Abstract
Maple syrup urine disease (MSUD) is an autosomal recessive disorder associated with impaired metabolism of branched-chain amino acids (BCAA) leucine, isoleucine, and valine. Children with MSUD suffer from bouts of metabolic decompensation, which may lead to neurological damage. Liver transplantation from unrelated deceased donors has been considered curative. The natural history of the disease following transplantation using a haploidentical (obligate heterozygous) living donor is still unclear, although previously described as favorable. We describe acute metabolic crises in a 20-month-old child with MSUD type II. The first well-documented one occurred 5 months after a successful liver transplantation from his mother. The patient developed encephalopathy with progressive lethargy and seizures after an episode of gastroenteritis with dehydration. Plasma levels of leucine, isoleucine, and valine were markedly elevated and alloisoleucine was detected. He promptly responded to dialysis and BCAA-free dietetic management and subsequently could resume a normal diet. Since then he has had another symptomatic metabolic crisis with seizures. This case strongly suggests that some recipients of liver transplantation from a haploidentical parent possess limited capacity to oxidize BCAA at the time of catabolic stress and dehydration and remain at risk of severe metabolic crises. Thus, careful metabolic monitoring and prompt treatment post liver transplantation are still required to avoid neurological sequelae of MSUD, particularly if the donor is heterozygous for MSUD.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
MSUD was first described in 1954 by Menkes et al. in four siblings with progressive infantile cerebral dysfunction and urine odor resembling maple syrup (Menkes et al. 1954). Markedly increased blood levels of leucine, isoleucine, and valine as well as ketonuria were then reported by Westall et al. (1957).The disease results from a deficiency in one of the three subunits of the branched-chain keto acid dehydrogenase (BCKDH) complex. This mitochondrial enzyme catalyzes the oxidative decarboxylation of branched-chain keto acids (BCKA) that are derived from the transamination of valine, leucine, and isoleucine. The management involves life-long dietary leucine restriction through a protein-restricted diet and BCAA-free medical foods, isoleucine and valine supplementation as needed, as well as careful clinical and biochemical monitoring. Metabolic decompensations can be complicated by brain edema and irreparable neurologic injury. They require aggressive management that includes treating the precipitating stress, maximizing calorie intake, and continuing the standard treatment of the disease. Dialysis is sometimes necessary to remove the excess BCAA (Westall et al. 1957).
Liver transplantation using deceased unrelated donors is considered potentially curative, offering complete protection from metabolic decompensations on a normal diet (Wendel et al. 1999; Bodner-Leidecker et al. 2000). Here, we report a child with MSUD type II who underwent a successful liver transplant from his mother but developed at least two episodes of severe symptomatic metabolic decompensation requiring intensive management. To the best of our knowledge, this is the first case of a well-documented occurrence of this complication.
Patient
The child of a consanguineous marriage presented with progressive irritability, lethargy, convulsions, and encephalopathy at 10 days of age and was diagnosed with MSUD type II (MIM 248610). Initial amino acid profile showed elevated leucine (1,950 μM, normal 75 to 163), isoleucine (316 μM, normal 40 to 88), valine (450 μM, normal 126 to 220), and alloisoleucine (detected). He was treated with dietary leucine restriction, BCAA-free medical foods, and isoleucine and valine supplementations. He recovered very well. DBT gene sequencing showed a homozygous splicing mutation c.1281+1G>T in exon 10. The parents were proven to be heterozygous for the same mutation. BCKDHA and BCKDHB genes were normal.
He had seven metabolic decompensations in the first 8 months of life with maximum leucine levels between 500 and 1,000 μM. He also had poor weight gain and mild hypotonia. His tolerated leucine level ranging between 48 and 272 μM (normal 75 to 163) whenever he was well prior to the liver transplantation.
At 15 months of age, liver transplantation was performed at King’s College Hospital, London UK. His mother, 24 years old, was the donor; she was healthy and had no history of metabolic decompensation. During the immediate postoperative period, his branched-chain amino acid levels settled slowly. However, he made good clinical progress and was discharged 11 days after the procedure on an unrestricted diet and immunosuppression with tacrolimus, mycophenolate mofetil and prednisolone. Shortly thereafter, he developed biochemical hepatitis (elevated transaminases) associated with CMV viremia. During follow-up, he suffered a brief episode of lethargy and seizure attributed to tacrolimus toxicity. It was treated with intravenous fluid and recovered completely within 2 days; an amino acid profile was not requested. Afterwards, while consuming a normal protein containing diet, he remained clinically stable; his hypotonia improved considerably and his seizures did not recur.
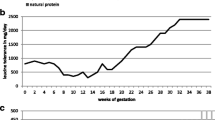
At 20 months of age, 5 months after the transplant, he presented to our center with symptoms of gastroenteritis and dehydration. He was admitted for intravenous rehydration for 1 day and went home. Three days later, he developed lethargy, convulsions, and encephalopathy requiring intensive management with high calories, BCAA-free medical foods, supplementation of isoleucine and valine, insulin administration and peritoneal dialysis. The amino acid profile of the initial admission for gastroenteritis reached us after a few days and showed elevated leucine (991 μM, normal range 73–161), isoleucine (521 μM, normal 46–90), valine (1,162 μM, normal 149–277) and alloisoleucine (detected). The amino acid profile at the time of encephalopathy and convulsions showed leucine (2,001 μM, normal range 73–161), isoleucine (877 μM, normal 46–90) and valine (1,653 μM, normal 149–277). His ALT was 30 IU/L (normal range 11–39) and his AST was 35 IU/L (normal range 22–58); the other liver tests were also normal. His ammonia level was 46 μmol/L. Liver Doppler study was normal, including the hemodynamic status of both arterial and venous vasculature. He recovered within 48 h and resumed his normal diet, without any protein restriction. His amino acid profiles during the illness are shown in Fig. 1.
Concentrations of branched-chain amino acids (BCAA) in plasma after liver transplantation in the index case. BCAA increased with acute dehydration evolving into encephalopathy (horizontal arrow) followed by normalization of BCAA on unrestricted diet after 48 h of intensive metabolic management (vertical arrow). The Y-axis indicates the BCAA plasma concentrations in μM/L and the X-axis days posttransplantation when patient presented with metabolic crisis
After that crisis, he remained well on a normal protein diet. His BCAA were done repeatedly and remained within the normal range.
At the age 26 months, 10 months after the transplantation, he presented again with an episode of seizures. This was his third one, after the one shortly after transplantation, which was attributed to tacrolimus toxicity, and the second one, 5 months after transplantation, while he was on unrestricted protein intake. This third episode was described as tonic movements of the right leg with uprolling of the eyes lasting for about 1 min. The amino acid profile on admission showed elevated leucine (990 μM, normal range 73–161), isoleucine (461 μM, normal 46–90), valine (892 μM, normal 149–277) and alloisoleucine (detected). Interestingly, a routine amino acid profile had been done 2 days prior to the admission. It was completely normal with leucine (66 μM, normal range 73–161), isoleucine (61 μM, normal 46–90) and valine (154 μM, normal 149–277). The patient recovered nearly completely within 24 h with IV fluids and his medical food. Since then, he again has been doing very well on normal diet. His last amino acid profile showed leucine, isoleucine, and valine values of 122, 53, and 145 μM, respectively; all are normal.
Discussion
The natural history of MSUD following liver transplant has been reported repeatedly. In all cases reported till now, the patients tolerated a normal diet and did not experience symptomatic metabolic crises after transplantation. These observations suggested that liver transplant sufficiently corrects BCAA metabolism in MSUD (Wendel et al. 1999).
Mazariegos et al. conducted a cohort study to assess the neurocognitive function of children with MSUD who had a liver transplant. Thirty-five patients (age, 9.9 ± 7.9 years) were followed between 2004 and 2009. They showed that liver transplant is an effective long-term treatment for MSUD and may arrest further brain damage (Mazariegos et al. 2012). However, they did report a child who developed hyperleucinosis during an episode of gastroenteritis and dehydration; this episode occurred 55 months post transplant from a non-related donor. The plasma leucine level reached 2,170 μM, isoleucine 1,009 μM and valine 1,483 μM. Surprisingly, this patient did not develop obvious neurological symptoms during this episode. His BCAA levels normalized within a few days of intravenous hydration, without specific metabolic treatment (Mazariegos et al. 2012).
Our patient, who received a graft from an asymptomatic carrier parent, showed some delay in normalizing BCAA levels despite very good early graft function. A minor episode of lethargy and brief seizures occurred 2 months after transplant, which, retrospectively, was probably due to a metabolic crisis. Both 5 and 10 months after transplant, he had a characteristic metabolic crisis with encephalopathy and seizures; his branched amino acid levels were very high, and he required emergency metabolic treatment. However, the patient then was able to return to a normal diet with normal BCAA levels.
As far as we know, this is the first time acute well-documented metabolic crises with severe neurological symptoms have occurred in a transplanted MSUD patient.
Mazariegos et al. suggested that visceral ischemia, hepatic vascular compromise, dehydration or factors restricting hepatic blood flow can compromise BCAA clearance by the graft (Mazariegos et al. 2012). It is not likely that any of these played a major role in our patient’s metabolic crises because both his liver echo-Doppler and liver function tests were normal at the time of the crisis at 5 months post transplant, which was the most serious one.
We hypothesized unknown factors in the metabolism of the transplant from the mother that would decrease its BCKDH branched-chain keto acid dehydrogenase complex activity more than would be expected in a heterozygote carrier. To gather evidence for this, we asked the mother to follow a high-protein diet for several days and then checked her branched amino acids. They remained in the low normal range (leucine 73 μM (normal range 97–161), isoleucine 38 μM (normal 46–90), and valine 145 μM (normal 178–284)). Thus, it appears that the mother has normal protein tolerance rather than an unusual dominant-negative mutation or any other factor leading to significant impairment of branched-chain amino acid metabolism, in spite of her heterozygous state.
Feier et al. reported a 2-year-old patient with MSUD who underwent liver transplant from his mother. The recipient’s leucine levels were normal by the second postoperative day without any dietary restriction and neither the donor nor the recipient experienced metabolic decompensations after the transplant, showing the utility of this management (Feier et al. 2014). However, the non-symptomatic metabolic crisis described by Mazariegos and the events described in our patient indicate that the capacity of a grafted liver to oxidize BCAA can be overwhelmed by catabolic stress and dehydration and that the protection afforded by the liver transplant may not be complete in some cases. The liver probably accounts for only 9 to 13% of the whole body BCKDH activity enzyme activity; the rest is found in other tissues, mainly in muscle (54–66%) (Mazariegos et al. 2012). It is reasonable to assume that heterozygous carriers of MSUD would provide less BCKDH activity in a live donated organ and therefore less protection during stress, leading to an increased risk for symptomatic metabolic crises post transplant. However, we do not know if the patient described by Mazariegos with an asymptomatic increase in leucine after transplant, received it from a heterozygous carrier or not. The case of our patient reinforces once more the importance of the following recommendations of Mazariegos et al.: (1) Clinicians should continue monitoring amino acids in post transplant patients, particularly those who develop serious catabolic illness or unexplained encephalopathy; (2) visceral ischemia, hepatic vascular compromise, dehydration, or factors restricting hepatic blood flow can compromise BCAA clearance by the graft (Mazariegos et al. 2012).
Abbreviations
- BCAA:
-
Branched-chain amino acids
- BCKDH:
-
Branched-chain keto acid dehydrogenase complex
- MSUD:
-
Maple syrup urine disease
References
Bodner-Leidecker A, Wendel U, Saudubray JM, Schadewaldt P (2000) Branched-chain L-amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation. J Inherit Metab Dis 23:805–818
Feier FH, Miura IK, Fonseca EA, Porta G, Pugliese R, Porta A, Schwartz IV, Margutti AV, Camelo JS Jr, Yamaguchi SN, Taveira AT, Candido H, Benavides M, Danesi V, Guimaraes T, Kondo M, Chapchap P, Neto JS (2014) Successful domino liver transplantation in maple syrup urine disease using a related living donor. Braz J Med Biol Res 47(6):522–526
Mazariegos GV, Morton DH, Sindhi R, Soltys K, Nayyar N, Bond G et al (2012) Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J Pediatr 160:116–121. doi:10.1016/j.jpeds.2011.06.033
Menkes J, Hurst P, Craig JM (1954) A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance. Pediatrics 14:462–466
Wendel U, Saudubray JM, Bodner A, Schadewaldt P (1999) Liver transplantation in maple syrup urine disease. Eur J Pediatr 158(Suppl 2):S60–S64
Westall RG, Dancis J, Miller S (1957) Maple syrup urine disease. Am J Dis Child 94:571–572
Acknowledgment
We want to thank the family. We also want to thank George Mazariegos from Pittsburgh, who contributed many useful ideas and kindly reviewed the article, as well as Fatma Al Jasmi and Abdul-kader Souid for reviewing the manuscript and useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Francois Feillet, MD, PhD
Compliance with Ethics Guidelines
Compliance with Ethics Guidelines
Conflict of Interest
The authors have declared that no conflict of interest exists.
Rights and permissions
Copyright information
© 2016 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Al-Shamsi, A., Baker, A., Dhawan, A., Hertecant, J. (2016). Acute Metabolic Crises in Maple Syrup Urine Disease After Liver Transplantation from a Related Heterozygous Living Donor. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 30. JIMD Reports, vol 30. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2016_532
Download citation
DOI: https://doi.org/10.1007/8904_2016_532
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53680-3
Online ISBN: 978-3-662-53681-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)