Abstract
Designing self-healing in metals is a challenging task. Self-healing concepts successfully applied in polymers cannot be directly transferred because of different energetics. This has detained the field of self-healing metals, as evidenced by absolute publication numbers. Yet, relative publication numbers indicate a rapidly increasing interest in recent years triggered by the potential economic impact of advanced metallic materials. This chapter reviews all currently available self-healing concepts in bulk metallic materials. We provide a classification into two conceptually distinct routes: (1) autonomous self-healing of nanovoids at the nanoscale, aiming at a prevention of large-scale damage and (2) non-autonomous self-healing of macrocracks by an external trigger such as heat. The general idea of each self-healing concept is comprehensibly introduced, relevant publications are reviewed, and the characteristics of the concepts are compared. Finally, we discuss current constraints and identify the most promising concepts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Crack closure
- Nanoparticles
- Phase transformation
- Precipitation
- Self-healing metals
- Shape-memory alloys
- Solder
1 Introduction: Status of Self-Healing Metals
The field of self-healing materials is dominated by polymer-based systems. This dominance has been stressed in other reviews (see, e.g., [1–6] and the accompanying reviews in this volume) and it also becomes apparent by analyzing publication numbers in the field of self-healing materials. Figure 1 shows an exponentially increasing number of polymer-based publications on self-healing (blue line) with an absolute number of 250 in 2014. A similar search for self-healing concrete and ceramics and self-healing metals reveals much smaller numbers (orange and gray lines). In fact, the gray line for metals is an overestimate. Optimization of the search keywords to rule out metallic ion-related self-healing polymers and surface oxides shows the true self-healing metal curve to be the black line.
Publication numbers from ISI Web of Knowledge for the last 14 years in the field of self-healing polymers, concrete and ceramics, and metals. The search term for metals (gray line) was TOPIC: (“self-healing”) AND TOPIC: (metal). Because the search term includes polymer self-healing studies employing “metal-ions” and also polymeric or oxide self-healing coating studies, a corrected search term was employed for metals, TOPIC: (“self-healing”) AND TOPIC: (metal) NOT TOPIC: (*polymer* OR *coating*). The latter results are shown by the black line (enlarged in the inset) and give a more representative publication curve for bulk metallic materials, which are the main focus of this review
The reason for this dominance of polymers is the fact that the self-healing concept is highly compatible with the energetic properties of polymers. Chemical reactions in polymers are very efficient, that is, they produce a significant energy release compared with the typical bonding strength. Relatively fast and massive diffusional processes are thus feasible, even at room temperature. These processes can be utilized to design self-healing agents that are autonomously activated and transported to sites of damage localization.
Atomic bonding is strong in concrete and ceramics and in metals. Diffusional processes that are needed to transport the self-healing agents to the damaged sites are therefore slow at ambient temperatures. The traditional concepts successfully applied in polymer-based self-healing materials cannot be directly transferred. The concepts must be modified or novel concepts must be developed. For that reason, the fields of self-healing concrete and ceramics and self-healing metals are less mature than that of self-healing polymers. In fact, judging by the absolute publication numbers (inset in Fig. 1), the field of self-healing in bulk metallic materials is in its infancy.
Yet, the black curve (inset of Fig. 1) shows a significant positive gradient over the last few years, indicating a rapidly increasing interest. This interest can be understood if one considers the role of metallic materials in the world today and the ecological and economic concerns associated with their production, use, and re-use. Although often well hidden, it is the discovery and employment of advanced metallic materials that undoubtedly trigger changes that revolutionize, for example, energy, transportation, health, safety, and infrastructure sectors. Examples are numerous: advanced high-strength steels enable lighter and safer cars, aluminum alloys enable larger and more fuel-efficient planes, creep-resistant nickel alloys enable more efficient power plants and long-life plane turbines, etc. All in all, metals-related industries account for ∼3.5 billion US$ of exports in 2013 in the world [7]. However, this high rate of industrialization is responsible for increasing natural problems, primarily regarding climate change [8]. Therefore, to avoid harmful effects the Intergovernmental Panel on Climate Change (IPCC) has recommended a challenging cut of 50–85% in global emission of greenhouse gases by 2050 [9]. Detailed investigations reveal that material efficiency options are compulsory in achieving this goal [8]. It is for this reason that, despite the inherent difficulties, successful self-healing in bulk metals would have enormously positive consequences, as such mechanisms have the potential to increase service lifetime significantly.
2 Classification of Self-Healing Metals
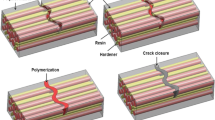
Schematics, features, and relevant publications for the presently available self-healing concepts in bulk metals are compiled in Fig. 2 and Tables 1 and 2, respectively. We found it useful to classify the concepts by employing the characteristic length scale of the healed damage. According to this classification there are two groups defined by (1) healing of nanoscale voids in the nanometer range (precipitation and nanoSMA-dispersoids concepts) and (2) healing of macroscale cracks in the millimeter range (SMA-clamp&melt, solder tubes/capsules, coating agent, and electro-healing concepts). The healing within each group occurs exclusively on the respective scale. Within the first group, only the nanoscale is accessible and, thus, if macrocracks appear, they cannot be healed and lead eventually to fracture. That stated, because the crack coalescence process involves interaction of spatially dispersed cracks, it can be safely proposed that these concepts would also be effective in slowing down the overall failure process. Within the second group, only the macroscale is observable and, thus, nanovoids are not healed until they grow or coalesce to form macrocracks. This could be seen as a disadvantage for the service life because the presence of unhealed nanovoids would cause fast growth of secondary macrocracks, even if the first macrocrack is healed. It should, as a general note, be stated that the success of any self-healing strategy needs to be considered in connection with the capabilities of the base microstructure. That is, introduction of a nanoscale self-healing mechanism could be more crucial in rendering an originally brittle microstructure sufficiently tough for application, compared to its effect in a microstructure that already had plenty of microstructural hardening mechanisms.
Our classification corresponds well with the classification into damage prevention and damage management introduced by van der Zwaag [10]. The self-healing concepts in the nanoscale group pursue the management of nanoscale damage in order to prevent macroscale damage (see Table 1, rows 1–3). In contrast, the self-healing concepts in the macroscale group pursue management of macroscale damage.
Manuel [11] has proposed a classification based on the type of phase transformation involved during healing. Row 4 of Table 1 shows that the self-healing concepts exhibiting solid-state healing according to Manuel’s classification correspond to the group of nanoscale concepts. Three of the macroscale concepts utilize liquid-assisted healing in the sense that the healing temperature must be close to the melting temperature of the solder (i.e., of the low-melting material used to “glue” the cracks together). The electro-healing concept does not fit well into Manuel’s original definitions (solid-state versus liquid-assisted) and we have therefore introduced a third class of electrolyte-assisted self-healing concepts.
The next section introduces the general idea of each of the available self-healing concepts, following the schematics in Fig. 2 along with relevant publications.
3 Proposed Self-Healing Concepts in Metals
3.1 High-T Precipitation
The self-healing concept using precipitation at high temperatures (high-$T$ precipitation) is the most intensively studied concept. Corresponding studies have been initiated and advanced by the group of Shinya from NIMS, Japan [12–16] (cf. Table 2). The group of van der Zwaag from TU Delft, The Netherlands has contributed several important investigations [17–21]. Besides these experimental studies, corresponding modeling efforts have recently been started by Karpov et al. [22]. A detailed introduction to the high-T precipitation concept can be found in Shinya’s review [23]. Here we concentrate on the general idea and on results obtained in recent years.
Figure 2a (first column of Fig. 2) shows the relevant requirements and processes of the high-T precipitation concept. The original microstructure (Fig. 2a, row 1) must contain a supersaturated amount of solute atoms. The supersaturation can be achieved by conventional metallurgical treatment, given that the phase diagram of the constituent atoms shows sufficient solubility of the solute atoms at high temperatures. Quenching from these temperatures produces a metastable, supersaturated solid solution, which upon additional aging tends to precipitate secondary phases. However, for the high-T precipitation concept to work properly, precipitation should not happen spontaneously throughout the microstructure, but only in localized regions where nanovoids are present. This is illustrated in Fig. 2a, row 2, where a nanovoid forms at a grain boundary during the damage phase and acts as an attractor for the solute atoms during the healing stage (Fig. 2a, row 3) and eventually as a nucleation site for the precipitation process (Fig. 2a, row 4).
An important feature of the high-T precipitation concept is that the temperature during the service lifetime must be sufficiently high. This is reflected by the name itself and is also highlighted in Fig. 2a by the red shaded background behind all four stages. An elevated temperature is required to enable lattice diffusion of the solutes toward the nanovoids, as indicated by the arrows in the healing stage. As mentioned above, the temperature must be not too high to prevent nucleation of precipitates at sites other than the nanovoids.
The described conditions on the phase diagram and for the service temperatures give significant constraints on the materials and applications for the high-T precipitation concept. One successful example was provided by Laha et al. [13], who applied this concept to improve the creep resistance of heat-resisting steels. The reference material studied by the authors was a standard 347 austenitic stainless steel, which is used in high temperature applications. A second modified steel with self-healing ability was prepared by adding boron to the standard 347 steel. The B atoms act as the solute healing agent by diffusing to the nanovoids and precipitating at the void surfaces. The precipitation was confirmed by Auger spectroscopy, and a significant improvement in creep properties was demonstrated by corresponding tests [13] (cf. Fig. 3). Further successful investigations were conducted by employing B together with N as solute atoms [14] and in Cu-modified heat-resisting steel [15].
Creep cavity growth rate and creep strain with creep exposure time in Ar at 750°C and 78 MPa. The growth rate in boron-modified 347 steel is slower by an order of magnitude than that of standard 347 steel. Figure based on data from Laha et al. [13]
A slightly different route toward understanding and optimizing the high-T precipitation concept was undertaken by the group of van der Zwaag from TU Delft. Rather than investigating complex industrial steel grades, they focused on high purity model systems of FeCu + BN for analysis of the fundamental mechanisms. In addition to considering model systems, high resolution techniques were employed for that purpose. For example, He et al. [17] used positron annihilation spectroscopy (PAS) to clearly confirm that the addition of B and N significantly accelerates Cu precipitation in a Fe–Cu alloy and that most open volume defects (nanovoids) can be closed. This result was corroborated by He et al. [18] using in-situ time-resolved small-angle neutron scattering (SANS) measurements. They found that Cu precipitation occurs in the form of spherical nanoscale precipitates inside the grains and in the form of dislocation and interface decoration. More recent investigations by the Delft group focused on replacing Cu by Au (i.e., FeAuBN) [20, 21]. The choice of Au was motivated by an atomic-scale analysis showing that homogenous nucleation can be prevented while enhancing nucleation at the damaged nanovoids sites [20]. SANS measurements indeed confirmed that Au strongly precipitates at nanovoids [20], and creep tests showed an improved creep lifetime [21].
3.2 Low-T Precipitation
The low-T precipitation concept is closely related to the high-T precipitation concept, with the main difference being that at all stages during service the temperature can be lower, as indicated by the blue background shading in Fig. 2b. The original idea was introduced by the group of Lumley from CSIRO, Australia [24–27] and further work was contributed by the TU Delft group [28–31] (Table 2). We give here a concise summary, while a detailed introduction can be found in reviews by Shinya [23] and Lumley [32].
As in the case of the high-T precipitation concept, the microstructure required for the low-T precipitation concept must contain supersaturated solute atoms. A distinctive and necessary feature for the healing process is that the solute atoms tend to segregate to dislocation cores, as indicated in Fig. 2b, row 1. In particular, the solute atoms within the dislocation cores can be considered mobile even at low temperatures, which is a consequence of pipe diffusion. When localization of dislocations in pile ups leads to stress concentration and formation of nanovoids, the mobile solute atoms are attracted and diffuse through the dislocations toward the stress region. Precipitation within the nanovoids eventually leads to void closure and healing of the damaged region.
Potential material systems with such a self-healing ability are Al alloys supersaturated with Cu solutes. A crucial requirement is that the Al alloys are prepared in an under-aged condition, that is, aging should be aborted before the peak strength of the material is reached. This condition guarantees that there are enough Cu atoms left in solution for performing the self-healing process in the form of precipitation.
Lumley studied under-aged Al alloys in detail and showed that they can posses enhanced creep behavior [24], fracture toughness [25], and fatigue resistance [26]. In particular, enhanced fatigue resistance (Fig. 4) is relevant in the present context because it can be explained in terms of the low-T precipitation concept, that is, Cu atoms are assumed to diffuse through dislocations by pipe diffusion toward the nanovoids and precipitate there, leading to void closure. This assumption is supported to some extent by positron annihilation spectroscopy investigations performed by Hautakangas et al. [28, 29]. Other possible mechanisms for the enhanced fatigue resistance are conceivable: (1) a general reduction in dislocation mobility caused by the Cu solutes and (2) localized matrix hardening as a result of dynamic precipitation at dislocations [27]. The actual contribution of nanovoid closure as a result of Cu precipitation to the enhanced fatigue resistance is not yet fully clarified.
Comparison between fatigue life of standard, peak-aged, and under-aged Al alloys for a stress ratio R = −1. Overall, the under-aged material shows a better fatigue resistance because it reaches larger cycles to failure at the same stress level. Figure based on data from Lumley et al. [26]
Besides this uncertainty in the actual mechanism, a technologically more important issue was raised by Wanhill [33]. By comparing various fatigue studies, he concluded that self-healing in commercially used Al alloys, particularly in aerospace applications, is inapplicable. The reasons are that most damage is either too large to be healed or is located on the surface of the component, where environmental effects could potentially hinder crack healing. These statements possibly explain the rather limited interest in the low-T precipitation concept in recent years (cf. Table 2).
3.3 NanoSMA-Dispersoids
Crack closure is a well-known phenomenon under cyclic loading conditions [34], and phase transformation is often discussed as one of the mechanisms responsible for it [35, 36]. Although direct experimental verification of the microstructural processes is scarce because of experimental challenges [37], results are available for shape memory alloys (SMA) [38], where the role of the transformation is more evident, clarifying the strength of this mechanism. Motivated by these observations, the nanoSMA-dispersoids self-healing concept was only very recently proposed by the present authors [39]. This concept belongs to the group of nanometer-scale healing mechanisms and the general idea is that nanovoids are closed by phase-transforming SMA nanoparticles. Note that the concept is presently in the development stage and that the self-healing ability has yet to be confirmed.
The nanoSMA-dispersoids self-healing idea is sketched in Fig. 2c. The original microstructure consists of a host matrix with embedded coherent SMA nanoparticles (Fig. 2c, row 1). The nanoparticles are stabilized by the host matrix in their austenite phase (i.e., high temperature phase). When damage is initiated in the form of dislocation localization and nanovoid formation (Fig. 2c, row 2), the nanoparticles are activated. In particular, the stress field of the nanovoid is thought to trigger phase transformation of the SMA nanoparticle from the austenite into the martensite phase. This phase transformation is accompanied by a strong change in the shape of the particle (Fig. 2c, row 3) that induces local strain fields on the host matrix and eventually leads to crack closure (Fig. 2c, row 4).
A crucial stage of the described self-healing process is crack closure by the phase-transforming nanoparticle. Recent theoretical simulations provide strong support that such a crack closure scenario is indeed realistic. Xu and Demkowicz [40] investigated the migration of a stress-driven grain boundary and found that close-by nanocracks are significantly affected. As illustrated in Fig. 5 , when a stress-driven grain boundary (GB2) moves toward the crack, the size of the crack is reduced and, depending on the stress magnitude, the crack can be completely closed leaving behind only geometrically necessary dislocations [40]. Detailed analysis showed that stress fields around the grain boundaries (see blue and red fields in Fig. 5b) generated image stress fields at the crack tip, eventually leading to crack healing.
Nanocrack healing by stress-driven grain boundary (GB) migration. The stress (τ) is applied such that GB1 does not move while GB2 moves toward the crack. (a) Atomistic picture, with the perfect bulk atoms removed for clarity. (b) Resulting strain fields (blue compressive, red tensile field). Figure adapted from [40] with permission
In the work of Xu and Demkowicz [40], crack closure was achieved by an externally driven (i.e., non-autonomous) grain boundary migration. In the nanoSMA-dispersoids self-healing concept, the moving grain boundary is replaced by the moving interface between host matrix and nanoparticle during its phase transformation. If similar stress fields can be achieved by the SMA nanoparticle transformation, as in the case of the moving grain boundary, autonomous nanovoid closure can be achieved.
A main challenge in implementing the nanoSMA-dispersoids concept in practice is production of the desired microstructure (Fig. 2c, row 1). For the nanoparticles, NiTi SMA is very well suited as it has been thoroughly investigated and shows significant shape changes during transformation. Present investigations focus on determining an ideal alloying addition to NiTi, with the condition that a double phase field connecting the NiTi phase with a solid solution is present in the phase diagram. Such a phase diagram would allow use of an annealing step, followed by an aging treatment to nucleate NiTi SMA nanoparticles inside the solid solution host matrix. To optimize the search for the alloying element, highly accurate finite temperature first-principles simulations are currently being developed [41–43] to provide the required phase stability dependencies.
3.4 SMA-Clamp&Melt
The SMA-clamp&melt concept (also called SMA reinforcement or SMA self-healing) is possibly the oldest self-healing concept for bulk metals. First investigations were started at the end of the 1990s by the group of Olson at the Northwestern University of Chicago, USA [44]. Manuel continued research at Northwestern [45, 46] and later at the University of Florida [47, 48] in collaboration with the Materials Science Division of NASA [48]. Recently, the group of Rohatgi at the University of Wisconsin-Milwaukee also began investigations [49, 50] (cf. Table 2). Because of the wide interest, the SMA-clamp&melt concept is currently the best-investigated macroscale concept. A detailed introduction can be found in the review by Manuel [11]. Here we discuss only the general idea of this concept.
Figure 2d illustrates the required microstructure and the self-healing process. The crucial difference from the self-healing concepts discussed so far relates to the characteristic length scale. The relevant microstructural features as well as the cracks to be healed are in the millimeter regime. In particular, a composite microstructure is required that consists of SMA reinforcement wires embedded in a solder matrix material (Fig. 2d, row 1). The solder material is the “glue”, and for that purpose it must have a melting point that is considerably lower than that of the SMA wires.
When the stress applied to a SMA–solder composite exceeds the ultimate tensile strength of the solder material, a crack is produced in the solder material (Fig. 2d, row 2). The SMA wires have a higher ultimate tensile strength and they respond to the applied stress by a transformation into the martensite phase. To achieve self-healing, the composite sample needs to be externally heated (cf. red shaded background in the healing stage; Fig. 2d, row 3) to a temperature above the austenite transition temperature. The increase in temperature leads to phase transformation of the SMA wires from the martensite to the high-temperature austenite phase. The transition is accompanied by a compressive stress that contracts the composite sample, bringing the cracked surfaces together. The temperature needs to be further increased toward the melting point of the solder. Once the cracked surfaces have started to melt, they can rejoin and the temperature can be reduced to the original, low value.
A unique advantage of this self-healing concept is that (in the ideal case) it can be repeated limitlessly. This is indicated in Fig. 2d by the fact that the original structure (Fig. 2d, row 1) is exactly the same as the healed structure (Fig. 2d, row 4). A marked disadvantage is the strong anisotropic response. As evident from the schematics, an applied horizontal stress causes a crack, leading to catastrophic fracture of the sample. Extensions of the present concept toward a three-dimensional SMA wire network are conceivable, preventing such material failure. Whether this idea can be implemented in practice remains to be shown.
A further disadvantage, common to all macroscale approaches, is the requirement for an externally applied trigger to activate the self-healing process. In the case of the SMA-clamp&melt concept, the external trigger is the heat transfer required to transform the SMA wires and melt the solder. This renders the process non-autonomous, such that possible industrial applications would require routine service intervals.
A successful example of the SMA-clamp&melt concept has been presented by Manuel and Olson [46]. The authors used the CALPHAD method [51] to select and optimize the material system and its properties. The finally selected material was a Sn-13at%Bi alloy matrix (Sn–Bi alloy with 13 Bi atoms and 87 Sn atoms per 100 atoms) containing continuous uniaxially oriented equiatomic TiNi SMA wires. The composition of the matrix was designed for a healing temperature of 169°C, with a 20% liquid fraction. This proof-of-concept composite was subjected to a tensile test, which confirmed more than 95% recovery of the ultimate tensile strength after self-healing [46]. Images of damaged and healed samples are presented in Fig. 6.
Demonstration of the SMA-clamp&melt self-healing concept. (a) Damaged SMA-matrix composite with a through-matrix crack. (b) Sample after self-healing. Figure taken from [46] with permission
Recent investigations have been directed toward industrially more relevant host matrix materials. For instance, Ferguson et al. [50] investigated the self-healing properties of a commercial ZnAlCu alloy enforced with TiNi SMA wires. A crucial insight was that encasing the samples in sand was necessary to maintain structural stability. However, even with this auxiliary technique, the retained ultimate tensile strength was only 30%. In another study, NASA [48] tested Al-based alloys with the focus on improving the damage tolerance of aeronautical structures. The authors [48] could show that an AlSi matrix reinforced with 2 vol% of SMA wires can retain more than 90% ultimate tensile strength after self-healing.
3.5 Solder Tubes/Capsules
The solder tubes/capsules concept is very interesting because it emulates the original self-healing concept employed in polymers [52]. It was tried by the group of Rohatgi at the University of Wisconsin-Milwaukee [53, 54] and is discussed in detail in the book by Nosonovsky and Rohatgi [55].
The central idea of the solder tubes/capsules concept is to encapsulate a solder material inside ceramic capsules or ceramic tubes, inside a host matrix of a higher melting material (Fig. 2e, row 1). Compared with the SMA-clamp&melt concept discussed in the previous section, the role of the solder is critically modified in the solder tubes/capsules concept. For the SMA-clamp&melt concept, the solder constitutes the host matrix and the crack to be healed appears in the solder itself (i.e., it is the ultimate tensile strength of the solder that determines crack initiation). For the solder tubes/capsules concept, the host matrix is composed of a different, high-melting material with an ultimate tensile strength that can be significantly larger than that of the solder. The solder is activated only when a crack in the host matrix has formed (Fig. 2e, row 2). Activation of the solder is achieved by increasing the temperature above the melting temperature of the solder (Fig. 2e, row 3). After activation, the solder wets the crack surfaces, fills the crack as a result of capillary pressure and surface tension, and solidifies, thereby closing the crack.
Despite the conceptual analogy to the successful self-healing concept in polymers, the solder tubes/capsules concept applied to metals involves many complications. Designing the original microstructure (Fig. 2e, row 1) is difficult when solder capsules are used. The ceramic capsules need to contain holes so that the solder can be filled in. The filling is easier for through-thickness tubes, but these introduce an anisotropy, as in the case of SMA reinforcement wires. Three, even more critical problems with the solder tubes/capsules concept occur during the damage and healing phases. First, for the solder to have any effect, the crack must not only hit a capsule, but must also destroy the ceramic shell such that the solder can escape. This condition is not easily fulfilled because the crack can spread along the interface of the host matrix and the ceramic shell. Second, even if the first condition is fulfilled and the solder can be activated to flow into the crack, the solder must wet the crack properly and, more importantly, bind strongly to the crack surface. Third, after the healing process new voids are created inside the bulk (cf. Fig. 2e, row 4), possibly leading to additional weakening of the sample.
In practice, the described conditions appear too difficult to be fulfilled in metallic systems. This probably explains why corresponding studies are very limited (Table 2). Lucci et al. [53] have investigated the possibility of embedding Al2O3 ceramic tubes filled with Sn3Pb2 solder inside an aluminum host matrix (cf. Fig. 7a). To simulate the self-healing process, an artificial crack was intentionally created such that one of the ceramic tubes was pierced. Although crack filling was achieved after heating above the melting temperature of the solder, the interface between solder and Al matrix was found to be very weak as a result of high porosity (Fig. 7b). In fact, more detailed scanning electron microscope studies revealed that no portion of the solder is in intimate contact with the aluminum and thus the bonding would not be sufficient to stop a realistic crack from growing [53].
(a) Al host matrix with embedded Al2O3 tubes (solder has not been filled in yet). (b) Solder filling a crack after the self-healing process. The interface between solder and Al matrix shows high porosity. Figure adapted from [55] with permission
3.6 Coating Agent
The coating agent concept was proposed in 2014 by Leser at North Carolina State University and Newman et al. from Langley Research Center, NASA [56]. This work was an effort to bring self-healing to “commonly used” metallic materials. Because the bulk microstructure is unchanged with respect to the usual material and only a coating is applied (Fig. 2f, row 1), the concept is expected to be applicable to a wide range of materials.
The central idea of the coating agent concept (i.e., utilization of a coating for self-healing) classifies it in principle into the family of self-healing coatings, as discussed in the review “Self-healing coatings” by Abdolah Zadeh et al. in this volume [57]. However, application of a solder as the self-healing agent renders the present concept similar to the previously discussed macroscale concepts and therefore it is useful to include this concept in the present discussion. To trigger self-healing with the solder, a heat treatment is required (indicated in Fig. 2f, row 3 by the red shaded background). After the application of the heat treatment, the crack should be filled with solder (Fig. 2f, row 4), as in the case of the solder tubes/capsules concept. The same crucial issue needs to be resolved, which is to guarantee good wetting of the crack surfaces and strong bonding between the host matrix and solder. An advantage of the coating agent concept over the solder tubes/capsules concept is that no voids occur after healing. It is only the solder coating that is locally reduced.
Based on the apparent applicability to aerospace components, Leser and coworkers [56] chose to carry out a proof-of-principle study of the coating agent concept on a titanium alloy with a 60% indium–40% tin (wt%) coating (near-eutectic composition). The melting temperature of this coating material is 124°C. Note that a low melting temperature is not only crucial for the healing heat treatment, but also for avoiding over-aging of the base material in the process. In this proof-of-principle case study, the thickness of the single-notch tension specimen and the coating were 2.03 mm and 0.01–0.02 mm, respectively. Fatigue crack growth testing results revealed that crack arrest is possible at lower crack-tip loads and that up to 50% reduction in crack growth rate is observed at higher crack-tip loads (where no full arrest is observed). A proof of full crack arrest was documented by X-ray tomography analysis, as shown in Fig. 8.
X-ray microtomography image of a healed crack using the coating agent concept. Figure taken from [56] with permission
3.7 Electro-healing
The electro-healing concept has been recently proposed and tested by Zheng et al. [58], who were inspired by a partially successful, electropulsing-based healing of microcracks [59]. The electro-healing concept is a macroscale concept that differs quite significantly from the other macroscale concepts. In particular, no composite matrix material and no solder as healing agent are required. Instead, the cracked sample needs to be immersed in an electrolyte solution and a voltage needs to be applied (indicated by the green shaded background in Fig. 2g, row 3). The subsequent electrochemical reaction leads to deposition of material inside the crack, eventually closing it.
The electro-healing concept is very appealing for several reasons: (1) The original microstructure needs no modification (cf. Fig. 2g, row 1). (2) Experience from the field of electrochemical metallic coatings can be utilized for design and optimization purposes. (3) As verified by the work of Zheng et al. [58], very strong bonding between the crack surfaces and the newly deposited material can be achieved, enabling good material properties in the healed stage. A present limitation is that a through-thickness crack is required for the self-healing process to work. The authors [58] argue that healing of single-sided cracks is possible, but this remains to be verified in future studies.
Zheng et al. [58] applied the electro-healing concept to polycrystalline plates of pure nickel, with grain sizes ranging from 0.1 to 0.15 mm. Pre-cracked tensile samples were prepared by creating a hole in cylindrical samples by electrodischarge machining (EMD), compression to close the hole into a crack, and then EDM cutting of the tensile sample. Crack healing was achieved as explained above and shown in Fig. 9, and then cracked, crack-free, and healed samples were tested in tension. The results revealed that through-thickness cracks could be successfully healed by the growth of healing crystals with finer grain sizes (and higher strength) than the pristine material. Full recovery of the tensile strength and partial recovery of ductility was observed, although success also depended on sample thickness.
(a) Schematic of the tension sample with a through-thickness crack in the center. (b) Image of a through-thickness crack. (c) Healed crack after electro-healing treatment. Figure taken from [58] with permission
4 Summary
We have reviewed the available self-healing concepts in metallic bulk materials. Generally, the concepts can be subdivided into a group of nano length-scale concepts and a group of macro length-scale concepts. The first group comprises concepts that autonomously self-heal nanovoids to prevent large-scale damage. The concepts falling into this group are the high-T precipitation concept, the low-T precipitation concept, and the nanoSMA-dispersoids concept. Of these, the most intensively studied and possibly the most promising concept is the high-T precipitation concept. It is applicable to heat-resisting steels and has been shown to considerably improve creep resistance.
The macroscale group comprises self-healing concepts that are designed to heal large-scale cracks (i.e., macroscopic cracks in the range of millimeters). These concepts are not autonomous because they require an external trigger to start the self-healing process, either heat treatment or an electrochemical reaction. The concepts falling into the macroscale group are the SMA-clamp&melt concept, the solder tubes/capsules concept, the coating agent concept, and the electro-healing concept. The SMA-clamp&melt concept is the most studied and possibly the most promising of the macroscale concepts. Its great advantage is that (in an ideal case) the self-healing process can be repeated limitlessly. The challenge is to design SMA reinforcement structures that avoid the strong anisotropy of currently available SMA–solder composites.
An important general characteristic of the available self-healing concepts is that they are bound by rather strict constraints. Mostly, these constraints relate to the choice of material. For example, for the precipitation concepts to work, the chosen elements must have specific phase diagrams and specific diffusion profiles. For the nanoSMA-dispersoids concept, the choice is restricted to material combinations that enable nanosized coherent particles showing the shape-memory effect. For the SMA-clamp&melt concept, an appropriate combination of an SMA and a solder is required. For the solder tubes/capsules concept and the coating agent concept, the host matrix and the solder must be carefully chosen to ensure good wetting and bonding properties at the crack surfaces. The possibly least restrictive concept with respect to material choice is the electro-healing concept, which “only” requires that the host matrix favors an electrochemical reaction with the electrolyte. However, for this latter concept, another restriction is the requirement of a through-thickness crack to enable sufficient flow of the electrolyte self-healing agent.
Given these constraints, the design of self-healing approaches in metals is challenging but, as evidenced by several successful examples, also very promising and, judging by the number of relevant publications in Fig. 1, becoming an increasingly active area of materials design research.
References
van der Zwaag S, van Dijk NH, Jonkers HM, Mookhoek SD, Sloof WG (2009) Self-healing behaviour in man-made engineering materials: bioinspired but taking into account their intrinsic character. Philos Trans A Math Phys Eng Sci 367:1689
Hager MD, Greil P, Leyens C, van der Zwaag S, Schubert US (2010) Self-healing materials. Adv Mater 22:5424
van der Zwaag S (2010) Routes and mechanisms towards self healing behaviour in engineering materials. Bull Pol Acad Sci Tech Sci 58:227
Kötteritzsch J, Schubert US, Hager MD (2013) Triggered and self-healing systems using nanostructured materials. Nanotechnol Rev 2:699
Drenchev L, Sobczak JJ (2014) Self-healing materials as biomimetic smart structures. Instytut Odlewnictwa, Kraków
Ferguson JB, Schultz BF, Rohatgi PK (2014) Self-healing metals and metal matrix composites. JOM 66:866
World Trade Organization (2014) International trade statistics. https://www.wto.org/english/res_e/statis_e/its2014_e/its2014_e.pdf
Allwood JM, Cullen JM, Carruth MA, Cooper DR, McBrien M, Milford RL, Moynihan M, Patel ACH (2012) Sustainable materials: with both eyes open. UIT, Cambridge
Intergovernmental Panel on Climate Change (2007) Fourth assessment report. https://www.ipcc.ch/report/ar4/
van der Zwaag S (2007) Self-healing materials: an alternative approach to 20 centuries of materials science. Springer, Dordrecht
Manuel MV (2009) Principles of self-healing in metals and alloys: an introduction. In: Ghosh G (ed) Self-healing materials: fundamentals, design strategies, and applications. Wiley-VCH, Chichester
Shinya N, Kyono J, Laha K (2003) Improvement of creep rupture properties of heat resisting steels by the self-healing of creep cavities. Mater Sci Forum 426–432:1107
Laha K, Kyono J, Kishimoto S, Shinya N (2005) Beneficial effect of B segregation on creep cavitation in a type 347 austenitic stainless steel. Scr Mater 52:675
Shinya N, Kyono J, Laha K (2006) Self-healing effect of boron nitride precipitation on creep cavitation in austenitic stainless steel. J Intell Mater Syst Struct 17:1127
Laha K, Kyono J, Shinya N (2007) An advanced creep cavitation resistance Cu-containing 18Cr–12Ni–Nb austenitic stainless steel. Scr Mater 56:915
Laha K, Kyono J, Shinya N (2012) Copper, boron, and cerium additions in type 347 austenitic steel to improve creep rupture strength. Metall Mater Trans A 43:1187
He SM, van Dijk NH, Schut H, Peekstok ER, van der Zwaag S (2010) Thermally activated precipitation at deformation-induced defects in Fe-Cu and Fe-Cu-B-N alloys studied by positron annihilation spectroscopy. Phys Rev B 81:094103
He SM, van Dijk NH, Paladugu M, Schut H, Kohlbrecher J, Tichelaar FD, van der Zwaag S (2010) In situ determination of aging precipitation in deformed Fe-Cu and Fe-Cu-B-N alloys by time-resolved small-angle neutron scattering. Phys Rev B 82:174111
He SM, Brandhoff PN, Schut H, van der Zwaag S, van Dijk NH (2013) Positron annihilation study on repeated deformation/precipitation aging in Fe–Cu–B–N alloys. J Mater Sci 48:6150
Zhang S, Kohlbrecher J, Tichelaar FD, Langelaan G, Brück E, van der Zwaag S, van Dijk NH (2013) Defect-induced Au precipitation in Fe–Au and Fe–Au–B–N alloys studied by in situ small-angle neutron scattering. Acta Mater 61:7009
S. Zhang (2015) Self healing of damage in Fe-based alloys. Ph.D. thesis, TU Delft
Karpov EG, Grankin MV, Liu M, Ariyan M (2012) Characterization of precipitative self-healing materials by mechanokinetic modeling approach. J Mech Phys Solids 60:250
Shinya N (2009) Self-healing of metallic materials: self-healing of creep cavity and fatigue cavity/crack. In: Ghosh G (ed) Self-healing materials: fundamentals, design strategies, and applications. Wiley-VCH, chichester
Lumley RN, Morton AJ, Polmear IJ (2002) Enhanced creep performance in an Al–Cu–Mg–Ag alloy through underageing. Acta Mater 50:3597
Lumley RN, Polmear IJ, Morton AJ (2003) Interrupted aging and secondary precipitation in aluminium alloys. Mater Sci Technol 19:1483
Lumley RN, O'Donnell RG, Polmear IJ, Griffiths JR (2005) Enhanced fatigue resistance by underageing an Al-Cu-Mg-Ag alloy. Mater Forum 29:256
Lumley RN, Polmear IJ (2007) Advances in self-healing of metals. In: Proceedings of the first international conference on self-healing materials, Noordwijk aan Zee, The Netherlands
Hautakangas S, Schut H, van der Zwaag S, Rivera Diaz del Castillo PEJ, van Dijk NH (2007) Self healing kinetics in an underaged AA2024 aluminium alloy. In: Proceedings of the first international conference on self-healing materials, Noordwijk aan Zee, The Netherlands
Hautakangas S, Schut H, van der Zwaag S, del Rivera Diaz Castillo PEJ, van Dijk NH (2007) Positron annihilation spectroscopy as a tool to develop self healing in aluminium alloys. physica status solidi C 4:3469
Hautakangas S, Schut H, van Dijk NH, del Rivera Díaz Castillo PEJ, van der Zwaag S (2008) Self-healing of deformation damage in underaged Al–Cu–Mg alloys. Scr Mater 58:719
Mahdavi Shahri M, Alderliesten RC, van der Zwaag S, Schut H (2014) Postponing crack nucleation in 2024 aluminium alloy by dynamic precipitation from the supersaturated state. Adv Mater Res 891–892:1577
Lumley RN (2007) Self healing in aluminium alloys. In: van der Zwaag S (ed) Self healing materials: an alternative approach to 20 centuries of materials science. Springer, Dordrecht
Wanhill R (2007) Fatigue crack initiation in aerospace aluminium alloys, components and structures. In: Proceedings of the first international conference on self-healing materials, Noordwijk aan Zee, The Netherlands
Ritchie RO (1999) Mechanisms of fatigue-crack propagation in ductile and brittle solids. Int J Fract 100:55
Vasudeven AK, Sadananda K, Louat N (1994) A review of crack closure, fatigue crack threshold and related phenomena. Mat Sci Eng A Struct 188:1
Mayer HR, Stanzl-Tschegg SE, Sawaki Y, Hühner M, Hornbogen E (2007) Influence of transformation-induced crack closure on slow fatigue crack growth under variable amplitude loading. Fatigue Fract Eng Mater Struct 18:935
James MN, Knott JF (1985) Critical aspects of the characterization of crack tip closure by compliance techniques. Mater Sci Eng 72:L1
Baxevanis T, Parrinello AF, Lagoudas DC (2013) On the fracture toughness enhancement due to stress-induced phase transformation in shape memory alloys. Int J Plasticity 50:158
Grabowski B, Tasan C (2014) Towards self-healing metals by employing optimally-dispersed Ti-Ni shape-memory nano-particles. Project within DFG SPP 1568: Design and generic principles of self-healing materials. DFG, Bonn
Xu GQ, Demkowicz MJ (2013) Healing of nanocracks by disclinations. Phys Rev Lett 111:145501
Glensk A, Grabowski B, Hickel T, Neugebauer J (2015) Understanding anharmonicity in fcc materials: from its origin toab initioStrategies beyond the quasiharmonic approximation. Phys Rev Lett 114:195901
Grabowski B, Wippermann S, Glensk A, Hickel T, Neugebauer J (2015) Random phase approximation up to the melting point: impact of anharmonicity and nonlocal many-body effects on the thermodynamics of Au. Phys Rev B 91:201103
Duff AI, Davey T, Korbmacher D, Glensk A, Grabowski B, Neugebauer J, Finnis MW (2015) Improved method of calculating ab initio high-temperature thermodynamic properties with application to ZrC. Phys Rev B 91:214311
Files BS (1997) Design of a biomimetic self-healing superalloy composite. Ph.D. thesis, Northwestern University, Chicago
Manuel MV (2007) Design of a biomimetic self -healing alloy composite. Ph.D. thesis, Northwestern University, Chicago
Manuel MV, Olson GB (2007) Biomimetic self-healing metals. In: Proceedings of the first international conference on self-healing materials, Noordwijk aan Zee, The Netherlands
Fisher CR, Manuel MV (2011) Design considerations for matrix compostions of self-healing metal-matrix composites. In: Proceedings of the third international conference on self-healing materials, Bath, UK
Wright MC, Manuel MV, Wallace T (2013) Fatigue resistance of liquid-assisted self- repairing aluminum alloys reinforced with shape memory alloys. NASA/TM-2013-216629. National Aeronautics and Space Administration, Hampton
Rohatgi PK (2014) Al-shape memory alloy self-healing metal matrix composite. Mat Sci Eng A Struct 619:73
Ferguson JB, Schultz BF, Rohatgi PK (2015) Zinc alloy ZA-8/shape memory alloy self-healing metal matrix composite. Mat Sci Eng A Struct 620:85
Kaufman L, Bernstein H (1970) Computer calculation of phase diagrams. Academic, New York
White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S (2001) Autonomic healing of polymer composites. Nature 409:794
Lucci JM, Rohatgi PK, Schultz B (2008) Experiment and computational analysis of self-healing in an aluminum alloy. In: Proceedings of the ASME international mechanical engineering congress and exposition, Boston, USA
Nosonovsky M, Amano R, Lucci JM, Rohatgi PK (2009) Physical chemistry of self-organization and self-healing in metals. Phys Chem Chem Phys 11:9530
Nosonovsky M, Rohatgi PK (2012) Biomimetics in materials science: self-healing, self-lubricating, and self-cleaning materials. Springer, New York
Leser PE, Newman JA, Smith SW et al. (2014) Mitigation of crack damage in metallic materials. NASA/TM-2014-218272. National Aeronautics and Space Administration, Hampton
Abdolah Zadeh M, van der Zwaag Z, Garcia SJ (2015) Self-healing coatings. Adv Polym Sci. doi:10.1007/12_2015_339
Zheng XG, Shi YN, Lu K (2013) Electro-healing cracks in nickel. Mat Sci Eng A Struct 561:52
Zhou Y, Guo J, Gao M, He G (2004) Crack healing in a steel by using electropulsing technique. Mater Lett 58:1732
Acknowledgements
Funding by the Deutsche Forschungsgemeinschaft (SPP 1568) and the European Research Council under the EU’s 7th Framework Programme (FP7/2007-2013)/ERC Grant Agreement No. 290998 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Grabowski, B., Tasan, C.C. (2016). Self-Healing Metals. In: Hager, M., van der Zwaag, S., Schubert, U. (eds) Self-healing Materials. Advances in Polymer Science, vol 273. Springer, Cham. https://doi.org/10.1007/12_2015_337
Download citation
DOI: https://doi.org/10.1007/12_2015_337
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32776-1
Online ISBN: 978-3-319-32778-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)