Abstract
Although sex differences in brain function and brain disorders are well documented, very few studies have had adequate number of women to address sex-related factors contributing to HIV-associated brain dysfunction. Compared to men living with HIV (MLWH), women living with HIV (WLWH) may be at greater risk for cognitive dysfunction and decline due to biological factors (e.g., hormonal, immunologic) and issues common in underserved communities including poverty, low literacy levels, mental health and substance abuse, barriers to health-care services, and environmental exposures. To address this issue, we review relevant cross-sectional and longitudinal findings from the Women’s Interagency HIV Study (WIHS), the largest study of the natural and treated history of WLWH, as well as other studies focusing on cognitive complications of HIV in women. We provide evidence that WLWH are more cognitively vulnerable than MLWH and that there are differences in the pattern of cognitive impairment. We next discuss factors that contribute to these differences, including biological factors (e.g., inflammation, hormonal, genetic) as well as common comorbidities (mental health, substance use, vascular and metabolic risk factors, coinfections and liver function, non-antiretroviral medications, and genetic markers). These findings demonstrate the importance of considering sex as a biological factor in studies of cognitive dysfunction and suggest avenues for future research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
In recent years, the field of neuroscience has emphasized the critical importance of biological sex in understanding healthy and abnormal brain function and recognizes that contrary to popular sentiment, sex differences in brain function and brain disorders are considerable (Cahill 2006). Our understanding of the influence of biological sex and gender-related factors in HIV-associated central nervous system (CNS) dysfunction is limited because few studies have focused on women living with HIV (WLWH) (Durvasula et al. 2001; Wojna et al. 2006; Cohen et al. 2001; Mason et al. 1998; Stern et al. 1998; Richardson et al. 2002, 2005; Maki et al. 2009). Yet, this subgroup comprises approximately 25% of HIV cases (Centers for Disease Control and Prevention 2017) in the USA and half of global cases (UN Women 2018).
CNS dysfunction including cognitive complications of HIV remains high, with estimates that 30 to 50% of individuals living with HIV will exhibit some form of neurocognitive impairment (NCI) during their lifetime (Grant 2008) even if they remain virally suppressed (VS) (Cysique et al. 2014). Notably, these estimates are based on studies either entirely or predominantly comprised of men. Few early studies had adequate numbers of women to sufficiently address questions about cognitive complications of HIV, but rates of NCI among women was estimated to be 42% (Richardson et al. 2002). WLWH may be at greater risk for cognitive NCI than men living with HIV (MLWH) due to poverty, low literacy levels, low educational attainment, substance abuse, poor mental health, barriers to health-care services, and adverse environmental exposures (Maki and Martin-Thormeyer 2009). Similarly, as in healthy women, WLWH are likely to show a different pattern of cognitive test performance than their male counterparts because of biological factors such as sex steroid hormones (e.g., estrogen, testosterone).
The Women’s Interagency HIV Study (WIHS), the largest multisite longitudinal study of the natural and treated history of WLWH and at-risk HIV-uninfected (HIV−) women, would have been the ideal cohort in which to understand cognitive complications of HIV in women earlier in the epidemic (mid-to-late 1990s and on). WIHS is a cohort that is representative of AIDS and HIV cases reported among women in the US (Barkan et al. 1998). Until 2009, the cognitive outcomes in the WIHS were limited to an abbreviated longitudinal cognitive battery. Initial neurocognitive studies conducted in the WIHS were limited by sample size (Richardson et al. 2002, 2005; Maki et al. 2009) or the number of neuropsychological tests (Rubin et al. 2014; Meyer et al. 2013). Until 2009, the cognitive outcomes in the WIHS were limited to an abbreviated longitudinal cognitive battery. The abbreviated battery only included measures of processing speed (Symbol Digit Modalities Test (SDMT)), attention (Trail Making Test (TMT) Part A), and executive function (TMT Part B). In the 2005 pilot study, the two measures administered were the Hopkins Verbal Learning Test (HVLT-R), a measure of learning and memory, and the Stroop Color-Word Test, a measure of attention/concentration (Word Reading Trial 1, Color Naming Trial 2) and executive function (Color-Word trial 3). In 2009, the WIHS Neurocognitive Working Group (NCWG) implemented the largest comprehensive evaluation of cognitive function in women living with HIV and controls to date and began to pursue a set of research aims directed at the uniqueness of an all-female HIV-infected cohort. The battery was explicitly designed to assess seven cognitive domains – executive function (TMT Part B, Stroop Color-Word trial 3), processing speed (SDMT, Stroop Color Naming Trial), attention and working memory (Letter Number Sequencing (LNS) control and experimental conditions), learning (HVLT-R total learning), memory (HVLT-R delayed free recall), language (Controlled Oral Word Association Test (COWAT) and category fluency), and motor function (Grooved Pegboard (GPEG)). In September 2011, the efforts of the NCWG were realized when the first cross-sectional dataset in approximately 1,400 WIHS participants became available and provided an unparalleled opportunity to begin to understand how HIV influences cognition in women. Findings from this all-female cohort have yielded novel and important findings regarding cognitive complications in WLWH. The purpose of this chapter will be to discuss these findings as well as highlight other studies focusing on the cognitive complications of WLWH outside of the WIHS.
1 Are WLWH More Cognitively Vulnerable Than MLWH?
Converging evidence suggests that WLWH may be more vulnerable to NCI than MLWH. Although not all studies demonstrate differences between males and females (Burlacu et al. 2018; Behrman-Lay et al. 2016), studies do find either that females show greater NCI than males overall or that females and males differ in the pattern of NCI (Royal et al. 2016; Robertson et al. 1996, 2014; Heaton et al. 2015; Failde-Garrido et al. 2008; Keutmann et al. 2016; Hestad et al. 2012; Maki et al. 2018). For example, in the two longest-running multisite, longitudinal studies of HIV progression in the United States (US), the WIHS and the Multicenter AIDS Cohort Study (MACS), performance was consistently worse among WLWH versus MLWH even after adjusting for HIV-related clinical characteristics (e.g., current and nadir CD4 count, viral load, ARV use). These differences were evident in measures of executive function (TMT Part B), attention (TMT Part A, psychomotor speed (SDMT), and motor function (GPEG) in a sample of 1,420 individuals (Maki et al. 2018). A recent large cross-sectional study of 1,361 people living with HIV (PLWH; 204 WLWH) and 702 HIV− (214 women) community-based individuals in the HIV Neurobehavioral Research Program (HNRP) demonstrated that the association between HIV seropositivity and a higher likelihood of NCI was stronger in women than men (Sundermann et al. 2018). A greater female vulnerability to NCI was also seen in among substance-dependent PLWH (Keutmann et al. 2016; Fogel et al. 2017; Martin et al. 2016).
2 Pattern of Cognitive Impairment Among Women Living with HIV
Cross-sectional results from the comprehensive cognitive test battery were first reported in a sample of 1,521 (1,019 WLWH) WIHS women (Maki et al. 2015). In this sample, 80% were ≥40 years of age, 21% self-identified as Latina or Hispanic and more than 60% as African American, and 45% were living below the federally defined poverty level. Unique to women, the greatest cognitive vulnerabilities in WLWH were on measures of verbal learning, memory, and attention (Fig. 1). Moreover, WLWH demonstrated impaired semantic clustering, a key component of verbal learning and memory reliant on the prefrontal cortex (PFC) (Becker and Lim 2003; Baker et al. 2001). The effect sizes for HIV serostatus were small (<0.20 standard deviations) and were smaller than effects of educational quality, chronological age, poverty, and depressive symptoms. These data suggested that memory, learning, and attention may be the cognitive domains most susceptible among WLWH. Interestingly, these findings contrast with studies in male-dominant HIV cohorts (MACS, CHARTER) where deficits are most prominent in executive function, complex attention, and learning (Heaton et al. 2011; Cysique et al. 2004).
In 2017, the first longitudinal analyses in the WIHS were published in WLWH to HIV− women at three time points over 4 years (Rubin et al. 2017c). WLWH showed persistent vulnerabilities in verbal learning, memory, attention/working memory, and executive function over time, while motor declined over time (Fig. 2). A functional magnetic resonance imaging (fMRI) study in WIHS implicated adverse alterations in the hippocampus (HI) and PFC with learning and memory deficits on the HVLT (Fig. 3). The data shown in Figs. 1 and 2 include a mixed group of VS and unsuppressed individuals. Our WIHS work (Rubin et al. 2017c) demonstrates that NCI persists in WLWH with consistent viral suppression on continuous ARV therapy.
Learning, memory, and attention remain a long-standing issue among WLWH. Att/WM, attention/working memory; EF, executive function; Δ, group difference in slopes at p < 0.05; ∗∗∗p < 0.001; ∗∗p < 0.01; p < 0.05; †(Norman et al. 2011); HVLT, Hopkins Verbal Learning Test
(a) During encoding, WLWH showed decreased HI activity vs. HIV− women. Decreased HI activity was associated with lower HVLT performance across groups (r’s > 0.54). (b) During recognition, HIV+ women showed increased HI activity vs. HIV− women. Increased HI activity was associated with lower HVLT performance across groups (r’s < −0.62)
3 Inflammatory Contributors to NCI Among WLWH
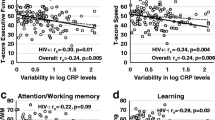
Similar to other studies (Burdo et al. 2013; Royal et al. 2016), evidence from WIHS indicates that cognitive vulnerabilities in VS WLWH are associated with soluble markers of monocyte-driven inflammatory markers (e.g., sCD163, sCD14) (Imp et al. 2017), as well as with more general markers of systemic low-grade inflammation (Rubin et al. 2017a), including interleukin (IL)-6, C-reactive protein (CRP), soluble tumor necrosis factor receptor (TNFR) I, matrix metalloproteinase (MMP)-9, but most significantly variability in CRP which had much stronger associations across cognitive domains in WLWH compared to HIV− women. S100 calcium-binding protein A9 (S100A9) has also been found to be decreased in Hispanic WLWH demonstrating NCI (Colon et al. 2016). Such findings show the importance of peripheral markers of inflammation as a key mechanism contributing to NCI in WLWH.
4 Common Comorbidities Among WLWH and Cognitive Correlates
Other factors that may contribute to the female-specific cognitive vulnerability in HIV and/or exacerbate the pattern of domain-specific NCI include mental health, substance use vascular and metabolic factors, female-specific risk factors (menopause), coinfections and liver function, non-ARV medications, genetic, and HIV-related factors.
4.1 Mental Health Comorbidities
Mental health risk factors and disorders are strongly associated with cognitive function in WLWH. In a series of cross-sectional epidemiological studies in the WIHS, we examined associations between post-traumatic stress, anxiety, perceived stress, depression, and cognitive function. Post-traumatic stress, anxiety, perceived stress, and depression were each associated with deficits in verbal learning and memory as well as attention and semantic clustering (Rubin et al. 2014, 2015a, 2016a; Maki et al. 2015). Notably, high levels of perceived stress and elevated anxiety were associated with decreased verbal learning and memory only among WLWH (Fig. 4) (Rubin et al. 2014, 2015a). In a longitudinal investigation of mental health factors and NCI in the WIHS, higher perceived stress and PTSD were associated with a greater cognitive decline in verbal fluency performance compared to those with lower stress and PTSD only among WLWH (Rubin et al. 2017b). Consistent with the cross-sectional analyses, regardless of time, perceived stress and PTSD were negatively associated with verbal learning and memory only among WLWH. Irrespective of time or HIV serostatus, depression, perceived stress, and PTSD were associated with lower processing speed, executive function, and global neuropsychological function. A longitudinal study in South Africa found less improvement over time in executive function (e.g., Wisconsin Card Sorting Test, Stroop) and fluency (COWAT, category fluency) among WLWH exposed to trauma compared to women not exposed to trauma (regardless of serostatus) (Spies et al. 2017).
HIV and mental health factors individually and in combination impair cognition in WLWH through effects on PFC. Among WLWH, those with higher levels of stress showed more PFC atrophy (including inferior frontal, middle frontal, and superior frontal gyri) and deactivation in the medial PFC and posterior cingulate cortex compared to those with lower stress (Rubin et al. 2015b, 2016b). Importantly, PFC atrophy and deactivation were associated with less semantic clustering; atrophy was also associated with worse verbal memory (Rubin et al. 2015b, 2016c). These studies point to the PFC as a region of particular vulnerability among WLWH particularly to mental health factors.
4.2 Substance Use Comorbidities
In the WIHS, the lifetime prevalence of any substance use disorder is 58%. Relatively few studies have examined the association between substance use and cognition among WLWH. Opioids, stimulants, and alcohol exacerbate the neurotoxic effects of HIV including acceleration of disease progression and increases in the risk of NCI (Nath et al. 2001; Carey et al. 2006). In the WIHS, WLWH who reported using crack, cocaine, and/or heroin in the past 6 months demonstrated lower performance on learning and memory compared to WLWH women who reported never using these substances (Meyer et al. 2013). There were no differences in memory performance by illicit substance use among the HIV− women. No interactions between illicit drug use and HIV serostatus were found on measures of attention or behavioral inhibition. Cocaine-associated deficits in verbal learning and memory among WLWH may in part be driven by alterations in PFC regions. In an fMRI study in WLWH, both current and former use of cocaine was associated with decreased activation in medial PFC during the encoding phase of a verbal memory task (Meyer et al. 2014). During the recognition phase of the verbal memory task, WLWH nonusers showed greater activation than current and former cocaine users in prefrontal regions (left dorsal medial PFC, right dorsal lateral PFC, anterior PFC). Other work in substance-dependent individuals shows greater impairment in risky decision-making and visual memory in WLWH compared with MLWH and HIV− individuals (Martin et al. 2016; Keutmann et al. 2016).
4.3 Vascular and Metabolic Risk Factors
The increasing effectiveness of ARVs has led to the aging of the HIV population and, with it, the increasing presence of age-related diseases including cardiovascular and cerebrovascular comorbidities. These comorbidities often result in the dysregulation of multiple systems including negative consequences for brain structure and function. In an early cross-sectional WIHS study, carotid lesion and carotid intima-media thickness (CIMT) were associated with lower performance on the Stroop interference trial but not with performance on the SDMT. Another WIHS study found an association between ultrasound-based measures of carotid artery stiffness and cognitive decline in TMT Parts A and B and SDMT (Huck et al. 2018). Specifically, greater baseline carotid stiffness was associated with greater decline on all outcomes over a median of 8.5 years, but these associations did not differ by HIV serostatus. In another early WIHS study, body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHR) were examined in relation to cognition and showed some stronger relationships with cognition in WLWH compared to HIV− women (Gustafson et al. 2013). On TMT A and B and SDMT, being underweight (BMI <18.5 kg/m2) was associated with worse performance compared to having a normal BMI in WLWH but not in HIV− women. For WLHV but not HIV− women, being obese (body mass index 30 kg/m2 or higher) was associated with better performance on TMT Part A compared to having a normal BMI. In WLWH only, higher WC was associated with better performance on the Stroop Color-Word Trial and SDMT. Higher leptin levels were associated with lower performance on TMT Part A in both WLWH and HIV− women (Gustafson et al. 2015). Three gut hormones (ghrelin, amylin, gastric inhibitory peptide) were examined to better understand the mechanisms underlying the obesity-cognition association (McFarlane et al. 2017). In general, lower gut hormone levels were associated with lower cognitive performance. Gastric inhibitory peptide and ghrelin were associated with cognitive performance among WLWH, whereas only ghrelin was associated with cognitive performance among HIV− women.
A number of WIHS studies have also examined the link between insulin resistance (IR) and cognition. In the initial cross-sectional study, insulin resistance as measured using the homeostasis model assessment (HOMA) was associated in the overall sample with Stroop Color Naming and not TMT Parts A and B, SDMT, or other Stroop trials (Valcour et al. 2012). There was also an interaction between HOMA-IR and HIV serostatus on the Stroop Color-Word Trial such that among those with average HOMA-IR, WLWH demonstrated worse performance than HIV− women. A follow-up study of HOMA in relation to performance on the comprehensive cognitive test battery (Valcour et al. 2015) revealed an association between increasing HOMA and worse performance on the LNS control condition (attention), HVLT-R recognition, and phonemic fluency across serostatus groups. Interactions were also noted on three measures of attention – LNS control condition, Stroop Word Reading, and Color Naming trials – with worse performance in WLWH versus HIV in women as HOMA values increased. In two non-WIHS studies conducted in Hispanic WLWH, levels of soluble and cell-associated insulin receptor levels, IR substrate-1 (IRS-1) levels, and IRS-1 tyrosine phosphorylation were assayed in plasma and CSF in association with NCI (Gerena et al. 2012). IR secretion was higher in WLWH than HIV− women, and higher IR secretion was associated with increasing NCI severity. Further, higher binding of free insulin to the soluble insulin receptor was also associated with NCI (Gerena et al. 2015).
4.4 Female-Specific Reproductive Risk Factors
Despite the wealth of literature on normative changes in women’s health across the menopausal transition, very little is known about the natural history of menopause in WLWH (Bull et al. 2018), particularly with regard to cognitive changes. In a cross-sectional WIHS study, the menopause stage was not associated with cognitive functioning on HVLT-R and Stroop (Rubin et al. 2014). However, across serostatus groups, depressive symptoms were associated with worse learning, memory, attention, and executive function, and anxiety symptoms were associated with worse learning and memory. Vasomotor symptoms were also associated with worse attention. Notably, there was an interaction between HIV serostatus and anxiety symptoms on verbal learning such that elevated anxiety was associated with worse verbal learning in WLWH only. Thus, menopause symptoms are associated with cognitive performance in WLWH.
4.5 Coinfections and Liver Function
Hepatitis C (HCV) infection is relatively common in WLWH and the WIHS cohort. There are inconsistent findings regarding the association between HCV infection and cognition. In an early WIHS study (n = 200), WLWH who were HCV positive demonstrated greater odds of NCI compared to women not infected with either (Richardson et al. 2005). In a later WIHS study, HCV was not associated with cognition in 1,338 women (Crystal et al. 2012). However, in a smaller non-WIHS sample of WLWH, hepatitis C virus coinfection was associated with lower motor function, processing speed, attention, working memory, and planning (Giesbrecht et al. 2014). Given the significant overlap between HCV and liver fibrosis, a follow-up study examined the association between liver function and cognition (Valcour et al. 2016). Liver fibrosis (APRI) was associated with worse performance in learning, executive function, memory, psychomotor speed, fluency, and fine motor skills in the overall sample. The severity of fibrosis measured via Fibroscan was associated with worse performance in attention, executive function, and fluency. These associations held after controlling for HCV and HIV status, and the associations were not moderated by these factors.
4.6 Non-ARV Medications
The use of non-ARV medications with adverse cognitive effects (NC-AE medications) is more common among WLWH compared to HIV− women (Radtke et al. 2018). Non-ARV medications are associated with worse cognition in the WIHS overall, but HIV serostatus did not moderate these associations (Rubin et al. 2018b). However, for women taking anticholinergic-acting medications, HIV serostatus differences were most pronounced (WLWH < HIV−) in global, learning, fluency, and motor function. For women taking anxiolytics/anticonvulsants or opioids, HIV serostatus differences were also more pronounced (WLWH < HIV−) in learning and processing speed, respectively.
4.7 Genetic Markers
To date, our understanding of the genetics of NCI is based primarily on male-dominant studies. To date, only two studies have been conducted in WLWH that have looked at genetic markers. One study in the WIHS examined the association between catechol-o-methyltransferase (COMT) Val158Met genotype and working memory as well as PFC function in WLWH (Sundermann et al. 2015). The COMT Val158Met (rs4680) single nucleotide polymorphism (SNP) influences executive function and PFC function through its effect on dopamine metabolism. Both HIV and the Val allele of the Val158Met SNP were associated with compromised executive function and inefficient PFC function. Among Val/Val but not Met allele carriers, WLWH performed worse than HIV− women on a measure of working memory. Val/Val carriers also showed greater PFC activations during performance of an n-back working memory task compared to HIV− Val/Val carriers. However, HIV− Met allele carriers demonstrated greater PFC activation versus WLWH Met allele carriers. Together the findings suggested that suboptimal dopamine levels associated with the Val/Val COMT genotype leads to working memory deficits and inefficient PFC function in WLWH. A study in Hispanic women examined apolipoprotein E (ApoE) allele status, HIV serostatus, and CSF APoE protein levels in relation to spatial learning and memory (measure of HI function) (Morales et al. 2012). The ApoE gene produces a protein responsible for the metabolism and transport of lipoproteins and cholesterol. The presence of the e4 allele is a well-known risk factor in Alzheimer’s disease and has been linked to NCI in some (Valcour et al. 2004; Wendelken et al. 2016) but not all (Morgan et al. 2013) studies in older PLWH (≥50 years of age). In the small sample of 20 WLWH and 16 controls approximately 40 years of age, the e4 allele was associated with cognitive performance on a standard neuropsychological test battery among HIV− but not WLWH (Morales et al. 2012). However, the e4 allele was associated with an experimental measure of spatial learning and memory (Memory Island task) in WLWH but not HIV− women.
5 Summary
Overall, findings from WIHS and other studies suggest that WLWH demonstrate persistent NCI despite ARV and that WLWH may be more cognitively vulnerable compared to MLWH. Two brain regions that appear to be particularly susceptible to HIV infection and female sex and may contribute to the prominent deficits in verbal learning and memory observed in WLWH are PFC-HI regions. Additional work is needed to understand brain regions that are susceptible to other domain-specific impairment that persists among WLWH including attention/working memory and executive function. Similar to MLWH, inflammation is a key contributor to NCI among WLWH. There are many factors (e.g., mental health, substance use) that contribute or exacerbate cognitive complications among WLWH.
Assessing sex differences in the context of HIV may help to elucidate novel therapeutics for CNS dysfunction in PLWH. We cannot assume that WLWH and MLWH will respond similarly to the same treatment or that the mechanisms underlying cognitive problems are the same for each sex (Rubin et al. 2017d, 2018a). Such results underscore the need to at least stratify cognitive analyses by sex to determine whether the patterns are the same between WLWH and MLWH or whether they qualitatively differ between the sexes (sex-dependent) or are present in one sex and not the other (sex-specific).
6 Future Directions
From the review above, we propose three key directions for future research. Arguably, the most critical research gap is a sufficiently powered cohort study of neuropsychological test performance in men and women living with HIV and matched controls. We were able to identify parallel neuropsychological measures between the WIHS and the MACS and had sufficient power to examine sex differences in HIV-seropositive participants versus HIV-seronegative controls (Maki et al. 2018). However, critical measures, including verbal memory, differed between the two cohorts so it is unknown whether WLWH show vulnerabilities in those measures as well. With the merging of the WIHS and the MACS into the MACS-WIHS Combined Cohort Study (MWCCS), we are now poised to directly compare a sufficiently large sample of men and women in a longitudinal prospective cohort study. With an estimated sample size 4,400 former MACS/WIHS recruits and 1,600–1,700 new recruits, the MWCCS will be unique in providing sufficient statistical power to determine whether such factors as depression, substance abuse, and trauma, as well as vascular, metabolic, and menopause-related risk factors, contribute to sex differences in cognitive performance. Similarly, the MWCCS will be able to draw on GWAS data to investigate genetic contributions to sex differences in cognitive function.
The second key direction is a longitudinal multimodal neuroimaging study comparing WLWH and MLWH in relation to seronegative controls. We are undertaking such a study in the Baltimore and Washington DC sites of the MACS-WIHS Combined Cohort Study (MWCCS). Building on our cross-sectional neuroimaging studies, we are using both task-based and resting state fMRI in HIV+ virally suppressed (HIV + VS) men and women and HIV-uninfected individuals to identify the neural circuitry contributing to deficits in two targeted domains – declarative memory and cognitive control. We are also using [11C]DPA-713 (DPA) PET to assess HIV-related alterations in chronic neuroinflammation and NCI. We are also assessing structural MRI and diffusion-weighted MRI. Imaging assessments will be conducting annually for 3 years. The longitudinal design allows an assessment of the reproducibility of key findings over time and the sensitivity of these neuroimaging measures to changes in cognitive performance. Such work will inform our understanding of the mechanisms linked to neurological comorbidity and to provide novel, more sensitive neuroimaging biomarkers to guide testing of new cognitive therapies for HIV+ individuals.
While it is critical to extend sex differences research in neuroAIDS in the USA, a third and critical question is whether any differences observed in American cohort studies are generalizable to international cohorts, including those in sub-Saharan Africa. Gender differences in factors such as mental health have been posited to contribute to differential cognitive impairment in WLWH compared to MLWH in sub-Saharan Africa, but sample sizes are limited to fewer than 210 people total (Royal et al. 2016; Hestad et al. 2012).
References
Baker JT, Sanders AL, Maccotta L, Buckner RL (2001) Neural correlates of verbal memory encoding during semantic and structural processing tasks. Neuroreport 12:1251–1256
Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J (1998) The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 9:117–125
Becker S, Lim J (2003) A computational model of prefrontal control in free recall: strategic memory use in the California Verbal Learning Task. J Cogn Neurosci 15:821–832
Behrman-Lay AM, Paul RH, Heaps-Woodruff J, Baker LM, Usher C, Ances BM (2016) Human immunodeficiency virus has similar effects on brain volumetrics and cognition in males and females. J Neurovirol 22:93–103
Bull L, Tittle V, Rashid T, Nwokolo N (2018) HIV and the menopause: a review. Post Reprod Health 24:19–25
Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC (2013) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 27:1387–1395
Burlacu R, Umlauf A, Luca A, Gianella S, Radoi R, Ruta SM, Marcotte TD, Ene L, Achim CL (2018) Sex-based differences in neurocognitive functioning in HIV-infected young adults. AIDS 32:217–225
Cahill L (2006) Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484
Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I (2006) Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav 10:185–190
Centers for Disease Control and Prevention (2017) Monitoring selected national HIV prevention and care objectives by using HIV surveillance data – United States and 6 dependent areas, 2015. HIV Surveill Suppl Rep 22(2)
Cohen RA, Boland R, Paul R, Tashima KT, Schoenbaum EE, Celentano DD, Schuman P, Smith DK, Carpenter CC (2001) Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS 15:341–345
Colon K, Perez-Laspiur J, Quiles R, Rodriguez Y, Wojna V, Shaffer SA, Leszyk J, Skolasky RL Jr, Melendez LM (2016) Macrophage secretome from women with HIV-associated neurocognitive disorders. Proteomics Clin Appl 10:136–143
Crystal H, Kleyman I, Anastos K, Lazar J, Cohen M, Liu C, Pearce L, Golub E, Valcour V, Ho A, Strickler H, Peters M, Kovacs A, Holman S, Kreek MJ, Manly J (2012) Effects of hepatitis C and HIV on cognition in women: data from The Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 59:149–154
Cysique LA, Maruff P, Brew BJ (2004) Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol 10:350–357
Cysique LA, Heaton RK, Kamminga J, Lane T, Gates TM, Moore DM, Hubner E, Carr A, Brew BJ (2014) HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuro HIV research. J Neurovirol 20:258–268
Durvasula RS, Miller EN, Myers HF, Wyatt GE (2001) Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol 23:149–163
Failde-Garrido JM, Alvarez MR, Simon-Lopez MA (2008) Neuropsychological impairment and gender differences in HIV-1 infection. Psychiatry Clin Neurosci 62:494–502
Fogel J, Rubin LH, Maki P, Keutmann MK, Gonzalez R, Vassileva J, Martin EM (2017) Effects of sex and HIV serostatus on spatial navigational learning and memory among cocaine users. J Neurovirol 23:855–863
Gerena Y, Skolasky RL, Velez JM, Toro-Nieves D, Mayo R, Nath A, Wojna V (2012) Soluble and cell-associated insulin receptor dysfunction correlates with severity of HAND in HIV-infected women. PLoS One 7:e37358
Gerena Y, Menendez-Delmestre R, Skolasky RL, Hechavarria RM, Perez S, Hilera C, Gonzalez C, Nath A, Wojna V (2015) Soluble insulin receptor as a source of insulin resistance and cognitive impairment in HIV-seropositive women. J Neurovirol 21:113–119
Giesbrecht CJ, Thornton AE, Hall-Patch C, Maan EJ, Cote HC, Money DM, Murray M, Pick N (2014) Select neurocognitive impairment in HIV-infected women: associations with HIV viral load, hepatitis C virus, and depression, but not leukocyte telomere length. PLoS One 9:e89556
Grant I (2008) Neurocognitive disturbances in HIV. Int Rev Psychiatry 20:33–47
Gustafson DR, Mielke MM, Tien PC, Valcour V, Cohen M, Anastos K, Liu C, Pearce L, Golub ET, Minkoff H, Crystal HA (2013) Anthropometric measures and cognition in middle-aged HIV-infected and uninfected women. The Women’s Interagency HIV Study. J Neurovirol 19:574–585
Gustafson DR, Mielke MM, Keating SA, Holman S, Minkoff H, Crystal HA (2015) Leptin, adiponectin and cognition in middle-aged HIV-infected and uninfected women. The Brooklyn Women’s Interagency HIV Study. J Gerontol Geriatr Res 4(5). pii: 240
Heaton RK, Franklin DR, Ellis RJ, Mccutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, Mcarthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16
Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, Mccutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, CHARTER Group (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 60:473–480
Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR Jr, Imasiku ML, Kalima K, Heaton RK (2012) Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: a pilot study in Zambia, Africa. J Nerv Ment Dis 200:336–342
Huck DM, Hanna DB, Rubin LH, Maki P, Valcour V, Springer G, Xue X, Lazar J, Hodis HN, Anastos K, Kaplan RC, Kizer JR (2018) Carotid artery stiffness and cognitive decline among women with or at risk for HIV infection. J Acquir Immune Defic Syndr 78:338–347
Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, Valcour VG (2017) Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis 215:114–121
Keutmann MK, Gonzalez R, Maki PM, Rubin LH, Vassileva J, Martin EM (2016) Sex differences in HIV effects on visual memory among substance-dependent individuals. J Clin Exp Neuropsychol:1–13
Maki PM, Martin-Thormeyer E (2009) HIV, cognition and women. Neuropsychol Rev 19:204–214
Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E (2009) Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology 72:1661–1668
Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K (2015) Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84:231–240
Maki PM, Rubin LH, Springer G, Seaberg EC, Sacktor N, Miller EN, Valcour V, Young MA, Becker JT, Martin EM, Neuropsychology Working Groups of the Womenʼs Interagency HIV Study and the Multicenter AIDS Cohort Study (2018) Differences in cognitive function between women and men with HIV. J Acquir Immune Defic Syndr 79:101–107
Martin E, Gonzalez R, Vassileva J, Maki PM, Bechara A, Brand M (2016) Sex and HIV serostatus differences in decision making under risk among substance-dependent individuals. J Clin Exp Neuropsychol 38:404–415
Mason KI, Campbell A, Hawkins P, Madhere S, Johnson K, Takushi-Chinen R (1998) Neuropsychological functioning in HIV-positive African-American women with a history of drug use. J Natl Med Assoc 90:665–674
Mcfarlane SI, Mielke MM, Uglialoro A, Keating SM, Holman S, Minkoff H, Crystal HA, Gustafson DR (2017) Ghrelin, amylin, gastric inhibitory peptide and cognition in middle-aged HIV-infected and uninfected women: The Women's Interagency HIV Study. J Neurol Neurophysiol 8(1). pii: 413
Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, Valcour V, Young MA, Crystal H, Anastos K, Aouizerat BE, Milam J, Maki PM (2013) HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr 63:67–76
Meyer VJ, Little DM, Fitzgerald DA, Sundermann EE, Rubin LH, Martin EM, Weber KM, Cohen MH, Maki PM (2014) Crack cocaine use impairs anterior cingulate and prefrontal cortex function in women with HIV infection. J Neurovirol 20:352–361
Morales D, Acevedo SF, Skolasky RL, Hechavarria R, Santiago S, De La Torre T, Maldonado E, Wojna V (2012) Translational spatial task and its relationship to HIV-associated neurocognitive disorders and apolipoprotein E in HIV-seropositive women. J Neurovirol 18:488–502
Morgan EE, Woods SP, Letendre SL, Franklin DR, Bloss C, Goate A, Heaton RK, Collier AC, Marra CM, Gelman BB, Mcarthur JC, Morgello S, Simpson DM, Mccutchan JA, Ellis RJ, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Grant I, Vaida F, Clifford DB, CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Group (2013) Apolipoprotein E4 genotype does not increase risk of HIV-associated neurocognitive disorders. J Neurovirol 19:150–156
Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR (2001) Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol 7:66–71
Norman MA, Moore DJ, Taylor M, Franklin D Jr, Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK (2011) Demographically corrected norms for African Americans and Caucasians on the Hopkins verbal learning test-revised, brief visuospatial memory test-revised, stroop color and word test, and wisconsin card sorting test 64-card version. J Clin Exp Neuropsychol 33:793–804
Radtke KK, Bacchetti P, Anastos K, Merenstein D, Crystal H, Karim R, Weber KM, Edmonds A, Sheth AN, Fischl MA, Vance D, Greenblatt RM, Rubin LH (2018) Use of nonantiretroviral medications that may impact neurocognition: patterns and predictors in a large, long-term HIV cohort study. J Acquir Immune Defic Syndr 78:202–208
Richardson JL, Martin EM, Jimenez N, Danley K, Cohen M, Carson VL, Sinclair B, Racenstein JM, Reed RA, Levine AM (2002) Neuropsychological functioning in a cohort of HIV infected women: importance of antiretroviral therapy. J Int Neuropsychol Soc 8:781–793
Richardson JL, Nowicki M, Danley K, Martin EM, Cohen MH, Gonzalez R, Vassileva J, Levine AM (2005) Neuropsychological functioning in a cohort of HIV− and hepatitis C virus-infected women. AIDS 19:1659–1667
Robertson K, Fiscus S, Wilkins J, van der Horst C, Hall C (1996) Viral load and neuropsychological functioning in HIV seropositive individuals: a preliminary descriptive study. J Neuro AIDS 1:7–15
Robertson K, Bayon C, Molina JM, Mcnamara P, Resch C, Munoz-Moreno JA, Kulasegaram R, Schewe K, Burgos-Ramirez A, De Alvaro C, Cabrero E, Guion M, Norton M, Van Wyk J (2014) Screening for neurocognitive impairment, depression, and anxiety in HIV-infected patients in Western Europe and Canada. AIDS Care 26:1555–1561
Royal W 3rd, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA (2016) Associations between cognition, gender and monocyte activation among HIV infected individuals in Nigeria. PLoS One 11:e0147182
Rubin LH, Sundermann EE, Cook JA, Martin EM, Golub ET, Weber KM, Cohen MH, Crystal H, Cederbaum JA, Anastos K, Young M, Greenblatt RM, Maki PM (2014) Investigation of menopausal stage and symptoms on cognition in human immunodeficiency virus-infected women. Menopause 21(9):997–1006
Rubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, Milam J, Anastos K, Young MA, Alden C, Gustafson DR, Maki PM (2015a) The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. J Neurovirol 21:422–432
Rubin LH, Meyer VJ, Conant R, Sundermann EE, Wu M, Weber KM, Cohen MH, Little DM, Maki PM (2015b) Prefrontal cortical volume loss is associated with stress-related deficits in verbal learning and memory in HIV-infected women. Neurobiol Dis 92(Pt B):166–174
Rubin LH, Pyra M, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, Milam J, Anastos K, Young MA, Alden C, Gustafson DR, Maki PM (2016a) Post-traumatic stress is associated with verbal learning, memory, and psychomotor speed in HIV-infected and HIV-uninfected women. J Neurovirol 22:159–169
Rubin LH, Wu M, Sundermann EE, Meyer VJ, Smith R, Weber KM, Cohen MH, Little DM, Maki PM (2016b) Elevated stress is associated with prefrontal cortex dysfunction during a verbal memory task in women with HIV. J Neurovirol 22(6):840–851
Rubin LH, Wu M, Sundermann EE, Meyer VJ, Smith R, Weber KM, Cohen MH, Little DM, Maki PM (2016c) Elevated stress is associated with prefrontal cortex dysfunction during a verbal memory task in women with HIV. J Neurovirol 22(6):840–851
Rubin LH, Benning L, Keating SM, Norris PJ, Burke-Miller J, Savarese A, Kumanan KN, Awadalla S, Springer G, Anastos K, Young M, Milam J, Valcour VG, Weber KM, Maki PM (2017a) Variability in C-reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study. J Neurovirol 24(1):41–51
Rubin LH, Cook JA, Springer G, Weber KM, Cohen MH, Martin EM, Valcour VG, Benning L, Alden C, Milam J, Anastos K, Young MA, Gustafson DR, Sundermann EE, Maki PM (2017b) Perceived and post-traumatic stress are associated with decreased learning, memory, and fluency in HIV-infected women. AIDS 31:2393–1401
Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, Villacres MC, Jiang X, Adimora AA, Waldrop-Valverde D, Vance DE, Bolivar H, Alden C, Martin EM, Valcour VG, Women’s Interagency HIV Study (2017c) Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 89:1594–1603
Rubin LH, Phan KL, Keating SM, Weber KM, Maki PM (2017d) Brief report: low-dose hydrocortisone has acute enhancing effects on verbal learning in HIV-infected men. J Acquir Immune Defic Syndr 75:e65–e70
Rubin LH, Phan KL, Keating SM, Maki PM (2018a) A single low-dose of hydrocortisone enhances cognitive functioning in HIV-infected women. AIDS 32:1983–1993
Rubin LH, Radtke KK, Eum S, Tamraz B, Kumanan KN, Springer G, Maki PM, Anastos K, Merenstein D, Karim R, Weber KM, Gustafson D, Greenblatt RM, Bishop JR (2018b) Cognitive burden of common non-antiretroviral medications in HIV-infected women. J Acquir Immune Defic Syndr 79(1):83–91
Spies G, Fennema-Notestine C, Cherner M, Seedat S (2017) Changes in cognitive function in women with HIV infection and early life stress. AIDS Care 29:14–23
Stern RA, Arruda JE, Somerville JA, Cohen RA, Boland RJ, Stein MD, Martin EM (1998) Neurobehavioral functioning in asymptomatic HIV-1 infected women. J Int Neuropsychol Soc 4:172–178
Sundermann EE, Bishop JR, Rubin LH, Little DM, Meyer VJ, Martin E, Weber K, Cohen M, Maki PM (2015) Genetic predictor of working memory and prefrontal function in women with HIV. J Neurovirol 21:81–91
Sundermann EE, Heaton RK, Pasipanodya E, Moore RC, Paolillo EW, Rubin LH, Ellis R, Moore DJ, HNRP Group (2018) Sex differences in HIV-associated cognitive impairment. AIDS 32:2719–2726
UN Women (2018) Facts and figures: HIV and AIDS [Online]. http://www.unwomen.org/en/what-we-do/hiv-and-aids/facts-and-figures#notes. Accessed 15 Aug 2018
Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, Grove J, Liu Y, Abdul-Majid KB, Gartner S, Sacktor N (2004) Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol 157:197–202
Valcour V, Maki P, Bacchetti P, Anastos K, Crystal H, Young M, Mack WJ, Cohen M, Golub ET, Tien PC (2012) Insulin resistance and cognition among HIV-infected and HIV-uninfected adult women: the Women’s Interagency HIV Study. AIDS Res Hum Retroviruses 28:447–453
Valcour V, Rubin LH, Tien P, Anastos K, Young M, Mack W, Cohen M, Golub ET, Crystal H, Maki PM (2015) Human immunodeficiency virus (HIV) modulates the associations between insulin resistance and cognition in the current combination antiretroviral therapy (cART) era: a study of the Women’s Interagency HIV Study (WIHS). J Neurovirol 21:415–421
Valcour VG, Rubin LH, Obasi MU, Maki PM, Peters MG, Levin S, Crystal HA, Young MA, Mack WJ, Cohen MH, Pierce CB, Adimora AA, Tien PC (2016) Liver fibrosis linked to cognitive performance in HIV and hepatitis C. J Acquir Immune Defic Syndr 72(3):266–273
Wendelken LA, Jahanshad N, Rosen HJ, Busovaca E, Allen I, Coppola G, Adams C, Rankin KP, Milanini B, Clifford K, Wojta K, Nir TM, Gutman BA, Thompson PM, Valcour V (2016) ApoE epsilon4 is associated with cognition, brain integrity, and atrophy in HIV over age 60. J Acquir Immune Defic Syndr 73:426–432
Wojna V, Skolasky RL, Hechavarria R, Mayo R, Selnes O, Mcarthur JC, Melendez LM, Maldonado E, Zorrilla CD, Garcia H, Kraiselburd E, Nath A (2006) Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol 12:356–364
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rubin, L.H., Maki, P.M. (2019). Neurocognitive Complications of HIV Infection in Women: Insights from the WIHS Cohort. In: Cysique, L.A., Rourke, S.B. (eds) Neurocognitive Complications of HIV-Infection. Current Topics in Behavioral Neurosciences, vol 50. Springer, Cham. https://doi.org/10.1007/7854_2019_101
Download citation
DOI: https://doi.org/10.1007/7854_2019_101
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80758-0
Online ISBN: 978-3-030-80759-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)