci M (2007) The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour Technol 98(1):183–190
Canakci M, Van Gerpen J (1999) Biodiesel production via acid catalysis. Trans ASAE 42(5):1203–1210
Canakci M, Van Gerpen J (2001) Biodiesel production from oils and fats with high free fatty acids. Trans Am Soc Agric Eng 44(6):1429–1436
Canakci M, Van Gerpen J (2003) A pilot plant to produce biodiesel from high free fatty acid feedstocks. Trans Am Soc Agric Eng 46(4):945–954
Canoira L, Rodrıguez-Gamero M, Querol E, Alcantara R, Lapuerta M, Oliva F (2008) Biodiesel from low-grade animal fat: production process assessment and biodiesel properties characterization. Ind Eng Chem Res 47:7997–8004
Cao W, Han H, Zhang J (2005) Preparation of biodiesel from soybean oil using supercritical methanol and co-solvent. Fuel 84:347–351
Carrapiso AI, Timón ML, Petrón MJ, Tejeda JF, García C (2000) In situ transesterification of fatty acids from Iberian pig subcutaneous adipose tissue. Meat Sci 56:159–164
CETESB (2006) Graxarias—Processamento de materiais de matadouros e frigoríficos bovinos e suínos. Guia técnico ambiental de graxarias (Série P+L), Governo de São Paulo, 2006
Chen W, Wang C, Ying W, Wang W, Wu Y, Zhang J (2009) Continuous production of biodiesel via supercritical methanol transesterification in a tubular reactor. Part 1: thermophysical and transitive properties of supercritical methanol. Energy Fuel 23:526–532
Chew TL, Bhatia S (2009) Effect of catalyst additives on the production of biofuels from palm oil cracking in a transport riser reactor. Bioresour Technol 100:2540–2545
Chiou BS, El Mashad HM, Avena-Bustillos RJ, Dunn RO, Bechtel PJ, McHugh TH, Imam SH, Glenn GM, Orts WJ, Zhang R (2008) Biodiesel from waste salmon oil. Trans ASABE 51(3):797–802
Chongkhong S, Tongurai C, Chetpattananondh P (2009) Continuous esterification for biodiesel production from palm fatty acid distillate using economical process. Renew Energy 34(4):1059–1063
Chua CBH, Lee HM, Choong JS (2010) Low life cycle emissions and energy study of biodiesel derived from waste cooking oil and diesel in Singapore. Int J Life Cycle Ass 15:417–423
Chung K-H, Chang D-R, Park B-G (2008) Removal of free fatty acid in waste frying oil by esterification with methanol on zeolite catalysts. Bioresour Technol 99:7438–7443
Deshmane VG, Gogate PR, Pandit AB (2009) Ultrasound-assisted synthesis of biodiesel from palm fatty acid distillate. Ind Eng Chem Res 16(3):345–350
Di Serio M, Tesser R, Pengmei L, Santacesaria E (2008) Heterogeneous catalysts for biodiesel production. Energy Fuel 22:207–217
FAO (2007) Food and Agricultural Organization of the United Nations. The state of world fisheries and aquaculture 2006. FAO, Rome. ftp://ftp.fao.org/docrep/fao/009/a0699e/a0699e.pdf
FAO (2010) The production of fish meal and oil. Fisheries and Aquaculture Department of FAO Corporate Document Repository. http://www.fao.org/docrep/003/x6899e/x6899e04.html
Ferroli PCM, Neto MF, Filho NC, Castro JEE (2001) Fábricas de Subprodutos de Origem Animal: a Importância do Balanceamento das Cargas dos Digestores de Vísceras. Produção 10(2):5–20
Firestone D, Reina RJ (1986) Authenticity of vegetable oils. In: Hamilton RJ, Rossel JB (eds) Analysis of oils and fats. Elsevier Applied Science Publishers, New York
Fjerbaek L, Christensen KV, Norddahl B (2009) A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol Bioeng 102(5):1298–1315
Freedman B, Pryde EH, Mounts TL (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. JAOCS 61:1638–1643
Georgogianni KG, Kontominas MG, Pomonis PJ, Avlonitis D, Gergis V (2008) Alkaline conventional and in situ transesterification of cottonseed oil for the production of biodiesel. Energy Fuel 22(3):2110–2115
Godiganur S, Murthy CS, Reddy RP (2010) Performance and emission characteristics of a Kirloskar HA394 diesel engine operated on fish oil methyl esters. Renew Energy 35:355–359
Gogate PR, Kabadi AM (2009) A review of applications of cavitation in biochemical engineering/biotechnology. Biochem Eng J 44:60–72
Guan G, Sakurai N, Kusakabe K (2009) Synthesis of biodiesel from sunflower oil at room temperature in the presence of various cosolvents. Chem Eng J 146:302–306
Gui MM, Lee KT, Bhatia S (2008) Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 33:1646–1653
Gunasekera RM, Turoczy NJ, De Silva SS, Gooley GJ (2002) An evaluation of the suitability of selected waste products in feeds for three fish species. J Aquat Food Prod Technol 11(1):57–78
Guru M, Artukoglu BD, Keskin A, Koca A (2009) Biodiesel production from waste animal fat and improvement of its characteristics by synthesized nickel and magnesium additive. Energy Convers Manage 50:498–502
Haas MJ, Scott KM, Foglia TA, Marmer WN (2007) The general applicability of in situ transesterification for the production of fatty acid esters from a variety of feedstocks. JAOCS 84:963–970
Han H, Cao W, Zhang J (2005) Preparation of biodiesel from soybean oil using supercritical methanol and CO2 as co-solvent. Process Biochem 40(9):3148–3151
Han M, Yi W, Wu Q, Liu Y, Hong Y, Wang D (2009) Preparation of biodiesel from waste oils catalyzed by a Brønsted acidic ionic liquid. Bioresour Technol 100:2308–2310
Hua T, Chunyi L, Chaohe Y, Honghong S (2008) Alternative processing technology for converting vegetable oils and animal fats to clean fuels and light olefins. Chin J Chem Eng 16(3):394–400
Issariyakul T, Kulkarni MG, Dalai AK, Bakhshi NN (2007) Production of biodiesel from waste fryer grease using mixed methanol/ethanol system. Fuel Process Technol 88(5):429–436
Kaieda M, Samukawa T, Kondo A, Fukuda H (2001) Effect of methanol and water contents on production of biodiesel, fuel from plant oil catalyzed by various lipases in a solvent-free system. J Biosci Bioeng 91(1):12–15
Kasteren JMN, Nisworo AP (2007) A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour Conserv Recy 50:442–458
Kiss A, Omota F, Dimian A, Rothenberg G (2006) The heterogeneous advantage: biodiesel by catalytic reactive distillation. Top Catal 40:141–150
Kotzamanis YP, Alexis MN, Andriopoulou A, Castritsi-Cathariou I, Fotis G (2001) Utilization of waste material resulting from trout processing in gilthead bream (Sparus aurata L.) diets. Aquacult Res 32:288–295
Kulkarni MG, Dalai AK (2006) Waste cooking oils—an economical source for biodiesel: a review. Ind Eng Chem Res 45:2901–2913
Kumar A, Sharma S (2008) An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Ind Crops Prod 28:1–10
Kusdiana D, Saka S (2001) Kinetics of transesterification in rapeseed oil to biodiesel fuels as treated in supercritical methanol. Fuel 80:693–698
Kusdiana D, Saka S (2004) Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour Technol 91:289–295
Lepper H, Friesenhagen L (1986) Process for the production of fatty acid esters of short-chain aliphatic alcohols from fats and/or oils containing free fatty acids. US Patent 4608202
Lertsathapornsuk V, Pairintra R, Aryusuk K, Krisnangkura K (2008) Microwave assisted in continuous biodiesel production from waste frying palm oil and its performance in a 100 kW diesel generator. Fuel Process Technol 89:1330–1336
Lin C-Y, Lee J-C (2010) Oxidative stability of biodiesel produced from the crude fish oil from the waste parts of marine fish. J Food Agric Environ 8(2):992–995
Liu Y, Lotero E, Goodwin JG Jr, Lu C (2007) Transesterification of triacetin using solid Brønsted bases. J Catal 246:428–433
Ma F, Clements LD, Hanna MA (1998) The effects of catalyst, free fatty acids, and water on transesterification of beef tallow. Trans ASAE 41(5):1261–1264
Madras G, Kolluru C, Kumar R (2004) Synthesis of biodiesel in supercritical fluids. Fuel 83:2029–2033
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energy Rev 14:217–232
Mata TM, Cardoso N, Ornelas M, Neves S, Caetano NS (2010) Sustainable production of biodiesel from tallow, lard and poultry and its quality evaluation. In: Buratti SS (ed) Chemical engineering transactions, vol 19. AIDIC Servizi Srl, pp 13–18
Melero JA, Clavero MM, Calleja G, Miravalles AGR, Galindo T (2010) Production of biofuels via the catalytic cracking of mixtures of crude vegetable oils and nonedible animal fats with vacuum gas oil. Energy Fuel 24:707–717
Mittelbach M, Pelkmans L, Rice B (2000) Waste oils and fats as biodiesel feedstocks: an assessment of their potential in the EU, ALTENER Program, NTB-NETT Phase IV, Task 4 Final Report, Teagasc, Agriculture and Food Development Authority, Crops Research Centre, Oak Park, Carlow, Ireland
Mondala A, Liang K, Toghiani H, Hernandez R, French T (2009) Biodiesel production by in situ transesterification of municipal primary and secondary sludges. Bioresour Technol 100:1203–1210
NBB (2010) Biodiesel—America’s First Advanced Biofuel, National Biodiesel Board (NBB). http://www.biodiesel.org/tools/calculator/default.aspx?AspxAutoDetectCookieSupport=1
Nebel BA, Mittelbach M (2006) Biodiesel from extracted fat out of meat and bone meal. Eur J Lipid Sci Technol 108:398–403
Noureddini H, Zhu D (1997) Kinetics of transesterification of soybean oil. J Am Oil Chem Soc 74(11):1457–1463
Oliveira ACM, Bechtel PJ (2005) Lipid composition of Alaska pink salmon (Oncorhynchus gorbuscha) and Alaska walleye pollock (Theragra chalcogramma) byproducts. J Aquat Food Prod Technol 14(1):73–91
Peng B-X, Shu Q, Wang J-F, Wang G-R, Wang D-Z, Han M-H (2008) Biodiesel production from waste oil feedstocks by solid acid catalysis. Process Saf Environ Protect 86:441–447
Perin G, Álvaro G, Westphal E, Viana LH, Jacob RG, Lenardão EJ, D’Oca MGM (2008) Transesterification of castor oil assisted by microwave irradiation. Fuel 87:2838–2841
Piu KC (2001) Study on a biodiesel fuel produced from restaurant waste animal fats. Master Thesis, University of Hong Kong
Predojevic ZJ (2008) The production of biodiesel from waste frying oils: a comparison of different purification steps. Fuel 87:3522–3528
Ranganathan SV, Narasimhan SL, Muthukumar K (2008) An overview of enzymatic production of biodiesel. Bioresour Technol 99:3975–3981
Refaat AA, El Sheltawy ST, Sadek KU (2008) Optimum reaction time, performance and exhaust emissions of biodiesel produced by microwave irradiation. Int J Environ Sci Technol 5(3):315–322
Rice B, Frohlich A, Leonard R, Korbitz W (1997) Bio-diesel production based on waste cooking oil: promotion of the establishment of an industry in Ireland, Final Report, ALTENER CONTRACT No. XVII/4.1030/AL/77/95/IRL
Robertson GP, Dale VH, Doering OC, Hamburg SP, Melillo JM, Wander MM, Parton WJ, Adler PR, Barney JN, Cruse RM, Duke CS, Fearnside PM, Follett RF, Gibbs HK, Goldemberg J, Mladenoff DJ, Ojima D, Palmer MW, Sharpley A, Wallace L, Weathers KC, Wiens JA, Wilhelm WW (2008) Sustainable biofuels redux. Science 322(5898):49–50
Sabudak T, Yildiz M (2010) Biodiesel production from waste frying oils and its quality control. Waste Manag 30:799–803
Sawangkeaw R, Bunyakiat K, Ngamprasertsith S (2007) Effect of co-solvents on production of biodiesel via transesterification in supercritical methanol. Green Chem 9(6):679–685
SCE (2007) A feasibility study for fish oil biodiesel production. Final Report prepared by Sustainable community enterprises (SCE) for Clayoquot Biosphere Trust, Vancouver Island, BC, Canada, November
Shaw JF, Chang SW, Lin SC, Wu TT, Ju HY, Akoh CC, Chang RH, Shieh CJ (2008) Continuous enzymatic synthesis of biodiesel with Novozym 435. Energy Fuel 22:840–844
Shimada Y, Watanabe Y, Sugihara A, Tominaga Y (2002) Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J Mol Catal B: Enzym 17:133–142
Siler-Marinkovic S, Tomasevic A (1998) Transesterification of sunflower oil in situ. Fuel 77(12):1389–1391
Soetaert W, Vandamme EJ (2009) Biofuels. Wiley, Chichester
Suwannakarn K (2008) Biodiesel production from high free fatty acid content feedstocks. PhD Thesis in Chemical Engineering, Clemson University
Tashtoush GM, Al-Widyan MY, Al-Jarrah MM (2004) Experimental study on evaluation and optimization of conversion of waste animal fat into biodiesel. Energy Convers Manage 45:2697–2711
Twaiq FAA, Mohamad AR, Bhatia S (2004) Performance of composite catalysts in palm oil cracking for the production of liquid fuels and chemicals. Fuel Process Technol 85:1283–1300
Van Gerpen J (2005) Biodiesel processing and production. Fuel Process Technol 86:1097–1107
Van Gerpen JV, Shanks B, Pruszko R, Clements D, Knothe G (2004) Biodiesel Production Technology, National Renewable Energy Laboratory, NREL/SR-510-36244, Colorado, USA
Watanabe Y, Shimada Y, Sugihara A, Tominaga Y (2001) Enzymatic conversion of waste edible oil to biodiesel fuel in a fixed-bed bioreactor. J Am Oil Chem Soc 78(7):703–707
Wu TH, Bechtel PJ (2008) Salmon by-product storage and oil extraction. Food Chem 111(4):868–871
Wyatt VT, Hess MA, Dunn RO, Foglia TA, Haas MJ, Marmer WN (2005) Fuel properties and nitrogen oxide emission levels of biodiesel produced from animal fats. JAOCS 82(8):585–591
Yin J-Z, Xiao M, Song J-B (2008) Biodiesel from soybean oil in supercritical methanol with co-solvent. Energy Convers Manage 49:908–912
Yuan X, Liu J, Zeng G, Shi J, Tong J, Huang G (2008) Optimization of conversion of waste rapeseed oil with high FFA to biodiesel using response surface methodology. Renew Energy 33:1678–1684
Zhang Y, Dubé MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol 89:1–16
Zhang Y, Dubé MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour Technol 90:229–240
Zheng S, Kate M, Dubé MA, McLean DD (2006) Acid-catalyzed production of biodiesel from waste frying oil. Biomass Bioenergy 30:267–272
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mata, T.M., Martins, A.A., Caetano, N.S. (2013). Valorization of Waste Frying Oils and Animal Fats for Biodiesel Production. In: Lee, J. (eds) Advanced Biofuels and Bioproducts. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3348-4_28
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3348-4_28
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-3347-7
Online ISBN: 978-1-4614-3348-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)