Abstract
Although converging epidemiological evidence links exposure to stressful life events with increased risk for affective spectrum disorders, there is extraordinary interindividual variability in vulnerability to adversity. The environmentally moderated penetrance of genetic variation is thought to play a major role in determining who will either develop disease or remain resilient. Research on genetic factors in the aetiology of disorders of emotion regulation has, nevertheless, been complicated by a mysterious discrepancy between high heritability estimates and a scarcity of replicable gene-disorder associations. One explanation for this incongruity is that at least some specific gene effects are conditional on environmental cues, i.e. gene-by-environment interaction (G × E) is present. For example, a remarkable number of studies reported an association of variation in the human serotonin (5-HT) transporter gene (SLC6A4, 5-HTT, SERT) with emotional and cognitive traits as well as increased risk for depression in interaction with psychosocial adversity. The results from investigations in non-human primate and mouse support the occurrence of G × E interaction by showing that variation of 5-HTT function is associated with a vulnerability to adversity across the lifespan leading to unfavourable outcomes resembling various neuropsychiatric disorders. The neural and molecular mechanisms by which environmental adversity in early life increases disease risk in adulthood are not known but may include epigenetic programming of gene expression during development. Epigenetic mechanisms, such as DNA methylation and chromatin modification, are dynamic and reversible and may also provide targets for intervention strategies (see Bountra et al., Curr Top Behav Neurosci, 2011). Animal models amenable to genetic manipulation are useful in the identification of molecular mechanisms underlying epigenetic programming by adverse environments and individual differences in resilience to stress. Therefore, deeper insight into the role of epigenetic regulation in the process of neurodevelopmental programmes is likely to result in early diagnosis of affective spectrum disorders and will contribute to the design of innovative treatments targeting neural pathways that foster resilience.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Research on genetic factors in the aetiology of neuropsychiatric diseases has been complicated by a mysterious discrepancy between high heritability estimates and a scarcity of replicable gene-disorder associations. This “missing heritability” is now either euphemized as the “dark matter” of gene-trait association or aggravated as the “looming crisis in human genetics”. Several explanations for this incongruity have been suggested (Manolio et al. 2009; Yang et al. 2010). These include larger numbers of variants with smaller effect size which are yet to be found; rarer variants with larger effects that are not detected by available single-nucleotide polymorphism arrays which ignore variants present in <5% of the population; other structural variations such as repeat length variants or short copy number variations which are not detected by existing arrays; inadequate power to detect gene-by-gene interactions (G × G) or, finally, inadequate accounting for the shared environment to be found among relatives.
Another plausible explanation for the missing heritability is that at least some specific gene effects are conditional on environmental cues, i.e. gene-by-environment interactions. Particularly stressful childhood experiences such as trauma, abuse, neglect or other forms of early life adversity are important risk factors for multiple diseases, including obesity, cardiovascular disease and neuropsychiatric illness later in life. Why one individual develops cardiovascular disease while another develops depression following early life stress is thought to be influenced by genetic susceptibility factors. Although converging epidemiological evidence links exposure to stressful life events with increased risk for neuropsychiatric disorders, there is remarkable interindividual variability in vulnerability to environmental cues. The environmentally moderated penetrance of genetic variation is therefore thought to play a major role in determining who will either develop disease or remain resilient. In this context, the ability to recover from trauma or crisis as well as resistance against biological and psychosocial risks – commonly called resilience – is not conceptualized as an innate trait but comprising a variable capacity initially acquired during development in the context of individual–environment interaction. A consolidated state of resilience is therefore rooted in specific resources of both individual genetic framework and social circumstances. There is considerable demand for research on the molecular mechanisms of genetic and epigenetic programming of motivational, attentional and emotional neural circuits underlying the development and consolidation of resilience.
Gene × environment interactions (G × E), involving specific gene polymorphisms, such as are found with the serotonin (5-HT) transporter gene (SLC6A4, synonyms: 5-HTT, SERT) for example, have been identified and replicated in humans and animal models. Research on the role of the 5-HTT in the pathophysiology of stress-linked disorders accompanied by emotional dysregulation has a history spanning more than half a century. Following the influential discovery of presynaptic neurotransmitter uptake by Hertting and Axelrod (1961) and shortly after its identification as the initial target of antidepressant drug action (Raisman et al. 1979), the 5-HTT was first linked with the pathogenesis of depression by Langer and associates (Langer et al. 1981). After cloning of the rat 5-HTT gene (Slc6a4) (Blakely et al. 1991), the era of molecular genetic studies of emotion regulation began with three seminal papers in 1996 that reported associations between 5-HTT variation and anxiety-related traits (Lesch et al. 1996) as well as depression (Collier et al. 1996; Ogilvie et al. 1996).
In the years following the first reports linking 5-HTT variation with anxiety- and aggression-related traits, numerous clinical entities have been studied for association with disorders characterized to a large extent by emotional dysregulation, including depression, bipolar affective disorder, attention-deficit/hyperactivity disorder, alcohol dependence, suicide, eating disorders and autism or disorders related to morphogenetic actions of 5-HT in other organ systems such as heart, blood vessels, bowel, and bone (Lesch and Mössner 2006; Murphy et al. 2004, 2008). Modest effect sizes typical of complex traits, polygenic patterns of inheritance, epistatic and epigenetic interactions and sample heterogeneity across studies are all factors which have led to inconsistent replication and have confounded attempts to reach agreement regarding the role of 5-HTT in the pathophysiology of all these diseases. Nevertheless, the impact of 5-HTT on complex traits in humans, non-human primates and genetically modified mice has become a model par excellence in cognitive, biosocial, and psychiatric neurosciences (Lesch 2007; Murphy and Lesch 2008; Suomi 2003).
The eye-opener that early life stress and other modes of G × E uniquely reinforce or even uncover links between 5-HTT variation, behaviour and psychopathology in humans and non-human primates has heralded a new era of behavioural genetics. Several recent studies suggest that 5-HTT variation interacts with deleterious early rearing experience in rhesus macaques and in mice to influence attentional, emotional and (social) cognitive processing (Canli and Lesch 2007). Thus, the identification of 5-HTT as a modifier of emotionality, and its interaction with environmental adversity, was a first step en route to an explanation of the molecular dimension of personality, emotion, (social) cognition and behaviour; suggests strategies to identify physiologic pathways and mechanisms that lead to other disorders of cognitive function and emotion regulation; provides tools to dissect the interactive effects of genes and environment in the development of affective disorders and holds the potential to predict response to treatment. It is anticipated that controlling environmental factors will eventually improve the reliability of genetic approaches.
This chapter focuses, from an epigenetic perspective, on the nature of an innate variability in brain 5-HTT function that predisposes to a wide spectrum of psychiatric disorders in which emotional dysregulation is a common denominator. The various psychobiological facets of 5-HTT variation and resulting phenomes will be critically reviewed with emphasis on neurodevelopmental programming. The relevance of G × E in emotional and (social) cognitive processes is also highlighted and an appraisal of morphofunctional imaging of G × E in emotionality is provided. Evidence for neural modularity of cognition and emotion is also taken into consideration. Finally, views of developmental programming by epigenetic mechanisms will be discussed in the perspective of the complex genetic architecture of emotional behaviour and social interaction in non-human primates and rodents. Better understanding of the role of epigenetic programming, particularly with respect to genome-wide modification of DNA and chromatin structure in complex tissues such as the brain, in the context of adverse life events, ageing processes, and resilience is likely to have far-reaching consequences for health, and mental health in particular.
2 5-HTT × Environmental Adversity Interaction in Humans
It is now well established that much of the impact of genetics on emotionality, including anxiety and depression, depends on interactions between genes and the environment. Such interactions imply that the expression of environmental effects occurs only in the presence of a permissive genetic background. There are several established environmental risk factors for disorders of emotional regulation. For example, numerous studies of G × E in humans have assessed the influence of childhood maltreatment and abuse, stressful events across the lifespan, socio-economic status and chronic somatic illness. Cumulative, repeated, or protracted exposures to adversity appear to exert a stronger effect than discrete acute events (Uher and McGuffin 2008, 2010 for reviews).
The work by Caspi and co-workers (Caspi et al. 2003) indicated that individuals carrying the low-expressing, short variant of a repetitive sequence in the upstream transcriptional control region of 5-HTT (Lesch et al. 1996), now commonly referred to as the 5-HTT-linked polymorphic region (5-HTTLPR), are up to twofold more likely to get depressed after stressful events such as bereavement, romantic disasters, illnesses or losing their job. Moreover, early trauma inflicted by childhood maltreatment significantly increased the probability of developing depressive syndromes in later life in individuals with the short allele of the 5-HTTLPR. A remarkable body of evidence suggests that emotionality and stress reactivity can be influenced by experiences early in life, and it has long been supposed that severe early life trauma may increase the risk for anxiety and affective disorders (Brown and Harris 2008). For example, adults experiencing four out of a possible seven severe early traumatic events showed a more than fourfold increased risk for depressive symptoms and about a 12-fold increased risk for attempted suicide (Felitti et al. 1998). No direct correlation between any specific childhood trauma and specific adult anxiety or mood disorder could be made, however, suggesting that other, possibly genetic, factors determine the precise pathology that is precipitated by the traumatic event. The observation that during early development individuals are particularly susceptible to adverse environmental influences is currently being confirmed by studies in non-human primates and mouse models that have demonstrated influential effects of the quality of maternal care on life-long emotional behaviour and brain functioning (see Sects. 3 and 4).
These results further support the notion that a combination of genetic disposition and specific life events may interact to facilitate the development of mental illness. What went largely unnoticed, however, were the implications for the genetics of personality. Depression is strongly associated with anxiety- and depression-related traits, the personality dimensions that have been linked with allelic variation of 5-HTT function. Given the high co-morbidity between anxiety and depression and the evidence for their modulation by common genetic factors (Kendler et al. 1993, 1995; Lesch 2003), it is likely that predisposition to disorders of emotional regulation will also be determined by environmental adversity whose impact on the brain is under genetic control.
Converging evidence from a large number of studies on the influence of 5-HTT × E established that stressful life events specifically contribute to the pathogenesis of anxiety and depression as well as of disorders in which anxiety and depression are co-morbid condition (Uher and McGuffin 2010) (Fig. 1). The effect however is variable, being closely related in time to the onset of disease and having more impact on first onset than on recurrences. Several caveats have to be kept in mind when G × E interaction is investigated in clinical cohorts. The assessment of stressful life events is generally retrospective and it is crucial to minimize recall bias, distortions and inaccuracies. As the temporal relationship between stressful life events and onset of anxiety disorders and depression is incompletely understood, a cohort of patients need to be followed over a sufficient period of time following an objectively recorded stressful event to establish the time course of G × E interactions. Moreover, there is evidence that experience and recall of stressful life events are partially under the control of both genes and Neuroticism as well as a personal history of anxiety and depression that predict reporting of life stress (Uher and McGuffin 2010). Although genetic factors that influence retrospective report of environmental cues overlap with those that affect personality traits, a causal relationship between adversity and affective symptomatology has, nevertheless, been demonstrated independent of individual differences in personality and recall.
While clinical evaluation and self-report of life events revealed that the effect of psychosocial adversity on depression and suicidal behaviour is modified by allelic variation of 5-HTT function, which renders carriers of the 5-HTTLPR short variant more vulnerable to depression, the neural mechanisms underlying this moderator effect are poorly understood. Although human and non-human primate functional imaging studies have begun to elucidate the neural circuits involved in the 5-HTT × E risk factor (see Sect. 5), a molecular understanding of this phenomenon is essentially lacking (Canli et al. 2006; Kalin et al. 2008).
In summary, these findings point toward molecular and cellular mechanisms by which early stressful experiences induce persistent changes in gene expression and neuronal function whose initiation or maintenance is influenced by 5-HTT. The molecular mechanisms by which early life stressors increase risk for disorders of emotional regulation in adulthood is not known but it is presumed to include epigenetic programming of gene expression during development and throughout the entire lifespan.
3 Interaction of 5-HTT and Maternal Separation in Rhesus Macaques
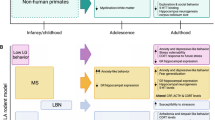
Animal models have become indispensable tools for studying the biological function of genes that are involved in the pathogenesis of neuropsychiatric disorders (Fig. 1). Since the neural and genetic basis of emotional and behavioural traits is already laid out in all mammalian species and may reflect selective forces among our remote ancestors, research efforts have recently been focussed on non-human primates, especially Macaca mulatta. Following the complete sequencing of the rhesus genome, this macaque species has become the “workhorse” of behavioural genetics of non-human primates. In this primate model environmental influences, while as complex as in humans, can be more easily controlled for and thus are less likely to confound gene-behaviour associations. In rhesus monkeys, all forms of emotionality and cognitive processing are moderated by environmental cues and marked disruptions to the mother–infant relationship confer increased risk for emotional dysregulation and cognitive impairment (Suomi 2003).
In rhesus monkeys, maternal separation (MS) and replacement of the mother by an inanimate surrogate mother during the first months of life results in long-term consequences for the functioning of the central 5-HT system, defects in peer interaction and social adaptation and is associated with increases in anxiety and depression-related behaviours such as rocking and excessive grooming (Higley et al. 1991). These behavioural studies already indicate that early life adversity can directly induce long-term neuroplastic changes in emotion circuits that alter anxiety and depression-related responses in adulthood.
One of the most replicated findings in psychobiology is the observation of lower 5-hydroxyindoleacidic acid (5-HIAA), the major metabolite of 5-HT, in the brain and cerebrospinal fluid (CSF) in impulsive aggression and suicidal behaviour. In rhesus monkeys brain 5-HT turnover, as measured by concentrations of 5-HIAA in the cisternal CSF, shows a strong heritable component and is trait-like, with confirmed stability over an individual’s lifespan (Higley et al. 1992; Kraemer et al. 1989). Early experiences have long-term consequences for the function of the central 5-HT system, as indicated by robustly altered CSF 5-HIAA levels, as well as anxiety, depression- and aggression-related behaviours in rhesus monkeys deprived of their mother at birth and raised only among peers. This animal model of MS was therefore used to study G × E by testing for associations between central 5-HT turnover and allelic variation of 5-HTT function based on a repeat length variation (rh5-HTTLPR) structurally and functionally orthologous to the 5-HTT-linked polymorphic region in humans (Lesch et al. 1997) (Fig. 2). The findings suggested that the rh5-HTTLPR genotype is predictive of CSF 5-HIAA concentrations, but that early experiences make unique contributions to variation in the functioning of the 5-HT system in later life and thus provides evidence of an environment-dependent association between the 5-HTT and a direct measure of brain 5-HT function (Bennett et al. 2002). The consequences of deleterious early experiences of MS seem consistent with the notion that the 5-HTTLPR may influence the risk for disorders of emotion regulation.
Studies were extended to the neonatal period of rhesus macaques, a time in early development when environmental influences are modest and least likely to confound gene-behaviour associations. Between postnatal days 7–30 mother-reared and maternally deprived neonates were assessed on a standardized neurobehavioural test designed to measure orienting, motor maturity, reflex functioning and temperament. Main effects of genotype and, in some cases, MS × genotype interactions, were demonstrated for items indicative of orienting, attention and temperament. In general, infants with the s form of the rh5-HTTLPR displayed higher behavioural stress-reactivity compared to one variant homozygote, as shown by diminished orientation, lower attentional capabilities and increased affective responding (Champoux et al. 2002). However, the genotype effects were more pronounced for animals raised in the neonatal nursery than for animals reared by their mothers. These results demonstrate the contributions of MS and genetic background, and their interaction, in a model of behavioural development during the neonatal phase.
Beyond the neonatal period, particularly during adolescence of rhesus monkeys, evidence for a complex interplay between inter-individual differences in the functions of the 5-HT system and social success has also been accumulated. The interactive effect of rh5-HTTLPR genotype and early rearing environment on social play and aggression was explored in infants and adolescents (Barr et al. 2003). Rhesus monkeys homozygous for the long variant were more likely to engage in rough play than those with the long/short variants, with a significant interaction between 5-HTT genotype and MS. Peer-reared infants carrying the short variant were less likely to play with peers than those homozygous for the long allele, whereas the rh5-HTTLPR genotype had no effect on the incidence of social play among mother-reared monkeys. Socially dominant mother-reared monkeys were more likely than their peer-reared counterparts to engage in aggression. In contrast, peer-reared but not mother-reared monkeys with the low-activity short allele exhibited more aggressive behaviours than their long/long variant counterparts. This genotype by rearing interaction for aggressive behaviour indicates that peer-reared subjects with the short allele, while unlikely to win in a competitive encounter, are more inclined to persist in aggression once it begins.
Since allelic variation of 5-HTT function is associated with anxiety-related traits as well as an increased risk for developing depression in the face of adversity, the impact of rh5-HTTLPR × MS interaction on stress-elicited endocrine responses was determined in infant rhesus macaques. Adrenocorticotropic hormone (ACTH) and cortisol plasma concentrations in monkeys reared with their mothers or in peer-only groups were determined at baseline and during separation stress at 6 months of age. Cortisol increased during separation and there was a main effect of rearing condition with decreased cortisol among peer-reared macaques. Monkeys carrying the rh5-HTTLPRs variant had higher ACTH. ACTH increased during separation, and there was a maternal deprivation × rh5-HTTLPR interaction, such that peer-reared short allele carriers had higher ACTH during separation than long variant homozygotes. A confirmatory study further revealed that this interaction is sexually dichotomous and the interactive effect may underlie the increased incidence of certain stress-related disorders of emotional regulation in women (Barr et al. 2004b). These findings confirm the data from studies in human populations that allelic variation of 5-HTT function affects hypothalamic–pituitary–adrenal (HPA) axis activity and that the influence of rh5-HTTLPR on hormonal responses during stress is modulated by early life adversity and displays sex specificity (Mannie et al. 2009; O’Hara et al. 2007; Wust et al. 2009).
Previous research also revealed that peer-reared primates display a higher preference for alcohol compared to other young adults. Furthermore, maternally deprived female rhesus macaques show exaggerated HPA axis responses to alcohol (Higley and Linnoila 1997). Because their environments can be controlled, use of the macaque model permits investigation of independent influences as well as potential interactions between 5-HT signalling pathway-related genes, MS, and other stressors in the aetiology of alcohol dependence. Given that 5-HT signalling and HPA axis hormones are involved in the reinforcement of alcohol intake and contribute to the risk for symptoms of withdrawal and relapse in alcohol dependence in a gender-specific manner, the interactive effect of rh5-HTTLPR genotype and early rearing environment on the patterns of preference and consumption across a 6-week alcohol consumption paradigm was examined (Barr et al. 2004a). Female rhesus macaques were reared with their mothers in social groups or in peer-only groups. As young adults, they were then given the choice of an alcohol solution or vehicle. Interactions between rearing condition and rh5-HTTLPR genotype, with dramatically higher levels of ethanol preference, were demonstrated in s variant carriers. An effect of rearing condition on alcohol consumption during the 6 weeks was found as well as a phase × MS interaction, such that peer-reared animals progressively increased their levels of consumption. This was especially evident for peer-reared females carrying the rh5-HTTLPR s variant. Thus, the high composite scores for alcohol intake and alcohol-elicited aggression associated with the low-expressing short rh5-HTTLPR variant in female rhesus monkeys confirm an interaction between the 5-HT system activity and early aversive experience in the vulnerability to alcohol dependence and represent a clinically valid model for type II alcoholism.
Taken together, these findings provide substantial evidence of an environment-dependent association between allelic variation of 5-HTT expression and central 5-HT function and illustrate the possibility that specific genetic factors play a role in regulating behaviours in primates which are modulated by 5-HT signalling pathways. Because rhesus monkeys exhibit temperamental and behavioural traits which parallel anxiety, depression- and aggression-related trait dimensions in humans associated with the low activity short 5-HTTLPR variant, it may be possible to search for evolutionary continuity in this genetic mechanism for inter-individual differences. Non-human primate studies may also be useful to help identify environmental circumstances that compound the vulnerability conferred by a particular genetic makeup or, conversely, act to improve behavioural outcomes associated with a distinct genetic disposition.
4 5-HTT-Deficient Mouse: A Model for Epigenetic Programming of Development
Quantitative genetic research on rodent models is based primarily on inbred strain and selection studies. While comparisons between different inbred strains of mice expose remarkable differences in behavioural measures, differences within strains can be attributed to environmental influences. Inbred and recombinant inbred strain studies are highly efficient for dissecting genetic influences, for investigating interactions between genotype and environment and for testing the disposition-stress model. Furthermore, investigations in rodents have shown that intra- and extra-uterine maternal signals have long-lasting consequences on anxiety-like behaviour in the offspring and can synergistically induce long-term plastic changes in anxiety- and depression-related neural circuits.
Since humans and mice have almost the same genome size and share many orthologous genes mapped to syntenic chromosomal regions, it is conceivable that gene variations which influence a behavioural trait in humans may be modelled in the mouse. Based on the close similarity in the genomes between the two species and the extensive knowledge derived from the sequencing of the murine genome, mouse mutants have become the standard model. With the introduction of efficient gene targeting techniques, the mouse is the only mammal uniting the top-down and bottom-up genetic approach, from phenotype to gene and from gene to phenotype, respectively (see also Gondo et al. 2011; O’Tuathaigh et al. 2011 for discussion). Moreover, these mouse models provide efficient ways to control and manipulate environmental factors and allow dissection of the molecular mechanisms of G × E interactions.
In mouse, there is no analogue to the human and macaque 5-HTTLPR, but it is possible to either inactivate the 5-HTT (Bengel et al. 1998) or to use transgenic approaches to increase its expression (Jennings et al. 2006). Inactivation of the murine 5-Htt and the resulting disturbance of brain 5-HT system homeostasis has considerably advanced our understanding of the neurobiological basis of anxiety and depression-related behaviour in mice (Lesch 2005; Murphy and Lesch 2008). 5-Htt−/− knockout mice show behaviours consistent with anxious and depressive traits; they appear reluctant to explore brightly lit spaces or elevated open platforms and give up struggling early when put in a stressful situation (Holmes et al. 2003). Remarkably, certain types of depression-like behaviour (e.g. behavioural despair) are manifest only after repeated exposure to stressors, which may be analogous to repeated or chronic stressful life events in humans (Wellman et al. 2007). 5-Htt−/− mice also show exaggerated neuroendocrine reactions to acute stress, similar to the increased HPA reactivity reported in some depressed patients. The effect of 5-Htt deficiency on anxiety and depression-like behaviours in mice may be mediated by reduced 5-HT clearance mechanism during a vulnerable developmental period but can still be selectively reversed by pharmacological 5-HT1A receptor inhibition later in life, suggesting an enduring modulatory involvement of the 5-HT system.
In addition, 5-Htt deficient mice provide a practical tool to study the impact of genetic mechanisms on the development and plasticity of the brain, (Altamura et al. 2006; Di Pino et al. 2004; Persico et al. 2001, 2003; Salichon et al. 2001). The anxious-depressive phenotype in 5-Htt−/− mice is associated with increased dendritic branching in fear associated circuits, including the medial prefrontal cortex and the amygdala (Wellman et al. 2007; Nietzer et al., in press). However, despite growing evidence for a critical role of the 5-Htt in the integration of synaptic connections in the mouse brain during critical periods of development and adult life, knowledge of the machinery involved in these fine-tuning processes remain incomplete.
Gene × enviromental adversity in the 5-HTT+/− mouse model based on the work by Caspi and coworkers (2003): (a), heterozygous 5-Htt+/− mice (displaying a 50% gene dose-dependent reduction of 5-Htt expression, thus representing a model for individuals with the short 5-HTTLPR variant) have used. These studies demonstrated that, although these mice do not show behavioural deficits at baseline, they developed increased anxiety and depression-like behaviour in adulthood when exposed to prenatal stressors (b), to early life adverse experiences including maternal neglect (c), or to psychosocial stress in adult life (d). These of modes of G × E serve as specific models for the increased vulnerability to environmental adversity across the lifespan in individuals with the low-expressing short 5-HTTLPR variant
Several studies used heterozygous 5-Htt+/− mice (displaying a 50% gene dose-dependent reduction of 5-Htt expression, thus representing a practical model for individuals with the short 5-HTTLPR variant). These studies found that, although the mice do not show behavioural deficits at baseline, they developed increased anxiety and depression-like behaviour in adulthood when exposed to prenatal stressors (Heiming et al. 2009; van den Hove et al. manuscript submitted), to early life adverse experiences including maternal neglect (Wellman et al. 2007) or to psychosocial stress in adult life (Bartolomucci et al. 2010; Jansen et al. 2010; Lewejohann 2010) (Fig. 3). It is proposed that such animal G × E paradigms serve as specific models for the increased vulnerability to environmental adversity across the lifespan in individuals with the low-expressing short 5-HTTLPR variant.
Interaction between rearing environment and 5-Htt function in mice: BDNF as a molecular substrate of both 5-Htt deficiency and maternal care (modified from Carola et al. 2008). (a) and (b), Epigenetic programming by early adverse environment in mice deficient for 5-Htt. The effects of maternal neglect on anxiety-like behaviour is modulated by 5-Htt inactivation in a way that mimics the interaction between early life stress and 5-HTTLPR genotype observed in rhesus macaques and humans. (c), heterozygous 5-Htt+/− mice experiencing maternal neglect showed increased anxiety-like behaviour. (d), Identification of neural and molecular substrates of G × E interaction: BDNF mRNA concentrations in hippocampus are elevated exclusively in heterozygous 5-Htt+/− mice experiencing poor maternal care, suggesting that developmental programming of hippocampal circuits may underlie the 5-HTT × E adversity risk factor
4.1 Prenatal Stress
Exposure to early life stress elicited by repeated mild electric footshock in the second postnatal week failed to further aggravate the increased anxiety and depression-like phenotype of 5-Htt deficient mice (Carroll 2007). Therefore, environmental adversity during early life may be more successfully modelled using ecologically relevant paradigms such as maternal neglect, simulation a threatening habitat for a female mouse and her pups or other species relevant adverse environmental challenges. Given the importance of early life stressors and their interaction with 5-HTT genotype in the development of affective disorders in humans, it is important to model the interaction of 5-Htt deficiency with prenatal or perinatal stress in mice.
Heiming and co-workers (Heiming et al. 2009) exposed pregnant and lactating 5-Htt+/− females to the olfactory cues of unfamiliar adult males, signalling the risk of infanticide. When mothers had lived in a threatening environment, their offspring showed increased anxiety-like and reduced exploratory behaviour compared to controls and the effects were most pronounced in 5-Htt−/− mice. It was concluded that the behavioural profile of the offspring was shaped in an adaptive way, preparing the young for an adverse environment. When modulated by 5-Htt genotype, which alters 5-HT neurotransmission, offspring may develop potentially pathological levels of avoidance behaviour, which are determined by the G × E interaction.
Van den Hove and associates (2010, manuscript submitted) developed a prenatal stress paradigm by restraining pregnant 5-Htt+/− mice three times a day for 45 min in transparent glass cylinders filled with water up to a height of 5 mm, whilst being exposed to bright light. Prenatal maternal stress was performed daily during the last week of pregnancy (E13–E17). The results indicate that the long-term effects of prenatal stress on anxiety and depression-like behaviour in the offspring are partly dependent on the 5-Htt genotype. Genome-wide expression analysis of the hippocampus of these mice revealed G × E effects on apoptotic and psychoimmunological processes.
4.2 Maternal Neglect
Another species-relevant adverse environmental stressor in rodents is deficient maternal care in the first postnatal weeks. Previous investigations in rats indicated that maternal behaviour has long-lasting consequences on anxiety-like behaviour of the offspring. MS for several hours a day during the early postnatal period results in increased anxiety-like behaviours as well as increased stress hormone reactivity in adult animals (Kalinichev et al. 2002). Similarly, pups that are raised by mothers that display low licking and grooming behaviour show higher levels of anxiety-like behaviour than pups raised by high licking and grooming mothers, and cross fostering studies show that these influences are primarily environmental (Caldji et al. 1998; Liu et al. 2000). Cross fostering offspring of low licking and grooming mothers to high licking and grooming mothers is able to impart low anxiety-like behaviour to the offspring, whereas the converse does not influence this behaviour. Offspring of high licking and high grooming mothers raised by low licking and grooming mothers do not show high anxiety-like behaviour, suggesting that specific genes inherited by the high licking and grooming offspring protect them from the effects of low licking and grooming mothering. Furthermore, Francis et al. (1999) have shown that the effect of high licking and grooming can be passed from one generation to the next. Females raised by high licking and grooming mothers themselves become high licking and grooming mothers and go on to produce low anxiety offspring regardless of whether their biological mother showed low or high licking and grooming. This epigenetic inheritance of anxiety-like behaviour underscores the power that environmental influences can exert to persistently remodel circuits in the brain during early development.
Studies using mice of defined genetic backgrounds have also begun to shed light on the molecular mechanisms of specific G × E interactions. Anisman et al. (1998) found that mice of the low licking and grooming Balb/c inbred strain cross-fostered at birth to the high licking and grooming C57BL/6 inbred strain display improvements in a hippocampus dependent memory task. Because the reverse cross fostering, where C57BL/6 pups are raised by Balb/c mothers, does not alter the behaviour of C57BL/6 mice, it appears that the C57BL/6 genetic background protects the pups from the effects of a Balb/c maternal environment. However, by transplanting C57BL/6 embryos into Balb/c foster mothers shortly after conception, Francis et al. (2003) were able to show that a combined prenatal and postnatal Balb/c maternal environment is sufficient to confer Balb/c behaviour on C57BL/6 offspring, demonstrating that intra- and extra-uterine maternal signals can synergistically induce long-term plastic changes in anxiety- and depression-related neural circuits.
To study the neural and molecular mechanisms underlying epigenetic programming by early adverse environment in an animal model amenable to genetic manipulation a G × E paradigm was developed in the mouse (Fig. 4a, b). It was shown that the effects of an adverse rearing environment on anxiety-related behaviour are modulated by mutations in 5-HTT in a way that mimics the interaction between early stress and 5-HTT seen in humans (Carola et al. 2008). Mice experiencing low maternal care showed deficient gamma-aminobutyric acid-A (GABA-A) receptor binding in the amygdala and heterozygous 5-Htt+/− mice showed increased anxiety and depression-like behaviour and decreased serotonin turnover in hippocampus and striatum (Fig. 4c). Strikingly, levels of brain-derived neurotrophic factor (BDNF) mRNA in hippocampus were elevated exclusively in 5-Htt+/− mice experiencing poor maternal care, suggesting that developmental programming of hippocampal circuits may underlie the 5-Htt × E risk factor (Fig. 4d). These findings demonstrate that 5-HT plays a similar role in modifying the long-term behavioural effects of rearing environment in mice as it does in non-human primates and identifies BDNF as a possible molecular substrate of this risk factor. It is therefore predicted that some of the principle neural and molecular mechanisms which underlie G × E interactions in mouse and non-human primate models are relevant to the aetiology of disorders of emotional regulation in humans.
The chronic social defeat mouse model (modified from Bartolomucci et al. 2010): Increased social avoidance (a), decreased locomotor activity, and increased serotonin concentrations (c) in the frontal cortex of mice in 5-Htt+/− mice receiving highest aggression in the resident-intruder paradigm, whereas both wildtype and 5-Htt+/− mice show increased body weight, body temperature, aggression-induced hyperthermia, and corticosterone plasma concentrations following social defeat (b)
4.3 Chronic Psychosocial Stress in Adulthood
Although epidemiological evidence links exposure to stressful life events with increased risk for disorders of emotional regulation, there is significant individual variability in vulnerability to environmental cues and the penetrance of genetic variation is thought to play a major role in determining who will develop disorders. Identifying the molecular mechanisms underlying this G × E risk factor will facilitate an understanding of the individual differences in resilience to stress. Therefore, a mouse model of the 5-HTT × adult life stress associated with winner or loser experience in a resident-intruder paradigm was recently generated.
Jansen et al. (2010) reported that male mice of all three 5-Htt genotypes experiencing social defeat on three consecutive days displayed increased anxiety-like behaviour and decreased exploration, irrespective of winning or losing. In losers, a distinct effect of genotype occurred. Homozygous 5-Htt−/− males showed more anxiety-like behaviour and less exploration than the other genotypes. In winners, no genotype-dependent variation was found. Genotypes did not differ in basal activation of the stress hormone system but there was a main effect of social experience with higher corticosterone levels in losers compared to winners. This effect was most pronounced in the heterozygous 5-Htt+/− mice, indicating that anxiety circuits and stress reactivity retain their plasticity throughout adulthood and can be shaped by genotype and social experiences during this phase of life.
Bartolomucci et al. (2010) subjected wild-type and heterozygous 5-Htt+/− male mice to 3 weeks of chronic psychosocial stress in a dominant/subordinate context of a resident-intruder paradigm (Fig. 5). The 5-Htt genotype did not affect the physiological consequences of stress as measured by changes in body temperature, body weight gain and plasma corticosterone. However, when compared with wild-type littermates, heterozygous 5-Htt+/− mice experiencing high levels of stressful life events, in the form of repetitive social defeat, showed significantly depressed locomotor activity and increased social avoidance toward an unfamiliar male in a novel environment. 5-Htt+/− mice exposed to high aggression stress also showed significantly lower levels of 5-HT turnover than wild-type littermates, selectively in the frontal cortex, which is a structure that is known to be involved in fear control and avoidance responses, and that is implicated in susceptibility to depression. These data point toward a useful animal model for better understanding the increased vulnerability to stress which has been reported in individuals carrying the low-expressing short 5-HTTLPR variant, and suggest that social avoidance represents a behavioural endophenotype of the interaction between 5-HTT and psychosocial stress.
To identify changes in mRNA expression of novel candidate genes associated with altered prenatal, rearing and adult psychosocial environment and/or 5-HTT genotype, genome-wide microarray-based expression profiling of mRNA extracted from brain region-specific tissue and laser-dissected single neurons will have to be performed in mice from the various G × E paradigms (Ichikawa et al. 2008) (Fig. 7). By determining differential gene expression resulting from chromatin modification, future experiments promise to identify genes that are epigenetically regulated by environmental adversity and 5-Htt in the mouse. These genes are likely to be candidate susceptibility genes for anxiety disorders and may serve as potential diagnostic and therapeutic targets. The evidence for epigenetic inheritance of anxiety and depression-like behaviour underscores the view that environmental influences can persistently remodel circuits in the brain during early development. 5-Htt deficient and other genetically modified mice will therefore be essential for the dissection of the molecular and neural mechanisms of epigenetic processes and of the neurodevelopmental–behavioural interface (Lesch and Mössner 2006).
The molecular cycle of epigenetics in the susceptibility of depression and related co-morbid disorders: Gene variation affecting transcriptional control is permissive for environmental adversity moderating epigenetic mechanisms that involve methylation of DNA and acetylation of histones which, in turn, impacts regulation of gene expression. Silencing chromatin by DNA methylation and histone deacetylation switches genes off, whereas activation of chromatin by DNA demethylation and histone acetylation switches genes on
5 Neural Mechanisms of Epigenetic Programming
As already outlined clinical evaluation and self-report of life events revealed that the effect of psychosocial stress on depression and suicidal behaviour is modified by allelic variation of 5-HTT function, which renders carriers of the short 5-HTTLPR variant more vulnerable to depression (see Sect. 2). As a first approximation toward identifying the neural circuits involved in controlling these epigenetic processes, individuals with self-reported life stress but no history of psychopathology were investigated with multimodal magnetic resonance imaging (MRI)-based imaging (functional, perfusion, structural). Based on functional MRI and perfusion data, support was found for a model by which life stress interacts with the effect of 5-HTTLPR genotype on amygdala and hippocampal resting activation, which may provoke a chronic state of vigilance, threat or rumination (Canli et al. 2006). Life events also differentially affected, as a function of 5-HTTLPR genotype, functional connectivity of the amygdala and hippocampus in response to emotional stimuli with a wide network of other regions, as well as grey matter structural features. These interactions may constitute a neural mechanism for epigenetic vulnerability or resilience against depressive illness and may have a morphological substrate in the increased dendritic branching and spine density within the fear circuit of 5-HTT deficient mice, including both the medial prefrontal cortex and the amygdala (Wellman et al. 2007; Nietzer et al. in press) (Fig. 6).
By the same token, the previously reported whole-brain analyses of activation, functional connectivity and grey matter density and volume also showed a moderation of the same prefrontal cortex-amygdala circuitry by 5-HTTLPR genotype × life stress interaction (Canli et al. 2006). In addition, the superior parietal lobule, superior temporal gyrus, inferior frontal gyrus, precentral gyrus and insula were affected by 5-HTT × E. The remarkable fact about these regions is that they belong to circuits that integrate imitation-related behaviour, from which social cognition and behaviour in a social world has evolved (Iacoboni 2005). Social cognition is a construct comprising representations of internal somatic states, interpersonal knowledge and motivations as well as procedures used to decode and encode the self relative to other people. This complex set of processes, which are carefully orchestrated to support skilled social functioning and communication-facilitated networking, has recently been associated with activity in distinct neural circuits (Adolphs 2009; Kitayama and Park 2010).
Regions involved in imitation, imitative learning, social cognition and communication skills (Amodio and Frith 2006; Carr et al. 2003), and affected by 5-HTT × life stress, include the superior parietal lobule, superior temporal gyrus, inferior frontal gyrus, precentral gyrus, insula, anterior cingulate and amygdala. Some of these regions contain mirror neurons (Rizzolatti and Craighero 2004; Uddin et al. 2005), which are activated during goal-directed behaviour or the observation of such behaviour in others, and Von Economo neurons, which are believed to play a role in social bonding. Dysfunction of both neural units is thought to cause social and communication disabilities associated with autistic syndromes (Allman et al. 2005; Dapretto et al. 2006). The morphological alterations in 5-HTT deficient mice in conjunction with data G × E functional MRI data suggests that social competence and behaviour involving mirror or Von Economo neurons are neural targets of epigenetic moderation may be subject to an interaction between psychosocial adversity and 5-HTTLPR genotype (Canli and Lesch 2007).
Future psychophysiological and morphofunctional imaging studies in both the animal model and the humans will have to address whether an interaction of 5-HTTLPR and life stress moderates neural activation during imitation or social processing tasks. As investigations have begun to refine the methodologies for capturing epigenetic effects on the brain, we may better understand the mechanisms that render some individuals susceptible and others resilient to depression and other affective spectrum disorders.
6 “To Be or Not to Be”: 5-HTT × Psychosocial Adversity Interaction Is Subject to Evolutionary Pressure
Recently, a series of studies have emerged showing that the short 5-HTTLPR variant is associated with improved cognitive functions. Therefore, these factors may counteract or completely offset the negative consequences of the anxiety and depression-related traits (for review: Homberg and Lesch in press). For instance, it has been shown that carriers of the 5-HTTLPR short-allele exhibited increased emotionally cued startle responses (Brocke et al. 2006) and a stronger attentional bias for anxious word stimuli (Beevers et al. 2007) along with greater difficulty disengaging their attention from stimuli with strong negative or positive valence (Beevers et al. 2009), and a bias to focus on positive images (Beevers et al. 2010). It has also been shown that in depression carriers of the short variant benefit most from social support (Brummett et al. 2008), indicating that sensitivity to positive stimuli can alleviate the negative consequences of sensitivity to adverse events. In a social context, short-allele carriers display more aggressive behaviours (Schwandt et al. 2010) and are characterized by an increased vulnerability to the adverse effects of psychosocial stress associated with subordinate status (Jarrell et al. 2008).
An analogous attentional bias in rhesus monkeys is particularly striking: The short variant of the rh5-HTTLPR has been associated with a reduction in time spent gazing at images of faces compared to non-face images, less time looking in the eye regions of faces and larger pupil diameters when gazing at photos of a high versus low status male macaques (Watson et al. 2009). The reluctance of rhesus macaques carrying the short allele to gaze directly at the eyes and faces of con-specifics, as well as their enhanced sympathetic response to the images of high-status males, suggests that these individuals experience greater anxiety than those homozygous for the long variant when viewing potential social threats. The greater pupil diameter indicates that the short-allele carrying monkeys found images of high-status male faces to be more arousing, which is in harmony with the observations of Beevers and associates (Beevers et al. 2010) that humans carrying the short-allele tend to exhibit a bias to focus on positive images and thus may experience more arousal when viewing rewarding pictures.
At the level of cognitive flexibility, it was found that the 5-HTTLPR short-allele is associated with improved performance in trials of attentional set shifting (Borg et al. 2009), which is in accord with increased attention towards task parameters (Roiser et al. 2007). The cognitive enhancement is also consistent with the observation that short-allele carriers show increased processing of negative feedback (Althaus et al. 2009). In rhesus monkeys, the short-allele of the rh5-HTTLPR was associated with superior performance on an array of cognitive tasks: the probability discounting task, the delay discounting task, the reversal learning task and the delayed match-to-sample task (Jedema et al. 2009). The better performance in the probability discounting task is consistent with the increased attention to high and low probabilities in the risky decision-making task in humans carrying the short 5-HTTLPR variant (Roiser et al. 2006) and may be explained by augmented cortico-limbic activation (Fallgatter et al. 1999, 2004) and superior ability to integrate feedback information over time to guide behaviour on subsequent choices (Althaus et al. 2009).
Connected to the medial prefrontal cortex-amygdala circuitry, other brain regions implicated in allelic 5-HTT function-mediated behavioural responses are the orbitofrontal cortex (OFC), the pulvinar nucleus of the thalamus and the bed nucleus stria terminalis. The OFC shows prominent morphological alterations and differential functional activation in response to environmental manipulation in rhesus monkeys carrying the short allele (Kalin et al. 2008). The OFC signals representations of expected outcomes and compares an expected with the actual outcome of behaviour. When incongruent, it modulates the activity of downstream brain areas that are involved in response selection and action, such as the amygdala and striatum. In that way, increased OFC activity may allow short-allele carriers to flexibly adapt behaviour when a mismatch is detected between the expected and actual outcome of behaviour, for instance during reversal learning in rhesus macaques (Jedema et al. 2009).
Taken together, the studies in non-human primates complement the findings in human on emotionality in social settings and suggest that the 5-HTTLPR short-variant is associated with increased attention (Roiser et al. 2007) to task parameters and may contribute to improved decision making and improved cognitive flexibility (Borg et al. 2009; Jedema et al. 2009; Roiser et al. 2006, 2009). Moreover, it may be linked with heightened social vigilance and, generally, sensitivity to environmental cues per se, which could result in maladaptive responsiveness when danger or social threats are absent. However, it may also be highly adaptive in distinct contexts or circumstances and consequently subject to positive selective pressure. That is, seizing opportunities and the simultaneous avoidance of potential harmful antagonistic interactions may lead to social (and thus, reproductive) success.
Heightened vigilance, i.e. high attentional biases directed towards motivationally relevant stimuli, may be the common denominator in the high emotionality and cognitive enhancement associated with allelic variation of 5-HTT function (Homberg and Lesch in press). The challenge is now to understand how the brain determines when to respond either emotionally or cognitively depending on environmental stimuli. Integration of these findings will provide novel hypotheses for the understanding of the mechanisms underlying the co-occurrence of anxiety-related traits and enhanced cognitive function in association with 5-HTT × E interaction.
7 Molecular Mechanisms of Epigenetic Programming
The molecular mechanisms by which early stress increases risk for disorders of emotional regulation in adulthood is not known, but is presumed to include epigenetic programming of gene expression (see Bountra et al. 2011; Weaver et al. 2004, 2006). Identifying the molecular mechanisms behind the long-term behavioural effects of altered rearing environments is a major goal of research in the field of developmental programming. Persistent changes in the expression of several genes have been documented in adult animals exposed to altered rearing environments. Decreased expression and function of the glucocorticoid receptor (GR) has been associated with a low maternal care environment and has been the subject of several studies (for review: Seckl and Meaney 2004). Decreased GABA-A receptor binding and subunit expression as well as increased corticotropin-releasing hormone (CRH) mRNA have been documented in the amygdala of offspring of low licking and grooming mothers and is proposed to be required for their increased anxiety-related and stress response behaviour, respectively (Calatayud et al. 2004; Caldji et al. 1998). Changes in hippocampal expression of NMDA receptor subunits, BDNF, neural cell adhesion molecule (NCAM), synaptophysin and acetylcholine esterase have also been reported in offspring of low versus high licking and grooming mothers (Liu et al. 2000). Recent work in rats has suggested that changes in the activity of the GR promoter play a critical role in the epigenetic programming of adult stress response by rearing environment. Decreased methylation and increased histone 3 acetyl-K9 binding of the GR I7 promoter was found in hippocampus of adult rats receiving low licking and grooming (Weaver et al. 2004). Treatment of these rats in adulthood with the histone deacetylase inhibitor (HDAC), tricostatin A, reversed the effects of low licking and grooming on GR expression. Remarkably, tricostatin treatment also reversed the effects of rearing environment on stress hormone responses, demonstrating a role for chromatin remodelling in maintaining the long-term effects of rearing environment. In a parallel study by the same group, supplementing the diet of high licking and grooming mothers with methionine in adulthood increased GR promoter methylation and stress response, suggesting that epigenetic programming of GR can be pharmacologically adjusted either up or down with corresponding changes in stress response activity (Weaver et al. 2005).
8 Conclusions and Outlook
Modest advances in behavioural genetics are contrasted by giant leaps in an epigenomic era still in its infancy. The application of paradigms novel to neurogenetic approaches including generation of genetically modified mice, validation of G × E models in non-human primates and rodents, application of functional neuroimaging and next-generation sequencing technologies in the quest for rare genetic variants with high effect size but variable penetrance and inclusion of a more extensive phenotypic spectrum (e.g. higher cognitive functions, social competence, resilience) have strengthened the connection between 5-HTT, (social) cognition and emotionality and continue to enable a more profound understanding of how common and rare genetic variation modulates human behaviour.
In this overview I have attempted to integrate findings from studies in G × E animal models which underscore the central role of 5-HT and its fine-tuning by 5-HTT functionality in embryonic patterning events, brain development, and synaptic plasticity, particularly in neural circuits related to (social) cognitive and emotional processes. Increasing evidence suggests that epigenetic mechanisms may play a crucial role in neurodevelopmental programming of behavioural abnormalities. Among a number of environmental cues, social environment can act as a primary risk for the development of anxiety and depression-related phenotypes (Fig. 8). The best evidence derives from findings of developmental outcomes associated with institutional deprivation that consistently highlight the increased rates of disorders of emotional regulation. Further support comes from the risk-attenuating role of alternative social environments through resilience-focused therapeutic intervention. Moreover, the social environment has been shown to determine the extent to which patients with affective disorders develop co-morbidity. Through modification of DNA and associated histones, epigenetic mechanisms translate environmental stimuli into changes in gene expression as neural correlates of epigenetic programming. Epigenetically influenced behavioural modifications are accompanied by changes in gene expression. Thus, these mechanisms are hypothesized to play an essential role in the interplay of genetic and environmental cues in determining anxiety and depression-related phenotypes. Epigenetic markers identified by genome-wide expression, DNA methylation, and histone modification profiles are dynamic and reversible and may also provide powerful targets for pharmacological intervention strategies. Therefore, deeper insight into the role of epigenetic regulation in the process of neurodevelopmental programmes, in particular in relation to depression and co-morbid disorders, will contribute to the establishment of early diagnosis and the development of innovative treatments targeting neural pathways that foster resilience.
Changes in mRNA expression of novel candidate genes associated with altered rearing environment and/or 5-Htt genotype may be identified by microarray-based expression profiling of mRNA extracted from brain region-specific tissue and laser-guided dissection of homogeneous neuron populations in mice from G × E paradigms. Whole-genome expression screening methods combined with high-throughput methylation and histone acetylation profiling microarray techniques both genome-wide and of selected genes in mice from stress-specific G × E paradigms promise to identify genes that are epigenetically regulated by 5-Htt × E interaction in the mouse. These genes are likely novel candidate susceptibility genes for disorders of emotion regulation and will serve as potential diagnostic and therapeutic targets
Finally, anxiety disorders and depression are known to be influenced not only by environmental stressors but also by each individual’s unique genetic background. The mouse models also permit analysis of synthetic mutant phenotypes or polygenic characteristics based on epistatic interaction and pleiotropy. Epistasis with genes with strong evidence-based rationale, such as BDNF, has a potential to further refine the concept and indicate mechanisms underlying the observed G × E interaction (Ren-Patterson et al. 2005). However, the majority of neural substrates and circuitries that regulate emotional processes or cause affective disorders remain remarkably elusive. Among the reasons for the lack of progress are remaining conceptual deficiencies regarding the genetic and neural architecture of emotionality and behavioural despair, which make it difficult to develop and validate reliable models of depression. As analyses of epigenomes in non-human primates and rodents will likely contribute fundamentally to our understanding how humans have evolved, the next stage of complexity concerns the nature of genetic variation, either common or rare, and epigenetic programming among humans and its influence on inter-individual differences at the level of physiology and disease pathogenesis, as well as the relative impact of genetic and environmental determinants on cognition, emotion and, ultimately, behaviour.
Abbreviations
- 5-HIAA:
-
5-Hydroxyindoleacidic acid
- 5-HT:
-
Serotonin
- 5-HTT:
-
Serotonin transporter
- 5-HTTLPR:
-
5-HTT gene-linked polymorphic region
- ACTH:
-
Adrenocorticotropic hormone
- BDNF:
-
Brain-derived neurotrophic factor
- CRH:
-
Corticotropin-releasing hormone
- CSF:
-
Cerebrospinal fluid
- G × E:
-
Gene-by-environment interactions
- GABA-A:
-
Gamma-aminobutyric acid-A receptor
- GR:
-
Glucocorticoid receptor
- HDAC:
-
Histone deacetylase inhibitor
- HPA:
-
Hypothalamic–pituitary–adrenal
- MRI:
-
Magnetic resonance imaging
- MS:
-
Maternal separation
- OFC:
-
Orbitofrontal cortex
- rh5-HTTLPR:
-
Rhesus macaque 5-HTT gene-linked polymorphic region
References
Adolphs R (2009) The social brain: neural basis of social knowledge. Annu Rev Psychol 60:693–716
Allman JM, Watson KK, Tetreault NA, Hakeem AY (2005) Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci 9:367–373
Altamura C, Dell’acqua ML, Moessner R, Murphy DL, Lesch KP, Persico AM (2006) Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb Cortex 17:1394–1401
Althaus M, Groen Y, Wijers AA, Mulder LJ, Minderaa RB, Kema IP, Dijck JD, Hartman CA, Hoekstra PJ (2009) Differential effects of 5-HTTLPR and DRD2/ANKK1 polymorphisms on electrocortical measures of error and feedback processing in children. Clin Neurophysiol 120:93–107
Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 7:268–277
Anisman H, Zaharia MD, Meaney MJ, Merali Z (1998) Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci 16:149–164
Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD (2003) The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav 2:336–340
Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD (2004a) Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry 61:1146–1152
Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD (2004b) Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA 101:12358–12363
Bartolomucci A, Carola V, Pascucci T, Puglisi-Allegra S, Cabib S, Lesch KP, Parmigiani S, Palanza P, Gross C (2010) Increased vulnerability to psychosocial stress in heterozygous serotonin transporter knockout mice. Dis Model Mech 3:459–470
Beevers CG, Gibb BE, McGeary JE, Miller IW (2007) Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J Abnorm Psychol 116:208–212
Beevers CG, Wells TT, Ellis AJ, McGeary JE (2009) Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol 118:670–681
Beevers CG, Ellis AJ, Wells TT, McGeary JE (2010) Serotonin transporter gene promoter region polymorphism and selective processing of emotional images. Biol Psychol 83:260–265
Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53:649–655
Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD (2002) Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry 7:118–122
Blakely RD, Berson HE, Fremeau RT Jr, Caron MG, Peek MM, Prince HK, Bradley CC (1991) Cloning and expression of a functional serotonin transporter from rat brain. Nature 354:66–70
Borg J, Henningsson S, Saijo T, Inoue M, Bah J, Westberg L, Lundberg J, Jovanovic H, Andree B, Nordstrom AL, Halldin C, Eriksson E, Farde L (2009) Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. Int J Neuropsychopharmacol 12:783–792
Bountra C, Oppermann U, Heightman TD (2011) Animal models of epigenetic regulation in neuropsychiatric disorders. Curr Top Behav Neurosci. doi:10.1007/7854_2010_104
Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch KP, Kirschbaum C, Strobel A (2006) Serotonin transporter gene variation impacts innate fear processing: acoustic startle response and emotional startle. Mol Psychiatry 11:1106–1112
Brown GW, Harris TO (2008) Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene–environment interaction. J Affect Disord 111:1–12
Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, Zuchner S, Collins A, Williams RB (2008) Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR). Behav Genet 38:34–43
Calatayud F, Coubard S, Belzung C (2004) Emotional reactivity in mice may not be inherited but influenced by parents. Physiol Behav 80:465–474
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95:5335–5340
Canli T, Lesch KP (2007) Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 10:1103–1109
Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP (2006) Neural correlates of epigenesis. Proc Natl Acad Sci USA 103:16033–16038
Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, Lesch KP, Gross C (2008) Identifying molecular substrates in a mouse model of the serotonin transporter × environment risk factor for anxiety and depression. Biol Psychiatry 63:840–846
Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL (2003) Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100:5497–5502
Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A (2007) Effects of mild early life stress on abnormal emotion related behaviors in 5-HTT knockout mice Behav Genet 37:214–222
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389
Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ (2002) Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry 7:1058–1063
Collier DA, Stober G, Li T, Heils A, Catalano M, Di Bella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Muller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP (1996) A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry 1:453–460
Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M (2006) Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci 9:28–30
Di Pino G, Mössner R, Lesch KP, Lauder JM, Persico AM (2004) Serotonin roles in neurodevelopment: more than just neural transmission. Curr Neuropharmacol 2:403–417
Fallgatter A, Jatzke S, Bartsch A, Hamelbeck B, Lesch K (1999) Serotonin transporter promoter polymorphism influences topography of inhibitory motor control. Int J Neuropsychopharm 2:115–120
Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis AC, Wagener A, Heidrich A, Ortega G, Zeng Y, Lesch KP (2004) Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology 29:1506–1511
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–258
Francis D, Diorio J, Liu D, Meaney MJ (1999) Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286:1155–1158
Francis DD, Szegda K, Campbell G, Martin WD, Insel TR (2003) Epigenetic sources of behavioral differences in mice. Nat Neurosci 6:445–446
Gondo Y, Murata T, Makino S, Fukumura R, Ishitsuka Y (2011) Mouse mutagenesis and disease models for neuropsychiatric disorders. Springer, Heidelberg. doi:10.1007/7854_2010_106
Heiming RS, Jansen F, Lewejohann L, Kaiser S, Schmitt A, Lesch KP, Sachser N (2009) Living in a dangerous world: the shaping of behavioral profile by early environment and 5-HTT genotype. Front Behav Neurosci 3:26
Hertting G, Axelrod J (1961) Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature 192:172–173
Higley JD, Linnoila M (1997) A nonhuman primate model of excessive alcohol intake. Personality and neurobiological parallels of type I- and type II-like alcoholism. Recent Dev Alcohol 13:191–219
Higley JD, Suomi SJ, Linnoila M (1991) CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology 103:551–556
Higley JD, Suomi SJ, Linnoila M (1992) A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry 32:127–145
Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL (2003) Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 28:2077–2088
Homberg JR, Lesch KP (2011) Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry (in press)
Iacoboni M (2005) Neural mechanisms of imitation. Curr Opin Neurobiol 15:632–637
Ichikawa M, Okamura-Oho Y, Shimokawa K, Kondo S, Nakamura S, Yokota H, Himeno R, Lesch KP, Hayashizaki Y (2008) Expression analysis for inverted effects of serotonin transporter inactivation. Biochem Biophys Res Commun 368:43–49
Jansen F, Heiming RS, Lewejohann L, Touma C, Palme R, Schmitt A, Lesch KP, Sachser N (2010) Modulation of behavioural profile and stress response by 5-HTT genotype and social experience in adulthood. Behav Brain Res 207:21–29
Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME (2008) Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav 93:807–819
Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, Hariri AR, Bradberry CW (2009) Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry 15:512–522
Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, Olverman HJ, Hastie ND, Harmar AJ, Shen S, Sharp T (2006) Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci 26:8955–8964
Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ (2008) The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry 13:1021–1027
Kalinichev M, Easterling KW, Holtzman SG (2002) Early neonatal experience of Long-Evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology 27:518–533
Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (1993) Major depression and phobias: the genetic and environmental sources of comorbidity. Psychol Med 23:361–371
Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ (1995) The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry 52:374–383
Kitayama S, Park J (2010) Cultural neuroscience of the self: understanding the social grounding of the brain. Soc Cogn Affect Neurosci 5:111–129
Kraemer GW, Ebert MH, Schmidt DE, McKinney WT (1989) A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology 2:175–189
Langer SZ, Zarifian E, Briley M, Raisman R, Sechter D (1981) High-affinity binding of 3H-imipramine in brain and platelets and its relevance to the biochemistry of affective disorders. Life Sci 29:211–220
Lesch KP (2003) Gene–environment interaction in generalized anxiety disorder. In: Nutt D, Rickels K, Stein D (eds) Generalized anxiety disorders: symptomatology, pathogenesis and management. Dunitz, London
Lesch KP (2005) Genetic alterations of the murine serotonergic gene pathway: the neurodevelopmental basis of anxiety. Handb Exp Pharmacol: 71–112
Lesch KP (2007) Linking emotion to the social brain. The role of the serotonin transporter in human social behaviour. EMBO Rep 8 Spec No S24–S29
Lesch KP, Mössner R (2006) Inactivation of serotonin (5HT) transport: modeling altered 5HT homeostasis implicated in emotional dysfunction, affective disorders, and somatic syndromes. In: Sitte H (ed.) Handbook of Experimental Pharmacology. Vol. 175, Anxiety and anxiolytic drugs. Springer, Berlin, Heidelberg, New York, pp. 417–456
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531
Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A (1997) The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm 104:1259–1266
Lewejohann L, Kloke V, Heiming RS, Jansen F, Kaiser S, Schmitt A, Lesch KP, Sachser N (2010) Social status and day-to-day behaviour of male serotonin transporter knockout mice. Behav Brain Res 211:220–228
Liu D, Diorio J, Day JC, Francis DD, Meaney MJ (2000) Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci 3:799–806
Mannie ZN, Barnes J, Bristow GC, Harmer CJ, Cowen PJ (2009) Memory impairment in young women at increased risk of depression: influence of cortisol and 5-HTT genotype. Psychol Med 39:757–762
Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM (2009) Finding the missing heritability of complex diseases. Nature 461:747–753
Murphy DL, Lesch KP (2008) Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci 9:85–96
Murphy DL, Lerner A, Rudnick G, Lesch KP (2004) Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv 4:109–123
Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR (2008) How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology 55:932–960
Nietzer SL, Bonn M, Jansen F, Heiming R, Lewejohann L, Sachser N, Asan ES, Lesch KP, Schmitt AG (2011) Serotonin transporter knockout and repeated social stress: impact on neuronal morphology and plasticity in limbic brain areas. Behav Brain Res (in press)
O’Hara R, Schroder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, Weiner M, Kraemer HC, Noda A, Lin X, Gray HL, Hallmayer JF (2007) Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry 12:544–555
O’Tuathaigh CMP, Desbonnet L, Moran PM, Kirby BP, Waddington JL (2011) Molecular genetic models related to schizophrenia and psychotic illness: heuristics and challenges. Springer, Heidelberg. doi:10.1007/7854_2010_111
Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, Smith CA (1996) Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet 347:731–733
Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F (2001) Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci 21:6862–6873
Persico AM, Baldi A, Dell’Acqua ML, Moessner R, Murphy DL, Lesch KP, Keller F (2003) Reduced programmed cell death in brains of serotonin transporter knockout mice. Neuroreport 14:341–344
Raisman R, Briley M, Langer SZ (1979) Specific tricyclic antidepressant binding sites in rat brain. Nature 281:148–150
Ren-Patterson RF, Cochran LW, Holmes A, Sherrill S, Huang SJ, Tolliver T, Lesch KP, Lu B, Murphy DL (2005) Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J Neurosci Res 79:756–771
Rizzolatti G, Craighero L (2004) The mirror-neuron system. Annu Rev Neurosci 27:169–192
Roiser JP, Rogers RD, Cook LJ, Sahakian BJ (2006) The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology (Berl) 188:213–227
Roiser JP, Muller U, Clark L, Sahakian BJ (2007) The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. Int J Neuropsychopharmacol 10:449–461
Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW, Dolan RJ (2009) A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci 29:5985–5991
Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer EE, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I (2001) Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase A and 5-HT transporter knock-out mice. J Neurosci 21:884–896
Schwandt ML, Lindell SG, Sjoberg RL, Chisholm KL, Higley JD, Suomi SJ, Heilig M, Barr CS (2010) Gene–environment interactions and response to social intrusion in male and female rhesus macaques. Biol Psychiatry 67:323–330
Seckl JR, Meaney MJ (2004) Glucocorticoid programming. Ann N Y Acad Sci 1032:63–84
Suomi SJ (2003) Gene–environment interactions and the neurobiology of social conflict. Ann N Y Acad Sci 1008:132–139
Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M (2005) Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. Neuroimage 25:926–935
Uher R, McGuffin P (2008) The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry 13:131–146
Uher R, McGuffin P (2010) The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry 15:18–22
Watson KK, Ghodasra JH, Platt ML (2009) Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS One 4:e4156
Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854
Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M (2005) Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054
Weaver IC, Meaney MJ, Szyf M (2006) Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA 103:3480–3485
Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A (2007) Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 27:684–691
Wust S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M (2009) Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology 34:972–982
Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, Visscher PM (2010) Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42:565–569
Acknowledgement
The work by the author is supported by the DFG (KFO 125, SFB 581/B9, SFB TRR 58/A1 and A5), BMBF (IZKF Wuerzburg, 01KS9603) and the EC (NEWMOOD LSHM-CT-2003-503474).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Lesch, KP. (2011). When the Serotonin Transporter Gene Meets Adversity: The Contribution of Animal Models to Understanding Epigenetic Mechanisms in Affective Disorders and Resilience. In: Hagan, J. (eds) Molecular and Functional Models in Neuropsychiatry. Current Topics in Behavioral Neurosciences, vol 7. Springer, Berlin, Heidelberg. https://doi.org/10.1007/7854_2010_109
Download citation
DOI: https://doi.org/10.1007/7854_2010_109
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-19702-4
Online ISBN: 978-3-642-19703-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)