Abstract
Trees serve countless functions for animals such as supplying food, offering cover from predators and the elements, providing substrates for locomotion, furnishing places to rest and sleep, etc. It is not surprising then, that the densities of many primary consumers such as primates, are related to the plant resources in their environment (Brown 1981), and specifically, to tree variables. These include the density and/or basal area of important tree species such as figs (Ficus spp.), palms (Palmae), and lianas, as well as forest structure indices such as total stem density; tree species richness, diversity, and equitability; stand basal area, mean patch size, and percent canopy cover (Table 11.1).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Trees serve countless functions for animals such as supplying food, offering cover from predators and the elements, providing substrates for locomotion, furnishing places to rest and sleep, etc. It is not surprising then, that the densities of many primary consumers such as primates, are related to the plant resources in their environment (Brown 1981), and specifically, to tree variables. These include the density and/or basal area of important tree species such as figs (Ficus spp.), palms (Palmae), and lianas, as well as forest structure indices such as total stem density; tree species richness, diversity, and equitability; stand basal area, mean patch size, and percent canopy cover (Table 11.1).
There are four endemic and endangered nonhuman primates inhabiting the Mentawai Islands of West Sumatra, Indonesia - Kloss’s gibbon (Hylobates klossii), the Mentawai pig-tailed macaque (Macaca pagensis), the Mentawai Island leaf langur/sureli (Presbytis potenziani), and the simakobu monkey (Simias concolor). Information on the way various factors, including forest vegetation, affect the population densities of these endemic and endangered primates is much needed. However, published data on the feeding behavior of habituated individuals exist for only one of the four species - Kloss’s gibbon (Whitten 1982).
While known food species can undoubtedly be good indicators of primate population numbers (Ficus spp.: Skorupa 1986; Eschweilera spp.: Stevenson 2001), general forest structure variables appear to be just as closely related to primate population patterns (total stem density: Skorupa 1986; canopy cover: Peres 1997). Therefore, in this study, the presence/absence, density, and basal areas of important food resources, as well as forest structure indices were computed from measures taken in nine forests on the Pagai, Mentawai Islands. In addition, primate surveys were conducted in the same forests and species’ densities were calculated. Subsequently, the relationship between primate densities and vegetation measures was examined to determine which variables, if any, were related to primate densities. Several basic hypotheses were tested.
Hypotheses and Predictions
Hylobates klossii: For 20 years, it has been speculated that gibbon densities are limited by Ficus densities (Leighton and Leighton 1983; Johns and Skorupa 1987). Sumatran gibbons feed on figs more than other hylobatids (60-70% of feeding time vs. 35-50%: Palombit 1997) and H. klossii seems to be one of the most avid fig eaters (72% fruit, 23% figs: Whitten 1982). Thus, it is predicted that Kloss gibbons will follow the patterns of other gibbons and have densities that are significantly positively correlated with (1) fig densities and (2) the presence, frequency, density, and basal area of known H. klossii food species.
Macaca pagensis: Macaques are known for their broad diet, high intelligence, extreme adaptability, and their propensity for taking advantage of unpredictable food resources at all levels of the canopy (Richard et al. 1989; Caldecott et al. 1996; Nakayama et al. 1999; Mastripieri 2007). In short, it seems that macaques have no trouble finding food wherever they are. Therefore, it is predicted that Mentawai pig-tailed macaque densities will not vary across sites.
Presbytis potenziani: P. melalophus, the species with which P. potenziani is most often grouped (Wilson and Wilson 1975; Tilson 1976; Brandon-Jones 1993), forages for food in all strata of the canopy (Curtin 1980; MacKinnon and MacKinnon 1980; Bennett and Davies 1994). In addition, many Presbytis species consume large amounts of seeds, as well as young leaf and fruit parts from common tree species (Bennett and Davies 1994). Thus, Presbytis may be able to maintain similar densities in varied forests by feeding on the most common species in those forests. Conversely, some colobine densities fluctuate with forest structure variables such as total stem density (Skorupa 1986), total tree stand basal area (Skorupa 1986; Mbora and Meikle 2004), and height of tree (Nijman 2004). P. potenziani densities may vary with these variables as well.
Simias concolor: Habituated simakobus have been observed spending roughly equal amounts of time feeding on young leaves, fruits, and flowers (Paciulli unpub. data). However, during the Indonesian drought of 1997, simakobus spent approximately 80% of their feeding time consuming keruing flowers (Dipterocarpus haselthii: Paciulli unpub. data). Thus, it seems that keruing flowers serve as a fallback food for simakobus, and it is predicted that their densities will vary with keruing densities. In addition, Simias densities may also vary with forest structure variables such as total stem density (Skorupa 1986), total tree stand basal area (Skorupa 1986; Mbora and Meikle 2004) and height of tree (Nijman 2004), as these affect other colobines.
Methods
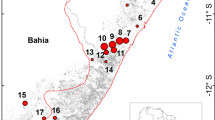
I conducted this study on the two southern Mentawai Islands - North Pagai (2°42′S 100°5′E) and South Pagai (3°00′S 100°20′E) (Fig. 11.1). The Pagai Islands span a length of approximately 110.5 km and a width of about 57.5 km (WWF 1980; Eudey 1987). Annual precipitation ranges from 2,655 to 6,383 mm (Tenaza and Fuentes 1995) and the maximum height above sea level is 368 m (Nelles Verlag 1999). A total of nine dipterocarp or mixed dipterocarp forests were selected for this study. The names of the nine forests are Kilometer 28, Kilometer 60, Kinumbu, Manganjo, Saumanganyak, Simpang G, Area I, Area III, and Area IV.
Map of the Mentawai Islands (WWF 1980) showing the nine areas surveyed on the Pagai Islands
The four primate species inhabiting the Pagai Islands are Kloss’s gibbon (Hylobates klossii), the Mentawai pig-tailed macaque (Macaca pagensis), the Mentawai Island langur/sureli (Presbytis potenziani), and the simakobu monkey (Simias concolor) (Eudey 1987; WWF 1980). H. klossii and P. potenziani are endangered while M. pagensis and S. concolor are critically endangered due to habitat loss, logging, and hunting (IUCN 2009).
I conducted primate and vegetation surveys between May 1999 and July 2000 in the nine forests. I used established line-transect methodology (Burnham et al. 1980; NRC 1981; Defler and Pintor 1985; Janson and Terborgh 1986; Brockelman and Ali 1987; Whitesides et al. 1988; Buckland et al. 1993). In each area, assistants cut three 4 km long transects, 300 m apart (Brockelman and Ali 1987), along cardinal directions. Each transect was surveyed randomly on three separate days during a 9 day period (Sterling and Rakotoarison 1998; Johnson and Overdorff 1999) at a rate of ∼0.9 km/h. The total line length sampled was 324 km.
During the surveys, I recorded all sights and sounds of primates. In addition, I wrote down the angle and distance of the first individual detected, as well as the time and location of the detection. I also noted the number of individuals and their age and sex. For a more detailed description of the methodology see Paciulli (2004).
I collected data on vegetation at 20 places along the primate survey trails. A 25 m by 5 m quadrat was demarcated every 200 m. Within each quadrat, I recorded the tree species, estimated total height, and diameter at breast height (DBH) for each tree ≥3 cm DBH to include more trees than the usual measurement of trees greater than 10 cm DBH would yield. I also gauged canopy coverage at 60 points per site (20 points × 3 trails) by estimating the percent of sky visible directly above the center of each quadrat. The total area I examined in each forest was 7,500 m2 or 0.75 ha, covering a total of 6.75 ha across all nine forests.
Data Analysis
I conducted analyses on primate densities using all detections and Kings’ method (observer-to-animal: King 1929 cited in Leopold 1933; Janson and Terborgh 1986). I used JMP 3.2.2 (SAS Institute 1989-1997) and SPSS 11.0.1 (SPSS 1989-2001) statistical programs for analyses. I used correspondence analysis (Sokal and Rohlf 1995) and hierarchical cluster analysis (using the average linkage method) (Everitt 1980) to identify co-occurring assemblages of trees, on the basis of species presence/absence, frequencies, and basal areas. First, the presence/absence, frequencies, and basal areas of all trees were organized by site and used in three separate correspondence analyses (CA). CA uses a graphical technique to show which rows or columns of tables have similar patterns. In addition, the CA output provides a plot and the coordinates (i.e., values) that were used to position the data points in the plot. The coordinates represent the variation of a single datum point in relation to all other data points in the plot. The three coordinates for each CA datum point were used in hierarchical cluster analyses (HCA). HCA groups species into clusters that have values that are close to each other relative to those of other clusters, with respect to their occurrence in distinct sampling sites.
I used multiple regressions (Sokal and Rohlf 1995) to establish which, if any, of the variables could predict primate densities. Multiple regressions were run on the densities of each species at the nine sites and the six presence/absence clusters, the six frequency clusters, and the six basal area clusters. To determine in which multiple regressions the variables from the other vegetation categories (i.e., the composite vegetation variables, the important tree variables, and the H. klossii food variables), could be included, I used Kendall’s correlations (Sokal and Rohlf 1995). I conducted the correlations to see which variables were correlated with one another and therefore, could not be used as independent variables in the same regression analysis. Furthermore, linear regressions between species’ densities and D. haselthii variables were run independently because this was thought of post-hoc. Significance levels were set at p ≤ 0.05 and tests were two-tailed unless noted.
Results
All four of the primate species were found in all of the forests surveyed in this study. Primate densities, forest structure variables, percent canopy cover, and the densities and basal areas of important tree species are listed in Appendix.
There were no significant relationships between any of the primate densities and the forest structure variables. However, two of the important tree variables predicted one of the species’ densities. When Ficus spp. densities were low, Mentawai Island leaf langur densities were high (r = −0.1390, F = 9.42, p = 0.037). In addition, when liana densities were high, leaf langur densities were high as well (r = 1.0755, F = 35.03, p = 0.004).
In addition, Kloss’s gibbon food variables seemed predictive of two species’ densities and overall primate densities. For instance, when the basal area of known gibbon food species was high, Presbytis densities were high too (r = 1.2155, F = 11.00, p = 0.045). Likewise, when the basal area (r = 0.6148, F = 19.05, p = 0.012) or presence (r = 0.9237, F = 29.50, p = 0.006) of known gibbon food species and genera were high, so were Presbytis densities. In contrast, when the presence of H. klossii food species was high, simakobu densities were low (r = −3.3052, F = 23.25, p = 0.017). Moreover, although primate densities were low when the presence or frequency of gibbon food species were high (r = −3.8941, F = 33.13, p = 0.01; r = −0.1959, F = 16.54, p = 0.027, respectively), primate densities were high when either the basal areas of known gibbon food species were high (r = 3.5982, F = 41.96, p = 0.008), or the presence of gibbon food genera was high (r = 3.2424, F = 43.40, p = 0.007).
Only one cluster variable in the hierarchical cluster analysis significantly predicted a single species’ density. When the presence of Cluster 6 trees was high, Hylobates’ densities were high as well (r = 1.4872, F = 19.41, p = 0.048).
Discussion
Relationship Between Vegetation Variables and Specific Hypotheses and Predictions
Of the four hypotheses and corresponding predictions proposed, only one was supported, that Mentawai pig-tailed macaque densities would not be affected by any of the vegetation and forest structure variables used in this study. In contrast, the three hypotheses regarding the gibbons, leaf langurs, and simakobus were rejected. Upon closer examination, it became apparent that other researchers have found that many vegetation and forest structure indices did not always correlate with primate densities, abundances, and/or biomass.
It was predicted that Kloss gibbon densities would be related to fig densities and the presence, frequency, and basal area of H. klossii food species. Likewise, it was expected that simakobu densities would vary with keruing densities. Neither of these predictions were supported by the data. Although these results were surprising at first, other studies have had similar outcomes. Stevenson (2001) found that indices such as the basal areas of figs were not good predictors of total primate biomass or of any primate guild. He reasoned that Ficus trees often occur at low densities and/or in hemiepiphitic form. As such, it is difficult to get good sample sizes for low-density species. Second, measures such as the presence, density, and/or basal area of trees may miss vital information on plants that grow on other plants, such as lianas (Stevenson 2001). In addition, there are other examples of important foods not being related to the population parameters of the primates that feed on them (leguminous plants: P. badius and C. polykomos: Davies et al. 1999).
Contrary to expectations, indices such as the basal area of Moraceae were not related to gibbon densities either. Stevenson (2001) found the same for the biomass of Alouatta spp. This was unanticipated considering that howlers frequently feed on Moraceae plants (Estrada et al. 1999; Serio-Silva et al. 2002). Likewise, after collecting and analyzing the nutritional content of leaves from numerous tree species at four sites, Chapman et al. (2002a) found that the average protein-to-fiber ratio of species eaten by red colobus (C. badius) did not improve the predictive power of tests for either red colobus biomass or total colobine biomass. This was not unexpected as the mean protein-to-fiber ratios of red colobus food species did not differ significantly from the protein-to-fiber ratios of nonfood species.
At first, it was perplexing to find that there was no relationship between simakobu densities and the presence, frequency, or basal area of Dipterocarpus haselthii. As stated earlier, in a time of a severe drought, simakobus were observed feeding on D. haselthii flowers approximately 80% of the time, every day, for a 2-month period (Paciulli unpub. data). This lack of a relationship between a primate species density and a keystone resource was unexpected. It is, however, not unprecedented. For instance, Stevenson (2001) did not find any significant correlations between the basal areas of suggested keystone resources (e.g., figs, palms) and the abundance of any primate guild (except for a weak effect of the basal area of figs on the biomass of large atelines) (Stevenson 2001). Perhaps one of the reasons for this apparent nonrelationship between primate densities and keystone food resources is the fact that many primates have broad ecological tolerances and can readily “switch” to less preferred resources, when the preferred ones are unavailable (e.g., P. b. rufomitratus switching from young to mature leaves: Mowry et al. 1996). Thus, although a single resource comprises a significant portion of a primates’ daily consumption, it does not necessarily mean that densities will be affected by this one species. For example, although Kloss gibbons on Siberut fed on figs 23% of the time, 77% of the time they ate other foods (Whitten 1982). This may be why low (or high) densities of figs do not appear to have much bearing on Kloss gibbon densities.
Although the case can be made for why the density of a primate species might not be strongly tied to its’ main food item, it is more difficult to explain why a primates’ density would not be strongly affected by all of the foods in its’ diet. More specifically, why do Kloss gibbon densities not vary at all with their foods? One possible reason is that the diets of individual groups of primates fluctuate significantly over time. For instance, Chapman et al. (2002b) found that food intake of the same red colobus (Procolobus badius) groups showed significant and consistent changes over a 4-year period. Not only were different plant species consumed, but the time spent feeding on different types of foods (i.e., leaves vs. fruits) as well as plant parts, varied. In addition, there were dietary differences between groups whose ranges overlapped by as much as 49% (Chapman et al. 2002b). Thus, although Whitten (1982) collected data on H. klossii food intake for approximately 650 h over a 2-year period, a study of one or two demographically changing groups occupying a single habitat during a brief period of time may not adequately represent the overall diet of the species (Chapman et al. 2002b:349). Diet differs significantly over both small spatial, as well as short temporal, scales within a single group, never mind, a species. This may explain why H. klossii foods seemed unrelated to H. klossii densities.
Another result that is difficult to explain is the significant positive relationship between gibbon densities and the presence of trees in Cluster 6. Cluster 6 was comprised of only two tree species - Trichosanthes sp. and Xylopia caudata - neither are known food species of Kloss’s gibbons. Therefore, the significant regression probably is spurious. Notwithstanding, it seems intriguing at the very least to mention that both Trichosanthes spp. and Xylopia spp. have curative value for humans (T. kirilowii: Akihisa et al. 2001; Campbell et al. 2002; Choi et al. 2002; Krishnan et al. 2002 and Xylopia: de Melo et al. 2001).
It was predicted that Mentawai Island leaf langur densities would be unrelated to vegetation and this was not the case. The significant negative relationship between P. potenziani densities and figs is difficult to explain because there have been no published accounts of the feeding habits of habituated Mentawai Island leaf langurs. Although some colobines consume a substantial amount of figs (e.g., Semnopithecus entellus: Hladik 1977; P. b. rufomitratus: Marsh 1986; P. pileata: Stanford 1991), others do not. For example, in some parts of Malaysia, banded leaf monkeys (P. melalophus) rarely eat figs (Lambert 1990). However, it is still unclear why Mentawai Island leaf langur densities would be significantly lower where Ficus spp. densities were high.
It is easier to explain the significant relationship between liana densities and leaf langur densities. Many colobines exploit liane resources (C. polykomos: Dasilva 1994; Oates 1994; Davies et al. 1999; P. melalophus: Bennett and Davies 1994; P. b. rufomitratus: Oates 1994; Mowry et al. 1996; P. rubicunda: Davies 1991). Analysis of protein, fiber, and energy values of foods selected versus items available (but not eaten), suggests that liana preference is related to protein and energy maximization (Dasilva 1994). This makes sense as in some areas, lianas generate young leaves more reliably and abundantly than trees do (Palombit 1997). In addition, legume vine shoots can contain up to 55% protein, whereas immature tree leaves usually contain only about 20% protein (Hladik 1978). Liana leaves also may not be as well defended chemically as tree-leaf shoots (Whitten 1982). It is probably for all of these reasons that some colobines, such as the Hanuman langurs (P. entellus) in southern Nepal, choose a climbing species, Spatholobus parviflorus, as their number one food resource, feeding on the leaves 42.2% of the time (Koenig et al. 1998). The Mentawai Island leaf langurs also may spend a significant proportion of their feeding time on liane species as well, and this may be the reason for the significant relationship between their densities and the densities of lianas.
When the basal area of known gibbon food species was high, overall primate and Presbytis densities were high too. In contrast, when the presence or frequency of gibbon food species was high, primate and simakobu densities were low. These seemingly conflicting results could be explained by the fact that the presence/absence of tree species is not a good predictor of tree size, which is more indicative of the potential resources a tree could provide. This also implies that P. potenziani and the other Pagai primates may be exploiting some of the same resources that Kloss’s gibbons do.
Relationship Between Other Vegetation Variables and Primate Densities
There were no significant relationships between any of the primate densities and the forest structure variables. There could be several reasons for this including; (1) the measures used in this study were too simple, (2) the botanical measures are not related to primate densities and/or have yielded insignificant results in similar studies, (3) other variables are better predictors of primate densities, and/or (4) the area sampled was not large enough.
One possible reason for the composite botanical indices not yielding significant results is that they may have been too crude. For example, Chapman et al. (2002a) found that resource quality was key in understanding determinants of colobine abundance and cautioned that a simple quantification of resource abundance might not be a good indicator of primate abundances (Chapman et al. 2002a). In the present study, finer analyses of resource quality, such as calculating the mean protein-to-fiber ratios of trees, were not undertaken. Collecting primate foods from several sites and analyzing nutritional composition yields important information on associations with primate population parameters (Ganzhorn 1995; Chapman et al. 2002a). However, such practices are both time-consuming (Chapman et al. 2002a) and expensive. In addition, where the foods of as yet unstudied primates are still largely unknown, the task of determining the relationship between single species of plants and primate populations can be daunting.
In addition, although each of the composite vegetation variables has been found to be associated with various primate abundances, densities, and biomasses (see section “Introduction”), each also has been found to be nonassociated with just as many primate population parameters. For example, Stevenson (2001) found that there was no significant relationship between total tree density per hectare and primate biomass. He proposed that one reason for this could be that measures such as density do not reflect size, which is an important determinant of reproductive output.
Likewise, basal area does not always turn out to be a good predictor of primate population sizes. For instance, in his broad analysis of New World sites, Stevenson (2001) found that total basal area per hectare was not correlated with the biomass of any primate guild. He extended his earlier observation that size is an important determinant of reproductive output by adding that other factors such as habitat quality and differences between plant families also affect plant reproduction. However, as Janson and Chapman (1999) point out, even measures of overall plant productivity can sometimes be negatively related to total primate biomass in some communities (Kay et al. 1997).
Species diversity is another less-than-perfect predictor of primate densities. For instance, Tutin (1999) compared fruit diversity and abundance in a forest fragment to a neighboring continuous forest. She found that despite the lower diversity and abundance of fruit in the fragment, the local density of primates in the fragment was equivalent to that of the adjoining continuous forest. Tutin (1999) proposed that the reason for the similar densities was that there were many benefits of living in such a small area (9 ha) such as having exceptional knowledge of the location and quality of food resources, reduced feeding competition between individuals through group fission, lower travel costs, etc.
Perhaps one of the clearest illustrations of how some vegetation indices at a single site can be correlated with some primate species, but not with others, comes from Skorupa (1986). Skorupa (1986) found that the strongest vegetation correlates were unique to each species. For instance, although the abundances of black-and-white colobus (C. guereza) and gray-cheeked mangabeys (C. albigena) were significantly related to total stem density, red colobus (C. badius) abundances were associated with stand basal area, and chimpanzee (P. troglodytes) and L’hoests’ guenon (C. l’hoesti) abundances were correlated with percent canopy cover. In other words, most species’ abundances covaried with a single vegetation variable (Skorupa 1986).
In addition, there may be variables other than those included in the current study that are better predictors of primate population parameters. For example, Peres (1997) found that latitude, total rainfall, and the degree of seasonality were all significantly related to howler (Alouatta spp.) densities. Stevenson (2001) also found that climatic variables were important in predicting total primate biomasses. Although it is not impossible, it is doubtful that variables such as latitude, total rainfall, and degree of seasonality affect Mentawai primate densities. These variables probably vary little across the tiny Mentawai archipelago, not to mention just the two Pagai Islands, which span a total length of approximately 110.5 km and a width of about 57.5 km.
Moreover, other biogeographic factors that affect primate populations such as fragment size were not examined in this study. Estimates of primate densities in southeastern Brazil were on the order of several hundreds to thousands of individuals/species in 20,000 ha size fragments and <50 individuals/species in 200 ha size fragments (Chiarello and de Melo 2001). Forest size could have affected primate densities in this study too.
An additional reason for the lack of significant results between primate densities and the botanical indices could be that the area sampled was not large enough. For instance, Stevenson (2001) found that basal areas reached stable values only after sampling at least 1.5 ha. In this study, 0.75 ha/site were sampled - half the area Stevenson (2001) reported was needed to reach a consistent value. Perhaps sampling a larger area would reflect the true basal areas at each of the sites and in turn, this might be more predictive of primate densities.
Conclusions
For conservation purposes, it is important to identify the general forest and tree indices, as well as the specific tree species that affect primate densities. The results of this study show that overall, none of the forest structure variables were consistently associated with primate densities. However, more meaningful vegetation measures such as liana densities and the plant species and genera consumed by at least one of the four primate species (H. klossii), were related to some primate densities. This makes sense because the forest structure indices were created from the overall data set from each site. In other words, there was no a priori reason to assume that one of the forest structure variables would be related to any one of the Mentawai primate densities, other than the fact that the same measures have been shown to be important indicators of some primate species’ densities in other regions. In contrast, only the plant species and genera compiled for the gibbon food variables and keruing trees (D. haselthii) were selected because they are important plant species for Mentawai primates. Therefore, it is not surprising that H. klossii foods were the strongest predictors of primate densities.
Additional data on the feeding habits of habituated Mentawai primates are needed to reveal the associations between Mentawai primate densities and vegetation variables. Once these are known, logging companies that practice selective logging can be informed of the tree species that are valuable to the Mentawai primates, with the hope that they will be left standing. It also is important to remember that trees are not only used by primates and other animals for food, but for cover from predators and the elements, as substrates for locomotion, and as places to rest and sleep. If all of these factors were taken into account in this and similar studies, then perhaps the relationship between nonhuman primate population parameters and the trees they use (i.e., not just the species on which they feed), would be more conspicuous.
References
Akihisa T, Tokuda H, Ichiishi E, Mukainaka T, Toriumi M, Ukiya M, Yasukawa K, Nishino H (2001) Anti-tumor promoting effects of multiflorane-type triterpenoids and cytotoxic activity of karounidol against human cancer cell lines. Cancer Lett 173:9–14
Arroyo-Rodríguez V, Mandujano S, Benítez-Malvido J, Cuende-Fanton C (2007) The Influence of Large Tree Density on Howler Monkey (Alouatta palliata mexicana) Presence in Very Small Rain Forest Fragments. Biotropica 39:760–766
Bennett EL, Davies AG (1994) Ecology of Asian colobines. In: Davies AG, Oates JF (eds) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge, pp 129–171
Brandon-Jones D (1993) The taxonomic affinities of the Mentawai Islands Sureli, Presbytis potenziani (Bonaparte, 1856) (Mammalia: Primata: Cercopithecidae). Raffles Bull Zool 41:331–357
Brockelman WY, Ali R (1987) Methods of surveying and sampling forest primate populations. In: Marsh CW, Mittermeier RA (eds) Primate conservation in the tropical rain forest. Alan R. Liss, New York
Brown JH (1981) Two decades of homage to Santa Rosalia - Toward a general theory of diversity. Am Zool 21:877–888
Buckland ST, Anderson DR, Burnham KP, Laake JL (1993) Distance sampling: estimating abundance of biological populations. Chapman and Hall, London
Burnham KP, Anderson DR, Laake JL (1980) Estimation of density from line transect sampling of biological populations. Wildl Monogr 72:1–202
Caldecott JO, Feistner ATC, Gadsby EL (1996) A comparison of ecological strategies of pig-tailed macaques, mandrills and drills. In: Fa JE, Lindburg DG (eds) Evolution and ecology of macaque societies. Cambridge University Press, Cambridge, pp 73–97
Campbell MJ, Hamilton B, Shoemaker M, Tagliaferri M, Cohen I, Tripathy D (2002) Antiproliferative activity of Chinese medicinal herbs on breast cancer cells in vitro. Anticancer Res 22:3843–3852
Chapman CA, Chapman LJ, Bjorndal KA, Onderdonk DA (2002a) Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. Int J Primatol 23:283–310
Chapman CA, Chapman LJ, Gillespie TR (2002b) Scale issues in the study of primate foraging: red colobus of Kibale National Park. Am J Phys Anthropol 117:349–363
Chiarello AG, de Melo FR (2001) Primate population densities and sizes in Atlantic forest remnants of Northern Espírito Santo. Braz Int J Primatol 22:379–396
Choi JH, Choi JH, Kim DY, Yoon JH, Youn HY, Yi JB, Rhee HI, Ryu KH, Jung K, Han CK, Kwak WJ, Cho YB (2002) Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthritis Cartilage 10:471–478
Curtin SH (1980) Dusky and banded leaf monkeys. In: Chivers DJ (ed) Malayan forest primates. Plenum, New York, pp 107–145
Dasilva GL (1994) Diet of Colobus polykomos on Tiwai Island: selection of food in relation to its seasonal abundance and nutritional quality. Int J Primatol 15:1–26
Davies AG (1991) Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo. Int J Primatol 12:119–144
Davies AG, Bennett EL, Waterman PG (1988) Food selection by two South-east Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biol J Linn Soc 34:33–56
Davies AG, Oates JF, Dasilva GL (1999) Patterns of frugivory in three West African colobine monkeys. Int J Primatol 20:327–357
De A. Moura AC (2007) Primate group size and abundance in the Caatinga Dry Forest, Northeastern Brazil. Int J Primatol 28:1279–1297
Defler TR, Pintor D (1985) Censusing primates by transect in a forest of known primate density. Int J Primatol 6:243–259
de Melo AC, Cota BB, de Oliveira AB, Braga FC (2001) HPLC quantitation of kaurane diterpenes in Xylopia species. Fitoterapia 72:40–45
Estrada A, Juan-Solano S, Martinez TO, Coates-Estrada R (1999) Feeding and activity patterns of a howler monkey (Alouatta palliata) troop living in a forest fragment at Los Tuxtlas, Mexico. Am J Primatol 48:167–183
Eudey AA (1987) Action Plan For Asian Primate Conservation: 1987–1991. UNEP, IUCN, WWF, Riverside
Everitt BS (1980) Cluster analysis. Halsted Press, New York
Felton AM, Engström LM, Felton A, Knott CD (2003) Orangutan population density, forest structure and fruit availability in hand-logged and unlogged peat swamp forests in West Kalimantan, Indonesia. Biol Conserv 114:91–101
Ganzhorn JU (1995) Low-level forest disturbance effects on primary production, leaf chemistry, and lemur populations. Ecology 76:2084–2096
Ganzhorn JU, Malcomber S, Andrianantoanina O, Goodman SM (1997) Habitat characteristics and lemur species richness in Madagascar. Biotropica 29:331–343
Hladik CM (1977) A comparative study of the feeding strategies of two sympatric species of leaf monkeys: Presbytis senex and Presbytis entellus. In: Clutton-Brock TH (ed) Primate ecology: studies of feeding and ranging behaviour in lemurs, monkeys, and apes. Academic, London, pp 323–353
Hladik A (1978) Phenology of leaf production in rain forest of Gabon: distribution and composition of food for folivores. In: Montgomery GG (ed) The ecology of arboreal folivores. Smithsonian Institution Press, Washington, DC, pp 51–71
IUCN (2009) IUCN Red List of Threatened Species. Version 2009.1. <www.iucnredlist.org>. Downloaded on 15 June 2009
Janson CH, Chapman CA (1999) Resources and primate community structure. In: Fleagle JG, Janson CH, Reed KE (eds) Primate communities. Cambridge University Press, Cambridge, pp 237–267
Janson CH, Terborgh JW (1986) Censando primates en el bosque lluvioso. In: Rios MA (ed) Reporte Manu. Centro Datos para la Conservacion, Lima, Peru, pp 1–48
Johns AD, Skorupa JP (1987) Responses of rain-forest primates to habitat disturbance: a review. Int J Primatol 8:157–191
Johnson SE, Overdorff DJ (1999) Census of brown lemurs (Eulemur fulvus spp.) in Southeastern Madagascar: methods-testing and conservation implications. Am J Primatol 47:51–60
Kay RF, Madden RH, van Schaik C, Higdon D (1997) Primate species richness is determined by plant productivity: implications for conservation. Proc Natl Acad Sci USA 94:13023–13027
Koenig A, Beise J, Chalise MK, Ganzhorn JU (1998) When females should contest for food - Testing hypotheses about resource density, distribution, size, and quality with Hanuman langurs (Presbytis entellus). Behav Ecol Sociobiol 42:225–237
Krishnan R, McDonald KA, Dandekar AM, Jackman AP, Falk B (2002) Expression of recombinant trichosanthin, a ribosome-inactivating protein, in transgenic tobacco. J Biotechnol 97:69–88
Lambert FR (1990) Some notes on fig-eating by arboreal mammals in Malaysia. Primates 31:453–458
Leighton DR, Leighton M (1983) Vertebrate responses to fruiting seasonality within a Bornean rain forest. In: Sutton SL, Whitmore TC, Chadwick AC (eds) Tropical rain forest: ecology and management. Blackwell, Oxford, pp 181–196
Leopold A (1933) Game management. The University of Wisconsin Press, Madison
MacKinnon K, MacKinnon J (1980) Niche differentiation in a primate community. In: Chivers DJ (ed) Malayan forest primates. Plenum, New York, pp 167–190
Marsh CW (1986) A resurvey of Tana primates and their forest habitat. Primate Conserv 7:72–81
Mastripieri D (2007) Macachiavellian intelligence: how rhesus macaques and humans have conquered the world. University of Chicago Press, Chicago
Mbora DNM, Meikle DB (2004) Forest fragmentation and the distribution, abundance and conservation of the Tana river red colobus (Procolobus rufomitratus). Biol Conserv 118:67–77
Mowry CB, Decker BS, Shure DJ (1996) The role of phytochemistry in dietary choices of Tana River red colobus monkeys (Procolobus badius rufomitratus). Int J Primatol 17:63–84
Nakayama Y, Matsuoka S, Watanuki Y (1999) Feeding rates and energy deficits of juvenile and adult Japanese monkeys in a cool temperate area with snow coverage. Ecol Res 14:291–301
Nelles Verlag (1999) Indonesia 4. Nelles Verlag GmbH, München, Germany
Nijman V (2004) Effects of habitat disturbance and hunting on the density and the biomass of the endemic Hose’s leaf monkey Presbytis hosei (Thomas, 1889) (Mammalia: Primates: Cercopithecidae) in east Borneo. Contrib Zool 73:283–291
NRC (1981) Techniques for the study of primate population ecology. National Academy Press, Washington, DC
Oates JF (1994) The natural history of African colobines. In: Davies AG, Oates JF (eds) Colobine monkeys: their ecology, behaviour and evolution. Cambridge University Press, Cambridge, pp 75–128
Paciulli LM (2004) The effects of logging, hunting, and vegetation on the densities of the Pagai, Mentawai Island primates (Indonesia). Dissertation thesis, State University of New York, Stony Brook
Palombit RA (1997) Inter- and intraspecific variation in the diets of sympatric siamang (Hylobates syndactylus) and lar gibbons (Hylobates lar). Folia Primatol 68:321–337
Peres CA (1997) Effects of habitat quality and hunting pressure on arboreal folivore densities in Neotropical forests: a case study of howler monkeys (Alouatta spp.). Folia Primatol 68:199–222
Richard AF, Goldstein SJ, Dewar RE (1989) Weed macaques: the evolutionary implications of macaque feeding ecology. Int J Primatol 10:569–594
SAS Institute (1989–1997) JMP, SAS Institute Inc
Serio-Silva JC, Rico-Gray V, Hernández-Salazar LT, Espinosa-Gomez R (2002) Ficus (Moraceae) in the diet and nutrition of a troop of Mexican howler monkeys, Alouatta palliata mexicana, released on an island in southern Veracruz, Mexico. J Trop Ecol 18(6):913–928
Skorupa JP (1986) Responses of rainforest primates to selective logging in Kibale Forest, Uganda: a summary report. In: Benirschke K (ed) Primates: the road to self-sustaining populations. Springer, New York, pp 57–70
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. W. H. Freeman and Company, Stony Brook, NY
SPSS (1989–2001) SPSS for Windows, SPSS Inc
Stanford CB (1991) The diet of the capped langur (Presbytis pileata) in a moist deciduous forest in Bangladesh. Int J Primatol 12:199–216
Sterling EJ, Rakotoarison N (1998) Rapid assessment of richness and density of primate species on the Masoala Peninsula, eastern Madagascar. Folia Primatol 69:109–116
Stevenson PR (2001) The relationship between fruit production and primate abundance in Neotropical communities. Biol J Linn Soc 72:161–178
Tenaza RR, Fuentes A (1995) Monandrous social organization of pigtailed langurs (Simias concolor) in the Pagai Islands. Indones Int J Primatol 16:295–310
Terborgh J (1986) Keystone plant resources in the tropical forest. In: Soule ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, MA, pp 330–344
Tilson RL (1976) Infant coloration and taxonomic affinity of the Mentawai Islands leaf monkey, Presbytis potenziani. J Mammal 57:766–769
Tutin CEG (1999) Fragmented living: behavioural ecology of primates in a forest fragment in the Lopé Reserve, Gabon. Primates 40:249–265
Tutin CEG, Williamson EA, Rogers ME, Fernandez M (1991) A case study of a plant-animal relationship - Cola lizae and lowland gorillas in the Lope Reserve. Gabon J Trop Ecol 7:181–199
van Roosmalen MGM, Mittermeier RA, Fleagle JG (1988) Diet of the Northern Bearded Saki (Chiropotes satanus chiropotes): a neotropical seed predator. Am J Primatol 14:11–35
Vidal MD, Cintra R (2006) Effects of forest structure components on the occurence, group size and density of groups of bare-face tamarin (Saguinus bicolor - Primates: Callitrichinae) in Central Amazonia. Acta Amazonica 36:237–248
Waterman PG, Ross JAM, Bennett EL, Davies AG (1988) A comparison in the floristics and leaf chemistry of the tree flora in two Malaysian rain forests and the influence of leaf chemistry on populations of colobine monkeys in the Old World. Biol J Linn Soc 34:1–32
Weghorst JA (2007) High population density of black-handed spider monkeys (Ateles geoffroyi) in Costa Rican lowland wet forest. Primates 48:108–116
White LJT, Rogers ME, Tutin CEG, Williamson EA, Fernandez M (1995) Herbaceous vegetation in different forest types in the Lopé Reserve, Gabon: implications for keystone food availability. Afr J Ecol 33:124–141
Whitesides GH, Oates JF, Green SM, Kluberdanz RP (1988) Estimating primate densities from transects in a west African rain forest: a comparison of techniques. J Anim Ecol 57:345–367
Whitten AJ (1982) Diet and feeding behaviour of Kloss gibbons on Siberut Island, Indonesia. Folia Primatol 37:177–208
Wilson WL, Wilson CC (1975) Species-specific vocalizations and the determination of phylogenetic affinities of the Presbytis aygula-melalophos group in Sumatra. In: Kondo S, Kawai M, Ehara A (eds) Contemporary primatology. S. Karger, Basel
Worman COD, Chapman CA (2006) Densities of two frugivorous primates with respect to forest and fragment tree species composition and fruit availability. Int J Primatol 27:203–225
WWF (1980) Saving Siberut: a conservation master plan. World Wildlife Fund, Bogor
Acknowledgments
Generous funding was provided by the Wildlife Conservation Society, Primate Conservation, Inc., and Conservation International/the Margo Marsh Biodiversity Foundation. Permits to conduct research were granted by the Indonesian Institute of Science (LIPI), the Indonesian Department of Forestry, and the project sponsor, Dr./Bapak Amsir Bakar of Universitas Andalas. Logistical help came from the Hutagalung family and Mr. Aurelius Napitupulu. Kathleen Donovan assisted with data collection and Amna Ali helped with the tables and appendices. Special thanks to Drs. Patricia C. Wright, Charles H. Janson, John G. Fleagle, Joshua Ginsberg, and two anonymous reviewers for commenting on and helping improve an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix. Primate Densities and Vegetation Variables. Key is at Bottom of Appendix
Key
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Paciulli, L.M. (2010). The Relationship Between Nonhuman Primate Densities and Vegetation on the Pagai, Mentawai Islands, Indonesia. In: Gursky, S., Supriatna, J. (eds) Indonesian Primates. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1560-3_11
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1560-3_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-1559-7
Online ISBN: 978-1-4419-1560-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)