Abstract
Radiostrontium is a common product of nuclear fission and was emitted into the environment in the course of nuclear weapon tests as well as from nuclear reactor accidents. The release of 90Sr and 89Sr into the environment can pose health threats due to their characteristics such as high specific activities and easy access in human body due to its chemical analogy to calcium. Radiostrontium enters the human food chain by the consumption of plants grown on sites comprising fission-derived radionuclides. For humans, Sr is not an essential element, but, due to solubility in water and homology with calcium, once interred in the body, it gets deposited in bones and in teeth. This concern has drawn the attention of researchers throughout the globe to develop sustainable treatment processes to remediate soil and water resources. Nowadays, phytoremediation has become a promising approach for the remediation of large extents of toxic heavy metals. Some of the plants have been reported to accumulate Sr inside their biomass but detailed mechanisms at genetic level are still to be uncovered. However, there is inadequate information offered to assess the possibility of this remediation approach. This review highlights phytoremediation approach for Sr and explains in detail the uptake mechanism inside plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
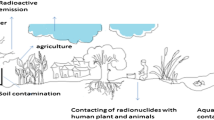
Strontium (Sr) is a soft, silver-grey metal of which about 17 different isotopes are known; four of them are stable isotopes, i.e. 84Sr, 86Sr, 87Sr, and 88Sr. The most abundant isotope is 88Sr which constitutes about 83% of natural strontium. Two major isotopes are of high concern in radioecology, strontium-89 (89Sr; T1/2 = 50.6 days) and strontium-90 (90Sr; T1/2 = 28.9 years) (Amano et al. 2016). Strontium isotope ratios (87Sr/86Sr) are also used as significant intrinsic markers in forensic sciences (West et al. 2009). During general studies on Sr, maximum researchers study in detail on stable Sr forms. Considering environmental impact of Sr and the need for utilising in field testing, stable form of Sr are mostly preferred and acceptable over radioactive form both theoretically and practically (Tatiana et al. 2010); 90Sr is considered to be harmful to humans due to its long half-life. Radiostrontium gets transmitted from nuclear accident sites to soil and water in close proximity which ultimately pollutes aquatic ecosystems and food products due to its high solubility in water and through this, pollution gets access into the human body (Ogawa et al. 2016). Land and water containing radiostrontium can exert adverse health effects on human, enhancing the risk of cancer and inducing skeletal abnormalities because of a prolonged exposure to 90Sr (Dahl et al. 2001). Strontium is preferentially accumulated in bones and teeth, replacing calcium in the biomineral apatite. 90Sr also easily accumulates within plants, depending on soil characteristics, climatic conditions, and particularly plant species (subspecies), etc. (Gupta et al. 2018). Both Sr and Ca alkaline earth metals exhibit similar behaviour in the environment (Voelkle et al. 1989). Like Ca, Sr is taken up by plants as many compounds are highly bioavailable for plants. 90Sr thereby enters in the food chain and may represent a radiological risk to the consumers. This radionuclide also migrates quite rapidly in the environment mostly due to its presence in the form of water-soluble complexes (Gupta and Walther 2018). Because of these hazardous characteristics, it has received the attention of scientists worldwide to propose an eco-friendly approach for eradication of contaminations from soil and water resources.
Under environmental conditions, the chemistry of strontium is exclusively governed by the divalent cationic form Sr2+ with the ionic radius of 2.00 Å and covalent radius of 1.92 Å, respectively, exemplifying the similarity to calcium (Ca2+) with 1.80 Å ionic radius (Crystalmaker 2015; Saniewski et al. 2016). Being a chemical homologue to Ca, Sr gets allocated with ease in bones and in teeth of humans. Strontium/calcium ions also can be absorbed by plant roots through soil and by leaves following airborne deposition. There are only minimal changes in Sr isotopes between associated trophic levels (Flockhart et al. 2015).
Bioavailability of radiostrontium in soil
The transfer of radionuclides from soils to plants is empirically quantified by the concentration ratio (or transfer factor) Fv, between the soil and the plant compartment of interest (IAEA 2009):
For Sr—as for other elements—these concentration ratios may vary by orders of magnitude, depending not only on the plant species but also on the specific soil characteristics and different experimental and environmental conditions (Frissel 1992).
The transfer of radiostrontium from contaminated soil to plants is a complex process. Before being intercepted by a plant root, Sr ions are subject to diverse exchange processes between different soil compartments rendering them un- or less available for plant root uptake. Furthermore, the fate of radiostrontium in pedosphere is closely linked to its ubiquitous and abundantly available chemical homologues—which is stable strontium in particular, calcium, and to a lesser extent magnesium as well (Bunde et al. 1997).
Entering in the soil—either through wet or dry deposition—radiostrontium is transported and distributed between different soil compartments via the soil solution. In this solution, Sr is generally transported as a free, hydrated divalent ion, or associated with small organic compounds. Strontium ions generally exhibit a comparably high affinity to organic matter (Valcke 1993; Guillén et al. 2015) and the binding of Sr to typical organic chelators was reported to significantly reduce transfer factors to plants (Van Bergeijk et al. 1992). At the same time, dissolved organic compounds have been found to significantly increase the amount of Sr in the mobile soil solution (Fedorkova et al. 2012). Sr is stronger associated with fulvic, than with humic acids, which can also partly explain its rather high mobility in soils (Lee and Lee 2000; Guillén et al. 2015). Sr2+ exhibits some affinity to cation exchange sites of clay minerals, which constitutes the main contributor for Sr sorption, for the majority of mineral soils in the temperate zone that are comparably poor in organic and sesquioxide content (Ehlken and Kirchne 2001). EXAFS studies by Fuller et al. (2016) revealed the chemical nature of the sorption on clay minerals to be an outer-shell complex of the hydrated Sr2+ ion to (regular) cation exchanger sites. On soils that have a high content of sesquioxides, sorption to these surfaces may also play a decisive role, and this effect seems to be enhanced by the presence of organic matter, through the formation of strong organic complexes at the sesquioxide surface (Chiang et al. 2010). On soils which contain (or are amended with) significant amounts of Ca-mineral phases, such as chalk/limestone, dolomite, or gypsum, incorporation or co-precipitation into these minerals may also play a role in its distribution (Robison et al. 2000; Gil-García et al. 2008). Sengupta et al. (2017) furthermore highlighted that Sr is also reversibly absorbed to calcite and aragonite phases. Al Attar et al. (2016) demonstrated that ageing of Sr, which would lead to an enhanced fixation of Sr on the soil does not significantly occur on pure mineral phases. Some studies reported that there are considerable ageing of radiostrontium in soils in the timeframe of several years (Eslava-Gomez and Brown 2013), while in other field-experiments, this effect was not observed (Noordijk et al. 1992; Corcho-Alvarado et al. 2016). Rigol et al. (1999) found a decrease in the available fraction of radiostrontium in podsols of the Chernobyl exclusion zone by 20–25% in the short term, but no further ageing was observed after 6 years of accident. It is not yet clear which process might mainly be responsible for the reported ageing effects. Strontium uptake is reported to be unaffected by depletion in the rhizosphere during plant growth by Casadesus et al. (2008).
The ratio of activity bound to the solid soil Am (Bq kg−1) is divided by the activity in the liquid soil solution Av (Bq L−1) is defined as the distribution coefficient KD:
KD values are widely used for the prediction of the mobility of Sr in soils. They also constitute a valuable first indicator of the potential for an interception of Sr by plants. However, mobility in the soil solution alone does not fully explain the potential for an interception of radionuclides by plant roots, because some of the mobile Sr may be strongly bound to colloidal or non-colloidal organic and inorganic complexes, while large parts of the uptake of minerals through root is dependent on the free metal ion activity (Zhao et al. 2016).
Furthermore, plants play an active, evolving, and dynamical role in the soil chemistry via the rhizosphere—a soil regime adjacent to the plant root that is predominantly controlled by the exudation and uptake of ions and organic agents by the plant root and microbiological symbionts. This exudation includes ions such as H+ and HCO3− as well as organic chelating agents that raise the mobility and availability of metals (Flora and Pachauri 2010). This complex competition between different and chemically diverse binding partners and the active role of plants in the uptake led to the development of the ‘bioavailability’ concept (Desmet et al. 1991). Bioavailability processes can be described as the ‘chemical ability to interact with the biological world, and they are quantifiable through the use of multiple tools’ (NRC 2003). Following ISO 17402, this tools can be divided into two categories, e.g. the bioavailability defined as a flux (in mol s−1 m−2) or in terms of content (in mol kg−1). Nowadays, for many non-radioactive heavy metal pollutants, flux-based approaches are in focus of scientific efforts, with modern tools for the measurement like DGT and DMT and comprehensive models, such as WHAM or NICA-Donnan model and Model VI being in use, a literature survey for strontium case reveals almost exclusively content-based approaches and yet little to no efforts to apply any of the above-mentioned methods in the case of strontium.
This may at the one hand partly be due to the fact that strontium—being of practically no concern as a stable element but only as a radioisotope—is being investigated only by rather small scientific community of radioecologists. On the other hand, when comparing different approaches for the assessment of bioavailability, Kim et al. (2015) concluded that a content-based approach using extraction techniques with neutral salt solutions is the best choice in the case of relatively highly mobile metals.
This concept of bioavailability considers those ions in soils that are readily available in soil liquid along with those ions that are easily desorbed from solid soil- and soil solution-binding partners—and can thus be ‘activated’ for uptake during plant growth—to constitute the ‘bioavailable fraction’. The strength of sorption that would distinguish between the bioavailable and the non-available fraction cannot strictly be defined in a physico-chemical way, due to the dynamical nature and active role of plants during uptake. Instead, it is commonly assessed by extracting soil with one or more specific extraction agents, which are assumed to have a comparable mobilising effect to that of the plant of interest. In general, typical extractants used include 1 M NH4OAc, 0.1 M NaNO3, and 0.01 M CaCl2 (Kennedy et al. 1997).
More in-depth information on the binding characteristics can be derived from sequential extraction procedures that would of times include water or solutions with a low ionic strength comparable to typical soil/water, such as 0.01 M CaCl2 as a first extraction step and 1 M NH4OAc as a second extraction step, to assess the amount of readily available and easily extracted analyte respectively (Comans et al. 1998).
In the case of Chernobyl soil, the inception of mobile radiostrontium into a soil system may additionally be delayed by fuel particle weathering and leaching, and in that case, a single or sequential extraction of only the (immediately) exchangeable fraction may lead to a strong underestimation of the mid-to-long-term amount of bioavailable radiostrontium (Oughton et al. 1993; Kashparov et al. 1999). In such cases, the use of sequential extraction patterns, including a strong extraction agent such as 6 M HCl (Comans et al. 1998) or 8 M HNO3 towards the end should be preferred (Askbrant et al. 1996). A more precise mathematical definition of bioavailability has been given by Tamponnet et al. (2008) as an outcome of the Bioavailability of Radionuclides in Soils (BORIS) program. Bioavailability (Btotal) is defined as ‘A quantity of the potential for radionuclides in bulk soil to be taken up by plants’ (Norden et al. 2005) and each chemical form of a radionuclide in soil compartment i with a relative abundance fi in this compartment is associated with a probability Bi of plant root interception from the given soil compartment. In this way, the total bioavailability can be defined as the weighted sum of the different partial bioavailabilities, ranging from 0 to 1.
While this model is theoretically well grounded, and both easily comprehensible and mathematically precise, it is somehow impractical because the needed input parameters (i.e. significant compartments in a given soil, distribution of the radionuclide in these compartments, probability of uptake from each compartment) are not easily and precisely obtained in the lab condition. In practice, they may be derived by (elaborate) sequential extraction procedures. Only if the contamination and soil are well enough characterised and comparable data already exist in the literature, the bioavailability can be estimated from literature data.
Many attempts have been made to break down the set of parameters governing Sr distribution to a minimum of pedological standard-parameters and derive a consistent, reliable, yet simple-enough model for the prediction of soil to plant transfer of contaminated sites (Gil-García et al. 2008). Proposed major parameters include exchangeable calcium (Prorok et al. 2014), exchangeable magnesium and cation exchange capacity (CEC) (Rauret and Firsakova 1996), electrical conductivity (Yasuda et al. 1995), and Ca distribution ratio (Ishikawa et al. 2008). It is in general agreement that the bioavailability of radiostrontium is completely governed by the availability of its abundantly available chemical homologues. The use of CEC and the addition of Ca and Mg concentrations in the soil solution (CaSS + MgSS) has proven to lead to rather reliable and easily obtainable predictions for Sr-KD (Gil-García et al. 2008) and was established as one method of choice, unique to the prediction of Sr-KD’s by the IAEA (IAEA-TECDOC-1616).
This distribution method gives a good first assumption of the amount of Sr that is available in any given soil, where CEC and Ca, as well as Mg-concentration, are known or can sufficiently will be assumed. Using CEC, this approach phenomenologically takes into account the impact of a whole range of parameters that have been reported to influence Sr availability and subsequent uptake, such as pH, clay content, and organic content and weighs it against the abundance of the main competitors of Sr in the environment.
Attempts have been made with some success to correlate KD values with transfer factors. Assuming a constant factor CF (concentration factor, conveyed as Bq kg−1 plant/Bq l−1 soil solution) for a given set of plants, Camps et al. (2004) showed that Sr concentration ratios may be in some cases well be predicted as follows:
Also, more complex models for the transfer of radionuclides, like the BioRUR model (Casadesus et al. 2008), are based on the KD value concept.
However, the main problem of the KD concept, as well as the derivation of transfer factors via concentration factors is that both concepts are rather static and do not consider the complexity of the biological system of a plant and the plant/soil interactions (Fig. 1). In addition, there is not one established standard method of measuring KD, which is why many of the literature data lack consistency. The use of KD values in predicting the bioavailability and subsequently the uptake would greatly profit from a standardisation and harmonisation of the process, a task that is being addressed by IAEA’s Modaria (IAEA 2017) project.
It seems though that, for the prediction of radionuclides transfer into plants, a modification of the original mobility-focused KD to a ‘bioavailable/exchangeable KD’ is advisable. Maskalchuk et al. (2015) implicitly targeted this issue by proposing the use of the exchangeable proportion αex that represents the fraction of 90Sr associated to the exchangeable fraction:
where [90Sr2+]ex represents the concentration of 90Sr in the exchangeable complex and [90Sr2+]s the total concentration of 90Sr in soil. Using some simplifications, they derived following prediction for the relocation of radiostrontium from soil to plant:
where Kc(90Sr2+/Ca2+) is the coefficient of exchange selectivity, a value that is generally considered to be close to 1 (Gil-García et al. 2008).Footnote 1 \( {\beta}_{{\mathrm{Ca}}^{2+}} \) represents the fraction of CEC, occupied by Ca2+. Now the fraction term of Eq. 8 represents the actual bioavailability that consists of parameters that can be quantified from standard lab experiments. The factor k in Eq. 8 represents the coefficient of proportion between the available and the uptake amount of radiostrontium. Thus, it reflects the physiologically regulated process of nutritional uptake of calcium together with Sr of a given plant. Maskalchuk et al. (2015) showed that the model is valid under confined experimental conditions where the plant physiology—and thus k—was held constant throughout an experiment in which they predicted and measured the effect of liming on an acidic podsolic soil, typical for the Chernobyl exclusion zone area.
However, plant physiology itself a complex process, which needs to be understood as well for a more precise prediction of radionuclide transfer to plants. Penrose et al. (2016) found considerable inter-cultivar variations of Sr transfer factors up to a factor of 23, which indicates that the k value itself is still largely variable, even when within one group of plants and in a confined experiment. Therefore, even under well-known and controlled pedological conditions, a best estimate of transfer factors might still be affected by an uncertainty at the range of an order of magnitude. As strontium is mainly reversibly sorbed to the solid soil it might potentially be completely extracted from a contaminated soil. However, the chemical homology to Ca (and Mg) and their high abundance in most soil means that a possible extraction through plants (phytoremediation) would need to exhibit significant uptake selectivity for Sr, as compared to Ca and Mg, because the selectivity of their environmental bioavailability in soil has shown to be close to 1.
Strontium uptake mechanism in plants
In the course of uptake of bioessential metals from soil by higher plants during normal growth, the reactions near the rhizosphere promote metal availability and ultimately uptake through roots. Metabolic processes continuously control the metals’ fate within plants and get it localised in reservoirs within the plants. Knowledge on detailed mechanisms for the uptake of particular metal and associated effects on plant growth and its environment are required. Many researchers have carried out studies regarding the mechanisms in plants which are responsible for uptake and translocation of Sr (Rediske and Selders 1953; Liu et al. 2016); however, our understanding of the effects on plants and the biogeochemical cycle of Sr is yet incomplete in terms of what other channels are responsible for Sr uptake via soil to plants. An overview of Sr uptake mechanisms is highlighted in Fig. 2. Uptake of Sr2+ occurs in three steps: (1) transport of metal from outer medium to the inside root—the xylem; (2) translocation within the root and/or from the root to shoot through the xylem; and (3) release of Sr2+ from the xylem vessels to the xylem-surrounding tissues (parenchymas) of the roots, stems, leaves, and flowers or fruits. The radial passage of Sr2+ within and out of xylem vessels can be governed by metabolic process (Russell and Squire 1958; Lauchli 1966). After reaching particular organs through transpiration, the mobility of Sr2+ is mostly inhibited (Rediske and Selders 1953; Isermann 1981). In an experiment, Oliver and Barber (1966) observed that the soybean root interception as pivotal mechanism for the supply of Sr2+ inside root in comparison through mass flow and diffusion way. They also noticed that the mobility of Sr2+ near roots was dependent on the percentage of soil used in soil-sand mixture and also suggest that the translocation of Sr2+ in soybean was inhibited in the presence of Ca2+. In homologous solution of Sr2+, in the presence of calcium, the uptake capability of Sr2+ by plants was parallel to the concentration of radiostrontium over an extensive concentration range (10−6–10−1 meq L−1), which reflects an inactive mode of uptake (Russell and Squire 1958). The radioactive ions contaminate plants mainly through uptake by roots from soil/water but also due to foliar deposition, the latter of which is more significant during fallout circumstances (Scotti and Carini 2000).
In plant roots, the uptake of Sr2+ is believed to occur through Ca2+ channels due to their chemical analogy (White et al. 2002). However, very few studies have specifically targeted Sr2+ uptake, and assumption that Sr2+ is taken up through the same corridors that account for Ca2+ that is based only on few data. Heuck et al. (2010) conducted an experiment on yeast cells (Saccharomyces cerevisiae) at genome level to check Ca2+ and Sr2+ uptake in cells and concluded that a cell does not discriminate between Ca2+ and Sr2+ under experimental conditions. Initially, Handley and Overstreet (1962) determined the absorption features of vacuolated and non-vacuolated sections of the primary roots of Zea mays L. in the presence of Sr2+ and they found out that the Sr2+ transport in the meristematic cells of the root tip was due to a non-metabolic process while in vacuolated tissue it was metabolism dependent. They also suggested that the presence of a obstruction, possibly lying closer to the cell surface which usually limits the non-metabolic uptake of Sr. On some occasions, discharge of H+ and HCO3− basically at rhizosphere, vigorously influence the pH in their direct neighbourhood of the plants, which may increase the availability of phosphorus and potassium to the plants (Gupta et al. 2016).
Distribution of radiostrontium between plant parts (experimental view)
Ding et al. (2016) studied the uptake mechanism of Sr in order to predict food safety in case of a radiostrontium contamination. In their experiment, stable 88Sr was utilised and introduced in Chinese cabbage and spinach grown in pots, in greenhouse under different range of Sr concentrations for 30 days. Results showed that both plants have different characteristics of uptake of Sr in leaves, stems, and in roots. Strontium concentrations in both plants were initially related to sodium and sulphur and later to calcium and magnesium concentrations. In another experiment, Kosior et al. (2016) give different levels of Sr treatment from 0.5 to 3.0 mM in hydroponic medium to Glycine max plants for 14 days to check phytoestrogens contents. From the results, it was concluded that introducing Sr ions to the culture media may be used to functionalise soybean plants with increased phytoestrogen content.
Zheng et al. (2016) utilised Tillandsia usneoides, also well-known as Spanish moss, and exposed it to different Sr concentrations (0.1–100 mmol L−1) and noticed that T. usneoides can sustain Sr stress for long time. The highest uptake ratio of Sr of about 82.21 ± 0.12% was observed after the plants were grown at 0.1 mmol L−1 Sr solutions. The Sr contents in the plant augmented linearly with applied concentration in medium. Hoeck et al. (2015) carried out dose-dependent experiment, to check interactions of β-radiation on Lemna minor plants exposed to different radiostrontium concentrations from 25 to 25,000 kBq L−1. Strontium accumulation was more in frond than roots ranging from 118.7 to 225.3 mg and in roots 5.7 to 38.8 mg; however, they observed that uptake in plants reached a maximum after 3 days exposure. Similar pattern of Sr uptake in large-flowered waterweed and also in hydroponically grown maize and in sunflower was observed (Soudek et al. 2006; Anamika et al. 2009). Lee and Sosulski (1965) established from their experiment that different varieties of barley plants had the lowest concentrations of Sr in the tissues of the leaf, stem, chaff, and seeds. Nishita et al. (1961) reported that the concentration of 90Sr was highest in the older leaves of red clovers through their experiment at Nevada test site. While in another experiment, Neel et al. (1953) reported that approx. one tenth of the total Sr was accumulated in the seeds of tested plants. The experiments of Sowa et al. (2014) revealed that soy has a distinct propensity concerning accumulation of Sr2+ in shoots, its content ranged from 87.4 to 90.4% of the total amount of the ions in the plants. Hoseini et al. (2012) reported percentage of Sr absorption in Cannabis sativa was found to be 45% in the roots, 40% in the stem, and the minimum absorption was found in the leaves (15%) respectively.
Genes responsible for strontium uptake
Strontium accumulation through calcium channels has already been acknowledged by other studies (White et al. 2003), and yet no significant role in cellular function has been identified. The existence of progressive correlation between Sr2+ and other divalent ions probably depends on the shared non-selective cation channels, comprising Ca2+-ATPase, ZIP, and YSL family of transport proteins, in xylem transport. However, still a number of studies are required for analysis at molecular level, what exact mechanism is exerting, which genes are responsible for accumulation inside plant biomass. Kanter et al. (2010) detected some of the genes responsible for Sr accumulation by QTL analysis. QTL SrFW1.1 incorporated the locus for CAX11, a cation/H+ antiporter. SrFW1.2 and SrDW1.2 harboured the locus of the alleged plasma membrane glutamate receptor channel GLR3.3 (Qi et al. 2006), SrFW2.1 comprised three loci for CNGCs (CNGC3, CNGC11, CNGC12) (Gobert et al. 2006). The locus for CAX3, an H+/Ca2+ antiporter, was found within the QTL SrFW3.1 (Cheng et al. 2005). SrFW4.1 exhibited the loci for two further CNGCs (CNGC9 and CNGC17) as well as for GLR3.2. Within SrDW5.1, the locus of another glutamate receptor channel GLR2.1 was found, playing a role in Ca2+ homeostasis (White 1998; White et al. 2002). QTL SrFW5.2 comprised CNGC1, which is recognised to participate in Ca2+ transport (Ali et al. 2006). Various authors suggested QTL analyses as a significant tool to ascertain the genetic factors underlying differences in ion accumulation within the closely related entities.
Regulation of strontium in plants
Strontium ions are not essential for plants, and they may be taken up by plants since they share the same transporting protein or channels or contend for the similar binding sites in the cell wall, with some vital elements (Chu et al. 2015). In plants, different parts exert differential intake capacity of Sr. Strontium content mostly increases in the upper part of the plants with respect to increase in soil/water matrix, but not in each case, after some point, it was shown to decrease (Isermann 1981). Glycine max (L.) Merr. grown at a level of 1.5 mM, Sr seemed to be a non-toxic and even stimulated plant growth. It has been described that Sr considerably affects the content of plant pigments depending on its quantity and time of introduction (Moyen and Roblin 2010; Sowa et al. 2014). Sowa et al. (2014) observed variations in chlorophyll a, b, and carotene content after 24 days of cultivation and the total sum of plant pigments reduced at 0.5 mM concentration of Sr, in contrast to control plants. In general, plants have different complex synchronised mechanism for metal tolerance which comprises both biochemical and physiological routes (Gupta and Sandalio 2012). Usually, plants have two mechanisms to cope with the toxic effects of radionuclides, first avoidance and second tolerance under stress (Chatterjee et al. 2017).
Interaction with calcium and other ions
Strontium exhibits a chemical resemblance with the vital plant macronutrient Ca and is taken up by plants in similar ways to Ca (Kabata-Pendias and Pendias 1989). Whereas Ca has an essential physiological function, Sr is not identified to have any role and was believed to be passively taken up and replaced for Ca (Miller et al. 1993). In terrestrial plants, uptake of Ca befalls mainly via Ca channels (White and Broadley 2003); calcium ATPases are also existing in the roots and catalyse the Ca influx and efflux through plasma membrane of endodermal cells and then transported to the shoots via the xylem where the surplus of Ca is stored in vacuoles to keep Ca concentration in the cytosol at a basal level. Also, kinetic studies of both ions approve the resemblance in uptake competences in plants (Moyen and Roblin 2010; Broadley and White 2012). White and Broadley (2003) reported that dicot and monocot plants have different Ca concentrations in different plant parts, i.e. dicot plants have more Ca in the shoots than monocots. It is also reported that phylogenetic variations in Ca both in the root/shoot can be used for the prediction of Ca movement in plants and also chemically similar elements like Sr and Mg (White and Broadley 2003). Beyond its toxic limit, Ca is reported to form calcium oxalate crystals in plants; however it was found that Sr also incorporated in Ca oxalate crystals when it enters inside plants instead of Ca in L. minor (Mazen et al. 2004). It is also possible that 90Sr, upon accumulation in plants, can consequently deliver a significant β-radiation dose to the plant organs. Therefore, a large quantity of studies were carried out to comprehend facts about connections between Ca and Sr in the soil-plant system (Camps et al. 2004; Broadley and White 2012; Guillaume et al. 2012). It has been described that the collaboration between Ca and Sr concentration in shoots was stable with reduced transport selectivity due to a larger influence of apoplastic movement of these elements in plants (White and Broadley 2000). Conversely, if the countermeasures to decrease Sr uptake are considered on the basis of straight proportional correlation between Ca and Sr, the intake of Ca by humans may be conceded in varieties with low Sr accumulation (Chu et al. 2015). Many investigators have exemplified a positive correlation between radiostrontium and Ca concentrations through studies on different plant varieties such as wheat, maize, and soybean (Malikov et al. 1981; James et al. 1995; Ariyama et al. 2006). The relationship between Sr and Ca is probable to be a constraint for exploiting varietal distinction as a basis for the remediation of Sr-polluted land as the concentration of Ca. Additionally, if intake of Ca by animals (including humans) is lowered, the amount of ingested Sr will be absorbed higher (Beresford et al. 2006).
Plant applications
Phytoremediator species
Phytoremediation is a prominent technique to battle environmental pollution with chemical compounds and radionuclides (Gupta 2013). Phytoremediation (involving both plants and associated microbes) is not only a potentially effective method; it allows the removal of pollutants with little detrimental environmental impact and at low cost. It is most effective at locations (involving both soil and water bodies) with a low or moderate level of contamination (Gupta 2013). In the course of phytoextraction, plants eliminate metals (Salt et al. 1995; Macek et al. 2011) and a variety of organic pollutants (Pilon-Smits 2005) from the environment. Phytoextraction of heavy metals from relies on plants that take up pollutants and accumulate them at elevated levels in the shoots or in the upper part of the plants. Metal hyperaccumulating plants not only take up higher-than-normal amounts of (heavy) metals from soil but also concentrate those pollutants in the shoots. As threshold value, 1% of the shoot dry mass basis has been defined (Qi et al. 2015). Recently, crops have been proposed as an alternative option to clean polluted soils. In the meantime, high biomass production and rapid growth may compensate for moderate accumulation characteristics (Qi et al. 2015; Ibeanusi et al. 2004; Hernandez-Allica et al. 2008). To date, Sr uptake and distribution in different plants was studied in rice, wheat, and sunflower, but few researches on oats (Tsukada et al. 2005; Soudek et al. 2006; Schimmack et al. 2007). Table 1 depicts some of the plants used for phytoremediation of Sr. Krouglov et al. (1997) did comparative studies and reported oats as better accumulators than wheat and rye. Since their roles as important agricultural crops, barley (Hordeum vulgare) and common wheat (Triticum aestivum) were the utmost studied species (Gerstmann and Schimmack 2006; Kanter et al. 2010). The study of Qi et al. (2015) was to examine the accumulation and dissemination of Sr in 26 cultivars of wheat (Triticum aestivum L.), husk oat (Avena sativa L.), naked oat (Avena nuda), and barley (Hordeum vulgare L.) for their prospective use in phytoremediation. From the above studies, Sr levels had no effect on the accumulation in shoot biomass at tillering or at maturity, which means that shoot Sr concentration of naked oat and barley at tillering was significantly (P < 0.05) higher than that of wheat; Neimengkeyimai-1, a naked oat cultivar, had the highest Sr concentrations and projected as model for auxiliary research to find more effective cultivars; and naked oat plants as effective candidate for phytoremediation to clean up polluted soil. A comparatively high ability of soybeans to accumulate strontium ions as well as non-appearance of toxicity or of undesirable effects up to 2.0 mM Sr concentrations on growth and physiological parameters were testified. The toxicity analysis for Cs+ and Sr2+ exhibited widely used Arabidopsis thaliana accession Ler-1 tolerated Sr2+ concentrations up to 1 mM without any observable symptoms (Kanter et al. 2010). For Sr2+, the genetic distinction for enrichment was monitored as well, within different wheat varieties; a 1.6–2.6-fold variation in Sr2+ concentration was identified (Gerstmann and Schimmack 2006). To date, no comparable data about Sr2+ concentration are available for A. thaliana.
Factors influencing strontium phytoremediation efficiency (biotic/abiotic)
In addition to differences in accumulation of Sr and Cs between species, exploiting within species (inter-varietal) variation in uptake has also been projected as a probable remediation approach to produce less-contaminated crops (White and Broadley 2000; White et al. 2003; IAEA 2012). Penrose et al. (2015) delivered the first comprehensive study analysis of inter-varietal variation (R) on Cs and Sr accumulation by plants. These data proposed that developing inter-varietal variation could decrease transfer of Cs and Sr in an analogous range, to the existing remediation strategies. Therefore, varietal assortment could be an effective economically and socially acceptable remediation method, which could be used in amalgamation with existing techniques such as fertilisation and ploughing. In some cases, analyses of the accessible data suggest that low Sr accumulating varieties also have lower absorption of Ca.
The parameter to analyse the uptake efficiency of radioactive compounds through roots in studied plant was by transfer factors (Fv) which are defined as the activity concentration ratio of the radionuclide per unit dry mass of the plant (Bq kg−1) to that in soil (Bq kg−1) (IAEA 2010; see Heading 2). In lettuce plants, through the germination stage in the presence of Sr2+, root mass propagates together with migrating Sr through soil layers. Sr2+ ions do not form strong complexes with the matrix of the soil due to the lack of organic materials that can act as ligands. Sr ions thus become available for root uptake in depths of 2–20 cm of soil throughout the crop development period (Choi et al. 1998; Forsberg et al. 2001; Al Attar et al. 2016). In any case, when the contamination occurs at leaf developmental stage (S2), crop exhibits its highest physiological activity, the transfer factors of 90Sr decrease by > 40% (GM values dropped from 1.20 to 0.70) compared to germination stage (S1). This may be caused by the dilution of the radionuclides in the increased plant biomass. Similar trends were observed by Choi et al. (1998) when exploring Fv of 85Sr (a gamma-emitting radionuclides of Sr) and 137Cs during four growing stages of Chinese cabbage and radish top, despite that their experiment was conducted in sandy soil matrix. In another study (Choi et al. 2011), transfer factors of radioactive Cs and Sr to soybean leafs during six growing stages exhibited similar patterns; 85Sr decreased, while 137Cs fluctuated, though to a lesser extent (Al Attar et al. 2016). In the case of straw, a slight increase of the transfer factors was observed for 90Sr at the tillering stage (S2) compared to the preceding stage, leaf development (S1). Transfer factors of 137Cs and 90Sr were investigated for contamination at four growing stages for lettuce and winter wheat (Al Attar et al. 2016). The results indicated that soil contaminated with 90Sr at early stages resulted in highest Fv for both crops, that being of potential radiological concern. Radiocesium deposited at the second plant growth stage resulted in higher Fv for lettuce and wheat grains (i.e. at leaf development for lettuce and tillering for wheat grains). However, contamination at the latest stage of plant growth of wheat straw (ripening, S4) resulted in the highest Fv. Several factors are responsible for these observations: (a) physiological activity of the plant at the time of deposition, (b) availability of radionuclide species to root uptake, and (c) its mobility through plant’s body (Al Attar et al. 2016). The extent of accumulation of these radionuclides by plants differs widely depending on physical and chemical properties of the soil (Tarsitano et al. 2011) and the plant species (IAEA 2012).
Biosorption of strontium
Mushrooms have great potential for uptake and bioaccumulation of heavy metals and radionuclides (Chatterjee et al. 2017). Mushrooms collected from Yunnan in south-western China in 2012 showed 90Sr activity concentration around 10 Bq kg−1 dry biomass, which was greater when compared to other mushrooms studies. The King Bolete Boletus edulis from China showed 90Sr activity in the caps of around 1.5 Bq kg−1 dry biomass (Saniewski et al. 2016). Concentration of 90Sr in Bay Bolete Royoporus badius (Xerocomus or Boletus) from the affected region (Chernobyl Power Plant blast) of Gomel in Belarus was about 2.1 Bq kg−1 dry biomass in 2010. The stable Sr isotopes (84Sr, 86Sr, 87Sr, and 88Sr) are weakly bioconcentrated by fungi in fruiting bodies and the value of bioconcentration (transfer) factor (BCF) for these element in caps of fruiting bodies is normally below 1, but could be above 1 for stipes (e.g. in Amanita muscaria) (Kojta et al. 2011). Guillen et al. (2009) reported on a weak transfer of 210Pb from soil substrate to mushroom and more or less alike in behaviour to this nuclide was 90Sr, while the amount of sequestered 210Pb in fruiting bodies was related to bioconcentration potential by given species of mushroom. Water-soluble radiostrontium (90Sr) was efficiently removed in carbonate form through micro-algal photosynthetic processes. The immobilisation of soluble 90Sr and production of highly perceptible radio-strontianite (90SrCO3) biomineral are achieved by using Chlorella vulgaris, and the biologically induced mineralisation drastically decreased 90Sr radioactivity in water to make the highest 90Sr removal ever reported (Lee et al. 2014); due to relatively high biological availability of 90Sr, they are transferred quickly into biotic systems and may enter in the food chain. An approach to study Sr2+ accumulation in the model system S. cerevisiae was also introduced, according to the candidates identified; strong evidence was given that the vacuole plays an important role in normal accumulation of Cs+ and Sr2+ in the cell (Heuck et al. 2010). Anupama et al. (2016) explored Sr uptake and subcellular compartmentation in the model filamentous fungus Neurospora crassa. Strontium uptake by the mycelium was found to be a function of time concerning three compartments corresponding in series to cell wall (52%), cytoplasm (19%), and vacuole (25%), when mycelia were exposed to a 10 mM Sr2+ concentration. At 4 °C, and in alkaline solution, only ~ 50% of the Sr2+ taken up by the mycelia was recuperated in cell wall-bound fraction, suggesting that it is metabolically mediated process. Inhibitors of the IP3 pathway and the CaM pathway inhibited Sr2+ accumulation in N. crassa mycelia by up to 88%. Strontium uptake into cytoplasm was through the Ca2+ transporters. Binding of Sr2+ onto cell walls, accumulation into the cells and displacement of calcium by Sr reveals the ability of the organism to substantiate Ca2+ by Sr2+. These data represents a first step concerning the understanding of the role of Sr2+ as a substitute in place of Ca2+ in fungi.
Conclusion and future perspective
Strontium is not an essential element for organisms and when it enters inside any organism, it employs toxicity to them at higher levels. In plants, as well as in soil, Sr pathways are primarily determined by their structural similarity with Ca. The literature on bioavailability in soil reveals that an almost complete uptake of radiostrontium from contaminated sites would be potentially possible, because of the reversible nature of the Sr binding in soil. However, a complete uptake is strongly hindered by the overabundance and omnipresence of calcium in soil. This leads to the need of plant species for phytoremediation that strongly discriminate between Ca vs. Sr uptake. Plants play an important role in cleaning aquatic and terrestrial habitats that are contaminated with heavy metals and/or radionuclides. Nowadays, numerous remediation tools are in existence (Gupta and Voronina 2018) but phytoremediation is an environmentally friendly and inexpensive method for the removal of pollutants from the environment. Plenty of scientific studies suggest that phytoremediation is an effective, economic, multipurpose, and ‘green’ way of remediate contaminated sites. This technique also has some downsides, in particular the rather high time demand for the removal of pollutants compared to chemical treatment or removal methods. In the nuclear application, phytoremediation is only applicable to low activities only. It also produces a large amount of contaminated phytomass; disposal of which may still represent a problem. With this, it also increases the possibility of toxin entry to the food chain. Yet, it is a powerful option for remediation because of its eco-friendliness and cost-effectiveness. Further detailed study needs to be done at genetic level to understand the mechanism how Sr uptake and fate inside plants and also feasibility of phytoremediation technique for Sr remediation in the case of any nuclear power plant decommissioning or leak.
Notes
Gil-Garcia et al. (2008) actually expand this approximation to both contributing homologues of Sr: \( K\mathrm{c}\left(\frac{\mathrm{Sr}}{\mathrm{Ca}-\mathrm{Mg}}\right)\approx 1 \).
References
Al Attar L, Al-Oudat M, Safia B, Abdul Ghani B (2016) Ageing impact on the transfer factor of 137Cs and 90Sr to lettuce and winter wheat. J Environ Radioact 164:19–25
Ali R, Zielinski RE, Berkowitz GA (2006) Expression of plant cyclic nucleotide-gated cation channels in yeast. J Exp Bot 57:125–138
Amano H, Sakamoto H, Shiga N, Suzuki K (2016) Method for rapid screening analysis of Sr-90 in edible plant samples collected near Fukushima, Japan. Appl Radiat Isot 112:131–135
Anamika S, Eapen S, Fulekar MH (2009) Phytoremediation technology for remediation of radiostrontium (90Sr) and radiocesium (137Cs) by Catharanthus roseus (L.) G. Don in aquatic environment. Environ Eng Manag J 8:527–532
Anupama M, Ashok KK, Latha JNL (2016) Mechanism of strontium uptake and transport in Neurospora crassa. Eur J Pharmaceut Med Res 3:379–386
Ariyama K, Nishida T, Noda T, Kadokura M, Yasui A (2006) Effects of fertilization, crop year, variety, and provenance factors on mineral concentrations in onions. J Agric Food Chem 54:3341–3350
Askbrant S, Melin J, Sandalls J, Rauret G, Vallejo R, Hinton T, Cremer A, Vandecastelle C, Lewyckyj N, Ivanov YA, Firsakova SK, Arkhipov NP, Alexakhin RM (1996) Mobility of radionuclides in undisturbed and cultivated soils in Ukraine, Belarus and Russia six years after the Chernobyl fallout. J Environ Radioact 31:287–312
Beresford NA, Barnett CL, Howard BJ, Rantavaara A, Rissanen K, Reales N, Gallay F, Papachristodoulou C, Ioannides K, Nisbet AF, Brown J, Hesketh N, Hammond D, Oatway W, Oughton D, Bay I, Smith JT (2006) Compendium of countermeasures for the management of food production systems, drinking waters and forests. EURANOS Version 1.3
Broadley MR, White PJ (2012) Some elements are more equal than others: soil-to plant transfer of radiocaesium and radiostrontium, revisited. Plant Soil 355:23–27
Bunde RL, Rosentreter JJ, Liszewski MJ, Hemming CH, Welhan J (1997) Effects of calcium and magnesium on strontium distribution coefficients. Environ Geol 32:219–229
Camps M, Rigol A, Hillier S, Vidal M, Rauret G (2004) Quantitative assessment of the effects of agricultural practices designed to reduce Cs-137 and Sr-90 soil-plant transfer in meadows. Sci Total Environ 332:23–38
Casadesus J, Sauras-Yera T, Vallejo VR (2008) Predicting soil-to-plant transfer of radionuclides with a mechanistic model. J Environ Radioact 99:864–871
Chatterjee S, Deb U, Datta S, Walther C, Gupta DK (2017) Common explosive (TNT, RDX, HMX) and their fate in the environment: emphasizing bioremediation. Chemosphere 184:438–451
Chatterjee S, Sarma MK, Deb U, Steinhauser G, Walther C, Gupta DK (2017) Mushrooms: from nutrition to mycoremediation. Environ Sci Pollut Res 24:19480–19493
Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138:2048–2060
Chiang PN, Wang MK, Huang PM, Wang JJ, Chiu CY (2010) Cesium and strontium sorption by selected tropical and subtropical soils around nuclear facilities. J Environ Radioact 101:472–481
Choi YH, Lee CW, Kim SR, Lee JH, Jo SJ (1998) Effect of application time of radionuclides on their root uptake by Chinese cabbage and radish. J Environ Radioact 39:183–198
Choi YH, Lim KM, Jun I, Keum DK, Han MH (2011) Time-dependent transfer of 54Mn, 60Co, 85Sr and 137Cs from a sandy soil to soybean plants. Nucl Sci Technol 1:392–395
Chu Q, Watanabe T, Sha Z, Osaki M, Shinano T (2015) Interactions between Cs, Sr, and other nutrients and trace element accumulation in Amaranthus shoot in response to variety effect. Agric Food Chem 63:2355–2363
Comans RNJ, Hilton J, Voitsekhovitch O, Laptev G, Popov V, Madruga MJ, Bulgakov A, Smith JT, Movchan N, Konoplev A (1998) A comparative study of radiocesium mobility measurements in soils and sediments from the catchment of a small upland oligotrophic lake (Devoke Water, U.K.). Water Res 32:2846–2855
Corcho-Alvarado JA, Balsiger B, Sahli H, Astner M, Byrde F, Röllin S, Holzer R, Mosimann N, Wüthrich S, Jakob A, Burger M (2016) Long-term behavior of 90Sr and 137Cs in the environment: case studies in Switzerland. J Environ Radioact 160(Supp C):54–63
Crystalmaker (2015) Elements, atomic radii and the periodic radii. http://www.crystalmaker.com/support/tutorials/crystalmaker/atomic-radii/index.html. Accessed 11 Sept 2015
Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, Tsouderos Y, Delmas PD, Christiansen C (2001) Incorporation and distribution of strontium in bone. Bone 28:446–453
Desmet GM, van Loon LR, Howard BJ (1991) Chemical speciation and bioavailability in the environment and their relevance to radioecology. Sci Total Environ 100:105–124
Ding K, Liu S, He Y, Yan D, Zhang F, Wang S, Guo J, Zhang W, Wang X, Jiang X (2016) Simulating the transfer of Strontium-90 from soil to leafy vegetables by using Strontium-88. Water Air Soil Pollut 227:414
Ehlken S, Kirchne K (2001) Environmental processes affecting plant root uptake of radioactive trace elements and variability of transfer factor data: a review. J Environ Radioact 58:97–112
Fedorkova MV, Pakhnenko EP, Sanzharova NI (2012) Chemical forms of radioactivestrontium interaction with organic matter of different soil types. Moscow Univ. Soil Sci Bull 67:133–136
Flockhart DTT, Kyser TK, Chipley D, Miller NG, Norris DR (2015) Experimental evidence shows no fractionation of strontium isotopes (87Sr/86Sr) among soil, plants, and herbivores: implications for tracking wildlife and forensic science. Isot Environ Health Stud 51:372–381
Flora SJS, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7:2745–2788
Forsberg S, Rosen K, Brechignac F (2001) Chemical availability of 137Cs and 90Sr in undisturbed lysimeter soils maintained under controlled and close-to-real conditions. J Environ Radioact 54:253–265
Frissel MJ (1992) An update of the recommended soil-to-plant transfer factors of Sr-90, Cs-137 and transuranics. In: International Union of Radioecologists. VIIIth report of the working group soil-to-plant transfer factors. pp 16–25
Fuller AJ, Shaw S, Peacock CL, Trivedi D, Burke IT (2016) EXAFS study of Sr sorption to illite, goethite, chlorite, and mixed sediment under hyperalkaline conditions. Langmuir 32:2937–2946
Gerstmann U, Schimmack W (2006) Soil-to-grain transfer of fallout 90Sr for 28 winter wheat cultivars. Radiat Environ Biophys 45:187–194
Gil-García CJ, Rigol A, Rauret G, Vidal M (2007) Radionuclide sorption–desorption pattern in soils from Spain. Appl Radiat Isot 66:126–138
Gil-García CJ, Rigol A, Vidal M (2008) New best estimates for radionuclide solid–liquid distribution coefficients in soils, part 1: radiostrontium and radiocaesium. J Environ Radioact 100:690–696
Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJ (2006) Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a nonselective ion transporter involved in germination and cation transport. J Exp Bot 57:791–800
Gomez AE, Brown J (2013) Determination of root uptake to vegetables grown in soil contaminated for twenty-five years. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/337136/HPA-CRCE-047_for_website.pdf. Accessed 27 Oct 2017
Guillaume T, Chawla F, Steinmann P, Gobat JM, Froidevaux P (2012) Disparity in 90Sr and 137Cs uptake in Alpine plants: phytogenetic effect and Ca and K availability. Plant Soil 355:29–39
Guillén J, Baeza A, Corbacho JA, Munoz-Munoz JG (2015) Migration of 137Cs, 90Sr, and 239+240Pu in Mediterranean forests: influence of bioavailability and association with organic acids in soil. J Environ Radioact 144(Supp. C):96–102
Guillen J, Baeza A, Ontalba MA, Míguez MP (2009) 210Pb and stable lead content in fungi: its transfer from soil. Sci Total Environ 407:4320–4326
Gupta DK (2013) Plant based remediation process. Springer, Germany
Gupta DK, Chatterjee S, Datta S, Voronina AV, Walther C (2016) Radionuclides: accumulation and transport in plants. Rev Environ Contam Toxicol 241:139–160
Gupta DK, Deb U, Walther C, Chatterjee S (2018) Strontium in the ecosystem: transfer in plants via root system. In: Gupta DK, Walther C (eds) Behaviour of strontium in plants and the environment. Springer, Germany
Gupta DK, Sandalio LM (2012) Metal toxicity in plants: perception, signaling and remediation. Springer, Germany
Gupta DK, Voronina A (2018) Remediation measures for radioactively contaminated areas. Springer, USA
Gupta DK, Walther C (2018) Behaviour of strontium in plants and the environment. Springer, USA
Handley R, Overstreet R (1962) Uptake of strontium by root of Zea mays. Plant Physiol 38:180–184
Hernandez-Allica J, Becerril JM, Garbisu C (2008) Assessment of the phytoextraction potential of high biomass crop plants. Environ Pollut 152:32–40
Heuck S, Gerstmann UC, Michalke B, Kanter U (2010) Genome-wide analysis of caesium and strontium accumulation in Saccharomyces cerevisiae. Yeast 27:817–835
Hoeck AV, Horemans N, Hees MV, Nauts R, Knapen D, Vandenhove H, Blust R (2015) β-Radiation stress responses on growth and antioxidative defense system in plants: a study with strontium-90 in Lemna minor. Int J Mol Sci 16:15309–15327
Hoseini PS, Poursafa P, Moattar F, Amin MM, Rezaei AH (2012) Ability of phytoremediation for absorption of strontium and cesium from soils using Cannabis sativa. Int J Environ Health Eng 1:1–5
IAEA (2009) Quantification of radionuclide transfer in terrestrial and freshwater environments for radiological assessments. TECTOC-1616. IAEA, Vienna
IAEA (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments. Technical Report Series No. 472. IAEA, Vienna
IAEA (2012) Guidelines for remediation strategies to reduce the radiological consequences of environmental contamination. Technical Report Series No.475. IAEA, Vienna
IAEA (2017) MODARIA II. Modelling and data for radiological impact assessments. http://www-ns.iaea.org/projects/modaria/default.asp?s=8&l=129. Accessed 24 Nov 2017
Ibeanusi VM, Grab DA, Jensen L, Ostrodka S (2004) Radionuclide biological remediation resource guide. U.S. Environmental Protection Agency. EPA 905-B-04-001
Isermann K (1981) Uptake of stable strontium by plants and effects on plant growth. In: Skoryna SC (ed) Handbook of stable strontium. Plenum Press, New York, pp 65–86
Ishikawa NK, Uchida S, Tagami K (2008) Estimation of soil-soil solution distribution coefficient of radiostrontium using soil properties. Appl Radiat Isot 67:319–323
James DW, Hurst CJ, Tindall TA (1995) Alfalfa cultivar response to phosphorus and potassium deficiency: elemental composition of the herbage. J Plant Nutr 18:2447–2464
Kabata-Pendias A, Pendias H (1989) Trace elements in soils and plants. CRC, Boca Raton
Kanter U, Hauser A, Michalke B, Draxl S, Schaffner AR (2010) Caesium and strontium accumulation in shoots of Arabidopsis thaliana: genetic and physiological aspects. J Exp Bot 61:3995–4009
Kashparov VA, Ougthon DH, Zvarich SI, Protsak VP, Levchuk SE (1999) Kinetics of fuel particle weathering and 90Sr mobility in the Chernobyl 30-km exclusion zone. Health Phys 76:251–259
Kennedy VH, Sanchez AL, Oughton DH, Rowland AP (1997) Use of single and sequential chemical extractants to assess radionuclide and heavy metal availability from soils for root uptake. Analyst 122:89R–100R
Kim RY, Yoon JK, Kim TS, Yang J, Owens G, Kim KR (2015) Bioavailability of heavy metals in soils: definitions and practical implementation—a critical review. Environ Geochem Health 37:1041–1061
Kojta AK, Gucia M, Jarzyńska G, Lewandowska M, Zakrzewska A, Falandysz J, Zhang D (2011) Phosphorous and metallic elements in parasol mushroom (Macrolepiota procera) and soil from the Augustowska Forest and Ełk regions in North-Eastern Poland. Fresenius Environ Bull 20:3044–3052
Kosior MW, Sowa I, Blicharski T, Strzemski M, Dresler S, Szymczak G, Wnorowski A, Kocjan R, Swieboda R (2016) The stimulatory effect of strontium ions on phytoestrogens content in Glycine max (L.) Merr. Molecules 21:90
Krouglov SV, Filipas AS, Alexakhin RM, Arkhipov NP (1997) Long-term study on the transfer of 133Cs and 90Sr from Chernobyl-contaminated soils to grain crops. J Environ Radioact 34:267–286
Lauchli A (1966) lonentransport durch Wurzeln intakter Keimpflanzen von Zea mays L. Ber Schweiz Bot Ges 75:5–19
Lee SY, Jung KH, Lee JE, Lee KA, Lee SH, Lee JY, Lee JK, Jeong JT, Lee SY (2014) Photosynthetic biomineralization of radioactive Sr via microalgal CO2 absorption. Bioresour Technol 172:449–452
Lee MH, Lee CW (2000) Association of fallout-derived 137Cs, 90Sr and 239,240Pu with natural organic substances in soils. J Environ Radioact 47:253–262
Lee CC, Sosulski FW (1965) Uptake of Sr-85 by cereal crops and varieties. Can J Plant Sci 45:13–17
Li G, Hu N, Ding D, Zheng Y, Liu Y, Wang Y, Nie X (2011) Screening of plant species for phytoremediation of uranium, thorium, barium, nickel, strontium and lead contaminated soils from a uranium mill tailing repository in South China. Bull Environ Contam Toxicol 86:646–652
Liu HC, Chung CH, You CF, Chiang YH (2016) Determination of 87Sr/86Sr and δ88/86Sr ratios in plant materials using MC-ICP-MS. Anal Bioanal Chem 408:387–397
Macek T, Francova K, Kochankova L, Lovecka P, Ryslava E, Rezek J, Mackova M (2011) Phytoremediation-biological cleaning of a polluted environment. Rev Environ Health 19:63–82
Malikov VG, Perepeliatnikova LV, Djukov BI (1981) Species and variety differences of plants in 137Cs and 90Sr accumulation from soil. Agrochem 8:94–100
Maskalchuk L, Baklay A, Leontieva T (2015) Modeling of radiostrontium migration in “mineral soil-plant” system in the performance of liming. J Chem Eng Chem Res 2:521–528
Mazen AMA, Zhang DZ, Franceschi VR (2004) Calcium oxalate formation in Lemna minor: physiological and ultrastructural aspects of high capacity calcium sequestration. New Phytol 161:435–448
Miller EK, Blum JD, Friedland AJ (1993) Determination of soil exchangeable cation loss and weathering rates using Sr isotopes. Nature 362:438–441
Moyen C, Roblin G (2010) Uptake and translocation of strontium in hydroponically grown maize plants, and subsequent effects on tissue ion content, growth and chlorophyll a/b ratio: comparison with Ca effects. Environ Exp Bot 68:247–257
National Research Council of the National Academies (NRS, 2003) Bioavailability of contaminants in soils and sediments: processes, tools and applications. The National Academies Press. ISBN 978-0-309-08625-7. doi:https://doi.org/10.17226/10523
Neel JW, Olafson JH, Steen AJ, Gillooly BE, Nishita H, Larson KH (1953) Soil-plant interrelationships with respect to the uptake of fission products. I. The uptake of 90Sr, 137Cs, 106 Ru, 144Ce, and 91Y. U. S. Atom Energy Comm UCLA-247
Nishita H, Romney EM, Larson KH (1961) Uptake of radioactive fission products by crop plants. J Agric Food Chem 9:101–106
Noordijk H, Van Bergeijk KE, Lembrechts J, Frissel MJ (1992) Impact of ageing and weather conditions on soil-to-plant transfer of radiocesium and radiostrontium. J Environ Radioact 15:277–286
Nordén M, Avila R, Gonze MA, Tamponnet C (2005) Bioavailability in the BORIS assessment model. Radioprotection 40:S107–S111
Ogawa K, Fukuda T, Han J, Kitamura Y, Shiba K, Odani A (2016) Evaluation of Chlorella as a decorporation agent to enhance the elimination of radioactive strontium from body. PLoS One 11:e0148080
Oliver S, Barber SA (1966) An evolution of the mechanisms governing the supply for Ca, Mg, K, and Na to soybean. Soil Sci Soc Am Proc 30:82–86
Oughton DH, Salbu B, Brand TL, Day JP, Aaskrog A (1993) Under-determination of strontium-90 in soils containing particles of irradiated uranium oxide fuel. Analyst 118:1101–1105
Penrose B, Beresford NA, Broadley MR, Crout NMJ (2015) Inter-varietal variation in caesium and strontium uptake by plants: a meta-analysis. J Environ Radioact 139:103–117
Penrose B, Johnson KA, Arkhipov A, Maksimenko A, Gaschak S, Meacham MC, Crout NJM, White PJ, Beresford NA, Broadley MR (2016) Inter-cultivar variation in soil-to-plant transfer of radiocaesium and radiostrontium in Brassica oleracea. J Environ Radioact 155–156:112–121
Pilon-Smits E (2005) Phytoremediation. Anul Rev Plant Biol 56:15–39
Prorok VV, Ganushevich AP, Makarenko TI, Ostashko VV, Poperenko LV, Melinchenko LY (2014) Strontium and calcium relations in plant and soil solution on Chornobyl-affected areas. Ukr J Phys 59:233–237
Qi L, Qin X, Li FM, Siddique K, Brandl H, Xu J, Li X (2015) Uptake and distribution of stable strontium in 26 cultivars of three crop species: oats, wheat, and barley for their potential use in phytoremediation. Int J Phytoremediation 17:264–271
Qi Z, Stephens NR, Spalding EP (2006) Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol 142:963–971
Rauret G, Firsakova S (1996) The transfer of radionuclides through the terrestrial environment to agricultural products, including the evaluation of agrochemical practices. EUR 16528 EN. European Commission, Luxembourg
Rediske JH, Selders AA (1953) The absorption and translocation of strontium by plants. Plant Physiol 28:594–605
Rigol A, Roig M, Vidal M, Rauret G (1999) Sequential extractions for the study of radiocesium and radiostrontium dynamics in mineral and organic soils from western Europe and Chernobyl areas. Environ Sci Technol 33:887–895
Robison WL, Conrado CL, Hamilton TF, Stoker AC (2000) The effect of carbonate soil on transport and dose estimates for long-lived radionuclides at a U.S. pacific test site. J Radioanal Nucl Chem 243:459–465
Russell RS, Squire HM (1958) The absorption and distribution of strontium in plants. J Exp Bot 9:262–272
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 13:468–474
Saniewski M, Zalewska T, Krasińska G, Szylke N, Wang Y, Falandy J (2016) 90Sr in King Bolete Boletus edulis and certain other mushrooms consumed in Europe and China. Sci Total Environ 543:287–294
Sasmaz A, Sasmaz M (2009) The phytoremediation potential for strontium of indigenous plants growing in a mining area. Environ Exp Bot 67:139–144
Schimmack W, Gerstmann U, Schultz W, Sommer M, Tschopp V, Zimmermann G (2007) Intra-cultivar variability of the soil-to-grain transfer of fallout 137Cs and 90Sr for winter wheat. J Environ Radioactiv 94:16–30
Scotti IA, Carini F (2000) Heavy metal effect on uptake and translocation of 134Cs and 85Sr in aubergine plants. J Environ Radioact 48:183–190
Sengupta P, Sanwal J, Mathi P, Mondal JA, Mahadik P, Dudwadkar N, Gandhi PM (2017) Sorption of Cs and Sr radionuclides within natural carbonates. J Radioanal Nucl Chem 312:19–28
Singh S, Eapen S, Thorat V, Kaushik CP, Raj K, D’Souza SF (2008) Phytoremediation of cesium and strontium from solutions and low level nuclear waste by Vetiveria zizanoides. Ecotoxicol Environ Saf 69:306–311
Soudek P, Valenova S, Vavrıkova Z, Vanek T (2006) 137Cs and 90Sr uptake by sunflower cultivated under hydroponic conditions. J Environ Radioact 88:236–250
Sowa I, Kosior MW, Strzemski M, Dresler S, Szwerc W, Blicharski T, Szymczak G, Kocjan R (2014) Biofortification of soy (Glycine max (L.) Merr.) with strontium ions. J Agric Food Chem 62:5248–5252
Tamponnet C, Martin-Garin A, Gonze MA, Parekh N, Vallejo R, Sauras-Year T, Casadeus J, Plassard C, Staunton S, Norden M, Avila R, Shaw G (2008) An overview of BORIS: bioavailability of radionuclides in soils. J Environ Radioact 99:820–830
Tarsitano D, Young SD, Crout NMJ (2011) Evaluating and reducing a model of radiocaesium soil-plant uptake. J Environ Radioact 102:262–269
Tatiana G, Levitskaia JA, Creim TL, Curry TL, Luders T, Morris JE, Peterson JM, Thrall KD (2010) Biomaterials for the decorporation of (85)Sr in the rat. Health Phys 99:394–400
Tsukada H, Takeda A, Takahashi T, Hasegawa H, Hisamatsu S, Inab J (2005) Uptake and distribution of 90Sr and stable Sr in rice plants. J Environ Radioact 81:221–231
Valcke E (1993) The behavior dynamics of radiocesium and radiostrontium in soils rich in organic matter. Ph. D. Thesis. Katholieke Universiteit Leuven, Belgium
Van Bergeijk KE, Noordijk H, Lembrechts J, Frissel MJ (1992) Influence of pH, soil type and soil organic matter content on soil-to-plant transfer of radiocesium and -strontium as analyzed by a nonparametric method. J Environ Radioact 15:265–276
Voelkle H, Murith C, Surbeck H (1989) Fallout from atmospheric bomb tests and releases from nuclear installations. Int J Radiat Appl Instrument Part C Rad Phy Chem 34:261–277
West JB, Hurley JM, Dudás FO, Ehleringer JR (2009) The stable isotope ratios of marijuana. II. Strontium isotopes relate to geographic origin. J For Sci 54:1261–1269
White PJ (1998) Calcium channels in the plasma membrane of root cells. Anal Bot 81:173–183
White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM (2002) Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta 1564:299–309
White PJ, Broadley MR (2000) Mechanisms of caesium uptake by plants. New Phytol 147:241–256
White PJ, Broadley MR (2003) Calcium in plants. Anal Bot 92:487–511
White PJ, Swarup K, Escobar-Gutierrez AJ, Bowen HC, Willey NJ, Broadley MR (2003) Selecting plants to minimise radiocaesium in the food chain. Plant Soil 249:177–186
Yasuda H, Uchida S, Muramatsu Y, Yoshida S (1995) Sorption of manganese, cobalt, zinc, strontium, and cesium onto agricultural soils: statistical analysis on effects of soil properties. Water Air Soil Pollut 83:85–96
Zhao CM, Campbell P, Wilkinson K (2016) When are metal complexes bioavailable. Environ Chem 13:425–433
Zheng G, Pemberton R, Li P (2016) Bioindicating potential of strontium contamination with Spanish moss Tillandsia usneoides. J Environ Radioact 152:23–27
Acknowledgements
We thank the Bundesministerium für Bildung und Forschung (02S9276D) for the financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Gupta, D.K., Schulz, W., Steinhauser, G. et al. Radiostrontium transport in plants and phytoremediation. Environ Sci Pollut Res 25, 29996–30008 (2018). https://doi.org/10.1007/s11356-018-3088-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3088-6