Abstract
Classical opioids (μ: mu, MOP; δ: delta, DOP and κ: kappa, KOP) variably affect immune function; they are immune depressants and there is good clinical evidence in the periphery. In addition, there is evidence for a central role in the control of a number of neuropathologies, e.g., neuropathic pain. Nociceptin/Orphanin FQ (N/OFQ) is the endogenous ligand for the N/OFQ peptide receptor, NOP; peripheral and central activation can modulate immune function. In the periphery, NOP activation generally depresses immune function, but unlike classical opioids this is in part driven by NOP located on circulating immune cells. Peripheral activation has important implications in pathologies like asthma and sepsis. NOP is expressed on central neurones and glia where activation can modulate glial function. Microglia, as resident central ‘macrophages’, increase/infiltrate in pain and following trauma; these changes can be reduced by N/OFQ. Moreover, the interaction with other glial cell types such as the ubiquitous astrocytes and their known cross talk with microglia open a wealth of possibilities for central immunomodulation. At the whole animal level, clinical ligands with wide central and peripheral distribution have the potential to modulate immune function, and defining the precise nature of that interaction is important in mitigating or even harnessing the adverse effect profile of these important drugs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Astrocytes
- Gliosis
- Immune function
- Lymphocytes
- Microglia
- N/OFQ receptor (NOP)
- Neuropathic pain
- Nociceptin/Orphanin FQ
- Sepsis
1 Introduction
Classical opioids (μ: mu, MOP; δ: delta DOP and κ: kappa, KOP) are immunomodulatory; this has been known for decades. Indeed, Hussey and Katz reported in 1950 that opioid addicts were more prone to infection and this was unlikely due to the injection itself (Hussey and Katz 1950). The site of this immunomodulation can be peripheral or central with the precise targets (especially peripheral) being disputed and highly controversial. Prescribing physicians are advised to consider and discuss immune modulation in chronic use decisions. Since its first de-orphanisation N/OFQ and NOP [non-classical opioid receptor (Lambert 2008)] have also been ascribed a role in immunomodulation, and in this chapter we review their roles at peripheral and central sites.
2 Peripheral Immune Actions
2.1 Classical Opioids
Opioid receptor expression on immune cells is still highly controversial. It is widely accepted that opioids have immunomodulatory properties, for example inhibition of T-cell activity or inhibition of B-cell antibody production (Manfredi et al. 1993; Morgan 1996). However, there is significant debate as to whether this action occurs through direct or indirect mechanisms. Evidence is strongly divided regarding the detection of classical opioid receptor (MOP, DOP and KOP) expression on immune cell types (Caldiroli et al. 1999; Bidlack 2000; Cadet et al. 2001; Al-Hashimi et al. 2013, 2016; Kadhim et al. 2018b). Some have posited that the action of morphine in immune responses is via the toll-like receptors (TLR), which have been shown to possess a morphine binding domain (Madden et al. 2001; Hutchinson et al. 2012).
2.2 N/OFQ-NOP
Conversely, there is significant evidence for expression of NOP receptors on immune cell subtypes. Several studies have identified the presence of ppN/OFQ and NOP mRNA (the precursor to N/OFQ) in polymorphonuclear cells, B cells, T cells and monocytes and mast cells (Peluso et al. 1998; Arjomand et al. 2002; Williams et al. 2008a; Singh et al. 2013; Al-Hashimi et al. 2016). Interestingly, screening of phytohemagglutinin (PHA)-activated human lymphocytes identified AT7-5EU cDNA, which encodes NOP, with divergent coding of a non-translated 5′ region in comparison to neuronal tissue. This message is encoded into B and T cell NOP mRNA, and suggests tissue-specific expression of the NOP receptor. Furthermore, these experiments indicated a tenfold increase in NOP mRNA expression after induction with PHA, implying NOP has an important role in immune function (Wick et al. 1995). Further studies have demonstrated similar levels of NOP mRNA in both immune cells and neuronal tissue (Peluso et al. 1998). Expression of functional NOP receptor has been identified in numerous continuous cell lines generated from immune cells. Using [125I]-N/OFQ, Horn and colleagues identified surface expression of NOP on Raji cells, a human B cell lymphoma line (Hom et al. 1999). NOP was further identified in CEM and MOLT-4 T cell leukemic lines and the monocyte lymphoma cell line U-937 using [3H]-N/OFQ to identify binding sites (Peluso et al. 1998). The addition of phorbol-12-myristate-13-acetate (PMA) to Mono Mac 6 cells, a monocyte leukemic cell line, led to increases in ppN/OFQ mRNA via the inhibition of mitogen-activated protein kinase signal transduction pathways (Zhang et al. 2016). Identification of NOP expression on primary immune cells has been challenging due to poorly selective antibodies for the NOP receptor and acquiring the necessary yield of protein to undertake a radioligand binding assay. Recently, a fluorescent marker for NOP, N/OFQATTO594, has been used to identify NOP receptor expression on human polymorphonuclear cells taken from healthy volunteers (Bird et al. 2018). Interestingly, not all polymorphonuclear cells expressed the NOP receptor protein; this is a cautionary note when assuming mRNA will always translate into protein.
Immune cells have also been shown to express N/OFQ. Human CD19+ B cells were amongst the first to be identified as expressing a novel N/OFQ mRNA transcript resulting in a truncated N/OFQ precursor lacking the signal peptide. Following mitogen-activation, N/OFQ mRNA transcripts, similar to that found in neuronal tissue, was upregulated in all lymphocytes (Arjomand et al. 2002). The mRNA transcript for ppN/OFQ has also been found in polymorphonuclear cells, which include neutrophils, eosinophils and granulocytes (Williams et al. 2008a). Furthermore, neutrophils stimulated with N-formyl-methionine-leucine-phenylalanine (FMLP) have been shown to release N/OFQ (Fiset et al. 2003).
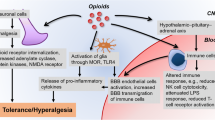
The presence of both N/OFQ and NOP in immune cells would strongly indicate a role in immunological function for this ligand-receptor pairing. An area where this pairing may have significant effect is in the trafficking of immune cells, with N/OFQ having significant effects on cell migration, both positive and negative. A significant example of the positive effects of N/OFQ was measured using monocytes taken from healthy volunteers. The monocytes were exposed to either FMLP or N/OFQ and chemotaxis measured (Trombella et al. 2005). FMLP caused robust migration of monocytes which was matched by N/OFQ, which displayed a high potency (pEC50 11.15) in producing migration. Confirmation of action through the NOP receptor was obtained through pharmacological characterisation using several NOP selective agonists, the inability of naloxone to block the function of N/OFQ at monocytes and through antagonism of migration via the NOP antagonist UFP-101 (Trombella et al. 2005). Neutrophil chemotaxis is also positively affected by the addition of N/OFQ. N/OFQ induced chemotaxis with maximal effect at 100 pM in ex vivo migration studies, and these findings were matched in mouse in vivo models whereby N/OFQ increased neutrophil migration into ad-hoc air pouches (Serhan et al. 2001). Conversely, both lung mast cells and eosinophils have been shown to be negatively affected by N/OFQ in regards to migration (Singh et al. 2016). Both human mast cell line-1 (HMC-1) and primary human lung mast cell migration produced by stem cell factor (SCF) were significantly inhibited by the addition of N/OFQ. Clearly there is a cell and tissue specific migratory response to NOP activation. In addition, and as reviewed by Thomas et al. (2014), N/OFQ induces vasodilation and increases the vascular permeability, actions that play a central role in immune response modulation (Fig. 1).
2.3 N/OFQ-NOP in Disease
The presence of NOP and/or N/OFQ in the immune system, as well as its ability to affect immune cell movement and function, identify a potential mediator of disease-related activity in immunity. NOP and N/OFQ activity has been demonstrated to show potential roles in several immune based diseases. Both NOP and N/OFQ have been implicated in the pathogenesis of colitis, an inflammatory bowel disease (Kato et al. 2005). NOP knockout mice demonstrated significant reduction in symptoms following treatment with dextran sulphate sodium (DSS), which is capable of producing acute colitis. In further studies, administration of SB612,111 (a high affinity NOP antagonist) to DSS-induced colitis also reduced symptoms of colitis as well as a reduction of the cytokines interferon-γ (IFN-γ), interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α). These cytokines are all known mediators of colitis (Alt et al. 2012). Increased levels of N/OFQ have also been detected in the synovial fluid of patients suffering with rheumatoid arthritis (Fiset et al. 2003). The increased level of N/OFQ was believed to be related to the high concentration of polymorphonuclear cells usually found in synovial fluid of patients suffering with this disease.
As previously noted, both lung eosinophils and mast cells express the NOP receptor. This is particularly relevant to asthma. Asthma is the result of obstruction of airflow (airway constriction, immune infiltration and remodelling) leading to difficulty in breathing (Haldar et al. 2008; Lotvall et al. 2011; Gough et al. 2015). Initial studies indicated that activation of NOP, via N/OFQ, led to inhibition of airway contraction and the release of the inflammatory peptide, substance P (Shah et al. 1998). This initial evidence for NOP receptor function in airway constriction was verified by work in ex vivo human bronchial tissue (Basso et al. 2005). Electric field stimulation produced contractions in the tissue, which was inhibited by N/OFQ in a concentration-dependent manner. Furthermore, the actions of N/OFQ could be blocked by the NOP antagonist, UFP-101, indicating action through the NOP receptor. In a more recent work, tissues from both healthy volunteers and asthmatic patients were screened for the presence of NOP and N/OFQ via PCR. In these studies, N/OFQ was identified in lung eosinophils and, in asthmatic patients, levels were found to be increased in sputum (Singh et al. 2016). In parallel experiments, N/OFQ was found to inhibit migration of immune cells through NOP receptor activation, as well as increasing wound healing in isolated human airway smooth muscle (HASM) cells. Using the same cells, it was found that N/OFQ led to relaxation of HASM cells in spasmogen-stimulated gel contraction experiments, a finding mirrored in Ovalbumin-sensitised mice. These findings suggest that NOP agonists could be potential therapeutic agents for asthma, with a spasmolytic and immune depressor profile.
Sepsis is the result of the immune system producing an overwhelming and potentially life-threatening response to an infection. Treatment options are limited to antibiotics, fluids and supportive care. Translation from the laboratory to the clinic has been poor and there is a real need for novel therapeutics. The mechanisms by which sepsis occurs are poorly understood, but NOP and N/OFQ have been implicated in this disease. Initial evidence for the role of N/OFQ-NOP in sepsis was found using rat models subjected to caecal ligation and puncture to induce sepsis. In these models, addition of N/OFQ to caecal ligation and puncture led to increased mortality, whereas addition of UFP-101 increased survival rates through inhibition of cell migration and modulation of pro-inflammatory cytokines and chemokines (Carvalho et al. 2008).
Both ppN/OFQ and NOP mRNA levels were decreased in peripheral blood taken from healthy volunteers exposed to varying concentrations of LPS. Furthermore, cytokines, such as TNF-α, IL-1β, IL-10 and IFN-γ, also demonstrated the ability to decrease ppN/OFQ and NOP mRNA levels in healthy volunteer blood (Zhang et al. 2013). While it was initially posited that this was a negative feedback loop downregulating N/OFQ and NOP expression, further data have demonstrated an increase in protein levels. In a small cohort of patients diagnosed with sepsis, plasma N/OFQ concentrations were measured; levels were higher in patients who died (3 pg mL−1) compared to survivors (1 pg mL−1) (Williams et al. 2008b). An inverse relationship was discovered with regards to ppN/OFQ mRNA in septic patients, with ppN/OFQ levels showing significant reduction when compared to healthy volunteers. Furthermore, this study demonstrated a correlation between increased levels of the septic inflammatory marker, procalcitonin and decreased levels of ppN/OFQ (Stamer et al. 2011). A larger prospective study was undertaken assessing 82 septic patients who were sex and age matched to healthy volunteers. Plasma N/OFQ was measured on the first 2 days after admission to the intensive care unit, with a follow-up sample taken in the recovery period. Radioimmunoassay and PCR data demonstrated an increase in plasma N/OFQ concentrations in Days 1 and 2 compared to recovery. Conversely, mRNA levels of ppN/OFQ and NOP decreased compared to healthy volunteers (Thompson et al. 2013).
3 Central Immune Actions
3.1 CNS Can Propagate an Immune Response Through Several Mechanisms
Despite a long history, the idea that the CNS is an immune-privileged organ is disappearing; the brain can mount immune responses and fight invading organisms (Galea et al. 2007). The meningeal lymphatic vasculature can transport cells and molecules resulting in cross-talk between the peripheral and central immune systems (Raper et al. 2016). According to clinically relevant studies, CNS innate immunity can be activated against pathogenic invasion (Carare et al. 2014). Beside microglia, resident central macrophages, meningeal macrophages and dendritic cells (namely in dura, arachnoid and pia mater, choroid plexus and perivascular spaces) can produce significant protective actions (Herz et al. 2017).
Several cellular components are involved in regulation of central immune response. Microglia are the central immune responders; they have specialised functions with higher reactivity and mobility than other cell populations in the CNS and respond to antigens and neuronal damage. When activated they can release proinflammatory mediators and undergo morphological changes (from round and small cell body with long processes to amoeboid with shorter processes) (Inoue and Tsuda 2018). In addition, they migrate to the site of injury, proliferate, perform phagocytic activity and change their protein expression profile (mainly express complement receptors and major histocompatibility complex proteins). Fully activated microglia resemble other macrophages (Hanisch and Kettenmann 2007; Davoust et al. 2008; Colton and Wilcock 2010).
Astrocytes are the most abundant cell population in the CNS and the term ‘astrocytes’ or ‘astroglia’ is attributed to their star-like shape with diverse processes and morphology depending on anatomical location (Raff et al. 1983; Bailey and Shipley 1993). Their processes cover synapses, contact nodes of Ranvier and form gap junctions between the processes of neighbouring astrocytes. Astrocytes are multifunctional elements participating in local blood flow regulation (Attwell et al. 2010), supplying neuronal nutrients and controlling brain haemostasis (Mulligan and MacVicar 2004; Magistretti 2006; Araque and Navarrete 2010). They form the majority of the blood–brain barrier and control its endothelial elements (Giaume et al. 2007). They can be precursors and are involved in neurogenesis and gliogenesis (Kettenmann and Verkhratsky 2008) along with detection process and guiding the growth of axons and development of certain neuroblasts when neuronal repair is required (Powell and Geller 1999; Araque and Navarrete 2010). Due to their high number of connection sites, astrocytes have high integration capacity and important roles in the regulation of neuronal activity (Smith 2010). They have a role to play in a number of central pathologies (Bundgaard and Abbott 2008). While neurons are able to propagate action potentials, astrocytes are not, and their excitability occurs through increasing the intracellular concentration of calcium ([Ca2+]i) and release of glutamate, purines, Gamma-aminobutyric acid and D-serine. These transmitters might be responsible for astrocyte–astrocyte communication and/or astrocyte–neuron cross-talk (Nedergaard et al. 2003; Seifert et al. 2006). In addition, these gliotransmitters control the dynamics of the synaptic cleft (Cornell-Bell et al. 1990; Volterra and Meldolesi 2005).
Cellular changes associated with microglial or astroglial activation (gliosis; microgliosis and astrogliosis, respectively) have been reported in models of inflammation and chronic pain (Beggs and Salter 2006; Ji and Suter 2007; Inoue and Tsuda 2018; Kohno et al. 2018). Regardless of the order, the sequence and the intensity of glial activation (due to infection, chronic or neuropathic pain and/or opioid tolerance), astrocytes and microglia have been found to be involved in the pathogenesis of the immunomodulation (e.g., in neuropathic pain) in terms of initiation and progress (Raghavendra et al. 2003; Tanga et al. 2004; Ledeboer et al. 2005; Hald et al. 2009). Following activation, glial cells produce and release pain mediators such as nitric oxide and prostaglandins (Watkins and Maier 2000) and proinflammatory cytokines such as IL-1 and TNF-α (Watkins et al. 2001; Marchand et al. 2005; Charo and Ransohoff 2006; Scholz and Woolf 2007).
Oligodendrocytes are well-known myelin producing cells, providing neurone ‘insulation’ and a propagated action potential. In addition, these cells are sensitive to the release of neurotransmitters and neural activity (Bakiri et al. 2009). They play an important role in the pathogenesis of different neurological diseases such as multiple sclerosis. They can release and/or respond to proinflammatory cytokines in response to brain injury (Jurewicz et al. 2005; Ramesh et al. 2012). On the other hand, and despite their specialised function, neurons have been found to release or respond to cytokines in different immunomodulatory conditions (Oh et al. 2001; Zhang et al. 2005).
In summary, complex interplay between neurons, immune cells, and glial cells are responsible for normal regulation and also initiation and maintenance of a number of neuropathologies of which neuropathic pain is an example.
3.2 The Effect of N/OFQ on the Central ‘Immune System’
NOP is expressed centrally by neurons in the brain and spinal cord (Pettersson et al. 2002). In addition, a range of glial cells (astrocytes, oligodendrocytes and microglia) have been found to express NOP receptor (Eschenroeder et al. 2012; Kadhim et al. 2018a). N/OFQ is also produced and released by N/OFQ releasing neurons as well as by a wide range of glial cells (Buzas et al. 1998; Buzas 2002; Eschenroeder et al. 2012; Bedini et al. 2017). N/OFQ-NOP therefore has the potential to modulate glial function.
N/OFQ has been found to play an important role in central immunomodulation but the underlying mechanisms remain to be fully understood. Several possible mechanisms have been proposed (Fig. 1). Proinflammatory cytokines, the main immune modulating molecules, are likely modulated by N/OFQ. Intrathecal administration of N/OFQ induced antagonist-reversed down-regulation of cytokine mRNA transcripts. It has been found that pain processing is accompanied by astrocyte activation, which is characterised by an elevated level of proinflammatory cytokines (Lai et al. 2018). Hence, the antinociceptive effect of N/OFQ might be related to its ability to inhibit cytokine expression and/or release in the CNS (Fu et al. 2007; Finley et al. 2008). In addition, infiltration of peripheral immune cells is an important event in the pathophysiology of immunomodulation and pain (Boddeke 2001). Zhao et al. (2002) reported that increased numbers of microglia induced by trauma were reduced by central administration of N/OFQ. N/OFQ-induced immunomodulation may be as a result of inhibition of the proliferation and migration of infiltrating and resident immune cells (note: in the periphery N/OFQ can both promote and inhibit migration). Furthermore, in the hypothalamic-pituitary-adrenal (HPA) axis, adrenocorticotrophic hormone (ACTH) is well known as a site of immunomodulation and there is controversial evidence with classical opioids (Al-Hashimi et al. 2013). N/OFQ has been found to activate HPA axis and increase the levels of ACTH (Devine et al. 2001).
Moreover, several neurotransmitters involved in the regulation of immune function are affected by N/OFQ-NOP system; these include dopamine, histamine, noradrenaline and glutamate. Dopamine is an immunomodulatory neurotransmitter and inhibition of its release can reduce immune activity (Tsao et al. 1997; Basu and Dasgupta 2000; Nakano et al. 2009). There is an extensive literature base demonstrating that dopamine release is inhibited by N/OFQ (Murphy et al. 1996; Murphy and Maidment 1999; Marti et al. 2004, 2005). Histamine release is an important event involved in the propagation of immune response; morphine-induced central histamine release is also affected by N/OFQ (Eriksson et al. 2000). Along with important roles in the pain pathway, noradrenaline is also an immunomodulator, and its release is inhibited by N/OFQ (Kappel et al. 1998). Given that glutamate and calcium signalling can be important players in immune activation (Watkins et al. 2001; Mattson and Chan 2003), N/OFQ-induced inhibition of glutamate (Nicol et al. 1996; Meis and Pape 2001; Kallupi et al. 2014; Meyer et al. 2017) and LPS-induced calcium signalling (Bedini et al. 2017) possibly affect the pattern of immune activation. The majority of these data are from work in neurones but as we note the brain is so much more than neurones. It can be concluded that the activation of NOP by N/OFQ can participate in central immunomodulation via multiple pathways; if there is disease specificity then this might open some new therapeutic options.
3.3 The Effect of Immunomodulation on the N/OFQ and NOP Receptor
The majority of the text above has covered immunomodulatory effect of NOP, but immune modulation can affect NOP and N/OFQ (the reverse) in the same ways as seen in the periphery in pathologies such as sepsis. As noted in Table 1, the expression profile, integrity and the activity of NOP and N/OFQ can be affected by a wide range of immunomodulatory conditions. These include bacterial products such as LPS, proinflammatory cytokines, ethanol traumatic brain injury, spinal cord injury in cultured neurons cultured glial cells or in whole animals.
4 Conclusions
Since its early description as a peptide receptor system involved in the modulation of pain processing, a plethora of biological functions, pathological indications and, importantly, therapeutic opportunities have been described. We know that classical opioids can modulate immune function and that immune pathologies can modulate opioid receptor, peptide and drug responsiveness. Here we have discussed both peripheral and central immune modulation by N/OFQ-NOP where there are similarities and differences in the brain and periphery. With the generally improved side effect profile for NOP activation with N/OFQ, and novel ligands such as cebranopadol close to the clinic, understanding the clinical consequences of the immune modulatory effects described above will be an area of research focus.
References
Acosta C, Davies A (2008) Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J Neurosci Res 86:1077–1086
Al-Hashimi M, Scott SW, Thompson JP, Lambert DG (2013) Opioids and immune modulation: more questions than answers. Br J Anaesth 111:80–88
Al-Hashimi M, McDonald J, Thompson JP, Lambert DG (2016) Evidence for nociceptin/orphanin FQ (NOP) but not micro (MOP), delta (DOP) or kappa (KOP) opioid receptor mRNA in whole human blood. Br J Anaesth 116:423–429
Alt C, Lam JS, Harrison MT, Kershaw KM, Samuelsson S, Toll L, D’Andrea A (2012) Nociceptin/orphanin FQ inhibition with SB612111 ameliorates dextran sodium sulfate-induced colitis. Eur J Pharmacol 683:285–293
Araque A, Navarrete M (2010) Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci 365:2375–2381
Arjomand J, Cole S, Evans CJ (2002) Novel orphanin FQ/nociceptin transcripts are expressed in human immune cells. J Neuroimmunol 130:100–108
Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243
Bailey MS, Shipley MT (1993) Astrocyte subtypes in the rat olfactory bulb: morphological heterogeneity and differential laminar distribution. J Comp Neurol 328:501–526
Bakiri Y, Burzomato V, Frugier G, Hamilton NB, Karadottir R, Attwell D (2009) Glutamatergic signaling in the brain’s white matter. Neuroscience 158:266–274
Basso M, Risse PA, Naline E, Calo G, Guerrini R, Regoli D, Advenier C (2005) Nociceptin/orphanin FQ inhibits electrically induced contractions of the human bronchus via NOP receptor activation. Peptides 26:1492–1496
Basu S, Dasgupta PS (2000) Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol 102:113–124
Bedini A, Baiula M, Vincelli G, Formaggio F, Lombardi S, Caprini M, Spampinato S (2017) Nociceptin/orphanin FQ antagonizes lipopolysaccharide-stimulated proliferation, migration and inflammatory signaling in human glioblastoma U87 cells. Biochem Pharmacol 140:89–104
Beggs S, Salter MW (2006) Neuropathic pain: symptoms, models, and mechanisms. Drug Dev Res 67:289–301
Bidlack JM (2000) Detection and function of opioid receptors on cells from the immune system. Clin Diagn Lab Immunol 7:719
Bird MF, Guerrini R, Willets JM, Thompson JP, Caló G, Lambert DG (2018) Nociceptin/Orphanin FQ (N/OFQ) conjugated to ATTO594; a novel fluorescent probe for the NOP receptor. Br J Pharmacol 175:4496
Boddeke EWGM (2001) Involvement of chemokines in pain. Eur J Pharmacol 429:115–119
Bundgaard M, Abbott NJ (2008) All vertebrates started out with a glial blood-brain barrier 4–500 million years ago. Glia 56:699–708
Buzas B (2002) Regulation of nociceptin/orphanin FQ gene expression in astrocytes by ceramide. Neuroreport 13:1707–1710
Buzas B, Rosenberger J, Cox BM (1998) Activity and cyclic AMP-dependent regulation of nociceptin/orphanin FQ gene expression in primary neuronal and astrocyte cultures. J Neurochem 71:556–563
Cadet P, Mantione K, Bilfinger TV, Stefano GB (2001) Real-time RT-PCR measurement of the modulation of Mu opiate receptor expression by nitric oxide in human mononuclear cells. Med Sci Monit 7:1123–1128
Caldiroli E, Leoni O, Cattaneo S, Rasini E, Marino V, Tosetto C, Mazzone A, Fietta AM, Lecchini S, Frigo GM (1999) Neutrophil function and opioid receptor expression on leucocytes during chronic naltrexone treatment in humans. Pharmacol Res 40:153–158
Carare RO, Hawkes CA, Weller RO (2014) Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav Immun 36:9–14
Carvalho D, Petronilho F, Vuolo F, Machado RA, Constantino L, Guerrini R, Calo G, Gavioli EC, Streck EL, Dal-Pizzol F (2008) The nociceptin/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Med 34:2284–2290
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621
Colton CA, Wilcock DM (2010) Assessing activation states in microglia. CNS Neurol Disord Drug Targets 9:174–191
Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247:470–473
D’Addario C, Caputi FF, Ekström TJ, Di Benedetto M, Maccarrone M, Romualdi P, Candeletti S (2013) Ethanol induces epigenetic modulation of prodynorphin and pronociceptin gene expression in the rat amygdala complex. J Mol Neurosci 49:312–319
Davoust N, Vuaillat C, Androdias G, Nataf S (2008) From bone marrow to microglia: barriers and avenues. Trends Immunol 29:227–234
Devine DP, Watson SJ, Akil H (2001) Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic–hypothalamic–pituitary–adrenal axis. Neuroscience 102:541–553
Eriksson KS, Stevens DR, Haas HL (2000) Opposite modulation of histaminergic neurons by nociceptin and morphine. Neuropharmacology 39:2492–2498
Eschenroeder AC, Vestal-Laborde AA, Sanchez ES, Robinson SE, Sato-Bigbee C (2012) Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of μ-opioid-and nociceptin/orphanin FQ receptors in cell development: implications for drug addiction treatment during pregnancy. Glia 60:125–136
Finley MJ, Happel CM, Kaminsky DE, Rogers TJ (2008) Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol 252:146–154
Fiset ME, Gilbert C, Poubelle PE, Pouliot M (2003) Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochemistry 42:10498–10505
Fu X, Zhu ZH, Wang YQ, Wu GC (2007) Regulation of proinflammatory cytokines gene expression by nociceptin/orphanin FQ in the spinal cord and the cultured astrocytes. Neuroscience 144:275–285
Galea I, Bechmann I, Perry VH (2007) What is immune privilege (not)? Trends Immunol 28:12–18
Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A (2007) Glia: the fulcrum of brain diseases. Cell Death Differ 14:1324–1335
Gough H, Grabenhenrich L, Reich A, Eckers N, Nitsche O, Schramm D, Beschorner J, Hoffmann U, Schuster A, Bauer CP, Forster J, Zepp F, Lee YA, Bergmann RL, Bergmann KE, Wahn U, Lau S, Keil T (2015) Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol 26:431–437
Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM (2009) Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain 13:138–145
Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH (2008) Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 178:218–224
Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J (2017) Myeloid cells in the central nervous system. Immunity 46:943–956
Hom JS, Goldberg I, Mathis J, Pan YX, Brooks AI, Ryan-Moro J, Scheinberg DA, Pasternak GW (1999) [(125)I]orphanin FQ/nociceptin binding in Raji cells. Synapse 34:187–191
Hussey HH, Katz S (1950) Infections resulting from narcotic addiction: report of 102 cases. Am J Med 9:186–193
Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32:11187–11200
Inoue K, Tsuda M (2018) Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19:138
Ji R-R, Suter MR (2007) p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain 3:33
Jurewicz A, Matysiak M, Tybor K, Kilianek L, Raine CS, Selmaj K (2005) Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain 128:2675–2688
Kadhim S, McDonald J, Lambert D (2018a) Nociceptin/Orphanin FQ (NOP) receptor is differentially expressed on glial cells. Br J Anaesth 120:e6
Kadhim S, McDonald J, Lambert DG (2018b) Opioids, gliosis and central immunomodulation. J Anesth 32:756–767
Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M (2014) Nociceptin/Orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology 39:1081–1092
Kappel M, Poulsen TD, Galbo H, Pedersen BK (1998) Effects of elevated plasma noradrenaline concentration on the immune system in humans. Eur J Appl Physiol Occup Physiol 79:93–98
Kato S, Tsuzuki Y, Hokari R, Okada Y, Miyazaki J, Matsuzaki K, Iwai A, Kawaguchi A, Nagao S, Itoh K, Suzuki H, Nabeshima T, Miura S (2005) Role of nociceptin/orphanin FQ (Noc/oFQ) in murine experimental colitis. J Neuroimmunol 161:21–28
Kettenmann H, Verkhratsky A (2008) Neuroglia: the 150 years after. Trends Neurosci 31:653–659
Kohno K, Kitano J, Kohro Y, Tozaki-Saitoh H, Inoue K, Tsuda M (2018) Temporal kinetics of microgliosis in the spinal dorsal horn after peripheral nerve injury in rodents. Biol Pharm Bull 41:1096–1102
Lai H-C, Lu C-H, Wong C-S, Lin B-F, Chan S-M, Kuo C-Y, Wu Z-F (2018) Baicalein attenuates neuropathic pain and improves sciatic nerve function recovery in rats with partial sciatic nerve transection. J Chin Med Assoc 81:955–963
Lambert DG (2008) The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7:694–710
Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR (2005) Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115:71–83
Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF Jr, Wardlaw AJ, Wenzel SE, Greenberger PA (2011) Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 127:355–360
Madden JJ, Whaley WL, Ketelsen D, Donahoe RM (2001) The morphine-binding site on human activated T-cells is not related to the mu opioid receptor. Drug Alcohol Depend 62:131–139
Magistretti PJ (2006) Neuron–glia metabolic coupling and plasticity. J Exp Biol 209:2304–2311
Manfredi B, Sacerdote P, Bianchi M, Locatelli L, Veljic-Radulovic J, Panerai AE (1993) Evidence for an opioid inhibitory effect on T cell proliferation. J Neuroimmunol 44:43–48
Marchand F, Perretti M, McMahon SB (2005) Role of the immune system in chronic pain. Nat Rev Neurosci 6:521–532
Marti M, Mela F, Veronesi C, Guerrini R, Salvadori S, Federici M, Mercuri NB, Rizzi A, Franchi G, Beani L (2004) Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior. J Neurosci 24:6659–6666
Marti M, Mela F, Fantin M, Zucchini S, Brown JM, Witta J, Di Benedetto M, Buzas B, Reinscheid RK, Salvadori S (2005) Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson’s disease. J Neurosci 25:9591–9601
Mattson MP, Chan SL (2003) Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium 34:385–397
Meis S, Pape HC (2001) Control of glutamate and GABA release by nociceptin/orphanin FQ in the rat lateral amygdala. J Physiol 532:701–712
Meyer LC, Paisley CE, Mohamed E, Bigbee JW, Kordula T, Richard H, Lutfy K, Sato-Bigbee C (2017) Novel role of the nociceptin system as a regulator of glutamate transporter expression in developing astrocytes. Glia 65:2003–2023
Minami M, Yamakuni H, Ohtani Y, Okada M, Nakamura J, Satoh M (2001) Leukemia inhibitory factor induces nociceptin mRNA in cultured rat cortical neurons. Neurosci Lett 311:17–20
Morgan EL (1996) Regulation of human B lymphocyte activation by opioid peptide hormones. Inhibition of IgG production by opioid receptor class (mu-, kappa-, and delta-) selective agonists. J Neuroimmunol 65:21–30
Mulligan SJ, MacVicar BA (2004) Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431:195–199
Murphy NP, Maidment NT (1999) Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem 73:179–186
Murphy NP, Ly HT, Maidment NT (1996) Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 75:1–4
Nakano K, Matsushita S, Saito K, Yamaoka K, Tanaka Y (2009) Dopamine as an immune-modulator between dendritic cells and T cells and the role of dopamine in the pathogenesis of rheumatoid arthritis. Nihon Rinsho Meneki Gakkai Kaishi 32:1–6
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530
Nicol B, Lambert DG, Rowbotham DJ, Smart D, McKnight AT (1996) Nociceptin induced inhibition of K+ evoked glutamate release from rat cerebrocortical slices. Br J Pharmacol 119:1081–1083
Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001) Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 21:5027–5035
Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gavériaux-Ruff C (1998) Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol 81:184–192
Pettersson LM, Sundler F, Danielsen N (2002) Expression of orphanin FQ/nociceptin and its receptor in rat peripheral ganglia and spinal cord. Brain Res 945:266–275
Popiolek-Barczyk K, Rojewska E, Jurga AM, Makuch W, Zador F, Borsodi A, Piotrowska A, Przewlocka B, Mika J (2014) Minocycline enhances the effectiveness of nociceptin/orphanin FQ during neuropathic pain. Biomed Res Int 2014:762930
Powell EM, Geller HM (1999) Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia 26:73–83
Raff MC, Abney ER, Cohen J, Lindsay R, Noble M (1983) Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci 3:1289–1300
Raghavendra V, Tanga F, DeLeo JA (2003) Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 306:624–630
Ramesh G, Benge S, Pahar B, Philipp MT (2012) A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J Neuroinflammation 9:72
Raper D, Louveau A, Kipnis J (2016) How do meningeal lymphatic vessels drain the CNS? Trends Neurosci 39:581–586
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10:1361
Seifert G, Schilling K, Steinhäuser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7:194
Serhan CN, Fierro IM, Chiang N, Pouliot M (2001) Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epi-lipoxin A4. J Immunol 166:3650–3654
Shah S, Page CP, Spina D (1998) Nociceptin inhibits non-adrenergic non-cholinergic contraction in guinea-pig airway. Br J Pharmacol 125:510–516
Singh S, Sullo N, Bradding P, Agostino B, Brightling C, Lambert D (2013) Role of nociceptin orphanin FQ peptide – receptor system in mast cell migration. Eur Respir J 42:P587
Singh SR, Sullo N, Matteis M, Spaziano G, McDonald J, Saunders R, Woodman L, Urbanek K, De Angelis A, De Palma R, Berair R, Pancholi M, Mistry V, Rossi F, Guerrini R, Calò G, D’Agostino B, Brightling CE, Lambert DG (2016) Nociceptin/orphanin FQ (N/OFQ) modulates immunopathology and airway hyperresponsiveness representing a novel target for the treatment of asthma. Br J Pharmacol 173:1286–1301
Smith K (2010) Neuroscience: settling the great glia debate. Nature 468:160–162
Stamer UM, Book M, Comos C, Zhang L, Nauck F, Stüber F (2011) Expression of the nociceptin precursor and nociceptin receptor is modulated in cancer and septic patients. Br J Anaesth 106:566–572
Tanga FY, Raghavendra V, DeLeo JA (2004) Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int 45:397–407
Thomas R, Stover C, Lambert DG, Thompson JP (2014) Nociceptin system as a target in sepsis? J Anesth 28:759–767
Thompson JP, Serrano-Gomez A, McDonald J, Ladak N, Bowrey S, Lambert DG (2013) The Nociceptin/Orphanin FQ system is modulated in patients admitted to ICU with sepsis and after cardiopulmonary bypass. PLoS One 8:e76682
Trombella S, Vergura R, Falzarano S, Guerrini R, Calo G, Spisani S (2005) Nociceptin/orphanin FQ stimulates human monocyte chemotaxis via NOP receptor activation. Peptides 26:1497–1502
Tsao C-W, Lin Y-S, Cheng J-T (1997) Effect of dopamine on immune cell proliferation in mice. Life Sci 61:PL361–PL371
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6:626
Watkins LR, Maier SF (2000) The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol 51:29–57
Watkins LR, Milligan ED, Maier SF (2001) Glial activation: a driving force for pathological pain. Trends Neurosci 24:450–455
Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH (1995) Expression of alternate forms of brain opioid ‘orphan’ receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Mol Brain Res 32:342–347
Williams JP, Thompson JP, Rowbotham DJ, Lambert DG (2008a) Human peripheral blood mononuclear cells produce pre-pro-nociceptin/orphanin FQ mRNA. Anesth Analg 106:865–866
Williams JP, Thompson JP, Young SP, Gold SJ, McDonald J, Rowbotham DJ, Lambert DG (2008b) Nociceptin and urotensin-II concentrations in critically ill patients with sepsis. Br J Anaesth 100:810–814
Witta J, Buzas B, Cox BM (2003) Traumatic brain injury induces nociceptin/orphanin FQ expression in neurons of the rat cerebral cortex. J Neurotrauma 20:523–532
Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ (2005) A proinflammatory chemokine, CCL3, sensitizes the heat-and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci 102:4536–4541
Zhang Y, Gandhi PR, Standifer KM (2012) Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Mol Pain 8:76
Zhang L, Stuber F, Stamer UM (2013) Inflammatory mediators influence the expression of nociceptin and its receptor in human whole blood cultures. PLoS One 8:e74138
Zhang L, Stuber F, Lippuner C, Schiff M, Stamer UM (2016) Phorbol-12-myristate-13-acetate induces nociceptin in human Mono Mac 6 cells via multiple transduction signalling pathways. Br J Anaesth 117:250–257
Zhao H, Huang HW, Wu GC, Cao XD (2002) Effect of orphanin FQ on interleukin-1beta mRNA transcripts in the rat CNS. Neuroscience 114:1019–1031
Acknowledgements
Work on immune effects of opioids in Leicester is funded by Biotechnology and Biological Sciences Research Council and British Journal of Anaesthesia. SK is funded by a scholarship from Higher Committee for Education Development in Iraq.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kadhim, S., Bird, M.F., Lambert, D.G. (2019). N/OFQ-NOP System in Peripheral and Central Immunomodulation. In: Ko, MC., Caló, G. (eds) The Nociceptin/Orphanin FQ Peptide Receptor. Handbook of Experimental Pharmacology, vol 254. Springer, Cham. https://doi.org/10.1007/164_2018_203
Download citation
DOI: https://doi.org/10.1007/164_2018_203
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20185-2
Online ISBN: 978-3-030-20186-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)