Abstract
Purpose of Review
The mechanisms involved in the TNF-mediated deregulated bone remodeling are little appreciated. This review will discuss and summarize the impact of TNF, Notch, and RBP-J signaling on bone remodeling.
Recent Findings
The integrity of the adult skeleton undergoes constant and dynamic remodeling throughout life to maintain a proper bone homeostasis, which is achieved by the essential tight control of coupling between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. The studies in this field include not only the differentiation and function of osteoblasts and osteoclasts, but also the mechanisms that simultaneously control both cell types during bone remodeling. Chronic inflammation is one of the most evident and common pathological settings that often leads to deregulated bone remodeling. The resounding success of TNF blockade therapy has demonstrated a key role for TNF in inflammation and the pathogenesis of inflammatory bone resorption associated with diseases such as rheumatoid arthritis and periodontitis.
Summary
Recent studies have highlighted the function of Notch and RBP-J signaling in both physiological and TNF-mediated inflammatory bone remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone destruction is a major cause of morbidity and disability in many inflammatory disorders, including rheumatoid arthritis (RA), psoriatic arthritis, periodontitis, and peri-prosthetic loosening [1,2,3,4,5]. Bone loss (osteolysis) significantly reduces the quality of life (QOL) of patients and increases their risk of mortality. Throughout life, bone tissues undergo constant remodeling and this provides a mechanism for adapting the skeleton to changing biomechanical influences and repairing bone damage. Bone remodeling requires a delicate balance between the activities of two major cell types: bone-resorbing osteoclasts and bone-forming osteoblasts/osteocytes. Crosstalk exists between these cells that coordinately couple their activities to ensure that osteoclast-generated resorption lacunae are filled with new bone produced by osteoblasts in order to maintain bone homeostasis during bone remodeling.
Bone remodeling is a complex and highly coordinated process that involves osteoclasts and osteoblasts, the major cellular participants, and diverse coupling factors. The studies in this field include not only the cell differentiation and function for each cell type, but also the mechanisms that simultaneously control both cell types during bone remodeling. Typical physiological coupling factors include local bone matrix-derived growth factors, e.g., transforming growth factor (TGF)-β and insulin-like growth factor (IGF)-1, osteoclast-derived factors, including bone morphogenetic protein (BMP)6, sphingosine-1-phosphate (S1P), Wnt10b and semaphorin-4D (sema4D), parathyroid hormone (PTH), the receptor activator of nuclear factor kappa-B ligand (RANKL)/ osteoprotegerin (OPG) axis, and cell membrane bound molecules ephrinB2/EphB4 [6,7,8,9,10,11,12,13,14]. Dickkopf-1 (DKK1) and sclerostin are two important factors that have been shown to uncouple bone remodeling in RA [15,16,17]. There is still a limited understanding of the mechanisms that impact deregulated bone remodeling in inflammatory conditions.
In pathological conditions, bone remodeling is deregulated, which results in unbalanced bone resorption and formation. Notably, the uncoupling of osteoclasts and osteoblasts in pathological bone remodeling synergizes to alter the rate and extent of bone loss [1, 2]. This is clearly evident in the uncoupled bone remodeling that occurs in RA and periodontitis, in which there is excessive osteoclast formation accompanied by extensive bone resorption but with a virtual absence of bone repair and formation [1, 2, 16]. In contrast, ankylosing spondylitis (AS), another form of inflammatory arthritis, is dominated by local bone formation with few signs of local bone erosions; whereas psoriatic arthritis (PsA) has unique features of increased pathologic bone resorption accompanied by aberrant new bone formation [18]. The underlying molecular mechanisms driving these differences in bone remodeling between these various inflammatory conditions are not well understood. Tumor necrosis factor (TNF) plays an important role in each of these inflammatory diseases; therefore, it is of great interest to elucidate how TNF regulates the differentiation of cell lineages involved in bone remodeling, including both osteoclasts and osteoblasts, and associated coupling factors.
Osteoclastogenesis induced by RANKL contributes to physiological bone development and remodeling throughout life. TNF plays a major role, mostly in synergy with RANKL, in promoting pathologic osteoclastogenesis and bone resorption; however, TNF alone does not effectively induce osteoclast differentiation [4, 5, 19,20,21,22,23,24]. TNF may function in vivo to increase osteoclast precursors [25,26,27,28], as well as indirectly increasing osteoclastogenesis through augmentation of RANK expression on osteoclast precursors and M-CSF and RANKL expression in stromal cells including osteoblasts and synovial fibroblasts [4, 29, 30].
A large body of literature shows that TNF inhibits bone formation through suppressing osteoblast differentiation. It does so, in part, by inhibiting the expression of IGF-1, osterix (OSX), and runt-related transcription factor 2 (Runx2) [31, 32]. Recent studies indicate that TNF promotes bone erosion and inhibits bone formation in rheumatoid arthritis (RA) by demonstrating that TNF induces the production of two potent inhibitors of the Wnt signaling, DKK1 and sclerostin (SOST), that control both osteoclast and osteoblast differentiation [2, 17, 18]. Thus, TNF promotes bone destruction by increasing osteoclastic bone resorption and generally decreasing osteoblastic bone formation.
The impact of TNF on the differentiation of mesenchymal stromal cells (MSC) to osteoblasts remains controversial. Some reports show that TNF inhibits MSC differentiation into osteoblasts via the WW domain containing E3 ubiquitin protein ligase 1 (Wwp1) or Notch signaling in mice expressing human TNF (TNF-Tg) [33, 34•]. Many other studies, however, reveal that TNF can induce osteogenic differentiation of MSC cells via upregulation of Runx2, Osx, osteocalcin (OCN), BMP-2, and alkaline phosphatase (ALP) levels [35,36,37,38,39,40,41]. The distinct roles of TNF in osteogenic differentiation of MSCs are presumably due to the differing cellular stages, the TNF concentration, and exposure time of TNF.
In this review, we will focus on and highlight recent discoveries of the function of Notch and recombination signal binding protein for immunoglobulin Kappa J region (RBP-J) signaling in both physiological and TNF-mediated inflammatory bone remodeling.
TNF and Notch/RBP-J Signaling-Mediated Bone Remodeling
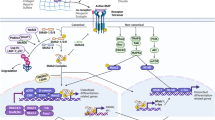
The canonical Notch signaling pathway regulates cell differentiation, proliferation, survival, and cell fate decisions during development and tissue homeostasis [42]. The Notch signaling pathway is evolutionarily conserved including four Notch receptors (Notch 1–4) and five Notch ligands (Jagged1, Jagged2, Delta-like 1 (DLL1), DLL3, and DLL4) in mammalian cells. Binding of the Notch ligands to Notch receptors induces a two-step cleavage of Notch by a disintegrin and metalloprotease (ADAM) family protease and the intracellular γ-secretase complex that releases the Notch intracellular domain (NICD) that translocates to the nucleus. In the model of canonical Notch signaling, RBP-J is bound to specific DNA binding sites ((C/T)GTGGAA) and is thought to act as a transcriptional repressor due to its ability to bind transcriptional corepressors (NCoRs) and histone deacetylases (HDACs) in the absence of NICD. Binding of NICD displaces corepressor complexes and recruits co-activators including mastermind proteins (MAML1–3), which in turn recruit transcription activation complexes in order to induce transcription of Notch target genes, such as Hes and Hey families.
Notch Signaling in Osteoblastic Differentiation and Skeletal Homeostasis
Notch signaling pathway plays a critical role in skeletal development and homeostasis. Skeletal cells mainly express Notch1 and 2 with low levels of Notch3 [43]. Genetic evidence using various genetic mutant mouse models targeting distinct Notch components show a stage-dependent role for Notch signaling pathway in osteoblast lineage differentiation [44]. Notch maintains bone marrow mesenchymal progenitors in an undifferentiated stage by repressing Runx2. Once committed to the osteoblastic lineage, Notch signaling induces osteoblast proliferation but suppresses osteoblast differentiation and maturation [45,46,47,48]. In contrast, activation of Notch1 in osteocytes dramatically increases bone mass by inducing OPG and increasing Wnt/β-catenin signaling via a suppression of DKK1 and SOST [43, 49,50,51]. Thus, activation of Notch signaling pathway functions differently and plays unique roles at distinct stages of osteoblastic lineage differentiation. These studies support a complex but important role for Notch signaling in cell fate and functional decision in osteoblastic lineage, which is tightly regulated temporally and spatially.
Notch Signaling Pathway Functions as a Crosstalk Between Bone Formation and Bone Resorption via Interaction with RANKL-OPG Axis and Wnt/β-Catenin Signaling Pathway
Notch activation in osteoblasts, osteocytes, and stromal cells decreases RANKL/OPG ratio and inhibits M-CSF gene expression, resulting in reduction of their ability to support osteoclast development and bone resorption. Consequently, Notch1-deficiency promotes osteoclastogenesis indirectly by enhancing the ability of osteoblast lineage cells to increase RANKL/OPG expression ratio [43, 51]. Notch also markedly suppresses the expression of Wnt antagonists, sclerostin, and DKK1, leading to an enhanced activation of Wnt/β-catenin signaling and increased osteoblastogenesis and bone formation [50, 51]. Additionally, the activated Wnt/β-catenin suppresses osteoclastogenesis and bone resorption by direct action on osteoclast precursors and also indirectly by increasing OPG expression [52,53,54].
Notch Signaling and TNF-Mediated MSC/Osteoblast Differentiation and Bone Formation
In a TNF-mediated inflammatory setting, TNF stimulation of mesenchymal stem cells (MSCs) induces the activation of canonical Notch signaling in and inhibits their differentiation into osteoblasts. TNF transgenic (TNF-Tg) mouse line 3647 exhibits low levels of TNF that drives chronic and persistent inflammation in mice, representing a good model for studying TNF effects on cell differentiation/function in an inflammatory condition similar to RA. Dr. Lianping Xing’s group took advantage of the CD45−SCA1+CD105+ MSCs isolated from TNF-Tg 3647 mouse line and wild-type littermates, performed RNA-seq followed by genome-wide screening, pathway analysis, and a series of elegant cellular and molecular experiments [34•]. They found an increase in the Notch signaling genes, including Hes1 and Hey1, in the MSCs from TNF-Tg mice or MSCs treated with recombinant TNF. MSCs isolated from TNF-Tg mice show markedly suppressed osteoblast differentiation. Consistently, the Notch inhibitors, DAPT (an inhibitor of γ-secretase) and thapsigargin, reverse the inhibition of osteoblast differentiation in in vitro cultures of TNF-Tg-MSCs and furthermore rescues bone loss, osteoblast numbers, and activity in TNF-Tg mice. TNF increases the expression levels of non-canonical NF-κB proteins p52 and RelB, which interact with NICD on the Notch target genes, such as Hes1, to enhance Notch target gene expression. Thus, Notch signaling contributes to the TNF-mediated inhibition of MSC differentiation into osteoblasts and suppresses bone formation through enhanced non-canonical NF-κB pathway. In the TNF-Tg model, Notch activation is driven by a chronic low-level of TNF signaling; this is different from genetic mouse models but closely mimics chronic inflammatory diseases such as RA. Exposure to different doses of TNF for different time periods may have distinct effects on Notch activity in MSCs. Taken into consideration of the complex role of Notch signaling in osteoblast lineage differentiation in physiological conditions, it would be of interest to investigate the regulation of Notch signaling by TNF and vice versa the impact of Notch signaling on TNF-mediated osteoblast lineage differentiation at various stages. This will help to obtain a broad view and insight into the pathological function of Notch signaling in osteoblast lineage and bone formation and to provide novel/alternative therapeutic option for treatment of inflammatory bone loss.
Notch Signaling and Osteoclastogenesis in Physiological Conditions
The role of Notch in RANKL-induced osteoclastogenesis is complex too. During bone marrow-derived osteoclastogenesis induced by RANKL, Notch1 and 2 are expressed, while the expression levels of Notch3 and 4 are low. Notch ligands, including Dll1, Jagged 1, and Jagged 2 are induced in the osteoclast precursors. The structure of Notch3 is diverged from that of Notch1 and 2, and although the structures of Notch1 and Notch2 are similar and the NICD functions of Notch1 and 2 seem to be equivalent, their biological functions are not redundant [43]. Notch1 and 3 inhibit osteoclastogenesis and bone resorption in vitro and in vivo, while Notch2 has opposite effects. Notch1 and 3 directly inhibit osteoclast precursor proliferation and differentiation as well as the expression of M-CSF receptor, c-Fms [55, 56]. Association of Notch2 and NF-κB protein p65 promotes RANKL-induced osteoclastogenesis [57, 58]. The nonsense mutations and deletions of NOTCH2 in Haidu-Cheney syndrome result in a truncated stably constitutively active Notch2 signaling in these patients [59]. Notch2 constitutive activation in mice enhances in vitro osteoclast differentiation [60]. Thus, the Notch1/Jagged1 axis seems to suppress while the Notch2/Dll1 axis enhances RANKL-induced osteoclast differentiation, presumably attribute to a preferential activation of Notch1 by Jagged 1 and Notch 2 by Dll1. Accumulating evidence shows the cell-type and context-dependent interaction between specific Notch receptors and ligands [61, 62]. For example, a preferential Notch1/Jagged1 interaction has been implicated in the maintenance of bone marrow hematopoietic stem cells [63, 64]. Notch2/Dll1 plays a key role in the marginal zone B cell development [65]. Dll1/4/Notch1/2 is specific to induce Th1 development, whereas Jagged 1/2/Notch1/2 is specific for the differentiation of Th2 or Treg [66,67,68,69]. The mechanisms that regulate this preferential combination between specific ligands and receptors are not clear. It is an enigma that the NICD functions of Notch1 and 2 seem to be equivalent, but their biological functions are not redundant. The underlying molecular mechanisms are far from well understood and additional complexity involves differing transcriptional partners that may contribute to the diverse biological effects of NICDs.
RBP-J Signaling and TNF-Mediated Osteoclast Differentiation and Bone Resorption
TNF is a key pathogenic factor driving inflammation and inflammatory bone resorption. It is well established that TNF plays a key role in RA pathogenesis and is a validated drug target of RA. Mechanisms that restrain TNF-induced osteoclast differentiation and bone resorption are not well understood [70]. We recently identified the transcription factor RBP-J that is activated by TNF stimulation in bone marrow-derived macrophages/osteoclast precursors and dramatically suppresses TNF-induced osteoclastogenesis and bone resorption in vitro and in vivo [71••, 72••].
RBP-J, a nuclear DNA-binding protein, can function as either a transcriptional repressor or activator depending on the partner proteins with which it interacts [42]. Most cell types express a basal level of RBP-J that can be activated by interaction with other proteins. RBP-J is originally identified and best known as a key transcription factor in the canonical Notch signaling pathway [42], where Notch receptor cytoplasmic domains translocate to the nucleus, bind to RBP-J, and induce RBP-J-mediated transcriptional activation of Notch target genes. Now, it is also established that RBP-J is a critical transcriptional regulator in other signaling pathways, such as the TNF [71••], TLR [73, 74], Wnt/β-catenin [75], NF-κB [76, 77], TAK1 [78], and ITAM signaling pathways [72••] and is also targeted by viral proteins [77, 79] and cellular proteins of unknown function [80, 81]. Thus, RBP-J functions as a central transcription factor that receives inputs from several signaling pathways. RBP-J regulates cell differentiation, proliferation, and survival, and plays important roles in cell fate decisions and diverse cellular functions, such as stem cell maintenance, neurogenesis, and lymphocyte development [42, 82]. In myeloid lineage cells, RBP-J has been implicated in inflammatory macrophage activation and function [73, 74, 83], dendritic cell (DC) differentiation, and maintenance of CD8− DC populations [62, 84]. Although many of these functions are related to its role in Notch signaling, RBP-J function is context-dependent, and under inflammatory conditions RBP-J plays a key role in expression of immune response genes not related to canonical Notch signaling [74].
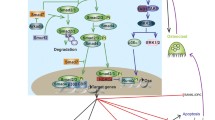
Notably, RBPJ allelic variants have been linked with RA. In parallel with these findings, we found that RBP-J expression level was suppressed in RA patients’ synovial fluid macrophages, which can function as osteoclast precursors, supporting a pathological relevance of RBP-J to RA. However, the role of RBP-J signaling in pathological osteoclastogenesis and bone resorption has not been well understood. RBP-J modestly suppresses RANKL-induced osteoclastogenesis in vitro [71••, 85]. Notch-RBP-J signaling plays a minor role in homeostatic bone resorption based on genetic evidence that mice with a myeloid-specific deletion of RBP-J (RBP-Jf/f LysM-Cre), mice with deletion of Notch 1/2/3, or mice with constitutively active NICD1 expression in the myeloid compartment (NICD1M) do not exhibit significant bone defects under physiological conditions [55, 71••]. In striking contrast, myeloid-specific deletion of RBP-J driven by LysMCre dramatically increases TNF-induced osteoclastogenesis, comparable to that induced by RANKL in control cells, and leads to severe bone destruction in a TNF-induced inflammatory bone resorption model. TNF can induce osteoclast differentiation and inflammatory bone resorption in RBP-J-deficient cells and mice even in the absence of RANK signaling. Thus, the absence of RBP-J reveals the full osteoclastogenic potential of TNF. Complementary evidence shows that forced activation of RBP-J suppresses inflammatory arthritic bone resorption. Mechanistically, RBP-J deficiency results in increased TNF-induced NFATc1 transcription and RNA Pol II occupancy at the NFATc1 gene locus. RBP-J suppresses NFATc1 induction by attenuating c-Fos activation and suppressing induction of Blimp1, thereby preventing downregulation of transcriptional repressor, IRF-8, that blocks osteoclast differentiation [71••] (Fig. 1). These studies shed new insight into a less well-characterized field of negative regulation of osteoclastogenesis, in which RBP-J functions as a critical transcriptional repressor to suppress inflammatory and TNF-mediated osteoclastogenesis and bone resorption.
RBP-J functions as a key negative regulator of osteoclastogenesis and inflammatory bone resorption. RBP-J suppresses NFATc1 induction by attenuating c-Fos activation and suppressing induction of Blimp1, thereby preventing downregulation of transcriptional repressor, IRF-8, that blocks osteoclast differentiation. RBP-J also restrains ITAM signaling and limits crosstalk between RANK or TNFR and ITAM-associated receptors by suppressing PLCγ2 expression and keeping PLCγ2-calcium signaling in a repressed basal state in a TGF-β-dependent pathway
Interestingly, our results revealed that RBP-J predominantly suppresses TNF-induced osteoclastogenesis compared to that induced by RANKL. Subsequent studies found an important clue for the relatively modest regulation of RANKL-induced osteoclastogenesis by RBP-J, which is likely attributed to immunoreceptor tyrosine-based activation motif (ITAM)-mediated calcium signaling in osteoclasts [72••]. ITAM-mediated signaling pathways play important roles in various cellular activities, including immune response and cancer activation. The main ITAM-containing adaptors expressed by myeloid osteoclast precursors are DNAX-activating protein 12 (DAP12) and Fc receptor common γ subunit (FcRγ). These adaptors associate with and mediate signaling by various receptors, including DAP12-associated triggering receptor expressed in myeloid cells 2 (TREM2) and signal-regulatory protein β 1 (SIRPβ1), FcRγ-associated osteoclast-associated receptor (OSCAR), paired immunoglobulin-like receptor-A (PIR-A), and FcRs. Osteoclasts require ITAM-mediated costimulation of RANK signaling for their appropriate differentiation during bone homeostasis [86]. RBP-J deficiency almost completely reverses the defects of this RANK/ITAM-driven osteoclast differentiation program and significantly rescues the osteopetrotic bone phenotype of Dap12 KO or Dap12/Fcrg double knockout (DKO) mice by bypassing the requirement for costimulation of osteoclastogenesis during bone homeostasis. In inflammatory settings, RBP-J limits crosstalk between RANK or TNFR and ITAM-associated receptors and restrains ITAM signaling by suppressing PLCγ2 expression and keeping PLCγ2-calcium signaling in a repressed basal state. RBP-J deficiency enables TNF to induce osteoclast formation and bone resorption in Dap12 KO mice. These data show that RBP-J deficiency allows osteoclast differentiation to occur independently of ITAM-mediated costimulation during homeostatic bone remodeling and inflammatory bone destruction. Thus, RBP-J imposes the requirement for ITAM-mediated costimulation. Our data suggests a model (Fig. 1) where the balance between ITAM-mediated induction and RBP-J-mediated suppression determines the level of basal PLCγ2/calcium signaling, which further determines whether stimulation of osteoclast precursors with factors such as RANKL or TNF induces sufficient calcium signaling to cross the threshold required to effectively activate NFATc1 and osteoclastogenesis. These studies provided an important inhibitory mechanism mediated by RBP-J that can at least partially explain the longstanding enigma in the field, why TNF alone is not able to induce osteoclast differentiation as effectively as the same superfamily member RANKL. The balance between these opposing pathways fine tunes osteoclastogenesis and determines the strength of activating signals required for osteoclastogenesis in physiological and various pathological settings. Accumulating evidence shows that the expression and function of RBP-J can be altered by various environmental cues. For example, we observed that RBPJ expression level was significantly suppressed in synovial fluid macrophages derived from RA patients [72••], indicating that RBP-J expression can be altered in response to environmental cues in pathological states.
Of interest, deficiency of Notch1/2/3 or AMAD10 in osteoclast precursors does not enhance TNF-induced osteoclastogenesis (Zhao B, unpublished data), suggesting that the canonical Notch-mediated osteoclastogenesis does not recapitulate RBP-J-suppressed osteoclastogenesis in response to TNF signaling. The pan-inhibition of canonical Notch signaling may compromise the function of each specific Notch receptor. Although we cannot exclude the possibility that specific individual Notch receptors may function upstream of RBP-J to inhibit TNF-induced osteoclastogenesis, RBP-J may also acts in a Notch-independent manner in this scenario. Furthermore, we did not find that TNF was able to induce osteoclast differentiation in the Hes1 and/or Hey1 deficient osteoclast precursors (Zhao B, unpublished data). This suggests that the targets of RBP-J other than the canonical Notch targets presumably control the negative regulation of TNF-induced osteoclastogenesis. Indeed, modern genome-wide analysis using RBP-J or NICD ChIPseq identifies a distinct set of sites where RBP-J recruits neither NICD nor p300 and binds DNA statically, irrespective of Notch activity [87,88,89]. These findings significantly modify the current paradigm of canonical Notch/RBP-J signaling pathway and provide genomic evidence of Notch-independent RBP-J signaling pathway.
Notch affects many cell types and is involved in primary and metastatic bone tumors, such as leukemias, lymphomas, and osteosarcoma [43, 90]. The important role of Notch signaling in the immune system is well established, for example, the differentiation of lymphoid T and B cell lineages as well as T cell activation. Recently, accumulating evidence show the close interaction between Notch signaling and innate immunity and inflammation [62]. Active Notch signaling has been observed in a variety of inflammatory conditions including RA where Notch receptors and ligands are expressed in synovial tissues [91, 92]. Compelling evidence suggest that Notch pathway is activated in RA and may modulate disease activities [62, 93, 94]. As their extensive expression and diverse functions, it is considered to be challenging to target the canonical Notch receptors/ligands or RBP-J. It would be of great interest and importance to identify the downstream targets of RBP-J in different cell types and context, including those NICD dependent and independent. Appropriate manipulation of Notch and RBP-J activity in inflammatory arthritis mouse models has significant impact on bone but discernable implications on TNF-mediated inflammation [34•, 71••], suggesting a possibility of selective control of Notch and RBP-J activities in inflammatory bone destruction without affecting inflammation induced by TNF. Identification of RBP-J targets induced by TNF would provide selective therapeutic strategy to prevent TNF-mediated bone resorption associated with inflammatory diseases, without significantly affecting physiological bone remodeling.
Conclusions
Similarly as in other systems, Notch effects in skeletal remodeling are highly dependent on cell types, cell differentiation stages, and context. Preferential activation of distinct Notch receptors seems to be a common phenomenon in various cell types and tissues; such as the distinct role of Notch1 and Notch2 in RANKL-induced osteoclast differentiation. Recent studies have highlighted novel functions of the Notch/RBP-J signaling pathway in TNF-mediated inflammatory bone remodeling. TNF-induced canonical Notch activation in mesenchymal stem cells (MSCs) inhibits their differentiation into osteoblasts and suppresses bone formation. RBP-J also plays a prominent inhibitory role in TNF-mediated osteoclastogenesis and inflammatory bone resorption. Interaction of RBP-J and ITAM-calcium signaling pathways fine tunes both physiological and inflammatory bone resorption. Appropriate manipulation of Notch and RBP-J activity in inflammatory arthritis mouse models selectively has significant impact on bone but discernable implications on TNF-mediated inflammation. Mounting evidence, particularly recent genome-wide analysis, show that Notch can signal independently of RBP-J, and RBP-J can be activated by alternative signaling pathways. Thus, identification of targets specific for different NICDs, RBP-J, or both NICD/RBP-J in physiological or TNF-mediated bone remodeling would shed insight into the mechanisms of the diversity of Notch effects and would obtain knowledge of non-canonical Notch- or RBP-J-dependent pathways. These targets would also have potential to provide selective therapeutic strategy to prevent TNF-mediated bone resorption associated with inflammatory diseases, without significantly affecting physiological bone remodeling.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–64.
Goldring SR, Purdue PE, Crotti TN, Shen Z, Flannery MR, Binder NB, Ross FP, McHugh KP. Bone remodelling in inflammatory arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii52–5.
Goldring SR. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology. 2003;42(Suppl 2):ii11–6.
Teitelbaum SL. Osteoclasts; culprits in inflammatory osteolysis. Arthritis research & therapy. 2006;8:201.
Boyce BF, Schwarz EM, Xing L. Osteoclast precursors: cytokine-stimulated immunomodulators of inflammatory bone disease. Curr Opin Rheumatol. 2006;18:427–32.
Hayden JM, Mohan S, Baylink DJ. The insulin-like growth factor system and the coupling of formation to resorption. Bone. 1995;17:93S–8S.
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–65.
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105:20764–9.
Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ. TGF-beta induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology. 2013;154:3745–52.
Matsuzaki E, Hiratsuka S, Hamachi T, Takahashi-Yanaga F, Hashimoto Y, Higashi K, Kobayashi M, Hirofuji T, Hirata M, Maeda K. Sphingosine-1-phosphate promotes the nuclear translocation of beta-catenin and thereby induces osteoprotegerin gene expression in osteoblast-like cell lines. Bone. 2013;55:315–24.
Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–80.
Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21.
Charles JF, Aliprantis AO. Osteoclasts: more than ‘bone eaters’. Trends Mol Med. 2014;20:449–59.
Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–46.
Choi Y, Arron JR, Townsend MJ. Promising bone-related therapeutic targets for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:543–8.
Schett G, Sieper J. Inflammation and repair mechanisms. Clin Exp Rheumatol. 2009;27:S33–5.
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–63.
Schett G. Joint remodelling in inflammatory disease. Ann Rheum Dis. 2007;66(Suppl 3):iii42–4.
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–8.
Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–71.
Schett G, Teitelbaum SL. Osteoclasts and arthritis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:1142–6.
Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–95.
Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–86.
Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–64.
Anandarajah AP, Schwarz EM, Totterman S, Monu J, Feng CY, Shao T, Haas-Smith SA, Ritchlin CT. The effect of etanercept on osteoclast precursor frequency and enhancing bone marrow oedema in patients with psoriatic arthritis. Ann Rheum Dis. 2008;67:296–301.
Yao Z, Li P, Zhang Q, Schwarz EM, Keng P, Arbini A, Boyce BF, Xing L. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem. 2006;281:11846–55.
Li P, Schwarz EM, O'Keefe RJ, Ma L, Looney RJ, Ritchlin CT, Boyce BF, Xing L. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50:265–76.
Zhang Q, Guo R, Schwarz EM, Boyce BF, Xing L. TNF inhibits production of stromal cell-derived factor 1 by bone stromal cells and increases osteoclast precursor mobilization from bone marrow to peripheral blood. Arthritis research & therapy. 2008;10:R37.
Kitaura H, Kimura K, Ishida M, Kohara H, Yoshimatsu M, Takano-Yamamoto T. Immunological reaction in TNF-alpha-mediated osteoclast formation and bone resorption in vitro and in vivo. Clinical & developmental immunology. 2013;2013:181849.
Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511.
Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-alpha on bone homeostasis. Front Immunol. 2014;5:48.
Algate K, Haynes DR, Bartold PM, Crotti TN, Cantley MD. The effects of tumour necrosis factor-alpha on bone cells involved in periodontal alveolar bone loss; osteoclasts, osteoblasts and osteocytes. J Periodontal Res. 2016;51:549–66.
Zhao L, Huang J, Zhang H, Wang Y, Matesic LE, Takahata M, Awad H, Chen D, Xing L. Tumor necrosis factor inhibits mesenchymal stem cell differentiation into osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells. 2011;29:1601–10.
• Zhang H, Hilton MJ, Anolik JH, Welle SL, Zhao C, Yao Z, Li X, Wang Z, Boyce BF, Xing L. NOTCH inhibits osteoblast formation in inflammatory arthritis via noncanonical NF-kappaB. J Clin Invest. 2014;124:3200–14. This study revealed that Notch signaling contributes to the TNF-mediated inhibition of MSC differentiation into osteoblasts and suppresses bone formation through enhanced non-canonical NF-κB pathway.
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J, Yang P. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44:420–7.
Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–90.
Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45:367–76.
Yu RY, Zeng BJ, Liu YS, Zhou YS. [Recombinant human tumor necrosis factor-alpha promotes human adipose-derived stromal cells transforming into osteoblast in vitro]. Beijing da xue xue bao. Yi xue ban = Journal of Peking University. Health sciences. 2012;44:475–80.
Lu Z, Wang G, Dunstan CR, Zreiqat H. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem Cells Dev. 2012;21:2420–9.
Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, Kim CD, Jung JS. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168–77.
Briolay A, Lencel P, Bessueille L, Caverzasio J, Buchet R, Magne D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-alpha in human mesenchymal stem cells. Biochem Biophys Res Commun. 2013;430:1072–7.
Kopan R, Ilagan MX. The canonical notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33.
Zanotti S, Canalis E. Notch signaling and the skeleton. Endocr Rev. 2016;37:223–53.
Regan J, Long F. Notch signaling and bone remodeling. Current osteoporosis reports. 2013;11:126–9.
Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–14.
Tu X, Chen J, Lim J, Karner CM, Lee SY, Heisig J, Wiese C, Surendran K, Kopan R, Gessler M, Long F. Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet. 2012;8:e1002577.
Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305.
Tao J, Chen S, Yang T, Dawson B, Munivez E, Bertin T, Lee B. Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:2175–83.
Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology. 2013;154:623–34.
Canalis E, Bridgewater D, Schilling L, Zanotti S. Canonical Notch activation in osteocytes causes osteopetrosis. Am J Physiol Endocrinol Metab. 2016;310:E171–82.
Canalis E, Adams DJ, Boskey A, Parker K, Kranz L, Zanotti S. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J Biol Chem. 2013;288:25614–25.
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18.
Regard JB, Zhong Z, Williams BO, Yang Y Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harbor Perspect Biol. 2012;4.
Albers J, Keller J, Baranowsky A, Beil FT, Catala-Lehnen P, Schulze J, Amling M, Schinke T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol. 2013;200:537–49.
Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–18.
Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, Kurino T, Hayashi S, Sakano S. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood. 2003;101:2227–34.
Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol. 2008;28:6402–12.
Sekine C, Koyanagi A, Koyama N, Hozumi K, Chiba S, Yagita H. Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes. Arthritis research & therapy. 2012;14:R45.
Canalis E, Zanotti S. Hajdu-Cheney syndrome, a disease associated with NOTCH2 mutations. Current osteoporosis reports. 2016;14:126–31.
Canalis E, Schilling L, Yee SP, Lee SK, Zanotti S. Hajdu Cheney mouse mutants exhibit osteopenia, increased osteoclastogenesis, and bone resorption. J Biol Chem. 2016;291:1538–51.
Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27.
Shang Y, Smith S, Hu X. Role of notch signaling in regulating innate immunity and inflammation in health and disease. Protein & cell. 2016;7:159–74.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6.
Weber JM, Calvi LM. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone. 2010;46:281–5.
Tan JB, Xu K, Cretegny K, Visan I, Yuan JS, Egan SE, Guidos CJ. Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity. 2009;30:254–63.
Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26.
Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–59.
Krawczyk CM, Sun J, Pearce EJ. Th2 differentiation is unaffected by Jagged2 expression on dendritic cells. J Immunol. 2008;180:7931–7.
Vigouroux S, Yvon E, Wagner HJ, Biagi E, Dotti G, Sili U, Lira C, Rooney CM, Brenner MK. Induction of antigen-specific regulatory T cells following overexpression of a Notch ligand by human B lymphocytes. J Virol. 2003;77:10872–80.
Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, Takayanagi H, Kamijo R. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med. 2009;15:1066–71.
•• Zhao B, Grimes SN, Li S, Hu X, Ivashkiv LB. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J Exp Med. 2012;209:319–34. This study for the first time identified RBP-J as a key negative regulator predominantly in TNF-induced osteoclastogenesis and inflammatory bone resorption.
•• Li S, Miller CH, Giannopoulou E, Hu X, Ivashkiv LB, Zhao B. RBP-J imposes a requirement for ITAM-mediated costimulation of osteoclastogenesis. J Clin Invest. 2014;124:5057–73. This study suggested a conceptually new model where ITAMs positively while RBP-J negatively regulates PLCγ-calcium signaling. The balance between these opposing pathways fine tunes osteoclastogenesis and determines the strength of activating signals required for osteoclastogenesis in physiological and various pathological settings.
Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, Outtz H, Kitajewski J, Shi C, Weber S, Saftig P, Li Y, Ozato K, Blobel CP, Ivashkiv LB, Hu X. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13:642–50.
Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji JD, Tateya T, Kang YJ, Han J, Gessler M, Kageyama R, Ivashkiv LB. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008;29:691–703.
Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, Taga T. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28:7427–41.
Plaisance S, Vanden Berghe W, Boone E, Fiers W, Haegeman G. Recombination signal sequence binding protein Jkappa is constitutively bound to the NF-kappaB site of the interleukin-6 promoter and acts as a negative regulatory factor. Mol Cell Biol. 1997;17:3733–43.
Izumiya Y, Izumiya C, Hsia D, Ellison TJ, Luciw PA, Kung HJ. NF-kappaB serves as a cellular sensor of Kaposi’s sarcoma-associated herpesvirus latency and negatively regulates K-Rta by antagonizing the RBP-Jkappa coactivator. J Virol. 2009;83:4435–46.
Swarnkar G, Karuppaiah K, Mbalaviele G, Chen TH, Abu-Amer Y. Osteopetrosis in TAK1-deficient mice owing to defective NF-kappaB and NOTCH signaling. Proc Natl Acad Sci U S A. 2015;112:154–9.
Hayward SD. Viral interactions with the Notch pathway. Semin Cancer Biol. 2004;14:387–96.
Taniguchi Y, Furukawa T, Tun T, Han H, Honjo T. LIM protein KyoT2 negatively regulates transcription by association with the RBP-J DNA-binding protein. Mol Cell Biol. 1998;18:644–54.
Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–30.
Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–74.
Foldi J, Shang Y, Zhao B, Ivashkiv LB, Hu X. RBP-J is required for M2 macrophage polarization in response to chitin and mediates expression of a subset of M2 genes. Protein & cell. 2016;7:201–9.
Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–64.
Ma J, Liu YL, Hu YY, Wei YN, Zhao XC, Dong GY, Qin HY, Ding Y, Han H. Disruption of the transcription factor RBP-J results in osteopenia attributable to attenuated osteoclast differentiation. Mol Biol Rep. 2013;40:2097–105.
Long CL, Humphrey MB. Osteoimmunology: the expanding role of immunoreceptors in osteoclasts and bone remodeling. BoneKEy Rep. 2012;1
Hamidi H, Gustafason D, Pellegrini M, Gasson J. Identification of novel targets of CSL-dependent Notch signaling in hematopoiesis. PLoS One. 2011;6:e20022.
Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by Notch signaling status. Genes Dev. 2013;27:1059–71.
Wang H, Zou J, Zhao B, Johannsen E, Ashworth T, Wong H, Pear WS, Schug J, Blacklow SC, Arnett KL, Bernstein BE, Kieff E, Aster JC. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc Natl Acad Sci U S A. 2011;108:14908–13.
Tao J, Jiang MM, Jiang L, Salvo JS, Zeng HC, Dawson B, Bertin TK, Rao PH, Chen R, Donehower LA, Gannon F, Lee BH. Notch activation as a driver of osteogenic sarcoma. Cancer Cell. 2014;26:390–401.
Yabe Y, Matsumoto T, Tsurumoto T, Shindo H. Immunohistological localization of Notch receptors and their ligands Delta and Jagged in synovial tissues of rheumatoid arthritis. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2005;10:589–94.
Ishii H, Nakazawa M, Yoshino S, Nakamura H, Nishioka K, Nakajima T. Expression of notch homologues in the synovium of rheumatoid arthritis and osteoarthritis patients. Rheumatol Int. 2001;21:10–4.
Ando K, Kanazawa S, Tetsuka T, Ohta S, Jiang X, Tada T, Kobayashi M, Matsui N, Okamoto T. Induction of Notch signaling by tumor necrosis factor in rheumatoid synovial fibroblasts. Oncogene. 2003;22:7796–803.
Okamoto T. The epigenetic alteration of synovial cell gene expression in rheumatoid arthritis and the roles of nuclear factor kappaB and Notch signaling pathways. Mod Rheumatol. 2005;15:79–86.
Acknowledgments
This work is supported by NIH grants AR062047, AR068970, and AR071463.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Baohong Zhao declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Osteoimmunology
Rights and permissions
About this article
Cite this article
Zhao, B. TNF and Bone Remodeling. Curr Osteoporos Rep 15, 126–134 (2017). https://doi.org/10.1007/s11914-017-0358-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-017-0358-z