Abstract
Chemical signals play an important role during various life stages of crustaceans. Settling of larvae, parent–offspring communication, mate finding, mate choice, aggressive contests, and dominance hierarchies are all mediated by chemical signals. Enormous advances have been made on understanding the function of chemical signals in crustaceans and we are on the doorstep of major advances in chemical characterization of pheromones. In many species urine is the carrier of chemical signals. Crustaceans control release and transfer direction of urine, but it is unknown whether crustacean senders can manipulate the composition of urineborne pheromones. Chemicals contained in the urine effectively convey information about conspecific properties such as sex, sexual receptivity, species identity, health status, motivation to fight, dominance, individual identity, and molt stage. In larger species (shrimp, crabs, lobsters, crayfish) signal delivery is often aided by self-generated fanning currents that flush chemicals towards receivers, which themselves might actively pull water towards their sensory structures. Antennal flicking also supports molecule exchange at the receptor level. Contact pheromones play a role in sex recognition in several crustacean taxa and in settlement of barnacles. Large crustacean species show little or no sexual dimorphism in receptor structures, but in smaller taxa, e.g. peracarids and copepods, males often have larger antennae than females. Whether differences in sexual roles have also resulted in sex-specific brain centers is not known at present. While pheromones play an important role in mate finding and species recognition, there are numerous examples from peracarids and copepods where males pursue or even form precopulatory pairs with females of closely related congeners. Differentiation of chemicals often appears to be insufficient to guarantee reproductive isolation. In many freshwater and coastal habitats, pollutants may also disrupt chemical communication in crustaceans, but the specific mechanisms of interference are not well understood. The chemical characterization of crustacean pheromones is viewed as a major step in improving our understanding of chemical communication. Knowing the chemical nature of pheromones in freshwater species will boost research on aquatic crustaceans. Interdisciplinary work between chemists (metabolomics), behavioral ecologists (bioassays), neurobiologists (chemoreception), and molecular biologists (genomics) promises to produce significant advances in our understanding of crustacean chemical communication during the coming decade.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
He drew up plans, made lists, experimented with smells, traced diagrams, built structures out of wood, canvas, cardboard, and plastic. There were so many calculations to be made, so many tests to be run, so many daunting questions to be answered. What was the ideal sequence of smells? How long should a symphony last, and how many smells should it contain? What was the proper shape of the symphony hall? … Should each symphony revolve around a single subject – food, for example, or female scents – or should various elements be mixed together?...What difference did it make if he didn’t fully understand? … It might not have served any purpose, but the truth was that it was fun.
From Timbuktu by Paul Auster (1999)
1 Introduction
Crustaceans are found in all major environments in the oceans and on land. Given the diversity of habitats, they face numerous challenges in communicating with conspecifics. How does a female crab that is ready to reproduce find a male in the murky waters of a shallow estuary? She could roam in search of a male or she could stay put and wait for a male to find her. In both cases, her success in finding a mating partner would be enhanced by a chemical guidance system. If she searches for a male, it would be advantageous to sniff out the environment for chemical cues that would indicate the presence of a male. And if she waits for a male to find her, she could guide him towards her by releasing attractive chemicals. Regardless of the strategy, chemical stimuli enhance the probability of mate finding which is only one of many benefits offered by chemical communication.
Chemical signals play an important role during various life stages of crustaceans. Settling of larvae, parent–offspring communication, mate finding, mate choice, and aggressive contests are all mediated by chemical signals. Chemicals are ubiquitous messengers because they can effectively convey information about conspecific properties such as sex, sexual receptivity, species identity, health status, motivation to fight, dominance, individual identity, and molt stage. Not surprisingly, many crustaceans employ chemical communication to coordinate important life processes. At first glance, crustaceans do not seem to differ from many other animals such as insects or mammals in which chemical communication plays an important role. However, crustaceans have conquered a wider range of habitats than most other animals, inhabiting the deep abyss of the oceans, wave-battered shores, calm freshwater lakes, dark forests, and even dry deserts. Furthermore, the range of crustacean body sizes and shapes is unparalleled in many other animal taxa. And finally, the diversity of crustacean life styles is mind boggling even to well-seasoned crustacean researchers; tiny planktonic species share a common history with bulky crabs, colorful shrimp, and strange parasitic forms that can only be recognized as crustaceans during their larval stages.
Many of these species, regardless of habitat or morphology, communicate with their conspecifics via chemical substances. The crustacean species that have been subject to chemical communication research were drawn from six of the 12 classes of crustacea including Branchiopoda (water fleas), Copepoda, Branchiura (including fish lice), Thecostraca (including barnacles), and Malacostraca (the largest class including stomatopods, peracarids, and decapods) (Fig. 1.1). By far the greatest contribution to our understanding of chemical communication comes from research on decapod crustaceans including crabs, lobsters, and shrimps.
Phylogeny of crustaceans, highlighting in bold the taxa that have been subject to research on chemical communication. Only those subtaxa of Malacostraca and Decapoda are shown that have been subject to chemical communication research. Phylogeny was modified after Tree of life (http://tolweb.org/Crustacea), and Dixon et al. (2003)
Many species from these groups employ chemical signals throughout or during parts of their lives. How do they do it and how have their phylogenetic histories and current environmental conditions shaped their communication systems? The contributions in this book offer answers to these questions and they also highlight fascinating challenges for the future.
2 Chemical Communication in Crustaceans – A Brief Literature Survey
2.1 Pheromone Signaling in Marine Invertebrates
In crustaceans, communication is mainly through the visual, chemical, and mechanical channels (see e.g., Mead and Caldwell, Chap. 11; Christy and Rittschof, Chap. 16; Clayton 2008). Whereas visual communication is mainly limited to species from terrestrial and clear-water environments, chemical communication can occur under most environmental conditions. Not surprisingly, studies on chemical communication dominate the literature. Of a total of 76 publications on crustacean communication (with the keywords communicat* and crustacea*) published between 1990 and 2010, 43 were on chemical communication, 24 on visual communication, and only 9 on mechanical/acoustic communication (Web of Science 2010).
Chemical communication may be prominent not only because it works under almost any environmental condition, but also because it may be subject to rapid evolutionary change (Symonds and Elgar 2008), possibly much more so than visual or mechanical communication, as was recently highlighted by Bargmann (2006): “The visual system and auditory system are stable because light and sound are immutable physical entities. By contrast, the olfactory system, like the immune system, tracks a moving world of cues generated by other organisms, and must constantly generate, test and discard receptor genes and coding strategies over evolutionary time.” The high potential for specificity has been one of the main reasons that many species communicate via chemical signals. These are often employed to attract conspecifics or to convey particular messages.
The first unequivocal demonstration of pheromone use by a crustacean was presented by Ryan (1966) who showed that male Pacific crabs Portunus sanguinolentus display a typical courtship response when stimulated with female premolt water. Males did not display when the female’s excretory pores were sealed. This paper was followed by several other studies confirming that crustaceans employ pheromones during mating interactions (e.g., Dahl et al. 1970, for amphipods; Atema and Engstrom 1971, for lobsters; Ameyaw-Akumfi and Hazlett 1975, for crayfish). Surprisingly, the first marine invertebrate for which the sex pheromone was chemically identified was the polychaete Platynereis dumerilii from the North Atlantic (Zeeck et al. 1988). Since then the chemical structure of pheromones has also been characterized for molluscs (Painter et al. 1998). Only during the past decade pheromones have been purified in several crustacean species (Kamio and Derby, Chap. 20; Hardege and Terschak, Chap. 19; Clare, Chap. 22; Rittschof and Cohen 2004).
Despite these advances, our knowledge about pheromone structure, production, and effects in marine invertebrates is scarce. A Boolean literature search from the past 20 years (1990–2009) showed that most pheromone studies with marine invertebrates have investigated crustaceans, polychaetes, and molluscs (Fig. 1.2). Especially during the pentad 2005–2009, there has been an increasing number of studies on crustacean pheromones, which most likely has been fostered by the beginning of the chemical characterization of pheromones in several species. Given recent advances in this field, it can be expected that this trend will continue in the future.
2.2 Crustaceans, Fish and Insects
Since most research on pheromones has been conducted in other taxa (e.g. insects, fish, and mammals), it is not surprising that crustacean researchers studying pheromones rely on this rich literature. Interestingly, not only do crustacean researchers cite a comparatively large number of studies on other taxa, but their own studies are also cited by researchers studying a diverse range of other taxa (Fig. 1.3). Traditionally, crustacean researchers studying pheromones have been inspired by research on fish (living in water) and insects (arthropod relatives of the crustaceans). Whereas crustacean studies often integrate information from studies on other taxa, the corresponding proportion in fish and insect studies is <10% (Fig. 1.3). Also, reciprocally, fish and insect studies are only rarely cited by pheromone studies on other taxa. Most likely, these differences between studies on crustaceans, fish, and insects are due to the fact that much more is known about pheromones in fish and insects than in crustaceans. Crustacean researchers might also cite studies on both aquatic (fish) and terrestrial (insects) taxa frequently because crustaceans have conquered both these environments. This integrative approach has always characterized studies on crustacean chemoreception (e.g., Weissburg 2000; Vickers 2000; Koehl 2001) and promises to do so in the future (see contributions in this volume).
3 Chemical Signals: Source and Identity
In crustaceans, chemical signals can be released to the surrounding liquid medium (soluble or volatile pheromones, “distance pheromones”) or bound to the body surface (“contact pheromones”; see e.g. Bauer, Chap. 14, and Snell, Chap. 23). In decapod crustaceans, the pheromones are released through the excretory pores (nephropores) located in the head region (Atema and Steinbach 2007; see also Kamio and Derby, Chap. 20; Hardege and Terschak, Chap. 19; Breithaupt, Chap. 13). Urine is often, but not always, the carrier of the chemical signal (see e.g. Kamio et al. 2002). Urine is predestined as a source of information molecules as it contains body metabolites that mirror the internal processes involved in sexual maturation, aggression, and illness. Many of the hormones underlying behavioral and developmental processes are well known in crustaceans (Chang, Chap. 21). Numerous studies on fish have shown that hormones, once released, assume a pheromonal role (Chung-Davidson et al., Chap. 24). Although this is likely the case in other animals as well, there are only few studies providing examples of hormonal pheromones in crustaceans (Chang, Chap. 21). The larger decapod crustaceans should be ideal model organisms to close the gap between endocrinology and chemical communication research.
Urineborne chemicals reveal crucial information about conspecifics that can provide the receiver with distinct advantages over competitors in feeding, reproduction, and dominance interactions. Early during the evolutionary history of chemical communication, individuals might have obtained information by spying on urine chemicals from conspecifics. If emitters of these chemicals had adaptive advantages in revealing their status to others, this may have led to the evolution of complex urine release pattern (see Fig. 2.4 in Wyatt, Chap. 2). An example would be the release of chemicals that permit individual recognition within dominance hierarchies, where senders and receivers benefit from recognizing conspecifics (Aggio and Derby, Chap. 12; Gherardi and Tricarico, Chap. 15).
It is unknown whether crustacean senders can manipulate the composition of urineborne pheromones. They do, however, have control over the timing of urine release (Breithaupt, Chap. 13) and are therefore able to adjust the signaling to their own benefit. This may include opportunities to manipulate the receiver by either falsely reporting or by withholding information (Christy and Rittschof, Chap. 16). Only in few examples has the chemical nature of distance pheromones been characterized. These studies employed behavioral assays that used a specific behavioral response in the receiver as an indicator for pheromonal activity (Kamio and Derby, Chap. 20; Hardege and Terschak, Chap. 19).
The slow progress in crustacean pheromone identification is due (1) to difficulties in designing appropriate bioassays for animals that are under conflicting motivational regimes such as fighting, mating, or escape (Breithaupt, Chap. 13; Hardege and Terschak, Chap. 19), (2) to the quick alteration and degradation of the chemical components by aquatic bacteria (Hay, Chap. 3; for a terrestrial example see Voigt et al. 2005), and (3) to analytical challenges particular to identification of marine pheromones such as the difficulty in extracting and separating small molecules from a salty medium (Hay, Chap. 3; Hardege and Terschak, Chap. 19).
The latter problem may also explain the bias towards freshwater species in fish pheromone studies. Hormonal pheromones (see Chung-Davidson et al., Chap. 24) were identified in goldfish, round goby, African catfish, and Atlantic salmons that all release the pheromones into a freshwater environment (Sorensen and Stacey 2004). Even in sea lampreys, the chemical nature of larval migratory pheromone attracting adults and of male sex pheromones attracting females was identified for components that are naturally emitted into the freshwater spawning environment (Chung-Davidson et al., Chap. 24). The difficulties inherent in identifying marine semiochemicals suggest that freshwater crustaceans such as amphipods and crayfish may be better model systems for chemical characterization of pheromone components (Fig. 1.4).
Examples of crustacean species (in their natural environment) that are suited as models to address particular questions on crustacean chemical communication. (a) Crayfish Austropotamobius torrentium (photograph courtesy of Dr. Michael van der Wall); (b) water flea Daphnia pulex (photograph courtesy of Linda C. Weiss); (c) amphipod Hyalella costera; (d) freshwater shrimp Cryphiops caementarius (photographs (c, d) courtesy of Iván A. Hinojosa)
Despite these medium-specific problems, recent progress in pheromone characterization in crustaceans including hair crabs (Asai et al. 2000), helmet crabs (Kamio et al. 2000, 2002), blue crabs (Kamio and Derby, Chap. 20), green crabs (Hardege and Terschak, Chap. 19), peppermint shrimp (Zhang et al. 2010a), and barnacles (Clare, Chap. 22) highlights the fact that some of the initial difficulties in chemical purification have now been overcome and that the door is open towards rapid progress in structural identification.
While almost all insect pheromones are fatty-acid-derived hydrocarbons (Baker, Chap. 27), crustacean pheromones are more diverse. They belong to various substance classes such as peptides (Rittschof and Cohen 2004), nucleotides (Hardege and Terschak, Chap. 19) or other small polar molecules (Kamio and Derby, Chap. 20), small nonpolar molecules (Ingvarsdóttir et al. 2002), and possibly to ceramids (Asai et al. 2000). The higher diversity of waterborne pheromones again reflects the physical differences between the two media, with solubility in water being much less restrictive for the evolution of signal molecules than volatility in air.
Contact pheromones were shown to play a role in sex recognition in copepods (Snell, Chap. 23) and in shrimp (Bauer, Chap. 14) as well as in inducing settlement in barnacles (Clare, Chap. 22). In copepods and barnacles, the molecules were identified as surface-bound glycoproteins (Snell, Chap. 23). Glycoproteins were also found on the surface of caridean shrimp from the genus Lysmata, but behavioral experiments on the role of these molecules as mate recognition pheromone in shrimps revealed contradictory results (Caskey et al. 2009; Zhang et al. 2010b), calling for additional studies. Chemical characterization of contact and distance pheromones remains one of the main challenges in crustacean chemical ecology and promises the greatest progress in this field.
4 Signal Transmission, Reception, and Processing
4.1 Signal Delivery
Pheromones released in an aquatic environment will be carried downstream by the ambient flow (see Weissburg, Chap. 4). In stagnant environments such as some lakes and ponds, odor dispersal will be slow. Signalers that are walking or swimming leave a scented trail behind that facilitates detection as it can be used by receivers to track and find the signaler (Yen and Lasley, Chap. 9; Weissburg, Chap. 4). Stationary senders generate their own water currents by ventilating or by fanning maxillipeds or pleopods to disperse the chemical signals (for lobsters see Atema and Steinbach 2007, and Aggio and Derby, Chap. 12; for crayfish see Breithaupt, Chap. 13; for blue crabs see Kamio and Derby, Chap. 20; for shrimp see Bauer, Chap. 14; for stomatopods see Mead and Caldwell, Chap. 11).
Actively flushing signals towards conspecifics appears to be a general strategy in many crustaceans as they are equipped with specialized fanning structures to generate water currents (see e.g., Breithaupt 2001; Cheer and Koehl 1987). Some insects (e.g., bees; Agosta 1992) and mammals (e.g., bats; Voigt and von Helversen 1999; and ring-tailed lemurs; Bradbury and Vehrencamp 1998) are also able to direct their chemical signals by using their wings (bees, bats) or tails (lemurs), but this strategy of dispersing odors is much less common in terrestrial animals than in aquatic organisms.
Terrestrial animals often display chemical signals by depositing gland excretions, urine, or feces to the substratum. There are numerous examples of terrestrial animals marking their territories using scent marks. Common examples are mammals such as badgers and mice where defecating or urinating appears to serve a territorial function (Roper et al. 1993; Hurst 2005), or female spiders giving away their reproductive status via chemicals in their web’s silk (Roberts and Uetz 2005). Interestingly, in terrestrial isopods, burrows or communal dwellings also carry kin- or species-specific scents, while observations of aquatic amphipods could find no evidence for the existence of scent marks on dwellings (Borowsky 1989). The lack of scent marks in aquatic environments may be a consequence of the high solubility of even large molecules such as proteins in water causing any scent marks to be rapidly diluted by water movements. In addition, the ubiquitous bacteria in water may quickly attack and degrade any scent marks.
4.2 Reception and Processing of Pheromone Signals
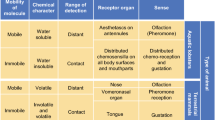
Crustaceans perceive chemical signals with olfactory receptors – limited to the aesthetasc hairs that only contain chemoreceptor neurons and are located on the antennae – or with other chemoreceptors situated in setae that are distributed over the body surface (“distributed chemoreceptors” including contact chemoreceptors, Schmidt and Mellon, Chap. 7; Hallberg and Skog, Chap. 6). “Contact chemoreceptors” contain both chemoreceptor neurons and mechanoreceptive neurons (Schmidt and Mellon, Chap. 7).
The evolutionary transition from water to land has resulted in an expansion of the chemoreceptor genes, most likely in response to the multitude of airborne odorants (Bargmann 2006). Organisms that frequently change between aquatic and terrestrial environments (e.g., amphibians) appear to have chemosensory systems for perception of both water-soluble as well as volatile odorants (Freitag et al. 1995). Soluble and volatile chemicals can also be perceived by aquatic and terrestrial crustaceans, respectively (e.g., Hansson et al., Chap. 8). However, at least in terrestrial peracarids, taste reception of odorants appears to be mediated by liquids (Seelinger 1983; Holdich 1984), just as food-smelling of terrestrial mammals under water is mediated by air bubbles (Catania 2006).
So far, in decapod crustaceans, the receptor-bearing structures have not been shown to display strong sexual dimorphism as is found in many insects (Hallberg and Skog, Chap. 6). In insects, particularly in moths, males generally have much larger chemoreceptor-bearing antennae than females (Lee and Strausfeld 1990; Baker, Chap. 27). This dimorphism reflects the direction of sexual communication, with females generally being the pheromone emitter and males being the pheromone receiver as reported or inferred for many species (Fig. 1.5). There is a strong selective pressure on the males to detect minor amounts of female pheromones and track down the female that usually remains stationary while signaling (Phelan 1997). Concordant with the dimorphism in olfactory organ morphology, the dimorphism extends to sex-specific differences in the brain. In most insect genera where adults are terrestrial, a sexual dimorphism was found in olfactory brain centers. In contrast to females, males often possess a system of sex-specific brain centers that make up the “macroglomerular complex”, which is involved in the processing of pheromone information (Strausfeld and Reisenman 2009). So far, no sexual dimorphism with respect to olfactory structures has been found in any decapod crustacean (Hallberg and Skog, Chap. 6). However, sexual dimorphism is evident in some peracarid crustaceans where males possess larger and more differentiated olfactory organs than females as well as exhibiting sex-specific olfactory centers (Johansson and Hallberg 1992; Hallberg and Skog, Chap. 6; Thiel, Chap. 10). It remains to be investigated whether the receptor dimorphism in peracarids is caused by sex-specific pheromones and whether it mediates sex-specific behaviors. In crustaceans with female sex pheromones and male-specific responses (Bauer, Chap. 14; Breithaupt, Chap. 13; Hardege and Terschak, Chap. 19; Kamio and Derby, Chap. 20; Yen and Lasley, Chap. 9), males may have specific adaptations for neural processing of female chemical signals.
Examples of mating interactions in several species of crustaceans where males are known or inferred to be receiver of female pheromones. (a) Rock shrimp Rhynchocinetes typus; (b) amphipod Parhyalella penai; (c) squat lobster Cervimunida johni; (d) barnacle Balanus laevis; (photographs courtesy of Iván A. Hinojosa)
Sex recognition may also involve multiple sensory channels in some (many?) crustacean species (see Hebets and Rundus, Chap. 17), requiring more complex central processing of multimodal information. One of the future challenges to research on crustacean chemical communication is to enhance our understanding of the neuronal processing underlying pheromone perception (see Schmidt and Mellon, Chap. 7; Hansson et al., Chap. 8). Most importantly, the pheromone receptors need to be identified. This will then facilitate further investigation of the central neural pathways mediating chemical communication. Knowledge of pheromone receptor proteins will also open the door to sequencing of olfactory receptor genes.
4.3 Signal Enhancement
Crustaceans can actively enhance odor acquisition by creating water currents that draw the molecules towards them (in lobsters: Atema and Steinbach 2007; crayfish: Breithaupt, Chap. 13, Denissenko et al. 2007; stomatopods: Mead and Caldwell, Chap. 11; copepods: Moore et al. 1999). Generation of microcurrents to obtain chemical information may be a strategy to enhance olfaction, as also described for some insects (silkworm moth: Loudon and Koehl 2000). While such activities that enhance odor transmission are limited to only few examples in insects and mammals, they are common strategies in crustaceans involving specific fanning appendages.
Active odor sampling by antennal flicking is an additional mechanism to enhance olfaction in crustaceans (see Koehl, Chap. 5). These active behavioral investments in fanning or flicking activities serving signal transmission and reception appear to be much more common in the aquatic environment than in the terrestrial realm, most likely due to the differences in density and viscosity. Given the importance of signal delivery and transport in chemical communication, this will be a promising research field for the future.
Aggregated individuals also produce stronger chemical signals than solitary individuals. This might explain density-dependent gregariousness in spiny lobsters (Ratchford and Eggleston 1998). This effect could also be at work in crustacean species that form large mating aggregations. Individuals might initially aggregate because concentrated chemical signals will then attract other conspecifics, including potential mates. For example, during the mating period male green crabs Carcinus maenas gather at particular mating grounds (van der Meeren 1994), to which females are then attracted. Male hotspots with larger numbers of males (and stronger chemical signals) might attract many receptive females in a given area, thus benefitting both females and males. Since various crustacean species are highly gregarious, it appears worthwhile to explore how density-dependent strength of chemical signals might affect the attraction of additional conspecifics to aggregations.
5 Speciation Processes and the Role of Chemical Communication
A variety of factors can modulate the pheromone blends produced by the signaler or the chemosensory system of the receiver, which may have important evolutionary implications (Symonds and Elgar 2008; Smajda and Butlin 2009). Divergence in chemical signals may be a driving force in speciation; variations in the composition of chemical blends lead to reproductive isolation as has been shown for a number of insect species (Smajda and Butlin 2009 and references therein). Ecological factors can play an important role in divergence of pheromone blends. For example, if pheromone communication is susceptible to eavesdropping and is selected to avoid exploitation by parasites or predators, this could provoke shifts in pheromone composition leading to divergence between populations frequently exposed to predators/parasites and those living in enemy-free environments (e.g., Symonds and Elgar 2008, and references therein). Compounds obtained with the diet also may influence the composition of pheromone blends (Bryant and Atema 1987; Symonds and Elgar 2008). Habitat or host preferences may affect the chemosensory system and result in divergent pheromone communication between populations from different habitats/hosts (e.g., Smajda and Butlin 2009, and references therein). It is likely that these processes are also at work in crustaceans, which are targets of diverse predators or parasites and utilize a variety of hosts as food or shelter (e.g., Poore et al. 2008).
Interestingly, several examples from crustaceans suggest that reproductive isolation via pheromones might be incomplete. For example, some congeneric amphipod species appear unable to distinguish between mates of closely related species (Kolding 1986). Also, in other peracarids species, males mistakenly pair up with closely related species or ecotypes (Mead and Gabouriaut 1977; Hargeby and Erlandsson 2006), which could be due to lack of species recognition via chemical stimuli. Possibly, the divergence of contact pheromones is lagging behind compared to other life-history traits that lead to reproductive isolation under natural conditions (e.g., size or habitat preferences). Sutherland et al. (2010) observed that males from a species complex of freshwater amphipods could only discriminate against females from genetically distinct clades, but not against those from more closely related clades. In an estuarine amphipod with populations from different habitats, males preferred females from their own habitat, but were unable to distinguish between female populations if these were raised on other food types (Stanhope et al. 1992). Interestingly, pheromone specificity is also low in planktonic copepods and males frequently pursue pheromone trails laid by females of other species; once reaching and grasping the female, most males recognized their mistake based on contact chemicals, but some males even placed a spermatophore on the female from a different species (Goetze 2008). In experiments with specific mate-attracting signals, Bublitz et al. (2008) found that males of several species of crabs were attracted in similar ways by uridine diphosphate (UDP). Similarly, cyprid larvae of the barnacle Balanus amphitrite settled preferentially on substrata treated with the settlement-inducing protein complex (SIPC) from conspecifics, but relatively high proportions of larvae also settled on surfaces treated with the SIPC of other barnacle species (Dreanno et al. 2007; Clare, Chap. 22).
These examples suggest that species recognition in many crustaceans is not based only on particular chemical stimuli. Species recognition may require specific pheromone blends as in many insects (e.g. Symonds and Elgar 2004), “combination-lock” cascades of chemical signals that must proceed in a specific order (Hay, Chap. 3), or multimodal communication where chemical signals are complemented by visual or mechanical signals (Hebets and Rundus, Chap. 17). Additionally, sex-specific differences in behavior may ensure reproductive isolation. For examples, males of two closely related species of spiders were unable to discriminate between chemical signals from females of their own and of a closely related species, but specific female responses to male courtship behaviors maintained reproductive isolation in these species (Roberts and Uetz 2004).
In summary, for chemical signals to play a relevant role in species recognition among crustaceans, they may have to be (1) complex blends, (2) arranged in particular sequences, or (3) complemented by other modes of communication. Given that chemical signals are not as easily recognizable by human observers as morphological (visual or mechanical) signals, their role in reproductive isolation may often be strongly underestimated. There is a high probability that closely related species that depend primarily on chemical signals to recognize conspecific mates are not distinguished by taxonomists (Bickford et al. 2006). This might be especially relevant for marine organisms (including crustaceans); marine animal taxonomists, unlike their terrestrial colleagues, have only limited chances to observe the living organisms (Bickford et al. 2006).
In addition to behavioral interactions mediated by chemicals, there is a wide range of chemical interactions at the sperm–egg level that can result in reproductive isolation. Divergence of proteins acting at the gamete level may be especially rapid (Swanson and Vacquier 2002). In Drosophila, the gametic interactions are mediated by accessory gland proteins (ACPs) released with the seminal fluids (Ravi Ram and Wolfner 2007). Similar interactions could be expected for many crustaceans, where spermatophores also contain appreciable quantities of seminal fluid. Especially in brachyuran crabs, seminal fluids that are injected together with sperm in the female’s spermatheca may interact with female reproductive processes, causing sexual conflict (see also Christy and Rittschof, Chap. 16). Sexual conflict is viewed as an important selective force in the divergence of reproductive proteins (Clark et al. 2009). Given the theoretical (species divergence) and applied (aquaculture and fishery) implications, it appears especially rewarding to explore chemical communication at the gametic level in crustaceans.
6 Anthropogenic Impact on Crustacean Chemical Communication
The contributions in this book demonstrate that chemical communication fulfills various crucial functions in reproduction, aggregation, and resolution of conflicts among crustaceans from a wide range of habitats. These functions are marred if pheromone production, transmission, or reception is disturbed by chemical or physical disturbances such as changes in salinity, acidity, temperature, or chemical pollution. Olsén (Chap. 26) explores how anthropogenic pollutants such as heavy metals, steroids, and pesticides disrupt different stages of chemical communication in fish and crustaceans. These interferences can produce morphological, physiological, and behavioral effects (e.g., Lürling and Scheffer 2007) that may entail dramatic consequences on reproduction and individual survival. Research on pheromone disruption is still in its infancy, but is direly needed to understand ecological consequences of anthropogenic disturbances of aquatic ecosystems and implement regulatory measures to protect aquatic species.
Variations in sex-specific population densities, e.g., due to extraction by fisheries, may also affect the dynamics of chemical communication in crustaceans (e.g., van Son and Thiel 2007). At low population densities, members from the mate-attracting sex may have to invest more in chemical advertisement than at high densities. On the other hand, individuals from the mate-searching sex that are efficient and rapid in responding to chemical signals may be at an advantage at low population densities.
7 Applied Aspects
Once identified, pheromones may be artificially synthesized to be used in mass attraction of target organisms. For crustaceans, this might be useful in the fisheries and aquaculture where desired individuals could be lured into traps (e.g., Barki et al., Chap. 25). Invasive crustaceans could also be caught by mass trapping, but it needs to be kept in mind that trapping is only efficient in combination with other measures aiming at suppressing or eliminating the populations of undesired species (see Baker, Chap. 27). Furthermore, pheromone trapping, if employed, may cause artificial selection for individuals responding to or producing other pheromone blends than the majority of the target population (see, e.g., Symonds and Elgar 2008).
Many of the contributions in this book are concerned with pheromones, i.e., chemical stimuli that are employed by crustaceans to attract conspecifics. However, chemical communication also includes substances that are used to repel other organisms. These repellents could be especially useful in the aquaculture context, e.g., to repel parasites or fouling organisms. Many crustaceans are parasites of commercially important fish species (e.g., salmon) and both traps and repellents could be used in controlling infection levels (e.g., Mordue and Birkett 2009). Similarly, barnacles are abundant fouling organisms in suspended structures or seawater systems and developing techniques to suppress their recruitment is one of the main motivations behind the identification of settlement factors (Clare, Chap. 22).
8 Outlook
The past two decades have seen enormous advances in our understanding of crustacean chemical communication. Nevertheless, our knowledge is still in its infancy when compared to insects, fish, or mammals. Most research on crustacean behavior in response to chemical stimuli has been conducted in controlled laboratory environments, where often only one stimulus context is tested. For example, the responses of numerous crustacean species to chemicals emitted by potential mates, by conspecific aggressors, or by interspecific enemies have been tested in isolation. However, in the natural environment, crustaceans are often exposed to multiple stimuli at the same time (see, e.g., Fig. 18.2 in Hazlett, Chap. 18). How an organism reacts in such a situation depends not only on external stimuli, but also on intrinsic factors (Hazlett, Chap. 18). Before drawing conclusions about the function of pheromones, it would be valuable to perform field tests in which organisms are confronted with a complex set of stimuli from all sensory modes (e.g., Johnson and Li 2010). While this is logistically challenging, it is essential if we are to understand how crustaceans employ pheromones in the natural environment.
In the field of behavioral ecology, a number of topics appear to be interesting. We consider it most rewarding to formally examine the presence of multimodal communication and of deception in crustaceans. Hebets and Rundus (Chap. 17) presented a number of examples that suggest that multimodal communication is much more common in crustaceans than previously assumed. In particular, chemical communication appears to be often combined with other sensory channels. Christy and Rittschof (Chap. 16) present several cases of deception in the visual channel, and they speculate on instances where crustaceans might deceive during chemical communication. Based on their overview, they suggest that it might be much easier to fake visual signals than chemical signals, but this hypothesis remains untested.
The major challenge ahead of us is the chemical identification of crustacean pheromones. We consider that this is the most crucial step because it not only will help us to understand the role of these pheromones in speciation processes, but will also permit a whole suite of experimental approaches. Insect biologists have utilized synthetically produced pheromones in experimental studies for several decades now (see Baker, Chap. 27). Once the first crustacean pheromone is synthetically produced, we expect a boost of research activities on chemical communication in crustaceans.
There are several crustacean taxa that appear to be at the doorstep of having the chemical structure of their pheromones identified. Probably the most promising approach to achieve pheromone identification will be to combine chemical analyses with genomics. Based on the fact that freshwater environments are so much more amenable to the chemical analysis of dissolved substances (see above), we suggest to focus on freshwater crustaceans during the next phase of chemical communication research (Fig. 1.4). Several crustacean taxa offer themselves for this approach. Similar to cladocerans (Daphnia spp.), freshwater amphipods and isopods are relatively small and can be easily reproduced in the lab without major space requirements. Short generation times permit the production of different strains under different selective pressures. Freshwater crayfish, in contrast, have longer generation times and require more space, but they have other important advantages. For example, emission of pheromone-containing urine can be made visible, neurological methods can be easily applied, and finally they are economically important fisheries and aquaculture resources, i.e., the need for applied research is high.
This next phase of research on chemical communication probably will also see more interdisciplinary work. Future interactions should involve behavioral ecologists, chemists, and geneticists. For example, recently 58 chemoreceptor genes were identified for Daphnia pulex, but at present it is not well known which particular chemicals these detect; females had some receptor genes that were lacking in males, but since there were no male-specific genes their role in mate finding is unclear (Peñalva-Arana et al. 2009). Future genomics studies should incorporate behavioral assays. Several of the contributions in this book highlight the importance of behavioral assays in the study of chemical communication (Breithaupt, Chap. 13; Hardege and Terschak, Chap. 19; Kamio and Derby, Chap. 20). It requires careful observations to identify well-suited behavioral assays that can be employed in tests of chemical compounds. Similarly, the selective mechanisms that ultimately lead to the divergence of signaling or chemoreceptor genes can only be uncovered with the help of behavioral studies. First advances in this next phase of interdisciplinary research have been achieved (see, e.g., Kamio and Derby, Chap. 20); more progress is expected in the near future. The next two decades of research on crustacean chemical communication promise to be very exciting and we look forward to what is to come.
References
Agosta WC (1992) Chemical communication – the language of pheromones. Scientific American Library, New York
Ameyaw-Akumfi C, Hazlett B (1975) Sex recognition in the crayfish Procambarus clarkii. Science 190:1225–1226
Asai N, Fusetani N, Matsunaga S, Sasaki J (2000) Sex pheromones of the hair crab Erimacrus isenbeckii. Part 1: isolation and structures of novel ceramides. Tetrahedron 56:9895–9899
Atema J, Engstrom DG (1971) Sex pheromone in the lobster, Homarus americanus. Nature 232:261–263
Atema J, Steinbach MA (2007) Chemical communication and social behavior of the lobster Homarus americanus and other decapod Crustacea. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems – crustaceans as model organisms. Oxford University Press, New York, pp 115–144
Bargmann CI (2006) Comparative chemosensation from receptors to ecology. Nature 444:295–301
Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I (2006) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155
Borowsky B (1989) The effects of residential tubes on reproductive behaviors in Microdeutopus gryllotalpa (Costa) (Crustacea: Amphipoda). J Exp Mar Biol Ecol 128:117–125
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication. Sinauer Associates, Sunderland
Breithaupt T (2001) Fan organs of crayfish enhance chemical information flow. Biol Bull 200:150–154
Bryant BP, Atema J (1987) Diet manipulation affects social behavior of catfish: importance of body odor. J Chem Ecol 13:1645–1661
Bublitz R, Sainte-Marie B, Newcomb-Hodgetts C, Fletcher N, Smith M, Hardege JD (2008) Interspecific activity of sex pheromone of the European shore crab (Carcinus maenas). Behaviour 145:1465–1478
Caskey JL, Watson GM, Bauer RT (2009) Studies on contact pheromones of the caridean shrimp Palaemonetes pugio: II. The role of glucosamine in mate recognition. Invertebr Reprod Dev 53:105–116
Catania KC (2006) Underwater “sniffing” by semi-aquatic animals. Nature 444:1024–1025
Cheer AYL, Koehl MAR (1987) Paddles and rakes: fluid flow through bristled appendages of small organisms. J Theor Biol 129:17–39
Clark NL, Gasper J, Sekino M, Springer SA, Aquadro CF, Swanson WJ (2009) Coevolution of interacting fertilization proteins. PLoS Genet 5(7):e1000570
Clayton D (2008) Singing and dancing in the ghost crab Ocypode platytarsus (Crustacea, Decapoda, Ocypodidae). J Nat Hist 42:141–155
Dahl E, Emanuelsson H, von Mecklenburg C (1970) Pheromone transport and reception in an amphipod. Science 170:739–740
Denissenko P, Lukaschuk S, Breithaupt T (2007) The flow generated by an active olfactory system of the red swamp crayfish. J Exp Biol 210:4083–4091
Dixon CJ, Ahyong ST, Schram FR (2003) A new hypothesis of decapod phylogeny. Crustaceana 76:935–975
Dreanno C, Kirby RR, Clare AS (2007) Involvement of the barnacle settlement-inducing protein complex (SIPC) in species recognition at settlement. J Exp Mar Biol Ecol 351:276–282
Freitag J, Krieger J, Strotmann J, Breer H (1995) Two classes of olfactory receptors in Xenopus laevis. Neuron 15:1383–1392
Goetze E (2008) Heterospecific mating and partial prezygotic reproductive isolation in the planktonic marine copepods Centropages typicus and Centropages hamatus. Limnol Oceanogr 53:33–45
Hargeby A, Erlandsson J (2006) Is size-assortative mating important for rapid pigment differentiation in a freshwater isopod? J Evol Biol 19:1911–1919
Holdich DM (1984) The cuticular surface of woodlice: a search for receptors. Symp Zool Soc Lond 53:9–48
Hurst JL (2005) Scent marking and social communication. In: McGregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge, pp 219–243
Ingvarsdóttir A, Birkett MA, Duce I, Mordue W, Pickett JA, Wadhams LJ, Mordue (Luntz) AJ (2002) Role of semiochemicals in mate location by parasitic sea louse, Lepeophtheirus salmonis. J Chem Ecol 28:2107–2117
Johansson KUI, Hallberg E (1992) Male-specific structures in the olfactory system of mysids (Mysidacea; Crustacea). Cell Tissue Res 268:359–368
Johnson NS, Li W (2010) Understanding behavioral responses of fish to pheromones in natural freshwater environments. J Comp Physiol A. 196:701–711
Kamio M, Matsunaga S, Fusetani N (2000) Studies on sex pheromones of the helmet crab, Telmessus cheiragonus. 1. An assay based on precopulatory mate-guarding. Zool Sci 6:731–733
Kamio M, Matsunaga S, Fusetani N (2002) Copulation pheromone in the crab Telmessus cheiragonus (Brachyura: Decapoda). Mar Ecol Prog Ser 234:183–190
Koehl MAR (2001) Fluid dynamics of animal appendages that capture molecules: Arthropod olfactory antennae. In: Fauci L, Gueron S (eds) Computational modeling in biological fluid dynamics. Springer, New York, pp 97–116
Kolding S (1986) Interspecific competition for mates and habitat selection in five species of Gammarus (Amphipoda: Crustacea). Mar Biol 91:491–495
Lee JK, Strausfeld NJ (1990) Structure, distribution and number of surface sensilla and their receptor cells on the olfactory appendage of the male moth Manduca sexta. J Neurocytol 19:519–538
Loudon C, Koehl MAR (2000) Sniffing by a silkworm moth: wing fanning enhances air penetration through and pheromone interception by antennae. J Exp Biol 203:2977–2990
Lürling M, Scheffer M (2007) Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol Evol 22:374–379
Mead F, Gabouriaut D (1977) Chevauchée nuptiale et accouplement chez l’isopode terrestre Helleria brevicornis Ebner (Tylidae). Analyse des facteurs qui contrólent ces deux phases du comportement sexuel. Behaviour 63:262–280
Moore P, Fields DM, Jen Y (1999) Physical constraints of chemoreception in foraging copepods. Limnol Oceanogr 44:166–177
Mordue (Luntz) AJ, Birkett MA (2009) A review of host finding behaviour in the parasitic sea louse, Lepeophtheirus salmonis (Caligidae: Copepoda). J Fish Dis 32:3–13
Painter SD, Clough B, Garden RW, Sweedler JV, Nagle GT (1998) Characterization of Aplysia attractin, the first water-borne peptide pheromone in invertebrates. Biol Bull 194:120–131
Peñalva-Arana DC, Lynch M, Robertson HM (2009) The chemoreceptor genes of the waterflea Daphnia pulex: many Grs but no Ors. BMC Evol Biol 9:79. doi:10.1186/1471-2148-9-79
Phelan PL (1997) Evolution of mate-signalling in moths: phylogenetic considerations and prediction from the asymmetric tracking hypothesis. In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and Arachnids. Cambridge University Press, Cambridge, pp 240–256
Poore AGB, Hill NA, Sotka EE (2008) Phylogenetic and geographic variation in host breadth and composition by herbivorous amphipods in the family Ampithoidae. Evolution 62:21–38
Ratchford SG, Eggleston DB (1998) Size- and scale-dependent chemical attraction contribute to an ontogenetic shift in sociality. Anim Behav 56:1027–1034
Ravi Ram K, Wolfner MF (2007) Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr Comp Biol 47:427–445
Rittschof D, Cohen JH (2004) Crustacean peptide and peptide-like pheromones and kairomones. Peptides 25:1503–1516
Roberts JA, Uetz GW (2004) Species-specificity of chemical signals: Silk source affects discrimination in a wolf spider (Araneae: Lycosidae). Insect Behav 17:477–491
Roberts JA, Uetz GW (2005) Discrimination of female reproductive state from chemical cues in silk by males of the wolf spider, Schizocosa ocreata (Araneae, Lycosidae). Anim Behav 70:217–223
Roper TJ, Conradt J, Butler JE, Ostler CJ, Schmid TK (1993) Territorial marking with faeces in badgers (Meles meles): a comparison of boundary and hinterland latrine use. Behaviour 127:289–307
Ryan EP (1966) Pheromone: evidence in a decapod crustacean. Science 151:340–341
Seelinger G (1983) Response characteristics and specificity of chemoreceptors in Hemilepistus reaumuri (Crustacea, Isopoda). J Comp Physiol A 152:219–229
Smajda C, Butlin RK (2009) On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102:77–97
Sorensen PW, Stacey NE (2004) Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. N Z J Mar Freshwater Res 38:399–417
Stanhope MJ, Connelly MM, Hartwick B (1992) Evolution of a crustacean chemical communication channel: behavioral and ecological genetic evidence for a habitat-modified, race-specific pheromone. J Chem Ecol 18:1871–1887
Strausfeld N, Reisenman CE (2009) Dimorphic olfactory lobes in the Arthropoda. Ann N Y Acad Sci 1170:487–496
Sutherland DL, Hogg ID, Waas JR (2010) Phylogeography and species discrimination in the Paracalliope fluviatilis species complex (Crustacea: Amphipoda): can morphologically similar heterospecifics identify compatible mates? Biol J Linn Soc 99:196–205
Swanson WJ, Vacquier VD (2002) The rapid evolution of reproductive proteins. Nat Rev Genet 3:137–144
Symonds MRE, Elgar MA (2004) The mode of pheromone evolution: evidence from bark beetles. Proc Biol Sci 271:839–846
Symonds MRE, Elgar MA (2008) The evolution of pheromone diversity. Trends Ecol Evol 23:220–228
Van der Meeren GI (1994) Sex- and size-dependent mating tactics in a natural population of shore crabs Carcinus maenas. J Anim Ecol 63:307–314
Van Son TC, Thiel M (2007) Anthropogenic stressors and their effects on the behavior of aquatic crustaceans. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems: Crustaceans as model organisms. Oxford University Press, New York, pp 413–441
Vickers NJ (2000) Mechanisms of animal navigation in odor plumes. Biol Bull 198:203–212
Voigt CC, von Helversen O (1999) Storage and display of odour by male Saccopteryx bilineata (Chiroptera, Emballonuridae). Behav Ecol Sociobiol 47:29–40
Voigt CC, Caspers B, Speck S (2005) Bats, bacteria, and bat smell: sex-specific diversity of microbes in a sexually selected scent organ. J Mammal 86:745–749
Weissburg MJ (2000) The fluid dynamical context of chemosensory behavior. Biol Bull 198:188–202
Zeeck E, Hardege JD, Bartels-Hardege HD, Wesselmann G (1988) Sex pheromone in a marine polychaete: determination of the chemical structure. J Exp Zool 246:285–292
Zhang D, Lin J, Harley M, Hardege JD (2010a) Characterization of a sex pheromone in a simultaneous hermaphroditic shrimp, Lysmata wurdemanni. Mar Biol 157:1–6
Zhang D, Zhu J, Hardege JD, Lin J (2010b) Surface glycoproteins are not the contact pheromones in the Lysmata shrimp. Mar Biol 157:171–176
Acknowledgments
We thank Iván A. Hinojosa for his help in preparing the final figures and Drs. Chuck Derby and Marc Weissburg for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Thiel, M., Breithaupt, T. (2010). Chemical Communication in Crustaceans: Research Challenges for the Twenty-First Century. In: Breithaupt, T., Thiel, M. (eds) Chemical Communication in Crustaceans. Springer, New York, NY. https://doi.org/10.1007/978-0-387-77101-4_1
Download citation
DOI: https://doi.org/10.1007/978-0-387-77101-4_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-77100-7
Online ISBN: 978-0-387-77101-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)