Abstract
The microenvironment plays an integral role in directing the differentiation of stem cells. The ability to control and manipulate systems on the microscale can be used to control the cellular microenvironment to direct stem cell behavior. For stem cells, this control greatly improves our ability to study cell–microenvironment interactions in a rapid and precise manner to regulate stem cell behaviors such as differentiation and proliferation. Combining microscale technologies with high throughput techniques could also greatly increase the possibility for probing the multivariable complexity of biological systems. In this chapter, microengineering approaches to control the cellular microenvironment and to influence embryonic stem cell (ESC) self-renewal and differentiation are introduced and specific examples of the use of microfabrication technologies for directing ESC fate decisions are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The establishment of embryonic stem cell (ESC) lines derived from both mouse and human cells has generated the possibility of cell therapies based on an unlimited and renewable source of cells [1, 2]. However, the use of human embryos to generate ESC lines is somewhat controversial [3]. A breakthrough to this problem was established when Yamanaka and co-workers demonstrated in vitro reprogramming of murine fibroblasts into induced pluripotent stem cell (iPS) [4]. These iPS cells were proven to be functionally and molecularly similar to ESCs [5, 6] offering new opportunities in regenerative cell therapy [7–9]. However, there are still many requirements to fulfill before these cells can be used clinically. One such challenge is to controllably direct stem cell differentiation [10].
Stem cells are sensitive to a variety of microenvironmental stimuli that regulate both self-renewal and differentiation [11]. Therefore stem cells can be characterized by their capacity to differentiate into specific cell lineages in response to temporally and spatially regulated extrinsic and intrinsic signals. Microengineering enables the regulation of cell–microenvironment interactions, such as cell–cell, cell–extracellular matrix (ECM), cell–soluble factors, and cell–mechanical stimuli interactions [12]. Thus, through microscale engineering, cell differentiation can be guided through controlled interplay between regulatory factors down to the level of individual cells. Also, microengineering can be used to generate three dimensional (3D) microenvironments for the development of physiologically relevant tissue models for tissue engineering [13–15]. This can be achieved through the merger of microfabrication techniques with biomaterials to create the desired tissue structures [16]. Advanced biomaterials are being used to study stem cells, interactions between cells and biomaterials, and as tissue engineering scaffolds. The field of tissue engineering is driven by the need to provide functional equivalents of native tissues that can be used for the in vitro study of tissue physiology and pathology, as well as for implantation where no native or artificial transplantable materials are currently available in sufficient quantities to repair damaged tissues [17]. This interdisciplinary field is at the intersection of engineering, biology, and medicine and aims to develop biological substitutes that restore, maintain, or improve tissue function.
Recent research has confirmed the existence of stem cells residing in niches, unique to the tissues and organs in which they reside that contain highly ordered microarchitectures, cellular compartmentalization, and arrangement [18]. The merger of microfabrication and advanced biomaterials are useful to recreate many of these complex features, to create in vitro microenvironments with the ability to effectively direct ESC behavior. These approaches are becoming powerful tools for the study of stem cells. The following chapter will highlight the current techniques for using microscale biomaterials both for investigating and directing stem cell behavior and for creating engineered tissues using 3D cell-laden scaffolds. These two applications of microscale engineering may be of great benefit to the future of regenerative medicine. The clinical success of regenerative medicine is highly dependent on successes in both tissue engineering and stem cell differentiation, suggesting that the development of technologies to achieve the desired outcome will hopefully shorten the time needed to bring these techniques to the clinic.
2 Control of the Cellular Microenvironment
The ability to control the cellular microenvironment is key to controlling cell viability, growth, migration and differentiation. Microenvironmental control can be performed at the level of interactions with surrounding cells, substrate mechanics, biomaterial chemistry, applied physical forces, and the degradation of surrounding materials [19–21]. For ESCs, self-renewal and differentiation pathways appear to be controlled by several interconnected interactions based on cell–cell interactions, cell–ECM interactions, and cell–soluble factors interactions (Fig. 1). We will discuss these interactions and subsequently discuss how microengineering techniques could control these interactions.
2.1 Cell–cell Contacts
Cell–cell contacts are major regulatory signals that control cell behavior [22]. For instance, the differentiation of skeletal muscle cells has been reported to depend on cell–cell contacts that induce cell cycle arrest and subsequent gene expression [23]. One representative factor that participates in and controls cell–cell adhesions are cadherins. Cadherins are a class of type I transmembrane protein with extracellular Ca2+-binding domains. There are several classes of cadherin molecules, and different classes are found in different cell types and tissues. For instance, E-cadherins are generally found in epithelial tissues, and preferentially bind to other E-cadherin molecules. This homophilic binding has been attributed to the NH2-terminal EC1 domain [24]. However, several observations have suggested that cadherins can also interact in a heterophilic fashion [25, 26]. In addition to E-cadherins, other cell types and tissues express other classes of cadherin: P-cadherin in the placenta, N-cadherin in neural cells, R-cadherin in renal tissue, VE-cadherin in vascular endothelial cells, M-cadherin in myotubules, OB-cadherin in osteoblasts, and K-cadherin in the kidney [27, 28]. Cadherin binding leads to specific signaling cascades that affect cellular behavior and function. Upon binding of the extracellular domain of cadherins, the intracellular domain binds p120-catenin and beta-catenin containing a highly phosphorylated region. Activated catenin also binds to alpha-catenin, resulting in regulation of actin-containing cytoskeletal filaments, ultimately leading to specific control of gene transcription [27, 29].
The loss or decrease in E-cadherin expression and function has been reported to be associated with high cancer cell progression and metastasis in which decreased strength of cellular adhesion within a tissue results in an increase in cellular motility and may allow cancer cells to cross the basement membrane and invade surrounding tissues [30–32]. For example, the addition of anti-E-cadherin antibodies restored cell migratory behavior by blocking cadherin-dependent adhesion [33]. Another study showed that transfecting fibroblasts with E-cadherin similarly suppressed cell infiltration of collagen gels in an E-cadherin-dependent manner [34]. Recently, it has been reported that E-cadherin regulates cell motility by both adhesion-dependent and adhesion-independent mechanisms in which E-cadherin-dependent reduction in epithelial cell motility depends both on the E-cadherin expression level and on the E-cadherin density on the migratory substratum [35]. These mechanisms are important in wound healing as cell–cell interactions, cell migration, and cell proliferation as it plays key roles in the tissue’s ability to repair a defect [36]. In addition to its modulation of wound healing mechanisms, similar cadherin modulated cell–cell interactions also play a key role in ESC differentiation and growth. For example, E-cadherin has been shown to induce cell to cell adhesion for the formation of embryoid bodies (EBs), which are 3D spherical aggregates containing differentiated cells of all three germ layers. Although E-cadherin is constitutively expressed in early stage embryos, it is down-regulated as the cells differentiate [37, 38]. Other factors that can similarly modulate stem cell attributes such as aggregation, migration, proliferation or differentiation can have a profound impact on the control of stem cell behavior.
2.2 Cell–soluble Factor Interactions
Soluble factors, such as growth factors, play an important role in influencing cell growth and differentiation. Growth factors are proteins that bind to receptors on the cell surface, and the binding activates a specific series of cellular mechanisms leading to events such as cellular proliferation and/or differentiation. These regulatory molecules are naturally derived, and can either be added to the culture medium or more specifically delivered to the targeted cells. Many growth factors are quite versatile, stimulating cellular responses in numerous cell types; while others act specifically on a particular cell-type. Based on their ability to control and direct cell behavior, the use of growth factors has been investigated for directing the lineage specific differentiation of ESCs. For example, Activin A was shown to mediate dorsoanterior mesoderm differentiation, while bone morphogenetic protein 4 (BMP-4) was shown to mediate formation of hematopoietic cells [39–41]. Also, it has been demonstrated that binding of IGF-II, a member of the insulin-like growth factor (IGF) family, to its signaling receptor, IGF1R, at the surface of mesoderm precursor cells increased mesoderm formation [42]. In addition, EGF, BMP-4 and FGF have been shown to induce ectodermal and mesodermal differentiation [43].
2.3 Cell–extracellular Matrix Interactions
The ECM consists of the structural proteins that surround cells in mammalian tissues. This serves to provide structural support and anchorage to the cells in addition to compartmentalizing cells and tissues, regulating intercellular communications, and sequestering growth factors [44, 45]. Cell–ECM interactions play a critical role in cell function and physiology through cell binding to components of the ECM. This cell–ECM adhesion is regulated by specific molecules of the surface of cells called cellular adhesion molecules (CAM), such as integrins. ECM provides a template for cell adhesion, proliferation, migration, differentiation and tissue formation. The phenotype of originated cells has also been observed to be sensitive to tissue elasticity and stiffness. For example, to direct the differentiation of mesenchymal stem cells (MSCs) soft matrices that mimic the brain are found to be neurogenic, while stiffer matrices that mimic muscle are myogenic, and comparatively rigid matrices that mimic collagenous bone were found to be osteogenic [46].
Recently, researchers have designed various biomaterials to provide more complex and biomimetic environments for ESC expansion and differentiation. For instance, Lammers and colleagues tested the ability of various biomaterials for ESC proliferation and differentiation, and reported that scaffolds made of insoluble collagenous bone matrix in combination with β-tricalciumphosphate were more effective in directing ESC differentiation into osteogenic lineage [47]. In addition, Langer and colleagues have demonstrated that rigid polymeric scaffolds supported ESC differentiation. When ESCs were surrounded by a soft biomaterial such as Matrigel, tissue was not formed, however, as Matrigel was combined with a rigid polymeric scaffold, tissue-like structures were formed [48]. These data suggested that the mechanical properties of polymeric scaffolds can be used to direct ESC differentiation and induce tissue formation. In addition, it was also found that fibronectin (FN) stimulated ESCs to differentiate into endothelial cells whereas laminin induced ESCs to differentiate into cardiomyocytes [49]. Thus, showing that the ECM chemistry works alongside mechanics in directing ESC fates.
3 Microengineering the Environment
Microengineering techniques can be used to regulate the cell’s interactions with its microenvironment [50]. For example, by restricting the cellular geometry through patterned substrates, controlled microenvironments were created to investigate specific cell behaviors [51–53]. Microengineering approaches have also been used to control the cellular microenvironment by using microscale channels [54], microwells [55], and cell-laden hydrogels [50, 56, 57] (Fig. 2). In addition, biomaterials have been engineered that are capable of controlling cell behavior [50, 58, 59]. These biomaterials have tunable property such as a controlled degradation rate, hydrophilicity, and mechanical properties. The ability to use microengineering to manipulate biomaterials is emerging as a powerful tool in regulating ESC behavior. These manipulations can involve methods of fabricating microscale units of biomaterials or spatial control of material properties. Furthermore, microarray techniques can be used to identify biomaterials that can direct ESC fate responses by enabling massively parallel synthesis and characterization of cell–materials interactions. These studies of cell behavior in response to various natural or synthetic stimuli have applications in a wide variety of fields ranging from basic biological studies to drug discovery. Here, we will review these techniques and where appropriate provide examples of their use in directing ESC differentiation.
3.1 Microfluidic Platforms for Controlling Cell–soluble Factor Interactions
The field of microfluidics involves the manipulation and processing of micro- and nanoliter volumes of fluids within small channels that are typically in the range of 10–100 μm [60]. Fluid behavior on the micron scale is different from that occurring on the macroscale [61]. For example, viscosity and molecular diffusion are of high importance at the microscale, while having less effect at the macroscale. This is because the small dimensions of microfluidic systems cause the Reynolds number to be low, making the flow in the laminar range. The Reynolds number is determined as Re = ρ*v*d/μ, where ρ is the fluid density, v is the fluid velocity, d is the hydraulic diameter of the channel, and μ is the fluid viscosity. Therefore small changes to viscosity is important at the microscale because of their substantial impact on the Reynolds number and whether the flow remains laminar. In addition to laminarly flowing fluids, microchannels offer other advantages compared to conventional systems. These include reduced analysis times, the need for lower sample/reagent volumes as well as the ability to run multiple assays on a single device [62, 63].
With the introduction of microfluidic platforms, stem cell research has entered a new era in which the cell–soluble factor interactions can be controlled in a much more controlled manner. Microfluidic technologies, which often use polydimethylsiloxane (PDMS) [62], have been used in number of different applications for studying cells which includes fluidic based cell patterning, subcellular localization of media components, high throughput drug screening, analysis of laminar flows on cells, and creation of soluble factor gradients [64–67].
One of the most valuable applications of microfluidic systems is the ability to expose cells to laminar flows to control mixing and shear stress. Kim et al. showed that microfluidic devices can produce a logarithmic scale of flow rates and logarithmic concentration gradients. It has been shown that cell morphology and proliferation for ESCs varies based on these gradients [68]. This ability to control the flow rate and shear stress has been utilized to study ESC self-renewal and proliferation, to show that high flow rates result in increased proliferation [69]. Since microfluidic systems use low sample/reagent volumes they are useful tools for the screening of soluble components such as serum components (growth factors), conditioned media, or different chemical formulations. In addition, microfluidic systems have been used for making gradients growth factors or oxygen concentrations to test their effects on cells in inefficient manner [70].
Microfluidic systems have also been used to mechanically stimulate cells. For example, Park et al. showed that compressive cyclic loading enhanced the osteogenic differentiation of human mesenchymal stem cells [71]. In another study, shear stress gradients where studied on hESC-derived endothelial cells to show that these cells are capable of responding to shear stress changes through varying gene expression [72]. The combination of microfluidic systems with controlled flow rates or concentration gradients have been shown to be important for regulating cell differentiation [64, 73]. For example, Chung et al. showed a microfluidics system for neural stem cell differentiation and proliferation, demonstrating that different growth factors combinations consisting of epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2) and platelet-derived growth factor (PDGF) could be used for monitoring differentiation towards astrocyte lineage in the laminar flow range. Microfluidic systems have also been used for analyzing single cell gene expression profiles to demonstrate a fivefold increased efficiency compared to bulk reactions [74]. Moreover, microfluidic devices can be used to deliver extremely precise concentrations of signaling molecules to cells [11]. With these systems, there is an improved ability to illustrate the complex in vivo environment with strict control over spatial and temporal resolution of soluble factors. This high degree of control on the microscale makes these systems ideal for studying the response of ESCs in a high throughput manner. In addition the volumes are much smaller than in traditional culture systems reducing the use of expensive soluble factors to enable testing of more complex interactions than is possible with traditional systems.
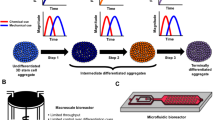
3.2 Controlled Microbioreactors
In a developing organism, tissues emerge from coordinated sequences of cell renewal, differentiation, and assembly within a dynamic environment characterized by spatial and temporal gradients of multiple factors. Thus, to direct cells to differentiate at the right time, in the right place, and into the right phenotype, one needs to recreate the appropriate microenvironment.
One way to accomplish this complicated feat is to characterize the native tissue environments and attempt to mimic these features in vitro. In tissues, cells are surrounded by other cells and embedded in an ECM that defines the architecture, signaling, and biomechanics of the microenvironment [75]. Cells respond to their immediate microenvironment, via phenotypic or heterotypic interactions with neighboring cells [76]. This can be difficult to recreate completely in vitro since much of the complex interplay of mechanical and molecular factors present in vivo are absent [77]. It has been argued that a new generation of 3D culture systems are needed that would be “something between a Petri dish and a mouse” to correctly present a cell’s environment in a living organism and be more predictive of in vivo systems [78].
For stem cells in particular, to unlock their full potential and obtain biologically sound and relevant data in vitro, at least some aspects of the dynamic 3D environments that are associated with their renewal, differentiation, and assembly into tissues need to be reconstructed. A fundamental approach to the generation of engineered tissues is to direct the 3D organization of cells (via biomaterial scaffolds) and to establish the conditions necessary for the cells to reconstruct a functional tissue structure (via bioreactors). This approach is based on a premise that cells’ responses to environmental factors are predicable, and that the cell function, in vitro can be modulated by the same complex factors known to play a role during development and remodeling.
ESCs can greatly benefit from being cultured in bioreactors that controllably recreate cell and tissue specific environmental conditions. Bioreactor systems may better facilitate the transition from laboratory to clinical scale production due to their scalability, while providing a means to quantitatively study cell behavior in response to various stimuli. Some of the important considerations during bioreactor design are: (1) rapid and controllable expansion of cells, (2) enhanced cell seeding of 3D scaffolds at a desired cell density, yield, kinetic rate, and spatial uniformity, (3) efficient exchange of oxygen, nutrients, and metabolites, and (4) provision of physiological stimuli [11]. Current designs incorporate cascades of biological and physical stimuli to exert greater influence over cellular differentiation and development into functional tissue constructs [79]. Bioreactors are typically custom engineered to account for specific mechanisms of nutrient transfer and specific physical factors inherent in the desired tissue. For instance, simple control of the transport rate of oxygen could monitor the pH of the environment in which stem cells are cultured [80, 81]. These bioreactors are often designed to be modular, mini-scaled, and multiparametric, to economize cells and reagents, and to increase the number of samples that can be analyzed.
3.3 Surface Micropatterning for Controlling Cell–cell Contacts
Microscale surface patterning techniques enable the control of cell–cell contacts on geometrically defined 2D surfaces [12, 82]. A variety of microscale technologies including microtopography [55, 56], microfabricated stencils [83], microcontact printing [84], and layer-by-layer deposition [85] have been developed to pattern the ESCs on 2D substrates. In one approach, hydrogel microwell arrays that generate low shear stress regions enabled the docking and positioning of ESCs. Such microwells could be seeded with feeder cells to maintain human ESCs in an undifferentiated state [86]. Microwell arrays which are fabricated from polyethyleneglycol (PEG) hydrogels have been demonstrated as a useful research tool for uniform cell seeding and EB formation [87] (Fig. 3). Similar microwell arrays were used to generate spatially and temporally synchronized beating human EBs [88]. Microwell arrays can also be used to screen and characterize ESCs, while homogeneous EBs obtained from this microfabricated microwell can direct EB-mediated differentiation [84]. In another example, a Bio Flip Chip (BFC) containing thousands of microwells with sizes optimized to trap single murine ESCs were fabricated to provide both incremental and independent control of contact-mediated signaling [89].
(Left) PEG microwell fabrication through UV cross-linking. (Right) Localizing cells within arrays of microwells using a wiping technique. This method produces cell seeding densities that vary consistently with microwell geometry and cell concentration [87]
Cell–cell communications in aggregates play a significant role in controlling ESC proliferation and differentiation. A microfabricated PDMS stencil can regulate the initial size of ESC aggregates, which is a critical factor for controlling ESC differentiation. By using this technique, it was demonstrated that larger aggregates resulted in more mesoderm and endoderm differentiation than small aggregates. Microfabricated parylene-C stencils were also used to micropattern co-cultures in both static and dynamic conditions by sequentially using several stencils with differently modified surface properties [90]. In addition to microfabricated parylene-C stencils, microcontact printing was used to study the differentiation of micropatterned human ESCs [84]. The gene and protein expression analysis of micropatterned human ESCs demonstrated that the endodermal and neuronal expression increased inversely with colony size. Moreover, greater mesoderm and cardiac induction was observed for larger EBs. This analysis demonstrated that heterogeneity in human ESC colony and aggregate size generated subsets of appropriate conditions for differentiation.
3.4 High-throughput Microarrays for Screening Microenvironments
ESC differentiation can be directed through different combinations of factors that influence the microenvironment. To study the combination of various influential stem cell differentiation factors is an enormous combinatorial problem that would be extremely difficult to analyze without the facilitation of high-throughput analysis, screening, and imaging. This goal can be achieved more readily through the effective use of microarray systems for analysis and screening of multiple factors individually and in combination. Because of the significant number of possible combinations, high-throughput approaches can be used to rapidly test and discover important combinations of parameters in ESC differentiation. Parameters such as ECM proteins, soluble molecules (e.g. cytokines, growth factors), and biomaterial interactions can influence the pathway and efficiency of ESC differentiation. To improve our understanding of ESC differentiation, studying the combined influence of parameters that may regulate stem cell expansion and specialization in an efficient and cost effective manner using high-throughput analysis, screening, and imaging may prove beneficial.
In addition to growth factors and cell-secreted morphogenetic factors, synthetic small molecules can direct ESC differentiation. Small permeable, naturally occurring molecules such as vitamin C, sodium pyruvate and retinoic acid have been used to regulate stem cell fate [91]. Similarly, new synthetic, heterocyclic small molecules that can alter stem cell fate have recently been studied to control stem cell differentiation. Ding et al. reported ESC differentiation into various cell types employing small molecules [92]. One manner by which synthetic molecules can be used to selectively control and regulate stem cell differentiation and proliferation is through adjusting the activities of proteins. Such processes have successfully been employed to cause controllable neurogenetic and cardiomyogenetic induction in murine ESCs, osteogenesis induction in MSCs, and skeletal muscle cell differentiation [91, 93]. Further investigation into small molecule discovery could play a substantial role in furthering the ability to reliably differentiate stem cells down specific pathways.
The interactions between ESCs and the surrounding matrix environment can profoundly influence stem cell behavior and direct ESC differentiation. A library of 576 different combinations of acrylate-based polymers was used to determine the effect on stem cell behavior in a biomaterial array format [94]. Combinations of the different polymers were mixed in 384-well plates and were robotically printed on coated glass slides. After printing, the slides were exposed to long wave UV to initiate polymerization, dried, sterilized with UV, and washed with PBS and cell culture media. ESCs were then seeded onto the slides and the influence of the materials on ESC differentiation was analyzed. Based on these observations, it was concluded that ESC differentiation may be induced toward epithelial cell lineage through interactions with specific combinations of materials. This study demonstrated the potential for creating a library of synthetic materials to direct ESC fate decisions.
3.5 Three Dimensional Scaffolds for Culturing ESCs
Biocompatible and biodegradable polymer scaffolds can be used to control growth and differentiation of ESCs [95]. Scaffolds provide structural support and physical cues for cell attachment, orientation, alignment and spreading. A fundamental approach to the generation of engineered tissues is to direct the 3D organization of cells (via biomaterial scaffolds) and to establish the conditions necessary for the cells to reconstruct a functional tissue structure (via bioreactors). This approach is based on the premise that the cellular response to environmental factors are predictable, and that the cell function, in vitro can be modulated by the same complex factors known to play a role during development and remodeling in vivo.
Various scaffold features, such as physical cues can have an impact on the differentiation of ESCs. ESCs cultured within 3D scaffolds can be differentiated into specific tissue lineages based on the scaffold characteristics. For example, ESC differentiation towards an osteogenic lineage was achieved through seeding cells onto appropriately designed biodegradable scaffolds as demonstrated by increased expression of osteo-specific markers and bone nodules [96]. The effect of the 3D environment on ESC growth and differentiation was further studied by culturing EBs in different polymer networks [49]. Various compositions and structures of polymer matrices were evaluated, demonstrating that both material structure and stiffness of the 3D scaffolds influenced ESC fate. Using analogous systems, ESC differentiation down a neuronal differentiation pathway, was also studied [97]. It was shown that the number and maturity of neural-like structures identified by neuronal marker (i.e. βIII-tubulin) were increased within these 3D tissue constructs. Therefore, specifically designed 3D scaffolds have the potential for creating 3D tissue constructs using stem cells differentiated into the desired tissue type.
3.6 Tissue Engineering Using Assembly of Microengineered Building Blocks
Similar to their use in controlling the behavior of stem cells, microscale technologies can be used to control and direct engineered tissue development by creating microgels with specific microarchitectural features containing stem cells. Tissue engineering, using a bottom-up approach, creates macroscale tissues with controlled microarchitectural features through assembly of microscale cell-laden building blocks. Through control of the microenvironment, the goal is to create macroscale tissues with specific microarchitectural features, amenable to directing tissue behavior.
One such technique employed a layer-by-layer approach, where arrays of cells and ECM/polymer were additively photocrosslinked [98–100]. In one example, rat hepatocytes were mixed with RGD functionalized PEG prepolymer and the resulting mixture was polymerized through exposure to light through one of three photomasks, the first of which resulted in a three pointed star alignment. Subsequent layers were created using complementary photomasks, creating a honeycomb enclosure around two-layer cell-laden tissue units, mimicking native hepatic tissue. Tissue from this technique demonstrated many benchmarks of hepatic tissue function, such as urea production. The next step would be to recreate this technique using stem cells to determine whether recreation of the native architecture using these protocols would lead to functional hepatic tissues with a more clinically relevant cell source.
A major challenge to overcome in engineered tissues is the creation of functional, integrated microvasculature. Previously, random packing technique was used to create tissues with perfusable capillary networks. To create these building blocks, HepG2 cells were mixed with collagen, allowed to gel in specific shapes and then mixed with HUVEC cells which formed an endothelial monolayer around the surfaces of the hydrogel blocks [101]. The HepG2-HUVEC modules were packed into unidirectional perfused tubing with a porous plug to contain and allow for module aggregation and compaction. The engineered tissues were successfully perfused with blood to demonstrate cell viability as well as endothelial function, as the tissues perfused without clotting. One major advantage of this technique was the ability to create perfusable tissues with functional endothelialized channels, with the potential to contain any number of cell types in the hydrogel module cores. Employing stem cells in this system could yield functional tissues designed primarily for filtration, such as the liver or kidney, while more tissue types could be possible if the culture conditions are optimized to allow for removal from the perfused tubing.
Recent work in our laboratory demonstrated the use of directed assembly to create tissue structures using cell-laden microgels. This technique harnesses the surface tension properties of hydrophilic hydrogels to assemble cell-laden microgels into tissue structures [102]. Cell-laden hydrogels of varying aspect ratios were placed into hydrophobic mineral oil to induce aggregation of hydrophilic microgels as they attempt to minimize surface energy. These assemblies were subsequently crosslinked into macroscale engineered tissues. The tissue shape and dimensions were controllable based on the building blocks’ aspect ratios, while even greater control, and the potential for making more complex structures, was demonstrated by using lock-and-key type geometries. These results introduce a useful approach to create larger and more complex tissues with controlled co-culture conditions, using a number of cell types.
These are just a few of the techniques that could be used to create engineered tissues with microscale features containing stem cells (Fig. 4). With the ability to not only create specific microarchitectural features to control and direct stem cell behavior, but also to assemble these microgels into macroscale structures while retaining control over the microenvironment, the future for creation of stem cell based engineered tissues for use in vivo looks bright.
Creating engineered tissues using directed assembly. Lock (a) and key (b) shaped hydrogel modules were created through UV exposure through a photomask, then aggregated and assembled in mineral oil, into single (c, d), double (e, f), triple (g, h) arrangements demonstrating control of co-cultured structures. Scale: 200 μm. Reprinted with permission from the National Academy of Science [102]
4 Conclusions
Although ESCs have been considered as a potential cell source for tissue engineering, some major challenges still remain in directing stem cell differentiation to specific cell types to create tissues with specific functions. It is believed that the merger of microengineering with biomaterials will lead to more precise understanding of cell biology and greatly contribute to the therapeutic potential of stem cells for tissue engineering. The combination of these fields contains great potential for the advancement of the field of regenerative medicine. The improvements in the fields mentioned above will aid in realizing the dream of regenerating degenerated or defective tissues to cure millions of current and future patients.
References
Martin, G.R.: Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78(12), 7634–7638 (1981)
Thomson, J.A. et al.: Embryonic stem cell lines derived from human blastocysts. Science 282(5391), 1145–1147 (1998)
Rolletschek, A., Wobus, A.M.: Induced human pluripotent stem cells: promises and open questions. Biol. Chem. 390(9), 845–849 (2009)
Takahashi, K., Yamanaka, S.: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4), 663–676 (2006)
Amabile, G., Meissner, A.: Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol. Med. 15(2), 59–68 (2009)
Hochedlinger, K., Plath, K.: Epigenetic reprogramming and induced pluripotency. Development 136(4), 509–523 (2009)
Ebert, A.D., et al.: Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457(7227), 277–280 (2009)
Tateishi, K., et al.: Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J. Biol. Chem. 283(46), 31601–31607 (2008)
Park, I.H., et al.: Disease-specific induced pluripotent stem cells. Cell 134(5), 877–886 (2008)
Wobus, A.M., Boheler, K.R.: Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol. Rev. 85(2), 635–678 (2005)
Burdick, J.A., Vunjak-Novakovic, G.: Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 15(2), 205–219 (2009)
Khademhosseini, A., et al.: Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 103(8), 2480–2487 (2006)
Bonassar, L.J., Vacanti, C.A.: Tissue engineering: The first decade and beyond. J. Cell. Biochem. 297 (1998)
Atala, A.: Tissue engineering of artificial organs. J. Endourol. 14(1), 49–57 (2000)
Ren, D.F., et al.: Evaluation of RGD modification on collagen matrix. Artif. Cells Blood Subst. Biotechnol. 34(3), 293–303 (2006)
Shin, H.: Fabrication methods of an engineered microenvironment for analysis of cell-biomaterial interactions. Biomaterials 28(2), 126–133 (2007)
Langer, R., Vacanti, J.P.: Tissue engineering. Science 260(5110), 920–926 (1993)
Murtuza, B., Nichol, J.W., Khademhosseini, A.: Micro- and nanoscale control of the cardiac stem cell niche for tissue fabrication. Tissue Eng. Part B Rev. 15(4), 443–454 (2009)
Alsberg, E., von Recum, H.A., Mahoney, M.J.: Environmental cues to guide stem cell fate decision for tissue engineering applications. Exp. Opin. Biol. Ther. 6(9), 847–866 (2006)
Lensch, M.W., Daheron, L., Schlaeger, T.M.: Pluripotent stem cells and their niches. Stem Cell Rev. 2(3), 185–201 (2006)
Metallo, et al. C.M.: Engineering the stem cell microenvironment. Biotechnol. Prog. 23(1), 18–23 (2007)
Tsai, R.Y., McKay, R.D.: Cell contact regulates fate choice by cortical stem cells. J. Neurosci. 20(10), 3725–3735 (2000)
Gavard, J., et al.: N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: involvement of p120 and beta-catenin. J. Biol. Chem. 279(35), 36795–36802 (2004)
Nose, A., Tsuji, K., Takeichi, M.: Localization of specificity determining sites in cadherin cell adhesion molecules. Cell 61(1), 147–155 (1990)
Niessen, C.M., Gumbiner, B.M.: Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J. Cell Biol. 156(2), 389–399 (2002)
Volk, T., et al.: Formation of heterotypic adherens-type junctions between L-cam-containing liver-cells and A-cam-containing lens cells. Cell 50(6), 987–994 (1987)
Halbleib, J.M., Nelson, W.J.: Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20(23), 3199–3214 (2006)
Takeichi, M.: The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102, 639–655 (1988)
Aberle, H., et al.: Cadherin-catenin complex: protein interactions and their implications for cadherin function. J. Cell Biochem. 61(4), 514–523 (1996)
Qureshi, H.S., et al.: E-cadherin status in breast cancer correlates with histologic type but does not correlate with established prognostic parameters. Am. J. Clin. Pathol. 125(3), 377–385 (2006)
Tlsty, T.D.: Cell-adhesion-dependent influences on genomic instability and carcinogenesis. Curr. Opin. Cell Biol. 10(5), 647–653 (1998)
Cowin, P., et al.: Cadherins and catenins in breast cancer. Curr. Opin. Cell Biol. 17(5), 499–508 (2005)
Chen, W.C., Obrink, B.: Cell-cell contacts mediated by E-cadherin (uvomorulin) restrict invasive behavior of L-cells. J. Cell Biol. 114(2), 319–327 (1991)
Frixen, U.H., et al.: E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 113(1), 173–185 (1991)
Silvestre, J., et al.: Cadherin and integrin regulation of epithelial cell migration. Langmuir 25(17), 10092–10099 (2009)
Potthoff, S., et al.: N-cadherin engagement provides a dominant stop signal for the migration of MDA-MB-468 breast carcinoma cells. Breast Cancer Res. Treat. 105(3), 287–295 (2007)
Bloor, D.J., et al.: Expression of cell adhesion molecules during human preimplantation embryo development. Mol. Hum. Reprod. 8, 237–245 (2002)
Dang, S.M., et al.: Controlled, scalable embryonic stem cell differentiation culture. Stem Cells 22, 275–282 (2004)
Huber, B.T.L., et al.: Cooperative effects of growth factors involved in the induction of hematopoietic mesoderm. Blood 92(11), 4128–4137 (1998)
Johansson, B.M., Wiles, M.V.: Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol. Cell Biol. 15(1), 141–151 (1995)
Valdimarsdotter, G., Mummery, C.: Functions of the TGF-b superfamily in human embryonic stem cells. APMIS 113, 773–789 (2005)
Morali, O.G. et al.: IGF-II promotes mesoderm formation. Dev. Biol. 227, 133–145 (2000)
M. Schuldiner, et al., Effect of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. 97(21), 11307–11312 (2000)
Dutta, R.C., Dutta, A.K.: Cell-interactive 3D-scaffold; advances and applications. Biotechnol. Adv. 27(4), 334–339 (2009)
Weisenberg, E.: Pocket companion to robbins pathologic basis of disease. Arch. Pathol. Lab. Med. 124(10), 1566 (2000)
Engler, A.J., et al.: Matrix elasticity directs stem cell lineage specification. Cell 126(4), 677–689 (2006)
Handschel, J., et al.: Compatibility of embryonic stem cells with biomaterials. J. Biomater. Appl. 0885328208094305 (2008)
Levenberg, S., et al.: Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. USA 100(22), 12741–12746 (2003)
Battista, S., et al.: The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials 26(31), 6194–6207 (2005)
Fukuda, J., et al.: Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials (2006)
Tien, J., Chen, C.S.: Patterning the cellular microenvironment. IEEE Eng. Med. Biol. Mag. 21(1), 95–98 (2002)
Tien, J., et al.: Fabrication of aligned microstructures with a single elastomeric stamp. Proc. Natl. Acad. Sci. USA 99(4), 1758–1762 (2002)
Nelson, C.M., Chen, C.S.: VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J. Cell Sci. Sep 1(116), 3571–3581 (2003)
Khademhosseini, A., et al.: Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip 4(5), 425–430 (2004)
Karp, J.M., et al.: Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 7, 786–794 (2007)
Khademhosseini, A., et al.: Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J. Biomed. Mater. Res. A 79(3), 522–532 (2006)
Napolitano, A.P., et al.: Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. Part A 13(8), 2087–2094 (2007)
Nichol, J.W., Khademhosseini, A.: Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5, 1312–1319 (2009)
Peppas, N.A., et al.: Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 18(11), 1345–1360 (2006)
Whitesides, G.M.: The origins and the future of microfluidics. Nature 442(7101), 368–373 (2006)
Purcell, E.M.: Life at low reynolds-number. Am. J. Phys. 45(1), 3–11 (1977)
McDonald, J.C., et al.: Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21(1), 27–40 (2000)
Park, T.H., Shuler, M.L.: Integration of cell culture and microfabrication technology. Biotechnol. Prog. 19(2), 243–253 (2003)
Rhee, S.W., et al.: Patterned cell culture inside microfluidic devices. Lab Chip 5(1), 102–107 (2005)
Tan, W., Desai, T.A.: Microfluidic patterning of cells in extracellular matrix biopolymers: effects of channel size, cell type, and matrix composition on pattern integrity. Tissue Eng. 9(2), 255–267 (2003)
Chiu, D.T., et al.: Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc. Natl. Acad. Sci. USA 97(6), 2408–2413 (2000)
Takayama, S., et al.: Subcellular positioning of small molecules. Nature 411(6841), 1016 (2001)
Kim, L., et al.: Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip 6(3), 394–406 (2006)
Meinel, L., et al.: Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann. Biomed. Eng. 32(1), 112–122 (2004)
Vollmer, A.P., et al.: Development of an integrated microfluidic platform for dynamic oxygen sensing and delivery in a flowing medium. Lab Chip 5(10), 1059–1066 (2005)
Park, S.H., et al.: An electromagnetic compressive force by cell exciter stimulates chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng. 12(11), 3107–3117 (2006)
Metallo, C.M., et al.: The response of human embryonic stem cell-derived endothelial cells to shear stress. Biotechnol. Bioeng. 100(4), 830–837 (2008)
Tourovskaia, A., et al.: Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip 5(1), 14–19 (2005)
Zhong, J.F., et al.: A microfluidic processor for gene expression profiling of single human embryonic stem cells. Lab Chip 8(1), 68–74 (2008)
Davey, R.E., Zandstra, P.W.: Spatial organization of embryonic stem cell responsiveness to autocrine Gp130 ligands reveals an autoregulatory stem cell niche. Stem Cells 24(11), 2538–2548 (2006)
Kirmizidis, G., Birch, M.A.: Microfabricated grooved substrates influence cell-cell communication and osteoblast differentiation in vitro. Tissue Eng. Part A. 15(6), 1427–1436 (2009)
Abbott, A.: Cell culture: biology’s new dimension. Nature 424(6951), 870–872 (2003)
Zhang, S.: Beyond the petri dish. Nat. Biotech. 22(2), 151–152 (2004)
Freed, L.E., et al.: Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA 94(25), 13885–13890 (1997)
Radisic, M., et al.: Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 286(2), H507–516 (2004)
Radisic, M., et al.: Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng. 93(2), 332–343 (2006)
Chung, B.G., et al.: Micro- and nanoscale approaches for tissue engineering and drug discovery. Exp. Opin. Drug Dis. 2(12), 1653–1668 (2007)
Moeller, H.-C., et al.: A microwell array system for stem cell culture. Biomaterials 29(6), 752–763 (2008)
Bauwens, C.L., et al.: Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 26(9), 2300–2310 (2008)
Khademhosseini, A., et al.: Layer-by-layer deposition of hyaluronic acid and poly-L-lysine for patterned cell co-cultures. Biomaterials 25(17), 3583–3592 (2004)
Mohr, J.C., et al.: 3-D microwell culture of human embryonic stem cells. Biomaterials 27(36), 6032–6042 (2006)
Kang, L., et al.: Cell confinement in patterned nanoliter droplets in a microwell array by wiping. J. Biomed. Mater. Res. A (2009)
Ungrin, M.D., et al.: Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE 3(2), e1565 (2008)
Rosenthal, A., Macdonald, A., Voldman, J.: Cell patterning chip for controlling the stem cell microenvironment. Biomaterials 28(21), 3208–3216 (2007)
Wright, D., et al.: Generation of static and dynamic patterned co-cultures using microfabricated parylene-C stencils. Lab Chip 7(10), 1272–1279 (2007)
Wu, X., et al.: Small molecules that induce cardiomyogenesis in embryonic stem cells. J. Am. Chem. Soc. 126(6), 1590–1591 (2004)
Xu, Y., Shi, Y., Ding, S.: A chemical approach to stem-cell biology and regenerative medicine. Nature 453(7193), 338–344 (2008)
Ding, S., Schultz, P.G.: A role for chemistry in stem cell biology. Nat. Biotechnol. 22(7), 833–840 (2004)
Urquhart, A.J., et al.: TOF-SIMS analysis of a 576 micropatterned copolymer array to reveal surface moieties that control wettability. Anal. Chem. 80(1), 135–142 (2008)
Levenberg, S., et al.: Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. U.S.A 100(22), 12741–12746 (2003)
Chaudhry, G.R., et al.: Osteogenic cells derived from embryonic stem cells produced bone nodules in three-dimensional scaffolds. J. Biomed. Biotechnol. 4, 203–210 (2004)
Levenberg, S., et al.: Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 11(3–4), 506–512 (2005)
Tsang, V.L., Bhatia, S.N.: Fabrication of three-dimensional tissues. Adv. Biochem. Eng. Biotechnol. 103, 189–205 (2007)
Tsang, V.L., Bhatia, S.N.: Three-dimensional tissue fabrication. Adv. Drug. Deliv. Rev. 56(11), 1635–1647 (2004)
Tsang, V.L., et al.: Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 21(3), 790–801 (2007)
McGuigan, A.P., Sefton, M.V.: Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl. Acad. Sci. USA 103(31), 11461–11466 (2006)
Du, Y., et al.: Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA 105(28), 9522–9527 (2008)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Spinger-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bae, H., Nichol, J.W., Foudeh, A., Zamanian, B., Kwon, C.H., Khademhosseini, A. (2010). Microengineering Approach for Directing Embryonic Stem Cell Differentiation. In: Roy, K. (eds) Biomaterials as Stem Cell Niche. Studies in Mechanobiology, Tissue Engineering and Biomaterials, vol 2. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8415_2010_7
Download citation
DOI: https://doi.org/10.1007/8415_2010_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-13892-8
Online ISBN: 978-3-642-13893-5
eBook Packages: EngineeringEngineering (R0)