Abstract
Application of radioactive elements or radionuclides for anthropogenic use is a widespread phenomenon nowadays. Radionuclides undergo radioactive decays releasing ionizing radiation like gamma ray(s) and/or alpha or beta particles that can displace electrons in the living matter (like in DNA) and disturb its function. Radionuclides are highly hazardous pollutants of considerable impact on the environment, food chain and human health. Cleaning up of the contaminated environment through plants is a promising technology where the rhizosphere may play an important role. Plants belonging to the families of Brassicaceae, Papilionaceae, Caryophyllaceae, Poaceae, and Asteraceae are most important in this respect and offer the largest potential for heavy metal phytoremediation. Plants like Lactuca sativa L., Silybum marianum Gaertn., Centaurea cyanus L., Carthamus tinctorius L., Helianthus annuus and H. tuberosus are also important plants for heavy metal phytoremediation. However, transfer factors (TF) of radionuclide from soil/water to plant ([Radionuclide]plant/[Radionuclide]soil) vary widely in different plants. Rhizosphere, rhizobacteria and varied metal transporters like NRAMP, ZIP families CDF, ATPases (HMAs) family like P1B-ATPases, are involved in the radio-phytoremediation processes. This review will discuss recent advancements and potential application of plants for radionuclide removal from the environment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Highlights
-
1.
Bioavailability of radionuclides in environment.
-
2.
Green plants for restoring balance/their application for radionuclide accumulation.
-
3.
Rhizosphere, rhizobacteria and metal transporters used in radionuclide remediation.
-
4.
Radionuclide exposure and plant responses.
-
5.
Radio-phytoremediation—an approach to decontaminate radionuclides using plants.
1 Introduction
With the discovery of X-rays in 1895 and radioactivity in the subsequent year, awareness of radiation and radioactivity became obvious and a new branch of science was started and subsequently developed into its deliberate use. However, the term radionuclide is much newer. Referring to an atomic species having precise constitution of its nucleus containing a certain number of nucleons (neutrons (N) and protons (Z) and its nuclear energy state), in 1947, Dr. T.P. Kohman of University of Chicago proposed the name nuclide. Thus, a nuclide consists of strongly bound protons and neutrons often referred to as nucleons (Kohman 1947). Nuclides may be stable and exist for an indefinite period of time (common material) or unstable. Usually, elements having atomic number higher than 83 (heavier than bismuth 83Bi), have only unstable nuclei with excess energy available. This surplus energy within a nucleus is either transformed internally or passed on to a newly formed radiation particle within the nucleus. This kind of nuclides with potential to undergo radioactive decays is called radionuclide. The decay goes together with the release of ionizing radiations like gamma ray(s) and/or subatomic, high speed alpha or beta particles. The nuclides are ultimately converted to stable nuclides. Ionizing radiation is capable of generating ions by displacing electrons in the living matter (like in DNA) and thereby disturbing its function (http://water.epa.gov/drink/contaminants/basicinformation/radionuclides.cfm, accessed 24.08.2015). However, exposure to radioactivity is a common and natural phenomenon. Apart from about 20 % anthropogenic sources (especially diagnostic imaging like X-rays, CT scans etc.) (Brenner and Hall 2007), most of the radiation exposures are usually natural (like cosmic radiation, radon gas from rocks and soil, or 40K through foods). Water from natural sources may contain radionuclides due to dissolution of such materials from the earth crust or sporadic release from laboratories or nuclear power plants. Though, in nature, unstable nuclides with long half-lives, so called primordial radionuclides, are also present. Especially 235U has been widely used for different applications in defence and civilian sectors. It is best used in nuclear power plants (due to low cost consumption), where, theoretically 1 kg of U (approx. 1500 tonnes of coal equivalent) can produce about 20 terajoules (2 × 1013 J) of energy.
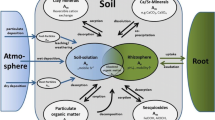
However, extensive use and indiscriminate or improper disposal of wastes, insufficient decontamination at mining sites, release of production tails or decommissioning sites of such material are a serious environmental concern and can lead to contamination by radioactive substances. And, radiation is produced by spontaneous decay of radioactive materials, the amount of which and power for penetration within the body may vary with each type. However, all kinds of ionizing radiations produce health effects that may vary in different tissues and even in different individuals. Exposure to radiation and radionuclides (radiation emitters) are well-known to affect the entire body while inhalation or ingestion may affect diverse tissues inside the body (http://www.medindia.net/patients/patientinfo/radiation-hazards.htm). Ionizing radiation is capable of ionizing many atoms or molecules and destroying molecular bonds. For example, due to a nuclear explosion or accident at a nuclear power plant, radioactive caesium or iodine can be released into the environment, which may directly or indirectly get accumulated within the body, for example through the food chain. For quantification of the radionuclide uptake efficiency the soil-to-plant transfer factor (TF) is widely used to analyse radiological human dose via the ingestion pathway (Chakraborty et al. 2013). The TF is basically the concentration of element in plant (Bq kg−1) divided by concentration of the same element in soil (Bq kg−1). The TF may vary depending upon the element, soil pH and its texture, solid/liquid distribution coefficient, exchangeable K+, organic matter and availability of the element at the root zone (Chakraborty et al. 2013). Starting from a given contamination level the total effective dose equivalent (TEDE) to individuals living in the neighbouring areas of waste-disposal/decommissioning sites can be calculated using models of biosphere and living habits. The Nuclear Regulatory Commission (NRC) has developed some sophisticated methodologies for the assessment of probable dose to man from evaluations of groundwater and soil contamination, irrigation processes, subsequent to food-chain pathways, of crops and forage and lifestyle of potentially exposed individuals (USNRC 2003; Napier et al. 2005, 2007). Above said chains, we want to focus following on soil to plant transfer of such radionuclides at contaminated sites (Fig. 1). Uptake of the radionuclides by plants depends upon several factors including mode of interaction with the materials and physiological characteristics of the species and factors like concentrations, bioavailability, and mobility of radionuclides in surface and subsurface geologic systems (Napier et al. 2005; Gupta and Walther 2014; Walther and Gupta 2015).
Potential radionuclide emissions and contamination pathways in the environment. Incorporation of radioactive materials may occur either as NORM (Naturally Occurring Radioactive Materials) or TENORM (Technologically Enhanced Naturally Occurring Radioactive Materials). Radioactive materials once enter into the environment gets absorbed into the soil or adsorbed to the different soil organic matter or mixed up with the water sources. Atmospheric emissions from different nuclear power plants, industries lead to deposition and pollution of the environment. Plants usually take up such elements leading to incorporation in the food chain. However, transfer factor of the radionuclides depend upon several factors including soil quality, texture and type, organic/inorganic materials, availability of elements etc
2 Natural Occurrence and Exposures
All types of soil and rocks contain uranium, the concentration of which is ranging from 0.003 mg kg−1 in meteorites to 120 mg kg−1 in phosphate rock. As a normal constituent of earth’s crust, rock phosphate (U resources in phosphate rocks are estimated at 9 × 106 MT) deposits usually contain several million tons of uranium (U), radium (Ra), and thorium (Th) which leads to potential exposure of the environment to radiation (Gupta et al. 2014). Commonly studied U decay series involves the following steps:


High levels of dose rate are found above soils predominated by granite rocks or mineral sand in comparison to the other soil types. Inhabitants of communities settled on this type of soil (like monazite based sand beaches of Espirito Santo and Rio de Janeiro in Brazil) receive doses many times the average global radiation level (Eisenbud and Gesell 1997). Additional sources of radiation are up-concentrated when earth crust products (oil, coal, coal ash, minerals, and phosphate based fertilizers etc.) are extracted refined or used, which is called naturally-occurring radioactive materials (NORM). Anthropogenic activities like sewage sludge treatment, mining of radioactive materials, concentrate or expose radioactive materials that occur naturally in ores, soils, water, or other natural materials are the sources of production of NORM (IAEA 2014) or technologically enhanced NORM. Radionuclides of concern found in NORM mainly include isotopes of U, Th, Ra, Rn, Pb and Po. Reports suggest that, based on samples collected from 15 different countries, the average concentrations of 40K, 238U and 232Th, in coal are 50, 20, and 20 Bq kg−1 (1.35, 0.54, and 0.54 nCikg−1), respectively (however, the concentration may vary considerably in different coal mines). Similarly, crude oil and natural gases are another source of radiation, where, approximately (average estimation), 40 pCiL−1 (1.5 Bq L−1) of Ra may be found at well heads of natural gas. Further, LPG (liquefied petroleum gas) processing and blending tends to enhance concentrations of radon and its products 210Pb, 210Po (Eisenbud and Gesell 1997). Uranium contamination in soil and water has been increased in a number of regions throughout the world due to technological progressions related to establishment of nuclear power plants and its fuel cycle (e.g., mining and milling of U ore and its waste), combustion of fossil fuels (e.g., coal), increased production of phosphate fertilizers through phosphate rock mining and other uses for warheads and present as in the form of TENORM, which leads to potential ecological risks to the biota through radiation as well as chemical toxicity (Sheppard 2001).
Phosphogypsum (mostly calcium sulfate) is a by-product of a wet-acid based process during the generation of phosphoric acid in the phosphate based fertilizer industry. Large quantities of deposits of phosphogypsum are a potential source of enhanced natural radiation and for heavy metals. Approximately 70 % of calcium phosphate along with different impurities like radionuclides is present in high grade ores of phosphate rock (PR) for the production of phosphoric acid and those impurities ultimately end up in the phosphogypsum. USEPA (http://www.epa.gov/radiation/neshaps/subpartr/about.html) reports suggest the concentration of 238U and 226Ra in phosphogypsum of central Florida were about 10 times for U and 60 times for 226Ra higher than the average background levels in soil. The concentration may vary according to the geographical area and quality of the PR ore. Mineral sands (sands with a specific gravity of more than 2.9), produced through the erosion of rocks (naturally or anthropogenically though blasting etc.), are also a major source of 232Th and 238U (UNSCEAR 1993: http://www.unscear.org/unscear/en/publications/1993.html).
3 Use of Green Plants for Restoring Balance and Its Applications
Phytoremediation (Ancient Greek: phyto- ‘plant,’ and Latin remedium- ‘restoring balance’), according to a recent definition by Landmeyer (2011), is the “application of plant-controlled interactions with groundwater and organic and inorganic molecules at contaminated sites to achieve site-specific remedial goals”. Cleaning up of the environment through plants is rendered by diverse environmental pollution problems through direct uptake of toxic chemical(s), followed by subsequent transformation, transport, and their accumulation in less toxic forms (Schnoor et al. 1995). Furthermore, remediation processes are being improved by plants, roots release of exudates and enzymes that induce microbial diversity at rhizosphere and biochemical activity in the bulk soil and mineralization (Macek et al. 2000; Chatterjee et al. 2013b).
Phytoremediation techniques are becoming a popular alternative to the conventional energy and instrument intensive, chemical-based expensive restoration techniques of vast polluted areas of land and water (Padmavathiamma and Li 2007; Lone et al. 2008). Use of green living systems for environmental remediation was first reported in 1948 for accumulation of nickel by the plant Alyssum bertolonii; however, the concept received momentum later. Since the last two decades phytoremediation work has got much attention throughout the globe (Gupta 2013).
After the reactor accident at Chernobyl, Ukraine in 1986 that caused the release of large quantities of radioactive particles into the environment and resulted in some 30 deaths mostly due to deterministic radiation effects and more due to long-term (stochastic) effects like thyroid cancers and leukaemia in the case of clean-up workers (UNSER report on ‘The Chernobyl accident’ from- http://www.unscear.org/unscear/en/chernobyl.html), the scientific community of the world started thinking to use plants for radionuclide decontamination of soil and water. In 1998, Consolidated Growers and Processors (CGP), PHYTOTECH, and the Ukraine’s Institute of Bast Crops, employed the phytoremediation process using industrial hemp (Cannabis) as most efficient plant useful for eliminating toxins such as metals, solvents, pesticides, explosives etc. from the contaminated topsoil. Continuing to this application of plant based green technology, US Department of Defense and USEPA jointly developed plant-based clean-up approaches to large-scale clean-up projects, where, specific plants were used to remove toxins and certain metals (Rai and Pal 1999).
Use of plants for removal of inorganic components or toxic elements like Hg, Cd, As, Cr, Cs, Pb and Sr involves extraction and translocation of toxic cations or oxyanions to above ground tissues by converting the element to a less toxic chemical species (Meagher 2000; Chatterjee et al. 2012). However, for organic compounds like polychlorinated biphenyl (PCBs), polycyclic aromatic hydrocarbons (PAH), dioxin, and trichloroethylene, the objective of phytoremediation processes is to totally mineralize them into relatively nontoxic constituents, such as carbon dioxides, nitrate and ammonia (Cunningham et al. 1996). Plants deal with such components through the strategies of stabilization, exclusion, detoxification and/or their storage in specific cells or cell organelles (vacuoles, cell walls). These strategies are variable with regard to the component characteristics and the processes termed as: phytostabilization, phytoextraction, phytovolatilization, rhizofiltration, phytodegradation, and phytostimulation (Marques et al. 2009; Chatterjee et al. 2013b). Furthermore, plants also develop defence mechanisms through the production of different anti-stress proteins like metallothioneins and phytochelatins etc.
Hyperaccumulator plants, that actively uptake exceedingly large amounts of one or more heavy metals (at concentrations 100–1000-fold higher than those found in non-hyperaccumulating species) from the soil, without exhibiting any symptoms of phytotoxicity, are especially suited candidates for phytoremediation (Reeves 2006). Plants belonging to the families of Brassicaceae, Papilionaceae, Caryophyllaceae, Poaceae and Asteraceae are most important and offer best potential for heavy metal phytoremediation. Amongst these, species belonging to the Asteraceae family show bio-removal potential of heavy metals and radionuclides, such as Sr, Cs and U. Plants like Lactuca sativa L., Silybum marianum Gaertn., Centaurea cyanus L., Carthamus tinctorius L. from Asteraceae, can uptake 134Cs very efficiently; high biomass producing varieties of Helianthus annuus and H. tuberosus are also suitable for the phytoremediation of polluted sites (Tang and Willey 2003). Phytoremediation of radionuclides has many advantages over the traditional treatments. Firstly, in phytoremediation the soil is treated in situ, which does not cause further disruption to the soil dynamics. Secondly, plants are established, they remain for consecutive harvests to continually remove the contaminants. Last but not least, phytoremediation reduces the time of workers who are supposed to expose to radionuclides. Finally, phytoremediation can be used as a long term treatment that can provide an affordable way to restore radionuclide contaminated areas (Gupta and Walther 2014). Table 1 summarizes the successful used radionuclide phytoremediator plant species (aquatic/terrestrial).
4 Rhizosphere, Rhizobacteria and Metal Transporters Used in Remediation Purposes
Plant roots secrete exudates in the adjacent soil matrix at the rhizosphere (region of soil at the root-soil interface, which is subjected to root secretions and related soil microorganisms). This mechanism helps in metal-chelation to control the entry of metal within plant through the root cell. Plants root exudates normally increase the abundance of soil microflora (bacterial and fungal communities) by 1-4 orders of magnitude compared to the surrounding bulk soil that helps to degrade diverse pollutants (Anderson et al. 1994). Root exudates include: diffusates (e.g. amino or organic acids, water, inorganic ions, and sugars etc.), excretions (e.g. bicarbonates, protons, and carbon dioxide etc.), secretions (e.g. mucilage, siderophores, and allelopathic compounds etc.) which help to modulate soil microflora community composition to have a better range of metabolic capabilities with unique gene pool (Hall 2002; LeDuc and Terry 2005). The availability of U and 137Cs to plants can be enhanced using citric acid and ammonium nitrate, respectively, in soil. However, soil augmentation may be done in a properly managed fashion, as for the inherent risks associated with the application which may in turn contaminate soil or groundwater (Prasad 2011). Plant cell walls operate as cation exchanger, sharing or excluding diverse metals, to retain the homeostasis in the cell (Rauser 1999).
On the other hand, plant growth-promoting rhizobacteria (PGPR) play a crucial role in plants growth by countering physiological stress in contaminated soils. PGPR generally performs rhizospheric colonization by stimulating the production of plant growth regulators like indole acetic acid, gibberellic acid, ethylene and cytokinins contributing to plant health and better response. Further, rhizobacteria may also secrete antibiotics, phosphate solubilising substances, hydrocyanic acid, siderophores, 1-aminocyclopropane-1-carboxylic acid (ACC) to increase bioavailability and root absorption of different metals (Meyer 2000; Davies et al. 2001). In a nickel-contaminated soil, Burd et al. (1998) reported that after the addition of Kluyvera ascorbata SUD165/26 an associated rhizobacteria, germination and growth of Indian mustard (Brassica juncea) seeds increase by 50–100 % with respect to the control plants.
Root cell plasma membranes harbour a number of metal transporter-proteins which play important roles in heavy metal homeostasis. Among different families of transporters, NRAMP (natural resistance-associated macrophage protein), ZIP (Zinc importer) families (ZRT, IRT-like Protein; [ZRT-Zinc regulated transporter, IRT-iron regulated transporter]), CDF (cation diffusion facilitator) family, heavy metal ATPases (HMAs) family like P1B-ATPases, copper transporter (COPT) family proteins, ATP-binding cassette (ABC) transporters, ABC transporters of the mitochondria (ATM), Ca2+ cation antiporter (CAX), multidrug resistance-associated proteins (MRP), yellow-stripe-like (YSL) pleiotropic drug resistance (PDR) transporters are well studied transporters (Dubey 2011; Huang et al. 2012; Gupta et al. 2013). Histidine-rich domain of ZIP family transporters is supposed to get activated in response to divalent metals and their uptake. As for example, AtZIP4 (Arabidopsis thaliana ZIP4) proteins help in Zn transport and Cd uptake from soil to root cells and Cd transport from root to shoot (Krämer et al. 2007). While, transport of Fe, and other metals like Mn2+, Zn2+, and Cd2+ in root cells was being carried out by IRT1 in A. thaliana (Nishida et al. 2008). HMAs family transporters (P1B-type ATPases) are basically internal transporters that load Cd and Zn metals from the surrounding tissues into the xylem and perform as an efflux pump (Krämer et al. 2007). AtHMA3 transporter of tonoplast membrane in A. thaliana sequesters a wide variety of heavy metals and its over-expression raises the tolerance to heavy metals like Cd, Co, Pb, and Zn (Manara 2012; Gupta et al. 2013). Strategies for plants to isolate metals from dynamic cytosol and metabolically active cellular compartments include vacuolar sequestration using proton pumps like vacuolar proton-ATPase (V-ATPase) and vacuolar proton-pyrophosphatase (V-PPase) (Dalcorso et al. 2010).
5 Radio-Phytoremediation: An Approach to Decontaminate Radionuclides Using Plants
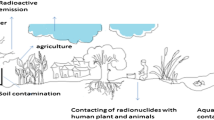
Radionuclides and/or toxic elements contaminated soils, sediments, surface water and groundwater’s remediation by using green plants is widely reported (Nishita et al. 1958; IAEA 1989; Soudek et al. 2004, 2006). However, radionuclide decontamination has its inherent problem of radioactivity itself. Plants require a long period of time to contact with a contaminant to evolve the ability to hyperaccumulate radioactive material (Fig. 2). The concentrations, mobility, and bioavailability of radionuclides depend upon several factors. These include the quality, quantity and the rate of release of radionuclides present at the source; hydrological factors, like dispersion, advection, and dilution; geochemical processes, such as complexation at aqueous phase, pH, adsorption/desorption, solid/liquid distribution coefficient, reduction/oxidation (redox), ion exchange, precipitation/dissolution, diffusion, colloid-facilitated transport, exchangeable potassium ion distribution, anion exclusion and organic matter contents (Albrecht et al. 2002; Napier et al. 2012; Chakraborty et al. 2013; Hegazy et al. 2013). Absorption and distribution of contamination in plants may take place either through direct (exposures at aerial organs like leaf, stem, and tendrils) or indirect (through root systems in soil related contamination) routes, which varies considerably in different plant species especially in case of long-lived radionuclides (Din et al. 2010). Further, soil properties/texture (like drying and subsequent cracking of soils) due to biological activity, colloid-facilitated transport in soil may augment the mobility and/or affectivity of radionuclides (Napier et al. 2012).
Radionuclide contamination pathways in plants. The root uptake of radionuclide depends upon various factors like the condition of metal with clay/soil particle/minerals and its availability to the plants. The translocation and deposition of the same may take place in the leaf or inflorescence. Again, metals present in the atmosphere also get deposited on different plant body surfaces (like leaf surfaces). The concentration of deposition varies and modulated through the factors like wind, rain etc. Wash-off metals further deposited into soil and sorbed by soil particles, and available for plants uptake
In the environment, a number of radionuclides (e.g. Tc, U and Pu) may be present in more than one oxidation state, and adsorption and precipitation behaviour of individual states may vary considerably. Thus, transfer pathways and efficiencies of radionuclides vary widely according to (1) chemical nature and reactivity of the isotope that may affect the solubility and transport of the isotope through soil pore (includes pore structures) water within the root (rhizosphere) of the plant, (2) exposure route (e.g. root versus foliar exposure) of the contaminant, (3) plant itself (species, physical stature, age, root structure, and root-shoot ratio etc.), (4) requirements and availability of nutrient(s) of the plant (chemical resemblance of the isotope to a nutrient) and (5) level of toxins and pathogens in the soil.
It is depending upon the water cycle and the compositions of the flora which are intrinsically coupled, thus interactions may occur within these water bodies or in terrestrial ecosystems. However, the distribution and productivity of terrestrial vegetation is mostly dependent upon the equilibrium of water. Plant communities and root zone (rhizosphere) of the plant along with the soil microorganisms play important roles in evapo-transpiration generation of runoffs (Gerten et al. 2004), metal uptake and remediation. Ehlken and Kirchner (2002) suggested that the concentration of a radionuclide in a plant or plant part is linearly related to its concentration in soil within the rhizosphere (same kind of relation is also true for transfer of heavy metals and pesticides in plants) (Gast et al. 1988; Trapp et al. 1990). However, interestingly, soil-to-plant transfer factors for a number of long-lived radionuclides (as for example, radiocesium uptake from agro-soil) may vary considerably up to three orders of magnitude (Frissel 1992; Nisbet and Woodman 2000; Ehlken and Kirchner 2002). Again significant variation is also present in case of longevity of plant roots in different plant species, their development and function is being responded by genetically determined range to their environment (Clausnitzer and Hopmans 1994).
6 Bioavailability of Radionuclides
In soil, most of the elements are usually present, from where plants acquire essential elements as micronutrients. Bioavailability of trace substances in soil is a complex phenomenon, where competitions of varied substances along with metal-metal interactions take place. Transfer dynamics from soil to plant is usually calculated for trace substances whose behaviour in the soil-plant-rhizosphere system largely depends on the concentrations of macro-nutrients present. It is reported that an activity concentration in a soil solution of 1 BqL−1 of 90Sr or 137Cs corresponds to ca. 2 × 10−15 MolesL−1 though the average concentrations of chemical homologes K, Ca, and Mg, respectively, in soil solution are in the order of 1 mMoles L−1 (Robson and Pitman 1983; Ehlken and Kirchner 2002). Uptake of radionuclides like radioactive caesium and strontium and heavy metals by plants is thus affected by several factors naturally present in the soil (Lorenz et al. 1994). Cation exchange reactions in the soil milieu determine the actual concentration of an ion in soil-solution which is competitive in nature. Accordingly, co-precipitation also depends on the concentrations of competing substances in solution (as for example, sorption of radiostrontium is dominated by reversible exchange with major cations like Ca2+). While strontium is exchanged in preference to Ca in minerals, the preference of the same metal reverses in the presence of organic matter (Valcke and Cremers 1994). The sorption of radiocaesium in soils is determined by ion exchange to a few sites, as for example, weathered mica which are accessible only to poorly hydrated cations and shows high selectivity for Cs+ over K+ and NH4+ (Comans and Hockley 1992; Ehlken and Kirchner 2002). In a recent report by Voronina et al. (2015), they reported a comparative study of selectivity and reversibility of radiocaesium and radiostrontium sorption by natural aluminosilicates (glauconite and clinoptilolite) as well as modified ferrocyanide sorbents, it was shown from their experiment that modification of natural aluminosilicates by ferrocyanides allows to increase distribution coefficients of caesium by 100–1000 times, to improve sorption capacity and to make sorption of caesium more selective and almost irreversible. They recommended the use of modified aluminosilicates for remediation of radioactively contaminated lands.
Some metals (like Zn, Cu) are essential for plant growth and sustenance, however, higher levels of essential or non-essential metals cause toxicity and inhibition of growth in most of the plants (Hall 2002; Lasat 2002). Absorption in plants is a vital issue, where the root zone (rhizosphere) region along with soil microorganisms interacts with the elements for bio-availability and uptake of the same (Wenzel et al. 2003). Elements are typically co-transported across the plasma membrane in the form of cations. Toxic heavy metals which do not have any known biological function (viz. As, Cd, and Pb) are also transported through the common transportation system (Manara 2012). Generally- in plants, root secrete exudates in adjacent soil matrix which may help in chelation of unwanted metals and to prevent transportation of these metals inside the cell (Marschner 1995). As for example, root exudates like histidine (His) prevent Ni uptake from the soil (Salt et al. 2000), whereas, pectic sites and different extracellular carbohydrate molecules present on the cell wall play an important role in immobilization of toxic heavy metal ions (Manara 2012). In metal contaminated soil, plants act upon as either “accumulators” or “excluders” for particular metal(s), where the excluders restrict metal uptake into their biomass and accumulators amass (high concentration) metals or contaminants into their aerial tissues like leaf tissues after breakdown of the same (Tangahu et al. 2011). However, chemical forms of metals may vary as bound component to soil particle and/or precipitated form, which mostly make a major portion of the metal(s) that is insoluble or unavailable to plants (Chatterjee et al. 2013a). Several factors involve in controlling uptake, translocation, and storage of different metals including toxic elements into the plant body. In soil microorganisms, plant induce pH changes and redox reactions, plants also produce chelating agents and exudates and a group of transporters embedded in plant cell plasma membrane, it is the major regulator for several functions. The metal transporter proteins like proton pumps –ATPases (that generate electrochemical gradients and consume energy), co-and anti-transporters proteins (that use the electrochemical gradients generated by –ATPases), and ion channel proteins (that facilitate the transport of ions into the cell) involved in metal uptake and translocation (Tangahu et al. 2011).
7 Radionuclide Exposure and Plant Responses
Plants have been observed to take up many cations present in their root region irrespective of biological requirement. When plants are exposed to ionizing radiation, molecular and cellular effects are induced directly through damage of macromolecules or indirectly through water radiolytic reactions producing reactive oxygen species (ROS) (Gupta and Walther 2014). By energy transfer from the radiation field to plant tissue, ionizing radiation can directly induce DNA strand breaks, lipid oxidation, or enzyme denaturation. ROS are also produced under natural metabolism and their functions in plants are as signaling molecules, regulating normal growth and development and they are useful in stress responses. Plants also possess an antioxidative defense system comprising enzymes (e.g., superoxide dismutase (SOD) and catalase (CAT)) and metabolites (e.g., ascorbate and glutathione) to regulate the amount of ROS in cells (Gupta and Sandalio 2012). Plant metal tolerance mechanisms requires the coordination of complex physiological and biochemical processes. Plants employ various strategies to cope with toxic effects of radionuclides and heavy metals or metalloids, in general, and radionuclides stress either by avoidance (restricting the metal uptake), or by tolerance (survive in the presence of high internal metal concentration). Plants also restrict metal stress by the mechanisms like reducing the concentration of metal entering into the cell by extracellular precipitation, biosorption to cell walls, reduced uptake, and/or by increased efflux (Gupta and Walther 2014). However, tolerating metal stress involves elaborate physiological responses like intracellular chelation through synthesis of amino acids, organic acids, glutathione (GSH), and/or by metal-binding ligands such as metallothioneins (MTs) and phytochelatins (PCs), vacuolar compartmentalization, and up-regulation of antioxidant defense and glyoxalase systems to counter the deleterious effects caused by ROS (Gupta and Sandalio 2012).
8 Phytoremediation of Selected Radionuclides
8.1 Caesium
Caesium (Cs) has the chemical similarity to potassium (K) and thus absorption and translocation by plants is common. Contamination of Cs (134Cs half-life:T1/2 = 2.06 years and 137Cs -T1/2 = 30.04 years) leads to long persistence in soil and environment (Koarashi et al. 2012; Kamei-Ishikawaa et al. 2013). This alkaline metal is present in solution as free hydrated cation Cs+. However, plant uptake of Cs was first reported by Collander in early 1940s (Collander 1941) and was thought to be coupled as a nutrient analogue with the same K+ uptake transporter, especially during low K concentrations (Menzel 1954; Zhu and Smolders 2000). It is apparent that K+ is the most significant cation amongst all other alkaline metals and compounds that competes with Cs+ uptake (Zhu and Shaw 2000). Plant families such as Brassicaceae, Amaranthaceae and Chenopodiaceae are the major Cs accumulators. For plant uptake of Cs, K+ concentration in soil is the most important factor. Thus, both selection of plant and potassium concentration in the soil should be taken into consideration for phytoremediation of Cs from soil (Buysse et al. 1996).
Cs uptake in plants is not yet been fully elucidated on a microscopic level. However, it has been observed that, low K concentration in soil helps to uptake Cs through K uptake system. Thus, higher K concentration suppresses the Cs uptake (Zhu and Smolders 2000). Studies on discrimination factor on Cs/K revealed that plant roots usually absorb Cs less ably than K like nutrient analogue (Smolders et al. 1996). Two K+ transport pathways (K+ channels and K+ transporters) are involved with Cs+ transportation, where multiple genes may be involved. As for example, in A. thaliana, high affinity K+ transporter family (e.g. AtHAK5) and voltage-insensitive cyclic nucleotide gated channels, AtCNGCs, are involved in the Cs+ uptake (Kanter et al. 2010; Kobayashi et al. 2010; Yamashita et al. 2014).
Specific absorption mechanism in soil by plant may depend upon several factors like availability and exchangeability of Cs along with the particular salt content and its interionic effects, soil type, water submergence time etc. (Tensho et al. 1961, Mimura et al. 2001). Salts like ammonium molybdophosphate (AMP) and rubidium chloride augment in uptake of Cs from soil. The application of AMP for partitioning and recovery of radioactive 137Cs from HNO3 and NaNO3 rich liquid waste is well documented (Mimura et al. 2001).
However, a number of conditions and physiological parameters of plants are involved that lead to differential uptake in different species. Among these factors, plants demand for potassium and its rooting pattern for its growth strategies are most important, along with the factors like presence of mycorrhizal fungi, rate of root growth, efficiency of ion transporters that are present on plasma membranes of root cells etc. (Broadley and Willey 1997; Zhu and Smolders 2000). Halophytes of family Chenopodiaceae are long been known as Cs accumulators, however, their capability to discriminate K and Na from Cs for uptake from salty soil is still discussed controversially (Flowers et al. 1986; Zhu and Smolders 2000).
To understand the genetic variations in plants for uptake of Cs is very important for selection of suitable crops grown in soils and their accumulation pattern within the edible parts. This kind of data may be helpful to minimize its transfer to food chain. Further, non-edible plants may also be explored to decontaminate the soil contaminated with this radionuclide (Zhu and Smolders 2000). Reports showed that red root pigweed (Amaranthus retroflexus) is a good accumulator of 137Cs with higher uptake rate and plant growth, as also shown during the phytoremediation practices for Cs‐contaminated soil in the vicinity of Chernobyl, Ukraine (Lasat et al. 1997, 1998; Dushenkov et al. 1999). Kang et al. (2012) showed that napier grass (Pennisetum purpureum Schum.) under hydroponic condition is also a good accumulator of Cs and may be applied for phytoremediation. To reduce the total time required for phytoremediation, various chemical and biological amendments may be useful in speeding up phytoextraction process. Reports suggest that the application of NH4NO3, (NH4)2SO4 could increase phytoextraction, while application of potassium based components to the soil should be minimized (Lasat et al. 1997, 1998; Dushenkov et al. 1999; Zhu and Smolders 2000). Further, genetic alteration and special plant breeding by selection of suitable plant taxa may be helpful to achieve the goal.
In a recent report by Yamashita et al. (2014) on the deposition of radionuclides (radiocesium- 134Cs, 137Cs) in different plant species after the accident of the Fukushima Daiichi Nuclear Power Station, Japan in March 2011, it is shown that the transfer factors of radiocesium from soil to plant([Cs]plant/[Cs]soil) vary widely in different plants. However, plants like Athyriumyo koscense, Dryopteris tokyoensis and Cyperus brevifolius exhibited relatively high TF values (more than 0.4).
8.2 Uranium
U has six naturally known isotopes 233U to 238U with half-lives varying between 69 years and 4.47 billion years. Among three primordial natural isotopes, 238U is the most abundant isotope (~99.275 % of the uranium found in nature) followed by 235U (~0.720 %), and 234U (~0.005 %). Radioactivity and metal toxicity of U makes it a pollutant of concern to the environment. Naturally, U is released into water or soil through the weathering of rocks in the oxidized zone of the terrestrial near-surface environment. In an almost constant isotopic ratio 238U(137.88):235U(1), release of U to water takes place through natural geochemical process, while 234U is produced by the radioactive decay of 238U. A range of physicochemical forms like free metal ion (U4+ or UO22+), inorganic compounds (e.g., uranyl carbonate or uranyl phosphate), mineralogical matrices and soil humic substances (e.g., uranyl fulvate or humate) in dissolved, colloidal, and/or particulate forms regulate the speciation and bioavailability of U (Markich 2002). Biologically dissimilatory metal reducing bacteria (DMRB) and sulfate reducing bacteria (SRB) play an important role in U(VI) reduction to U(IV) in anaerobic environments (Liu et al. 2002; Payne et al. 2002). Though U has no known essential biological functions, it is taken up by a variety of mineral and other surfaces by the presence of dissolved calcium by inducing the formation of ternary uranyl-calcium carbonate complexes. However, redox state of U distribution is a consequence of the redox potential in solution, predominantly the U(VI) to U(IV) ratio, usually regulate its solubility and potential for migration and formation of soluble UO2 (Fredrickson et al. 2000; Stewart 2008). Organic acids like citric acid were found to be most effective in increasing U accumulation in plants. Huang et al. (1998) showed that U accumulation in shoot of Brassica juncea and B. chinensis increased from less than 5 mg kg−1 to more than 5000 mg kg−1 in citric acid-treated soils, where soil concentration of total U was 750 mg kg−1.
Chemical and civil engineering based U remediation have several apprehensions for its high economic and energy implications, chemicals that are used for the process and their fates, specificity on the characteristics of contaminant, etc. On the other hand, phytoextraction of uranium contaminated soil and water have recently gained importance as it provides a low-cost alternative to currently employed remediation procedures. Phytoremediation processes of uranium generate a minimum amount of secondary waste(s) that may otherwise produce large amounts of heavy metal laden leachate. Results of the study by Huang and his colleagues for phytoextraction of uranium through amendment of soil using citric acid, however, showed the process to be highly effective in triggering U hyperaccumulation in selected plant species (Huang et al. 1998). The application of citric acid transiently reduced the soil pH that enhances desorption of soil U leading to more root absorption. Favas and Pratas (2013) reported that plants like Callitriche stagnalis, Apium nodiflorum and Fontinalis antipyretica have the capacity to accumulate significant amounts of U. These authors also suggested C. stagnalis, A. nodiflorumas are keystone species which can be used for different phytoremediation applications for removal of U from the contaminated sites for their efficient rooting capability, high biomass and bio-productivity and the positive correlation with the uranium concentration in waters (Favas and Pratas 2013; Pratas et al. 2012). Duckweed (Lemna gibba and L. minor) was also reported to have good ability to accumulate U in the standing water sources (Mkandawire and Dudel 2005; Favas and Pratas 2013). Further, greenhouse experiments with B. juncea and H. annuus showed the phytoremediation of uranium-contaminated soil is enhanced in B. juncea when the soil is augmented with citric acid buffer (pH 4.8) that caused an enhancement of the desorption of U in the soil (Dushenkov 2003; Huhle et al. 2008).
8.3 Radium
Radium (226Ra), discovered by Marie Curie and Pierre Curie in 1898, is the product of 238U decay series but is 2.7 million times more radioactive than the same molar amount of natural U due to its shorter half-life. In the last nine steps of the fourteen steps 238U decay series, Ra decay (Ra emanation) occurs producing exradio (222Ra gas), Ra A (218Po), Ra B (214Pb), Ra C (214Bi) etc. until stable 206Pb is reached. Ra is a very important radionuclide due to its (inclusive isotopes 226Ra and 228Ra) presence in all three natural decay series, relatively long half-lives. It has a high mobility in different environmental conditions like uranium mining and processing industries, phosphate and gold mining areas. Ra is a well-known stressors of human body and accumulates in bone (IAEA 2002, 2014). In general, naturally four Ra isotopes are present in the environment: uranium decay series- 226Ra, thorium decay series 228Ra and 224Ra and actinium decay series 223Ra. Among these, 226Ra and 228Ra have half-lives of 1600 and 5.75 years, respectively (IAEA 2002, 2014). Alkaline earth metal Ra is a readily reactive element that reacts with soil and its organic matter. However, absorption of Ra by plants from root zone decreases with high soil sulphate content, increasing pH and increased exchangeable Ca (IAEA 2014). 226Ra is mostly studied among its other isotopes. In plants, grown in natural environment, Ra concentrations vary from 0.0044 to 52 Bq kg−1 (DW), with higher concentrations reported in Brazil nut (Simon and Ibrahim 1990).
Wetland plants like Typha latifolia, Phragmites australis, Juncus inflexus, Carex buxbaumii and, Iris pseudacorus showed interesting result in the accumulation of Ra from a constructed wetland contaminated with U/Ra heavy metals (Soudek et al. 2006, 2007). Macrophytic algae, members of Characeae family have also been shown as hyper-accumulators of radium, the mechanism of which needs to be ascertained properly (Kalin et al. 2002). However, Ra may be loaded into the calcium-rich lattice and/or form of BaSO4 substitute crystals in algal tissue (Kalin et al. 2002; Kunze et al. 2007).
9 Summary and Conclusion
The two most crucial factors for successful implementation of specific plants for restoration of radionuclide contaminated sites are (1) ability to uptake the radioactive material to relatively high levels (2) without affecting the growth or high biomass production. Potential plants should usually accumulate radionuclide in the above ground parts at a level that exceed the soil concentration. There are several factors that limit the application of plant based techniques for remediation, like the rapid incorporation of the metal within/in clay/soil particle/minerals which may limits its availability to the plants. However, phytoremediation might be the most valuable easy technique for restoration of radionuclide contaminated soil if selected cultivars are used. Further, selection of plants, genetic manipulation of different transporters, elucidation of anti-stress factors in plant physiology are the main future area of comprehensive research that will prove useful to counter our own poised nuclear age.
References
Albrecht A, Schultze U, Liedgens M, Flühler H, Frossard E (2002) Incorporating soil structure and root distribution into plant uptake models for radionuclides: toward a more physically based transfer model. J Environ Radioact 59:329–350
Anderson TA, Kruger EL, Coats JR (1994) Enhanced degradation of a mixture of three herbicides in the rhizosphere of a herbicide-tolerant plant. Chemosphere 28:1551–1557
Brenner DJ, Hall EJ (2007) Computed tomography—an increasing source of radiation exposure. New Engl J Med 357:2277–2284
Broadley MR, Willey NJ (1997) Differences in root uptake of radiocaesium by 30 plant taxa. Environ Pollut 95:311–317
Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64:3663–3668
Buysse J, Van de Brande K, Merckx R (1996) Genotypic differences in the uptake and distribution of radiocaesium in plants. Plant Soil 178:265–271
Chakraborty SR, Azim R, Rahman AKMR, Sarker R (2013) Radioactivity concentrations in soil and transfer factors of radionuclides from soil to grass and plants in the Chittagong city of Bangladesh. J Phys Sci 24:95–113
Chatterjee S, Datta S, Mallick PH, Mitra A, Veer V, Mukhopadhyay SK (2013a) Use of wetland plants in bioaccumulation of heavy metals. In: Gupta DK (ed) Plant based remediation processes, soil biology, vol 35. Springer, Berlin Heidelberg
Chatterjee S, Mitra A, Datta S, Veer V (2013b) Phytoremediation protocols: an overview. In: Gupta DK (ed) Plant based remediation processes, soil biology, vol 35. Springer, Berlin Heidelberg
Chatterjee S, Singh L, Chattopadhyay B, Datta S, Mukhopadhyay SK (2012) A study on the waste metal remediation using floriculture at East Calcutta Wetlands, a Ramsar site in India. Environ Monit Assess 184:5139–5150
Clausnitzer V, Hopmans JW (1994) Simultaneous modelling of transient three dimensional root growths and soil water flow. Plant Soil 164:299–314
Collander R (1941) Selective absorption of cations by higher plants. Plant Physiol 16:691–720
Comans RNJ, Hockley DE (1992) Kinetics of cesium sorption on illite. Geochim Cosmochim Acta 56:1157–1164
Cunningham SD, Anderson TA, Schwab P, Hsu FC (1996) Phytoremediation of soils contaminated with organic pollutants. Adv Agron 56:55–114
Dalcorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:1–5
Davies FT Jr, Puryear JD, Newton RJ (2001) Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J Plant Physiol 158:777–786
Din KS, Harb S, Abbady A, Saad N (2010) The distribution of the radionuclides Ra-226, Th-232 and K-40 in various parts of the Alfalfa plant. Tenth radiation physics and protection conference, 27–30 November, Nasr City-Cairo, Egypt. http://www.rphysp.com/s7/distribution.pdf
Dubey RS (2011) Metal toxicity, oxidative stress and antioxidative defense system in plants. In: Gupta SD (ed) Reactive oxygen species and antioxidants in higher plants. CRC Press, Boca Raton, FL
Dushenkov S (2003) Trends in phytoremediation of radionuclides. Plant Soil 249:167–175
Dushenkov S, Mikheev A, Prokhnevsky A, Ruchko M, Sorochisky B (1999) Phytoremediation of radiocaesium‐contaminated soil in the vicinity of Chernobyl, Ukraine. Environ Sci Technol 33:469–475
Eapen S, Singh S, D’Souza SF (2007) Phytoremediation of metals and radionuclides. In: Singh SN, Tripathi RD (eds) Environmental bioremediation technologies. Springer, Germany
Ehlken S, Kirchner G (2002) Environmental processes affecting plant root uptake of radioactive trace elements and variability of transfer factor data: a review. J Environ Radioact 58:97–112
Eisenbud M, Gesell T (1997) Environmental radioactivity (fourth edition) from natural, industrial and military sources. Academic, USA
Favas PJC, Pratas J (2013) Uptake of uranium by native aquatic plants: potential for bio indication and phytoremediation. E3S Web of Conferences1.13007. doi: 10.1051/e3sconf/20130113007; Article available at http://www.e3s-conferences.org or http://dx.doi.org/10.1051/e3sconf/20130113007
Favas PJC, Pratas J, Varun M, D’Souza R, Paul MS (2014) Accumulation of uranium by aquatic plants in field conditions: prospects for phytoremediation. Sci Total Environ 470–471:993–1002
Flowers TJ, Hajibagheri MA, Clipson NJW (1986) Halophytes. Quart Rev Biol 61:313–337
Fredrickson JK, Zachara JM, Kennedy DW, Duff MC, Gorby YA, Li SMW, Krupka KM (2000) Reduction of U(VI) in goethite (alpha-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim Cosmochim Acta 64:3085–3098
Frissel MJ (1992) An update of the recommended soil-to-plant transfer factors of Sr-90, Cs-137 and transuranic. In: International Union of Radio Ecologists (ed) VIIIth report of the working group soil to-plant transfer factors. Balen, Belgium, pp 16–25, IUR Pub R-9212-02
Fukuda S, Iwamoto K, Atsumi M, Yokoyama A, Nakayama T, Ishida K, Inouye I, Shiraiwa Y (2014) Global searches for microalgae and aquatic plants that can eliminate radioactive cesium, iodine and strontium from the radio-polluted aquatic environment: a bioremediation strategy. J Plant Res 127:79–89
Gast CH, Jansen E, Bierling J, Haanstra L (1988) Heavy metals in mushrooms and their relationship with soil characteristics. Chemosphere 17:789–799
Gerten DS, Schaphoff S, Haberlandt U, Lucht W, Sitch S (2004) Terrestrial vegetation and water balance–hydrological evaluation of a dynamic global vegetation model. J Hydrol 286:249–270
Gupta DK (2013) Plant based remediation process. Springer, Germany. ISBN 978-3-642-35563-9
Gupta DK, Sandalio LM (2012) Metal toxicity in plants: perception, signaling and remediation. Springer, Germany. ISBN 978-3-642-22080-7
Gupta DK, Huang HG, Corpas FJ (2013) Lead tolerance in plants: strategies for phytoremediation. Environ Sci Pollut Res 20:2150–2161
Gupta DK, Chatterjee S, Datta S, Veer V, Walther C (2014) Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 108:134–144
Gupta DK, Walther C (2014) Radionuclide contamination and remediation through plants. Springer, Germany. ISBN 978-3-319-07664-5
Hall JL (2002) Cellular mechanism for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Hegazy AK, Afifi SY, Alatar AA, Alwathnani HA, Emam MH (2013) Soil characteristics influence the radionuclide uptake of different plant species. Chem Ecol 29:255–269
Hu N, Ding D, Li G, Zheng J, Li L, Zhao W, Wang Y (2014) Vegetation composition and 226Ra uptake by native plant species at a uranium mill tailings impoundment in South China. J Environ Radioact 129:100–106
Huang J, Zhang Y, Peng JS, Zhong C, Yi HY, Ow DW, Gong JM (2012) Fission yeast HMT1 lowers seed cadmium through phytochelatin-dependent vacuolar sequestration in Arabidopsis. Plant Physiol 158:1779–1788
Huang JW, Blaylock MJ, Kapulnik Y, Ensley AD (1998) Phytoremediation of uranium-contaminated soils: role of organic acids in triggering uranium hyperaccumulation in plants. Environ Sci Technol 32:2004–2008
Huhle B, Heilmeier H, Merkel B (2008) Potential of Brassica juncea and Helianthus annuus in phytoremediation for uranium. In: Merkel BJ, Hasche-Berger A (eds) Mining and hydrogeology. pp 307–318
IAE (International Atomic Energy) (1989) Clean-up of large areas contaminated as a result of a nuclear accident. Technical reports series no. 300
IAEA (International Atomic Energy Agency) (2002) Non-technical factors impacting on the decision making processes in environmental remediation, IAEA-TECDOC- 1279. IAEA, Vienna
IAEA (International Atomic Energy Agency) (2014) The environmental behaviour of radium: revised edition, technical reports. Series no 476. IAEA, Vienna
Kalin M, Kießig G, Küchler A (2002) Ecological water treatment processes for underground uranium mine water: Progress after three years of operating a constructed wetland. In: Merkel BJ, Planer-Friedrich B, Wolkersdorfer C (eds) Uranium in the aquatic environment. Proceedings of the International Conference Uranium Mining and Hydrogeology III and the International Mine Water Association Symposium, Freiberg, Germany, 15-21, September 2002, Springer Verlag, Berlin, Heidelberg, New York, pp 587
Kamei-Ishikawaa N, Itob A, Tagamic K, Umitaa T (2013) Fate of radiocesium in sewage treatment process released by the nuclear accident at Fukushima. Chemosphere 93:689–694
Kang DJ, Seo YJ, Saito T, Suzuki H, Ishii Y (2012) Uptake and translocation of cesium-133 in napier grass (Pennisetum purpureum Schum.) under hydroponic conditions. Ecotoxicol Environ Saf 82:122–126
Kanter U, Hauser A, Michalke B, Draxl S, Schaffner AR (2010) Caesium and strontium accumulation in shoots of Arabidopsis thaliana: genetic and physiological aspects. J Exp Bot 61:3995–4009
Koarashi J, Moriya K, Atarashi-Andoh M, Matsunaga T, Fujita H, Nagaoka M (2012) Retention of potentially mobile radiocesium in forest surface soils affected by the Fukushima nuclear accident. Sci Rep 2:1005
Kobayashi D, Nozomi N, Hisamatsu S, Yamagami M (2010) At KUP/HAK/KT9, a K? Transporter from Arabidopsis thaliana, mediates Cs? Uptake in Escherichia coli. Biosci Biotechnol Biochem 74:203–205
Kohman TP (1947) Proposed new word: nuclide. Am J Phys 15:356–357
Krämer U, Talke IN, Hanikenne M (2007) Transition metal transport. FEBS Lett 581:2263–2272
Kunze C, Kießig G, Küchler A (2007) Management of passive biological water treatment systems for mine effluents. In: Marmiroli N, Borys S, Marmiroli M (eds) Advanced science and technology for biological decontamination of sites affected by chemical and radiological nuclear agents. Springer, Germany
Landmeyer JE (2011) Introduction to phytoremediation of contaminated groundwater. Springer, London
Lasat MM (2002) Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual 31:109–120
Lasat MM, Ebbs SD, Kochian LV (1997) Potential for phytoextraction of 137Cs from contaminated soils. Plant Soil 195:99–106
Lasat MM, Ebbs SD, Kochian LV (1998) Phytoremediation of a radiocaesium‐contaminated soil: evaluation of caesium‐137 bioaccumulation in shoots of three plant species. J Environ Qual 27:165–169
LeDuc DL, Terry N (2005) Phytoremediation of toxic trace elements in soil and water. J Ind Microbiol Biotechnol 32:514–520
Liu CX, Gorby YA, Zachara JM, Fredrickson JK, Brown CF (2002) Reduction kinetics of Fe(III), Co(III), U(VI), Cr(VI), and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol Bioengg 80:637–649
Lone MI, He ZH, Stoffella J, Yang X (2008) Phytoremediation of heavy metal polluted soils and water: progresses and perspectives. J Zhejiang Univ Sci B 9:210–220
Lorenz SE, Hamon RE, McGrath SP, Holm PE, Christensen TH (1994) Applications of fertilizer cations affect cadmium and zinc concentrations in soil solutions and uptake by plants. Eur J Soil Sci 45:159–165
Macek T, Mackova M, Kas J (2000) Exploitation of plants for the removal of organics in environmental remediation. Biotechnol Adv 18:23–34
Manara A (2012) Plant responses to heavy metal toxicity. In: Furini A (ed) Plants and heavy metals, Springer briefs in biometals. Springer, New York, pp 27–53
Markich SJ (2002) Uranium speciation and bioavailability in aquatic systems: an overview. Sci World J 2:707–729
Marques APGC, Rangel AOSS, Castro PML (2009) Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Crit Rev Environ Sci Technol 39:622–654
Marschner H (1995) Mineral nutrition for higher plants, 2nd edn. Academic Press and Harcourt Brace and Co., London-San Diego-New York-Boston-Sydney-Tokyo-Toronto
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3:153–162
Menzel RG (1954) Competitive uptake by plants of potassium, rubidium, cesium, and calcium, strontium, barium from soils. Soil Sci 77:419–426
Meyer JM (2000) Pyoverdines: pigments siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol 174:135–142
Mimura H, Saito M, Akiba K, Onodera Y (2001) Selective uptake of cesium by ammonium molybdophosphate (AMP)-calcium alginate composites. J Nucl Sci Technol 38:872–878
Mkandawire M, Dudel EG (2005) Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci Total Environ 336:81–89
Napier BA, Krupka KM, Valenta MM, Gilmore TJ (2005) Soil and groundwater sample characterization and agricultural practices for assessing food chain pathways in biosphere model. U.S. Nuclear Regulatory Commission, Washington, DC, NUREG/CR-6881
Napier BA, Fellows RJ, Krupka KM (2007) Soil-to-plant concentration ratios for assessing food chain pathways in biosphere models. U.S. Nuclear Regulatory Commission, Washington, DC, NUREG/CR-6941
Napier BA, Fellows RJ, Krupka KM (2012) Soil-to-plant concentration ratios for assessing food chain pathways in biosphere models. U.S. Nuclear Regulatory Commission, Washington, DC, NUREG/CR-7120
Nisbet AF, Woodman RFM (2000) Soil-to-plant transfer factors for radiocesium and radiostrontium in agricultural systems. Health Phys 78:279–288
Nishida S, Mizuno T, Obata H (2008) Involvement of histidine-rich domain of ZIP family transporter TjZNT1 in metal ion specificity. Plant Physiol Biochem 46:601–606
Nishita H, Steen AJ, Larson KH (1958) Release of strontium-90 and cesium-137 from vina loam upon prolonged cropping. Soil Sci 86:195–201
Padmavathiamma PK, Li LY (2007) Phytoremediation technology: hyper-accumulation metals in plants. Water Air Soil Pollut 184:105–126
Payne RB, Gentry DA, Rapp-Giles BJ, Casalot L, Wall JD (2002) Uranium reduction by Desulfovibrio desulfuricans strain G20 and a cytochrome C3 mutant. Appl Environ Microbiol 68:3129–3132
Prasad MNV (2011) A state-of-the-art report on bioremediation, its applications to contaminated sites in India. Ministry of Environment & Forests, Government of India. http://www.moef.nic.in/downloads/public-information/BioremediationBook.pdf
Pratas J, Favas PJC, Paulo C, Rodrigues N, Prasad MNV (2012) Uranium accumulation by aquatic plants from uranium-contaminated water in Central Portugal. Int J Phytorem 14:221–234
Rai UN, Pal A (1999) Toxic metals and phytoremediation. Enviro News, Newsletter of International Society of Environmental Botanists, India, vol 5(4)
Rauser WE (1999) Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin and metallothioneins. Cell Biochem Biophys 31:19–48
Reeves RD (2006) Hyperaccumulation of trace elements by plants. In: Morel JL, Echevarria G, Goncharova N (eds) Phytoremediation of metal-contaminated soils, NATO Science series IV: Earth and Environmental Sciences. Springer, New York
Robson AD, Pitman JB (1983) Interactions between nutrients in higher plants. In: Lðuchli A, Bieleski RL (eds). Inorganic plant nutrition. New York: Springer, pp 147–180. (Encyclopedia of Plant Physiology, 1)
Salt DE, Kato N, Kräme U, Smith RD, Raskin I (2000) The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and nonaccumulator species of Thlaspi. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press LLC, Boca Raton
Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29:318–323
Sheppard SC (2001) Toxicants in the environment: bringing radioecology and ecotoxicology together. In: Brechignac F, Howard BJ (eds) Radioactive pollutants: impact on the environment. EDP Sciences, Les UlisCedex A, France
Simon SL, Ibrahim SA (1990) Biological uptake of radium by terrestrial plants, vol 1, The environmental behaviour of radium. IAEA, Vienna, pp 545–599, Technical reports series no. 310
Smolders E, Kiebooms L, Buysse J, Merckx R (1996) 137Cs uptake in spring wheat (Triticum aestivum L. Cv. Tonic) at varying K supply. II: The effect in solution culture. Plant Soil 18:211–220
Soudek P, Tykva R, Vaková R, Vank T (2006) Accumulation of radioiodine from aqueous solution by hydroponically cultivated sunflower (Helianthus annuus L.). Environ Exp Bot 57:220–225
Soudek P, Petrová S, Benesová D, Tykva R, Vanková R, Vanek T (2007) Comparison of 226Ra nuclide from soil by three woody species Betula pendula, Sambucus nigra and Alnus glutinosa during the vegetation period. J Environ Radioact 97:76–82
Soudek P, Tykva R, Vank T (2004) Laboratory analyses of Cs uptake by sunflower, reed and poplar. Chemosphere 55:1081–1087
Stewart BD (2008) The dominating influence of calcium on the biogeochemical fate of uranium. Ph.D. thesis, Stanford University, USA
Tang S, Willey NJ (2003) Uptake of 134Cs by four species from the Asteraceae and two varieties from the Chenopodiaceae grown in two types of Chinese soil. Plant Soil 250:75–81
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 939161
Tensho K, Yeh KL, Mitsui S (1961) The uptake of strontium and cesium by plants from soil with special reference to the unusual cesium uptake by lowland rice and its mechanism. Soil Sci Plant Nutr 6:176–183
Trapp S, Matthies M, Scheunert I, Topp EM (1990) Modelling the bioconcentration of organic chemicals in plants. Environ Sci Technol 24:1246–1252
USNRC: U.S. Nuclear Regulatory Commission, Washington, DC (2003) Literature review and assessment of plant and animal transfer factors used in performance assessment modelling. Pacific Northwest National Laboratory. NUREG/CR-6825, PNNL-14321
Valcke E, Cremers A (1994) Sorption-desorption dynamics of radiocesium in organic matter soils. Sci Total Environ 157:275–283
Voronina AV, Blinova MO, Semenischev VS, Gupta DK (2015) Returning lands, contaminated as a result of radiation accidents, to farming use. J Environ Radioact 144:103–112
Walther C, Gupta DK (2015) Chemistry of radionuclides in the environment: influence of chemical speciation and plant uptake on radionuclide migration. Springer, Germany. ISBN 978-3-319-22170-0
Wenzel WW, Unterbrunner R, Sommer P, Sacco P (2003) Chelate assisted phytoextraction using canola (Brassica napus L.) in outdoors pot and lysimeter experiments. Plant soil 249: 83–89
Yamashita J, Enomoto T, Yamada M, Ono T, Hanafusa T, Nagamatsu T, Shoji Sonoda S, Yamamoto Y (2014) Estimation of soil-to-plant transfer factors of radiocesium in 99 wild plant species grown in arable lands 1 year after the Fukushima 1 Nuclear Power Plant accident. J Plant Res 127:11–22
Zhu YG, Shaw G (2000) Soil contamination with radionuclides and potential remediation. Chemosphere 41:121–128
Zhu YG, Smolders E (2000) Plant uptake of radiocaesium: a review of mechanisms, regulation and application. J Exp Bot 51:1635–1645
Acknowledgement
Authors are thankful to Mrs. Swagata Chatterjee for making handmade figures. S.C. and S.D. are thankful to Director, DRDO, Assam, India. The authors apologize for the many colleagues who are not referenced in this work due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gupta, D.K., Chatterjee, S., Datta, S., Voronina, A.V., Walther, C. (2016). Radionuclides: Accumulation and Transport in Plants. In: de Voogt, P. (eds) Reviews of Environmental Contamination and Toxicology Volume 241. Reviews of Environmental Contamination and Toxicology, vol 241. Springer, Cham. https://doi.org/10.1007/398_2016_7
Download citation
DOI: https://doi.org/10.1007/398_2016_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46944-7
Online ISBN: 978-3-319-46945-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)