Abstract
This study investigates thermogravimetric analysis (TGA) and kinetics study (KS) of the co-gasification of municipal solid waste (MSW) and wood pellets (WP) using flue gas as the gasification medium. Our novel decoupling gasifier design facilitates a gasification process utilizing flue gas as the reaction medium. Therefore, studying the thermal decomposition characteristics of MSW and WP during this unique gasification process is crucial. Thermal decomposition exhibited two stages based on mass loss and the rate of mass loss. The maximum mass loss rate occurred during the first stage for all samples at temperatures of 286.5/318 (two peaks), 286.5, and 293.4 ºC for 0WP100MSW, 10WP90MSW, and 20WP80MSW, respectively. The addition of WP to MSW significantly increased the DTG maximum value and eliminated the second decomposition peak of MSW. KS illustrates that the E value decreased from 19.70 to 3.35 kJ/mol under air and 64.05 to 2.12 kJ/mol under flue gas with the addition of 20%wt wood pellets.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The thermochemical conversion process has been recognized as an effective method of transforming solid waste into valuable products [1]. It is typically done by heating the feedstock in an oxygen-deprived environment to convert it into gas, ash, and tar. It is a very enticing approach to be considered for the treatment of waste such as municipal solid waste (MSW) [2]. A multitude of research has been done in this field to improve the conversion efficiency of these processes. Phatavee et al. [3] investigated the fuel properties and thermal decomposition of hydrothermally treated MSW (HTT-MSW) during combustion, utilizing a hydrothermal process combined with thermogravimetric analysis (TGA). Their findings revealed that HTT-MSW exhibited a higher activation energy than raw MSW, suggesting enhanced combustion characteristics. Areeprasert et al. [4] explored the impact of hydrothermal treatment (HTT) on the co-firing of paper sludge (PS) with coal, analyzing thermal degradation through TGA. The study demonstrated that the treated paper sludge significantly reduced emissions by approximately 26‒31%, indicating its potential as an environmentally friendly fuel source. Ayyadurai et al. [5] conducted an experiment on gasifying heterogeneous feedstock with MSW using a downdraft gasifier. Their work improved efficiency in hot gas generation and a remarkable reduction in thermal energy consumption, highlighting the significance of optimizing gasification processes for enhanced performance.
Gasification is particularly effective in producing valuable synthesis gas or syngas from MSW. Syngas holds great potential for various applications, including heat generation, power generation, and chemical synthesis [6]. One of the novel designs in gasification technology is decoupling gasification, which is often defined as gasification process that physically separates combustion and gasification zones [7, 8]. The heating of the gasification zone is done by using flue gas from the separated combustion chamber. Therefore, the reacting medium of the decoupling gasification is the flue gas. This is expected to improve the performance of the gasifier.
Another approach in improving gasification performance, especially for MSW, is by mixing it with biomass such as wood pellets (WP). Since this is also a novel approach, there is a significant gap and a lack of comprehensive research on the kinetics of MSW and WP/MSW gasification. This paper addresses this research gap by conducting a comprehensive thermogravimetric analysis (TGA) on both MSW and WP/MSW during the gasification process. Flue gas was employed as the medium to study kinetic parameters and gained insights into the thermal degradation of MSW and WP/MSW during gasification. By investigating the gasification behavior of MSW and WP/MSW, this study aims to contribute to understanding thermal decomposition of the feedstock under flue gas, a medium representing the fixed-bed decoupling gasification process. The obtained kinetic parameters and thermal degradation characteristics are expected to provide more insight into the body of knowledge on this new gasifier configuration.
2 Methodology
2.1 Raw Materials
In this study, MSW was collected and sorted to remove harmful and non-combustible elements. The sorted MSW samples were categorized into four types: Type 1 (paper and cardboard), Type 2 (mixed plastics), Type 3 (rubber and textile), and Type 4 (wood waste), representing 45%, 33%, 12.7%, and 9.3% of the total MSW mass, respectively. WP produced from sawdust was selected as the co-firing material to enhance the decoupling gasification efficiency of the sorted MSW. MSW and WP were then mixed in three mass ratios: 0%WP: 100%MSW, 10%WP: 90%MSW, and 20%WP: 80%MSW. To facilitate the experiments, MSW samples were cut into tiny pieces approximately 0.5‒1 cm in length, while WP was pulverized to achieve a finer particle size. These materials and methods were implemented to evaluate the impact of WP on the gasification process of MSW, ensuring the accuracy and consistency of the experimental analysis.
2.2 Material Characterizations
The heating value was assessed by the bomb calorific technique (bomb calorimeter, LECO-AC-500, USA) under the ASTM D5865 standard. The proximate analysis was executed by a Thermogravimetric analyzer (LECO-TGA801, USA) under the ASTM D7582 standard. Meanwhile, an element analyzer (CHNS/O Analyzer, LECO-Model 628 series, USA) carried out the ultimate analysis under ASTM D3176 standard. The material properties are given in Table 1.
2.3 Kinetic Study
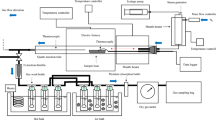
A thermogravimetric analyzer (LECO-TGA801, USA) was utilized to examine the thermal behavior of the prepared materials during gasification. The TGA analysis was conducted at a temperature of 900 °C with a heating rate of 10 °C/min under flue gas conditions. In this study, mass loss (TG) and the rate of mass loss (DTG) were determined, and the kinetics study was performed based on non-isothermal thermogravimetric data. The kinetics parameters, including the apparent activation energy (E) and the pre-exponential factor (A), were obtained through data calculations from the TGA analysis. The thermal decomposition of MSW and WP/MSW during the combustion process could be represented by the following Eq. (1):
Where k(T) is representing temperature-dependent rate constant, \(\alpha\) is the extent of the conversion of the sample at t time, \(f(\alpha )\) is a function of conversion, as defined by Eq. (2).
where \(m_{i}\) is the initial mass of the sample, \(m_{t}\) is the mass of the sample at time t, and \(m_{f}\) is the final mass of the sample. The temperature-dependent rate constant k(T) is conventionally expressed through the Arrhenius equation, Eq. (3).
where A is the pre-exponential factor, E is the apparent activation energy, and R is the universal gas constant (8.314 \(J/{\text{mol}} \cdot K\)). A mathematical term for constant heating rate \(\beta\) is described as Eq. (4).
Then, (3) and (4) were substituted to (1), methodized in general form, and integrated: which gives Eq. (5).
where \(g(\alpha )\) is defined as an integral of the reaction model [3]. To estimate the kinetic parameters, the temperature integral term in (5) was calculated by the coats Redfern approximation [9], applying natural logarithms and rearranging yields Eq. (6).
Since (RT/E) << 1, the term (1-2RT/E) was approximately equal to unity (1-2RT/E \(\approx\) 1). Thus,
Plotting, (\(\ln g(\alpha )/T^{2}\)) versus (\(1/T\)) will carry out a straight line that slope equals to −E/R. Therefore, the E and the A can be acquired by slope and the intercept, respectively.
3 Results and Discussion
3.1 Thermogravimetric Analysis
The TG profiles of all samples using air as the medium (Fig. 1a) exhibited similar characteristics. Decomposition of a significant portion of the samples occurred during the initial combustion period (260‒310 °C) and a subsequent period (400‒800 °C). When flue gas was used as the medium (Fig. 1c), slight differences in the decomposition characteristics were observed. The first-period temperature range decreased slightly to (220‒300 °C), particularly noticeable in the F-20%WP80%MSW sample. This could have been due to combustible elements. The second period of decomposition occurred between 340 and 790 °C.
From the TG profile data, it can be deduced that transitioning the medium from air to flue gas resulted in a subtle modification of the combustion mechanism. This observation aligns with prior research by Kwon et al., which demonstrated that CO2 improved gasification performance by enhancing volatile chemicals cracking during thermal degradation [10]. This is supported by the lower E value of gasification under flue gas compared to air in the temperature range of 350‒460 °C.
Additionally, the introduction of WP accelerated the combustion rate of MSW compared to raw MSW. Previous studies have explored various catalysts to improve biomass gasification efficiency [11, 12]. Research by Soomro et al. demonstrated that adding dolomite, alkaline metal, nickel, or olivine reduced tar generation and increased gas production [13]. However, these catalysts often have a short active life, high cost, and potential regeneration difficulty. Based on our findings, the use of WP as additives showed promise for improving the performance of the gasification process. However, its potential drawback of increased tar generation during gasification should also be considered.
The TGA profiles of mixed samples using air as the medium (see Fig. 1b) displayed a major peak (220‒350 ºC) and a minor peak (400‒480 ºC). In pure MSW, the major peak occurred within the range of (210‒320 °C), with the minor peak appearing the range of (330‒480 ºC). The DTG rates for the major peak were 20%, 16%, and 14.1% for A-20WP80MSW, A-0WP100MSW, and A-10WP90MSW, respectively. The corresponding DTG values for the minor peak were 2.2%, 2.5%, and 3%, respectively.
When flue gas was used as the medium (Fig. 1d), the DTG results showed changes. The temperature profiles exhibited a similar trend, with the major peak appearing within the range of (220‒340 ºC), and the minor peak from (330‒450 ºC). The DTG rates for the major peak decreased to 18.1% for F-20WP80MSW, increased to 16% for F-10WP90MSW, and decreased to 14% for F-0WP100MSW. The DTG values for the minor peak were 2%, 2.5%, and 2.6% for the respective samples.
3.2 Kinetic Parameters
Kinetic parameters were determined using the first-order reaction model, and the obtained parameter results are summarized in Table 2. The temperature range for the analysis was divided into two major decomposition regions, and corresponding minor decomposition peaks were identified. In the first temperature range, where air was used as the medium, temperatures between 210 and 380 ºC were selected. For A-0WP100MSW, the activation energy (E) was found to be 32.85 kJ/mol. However, with the addition of WP, the E values for the WP/MSW samples, namely A-10WP90MSW and A-20WP80MSW, slightly increased to 48.74 and 58.19 kJ/mol, respectively.
When considering the first temperature range with flue gas as the medium, the selected temperature range was between 245 and 355 ºC. For F-0WP100MSW, the E was notably higher at 60.34 kJ/mol compared to A-0WP100MSW. With the addition of WP, the E values for the mixed samples, F-10WP90MSW and F-20WP80MSW, increased slightly to 63.11 and 64.25 kJ/mol, respectively. In the second temperature range, which was within the range of 350 to 460 ºC, the E value for F-0WP100MSW was noticeably lower than A-0WP100MSW. It indicated that at lower temperatures, the addition of WP caused the conversion of feedstock to require more energy than feedstock without WP. However, at higher temperatures (range 2), adding WP lowered the E value significantly. It indicated that WP caused the system to require less energy to achieve conversion. This relation between E and energy required for conversion was reported by Sarabhorn et al. [7]. This result suggests that MSW started to combust at a lower temperature than WP, which could be the result of components with relatively low ignition temperature such as plastics in MSW [14, 15]. However, when the system reached higher temperatures at range 2 and WP also spontaneously combusted, its presence appeared to assist in the spontaneousness of the feedstock conversion, as shown by the much lower E values. This provides evidence that the addition of WP could assist in converting MSW, but only at temperatures above 350 ºC. This was probably caused by the degradation of cellulose, which is the main component of WP, at around this temperature [16].
The same phenomenon was also observed when the atmosphere was replaced with flue gas, supporting the notion that it was driven more by the feedstock than the atmosphere. However, it was noticed that the E values under flue gas were higher than under air. This was in line with the finding in the study by Sarabhorn et al. [7], which explained that WP required more energy to thermally decompose under flue gas than under air. This might be because of the higher oxygen content in the air compared to flue gas, promoting more degradation of cellulose through oxidation [17].
4 Conclusion
In summary, the TGA analysis identified two stages of thermal decomposition based on the mass loss and rate of mass loss. The maximum mass loss occurred at approximately 286/318 ºC (two peaks) under air and 260/310 ºC (two peaks) under flue gas conditions. When WP was mixed with MSW, the DTG value significantly improved, indicating a higher rate of mass loss during thermal decomposition. Moreover, the second decomposition peak of MSW was eliminated; therefore enhancing the mixed material's thermal behavior.
Kinetic study found that adding WP to MSW increased the E value at a temperature lower than 350 ºC. When the temperature reaches over 350 ºC, the WP combusted, enhancing the combustion and conversion rate as indicated by the E value decrease from 19.70 to 3.35 kJ/mol under air and 64.05 to 2.12 kJ/mol under flue gas with the addition of 20%wt wood pellets. It was also observed that the E values for gasification under flue gas atmosphere were higher than under air, indicating that the thermal degradation of material required a higher amount of energy under flue gas.
References
Siwal SS, Zhang Q, Devi N, Saini AK, Saini V, Pareek V, Gaidukovs S, Thakur VK (2021) Recovery processes of sustainable energy using different biomass and wastes. Renew Sustain Energy Rev 150:111483
Nandhini R, Berslin D, Sivaprakash B, Rajamohan N, Vo D-VN (2022) Thermochemical conversion of municipal solid waste into energy and hydrogen: a review. Environ Chem Lett 20(3):1645–1669
Phasee P, Areeprasert C (2017) Thermal decomposition behavior during combustion of hydrothermally treated MSW by thermogravimetric analysis. Energy Proc 138:616–621
Areeprasert C, Chanyavanich P, Ma D, Shen Y, Yoshikawa K (2017) Effect of hydrothermal treatment on co-combustion of paper sludge with coal: thermal behavior, NO emissions, and slagging/fouling tendency. Biofuels 8(2):187–196
Saravanakumar A, Chen W-H, Arunachalam KD, Park Y-K, Ong HC (2022) Pilot-scale study on downdraft gasification of municipal solid waste with mass and energy balance analysis. Fuel 315:23287
Seo YC, Alam MT, Yang WS (2018) Gasification of municipal solid waste. Gasification for low-grade feedstock, IntechOpen, London, UK
Sarabhorn P, Sitthichirachat P, Siripaiboon C, Khaobang C, Palay P, Thapsamut T, Wibowo H, Areeprasert C, Scala F (2023) Investigation of wood pellet gasification in a novel pilot-scale fixed-bed decoupling gasifier. Fuel 352:129025
Khaobang C, Sarabhorn P, Siripaiboon C, Scala F, Areeprasert C (2022) Pilot-scale combined pyrolysis and decoupling biomass gasification for energy and metal recovery from discarded printed circuit board and waste cable. Energy 245:123268
Coats AW, Redfern J (1964) Kinetic parameters from thermogravimetric data. Nature 201(4914):68–69
Kwon EE, Jeon YJ, Yi H (2012) New candidate for biofuel feedstock beyond terrestrial biomass for thermo-chemical process (pyrolysis/gasification) enhanced by carbon dioxide (CO2). Biores Technol 123:673–677
Abu El-Rub Z, Bramer EA, Brem G (2004) Review of catalysts for tar elimination in biomass gasification processes. Ind Eng Chem Res 43(22):6911–6919
Anis S, Zainal Z (2011) Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: a review. Renew Sustain Energy Rev 15(5):2355–2377
Soomro A, Chen S, Ma S, Xiang W (2018) Catalytic activities of nickel, dolomite, and olivine for tar removal and H2-enriched gas production in biomass gasification process. Energy and Environ 29(6):839–867
Thomson H, Drysdale D (1987) Flammability of plastics I: ignition temperatures. Fire Mater 11(4):163–172
Grotkjær T, Dam-Johansen K, Jensen AD, Glarborg P (2003) An experimental study of biomass ignition☆. Fuel 82(7):825–833
Benítez-Guerrero M, López-Beceiro J, Sánchez-Jiménez PE, Pascual-Cosp J (2014) Comparison of thermal behavior of natural and hot-washed sisal fibers based on their main components: cellulose, xylan and lignin. In: TG-FTIR analysis of volatile products. Thermochimica Acta, vol 581. pp 70–86
Parascanu M, Sandoval-Salas F, Soreanu G, Valverde JL, Sanchez-Silva L (2017) Valorization of Mexican biomasses through pyrolysis, combustion and gasification processes. Renew Sustain Energy Rev 71:509–522
Acknowledgements
P. Sitthichirachat received a scholarship from the Faculty of Engineering, Kasetsart University, the Department of Mechanical Engineering, Faculty of Engineering, Kasetsart University, and the Graduate School of Kasetsart University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sitthichirachat, P., Siripaiboon, C., Sarabhorn, P., Khaobang, C., Wibowo, H., Areeprasert, C. (2024). Thermogravimetric Analysis and Kinetics Study of MSW and Wood Pellet Co-Gasification Using Flue Gas as a Medium. In: Ong, H.L., Yusof, S.J.H.M., Kasim, K.F., Gunny, A.A.N., Othman, R. (eds) Proceedings of the 3rd International Conference on Biomass Utilization and Sustainable Energy; ICoBiomasSE 2023; 4–5 September; Kuala Lumpur, Malaysia. ICoBiomasSE 2023. Green Energy and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-9164-8_4
Download citation
DOI: https://doi.org/10.1007/978-981-99-9164-8_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-9357-4
Online ISBN: 978-981-99-9164-8
eBook Packages: EnergyEnergy (R0)