Abstract
Osteoarthritis (OA) is the most common chronic disabling condition effecting the elderly, significantly impacting an individual patient’s quality of life. Current treatment options for OA are focused on pain management and slowing degradation of cartilage. Some modern surgical techniques aimed at encouraging regeneration at defect sites have met with limited long-term success. Mesenchymal stem cells (MSCs) have been viewed recently as a potential tool in OA repair due to their chondrogenic capacity. Several studies have shown success with regards to reducing patient’s OA-related pain and discomfort but have been less successful in inducing chondrocyte regeneration. The heterogeneity of MSCs and their limited proliferation capacity also raises issues when developing an off-the-shelf treatment for OA. Induced pluripotent stem cell (iPSC) technology, which allows for the easy production of cells capable of prolonged self-renewal and producing any somatic cell type, may overcome those limitations. Patient derived iPSCs can also be used to gain new insight into heredity-related OA. Efforts to generate chondrocytes from iPSCs through embryoid bodies or mesenchymal intermediate stages have struggled to produce with optimal functional characteristics. However, iPSCs potential to produce cells for future OA therapies has been supported by iPSC-derived teratomas, which have shown an ability to produce functional, stable articular cartilage. Other iPSCs-chondrogenic protocols are also improving by incorporating tissue engineering techniques to better mimic developmental conditions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Articular cartilage is essential for the pain-free and easy movement of diarthrodial joints, as it provides shock absorbance and lubrication. Unfortunately, once formed, articular cartilage has a very low capacity for self-repair in part due to its lack of vascularisation. Damage to the cartilage from excess mechanical stress can begin a progressive degradation that can lead into a condition known as osteoarthritis (OA), the most common chronic disabling condition effecting adults in later life (Loeser 2011). This condition begins with a sense of stiffness (crepitus) in the affected joints those progresses on to pain and a reduction in function and significantly impact a sufferer’s quality of life. The areas most commonly affected are the highly-used joints in the hand and load bearing joints of the hips and knees (Neogi 2013). While there can be some genetic predisposition for OA, obesity and old age (Lawrence et al. 2007) can play a large role in the development of the condition due to extra strain being but on the major joints and changes in the aging cartilage matrix respectively. A Dutch population study show signs of OA in over 60% of those over 60 and found similar rates when they compared them to other populations worldwide (Saase et al. 1989). The impact of OA is set to rise further wit increasingly aging populations and rising obesity levels across the developed world. Projections in the US estimate that by 2030 over 20% of the population will have arthritis with OA being by far the most prevalent form (Murphy and Helmick 2012).
The WHO has placed OA as one of its top ten most disabling diseases in developed countries (Neogi 2013) with loss of work days and productivity due to OA valued at 10 billion dollars a year in the US (Muchmore et al. 2003) as well as placing heavy economic burdens on health care systems. Currently treatment option for OA are limited and focused on pain management, slowing degradation and reducing inflammation. Artificial joint replacement surgery for knees and hips make up much of the medical costs linked to OA. These implants are effective at returning mobility to a patient with severe OA and have very low failure rates up to 10 years after implantation (Herberts and Malchau 2000). However, this condition, so predominantly associated with older adults, has been occurring at increasing rates in people below the age of 65, providing challenge for the current strategies, where patients may be faced with managing their painful condition for decades and progressive bone loss around the site of the prosthesis is a significant concern for long-term cases. For these reasons, new therapies focused on joint repair and preservation will be important to maintain pain free mobility in into old age for the many adults. Interest has been growing in stem cell cell-based regenerative techniques as a potential source of these innovative therapies.
2 The Articular Cartilage
2.1 Overview
Diarthrodial joints are formed from a highly specialized connective tissue, the articular cartilage (AC). The principal function of AC is to supply a lubricated and smooth surface for articulation and to facilitate the transmission of loads with a low frictional coefficient. It is created by a specialised cell type, the chondrocytes, which are able to produce its exceptional collagenous extracellular matrix (ECM) composed mainly from proteoglycans, collagen, water and non-collagenous proteins and glycoproteins. AC is a hyaline-type cartilage, which differs from the other two cartilage types, the elastic and fibrocartilage in relative amounts of collagen and proteoglycan. One specialty of the AC tissue is that it does not contain blood vessels or nerves, therefore, the nutrition happens through diffusion which is powered by a fluid flow (synovial fluid) generated by the joint movements (compression or flexion). This is one reason behind the very slow turnover of its extracellular matrix and the fact that AC does not able to repair. To understand the chronic conditions that affects AC and their potential treatment possibilities, first, we have to review its development and maintenance.
2.2 Articular Cartilage Development
Chondrocyte formation begins following the condensations with the dynamic expression of cartilage specific genes such as collagen type II, type IX and type XI and Aggrecan under the regulation of SOX transcription factors, SOX9 (SRY-box 9) being the so-called master regulator of chondrogenesis (Wehrli et al. 2003), while the cells proliferate and secrete a cartilage matrix (Iwamoto et al. 2007). Early in endochondral bone development the cells in the cartilage template at the site of a future synovial joint are directly connected to each other. The separation of the long bones at the joint site begin when the chondrocytes at the site become more densely packed and form an area known as an interzone. During interzone formation the chondrocytes show a downregulation in SOX9 and collagen type II (Ito and Kida 2000). The interzone is made up of three layers: two chondrogenic, perichondrium-like layers and one intermediate layer of densely packed cells. Shortly after the formation of the interzone an apoptosis induced cavitation will occur at the site of the future synovial joint within the dense intermediate interzone, with some cells from this region going on to form the synovial tissues of the joint (Caldwell and Wang 2015). The two outer interzone layer are incorporated into the epiphysis of the cartilage growth plate to contribute to the postnatal growth of the long bone through proliferation and hypertrophy. The remaining cells form the intermediate interzone layer and are not included in this process and will separate from the epiphyseal growth plate cartilage to form a layer of chondrocytes expressing collagen type X and assembling matrices of vesicles and proteoglycans to promote the formation of the permanent articular cartilage found in the mature joint. Biochemical signals from transcription factors like WNT family member 4 (WNT4), Catenin B1 (CTNNB1) and transforming growth factor beta (TGF-β) promote the development and maintenance of articular cartilage (Hill et al. 2005). TGF-β is of particular importance to keep articular cartilage in its proper state, as seen in transgenic mice with defective TGF-β receptors where articular cartilage is replaced by hypertrophic cartilage and bone (Spagnoli et al. 2007). However, theses biochemical signals are not the only ones to have an impact on the development of articular cartilage as there is also evidence to suggest that mechanical stimulation during development may play an important role in the development of the future joint.
As the pre-chondrogenic cells are differentiating at the sites of the future bones, progenitors of muscles and tendons are also being defined (Rodríguez et al. 1988). This forming muscle mass begins contracting at the same time as the cartilaginous template is taking shape. This connection is hinted at by the severe bone and cartilage malformations seen in children born with the congenital neuro-muscular disorders (Amthor et al. 1998). Experiments with chemically paralysed chick embryos (Nowlan et al. 2010) and mutant mice with muscle-less limbs have shown that a lack of mechanical stimulation can result in serious failures in interzone development resulting in fused joints with no synovial cavity and a lack of articular cartilage.
2.3 Articular Cartilage Maintenance
Many of these factors that play important roles in the development of articular cartilage, such as Bone Morphogenic Protein (BMP) and TGF-β, are also essential for maintaining it’s healthy permanent state in adulthood. The homeostasis required for this maintenance can be disrupted by excessive damage to the cartilage and can result in the over expression of catabolic factors beginning the tissue degradation seen in OA (Fukui et al. 2001). Chondrocytes from osteoarthritic articular cartilage have also been seen to express early and late stage differentiation markers (Pfander et al. 2001) suggesting it has taken on a transient form that could differentiate into undesired forms of cartilage or calcify resulting in greater wear on the joint. Genetic variations in the strength of receptor signalling for genes related to the development and maintenance of articular cartilage are thought to be an important risk factor for the development of OA. These complex processes that develop and maintain articular cartilage also contribute to the difficulties and limitations faced by current treatments aiming to regenerate the cartilage damaged in OA.
3 Current Regeneration Based Treatments and Their Limitations
3.1 Overview
Non-surgical treatment possibilities are considered in the early stages of OA, however, their effect on the restoration of the normal tissue function has not been demonstrated convincingly (Browne and Branch 2000). Surgical methods such as arthroscopy, subchondral drilling, abrasion arthroplasty, microfracture, autologous chondrocyte implantation (ACI) or its second generation version the matrix-assisted autologous chondrocyte implantation (MACI) aim to restore the damaged cartilage itself. However, these technologies have limitations as well, mainly the formation of fibrocartilage, which is not as effective as hyaline cartilage in AC to respond frictional, compressive, shear and tensile loading. Below, we will concentrate on microfracture and ACI techniques and review their applications and major limitations.
3.2 Microfracture
Microfracture surgery arose from investigations into surgical bone marrow stimulation in the late 80s and early 90s (Freitag et al. 2016). The technique involves the drilling of small holes into the subchondral bone plate at the site where the cartilage has diminished. The aim is to allow blood and bone marrow to seep out of these fractures as with the hopes that the mesenchymal stem cells also known as mesenchymal stromal cells (MSCs) (Dominici et al. 2006) contained within the bone marrow will differentiate and form new healthy cartilage. The procedure itself is quite short and the recovery time is much less than that of joint replacement surgery, as such it has become a very popular treatment option in the world of sports medicine. However, the cartilage formed by these released MSCs will most often take the form of fibrocartilage which has a different biochemical make up from articular, also called hyaline, cartilage (Freitag et al. 2016). Fibrocartilage contains both collagen I and collagen II and form white fibrous tissues unlike articular cartilage which contains only collagen II and has a smooth, glass like appearance (Pearle et al. 2005). This difference in composition means that the biomechanical properties of fibrocartilage are less suited to the mechanical forces placed on cartilage in the joints and the new cartilage is effectively mechanically inferior. In addition, the microfractures in the subchondral bone can result in the formation of lesions. Efforts have been made to refine the procedure and reduce the fracture size, but long-term studies (Freitag et al. 2016) have found that the relief the procedure provides is reversed 5 years after surgery regardless of fracture size. The changes to the subchondral bone surface also increase the failure rate of a more recently developed regenerative therapy, autologous cartilage transplantation (ACT) up to seven-times if applied after microfracture.
3.3 Autologous Cartilage Transplantation
The ACT procedure involves taking a biopsy of a patient’s own cartilage from a non-loadbearing site on the joint. These cells are then cultured in vitro to expand a population of a patient’s own chondrocytes with the aim to implant these healthy cells into the damaged area of the joint, where they are covered with a membrane and sutured in place. Unlike microfracture, pre-clinical and clinical trial of ACT show the formation of new hyaline-like cartilage in the joints and a study following 61 patients found the clinical outcomes rate good to excellent for 83% of the group after 5 years (Browne et al. 2005). Unfortunately, ACT is not without drawbacks of its own. Two separate operations are required with time between needed for the expansion of the chondrocytes, increasing a patient’s recovery time, and the harvesting of the cartilage is an invasive and painful procedure and can cause damage to the donor site. The low cell number in native cartilage tissue and the limited amount of suitable non-loadbearing donor tissue restricts the number of cells that can be produced for implantation. The most reported cause of failure for ACTs is the hypertrophy of the membrane or periosteal flap used to secure the implanted cells (Peterson et al. 2010). A number of artificial and porcine based (Makris et al. 2015) membranes have been tried to correct this but they can cause an immune response negating the key advantage of using autologous cells in the first place. Studies have also shown that up to 40% of ACTs show signs of cartilage “dedifferentiation”, with the autologous cartilage turning into fibrocartilage (Caplan and Kader 2013). This could be due to changes the cells undergo when they are being cultured ex vivo or the failure of these cells to properly integrate into the normal extracellular matrix of cartilage.
Both microfracture surgery and ACT are effective treatments that have been shown temporarily restore normal function to patients with cartilage damage and delay the need for drastic joint replacement surgery. However, both procedures are more suited for treating isolated defects in cartilage and not the more generalized degradation seen in OA. Nevertheless, recent efforts in generating functional chondrocytes from pluripotent and multipotent stem cells may bypass some of the short-comings of current regenerative treatment and shed new insight into the pathology of OA.
4 Using Stem Cells in Cartilage Replacement
4.1 Overview
Interest has been growing in the use of stem cell technologies to both offer new methods for studying the mechanisms of OA and new treatments offering more effective and longer-term solutions than the options currently available to patients.
4.2 Stem Cell Types Available for Cartilage Replacement
The chondrogenic capabilities of adult MSCs have been extensively investigated for the last decade as a possible source of replacement cartilage. MSCs can be easily harvested in large numbers from several sources including a patient’s bone marrow and adipose tissue (Kern et al. 2006), avoiding the potential damage that can be done to the patient’s existing cartilage inherent in current cell therapies.
Another cell source would be pluripotent stem cells. Embryonic stem cells (ESCs) derived from the Inner Cell Mass (ICM) of the blastocyst are pluripotent, having the ability to form tissues from any of the three germ layers, and have also been used to produce hyaline cartilage in vitro (Diekman et al. 2012). Unlike MSCs, ESCs have the ability to self-renew, making them a potentially unlimited replacement cartilage (Koch et al. 2009). However, the ethical issues related to the derivation of ESCs from preimplantation embryos limits their clinical applications. Induced pluripotent stem cells (iPSCs) derived from adult somatic cells using a combination of reprogramming factors offer an alternative source of self-renewing pluripotent cells that avoid these ethical issues. Since its development by Yamanka (Takahashi et al. 2007) iPSC technology has garnered massive attention in the field of regenerative medicine with ambitions to develop new therapies with a patient patent specific pluripotent cells. While the reprogramming factors used to produce the first iPSCs, Oct3/4, Klf4, Sox2 and c-Myc (also called as ‘OSKM factors’) caused some concern for future clinical use due to the oncogenic nature of c-Myc and Klf4 but more recently it has been found that they can be replaced with Nanog and Lin28 (Shi et al. 2016). New methods for introducing these reprogramming factors to the cells including non-integrating viral vectors, such as a Sendai and the development of several non-viral methods using microRNA, synthetic messenger RNA and proteins have also increased the safety of iPSC derived cells. Early animal studies have been promising with iPSCs derived cardiomyocytes, able to repair cardiac defects in a porcine model (Shiba et al. 2012). Safety trials of iPSC derived cells in humans are already underway (Trounson and DeWitt 2016).
In addition to the potential for iPSCs to form replacement tissues, the ability to generate pluripotent cell from a patient’s own tissue has opened up new avenues in personalised medicine and the modelling of genetic diseases. Two studies published in 2017 have found altered expressions of genes associated with some forms of OA and phenotypic differences in MSCs and osteoblast generated from iPSCs derived from patients with disorder related to bone growth. Esseltine et al. (2017) generated iPSCs from a patient with the developmental disorder oculodentodigital dysplasia, linked to a Connexin-mutation that commonly results in malformations of the facial bones. Connexin is a gap junction protein and has been shown to both be upregulated in cells at the joints during OA and to enhance the expression of several other OA-related genes (Gupta et al. 2014). This study found that connexion had a reduced expression in the patient-derived iPSCs when compared to healthy control iPSCs. The patient-derived iPSCs also showed delayed osteogenic differentiation. The osteoblast generated showed reduced levels of connexin which could negatively impact their future maturation and mineralization. Layh-Schmitt et al. (2016) produced iPSCs from patients with axial spondyloarthritis, a genetic disease that results in abhorrent bone formation at the joints and spine. They found that MSCs derived from these iPSCs shown elevated expression of number of genes related to bone formation. Mutations in one of these genes, HAPLN1 has been associated with spinal osteophyte formation in OA. In both studies iPSC derived from patient cells gave fresh insights into mechanisms of their rare conditions while also showing how mutations in genes related to OA can be successfully modelled by iPSCs. Similar studies that established in vitro disease models from patient derived iPSCs, such as a recent study (Cao et al. 2016) of patients with inherited erythromelalgia, have been able to use these models to test an array of drug compounds for their effectiveness in correcting or reducing the phenotypic expression of the disease mutation in these cells. Some small trials have gone on to show drugs that had been found effective on these patient-derived iPSC disease models in vitro, to in turn be effective at alleviating the related diseases symptoms when given those patients (Cao et al. 2016). This potential to provide models for diseases which currently lack representative animal models and creating an easily expandable population of cells that show a diseases phenotype to test the efficacy of arrays of drug compounds represents major advantages iPSCs have over MSCs and other stem cells. However, in order to realise this potential for OA researcher must show that iPSCs can be differentiated into functional chondrocytes.
4.3 Producing Cartilage from Mesenchymal Stem Cells
Several in vitro techniques have been investigated to induce the differentiation of chondrocytes from MSCs with TGF-β1 and Insulin-Like Growth Factor 1 (IGF-1) commonly used together to stimulate chondrogenesis (Longobardi et al. 2005). Some other compounds including dexamethasone and BMP-7 have been found to assist in directing the cells down a chondrogenic lineage.
A number of therapies for OA based on introducing MSCs to sites of damaged cartilage have gone through preclinical and clinical trials in recent years. Some trials used a technique similar to ACT, transplanting a cellular scaffold containing MSCs instead of ACs to the site of damage (Grigolo et al. 2009). While this technique has shown some success in repairing cartilage defects in both the preclinical models and human patients, a direct comparison study showed that there was no significant difference in clinical outcome between MSC scaffold transplantation and ACT (Nejadnik et al. 2010) including the risk of non-hyaline cartilage formation. Another MSC based therapy for OA currently under investigation is the injection of MSCs into the inter-articulated region. One advantage of this approach is its potential to affect the entire joint, rather than just the site of a specific defect, making it better suited for treating OA which causes a diffuse degradation of cartilage across the joint. There are currently a number of active and recruiting phase I/II clinical trials testing the safety and efficacy of MSC-based therapies for knee OA.
The sources of MSCs most commonly used in these therapies are autologous adipose (https://clinicaltrials.gov/ct2/ MSC) MSCs or allogenic MSCs obtained from umbilical cord blood (https://clinicaltrials.gov/ct2/ UCB). In most of these cases the MSCs are applied trough an intra-articular injection either in a single dose or in 2–3 doses over a 6-months period. This single or repeated dose strategy may impact the products safety outcomes depending on the source of MSCs used as a 2017 equine model study (Joswig et al. 2017) suggested that repeated intra-articular injection of allogeneic MSCs causes an adverse response compared to autologous MSCs. Recently two phase I/II trial reports have been published for therapies using autologous MSCs and a product using allogenic cell intra-articularly to treat OA. Soler et al. (2016), expanded autologous bone marrow derived MSCs ex vivo and infused them in a single dose for 15 patients. They found a few patients experienced some discomfort which diminished 8 days after injection. Twelve month follow ups showed improvements in bodily pain and function and magnetic resonance T2 mapping indicated signs of cartilage regeneration. Stempeucel®, an allogenic, pooled MSC product, was administered to 60 OA patients (Gupta et al. 2016) in a single dose. While no adverse effect was observed over a 12-months period, reports of pain reduction by patients was not statically significant when compared to the placebo group.
A number of other clinical trials of MSC injections for OA have shown that a majority of patients get some pain relief following the injections (Centeno et al. 2011). However, the evidence for disease modification or cartilage regeneration resulting from this technique are inconsistent and unclear (Freitag et al. 2016). Many of the trials are unblinded and have small numbers of patients with some concerns being raised about potential bias in a number of trials. Additionally, other trials for a number of conditions have shown paracrine secretions of MSCs to have immunomodulatory and anti-inflammatory properties (Aggarwal and Pittenger 2005) and these may be responsible for the pain relief the OA patients received.
While these trials show promise in terms of slowing OA related degradation and improving patients’ quality of life, MSCs have a number of drawbacks that limit their capacity as a source of cartilage for regeneration or repair. While MSCs can be obtained from many source tissues, there is a lot of heterogeneity in the differentiation capabilities of these various stem cell populations. Bone marrow derived MSCs are commonly used to produce chondrocytes however, chondrogenic differentiation in these cells normally follows an endochondral pathway, producing transient cartilage not suitable to replace articular cartilage (Pelttari et al. 2006). Additionally, primary autologous MSCs obtained from any adult tissue have a limited proliferation capacity, limiting the amount of replacement chondrocytes that they can produce. The heterogeneity even within a population of MSCs from the same tissue means that not all of the primary cells will be capable of chondrocyte differentiation (Russell et al. 2010). This heterogeneity among adult MSC populations, may contribute to variable clinical outcomes when using autologous MSCs for cartilage repair. These limitations have caused some to look to induced pluripotent stem cells (iPSCs) made from patient derived tissue to possible provide both large numbers of autologous cells with powerful chondrogenic capabilities and provide new ways to study the molecular and genetic aspects of OA.
4.4 Chondrogenesis from iPSCs
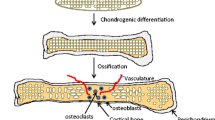
While there is currently no generally accepted efficient protocol for differentiation chondrocytes from iPSC (Lietman 2016) (Fig. 1), the methods that have been commonly tried produce some to the conditions of cartilage development with most using one of three main approaches, (i) the induction of MSC-like iPSCs and the differentiation of these cells into chondrocytes using the growth factors used in normal bone marrow MSC in vitro chondrogenesis (Nejadnik et al. 2015), (ii) the co-culture of iPSCs derived MSCs with primary chondrocytes or other feeder layer cells (Qu et al. 2013) or (iii) the culturing of embryoid bodies (EB) from iPSCs, followed by the differentiation of the mesodermal cells in the EBs into chondrocytes by treatment with growth factors (Nakagawa et al. 2009). All of these techniques have had some success producing chondrocyte cells but have had some limitations, often producing very heterogeneous populations of cells, very few of which were able to generate healthy hyaline cartilage.
A Summary of recent attempts to generate cartilage from IPSCs. Induced pluripotent stem cells (iPSCs) can be generated from any somatic cell via induction with the Yamanaka factors. The great potential of iPSCs lies in the capacity to differentiate into any cell type if they are subjected to the right conditions A number of methods have recently been attempted to generate stable homogenous cartilage from iPSC: (A) the induction of an MSC-like intermediate stage and the differentiation of these cells into chondrocytes using the growth factors used in normal bone marrow MSC in vitro chondrogenesis, (B) Co-culturing of iPSC-derived MSCs with primary chondrocytes or other feeder layer cells to promote chondrogenic differentiation or (C) culturing aggregates of iPSCs to form embryoid bodies (EB), encouraging a spontaneous differentiation toward the three germ layers followed by the differentiation of the mesodermal cells in the EBs into chondrocytes by treatment with growth factors. While all of these techniques have had some success producing chondrocyte cells but have had some limitations, often producing very heterogeneous populations of cells, very few of which were able to generate healthy hyaline cartilage. However, stable homogenous hyaline cartilage has been produced, (D) by forming in teratomas in immunodeficient mice using a line of hiPSC that expressed GFP in cartilage. This expression was used to purify a population of homogenous cartilaginous particles from the teratoma tissues, that formed hyaline cartilage tissue when cultured in a scaffold-free suspension (Figure was created by Roxana Mobasheri based on the author’s original concept)
To discuss the issues involved in these methods we must first look at how comparable MSCs derived from iPSCs are to bone marrow derived MSCs. Diederichs and Tuan (2014) performed side-by-side genomic and functional comparisons of adult bone marrow derived MSCs and MSCs generated from human iPSCs. The iPSCs themselves were also derived from bone marrow MSCs from the same donors and the MSCs were differentiated from them were generated using several different methods including MSC growth factors, EBs and co-culture with primary MSCs. The comparative analyses showed distinct transcriptomic and functional differences between bone marrow and iPSC derived MSCs. The iPSC derived MSCs were generally found to be less responsive to chondrogenic differentiation protocols commonly used on MSCs. Diederich and colleagues recently investigated further (Diederichs et al. 2016) the chondrogenic discrepancies between iPSCs and bone marrow derived MSCs, focusing on the regulation of SOX9 in the cell, due to this protein’s essential nature in cartilage development. When intermediate mesenchymal progenitor cells (iMPCs) were generated from the iPSCs SOX9 was induced and reached varying protein levels compared to bone marrow MSCs cultured under the same conditions. The iMPCs also produced less robust cartilage compared to the MSCs, though iMPCs with high levels of SOX9 produced better cartilage than those with low levels. SOX9 levels in the iMPCs were actually downregulated by the standard TGF-β based protocol for MSC chondrogenesis though this effect could be mitigated somewhat by a co-treatment of BMP-4. These results seem to indicate that there are some underlying differences between MSCs and iPSC-derived iMPCs, maybe an epigenetic memory retained from the iPSCs tissue of origin that impacts their chondrogenic potential. It is also known that differences exist between iPSC lines from various donors, which can affect the outcome of differentiation experiments.
However, these limitations in current differentiation strategies do not mean that iPSCs are incapable of producing functional chondrocytes from patient derived cells. A key piece of evidence for early iPSCs’ pluripotent capability was their ability to form teratomas with tissues from all three germ layers when implanted in vivo (Shi et al. 2016). Yamashita et al. (2015) produced scaffold-less hyaline cartilaginous tissue from human iPSCs, by generating a line of hiPSC that expressed GFP in cartilage when it formed in teratomas in immunodeficient mice. They then used this expression to purify a population of homogenous cartilaginous particles from the teratoma tissues, culturing them in a scaffold-free suspension culture. These cartilaginous particles formed hyaline cartilage when implanted subcutaneously in immunodeficient mice and integrated with the native cartilage transplanted to the site of joint defects in mice. A similar method using teratoma formation to derive cartilage tissue has also been used to model genetic cartilage conditions. Xu et al. (2016) produced iPSCs from skin fibroblasts taken from patients with the inherited skeletal defect, familial osteochondritis dissecans (FOCD) which is characterised by the development of large cartilage lesions in multiple joints and early onset of severe OA. Xu injected these patient-derived iPSCs subcutaneously in immunodeficient mice and harvested teratoma tissue after 2–3 months, using Safranin-O staining to identify cartilage tissues. This teratoma derived cartilage tissue displayed irregularities that could helped explain why these patients are so susceptible to cartilage damage. The ECM around the cells was largely depleted and cells were densely packed indicating poor matrix formation. Finally, large amounts of aggrecan accumulated within the endoplasmic reticulum of the differentiated chondrocytes together with a marked absence of aggrecan in the ECM, a site it would normally be found in abundance and play a crucial role in the ECM’s structural integrity.
Teratoma formation in an immunodeficient animals is not a suitable method for producing large number of cells to be used for replacement and regenerative therapies for OA patients due to several issues such as risks related to transplant animal grown or transgenic tissues into patients, the long timeframes needed to produce the final cartilage product, and the expenses and ethical issues involved with raising large numbers of animals in which to generate the teratoma. However, these experiments have shown that human iPSCs can fulfil their potential of growing hyaline cartilage that can integrate with a joint and modelling genetic diseases that can contribute to OA development.
5 Concluding Remarks
While current efforts to produce iPSC-derived cartilage that can be used to benefit OA patients in vitro have some ways to go, the functional cartilage produced from human iPSCs in teratomas suggests that success lies in the right combination of environmental factors. As our understanding of the developmental process necessary for chondrogenesis and the development of specialised articular cartilage grows we can develop new strategies to better replicate those processes in vitro. Efforts are made to replicate the mechanical stimulation that play such an important role in the cartilage and interzone development in cultured cells culture. Mechanical micro-bioreactors have recently developed to exert compressive pressure and shear stress on MSC during chondrogenesis and have produced stable cartilage with good biomechanical properties (Halvaei et al. 2016). The frequency and intensity of the mechanical stimulation applied by these bioreactors can easily be modified to test chondrogenesis in MSC and iPSC derived cells using a range of conditions best matching the natural development of specialised types of cartilage. And while iPSC still have a long way to go before they can be used for therapy, step are being made with the lessons learned from the previous attempts to produce iPSC-derived chondrocytes and surgically repair cartilage. Diederichs et al. (2016) suggested screening iPSC colonies for SOX9 expression to start the refinement of the chondrogenic process and recent papers have already been doing this to produce osteochondrogenic-progenitor from iPSCs (Wang et al. 2017). Nguyen et al. (2017) have co-cultured iPSCs into a 3D–bioprinted scaffold alongside irradiated primary chondrocytes and produced some cartilaginous-like tissue in a system that can be easily place at a joint defect site much like ACT. Finally, in an interesting twist, a very recent paper has suggested another role iMSCs can play in the treatment of OA. As discussed earlier in this review the paracrine secretions of MSCs to have immunomodulatory and anti-inflammatory properties and may provide effective relief from the pain and discomfort caused by OA. Zhu et al. (2017) have found that exosomes taken from the paracrine secretions of iMSCs had a superior therapeutic effect on a mouse OA model when injected intra-articularly than those taken from adult MSCs derived from the synovial membrane.
In summary, while more work still needs to be done to establish the standard, reliable, reproducible method of chondrocyte production from iPSCs for therapeutic applications, the success of deriving hyaline-cartilage by the iPSC-teratoma method and continuously improving iPSC-chondrogenic protocols show the attainable promise of this technology and its potential to meet the needs of the growing numbers of OA patients around the world.
Abbreviations
- AC:
-
articular cartilage
- ACT:
-
autologous cartilage transplantation
- BMP:
-
Bone Morphogenetic Protein
- ECM:
-
extracellular matrix
- ESC:
-
Embryonic Stem Cell
- FOCD:
-
Familial osteochondritis dissecans
- HALPN1:
-
Hyaluronan and proteoglycan link protein 1
- ICM:
-
Inner Cell Mass
- IGF-1:
-
Insulin-Like Growth Factor 1
- iMPC:
-
intermediate Mesenchymal Progenitor Cell
- iPSC:
-
induced Pluripotent Stem Cell
- KLF4:
-
gut-enriched Krüppel-like factor
- MSC:
-
Mesenchymal Stem Cell
- OA:
-
Osteoarthritis
- Oct-4:
-
octamer-binding transcription factor 4
- SOX:
-
Sry-related HMG box
- SRY:
-
Sex-Determining Region Y-Box
- TGF-β:
-
Transforming Growth Factor-beta
- WHO:
-
Word Health Organisation
- WNT4:
-
WNT Family Member 4
References
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822. https://doi.org/10.1182/blood-2004-04-1559
Amthor H, Christ B, Weil M, Patel K (1998) The importance of timing differentiation during limb muscle development. Curr Biol 8(11):642–652. https://doi.org/10.1016/s0960-9822(98)70251-9
Browne JE, Branch TP (2000) Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg 8(3):180–189. https://doi.org/10.5435/00124635-200005000-00005
Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB, Micheli LJ, Lyle J, Fu F, Erggelet C (2005) Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res 436:237–245
Caldwell K, Wang J (2015) Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthr Cartil 23(3):351–362. https://doi.org/10.1016/j.joca.2014.11.004
Cao L, McDonnell A, Nitzsche A, Alexandrou A, Saintot PP, Loucif AJC, Brown AR, Young G, Mis M, Randall A, Waxman SG, Stanley P, Kirby S, Tarabar S, Gutteridge A, Butt R, McKernan RM, Whiting P, Ali Z, Bilsland J, Stevens EB (2016) Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med 8(335). https://doi.org/10.1126/scitranslmed.aad76533
Caplan N, Kader DF (2013) Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. In: Classic papers in orthopaedics. Springer, London, pp 165–168. https://doi.org/10.1007/978-1-4471-5451-8_40
Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, Hanson R (2011) Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther 6(4):368–378. https://doi.org/10.2174/157488811797904371
Clinical Study of Umbilical Cord Tissue Mesenchymal Stem Cells (UC-MSC) for Treatment of Osteoarthritis – Full Text View – ClinicalTrials.gov. (n.d.) https://clinicaltrials.gov/ct2/show/NCT02237846?term=Mesenchymal+Stem+Cell+osteoarthritis&recr=Active%2C+not+recruiting&rank=1. Accessed 10 May 2017
Diederichs S, Tuan RS (2014) Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev 23(14):1594–1610. https://doi.org/10.1089/scd.2013.0477
Diederichs S, Gabler J, Autenrieth J, Kynast KL, Merle C, Walles H, Utikal J, Richter W (2016) Differential regulation of SOX9 protein during Chondrogenesis of induced pluripotent stem cells versus mesenchymal stromal cells: a shortcoming for cartilage formation. Stem Cells Dev 25(8):598–609. https://doi.org/10.1089/scd.2015.0312
Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F (2012) Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci 109(47):19172–19177. https://doi.org/10.1073/pnas.1210422109
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. https://doi.org/10.1080/14653240600855905
Esseltine JL, Shao Q, Brooks C, Sampson J, Betts DH, Séguin CA, Laird DW (2017) Connexin43 mutant patient-derived induced pluripotent stem cells exhibit altered differentiation potential. J Bone Miner Res. https://doi.org/10.1002/jbmr.3098
Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A (2016) Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy – a review. BMC Musculoskelet Disord 17(1). https://doi.org/10.1186/s12891-016-1085-9
Fukui N, Purple CR, Sandell LJ (2001) Cell biology of osteoarthritis: the chondrocyte’s response to injury. Curr Rheumatol Rep 3(6):496–505. https://doi.org/10.1007/s11926-001-0064-8
Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, Tschon M, Giavaresi G, Fini M, Giardino R, Facchini A (2009) Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods 15(4):647–658. https://doi.org/10.1089/ten.TEC.2008.0569
Gupta A, Niger C, Buo AM, Eidelman ER, Chen RJ, Stains JP (2014) Connexin43 enhances the expression of osteoarthritis-associated genes in synovial fibroblasts in culture. BMC Musculoskelet Disord 15(1). https://doi.org/10.1186/1471-2474-15-425
Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, Majumdar AS (2016) Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther 18(1). https://doi.org/10.1186/s13075-016-1195-7
Halvaei M, Abolfathi N, Shokrgozar M, Eskandari M, Haghighipour N, Navaee F (2016) A new mechanical micro-bioreactor for cartilage tissue. Tissue Eng 22(Suppl 1):94–95. doi:EPFL-CONF-224956
Herberts P, Malchau H (2000) Long-term registration has improved the quality of hip replacement: a review of the Swedish THR register comparing 160,000 cases. Acta Orthop Scand 71(2):111–121. https://doi.org/10.1080/0001
Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C (2005) Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8(5):727–738. https://doi.org/10.1016/j.devcel.2005.02.013
Ito MM, Kida MY (2000) Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J Anat 197(4):659–679. https://doi.org/10.1046/j.1469-7580.2000.19740659
Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, Williams JA, Pacifici M (2007) Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol 305(1):40–51. https://doi.org/10.1016/j.ydbio.2007.01.037
Joswig A, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R, Watts AE (2017) Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther 8(1). https://doi.org/10.1186/s13287-017-0503-8
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301. https://doi.org/10.1634/stemcells.2005-0342
Koch P, Opitz T, Steinbeck JA, Ladewig J, Brustle O (2009) A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci 106(9):3225–3230. https://doi.org/10.1073/pnas.0808387106
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA (2007) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum 58(1):26–35. https://doi.org/10.1002/art.23176
Layh-Schmitt G, Lu S, Navid F, Brooks SR, Lazowick E, Davis K, Montag C, Gadina M, Colbert RA (2016) Generation and differentiation of induced pluripotent stem cells reveal ankylosing spondylitis risk gene expression in bone progenitors. Clin Rheumatol 36(1):143–154. https://doi.org/10.1007/s10067-016-3469-5
Lietman SA (2016) Induced pluripotent stem cells in cartilage repair. World J Orthop 7(3):149. https://doi.org/10.5312/wjo.v7.i3.149
Loeser RF (2011) Aging and osteoarthritis. Curr Opin Rheumatol 23(5):492–496. https://doi.org/10.1097/BOR.0b013e3283494005
Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A (2005) Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J Bone Miner Res 21(4):626–636. https://doi.org/10.1359/jbmr.051213
Makris EA, Gomoll AH, Malizos KN, JC H, Athanasiou KA (2015) Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11(1):21–34. https://doi.org/10.1038/nrrheum.2014.157
Muchmore L, Lynch WD, Gardner HH, Williamson T, Burke T (2003) Prevalence of arthritis and associated joint disorders in an employed population and the associated healthcare, sick leave, disability, and workers’ compensation benefits cost and productivity loss for employers. J Occup Environ Med 45(4):369–378. https://doi.org/10.1097/01.jom.0000063621.37065.26
Murphy L, Helmick CG (2012) The impact of osteoarthritis in the United States. Orthop Nurs 31(2):85–91. https://doi.org/10.1097/nor.0b013e31824fcd42
Nakagawa T, Lee SY, Reddi AH (2009) Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor β1. Arthritis Rheum 60(12):3686–3692. https://doi.org/10.1002/art.27229
Nejadnik H, Hui JH, Choong EP, Tai B, Lee EH (2010) Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation. Am J Sports Med 38(6):1110–1116. https://doi.org/10.1177/0363546509359067
Nejadnik H, Diecke S, Lenkov OD, Chapelin F, Donig J, Tong X, Derugin N, Chan RC, Gaur A, Yang F, JC W, Daldrup-Link HE (2015) Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev Rep 11(2):242–253. https://doi.org/10.1007/s12015-014-9581-5
Neogi T (2013) The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil 21(9):1145–1153. https://doi.org/10.1016/j.joca.2013.03.018
Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M, Lindahl A, Gatenholm P, Enejder A, Simonsson S (2017) Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep 7(1). https://doi.org/10.1038/s41598-017-00690-y
Nowlan NC, Bourdon C, Dumas G, Tajbakhsh S, Prendergast PJ, Murphy P (2010) Developing bones are differentially affected by compromised skeletal muscle formation. Bone 46(5):1275–1285. https://doi.org/10.1016/j.bone.2009.11.026
Pearle AD, Warren RF, Rodeo SA (2005) Basic science of articular cartilage and osteoarthritis. Clin Sports Med 24(1):1–12. https://doi.org/10.1016/j.csm.2004.08.007
Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Ainger T, Richter W (2006) Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54(10):3254–3266. https://doi.org/10.1002/art.22136
Peterson L, Vasiliadis HS, Brittberg M, Lindahl A (2010) Autologous chondrocyte implantation a long-term follow-up. Am J Sports Med 38(6):1117–1124. https://doi.org/10.1177/0363546509357915
Pfander D, Swoboda B, Kirsch T (2001) Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am J Pathol 159(5):1777–1783. https://doi.org/10.1016/s0002-9440(10)63024-6
Qu C, Puttonen KA, Lindeberg H, Ruponen M, Hovatta O, Koistinaho J, Lammi MJ (2013) Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int J Biochem Cell Biol 45(8):1802–1812. https://doi.org/10.1016/j.biocel.2013.05.029
Rodríguez JI, Palacios J, García-Alix A, Pastor I, Paniagua R (1988) Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tissue Int 43(6):335–339. https://doi.org/10.1007/bf02553275
Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, Oconnor KC (2010) In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 28(4):788–798. https://doi.org/10.1002/stem.312
Saase JL, Romunde LK, Cats A, Vandenbroucke JP, Valkenburg HA (1989) Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis 48(4):271–280. https://doi.org/10.1136/ard.48.4.271
Shi Y, Inoue H, Wu JC, Yamanaka S (2016) Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16(2):115–130. https://doi.org/10.1038/nrd.2016.245
Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA (2012) Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489(7415):322–325. https://doi.org/10.1038/nature11317
Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, Garcia-Lopez J (2016) Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 23(4):647–654. https://doi.org/10.1016/j.knee.2015.08.013
Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL (2007) TGF-β signaling is essential for joint morphogenesis. J Cell Biol 177(6):1105–1117. https://doi.org/10.1083/jcb.200611031
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. https://doi.org/10.1016/j.cell.2007.11.019
Trounson A, DeWitt ND (2016) Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 17(3):194–200. https://doi.org/10.1038/nrm.2016.10
Use of Adipose Tissue Derived Mesenchymal Stem Cells for Knee Osteoarthrosis – Full Text View-ClinicalTrials.gov. (n.d.) https://clinicaltrials.gov/ct2/show/NCT02966951term=Mesenchymal+Stem+Cell+osteoarthritis recr=Open rank=2. Accessed 10 May 2017
Wang Y, Wu M, Cheung MP, Sham MH, Akiyama H, Chan D, Cheah KS, Cheung M (2017) Reprogramming of dermal fibroblasts into osteo-chondrogenic cells with elevated osteogenic potency by defined transcription factors. Stem Cell Rep 8(6):1587–1599. https://doi.org/10.1016/j.stemcr.2017.04.018
Wehrli BM, Huang W, De Crombrugghe B, Ayala AG, Czerniak B (2003) Sox9, a master regulator of chondrogenesis, distinguishes mesenchymal chondrosarcoma from other small blue round cell tumors. Hum Pathol, 34(3):263–269. http://dx.doi.org/10.1053/hupa.2003.41
Xu M, Stattin E, Shaw G, Heinegård D, Sullivan G, Wilmut I, Colman A, Önnerfjord P, Khabut A, Aspberg A, Dockery P, Hardingham T, Murphy M, Barry F (2016) Chondrocytes derived from mesenchymal stromal cells and induced pluripotent cells of patients with familial osteochondritis dissecans exhibit an endoplasmic reticulum stress response and defective matrix assembly. Stem Cells Transl Med 5(9):1171–1181. https://doi.org/10.5966/sctm.2015-0384
Yamashita A, Morioka M, Yahara Y, Okada M, Kobayashi T, Kuriyama S, Matsuda S, Tsumaki N (2015) Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep 4(3):404–418. https://doi.org/10.1016/j.stemcr.2015.01.016
Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y (2017) Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther 8(1). https://doi.org/10.1186/s13287-017-0510-9
Acknowledgements
The authors would like to acknowledge Miss Roxana Mobasheri for the artwork in Fig. 1 and current and previous members of their laboratories and their internal and external collaborators for their contributions.
Funding
This work was supported by grants from EU FP7 projects (D-BOARD, HEALTH-F2-2012-305815; EpiHealthNet, PITN-GA-2012-317146). A.M. is coordinator of the D-BOARD Consortium funded by European Commission Framework 7 programme (EU FP7; HEALTH.2012.2.4.5-2, project number 305815, Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases, awarded to AM), and member of the Arthritis Research UK Centre for Sport, Exercise, and Osteoarthritis, funded by Arthritis Research UK (Grant Reference: 20194). A.M. are members of the Applied Public-Private Research enabling OsteoArthritis Clinical Headway (APPROACH) consortium, a 5-year project funded by the European Commission’s Innovative Medicines Initiative (IMI). APPROACH is a public-private partnership directed towards osteoarthritis biomarker development through the establishment of a heavily phenotyped and comprehensively analyzed longitudinal cohort. A.M. has received partial support from the Innovative Medicines Initiative (IMI) Joint Undertaking under grant agreement no. 115770, resources of which are composed of financial contribution from the European Union’s Seventh Framework programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. A.M. has also received funding from the European Union through a Marie Skłodowska-Curie scheme (project number 625746; acronym: CHONDRION; FP7-PEOPLE-2013-IEF). This work has also received financial support from the European Social Fund according to the activity ‘Improvement of researchers’ qualification by implementing world-class R&D projects’ of Measure No. 09.3.3-LMT-K-712.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Competing Interests
AM declares that he has served as a scientific Advisory Board Member for AbbVie and has received honoraria from AbbVie and Bioiberica. The other authors declare that they have no competing interests.
Authors’ contributions
CM, ZT and JK proposed the concept and wrote the manuscript, AD read and approved the paper. AM read and edited the manuscript before submission. All authors read and approved the final version of the paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Murphy, C., Mobasheri, A., Táncos, Z., Kobolák, J., Dinnyés, A. (2017). The Potency of Induced Pluripotent Stem Cells in Cartilage Regeneration and Osteoarthritis Treatment. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 1. Advances in Experimental Medicine and Biology(), vol 1079. Springer, Cham. https://doi.org/10.1007/5584_2017_141
Download citation
DOI: https://doi.org/10.1007/5584_2017_141
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93866-0
Online ISBN: 978-3-319-93867-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)