Abstract
TNF-ligand and TNF-receptor family members have established essential roles in the regulation of bone remodelling. We showed recently that TWEAK is a novel mediator in a mouse model of inflammatory bone destruction and that osteoblast lineage cells expressed the TWEAK receptor, fibroblast growth factor-inducible gene 14 (Fn14). Subsequent studies have revealed that TWEAK has a number of effects on human primary osteoblasts, including the strong induction of cell proliferation and the inhibition of in vitro mineralisation. Most notably, TWEAK induced the expression of the negative regulator of bone mass, sclerostin. TWEAK and TNF-induced sclerostin expression synergistically and these effects were dependent on extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK) phosphorylation. While sclerostin expression was induced by TWEAK in proliferating cells, there was an inverse relationship between sclerostin expression and the proliferative potential in the responding cells. TWEAK and TNF were found to have divergent effects on other aspects of osteoblast behaviour, including the expression of a number of key genes involved in osteoblastogenesis and osteoblast function. These findings suggest that the roles of TWEAK and TNF need to be considered together in the regulation of physiologic as well as inflammation-driven osteoblast differentiation. In particular, the ways in which these cytokines may synergize or antagonize each other’s activity need to be elucidated further.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Bone remodelling is the critical process, by which skeletal mass and integrity are controlled. This process is disrupted in a number of bone pathologies, such as osteoporosis, and the focal bone loss that occurs in rheumatoid arthritis, around artificial joint prostheses or that induced by osteolytic tumours. A common feature of conditions that result in a net loss of bone is the presence of elevated levels of pro-inflammatory mediators and evidence has accumulated to show that a chronic inflammatory environment is both catabolic for bone and may also suppress bone formation. Here, we focus on the possible anti-anabolic roles in bone of TNFα and TNF-like weak inducer of apoptosis (TWEAK).
TWEAK
TWEAK, a member of the TNF ligand superfamily designated TNFSF12 [1], has pleiotropic effects, which include induction of pro-inflammatory mediators in a number of target cell types, including fibroblasts and synoviocytes obtained from RA and advanced OA patient tissues [2]. The receptor for TWEAK, fibroblast growth factor-inducible gene-14 (Fn14/TNFRSF12/TWEAK R), is widely expressed [3] and is up-regulated in the context of tissue injury and inflammatory responses [4–7]. Ligation of TWEAK to Fn14 has been shown to activate a limited set of signalling pathways involved in cell proliferation and differentiation [8], including NFκB activation, which is associated with its pro-inflammatory effects [3, 9]. TWEAK has also been shown to activate the mitogen-activated protein kinases (MAPK), JNK [10], ERK [10, 11] and p38 MAPK [5].
A Role for TNF Family Members in Bone Remodeling
The TNF family members and their cognate receptors, RANKL/RANK/OPG and TNF/TNFR1, have central and well-established actions in bone remodelling [12]. Physiologically, RANKL expression by osteoblastic stromal cells mediates osteoclast formation by binding to RANK expressed by osteoclast precursors. Evidence suggests that RANKL and TNF are together involved in inflammatory bone remodelling [13–15]. Although the mechanisms remain to be fully elucidated, TNF can both enhance RANKL expression and sensitize preosteoclasts to RANKL [12]. TNF expression is markedly up-regulated in peri-prosthetic osteolysis [16], in association with polyethylene (PE) particles produced by wear of the prosthesis [17]. We [18] and others [19] have reported a significant role for TWEAK in the inflammatory bone remodelling seen in the mouse CIA model of rheumatoid arthritis. Serum TWEAK was elevated in CIA mice [18] and a neutralising TWEAK antibody significantly reduced the disease severity. Together, the data suggest that TWEAK may play roles in both joint inflammation and tissue damage in the context of RA. The extent to which TWEAK and TNF, also a known mediator of joint pathology in inflammation-driven bone remodelling, each contribute to the pathogenesis of CIA remains an open question. We have reported that human osteoblasts express Fn14 and that TWEAK exposure inhibited their expression of the key osteoblast gene, osteocalcin [18, 20], implying a role for TWEAK in osteogenesis. Ando and colleagues [11] reported that an initial effect of TWEAK on the mouse osteoblast cell line, MC3T3-E1, is to induce RANTES production and RANKL expression. The work described below suggests that TWEAK may contribute to physiologic human osteoblast function and/or their differentiation and so may be a key regulator of bone remodelling.
Human Osteoblasts and Osteoblast-Like Cell Lines Express Fn14 and TWEAK
We have found that all human primary osteoblast [20] and osteoblast-like cell lines (Fig. 34.1a–c) tested express high basal levels of cell surface Fn14. Human osteoblasts also express intracellular TWEAK, suggesting that TWEAK could have an autocrine role in osteoblast activity [20]. In addition, TWEAK mRNA levels may be regulated in an inflammatory milieu, since exposure of human osteoblasts to TNF and IL-1β decreased the expression of TWEAK mRNA in this cell type (Fig. 34.1d).
Human osteoblast expression of Fn14 and TWEAK. All human osteoblastic-like cell lines examined expressed high levels of Fn14, including (a) SAOS2, (b) HOS and (c) MG63 cells. Immunofluorescence and flow cytometry were performed essentially as described previously [20]. (d) TWEAK expression in NHBC was regulated by a 48-h incubation with either recombinant human TNFα or IL-1β (R&D Systems). Real-time RT-PCR was performed as described previously [20]. Oligonucleotide primers designed in-house to amplify human TWEAK were forward primer: 5'-ATCGCTGTCCGCCCAGGAGC-3' and reverse primer 5'-CTGTCTGGGGATTCAGTTCCG-3', which amplify a 86-bp product. Data shown are means of triplicate reactions ± SD, and are representative of two independent experiments. Asterisks denote significant difference compared to untreated (UT) (p < 0.01)
TWEAK Inhibits In Vitro Mineralisation and Antagonizes the Osteogenic Effect of TNF
The ability to form a mineralised matrix over time is a key functional indicator of osteoblast activity and osteogenesis. We have shown that TWEAK inhibits in vitro mineralisation by human osteoblasts and that this inhibitory effect could be reversed by concomitant incubation with a TWEAK neutralizing antibody. Conversely, TNF promoted in vitro mineralisation by human osteoblasts. Interestingly, TWEAK antagonized this effect in a dose-dependent fashion [20]. These results suggest that TWEAK is a negative regulator of osteoblast differentiation and osteogenesis.
Effect of TWEAK and TNFα on Osteoblast Proliferation
Osteoblast proliferation is another important determinant of the osteogenic effect and is inversely related to osteoblast maturation. We have described the use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to measure proliferation of human osteoblasts [21, 22]. CFSE-labelled human osteoblasts exhibited enhanced proliferation in response to TWEAK, showing reduced numbers of cells in the parental population and increased numbers of cells having three or more cell divisions. TNFα is also mitogenic for NHBC and this effect is enhanced in the presence of TWEAK, to the extent seen with TWEAK alone [20].
Effect of TWEAK and TNF on Osteoblast Osteogenic Gene Transcription
We have also investigated the effects of TNF and TWEAK on the expression of a number of osteoblast-associated genes under two sets of conditions, viz. basal conditions for 3 days and conditions permissive of mineralization (the latter containing supplemented phosphate levels) over 3 weeks. Under basal conditions, the osteoblast transcription factors, RUNX2 and osterix, together govern osteoblast lineage commitment and their subsequent differentiation [23]. We found that TWEAK significantly inhibits RUNX2 mRNA expression, in the co-presence or absence of TNF, although TNF alone had no discernible effect on RUNX2 levels [20]. TWEAK dose responsively increased osterix mRNA levels by up to ninefold, consistent with it holding osteoblasts at an immature stage of differentiation. In contrast, TNF alone had no apparent effect on osterix expression, the combined effect of TWEAK/TNF on osterix being similar to the effect of TWEAK alone [20]. These data implicate TWEAK as an important controller of osteoblast differentiation. The effect of TWEAK exposure on RUNX2 is consistent with the emerging negative role of RUNX2 in controlling osteoblast proliferation [24, 25]. Also consistent with the effect of TWEAK on osteoblast proliferation is the stimulatory effect on osterix expression, which has been shown itself to promote cell proliferation and decrease expression of osteoblast genes such as osteocalcin and alkaline phosphatase [26]. TWEAK dose-dependently down-regulated the expression of genes associated with osteoblast differentiation and mineralisation, including osteocalcin, BSP1 [20], alkaline phosphatase and osteopontin (Atkins, unpublished data). TNF had qualitatively similar effects on these genes, although quantitatively it varied from considerably more potent than TWEAK (e.g. on the osteocalcin gene) to approximately equipotent with respect to other genes (e.g. BSP1) [20].
Under conditions permissive of osteoblast differentiation and in vitro mineralization, we found that, while osteogenic genes such as OCN, BSP1 and OPN were more potently down-regulated by TNF in short-term dose–response studies compared with TWEAK, a different pattern of gene responsiveness emerged during a 3-week differentiation period. Early strong suppression of OCN transcription by TNF, for example, was followed by partial release from this suppression, consistent with our finding of an overall positive effect of TNFα on mineral apposition by human osteoblasts (Fig. 34.2a). TWEAK, on the other hand, suppressed OCN transcription throughout the culture period (Fig. 34.2a), in fact antagonizing the TNF effect on OCN transcription. Similarly, both TWEAK and TNF suppressed BSP1 mRNA expression, a gene associated with mineral deposition, in the early phase of the mineralising cultures. However, expression was later induced by TNF to a level above that seen in control cultures, again consistent with an osteogenic effect of TNF, but this was not seen in cultures exposed to either TWEAK or TWEAK/TNF (Fig. 34.2b). Thus, while the short-term effects of TWEAK resemble those of TNF, these cytokines have divergent effects on osteoblast differentiation, with TWEAK exerting the more dominant inhibitory effect.
Effect on osteogenic gene expression as a function of time of recombinant human (rh) TWEAK (50 ng/ml) [18], rhTNFα (1 ng/ml; R&D Systems, MN, USA) or a combination of both cytokines. Human osteoblasts were cultured for up to 3 weeks under mineralising conditions and analysed at the times indicated by real-time RT-PCR for (a) OCN and (b) BSP1 using a published protocol [20]. Significant differences to control (untreated) levels are indicated by a for TWEAK, b for TNF and c for the combination treatment (p < 0.05). Data shown are normalised to GAPDH mRNA levels and are means ± SD of triplicate reactions. Similar results were obtained in two independent experiments
Interaction Between TWEAK and TNF
TNF ligand family members are capable of modulating each others’ activities. For example, TNF synergizes with RANKL in the induction of osteoclast formation [16, 27]. Given that the microenvironment encountered by cells during physiologic or pathologic bone remodelling, for example in RA, consists of a complex cytokine milieu, such interactions are likely highly relevant and important. For example, we recently reported that TWEAK is a mediator of joint erosion in the mouse CIA model, a model in which TNF is also expressed and is implicated in the mechanism [18]. Our data indicate that TWEAK and TNF modulate each other’s activities with respect to human osteoblast behaviour, and the functional outcome likely depends on the relative expression of each cytokine and their respective receptors. It is also likely that there are species-specific effects of TWEAK, as neither TWEAK nor TNF caused detectable phosphorylation of p38 MAPK, or activation of the Akt survival pathway, in human osteoblasts in contrast to effects of TWEAK in mouse MC3T3-E1 osteoblast-like cells [11].
The Wnt Signalling Pathway and Inhibitors: Sclerostin
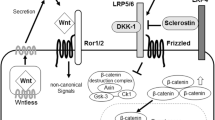
In investigating the mechanisms for the inhibitory effects of TWEAK on osteoblast differentiation and function, it is important to consider the possibility of interaction with a central regulator of this process, the Wnt signalling pathways. The canonical Wnt-signalling pathway is the best described of these [28, 29]. In this pathway, Wnt ligands bind to frizzled (Fzd) and LRP5/6 co-receptors on target cells, preventing the proteosomal degradation of β-catenin and promoting the formation of transcription complexes with TCF/LEF transcription factors, resulting in the downstream transcription of osteogenesis-related genes. Several inhibitors of the Wnt pathway have been identified, including Dikkopf 1 (DKK1), secreted Frizzled-related protein (sFRP) and sclerostin [28]. It is noteworthy that impaired osteoblast function on bone surfaces adjacent to sites of inflammation, in a murine model of rheumatoid arthritis, was associated with up-regulated expression of a number of Wnt inhibitors, including DKK1 and sFRP1 [30]. Sclerostin is the product of the SOST gene, mutations in which cause conditions with high bone mass, and is a key negative regulator of bone formation [29, 31, 28]. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength [32]. A major activity of sclerostin appears to be the inhibition of Wnt/β-catenin signalling [33].
TWEAK Alone and in Combination with TNF Induce Sclerostin Expression
We have reported that TWEAK can induce the expression, at both the mRNA and protein levels, of sclerostin in a dose-dependent fashion [20]. Sclerostin mRNA levels remained elevated relative to control conditions throughout the mineralisation period, increasing further towards the end of the 3-week differentiation period. TNF by itself had a mild effect but combined with TWEAK to further increase sclerostin expression. The induction of sclerostin transcription by TWEAK and TWEAK/TNF was highly dependent on JNK and ERK1/2 phosphorylation [20]. Sclerostin expression in normal bone is virtually restricted to mature osteocytes embedded in mineralised bone, with some expression by osteoblasts [31]. Our unpublished observations concur with this, that sclerostin expression normally coincides with in vitro mineralisation by NHBC (Atkins et al., unpublished), and in some cases where mineralisation is increased, for example by the anabolic drug, strontium ranelate [34], sclerostin levels also increase both as a marker for osteocytes arising in the cultures and in an apparent bid to regulate the anabolic effect. However, the expression pattern of sclerostin in vivo has not been tested under inflammatory conditions. It is possible that less mature osteoblasts may be induced to express sclerostin under inflammatory conditions. Notably, while TWEAK induced proliferating cells to express sclerostin, as judged by their expression of the nuclear proliferation antigen Ki67 [20], there was an inverse relationship between intracellular sclerostin expression and the proliferative potential of primary osteoblasts following TWEAK treatment (Fig. 34.3). It is possible that by inducing sclerostin expression TWEAK may cause premature differentiation into a mature osteocyte-like phenotype, thereby bypassing the mineral apposition phase of osteoblast differentiation [35] and explaining the observed inhibitory effect on mineralisation [20]. This possibility is the subject of ongoing research.
Sclerostin (SCL) expression is inversely related to cell proliferation. NHBC were labelled with CFSE and cultured for 3 days in the presence or absence of recombinant human TWEAK (50 ng/ml), as described [20]. Cells were analysed by flow cytometry for intracellular expression of sclerostin by a published method [20], using a commercially available monoclonal antibody (MAb) against human sclerostin (R&D Systems) or an isotype-matched negative control MAb. Data were analysed using Modfit™ software (Verity, NJ, USA). (a) Cells were gated on low, mid and high levels of expression of sclerostin, the ‘low’ gate completely overlapping the level of staining generated by the negative control MAb. (b) Cell divisions were calculated as described [20], the proportion of cells having undergone successive divisions represented by coloured peaks, with the dark blue peak representing undivided cells and the light blue peak the cells that had undergone four divisions. Even though TWEAK induces sclerostin expression [20], cells expressing high levels (SCLHIGH) proliferated the least while the cells expressing low levels of intracellular sclerostin (SCLLOW) displayed the highest proliferative potential
Our preliminary data indicated that the exposure of human osteoblasts to recombinant human sclerostin has remarkably similar effects to treatment with TWEAK and TWEAK/TNF, with respect to the suppression of osteocalcin and RUNX2 expression [20]. We are currently exploring in greater detail the involvement of sclerostin in TWEAK-mediated effects on osteoblasts, using human cell line models. Notably, blockage of sclerostin activity is currently being explored as a novel treatment option for osteoporosis [36]. A recent report [37] identified TNF-mediated induction of DKK1 expression in a mouse model of inflammatory arthritis and in human RA.
Concluding Remarks
Taken together, these data provide a number of potential mechanisms for the anti-anabolic effects of an inflammatory environment in bone. The induction of expression of inhibitors of the Wnt and/or BMP signalling pathways is a novel potential mechanism, by which TWEAK alone, or in concert with TNF, might regulate physiologic osteoblast differentiation and mineralisation, and suppress these processes in chronic inflammatory disease states. In an inflammatory setting, TWEAK or TWEAK/TNF may act to regulate osteoblast proliferation, inhibit the osteogenic activity of osteoblasts and perhaps also promote the formation and activity of osteoclasts, as depicted in (Fig. 34.4). TNF has been shown to both directly and indirectly activate osteoclast activity [12, 27]. A single report has implicated TWEAK as a direct regulator of osteoclast activity in the absence of evidence for Fn14 expression [38]. This has yet to be confirmed by others, and evidence from our laboratories instead implies that the effect is indirect, via effects on osteoblastic stromal cells (Atkins, unpublished data). Together, these observations give new insight into the mechanism of physiologic bone remodelling and the bone loss and lack of repair that are observed in a number of clinically important bone loss pathologies, including osteoporosis and RA.
Cartoon depicting the anti-anabolic actions of TWEAK in bone remodelling, here divided into the phases of bone resorption, osteoblast proliferation and osteoblast differentiation accompanying bone formation, as inferred from the work described in this manuscript and that reported by others and discussed in the text. In an inflammatory environment, TWEAK may act (solid arrows) on cells of the osteoblast lineage to stimulate osteoblast proliferation and also produce inhibitors of bone formation, including sclerostin (curved arrows), which acts back on bone forming osteoblasts to limit both osteoblast differentiation and the production of new bone. TNFα also has mitogenic effects on osteoblasts and induces the production of sclerostin (dashed arrows) but the overall effect is positive on bone formation perhaps because of the transient nature of these effects. TNFα together with TWEAK modulate each other’s activity and the net effect would depend on their relative abundance. TWEAK may also activate bone resorption by promoting the formation and activity of osteoclasts, as has been reported for TNFα
References
Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL (1997) TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem;272(51):32401–32410
Chicheportiche Y, Chicheportiche R, Sizing I, Thompson J, Benjamin CB, Ambrose C, Dayer JM (2002) Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res 4(2):126–133
Wiley SR, Winkles JA (2003) TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev 14(3–4):241–249
Feng SLY, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, Peifley KA, Winkles JA (2000) The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol 156(4):1253–1261
Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich PY (2000) TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia 32(1):102–107
Yepes M, Brown SAN, Moore EG, Smith EP, Lawrence DA, Winkles JA (2005) A soluble fn14-fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia 1. Am J Pathol 166(2):511–520
Campbell S, Michaelson J, Burkly L, Putterman C (2004) The role of TWEAK/Fn14 IN the pathogenesis of inflammation and systemic autoimmunity. Front Biosci 9:2273–2284
Meighan-Mantha RL, Hsu DK, Guo Y, Brown SA, Feng SL, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, Richards CM, Winkles JA (1999) The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem 274(46):33166–33176
Brown SA, Richards CM, Hanscom HN, Feng SL, Winkles JA (2003) The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J 371(Pt 2):395–403
Donohue PJ, Richards CM, Brown SA, Hanscom HN, Buschman J, Thangada S, Hla T, Williams MS, Winkles JA (2003) TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler Thromb Vasc Biol 23(4):594–600
Ando T, Ichikawa J, Wako M, Hatsushika K, Watanabe Y, Sakuma M, Tasaka K, Ogawa H, Hamada Y, Yagita H, Nakao A (2006) TWEAK/Fn14 interaction regulates RANTES production, BMP-2-induced differentiation, and RANKL expression in mouse osteoblastic MC3T3-E1 cells. Arthritis Res Ther 8(5):R146
Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y (2004) IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 15(1):49–60
Schett G, Redlich K, Hayer S, Zwerina J, Bolon B, Dunstan C, Gortz B, Schulz A, Bergmeister H, Kollias G, Steiner G, Smolen JS (2003) Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum 48(7):2042–2051
Crotti TN, Smith MD, Findlay DM, Zreiqat H, Ahern MJ, Weedon H, Hatzinikolous G, Capone M, Holding C, Haynes DR (2004) Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials 25(4):565–573
Crotti TN, Ahern MJ, Lange K, Weedon H, Coleman M, Roberts-Thomson PJ, Haynes DR, Smith MD (2003) Variability of RANKL and osteoprotegerin staining in synovial tissue from patients with active rheumatoid arthritis: quantification using color video image analysis. J Rheumatol 30(11):2319–2324
Holding CA, Findlay DM, Stamenkov R, Neale SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ, Howie DW, Haynes DR (2006) The correlation of RANK, RANKL and TNFalpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials 27(30):5212–5219
Howie DW, Neale SD, Stamenkov R, McGee MA, Taylor DJ, Findlay DM (2007) Progression of acetabular periprosthetic osteolytic lesions measured with computed tomography. J Bone Joint Surg Am 89(8):1818–1825
Perper SJ, Browning B, Burkly LC, Weng S, Gao C, Giza K, Su L, Tarilonte L, Crowell T, Rajman L, Runkel L, Scott M, Atkins GJ, Findlay DM, Zheng TS, Hess H (2006) TWEAK is a novel arthritogenic mediator. J Immunol 177(4):2610–2620
Kamata K, Kamijo S, Nakajima A, Koyanagi A, Kurosawa H, Yagita H, Okumura K (2006) Involvement of TNF-like weak inducer of apoptosis in the pathogenesis of collagen-induced arthritis. J Immunol 177(9):6433–6439
Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR, Fazzalari NL, Evdokiou A, Atkins GJ (2009) Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFalpha induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Miner Res 24(8):1434–1449
Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O’Loughlin P D, Morris HA (2007) Metabolism of vitamin D(3) in human osteoblasts: Evidence for autocrine and paracrine activities of 1alpha,25-dihydroxyvitamin D(3). Bone 40(6):1517–1528
Lyons AB, Parish CR (1994) Determination of lymphocyte division by flow cytometry. J Immunol Methods 171(1):131–137
Komori T (2006) Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99(5):1233–1239
Galindo M, Pratap J, Young DW, Hovhannisyan H, Im HJ, Choi JY, Lian JB, Stein JL, Stein GS, van Wijnen AJ (2005) The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J Biol Chem 280(21):20274–20285
Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi JY, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen AJ (2003) Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 63(17):5357–5362
Kim YJ, Kim HN, Park EK, Lee BH, Ryoo HM, Kim SY, Kim IS, Stein JL, Lian JB, Stein GS, van Wijnen AJ, Choi JY (2006) The bone-related Zn finger transcription factor Osterix promotes proliferation of mesenchymal cells. Gene 366(1):145–151
Boyce BF, Li P, Yao Z, Zhang Q, Badell IR, Schwarz EM, O’Keefe RJ, Xing L (2005) TNF-alpha and pathologic bone resorption. Keio J Med 54(3):127–131
Baron R, Rawadi G, Roman-Roman S (2006) Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol 76:103–127
Ott SM (2005) Sclerostin and Wnt signaling–the pathway to bone strength. J Clin Endocrinol Metab 90(12):6741–6743
Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, Burr DB, Gravallese EM (2009) Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res 24(9):1572–1585
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. Faseb J 19(13):1842–1844
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23(6):860–869
van Bezooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperien M, Quax PH, Vrieling H, Papapoulos SE, ten Dijke P, Lowik CW (2007) Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res 22(1):19–28
Atkins GJ, Welldon KJ, Halbout P, Findlay DM (2009) Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos Int 20(4):653–664
Barragan-Adjemian C, Nicolella D, Dusevich V, Dallas MR, Eick JD, Bonewald LF (2006) Mechanism by which MLO-A5 late osteoblasts/early osteocytes mineralize in culture: similarities with mineralization of lamellar bone. Calcif Tissue Int 79(5):340–353
Chan A, van Bezooijen RL, Lowik CW (2007) A new paradigm in the treatment of osteoporosis: Wnt pathway proteins and their antagonists. Curr Opin Investig Drugs 8(4):293–298
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163
Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T (2003) TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR. Evidence for a second TWEAK receptor. J Biol Chem 278(34):32317–32323
Acknowledgments
The authors wish to acknowledge the contributions to this work by the co-authors of our recent publication [20]. We continue to be extremely grateful to the surgeons and nursing staff of the Department of Orthopaedics and Trauma, Royal Adelaide Hospital. This work was supported by the National Health and Medical Research Council of Australia (NHMRC). GJA was supported by a NHMRC R Douglas Wright Fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this paper
Cite this paper
Findlay, D.M., Atkins, G.J. (2011). TWEAK and TNF Regulation of Sclerostin: A Novel Pathway for the Regulation of Bone Remodelling. In: Wallach, D., Kovalenko, A., Feldmann, M. (eds) Advances in TNF Family Research. Advances in Experimental Medicine and Biology, vol 691. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6612-4_34
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6612-4_34
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6611-7
Online ISBN: 978-1-4419-6612-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)