Abstract
Human exposure to arsenic (As) can lead to oxidative stress that can become evident in organs such as the skin, liver, kidneys and lungs. Several intracellular antioxidant defense mechanisms including glutathione (GSH) and metallothionein (MT) have been shown to minimize As cytotoxicity. The current review summarizes the involvement of MT as an intracellular defense mechanism against As cytotoxicity, mostly in blood. Zinc (Zn) and selenium (Se) supplements are also proposed as a possible remediation of As cytotoxicity. In vivo and in vitro studies on As toxicity were reviewed to summarize cytotoxic mechanisms of As. Intracellular antioxidant defense mechanisms of MT are linked in relation to As cytotoxicity. Arsenic uses a different route, compared to major metal MT inducers such as Zn, to enter/exit blood cells. A number of in vivo and in vitro studies showed that upregulated MT biosynthesis in blood components are related to toxic levels of As. Despite the cysteine residues in MT that aid to bind As, MT is not the preferred binding protein for As. Nonetheless, intracellular oxidative stress due to As toxicity can be minimized, if not eliminated, by MT. Thus MT induction by essential metals such as Zn and Se supplementation could be beneficial to fight against As toxicity.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Several factors contribute to human exposure to Arsenic (As), such as As-compounds in ground water, sodium arsenate (Na-AsV) in pesticides, and cigarette smoke. In natural waters, As is mostly found in inorganic forms as oxyanions of trivalent arsenite (AsIII) or pentavalent arsenate (AsV) (Smedley and Kinniburgh 2002). Millions of people are exposed to As concentrations that are above the WHO recommended limit (Garelick et al. 2008; Hashim and Boffetta 2014). In the environment, oxidation states of As vary such as −3, 0, +3 and +5. Organic forms of As are mainly produced by biological activities. Depending upon the chemical form, the acute toxicity of arsenicals decreases from inorganic AsIII > inorganic AsV ≫ organic arsenicals (e.g., monomethyl AsV) (Klaassen 1990).
Various tissues and organs are affected by As toxicity, such as the skin, liver, kidneys and lungs. In those tissues/organs, a number of mechanisms have been identified resulting in cytotoxicity (Table 1). At the same time arsenicals were found to be associated with both in vitro and in vivo induced expression of several antioxidant defense systems including glutathione (GHS) and metallothionein (MT) (Table 2). It is important to note that MT is a family of proteins in which 12 members (isoforms) were reported to be actively expressed in mammals (Mehus et al. 2014).
The most important physiological importance of MT, the SH-containing family of low molecular weight proteins, lies in its toxic heavy metal detoxification and essential heavy metal homeostasis (reviewed by Hamer 1986). At the same time MT provides protection against reactive oxygen species (ROS) (Chiaverini and De Ley 2010; Kassim et al. 2013), which is one of the major primary toxic impacts of As exposure (Pi et al. 2002; Kitchin and Ahmad 2003; Valko et al. 2005). Elevated levels of ROS cause oxidative damage within a cell. Such oxidative stress is often directly associated with Zn deficiency (Kloubert and Rink 2015). Zn plays its antioxidant role as a cofactor of the superoxide dismutase (SOD) by modulating the glutathione (GSH) metabolism and MT expression, by competing with iron and copper in the cell membrane and also by inhibiting the nicotinamide adenine dinucleotide phosphate-oxidase enzyme (Cruz et al. 2015). Thus MTs, a major Zn homeostatic group of proteins, participate in controlling intracellular oxidative stress.
Once ingested through drinking water or food, As travels to organs such as the skin, kidneys and the liver through the circulatory system where MTs play an important role in response to toxic levels of As. However, little is known about the association of As toxicity and blood MT. It is expected that the plasma and cells of the circulatory systems including erythrocytes, thrombocytes, lymphocytes and their precursors are well known reservoirs as well as producers of MT (Vandeghinste et al. 2000; Rahman and De Ley 2001; Rahman and De Ley 2008; Maghdooni Bagheri et al. 2011).
The current review highlights the involvement of MT, with more focus on MT synthesis in blood, and in response to As. Finally, we propose how Zn inducible MT might provide protection against As toxicity in blood.
2 Metallothionein Induction and the Role of As
The increase of MT in plasma, bone marrow, erythrocytes, liver, kidneys differs markedly depending on the type and/or the dose of inducer(s). Dietary Zn for example, increases erythrocyte MT more than plasma MT (Grider et al. 1990), again endotoxins induce plasma MT more than the erythrocyte MT (Bremner et al. 1987). Huber and Cousins (1993) have shown that bone marrow MT expression is highly responsive to the amount of Zn in the diet.
AsIII enters mammalian cells through multiple routes such as aquaglyceroporins (AQP), organic anion transporting polypeptides (OATP) as well as the glucose permeases namely, GluT1, GluT2 and GluT5 (Porquet and Filella 2007; Reviewed by Maciaszczyk-Dziubinska et al. 2012). Erythrocytes and lymphocytes, the two most abundant cell populations among all the cellular components of the circulatory system, express the highest level of GluT1 (Mueckler et al. 1985; Rathmell et al. 2000). Leukocytes as well as liver, spleen, and testis express AQP9, thus they mediate most AsIII uptake from blood to liver (Ishibashi et al. 2009). In eukaryotic cells, AsV uptake is mediated by the high-affinity phosphate transporter, namely sodium-phosphate cotransporter (NaPiIIb), which is expressed in a variety of cells, such as the brush borders of enterocytes apical pole of alveolar type II cells in the lung, apical membrane of the mammary glands, epididymis cells of the testis, hepatocytes and apical cells of the renal proximal tubule (Murer et al. 2004).
Once AsIII enters the cytoplasm, it is sequestrated by GSH and transported through ATP-binding cassette (ABC) transporters present at the plasma membranes. In mammals, inorganic AsIII is methylated, the methylated forms are then exported from the cells by multiple ABC transporters, AQP and glucose permeases (Drobná et al. 2010; McDermott et al. 2010; Carew et al. 2011). In addition, multidrug resistance-associated protein (MRP), also called canalicular multispecific organic anion transporter (MRP1 and MRP2), transports GSH-AsIII complex (Leslie et al. 2004). MRP2 mediates the efflux of seleno-bis(S-glutathionyl) arsinium ion. AsV in cytoplasm undergoes a rapid reduction to AsIII and follows the similar fate of exportation through glucose permeases or MRP1 and 2 (Carew and Leslie 2010).
Major metal MT inducers such as Zn transportation and homeostasis are strictly regulated by Zn binding proteins and Zn transporters (Gaither and Eide 2000) which are different from the As transporters. In circulating erythrocytes, major Zn transporter proteins are ZnT1, Zip8, and Zip10 (Ryu et al. 2008), while in leukocytes they are hZnT-1-9 (Overbeck et al. 2008).
Compared to the extent of research investigating MT expression in relation to As cytotoxicity in different cells and organs (Table 2), studies in the hematopoietic system are scarce. In arsenicosis patients, MT mainly MT-1A and 2A transcripts levels in blood and buccal cells were found positively correlated. When compared to healthy subjects, MT levels are significantly lower in arsenicosis patients (Liu et al. 2007). Inorganic AsIII resistant multiple myeloma (MM) cells have shown an increased expression of the MT-2A, which was found to chelate intracellular inorganic AsIII (Zhou et al. 2005). In our laboratory, we have found that in vitro human cord blood mononuclear cells (MNC) expressed MT in response to 50 μM of Na-AsV (Fig. 1b), albeit, the level of MT expression was higher when MNCs were treated with 100 μM of Zn (Fig. 1a) (Rahman 2001).
MT expression in human cord blood MNC in response to in vitro treatment with Na-AsV. MT (red at the peri-cytoplasmic spaces) is expressed in MNCs treated with 100 μM of Zn (a) and 50 μM of Na-AsV (b). Control cultures (c), without Zn or AsV, did not show any detectable MT. (d) Amplified retro-transcripts of total MT isogenes was similar in MNC cultures treated with 100 μM of Zn (lane 3) and 50 μM of AsV (lane 4) but higher compared to the control culture (lane 2), no band (lane 1) was detected after RT-PCR using deionized water instead of mRNA. (e) Band intensity for amplified G3PDH retro-transcripts was similar in control (lane 1), Na-AsV (lane 2) and 100 μM of Zn (lane 3) treated cultures. bp, 100 base pair DNA size marker (Rahman 2001)
3 Interaction of As with Blood Proteins
Binding of As to blood proteins such as plasma proteins is a complex phenomenon as it varies from species to species in animals, the route of administration, as well as the proteins involved in the binding. Among different reactive forms of the arsenicals, AsIII has a high affinity for thiolates of Cys and the imidazolium nitrogen of histidine (His) residues. Typically AsIII forms three-coordinate trigonal–pyramidal complexes with three Cys in proteins. In hemoglobin (Hb), AsIII binds with Cys13α and was found responsible for As accumulation in blood (rats fed with As). The relative reactivity of the Cys in rat Hb was suggested in the decreasing order of: Cys13α ≫ Cys111α > Cys104α and Cys13α ≫ Cys125β > Cys93β (Lu et al. 2008). Thus, it is expected that As is bound to Hb through vicinal thiol groups in spleen, bone marrow, plasma and in packed cells.
Besides Hb, As also binds to proteins which have a Mr of 100 kDa, 450 kDa or >2000 kDa in liver cytosol. It has been shown by de novo peptide synthesis that AsIII -Cys interactions stabilise three-helix bundles found in aqueous solutions (Farrer et al. 2000). When analysing the serum proteins in patients on continuous ambulatory peritoneal dialysis, only the inorganic As species were found to be able to bind to serum proteins, where transferrin is the main carrier (Zhang et al. 1998). It was also shown that, after in vitro incubation in human serum, inorganic AsV binds with serum transferrin (De Kimpe et al. 1993).
Intravenous administration of AsIII/AsV to mice and rabbits has shown that the percentage of As bound to plasma proteins was 20 % (Vahter and Marafante 1983). When given an intraperitoneal (IP) administration of AsIII (1 μg of AsIII/kg mass of the rabbit), the binding of As in plasma proteins increases by about 10 % and 50 % at the 5th and 48th hour (Bertolero et al. 1981). However, prolonged time lapse resulted in gradual decrease of As after IP administration in rabbits. When protein-bound As reached a maximum of 18 % of the total administered As within 20 h, it is then reduced to about 10 % at 120 h (De Kimpe et al. 1996). However, while the percentage of plasma proteins bound As in marmoset monkeys could be as high as 70 % (Vahter et al. 1982), it can also be very low in dogs (Neiger and Osweiler 1989).
4 Interaction of As with Metallothionein
Generally, the two-domain (α and β) MT binds to divalent metals (M) to form two metal–thiolate clusters with stoichiometries of: M4SCys11 in α-domain and M3SCys9 in β-domain (Fig. 2a). Using human recombinant MT 1A, Ngu and Stillman (2006) reported that AsIII binds with stoichiometries of As3 SCys9 in both β domain and As3 SCys11 in α domain (Fig 2b).
Metal binding of cysteine (Cys) residues of MT. (a) Four divalent metal ions (M2+) such as Cd2+ or Zn2+ (filled circles) generally binds with α-domain while the number of that in β-domain is 3. M2+ are bonded with SH (-S-) group of Cys (circles). (b) Six As atoms (filled circles) are bound to 18 Cys (white circles) in MT. Polypeptide backbone is shown in black ribbon with an approximate location of M2+, As and Cys. Figure a and b are simplified from the corresponding models reported by Ruttkay-Nedecky et al. (2013); and Ngu and Stillman (2006) respectively
Size exclusion chromatography with inductively coupled plasma mass spectrometry analysis of reaction mixtures between AsIII and MT clearly demonstrated the formation of complexes of arsenic with MT. Analysis of the complexes using electrospray quadrupole time-of-flight tandem mass spectrometry revealed the detailed binding stoichiometry between As and the 20 Cys residues in the MT molecule (Jiang et al. 2003). Inorganic AsIII and its two trivalent methylation metabolites, monomethylarsonous acid (MMAIII) and dimethylarsinous acid (DMAIII), readily bind with MT (Jiang et al. 2003). Each MT molecule could bind with up to six AsIII, 10 MMAIII, and 20 DMAIII molecules, consistent with the coordination chemistry of these arsenicals (Jiang et al. 2003).
The time- and temperature-resolved electrospray ionization mass spectrometry, Ngu et al. (2008) demonstrated that AsIII binds to MT in a non-cooperative manner involving six sequential reactions in which binding begins with the α-domain followed by the β-domain. Compared to the single domain MT present in cyanobacteria, the two-domain structures allow MT to bind metals faster, and thus make it an efficient metal scavenger (Ngu et al. 2008).
At neutral pH (pH 7), where free AsIII is not stable, AsIII that is bound to the recombinant human MT-1A is stable and translocates via protein-protein interactions to other MTs. In vitro studies also confirms that AsIII transfers from the two-domain β-α-hMT-1A to the isolated apo-β-hMT and apo-α-hMT, where demetallation of the As(6)-βα-hMT occurs in noncooperative manner as apo- and partially-metallated species coexisting in equilibrium conditions (Ngu et al. 2010).
Studies on binding of different metals with MT reveal that mercury (Hg) has the highest affinity for MT while As, Ca and Mo had a limited affinity. When different metals were added to Zn-MT complex, Zn that bound to MT could be replaced in the order of the following affinity: Cd (1.33 μM) > Pb (1.46 μM) > Cu (1.93 μM) > Hg (3.93 μM) > Zn (8.06 μM) > Ag (10.4 μM) > Ni (474 μM) > LCo (880 μM). Al, Cr, Fe, Mg, Mn, Tl and V had no effect on Zn binding even at 1.0 mM (Waalkes et al. 1984). Later Nielson et al. (1985) proposed the metal binding affinity to thiol in the order of: Hg > Cu > Cd > Zn > Ni = Co. Again, Hamer (1986) proposed the affinity in the order of: Hg > Ag > Cu > Cd > Zn.
When partially metallated Cd-MT and Zn-MT were considered the more stable form of metal-apoMT complex, AsIII transfer at pH 7 is found to be dependent on protein-protein interaction (Ngu et al. 2010). Nonetheless, the cellular redox state as well as the concentration of other biological metal chelators determines the Zn transfer from and to MTs (Jacob et al. 1998). Although initially, Cd or Zn binding to apo-MT is reported to be cooperative (Nielson and Winge 1983), recently, by using recombinant human MT-1A, Sutherland et al. (2012) concluded that the metalation of apo MT occurs in a non-cooperative fashion for both Zn2+ and Cd2+. The binding of As to MT was also reported to be non-cooperative (Ngu et al. 2008). These lines of evidence suggest that even though As toxicity such as increased ROS could induce MT synthesis, the apoprotein might prefer free Zn2+ in the cytosol.
Notably, free Zn2+ maintains equilibrium in blood through different routes of exchange such as through the erythrocyte membrane permeability. Intracellular Zn2+ was found to maintain about 129 μmole/1013 erythrocytes while the main component of Zn2+ buffering is Hb, with a dissociation constant of about 2 × 10−8 M (Simons 1991).
5 Accumulation and Metabolism of As
Multiple myeloma cells of bone marrow treated with inorganic AsIII show intracellular biotransformation from AsIII to AsV. Such biological oxidation of AsIII was described as a protective mechanism of the cell against As cytotoxicity (Falnoga et al. 2007). AsV is reduced to AsIII by CDC25 phosphatases or arsenate reductases (Bhattacharjee et al. 2010). However, biomethylation, particularly the production of AsIII containing methylated metabolites, is a process that activates As both as a toxin and a carcinogen (Smith et al. 2009). In liver, the intracellular AsIII-methyltransferase methylates As resulting in formation of both mono and dimethyl AsV and AsIII, and is eventually excreted through bile and urine (Thomas et al. 2007). AQP9 is found to be involved not only in AsIII uptake from blood to liver, but also in the removal of methylated forms of As down the concentration gradient from hepatocytes to the blood flow to end up in urine (Liu et al. 2006; Carbrey et al. 2009; McDermott et al. 2010). Furthermore, clinical and epidemiological studies have proven that affinity for thiol groups renders As binding to SH moieties of critical proteins like keratin (Lindgren et al. 1982; De Kimpe et al. 1999). Therefore, a variety of skin lesions were linked to As-toxicity (Chen et al. 1988; Brown et al. 1997; Yu et al. 2006).
6 As Cytotoxicity and the Role of Essential Metals
It can be expected that cellular MT induction might act as protective mechanism against As toxicity. This is because production of ROS is one of the major toxic impact of As exposure while MT provides protection against such oxidative stress (Chiaverini and De Ley 2010; Kassim et al. 2013). Induction of MT is achieved through a variety of mechanisms which includes activation of: (1) metal response elements (MRE) by the Zn binding metal-responsive transcription factor (MTF-1) (2) glucocorticoid response elements (GRE) (Kelly et al. 1997), and (3) antioxidant (or electrophile) response element (ARE), in response to the redox status (Andrews 2000). Zn is also an important regulator of GSH synthesis, where GSH is involved in As excretion (Kala et al. 2000). Zn deficiency is accompanied by the increase in ROS (Kraus et al. 1997; Kojima-Yuasa et al. 2005). In vitro treatment of tARPE-19 cells with 150 μM Zn caused 70 % increase in GSH levels through ARE activated de novo synthesis. ARE activation and GSH synthesis could be inhibited by silencing Nrf2 expression (Ha et al. 2006). Thus, activation of MRE and ARE, by essential nutrients such as Zn might prove beneficial in reducing As toxicity specially to minimize the ROS mediated cytotoxicity.
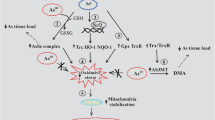
Cellular metabolism consistently generates ROS, where intracellular GPx plays an important role to reduce ROS such as H2O2 to water, hence limiting the harmful effects of the ROS. GPx is a selenocysteine-containing enzyme, expression of which is strictly regulated by the supply of Se and selenocysteine (reviewed by Lubos et al. 2011). Therefore, the in vivo acceptable range of Se supplement to induce antioxidant mechanisms such as GPx and GSH might be beneficial in the reduction of As toxicity, which is mostly linked with ROS. Notably, several lines of evidence have proven the beneficial impact of essential metals such as Zn and Se supplement against metal toxicity, such as cadmium and chromium (Table 3). Cd toxicity on sperm motility and the testicular antioxidant status could be restored by Se and Zn supplement (Saïd et al. 2010). Again Se supplements improved renal toxicity biomarkers’ levels and antioxidant enzyme activities in K2Cr2O7 administered renal damages (Soudani et al. 2010). Similarly, co-administration of Se with K2Cr2O7 restored hematological dysfunction related to the Cr exposure to near-normal values (Soudani et al. 2011). Furthermore, based on a number of in vivo and in vitro studies, McCarty (2012) proposed Zn supplement to ameliorate pathogenic impact of Cd toxicity as Zn is known to have protective anti-inflammatory, antioxidant, and immunosupportive effects. Therefore, it is not unexpected that Zn and Se supplement could be beneficial to minimize cytotoxicity exerted by As (Fig. 3).
Intracellular As toxicity and its possible remediation. Once internalized, AsIII and AsV via AsIII will generate reactive oxygen species (ROS). AsIII and AsV via AsIII can also induce metallothionein (MT) which in turn will reduce ROS and block intracellular transport of MT respectively. If given Zn and Se supplement, AsIII inducible ROS can be further reduced by induction of additional MT and glutathione peroxidase (GPx) or glutathione (GSH). [AQP aquaglyceroporins, ARE antioxidant response elements, AsT As transporter, GluT glucose permease, M-As methylated As, MRE metal response elements, MRP multidrug resistant protein, NaPiIIb sodium-phosphate cotransporter, QATP organic anion transporting polypeptides, ZnT/ZIP Zn transporter proteins]
7 Conclusion
As, commonly known as a metalloid, has been used to treat cancer, such as the acute promyelocytic leukaemia (Dilda and Hogg 2007), infectious diseases (Frézard and Demicheli 2010) and sleeping sicknesses (Chappuis 2007), the same metalloid is also able to cause cancer and damage the liver and the kidneys. As a means of treatment to As induced damages, essential metals such as Zn and Se could be beneficial, as these metals can induce both intracellular MT and antioxidant mechanisms.
8 Summary
Millions of people are exposed to a toxic level of arsenic (As). Oxidative stress due to As is evident in organs such as the skin, liver, kidneys and lungs. Several intracellular antioxidant defense mechanisms including glutathione (GSH) and metallothionein (MT) have been shown to minimize As cytotoxicity. The current review summarizes and the involvement of MT as an intracellular defense mechanism against As cytotoxicity, mostly in blood. Zinc (Zn) and selenium (Se) supplements are also proposed as a possible remediation of As cytotoxicity. In vivo and in vitro studies on As toxicity were reviewed to summarize cytotoxic mechanisms of As. Intracellular antioxidant defense mechanisms of MT are linked in relation to As cytotoxicity. In addition, in vitro potential of pentavalent inorganic As to induce MT biosynthesis was evaluated in human peripheral blood mononuclear cells. Arsenic uses a different route, compared to major metal MT inducers such as Zn, to enter/exit blood cells. However, a number of in vivo and in vitro studies showed that upregulated MT biosynthesis in blood components are related to toxic levels of As. Despite the cysteine residues in MT that aid to bind As, MT is not the preferred binding protein for As. Nonetheless, intracellular oxidative stress due to As toxicity can be minimized, if not eliminated, by MT. Thus MT induction by essential metals such as Zn and Se supplementation could be beneficial to fight against the global As toxicity.
References
Agrawal S, Flora G, Bhatnagar P, Flora SJ (2014) Comparative oxidative stress, metallothionein induction and organ toxicity following chronic exposure to arsenic, lead and mercury in rats. Cell Mol Biol (Noisy-le-Grand) 60:13–21
Albores A, Koropatnick J, Cherian MG, Zelazowski AJ (1992) Arsenic induces and enhances rat hepatic metallothionein production in vivo. Chem Biol Interact 85:127–140
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59:95–104
Bertolero F, Marafante E, Rade J, Pietra R, Sabbioni E (1981) Biotransformation and intracellular binding of arsenic in tissues of rabbits after intraperitoneal administration of 74As labelled arsenite. Toxicology 20:35–44
Bhattacharjee H, Sheng J, Ajees AA, Mukhopadhyay R, Rosen BP (2010) Adventitious arsenate reductase activity of the catalytic domain of the human Cdc25B and Cdc25C phosphatases. Biochemistry 49:802–809
Bhuvaneswaran C (1979) The influence of phosphorylation state ratio on energy conservation in mitochondria treated with inorganic arsenate. Biochem Biophys Res Commun 90:1201–1206
Bremner I, Morrison JN, Wood AM, Arthur JR (1987) Effects of changes in dietary zinc, copper and selenium supply and of endotoxin administration on metallothionein I concentrations in blood cells and urine in the rat. J Nutr 117:1595–1602
Brown KG, Guo HR, Kuo TL, Greene HL (1997) Skin cancer and inorganic arsenic: uncertainty-status of risk. Risk Anal 17:37–42
Brown MM, Rhyne BC, Goyer RA, Fowler BA (1976) Intracellular effects of chronic arsenic administration on renal proximal tubule cells. J Toxicol Environ Health 1:505–514
Carbrey JM, Song L, Zhou Y, Yoshinaga M, Rojek A, Wang Y, Liu Y, Lujan HL, DiCarlo SE, Nielsen S (2009) Reduced arsenic clearance and increased toxicity in aquaglyceroporin-9-null mice. Proc Natl Acad Sci U S A 106:15956–15960
Carew MW, Leslie EM (2010) Selenium-dependent and -independent transport of arsenic by the human multidrug resistance protein 2 (MRP2/ABCC2): implications for the mutual detoxification of arsenic and selenium. Carcinogenesis 31:1450–1455
Carew MW, Naranmandura H, Shukalek CB, Le XC, Leslie EM (2011) Monomethylarsenic diglutathione transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Drug Metab Dispos 39:2298–22304
Chappuis F (2007) Melarsoprol-free drug combinations for second-stage Gambian sleeping sickness: the way to go. Clin Infect Dis 45:1443–1445
Chen CJ, Kluo TL, Wu MM (1988) Arsenic and cancers. Lancet 1:414–415
Chiaverini N, De Ley M (2010) Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic Res 44:605–613
Cruz KJ, de Oliveira AR, Marreiro DN (2015) Antioxidant role of zinc in diabetes mellitus. World J Diabetes 6(2):333–337
De Kimpe J, Cornelis R, Mees L, Vanholder R (1993) Arsenate–transferrin binding is a possible contributor to elevated arsenic-levels in the serum of chronic HD patients. In: Anke M, Meissner D, Mills CF (eds) Trace elements in man and animals, vol 8. Verlag Media Touristik Gersdorf, Germany, pp 845–848
De Kimpe J, Cornelis R, Mees L, Vanholder R (1996) Basal metabolism of intraperitoneally injected carrier-free 74As-labelled arsenate in rabbits. Fundam Appl Toxicol 34:240–248
De Kimpe J, Cornellis R, Mees L, Vanholder R, Verhoeven G (1999) 74As-arsenate metabolism in Flemish Giant rabbits with renal insufficiency. J Trace Elements Med Biol 13:7–14
Dilda PJ, Hogg PJ (2007) Arsenical-based cancer drugs. Cancer Treat Rev 33:542–564
Drobná Z, Walton FS, Paul DS, Xing W, Thomas DJ, Stýblo M (2010) Metabolism of arsenic in human liver: the role of membrane transporters. Arch Toxicol 84:3–16
Falnoga I, Slejkovec Z, Pucer A, Podgornik H, Tusek-Znidaric M (2007) Arsenic metabolism in multiple myeloma and astrocytoma cells. Biol Trace Elem Res 116:5–28
Falnoga I, Zelenik Pevec A, Šlejkovec Z, Žnidarič MT, Zajc I, Mlakar SJ, Marc J (2012) Arsenic trioxide (ATO) influences the gene expression of metallothioneins in human glioblastoma cells. Biol Trace Elem Res 149:331–339
Farrer BT, Maclure CP, Penner-Hahn JE, Pecoraro VL (2000) Arsenic(III)-cysteine interactions stabilize three-helix bundles in aqueous solution. Inorg Chem 39:5422
Frézard F, Demicheli C (2010) New delivery strategies for the old pentavalent antimonial drugs. Expert Opin Drug Deliv 7:1343–1358
Gaither LA, Eide DJ (2000) Functional expression of the human hZIP2 zinc transporter. J Biol Chem 275:5560–5564
Garelick H, Jones H, Dybowska A, Valsami-Jones E (2008) Arsenic pollution sources. Rev Environ Contam Toxicol 197:17–60
Grider A, Bailey LB, Cousins RJ (1990) Erythrocyte metallothionein as an index of zinc status in humans. Proc Natl Acad Sci U S A 87:1259–1262
Ha K-N, Chen Y, Cai J, Sternberg P Jr (2006) Increased glutathione synthesis through an ARE-Nrf2–dependent pathway by zinc in the RPE: implication for protection against oxidative stress. Invest Ophthalmol Vis Sci 47:62709–62715
Hamer DH (1986) Metallothionein. Annu Rev Biochem 55:913–951
Hashim D, Boffetta P (2014) Occupational and environmental exposures and cancers in developing countries. Ann Glob Health 80:393–411
He X, Ma Q (2009) Induction of metallothionein I by arsenic via metal-activated transcription factor 1: critical role of C-terminal cysteine residues in arsenic sensing. J Biol Chem 284:12609–12621
Huber KL, Cousins RJ (1993) Zinc metabolism and metallothionein expression in bone marrow during erythropoiesis. Am J Physiol 264:E770–E775
Huerta-Olvera SG, Macías-Barragán J, Ramos-Márquez ME, Armendáriz-Borunda J, Díaz-Barriga F, Siller-López F (2010) Alpha-lipoic acid regulates heme oxygenase gene expression and nuclear Nrf2 activation as a mechanism of protection against arsenic exposure in HepG2 cells. Environ Toxicol Pharmacol 29:144–149
Ishibashi K, Hara S, Kondo S (2009) Aquaporin water channels in mammals. Clin Exp Nephrol 13:107–117
Jacob C, Maret W, Vallee BL (1998) Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc Natl Acad Sci U S A 95(7):3489–3494
Jiang G, Gong Z, Li XF, Cullen WR, Le XC (2003) Interaction of trivalent arsenicals with metallothionein. Chem Res Toxicol 16:873–880
Jimi S, Uchiyama M, Takaki A, Suzumiya J, Hara S (2004) Mechanisms of cell death induced by cadmium and arsenic. Ann N Y Acad Sci 1011:325–331
Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW (2000) The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem 275:33404–33408
Kassim R, Ramseyer C, Enescu M (2013) Oxidation reactivity of zinc-cysteine clusters in metallothionein. J Biol Inorg Chem 18:333–342
Kelly EJ, Sandgren EP, Brinster RL, Palmiter RD (1997) A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc Natl Acad Sci U S A 94(19):10045–10050
Kessel M, Liu SX, Xu A, Santella R, Hei TK (2002) Arsenic induces oxidative DNA damage in mammalian cells. Mol Cell Biochem 234–235:301–308
Kitchin KT, Ahmad S (2003) Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett 137(1–2):3–13
Klaassen CD (1990) Heavy metals and heavy-metal antagonists. In: Gilman AG, Rall TW, Nies AS, Taylor P (eds) Goodman and Gilman’s: the pharmacological basis of therapeutics, 8th edn. Macmillan, New York, pp 1592–1614
Kojima-Yuasa A, Umeda K, Olikita T, Kennedy DO, Nishiguchi S, Matsui-Yuasa I (2005) Role of reactive oxygen species in zinc deficiency-induced hepatic stellate cell activation. Free Radic Biol Med 39:631–640
Kloubert V, Rink L (2015) Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct 6(10):3195–3204
Kraus A, Roth HP, Kirchgessner M (1997) Supplementation with vitamin C, vitamin E or beta-carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc-deficient rats. J Nutr 127:1290–1296
Kreppel H, Bauman JW, Liu J, McKim JM Jr, Klaassen CD (1993) Induction of metallothionein by arsenicals in mice. Fundam Appl Toxicol 20:184–189
Leslie EM, Haimeur A, Waalkes MP (2004) Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J Biol Chem 279:32700–32708
Lindgren A, Vahter M, Dencker L (1982) Autoradiographic studies on the distribution of arsenic in mice and hamsters administered 74As-arsenite or -arsenate. Acta Pharmacol Toxicol 51:253–265
Liu J, Cheng ML, Yang Q, Shan KR, Shen J, Zhou Y, Zhang X, Dill AL, Waalkes MP (2007) Blood metallothionein transcript as a biomarker for metal sensitivity: low blood metallothionein transcripts in arsenicosis patients from Guizhou, China. Environ Health Perspect 115:1101–1106
Liu Z, Styblo M, Rosen BP (2006) Methylarsonous acid transport by aquaglyceroporins. Environ Health Perspect 114:527–531
Lu M, Wang H, Wang Z, Li XF, Le XC (2008) Identification of reactive cysteines in a protein using arsenic labeling and collision-induced dissociation tandem mass spectrometry. J Proteome Res 7:3080–3090
Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15:1957
Maciaszczyk-Dziubinska E, Wawrzycka D, Wysocki R (2012) Arsenic and antimony transporters in eukaryotes. Int J Mol Sci 13:3527–3548
Maghdooni Bagheri P, Govaerts I, De Ley M (2011) Role of metallothionein in differentiation of leukemia cells. Mol Biol Rep 38:3017–3022
McCarty MF (2012) Zinc and multi-mineral supplementation should mitigate the pathogenic impact of cadmium exposure. Med Hypotheses 79:642–648
McDermott JR, Jiang X, Beene LC, Rosen BP, Liu Z (2010) Pentavalent methylated arsenicals are substrates of human AQP9. Biometals 23:119–127
Mehus AA, Muhonen WW, Garrett SH, Somji S, Sens DA, Shabb JB (2014) Quantitation of human metallothionein isoforms: a family of small, highly conserved, cysteine-rich proteins. Mol Cell Proteomics 13:1020–1033
Milnerowicz H, Ściskalska M, Dul M (2015) Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke. J Trace Elem Med Biol 29:1–10
Mueckler M, Caruso C, Baldwin SA et al (1985) Sequence and structure of a human glucose transporter. Science 229:941–945
Murer H, Forster I, Biber J (2004) The sodium phosphate cotransporter family SLC34. Pflugers Arch 447:763–767
Neiger RD, Osweiler GD (1989) Effect of subacute low level dietary sodium arsenite on dogs. Fundam Appl Toxicol 13:439–451
Ngu TT, Stillman MJ (2006) Arsenic binding to human metallothionein. J Am Chem Soc 128(38):12473–12483
Ngu TT, Dryden MD, Stillman MJ (2010) Arsenic transfer between metallothionein proteins at physiological pH. Biochem Biophys Res Commun 401:69–74
Ngu TT, Easton A, Stillman MJ (2008) Kinetic analysis of arsenic-metalation of human metallothionein: significance of the two-domain structure. J Am Chem Soc 130:17016–17028
Nielson KB, Atkin CL, Winge DR (1985) Distinct metal-binding configurations in metallothionein. J Biol Chem 260:5342–5350
Nielson KB, Winge DR (1983) Order of metal binding in metallothionein. J Biol Chem 258:13063–13069
Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L (2008) Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol 83:368–380
Peng Z, Peng L, Fan Y, Zandi E, Shertzer HG, Xia Y (2007) A critical role for IkappaB kinase beta in metallothionein-1 expression and protection against arsenic toxicity. J Biol Chem 282:21487–21496
Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H, Hopenhayn-Rich C, Shimojo N (2002) Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ Health Perspect 110(4):331–336
Porquet A, Filella M (2007) Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: implications for their uptake. Chem Res Toxicol 20:1269–1276
Qu W, Waalkes MP (2014) Metallothionein blocks oxidative DNA damage induced by acute inorganic arsenic exposure. Toxicol Appl Pharmacol 282:267–274
Rahman MT, De Ley M (2001) Metallothionein isogene transcription in red blood cell precursors from human cord blood. Eur J Biochem 268:849–856
Rahman MT, De Ley M (2008) Metallothionein in human thrombocyte precursors, CD61+ megakaryocytes. Cell Biol Toxicol 24:19–25
Rahman MT (2001) Metallothionein isogene expression in precursors of erythrocytes and platelets from human cord blood. PhD Dissertation, Katholieke Universiteit Leuven, Leuven, Belgium
Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB (2000) In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell 6:683–692
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14(3):6044–6066
Ryu MS, Lichten LA, Liuzzi JP, Cousins RJ (2008) Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J Nutr 138:2076–2083
Saïd L, Banni M, Kerkeni A, Saïd K, Messaoudi I (2010) Influence of combined treatment with zinc and selenium on cadmium induced testicular pathophysiology in rat. Food Chem Toxicol 48:2759–2765
Sakurai T, Kaise T, Matsubara C (1998) Inorganic and methylated arsenic compounds induce cell death in murine macrophages via different mechanisms. Chem Res Toxicol 11:273–283
Simons TJ (1991) Intracellular free zinc and zinc buffering in human red blood cells. J Membr Biol 123:63–71
Sutherland DEK, Summers KL, Stillman MJ (2012) Modeling the Zn2+ and Cd2+ metalation mechanism in mammalian metallothionein 1a. Biochem Biophysic Res Commun 426(4):601–607
Slusser A, Zheng Y, Zhou XD, Somji S, Sens DA, Sens MA, Garrett SH (2014) Metallothionein isoform 3 expression in human skin, related cancers and human skin derived cell cultures. Toxicol Lett 232:141–148
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith AH, Ercumen A, Yuan Y, Steinmaus CM (2009) Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J Expo Sci Environ Epidemiol 19(4):343–8
Soudani N, Ben Amara I, Troudi A, Hakim A, Bouaziz H, Ayadi Makni F, Zeghal KM, Zeghal N (2011) Oxidative damage induced by chromium (VI) in rat erythrocytes: protective effect of selenium. J Physiol Biochem 67:577–588
Soudani N, Sefi M, Ben Amara I, Boudawara T, Zeghal N (2010) Protective effects of selenium (Se) on chromium (VI) induced nephrotoxicity in adult rats. Ecotoxicol Environ Saf 73:671–678
Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M (2007) Arsenic (+3 oxidation state) methyltransferase and methylation of arsenicals. Exp Biol Med 232:3–11
Tokar EJ, Person RJ, Sun Y, Perantoni AO, Waalkes MP (2013) Chronic exposure of renal stem cells to inorganic arsenic induces a cancer phenotype. Chem Res Toxicol 26:96–105
Vahter M, Marafante E, Lindgren A, Dencker L (1982) Tissue distribution and subcellular binding of arsenic in marmoset monkeys after injection of 74As-arsenite. Arch Toxicol 51:65–77
Vahter M, Marafante E (1983) Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits. Chem Biol Interact 47:29–44
Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Vandeghinste N, Proost P, De Ley M (2000) Metallothionein isoform gene expression in zinc-treated human peripheral blood lymphocytes. Cell Mol Biol 46:419–433
Waalkes MP, Harvey MJ, Klaassen CD (1984) Relative in vitro affinity of hepatic metallothionein for metals. Toxicol Lett 20:33–39
Yu HS, Liao WT, Chai CY (2006) Arsenic carcinogenesis in the skin. J Biomed Sci 13(5):657–666
Zhang X, Cornelis R, De Kimpe J, Mees L, Lameire N (1998) Study of arsenic–protein binding in serum of patients on continuous ambulatory peritoneal dialysis. Clin Chem 44:141–147
Zhao Y, Toselli P, Li W (2012) Microtubules as a critical target for arsenic toxicity in lung cells in vitro and in vivo. Int J Environ Res Public Health 9:474–495
Zhou P, Kalakonda N, Comenzo RL (2005) Changes in gene expression profiles of multiple myeloma cells induced by arsenic trioxide (ATO): possible mechanisms to explain ATO resistance in vivo. Br J Haematol 128:636–644
Acknowledgement
This work was supported by a research grant from the Fonds voor Wetenschappenlijk Onderzoek-Vlaanderen (G.0410.98) and Basic Applied Research Cluster Unit, RMC, IIUM (RCG 22-05). Authors wish to acknowledge the kind assistance of Marzouq Abedur Rahman and Jan Czernuszka (Oxford University) for language editing.
Competing Financial Interest Declaration: The authors declare no conflict of financial interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing
About this chapter
Cite this chapter
Rahman, M.T., De Ley, M. (2016). Arsenic Induction of Metallothionein and Metallothionein Induction Against Arsenic Cytotoxicity. In: de Voogt, P. (eds) Reviews of Environmental Contamination and Toxicology Volume 240. Reviews of Environmental Contamination and Toxicology, vol 240. Springer, Cham. https://doi.org/10.1007/398_2016_2
Download citation
DOI: https://doi.org/10.1007/398_2016_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42299-2
Online ISBN: 978-3-319-42300-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)