Abstract
The use of natural resources has increased by 254% from 1970 to 2017 globally. Similarly, the consumption of fish and fish products has also gained popularity over the last few years. As per available records and data, it has been projected that about 75% of the fish biomass is discarded as biological waste which can be explored, recycled, and processed for further validation. Fish waste as a rich source of enzymes, bioactive peptides, polymers, and many other bioactive compounds has been studied by several researchers in the last decades. In this chapter, we would focus only on chitin, chitosan, its derivatives, and processing by-products in advanced applications dimensions. They have a huge potential for use in the field of biomedical engineering, and in this article, we will concentrate on their usage in the areas of growth factor delivery, cancer diagnostics, cartilage and tendon repair, dentistry, drug administration, gene delivery, and bone tissue creation. Besides, they have also got used in food and cosmetic industries, nutraceuticals, and bioremediation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Recent significant increases in several diseases concluding cardiovascular system, the change in lifestyle and food habits are the major advised prescription. As a part of this, the consumption of fish and fish products have been increased dramatically (Coppola et al. 2021; FAO 2018). As a result of it, the fish byproduct and biomass have also increased which environmentally, socially, and economically can best be utilized with effective fish waste management strategies (Ferraro et al. 2010; Mo et al. 2018). Among the by-products of the fish industry, different important bioactive and valuable components have been reviewed for use in the biomedical and pharmaceutical fields like enzymes, chitin, polyunsaturated fatty acids, minerals, collagen, peptides, etc. (Shahidi et al. 2019; Shavandi et al. 2019).
In this chapter, we would exclusively discuss only on chitin, chitosan, and their derivatives in advanced application dimensions. Because they are natural biopolymers, chitin and chitosan are non-toxic, biocompatible, and biodegradable. Chitosan is a chitin derivative produced by deacetylating chitin through enzymatic hydrolysis. They can be used to make a variety of forms, such as membranes, gels, nanoparticles, microparticles, nanofibers, beads, scaffolds, and sponges.
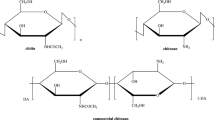
Chitin, which is the second most prevalent polymer after cellulose and is a white, nonelastic, rigid, nitrogenous polymer of natural origin, was first discovered in 1884. It is a part of the exoskeleton of arthropods and a component of yeast and fungi's cell walls. Chitosan, which is produced by enzymatic hydrolysis or deacetylation, is the primary chitin derivative. Although it cannot be dissolved in water or organic solvents, it can be dissolved in a variety of acids, such as acetic, hydrochloric, nitric, and perchloric (Rinaudo 2006; Sankararamakrishnan and Sanghi 2006; Kurita 2006). Chitosan has an electrically positive charge and is hence able to adhere to negatively charged surfaces. As the cell surface is anionic, chitosan is believed to adhere, due to electrostatic interactions (Dash et al. 2011). Three different group types in chitosan make it possible to produce copolymerized components specifically for use in tissue engineering. Different modified chitosan can be created with various targeted effects depending on the level of deacetylation (DDA) and molecular weight (MW). Researchers have created a variety of chitin and chitosan composites that have shown to be future attractive candidates in the biomedical fields.
2 Chitosan Processing
3 Chitin/Chitosan from Aquatic Ecosystem
Chitin may be isolated from shrimps, crabs, lobsters, and crayfish in the form of granules, sheets, and powders (Khor and Lim 2003; Khor 2014; Ehrlich et al. xxxx). Chitin has also been isolated from sponges and found to be effective in biomedical engineering (Ehrlich et al. 2007; Brunner et al. 2009a, 2009b). Chitin is been documented to be obtained from protozoa or alga, foraminifera (Jeuniaux and Voss-Foucart 1991). Diatoms, hydroids, coelenterates, brachiopods, polychaetes, pogonophorans, mollusks, and crustaceans have also been documented to yield chitin (Kurita 2006; Brunner et al. 2009a). The shell of all crabs and shrimps has been enlisted to provide the α-chitin (Stepnowski et al. 2004).
4 Different Composite of Chitin and Chitosan
Base | Composite and type | Reference |
|---|---|---|
Chitin | The sponge-like 3D chitin | |
Chitin | Electrospun water-soluble carboxymethyl | Menon et al. xxxx) |
Chitin | Fiber | Mikhailov et al. 2001) |
Chitin | Electrospun transparent nano mats | Shamshina et al. 2018) |
Chitosan | Heparin-like composite fibrous membranes | Li et al. 2018) |
Chitosan | Nanofibers with carbon nanotubes, Fe3O4, and TiO2 | Bahmani et al. 2020) |
Chitosan | Cellulose chitosan multifilament fiber | Zhu et al. 2019) |
Chitosan | Films and membranes | |
Chitin | Cellulose hydrogels | Shamshina et al. 2014) |
Chitin | MCC aerogels | Shen et al. 2016) |
Chitosan | CMC and Glycerol 2 phosphate hydrogels | Azadi et al. 2018) |
Chitosan | Hydroxypropylmethylcellulose and glycerol thermosensitive hydrogel | Wang et al. 2016) |
Chitosan | Cellulose hydrogel | Kabir et al. 2018) |
Biomedical applications in the area of.
-
1.
Bone Tissue Engineering
For effective bone tissue engineering, any implant/scaffold must be biocompatible, biodegradable, and bioactive. Chitin and chitosan along with these properties also possess flexibility and porosity, but they lack mechanical strength and are unstable too (Mathur and Narang 1990). Hence different composites of chitin and chitosan have evolved to mitigate the disadvantages and have been used in bone tissue engineering. Enhancement of mechanical strength with different composites like alginate for resisting compressive force (Li et al. 2005) and poly(lactic-co-glycolic)acid(PLAGA) (Jiang et al. 2006) composite was investigated with promising results. Further in one study the efficacy of heparin-modified chitosan-PLGA scaffold and recombinant human bone morphogenic protein (rhBMP-2)-heparin chitosan-PLGA scaffold was investigated for osteointegration, osteoblastic proliferation, and differentiation, and rapid bone formation with a promising result (Jiang et al. 2010). Additionally, different composites have also been examined in bone tissue engineering like arginine–glycine–aspartic acid conjugated UV-cross-linked chitosan (Tsai et al. 2012), poly-caprolactone-chitosan scaffolds (Wu et al. 2010; Thuaksuban et al. 2011), growth factors releasing porous poly-caprolactone-chitosan scaffolds (Im et al. 2003), bone morphogenic protein-2 and 7 incorporated chitosan scaffold (Yilgor et al. 2009), chitosan-collagen scaffold incorporated with rhBMP-2-PLGA (Shi et al. 2009), chitosan scaffold functionalized with heparin (Gümüşderelioğlu and Aday 2011). In one study poly L Lactic acid-chitosan scaffold was soaked in Ca2+ and PO43−solutions for increasing the osteoconductive properties of this scaffold and the same was investigated to facilitate bone regeneration (Prabaharan et al. 2007). All of the above studies yield better results in terms of maintaining scaffold porosity, increasing osteoconductivity, improving mechanical attributes, reducing the rate of degradation of materials, and finally augmenting bone formation.
Apart from this, nano-hydroxyapatite (nHAp), being osteoinductive and conductive, also have been investigated by incorporating chitin (Chang et al. 2013). Similarly, the incorporation of nHAp with α-chitin and β-chitin, chitosan hydrogels for bone tissue engineering is investigated (Kumar et al. 2011; Sudheesh Kumar et al. 2011; Madhumathi et al. 2009). Studies on HAp-incorporated chitin and chitosan, different polymeric and ceramic-like silk fibroin, carboxymethyl cellulose, gelatin, and carbon nanotube-incorporated chitin and chitosan have been reviewed and found to have improved mechanical strength with better and uniform mineralization (Fig. 2) (Li et al. 2006; Frohbergh et al. 2012). Osteoblast obtained from mesenchymal stem cells was seeded into a HAp-chitin scaffold and was studied for osteogenic properties (Ge et al. 2004). Similarly, goat mesenchymal stem cells were also studied in chitin composite for new bone tissue regeneration with good results (Liu et al. 2010). Reports on bioactive glass (BGC)-chitin/chitosan composite for use in load-bearing areas including nanocomposite, nano-silvers, solvent cast bioactive glass, the hybrid scaffold of Bioactive glass, surface modified BGC is recorded for better mechanical strength, biocompatibility in the bone and dentistry (EBSCOhost xxxx; Hench et al. 1971; Jones 2013). Hybrid Chitin/chitosan-silica/titania and zirconia composite for bone formation and regeneration is also demonstrated by different researchers (Toskas et al. 2013; Jongwattanapisan et al. 2011).
Examination of electrospun scaffolds morphology by atomic force and scanning electron microscopy. SEM images of chitosan nanofibers with 7% HA content and 0.1% genipin cross-linked CTS-GP a and 0.1% genipin cross-linked 1.0% GP b show distinctive nanofiber shapes at low magnification. (Reproduced from Biomaterials Journal, Frohbergh, et al., 2012, with permission)
-
2.
Cartilage Repair
Most of the time cartilage damage demands surgical interventions especially replacement owing to the fact of degenerative changes that may occur due to disease, trauma, or genetic irregularities. Conventional surgeries do not guarantee all the time a scarless operative site and hence suffer from loss of mobility and reduced functionality. Here lies the need for a cell scaffold composite that can stimulate, regenerate, and remain active in the scaffold environment. Chitosan has been found to have chondrogenesis properties. Porcine chondrocyte-seeded chitosan scaffold was studied with a positive outcome for chondrogenesis (Use and of Chitosan as a Cell Scaffold Material for Cartilage Tissue Engineering xxxx; Griffon et al. 2006). Chitosan microspheres incorporated with transforming growth factor β showed improved chondrocyte growth (Kim et al. 2003; Lee et al. 2004). Chitosan-collagen-genipin scaffold demonstrated better viability of chondrocytes in rabbits (Yan et al. 2010). Utilizing the hemostatic characteristic of chitosan composite in cartilage regeneration resulted in the development of hyaline cartilage and the variation of pluripotent cells into chondrocytes (Hoemann et al. 2007; Hao et al. 2010). Chitin and chitosan hydrogels showed significant achievement in terms of regeneration of damaged cartilage and wound healing (Xi et al. 1999; Jin et al. 2009; Tan et al. 2009). Different hydrogel composite of chitosan is demonstrated to provide better chondrocyte survival (Park et al. 2013). The chitosan-based fibrous scaffold has been studied with better deposition of the extracellular matrix (Subramanian et al. 2004). The hybrid scaffold of chitosan was evaluated for the mechanical integrity of cartilage formation (Neves et al. 2011).
-
3.
Tendon and Ligament Repair
As with cartilage, tendon and ligament repair also have drawbacks of scar tissue formation. For any technology to be used in the repair process must meet high tensile strength along with regenerative capacity. A polyelectrolyte complex of alginate-chitosan gave better adhesion attributes (Majima et al. 2005). Similar findings along with enhanced strength were found in the hyaluronic acid chitosan complex (Funakoshi et al. 2005a). Other studies using hyaluronic acid chitosan complexes with osteoblast seeds also produced results that were more favorable in terms of increased mechanical strength (Fig. 3) (Funakoshi et al. 2005b; Irie et al. 2011). As chitin is degraded first, hence alone chitin fabric sometimes gave poor regeneration of non-healed ligament with evidence of scar tissue, but if a composite is formed with PCL the outcome was found to be encouraging (Funakoshi et al. 2006; Sato et al. 2000). The role of chitosan in treating ligament injury lies in favoring the deposition of collagen type I (Tendon healing in vivo and in vitro: chitosan improves range of motion after flexor tendon repair 2013). Similar studies were also carried out by other scientists indicating the outcome at per (Shao et al. 2010a, 2010b).
-
4.
Skin Regeneration
As already discussed the hemostatic properties of chitin and chitosan, may be well employed for the healing of varied types of dermatological conditions (Taravel and Domard 1995, 1996; Ma et al. 2001). Cotton fiber type chitosan with rapid healing and presence of polymorphonuclear cells (Ueno et al. 1999), growth factor incorporated chitosan with better healing (Mizuno et al. 2003), chitosan-alginate polyelectrolyte complex with better wound stability (Yan et al. 2000; Wang et al. 2002), chitosan-collagen scaffold providing more fibroblast infiltration (Ma et al. 2003), chitosan acetate bandaging with improved antibacterial and anti-inflammatory property (Burkatovskaya et al. 2008), growth factor incorporated collagen-chitosan complex in burn wound with enhanced neo-angiogenesis (Guo et al. 2011), atelocollagen-chitosan in excisional wound healing198, chitosan hydrogels for skin tissue healing (Kumar et al. 2013) have been investigated and documented for ease of ready to hand reference.
-
5.
Liver Regeneration
As the liver’s extracellular matrix contains glycosaminoglycans, hence chitin and chitosan are used in different hepatic engineering and regeneration because these glycosaminoglycans are also a part of chitin and chitosan (Li et al. 2003a) and have been recorded to modulate the actions of vascular endothelial cells (Chupa et al. 2000). Different composites along with their applications have been listed below.
Composites | Application | References |
|---|---|---|
Chitosan-collagen | Increased hepatocyte compatibility | Wang et al. 2003) |
Chitosan-collagen-heparin | Artificial liver with more blood compatibility | Wang et al. 2005) |
Chitosan-galactose | Increased hepatocyte attachment | Park et al. 2003) |
Chitosan-fructose | Increased liver cell metabolic activities | |
Chitosan-fibroin | Increased hepatocyte attachment | She et al. 2009) |
Chitosan-galactosylated hydroxyapatite | More albumin secretion and elimination of urea | Fan et al. 2010) |
Chitosan-poly ether-ether ketone | The proliferation of progenitor cells | Piscioneri et al. 2011) |
-
6.
Nerve Regeneration
Peripheral nerve regeneration and repair frequently involve chitin and chitosan. Schwann cell, one of the key cells in nerve tissue engineering showed better migration, adhesion, and proliferation with chitosan fiber (Yuan et al. 2004). The effectiveness of the chitosan complex in additional research on cell adhesion, proliferation, and migration has been evaluated (Cao et al. 2005; Mingyu et al. 2004; Cheng et al. 2003; Chiono et al. 2008). The viability and proliferation of different neural cells were studied in chitosan-collagen composite (Yang et al. 2010) and laminin-coated chitosan membrane (Guo et al. 2012).
5 Application in the Field of the Delivery System
Any kind of regeneration, repair, and healing is always associated with growth factors and can be influenced by different drugs and gene therapy. In these domains, chitin and chitosan are crucial. Here we will briefly review the effect of these in tabular form.
Different drug delivery implant has been prepared like chitosan scaffold for 5-Fluorouracil (Denkbaş et al. 2000), chitosan-pectin cross-linked scaffold with pentoxifylline incorporated films (Lin and Yeh 2010a), pentoxifylline loaded chitosan-alginate scaffold (Lin and Yeh 2010b), ketoprofen loaded Chitosan-carboxymethyl β–CD (Prabaharan and Jayakumar 2009), ampicillin-loaded alginate microspheres in chitosan-nano-hydroxyapatite scaffolds (Shi et al. 2007), dexamethasone impregnated Chitosan scaffold (Duarte et al. 2009), tetracycline loaded chitosan-hydroxyapatite scaffolds (Teng et al. 2009), amikacin and vancomycin loaded chitosan sponge (Noel et al. 2010), collagen-chitosan for transdermal drug delivery (Thacharodi and Panduranga Rao 1996), vitamin B2 as a model drug in glucose-cross-linked N-alkylated chitosan membranes (Li et al. 2002), nifedipine incorporated chitosan membrane (Thacharodi and Rao 1993), collagen-chitosan as a carrier of propranolol (Thacharodi and Panduranga Rao 1995) and the results demonstrated better release, stability with lowered enzymatic degradation. Carboxymethyl chitin has also been used for drug delivery (Jayakumar et al. 2010).
Besides, the delivery of growth factors may be attributed to chitin and chitosan. The incorporation of bFGF into chitosan was in periodontal regeneration (Tığlı et al. 2009). bFGF was also incorporated in chitosan-alginate scaffold and found to be a potential carrier system for tissue repair and regeneration (Ho et al. 2009). Chitosan microsphere scaffolds have been used for the evaluation of carriers of ALP and BMP-2 (Reves et al. 2009). CS composite has also been used for the delivery of VEGF for hard tissue regeneration (Riva et al. 2010). The administration of rhBMP-2 for bone regeneration has been successfully shown using the chitosan-collagen scaffold (Shi et al. 2009).
Chitosan-based DNA/siRNA complexes sometimes have the problem of early and immature release of nucleic acids (Buyens et al. 2012) and less penetration to cells. Hence, complexes with improved performances have been investigated with promising results. Enhanced cell penetration has been achieved by surface modifications of chitosan by ligands like transferrin (Mao et al. 2001; Chan et al. 2007), folate (Kim et al. 2006), mannose (Gao et al. 2003), and galactose (Gao et al. 2003; Park et al. 2001) have yielded good results. For increased stability, chitosan has been modified by Quaternization (Kean et al. 2005; Thanou et al. 2002), glycosylation (Thanou et al. 2002; Strand et al. 2008), and hydrophobic modification (Lee et al. 2012). Fusogenic peptides and pH-sensitive neutral lipids have been added to several research projects to improve DNA/siRNA. Plans to mute RANK signaling utilizing chitosan hydrogel as a siRNA reservoir and vector have also been examined (Kang et al. 2017).
6 Application in Wound Healing
Chitin and different composite membranes have been tested from the perspective of wound healing and found to possess excellent biocompatibility, minimal or less tissue reaction, and regenerative and antibacterial properties (Singh et al. 2008; Azad et al. 2004). Similar results were also obtained with chitosan membrane (Santos et al. 2013), chitosan composites (Pang et al. 2008), silver sulfadiazine incorporated chitosan alone (Mi et al. 2003), and chitosan-alginate composite (Meng et al. 2010), argon plasma treated chitosan membranes in fibroplasia (Zhu et al. 2005). Different wound dressing materials based on chitosan hydrogel composite gave excellent results (Queiroz et al. 2003). Porous chitosan membrane proved to possess good hemostatic as well as excellent epithelialization properties (Mi et al. 2001). The different composite sheets of chitosan (Wang et al. 2012), diverse sponges of it (Lee et al. 2000), and composite bandages in non-cytotoxic in nature (Sudheesh Kumar et al. 2012). According to all of the aforementioned studies, chitin and chitosan, as well as their composites, have excellent bioacceptability, good regenerative properties, epithelialization, hemostasis, adhesion, stability, antibacterial and anti-inflammatory properties, and, most importantly, have improved wound healing.
7 Application in Cancer Diagnosis
Chitin and chitosan are frequently employed in the diagnosis and treatment of cancer among other purported biological purposes. In cancer imaging heavy metal-free luminescent zinc sulfide (ZnS) is considered bio-friendly to healthy and cancer cells over the use of heavy metal-containing nanocrystals such as cadmium sulfide, cadmium selenide, and zinc selenide (Derfus et al. 2004). However, in an in-vitro study chitosan encapsulated ZnS nanoparticle is used in cancer imaging (Higuchi et al. 2008) and the study shows the yield of mannosylated ZnS of size 120 nm after functionalizing with D-Mannose and its low cytotoxicity toward healthy and cancer cells. Moreover, its specificity of the binding property toward mannose-bearing KB cells under fluorescence microscopy encourages receptor-mediated imaging with nanoparticles as well as in cancer therapy (Higuchi et al. 2008).
The report of various researchers from across the world clearly demonstrates the importance of chitin and chitosan as therapeutic agent in cancer biology through the direct death of malignant cell lines and as a vehicle for anticancer medication delivery systems. Chitosan's anti-cancerous properties are mostly attributed to the maturation and infiltration of cytolytic T-lymphocytes via enhanced interleukin-1 and interleukin-2 production (Lin et al. 2007). Some researchers have shown anticancer efficacy through the direct destruction of tumor cells by triggering apoptosis (Gibot et al. 2015).
Chitin inhibits an elevated serum level of proinflammatory mediators chitinase-3 like protein-1 (CHI3L1) in breast cancer, colorectal cancer, ovarian cancer, leukemia, lymphoma, metastatic prostate cancer, lung cancer, and glioblastoma by blocking the synthesis of vascular endothelial growth factor C. (VEGF-C). Additionally, other forms of chitin, such as chitin-glucan-aldehyde-quercetin conjugation and silver-embedded chitin nanocomposites, exhibit biocompatible cytotoxicity in human breast cancer (MCF-7) cells (Solairaj et al. 2017) and in macrophage cancer cell line (J774) respectively (Singh et al. 2018). Further, in the drug delivery system doxorubicin coated with chitin-based poly L lactic acid composite nano gel and ellagic acid in chitin nanoparticle shows cytotoxic effect in the liver (Arunraj et al. 2014) and breast (Pirzadeh-Naeeni et al. 2020) carcinoma respectively. Similarly, chitin, chitosan, and chitosan oligosaccharides are also used in cancer therapy. A 2015 study by Gibot et al. found that the apoptotic impact increased in RPMI7951 cells, cell proliferation decreased in SKMEL38 cells, and cell adhesion quality decreased in A 375 cells (Gibot et al. 2015). Moreover, chitosan oligosaccharide in colorectal cancer abolished tumor progression (Mattaveewong et al. 2016), and modulated cell autophagy in the A549 lung cancer cell line in oral squamous cell carcinoma producing the cytotoxic effect by arresting cell cycle and apoptosis without any adverse effect on adjacent noncancerous cells (Wimardhani et al. 2014). This chitosan also shows anti-cancerouse effects in liver carcinoma in mice (Jiang et al. 2015), and lung cancer A549 cell line (Gao et al. 2020). In lung cancer, the A549 cell line chitosan selenate shows its potent activity in the apoptosis of cancer cells. In addition, many chitosan nanoparticles are employed in anti-cancerous drug delivery systems, including chitosan oligosaccharide conjugated to 5-fluorouracil and vanillin, chitosan oligosaccharide conjugated to indomethacin, and chitosan graphene oxide attached to thioguanine (Hasanzade and Raissi 2019; Lee et al. 2017; Li et al. 2016).
8 Application in Dentistry
Chitosan is been widely studied in dental engineering for its biocompatibility, non-toxicity, biodegradability, and antimicrobial activities. Apart from that, it has the property to form film and gel which is very helpful for application in the dental field (Fiorillo 2019; Husain et al. 2017). Chitosan has widely been used for caries prevention as well as in conservative dentistry (Ortiz and Boyce 2008). One remarkable study report denotes better periodontal health and condition with chitosan brushing (Zeza et al. 2017). Chitosan has also been proven to reduce the bleeding time after the extraction of teeth (Pippi et al. 2017). It has been investigated for anti-inflammatory and pain relief properties with significant outcomes in dental affection and engineering (Lope-Lopez et al. 2015). Chitosan also proved to combat demineralization in dental affection cases (Uysal et al. 2011). The form of toothpaste of chitosan has been used to reduce the oral bacterial count (Mohire and Yadav 2010).
9 Other Applications of Chitin and Chitosan Apart from Biomedical Spheres
Chitin and chitosan are employed in a variety of industries, including food and cosmetics, nutraceuticals, water treatment, and bioremediation, in addition to biomedicine. The usage of chitin and chitosan in bioremediation is significant due to the conversion of contaminants and interaction with heavy metals and removal in an aqueous solution (Hayes et al. 2008; Barriada et al. 2007). Papayafish scale collagen has been found to have iron-clearing properties from groundwater (Irawan et al. 2018). Chitin and chitosan are utilized in the food industry as food additives and have also been included in food packaging materials because of their antioxidant and antibacterial properties. Silver thiosulfate and actinides are two industrial pollutants that have been treated using chitin-based biomaterials (Kosyakov et al. 2002). Chitin has also been used in the paper industry for increasing strength.
10 Conclusion and Future Scope
Fish processing is now becoming a major industry in many countries and fish by-products are being widely used in many sectors including biomedical applications. The vast source of collagen, proteins, oils, chitin, and chitosan is derived from the fish industry. The encouraging biomedical applications of chitin and chitosan have attracted the attention of researchers to explore their use in a more intensive and easier application, as well as making the research into a new dimension for application in biomedical spheres. Still, the user has yet to be investigated in light of biocompatibility, degradability, acceptability, and bioactivity. Further, the research program should be oriented to develop composites of chitin and chitosan more fruitfully in various biomedical applications. The same has to reach the hand of clinicians at a reachable price. The research should be aimed to transfer from laboratories to clinics.
References
Coppola D, Lauritano C, Palma Esposito F, Riccio G, Rizzo C, de Pascale D (2021) Fish Waste: From Problem to Valuable Resource. Mar Drugs 19:116. https://doi.org/10.3390/md19020116
FAO (2018) The state of world fisheries and aquaculture 2018: Meeting the sustainable development goals. FAO, Rome, Italy
Ferraro V, Cruz IB, Jorge R, Malcata F, Pintado M, Castro P (2010). Valorisation of natural extracts from marine source focused on Marine by-Products: a review. https://doi.org/10.1016/J.FOODRES.2010.07.034
Mo WY, Man YB, Wong MH (2018) Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci Total Environ 613–614:635–643. https://doi.org/10.1016/j.scitotenv.2017.08.321
Shahidi F, Varatharajan V, Peng H, Senadheera R (2019) Utilization of marine by-products for the recovery of value-added products. J Food Bioact 6:. https://doi.org/10.31665/JFB.2019.6184
Shavandi A, Hou Y, Carne A, McConnell M, Bekhit A (2019) Marine Waste utilization as a source of functional and health compounds. Adv Food Nutr Res. https://doi.org/10.1016/bs.afnr.2018.08.001
Rinaudo M (2006) Chitin and chitosan: Properties and applications. Prog Polym Sci 31:603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Sankararamakrishnan N, Sanghi R (2006) Preparation and characterization of a novel xanthated chitosan. Carbohydr Polym 66:160–167. https://doi.org/10.1016/j.carbpol.2006.02.035
Kurita K (2006) Chitin and chitosan: functional biopolymers from marine crustaceans. Mar Biotechnol N Y N 8:203–226. https://doi.org/10.1007/s10126-005-0097-5
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 36:981–1014. https://doi.org/10.1016/j.progpolymsci.2011.02.001
Roberts GAF (1992) Chitin chemistry. Macmillan, London
Khor E, Lim LY (2003) Implantable applications of chitin and chitosan. Biomaterials 24:2339–2349. https://doi.org/10.1016/S0142-9612(03)00026-7
Khor E (2014) Chitin: Fulfilling a Biomaterials Promise. Elsevier
Ehrlich H, Maldonado M, Hanke T, Meissner H, Born R, Scharnweber D, Worch H Spongins: nanostructural investigations and development of biomimetic material model. 4
Ehrlich H, Maldonado M, Spindler K, Eckert C, Hanke T, Born R, Goebel C, Simon P, Heinemann S, Worch H (2007) First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J Exp Zoolog B Mol Dev Evol 308B:347–356. https://doi.org/10.1002/jez.b.21156
Brunner E, Ehrlich H, Schupp P, Hedrich R, Hunoldt S, Kammer M, Machill S, Paasch S, Bazhenov VV, Kurek DV, Arnold T, Brockmann S, Ruhnow M, Born R (2009a) Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J Struct Biol 168:539–547. https://doi.org/10.1016/j.jsb.2009.06.018
Brunner E, Richthammer P, Ehrlich H, Paasch S, Simon P, Ueberlein S, van Pée K-H (2009b) Chitin-Based organic networks: an integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew Chem Int Ed 48:9724–9727. https://doi.org/10.1002/anie.200905028
Jeuniaux C, Voss-Foucart MF (1991) Chitin biomass and production in the marine environment. Biochem Syst Ecol 19:347–356. https://doi.org/10.1016/0305-1978(91)90051-Z
Stepnowski P, Olafsson G, Helgason H, Jastorff B (2004) Recovery of astaxanthin from seafood wastewater utilizing fish scales waste. Chemosphere 54:413–417. https://doi.org/10.1016/S0045-6535(03)00718-5
Kim I-Y, Seo S-J, Moon H-S, Yoo M-K, Park I-Y, Kim B-C, Cho C-S (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1–21. https://doi.org/10.1016/j.biotechadv.2007.07.009
Rinaudo M (2008) Main properties and current applications of some polysaccharides as biomaterials. Polym Int 57:397–430. https://doi.org/10.1002/pi.2378
Abe M, Takahashi M, Tokura S, Tamura H, Nagano A (2004) Cartilage-scaffold composites produced by bioresorbable beta-chitin sponge with cultured rabbit chondrocytes. Tissue Eng 10:585–594. https://doi.org/10.1089/107632704323061942
Menon D, Vidyapeetham AV, Profile S, Furuike T, Profile S, Rangasamy J 144 Publications 5,749 Citations
Mikhailov G, Lebedeva M, Nud’ga L, Petrova VA, (2001) Composite fibers based on chitin and cellulose. Russ J Appl Chem 74:1573–1576. https://doi.org/10.1023/A:1013734008473
Shamshina JL, Zavgorodnya O, Choudhary H, Frye B, Newbury N, Rogers RD (2018) In search of stronger/cheaper chitin nanofibers through electrospinning of Chitin-Cellulose composites using an ionic liquid platform. ACS Sustain Chem Eng 6:14713–14722. https://doi.org/10.1021/acssuschemeng.8b03269
Li Z, Ma J, Li R, Yin X, Dong W, Pan C (2018) Fabrication of a blood compatible composite membrane from chitosan nanoparticles, ethyl cellulose and bacterial cellulose sulfate. RSC Adv 8:31322–31330. https://doi.org/10.1039/C8RA05536J
Bahmani E, Zonouzi H, Koushkbaghi S, Hafshejani F, Fassadi Chimeh A, Irani M (2020) Electrospun polyacrylonitrile/cellulose acetate/MIL-125/TiO2 composite nanofibers as an efficient photocatalyst and anticancer drug delivery system. Cellulose 27:1–17. https://doi.org/10.1007/s10570-020-03459-1
Zhu K, Wang Y, Lu A, Fu Q, Hu J, Zhang L (2019) Cellulose/Chitosan composite multifilament fibers with Two-Switch shape memory performance. ACS Sustain Chem Eng 7:6981–6990. https://doi.org/10.1021/acssuschemeng.8b06691
Hu D, Wang H, Wang L (2016) Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT - Food Sci Technol 65:398–405. https://doi.org/10.1016/j.lwt.2015.08.033
Youssef AM, El-Sayed SM, El-Sayed HS, Salama HH, Dufresne A (2016) Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr Polym 151:9–19. https://doi.org/10.1016/j.carbpol.2016.05.023
Shamshina JL, Gurau G, Block LE, Hansen LK, Dingee C, Walters A, Rogers RD (2014) Chitin–calcium alginate composite fibers for wound care dressings spun from ionic liquid solution. J Mater Chem B 2:3924–3936. https://doi.org/10.1039/C4TB00329B
Shen X, Shamshina JL, Berton P, Bandomir J, Wang H, Gurau G, Rogers RD (2016) Comparison of hydrogels prepared with Ionic-Liquid-Isolated versus Commercial Chitin and Cellulose. ACS Sustain Chem Eng 4:471–480. https://doi.org/10.1021/acssuschemeng.5b01400
Azadi MDA, Hassanjili S, Zarrabi K, Sarkari B (2018) Solidification of hydatid cyst fluid with an injectable chitosan/carboxymethylcellulose/β-glycerophosphate hydrogel for effective control of spillage during aspiration of hydatid cysts. Prog Biomater 7:35–54. https://doi.org/10.1007/s40204-018-0082-5
Wang T, Chen L, Shen T, Wu D (2016) Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int J Biol Macromol 93:775–782. https://doi.org/10.1016/j.ijbiomac.2016.09.038
Kabir SMF, Sikdar PP, Haque B, Bhuiyan MAR, Ali A, Islam MN (2018) Cellulose-based hydrogel materials: chemistry, properties and their prospective applications. Prog Biomater 7:153–174. https://doi.org/10.1007/s40204-018-0095-0
Mathur NK, Narang CK (1990) Chitin and chitosan, versatile polysaccharides from marine animals. J Chem Educ 67:938. https://doi.org/10.1021/ed067p938
Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M (2005) Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 26:3919–3928. https://doi.org/10.1016/j.biomaterials.2004.09.062
Jiang T, Abdel-Fattah WI, Laurencin CT (2006) In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials 27:4894–4903. https://doi.org/10.1016/j.biomaterials.2006.05.025
Jiang T, Nukavarapu SP, Deng M, Jabbarzadeh E, Kofron MD, Doty SB, Abdel-Fattah WI, Laurencin CT (2010) Chitosan-poly(lactide-co-glycolide) microsphere-based scaffolds for bone tissue engineering: in vitro degradation and in vivo bone regeneration studies. Acta Biomater 6:3457–3470. https://doi.org/10.1016/j.actbio.2010.03.023
Tsai W-B, Chen Y-R, Li W-T, Lai J-Y, Liu H-L (2012) RGD-conjugated UV-crosslinked chitosan scaffolds inoculated with mesenchymal stem cells for bone tissue engineering. Carbohydr Polym 89:379–387. https://doi.org/10.1016/j.carbpol.2012.03.017
Wu H, Wan Y, Dalai S, Zhang R (2010) Response of rat osteoblasts to polycaprolactone/chitosan blend porous scaffolds. J Biomed Mater Res A 92:238–245. https://doi.org/10.1002/jbm.a.32376
Thuaksuban N, Nuntanaranont T, Pattanachot W, Suttapreyasri S, Cheung L (2011) Biodegradable polycaprolactone-chitosan three-dimensional scaffolds fabricated by melt stretching and multilayer deposition for bone tissue engineering: Assessment of the physical properties and cellular response. Biomed Mater Bristol Engl 6:015009. https://doi.org/10.1088/1748-6041/6/1/015009
Im SY, Cho SH, Hwang JH, Lee SJ (2003) Growth factor releasing porous poly (epsilon-caprolactone)-chitosan matrices for enhanced bone regenerative therapy. Arch Pharm Res 26:76–82. https://doi.org/10.1007/BF03179936
Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V (2009) Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 30:3551–3559. https://doi.org/10.1016/j.biomaterials.2009.03.024
Shi S, Cheng X, Wang J, Zhang W, Peng L, Zhang Y (2009) RhBMP-2 microspheres-loaded chitosan/collagen scaffold enhanced osseointegration: an experiment in dog. J Biomater Appl 23:331–346. https://doi.org/10.1177/0885328208090013
Gümüşderelioğlu M, Aday S (2011) Heparin-functionalized chitosan scaffolds for bone tissue engineering. Carbohydr Res 346:606–613. https://doi.org/10.1016/j.carres.2010.12.007
Prabaharan M, Rodriguez-Perez MA, de Saja JA, Mano JF (2007) Preparation and characterization of poly(L-lactic acid)-chitosan hybrid scaffolds with drug release capability. J Biomed Mater Res B Appl Biomater 81:427–434. https://doi.org/10.1002/jbm.b.30680
Chang C, Peng N, He M, Teramoto Y, Nishio Y, Zhang L (2013) Fabrication and properties of chitin/hydroxyapatite hybrid hydrogels as scaffold nano-materials. Carbohydr Polym 91:7–13. https://doi.org/10.1016/j.carbpol.2012.07.070
Kumar P, Srinivasan S, Lakshmanan V-K, Tamura H, Nair S, Jayakumar R (2011) Synthesis, characterization and cytocompatibility studies of α-chitin hydrogel/nano hydroxyapatite composite scaffolds. Int J Biol Macromol 49:20–31. https://doi.org/10.1016/j.ijbiomac.2011.03.006
Sudheesh Kumar PT, Srinivasan S, Lakshmanan V-K, Tamura H, Nair SV, Jayakumar R (2011) β-Chitin hydrogel/nano hydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr Polym 85:584–591. https://doi.org/10.1016/j.carbpol.2011.03.018
Madhumathi K, Binulal NS, Nagahama H, Tamura H, Shalumon KT, Selvamurugan N, Nair SV, Jayakumar R (2009) Preparation and characterization of novel β-chitin–hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol 44:1–5. https://doi.org/10.1016/j.ijbiomac.2008.09.013
Li X, Feng Q, Cui F (2006) In vitro degradation of porous nano-hydroxyapatite/collagen/PLLA scaffold reinforced by chitin fibres. Mater Sci Eng C 26:716–720. https://doi.org/10.1016/j.msec.2005.06.062
Frohbergh ME, Katsman A, Botta GP, Lazarovici P, Schauer CL, Wegst UGK, Lelkes PI (2012) Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 33:9167–9178. https://doi.org/10.1016/j.biomaterials.2012.09.009
Ge Z, Baguenard S, Lim LY, Wee A, Khor E (2004) Hydroxyapatite–chitin materials as potential tissue engineered bone substitutes. Biomaterials 25:1049–1058. https://doi.org/10.1016/S0142-9612(03)00612-4
Liu X, Li X, Fan Y, Zhang G, Li D, Dong W, Sha Z, Yu X, Feng Q, Cui F, Watari F (2010) Repairing goat tibia segmental bone defect using scaffold cultured with mesenchymal stem cells. J Biomed Mater Res B Appl Biomater 94B:44–52. https://doi.org/10.1002/jbm.b.31622
EBSCOhost 65959103 Biocompatible β-chitin Hydrogel/Nanobioactive Glass Ceramic Nanocomposite Scaffolds for Periodontal Bone Regeneration. https://web.p.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=09711198&AN=65959103&h=ODSD8capDjdUQBltuvKLKIRbAbe2kFIu%2fbfN7tlNnCZfDfwJiqSyEBXOWgh%2bqDo%2fjHok8mu61W5D9Zn4ZKCYxQ%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d09711198%26AN%3d65959103. Accessed 10 Jul 2022
Hench LL, Splinter RJ, Allen WC, Greenlee TK (1971) Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 5:117–141. https://doi.org/10.1002/jbm.820050611
Jones JR (2013) Review of bioactive glass: From Hench to hybrids. Acta Biomater 9:4457–4486. https://doi.org/10.1016/j.actbio.2012.08.023
Toskas G, Cherif C, Hund R-D, Laourine E, Mahltig B, Fahmi A, Heinemann C, Hanke T (2013) Chitosan(PEO)/silica hybrid nanofibers as a potential biomaterial for bone regeneration. Carbohydr Polym 94:713–722. https://doi.org/10.1016/j.carbpol.2013.01.068
Jongwattanapisan P, Charoenphandhu N, Krishnamra N, Thongbunchoo J, Tang I-M, Hoonsawat R, Smith SM, Pon-On W (2011) In vitro study of the SBF and osteoblast-like cells on hydroxyapatite/chitosan–silica nanocomposite. Mater Sci Eng C 31:290–299. https://doi.org/10.1016/j.msec.2010.09.009
Potential Use of Chitosan as a Cell Scaffold Material for Cartilage Tissue Engineering Tissue Engineering. https://www.liebertpub.com/doi/abs/https://doi.org/10.1089/107632702320934100. Accessed 10 Jul 2022
Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL (2006) Chitosan scaffolds: Interconnective pore size and cartilage engineering. Acta Biomater 2:313–320. https://doi.org/10.1016/j.actbio.2005.12.007
Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC (2003) Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: Implications for cartilage tissue engineering. J Controlled Release 91:365–374. https://doi.org/10.1016/S0168-3659(03)00274-8
Lee JE, Kim KE, Kwon IC, Ahn HJ, Lee S-H, Cho H, Kim HJ, Seong SC, Lee MC (2004) Effects of the controlled-released TGF-β1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 25:4163–4173. https://doi.org/10.1016/j.biomaterials.2003.10.057
Yan L-P, Wang Y-J, Ren L, Wu G, Caridade SG, Fan J-B, Wang L-Y, Ji P-H, Oliveira JM, Oliveira JT, Mano JF, Reis RL (2010) Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J Biomed Mater Res A 95A:465–475. https://doi.org/10.1002/jbm.a.32869
Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard G-E, Hurtig M, Buschmann MD (2007) Chitosan–glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage 15:78–89. https://doi.org/10.1016/j.joca.2006.06.015
Hao T, Wen N, Cao J-K, Wang H-B, Lü S-H, Liu T, Lin Q-X, Duan C-M, Wang C-Y (2010) The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthritis Cartilage 18:257–265. https://doi.org/10.1016/j.joca.2009.08.007
Xi LuJ, Prudhommeaux F, Meunier A, Sedel L, Guillemin G (1999) Effects of chitosan on rat knee cartilages. Biomaterials 20:1937–1944. https://doi.org/10.1016/S0142-9612(99)00097-6
Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, Feijen J (2009) Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 30:2544–2551. https://doi.org/10.1016/j.biomaterials.2009.01.020
Tan H, Chu CR, Payne KA, Marra KG (2009) Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 30:2499–2506. https://doi.org/10.1016/j.biomaterials.2008.12.080
Park H, Choi B, Hu J, Lee M (2013) Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater 9:4779–4786. https://doi.org/10.1016/j.actbio.2012.08.033
Subramanian A, Lin H-Y, Vu D, Larsen G (2004) Synthesis and evaluation of scaffolds prepared from chitosan fibers for potential use in cartilage tissue engineering. Biomed Sci Instrum 40:117–122
Neves SC, Moreira Teixeira LS, Moroni L, Reis RL, Van Blitterswijk CA, Alves NM, Karperien M, Mano JF (2011) Chitosan/Poly(ɛ-caprolactone) blend scaffolds for cartilage repair. Biomaterials 32:1068–1079. https://doi.org/10.1016/j.biomaterials.2010.09.073
Majima T, Funakosi T, Iwasaki N, Yamane S-T, Harada K, Nonaka S, Minami A, Nishimura S-I (2005) Alginate and chitosan polyion complex hybrid fibers for scaffolds in ligament and tendon tissue engineering. J Orthop Sci 10:302–307. https://doi.org/10.1007/s00776-005-0891-y
Funakoshi T, Majima T, Iwasaki N, Yamane S, Masuko T, Minami A, Harada K, Tamura H, Tokura S, Nishimura S-I (2005a) Novel chitosan-based hyaluronan hybrid polymer fibers as a scaffold in ligament tissue engineering. J Biomed Mater Res A 74A:338–346. https://doi.org/10.1002/jbm.a.30237
Funakoshi T, Majima T, Iwasaki N, Suenaga N, Sawaguchi N, Shimode K, Minami A, Harada K, Nishimura S (2005b) Application of tissue engineering techniques for rotator cuff regeneration using a Chitosan-Based hyaluronan hybrid fiber scaffold. Am J Sports Med 33:1193–1201. https://doi.org/10.1177/0363546504272689
Irie T, Majima T, Sawaguchi N, Funakoshi T, Nishimura S, Minami A (2011) Biomechanical and histologic evaluation of tissue engineered ligaments using chitosan and hyaluronan hybrid polymer fibers: A rabbit medial collateral ligament reconstruction model. J Biomed Mater Res A 97A:111–117. https://doi.org/10.1002/jbm.a.32938
Funakoshi T, Majima T, Suenaga N, Iwasaki N, Yamane S, Minami A (2006) Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg 15:112–118. https://doi.org/10.1016/j.jse.2005.05.012
Sato M, Maeda M, Kurosawa H, Inoue Y, Yamauchi Y, Iwase H (2000) Reconstruction of rabbit Achilles tendon with three bioabsorbable materials: histological and biomechanical studies. J Orthop Sci 5:256–267. https://doi.org/10.1007/s007760050161
Tendon healing in vivo and in vitro: chitosan improves range of motion after flexor tendon repair—Minerva Ortopedica e Traumatologica 2013 August;64(4):445–52. https://www.minervamedica.it/en/journals/minerva-orthopedics/article.php?cod=R14Y2013N04A0445. Accessed 11 Jul 2022
Shao H-J, Chen CS, Lee Y-T, Wang J-H, Young T-H (2010a) The phenotypic responses of human anterior cruciate ligament cells cultured on poly(ϵ-caprolactone) and chitosan. J Biomed Mater Res A 93A:1297–1305. https://doi.org/10.1002/jbm.a.32629
Shao H-J, Lee Y-T, Chen C-S, Wang J-H, Young T-H (2010b) Modulation of gene expression and collagen production of anterior cruciate ligament cells through cell shape changes on polycaprolactone/chitosan blends. Biomaterials 31:4695–4705. https://doi.org/10.1016/j.biomaterials.2010.02.037
Taravel MN, Domard A (1995) Collagen and its interaction with chitosan: II. Influence of the physicochemical characteristics of collagen. Biomaterials 16:865–871. https://doi.org/10.1016/0142-9612(95)94149-F
Taravel MN, Domard A (1996) Collagen and its interactions with chitosan: III. some biological and mechanical properties. Biomaterials 17:451–455. https://doi.org/10.1016/0142-9612(96)89663-3
Ma J, Wang H, He B, Chen J (2001) A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan bilayer material as a scaffold of human neofetal dermal fibroblasts. Biomaterials 22:331–336. https://doi.org/10.1016/S0142-9612(00)00188-5
Ueno H, Yamada H, Tanaka I, Kaba N, Matsuura M, Okumura M, Kadosawa T, Fujinaga T (1999) Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 20:1407–1414. https://doi.org/10.1016/S0142-9612(99)00046-0
Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, Sakurai T, Nimura Y (2003) Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A 64A:177–181. https://doi.org/10.1002/jbm.a.10396
Yan X, Khor E, Lim L-Y (2000) PEC Films Prepared from Chitosan-Alginate Coacervates. Chem Pharm Bull (tokyo) 48:941–946. https://doi.org/10.1248/cpb.48.941
Wang L, Khor E, Wee A, Lim LY (2002) Chitosan-alginate PEC membrane as a wound dressing: Assessment of incisional wound healing. J Biomed Mater Res 63:610–618. https://doi.org/10.1002/jbm.10382
Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, Han C (2003) Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 24:4833–4841. https://doi.org/10.1016/S0142-9612(03)00374-0
Burkatovskaya M, Castano AP, Demidova-Rice TN, Tegos GP, Hamblin MR (2008) Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen 16:425–431. https://doi.org/10.1111/j.1524-475X.2008.00382.x
Guo R, Xu S, Ma L, Huang A, Gao C (2011) The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen–chitosan dermal equivalents. Biomaterials 32:1019–1031. https://doi.org/10.1016/j.biomaterials.2010.08.087
Kumar PTS, Raj NM, Praveen G, Chennazhi KP, Nair SV, Jayakumar R (2013) In vitro and in vivo evaluation of microporous chitosan Hydrogel/Nanofibrin composite bandage for skin tissue regeneration. Tissue Eng Part A 19:380–392. https://doi.org/10.1089/ten.tea.2012.0376
Li J, Pan J, Zhang L, Guo X, Yu Y (2003a) Culture of primary rat hepatocytes within porous chitosan scaffolds. J Biomed Mater Res A 67A:938–943. https://doi.org/10.1002/jbm.a.10076
Chupa JM, Foster AM, Sumner SR, Madihally SV, Matthew HWT (2000) Vascular cell responses to polysaccharide materials: in vitro and in vivo evaluations. Biomaterials 21:2315–2322. https://doi.org/10.1016/S0142-9612(00)00158-7
Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH, van der Werf M (2003) Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials 24:3213–3220. https://doi.org/10.1016/S0142-9612(03)00170-4
Wang X, Yan Y, Lin F, Xiong Z, Wu R, Zhang R, Lu Q (2005) Preparation and characterization of a collagen/chitosan/heparin matrix for an implantable bioartificial liver. J Biomater Sci Polym Ed 16:1063–1080. https://doi.org/10.1163/1568562054798554
Park I-K, Yang J, Jeong H-J, Bom H-S, Harada I, Akaike T, Kim S-I, Cho C-S (2003) Galactosylated chitosan as a synthetic extracellular matrix for hepatocytes attachment. Biomaterials 24:2331–2337. https://doi.org/10.1016/S0142-9612(03)00108-X
Li J, Pan J, Zhang L, Yu Y (2003b) Culture of hepatocytes on fructose-modified chitosan scaffolds. Biomaterials 24:2317–2322. https://doi.org/10.1016/S0142-9612(03)00048-6
She Z, Liu W, Feng Q (2009) Self-assembly model, hepatocytes attachment and inflammatory response for silk fibroin/chitosan scaffolds. Biomed Mater 4:045014. https://doi.org/10.1088/1748-6041/4/4/045014
Fan J, Shang Y, Yuan Y, Yang J (2010) Preparation and characterization of chitosan/galactosylated hyaluronic acid scaffolds for primary hepatocytes culture. J Mater Sci Mater Med 21:319–327. https://doi.org/10.1007/s10856-009-3833-y
Piscioneri A, Campana C, Salerno S, Morelli S, Bader A, Giordano F, Drioli E, Bartolo LD (2011) Biodegradable and synthetic membranes for the expansion and functional differentiation of rat embryonic liver cells. Acta Biomater 7:171–179. https://doi.org/10.1016/j.actbio.2010.07.039
Yuan Y, Zhang P, Yang Y, Wang X, Gu X (2004) The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 25:4273–4278. https://doi.org/10.1016/j.biomaterials.2003.11.029
Cao W, Cheng M, Ao Q, Gong Y, Zhao N, Zhang X (2005) Physical, mechanical and degradation properties, and Schwann cell affinity of cross-linked chitosan films. J Biomater Sci Polym Ed 16:791–807. https://doi.org/10.1163/1568562053992496
Mingyu C, Kai G, Jiamou L, Yandao G, Nanming Z, Xiufang Z (2004) Surface modification and characterization of chitosan film blended with Poly-L-Lysine. J Biomater Appl 19:59–75. https://doi.org/10.1177/0885328204043450
Cheng M, Deng J, Yang F, Gong Y, Zhao N, Zhang X (2003) Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials 24:2871–2880. https://doi.org/10.1016/S0142-9612(03)00117-0
Chiono V, Pulieri E, Vozzi G, Ciardelli G, Ahluwalia A, Giusti P (2008) Genipin-crosslinked chitosan/gelatin blends for biomedical applications. J Mater Sci Mater Med 19:889–898. https://doi.org/10.1007/s10856-007-3212-5
Yang Z, Mo L, Duan H, Li X (2010) Effects of chitosan/collagen substrates on the behavior of rat neural stem cells. Sci China Life Sci 53:215–222. https://doi.org/10.1007/s11427-010-0036-1
Guo X, Zahir T, Mothe A, Shoichet MS, Morshead CM, Katayama Y, Tator CH (2012) The effect of growth factors and soluble Nogo-66 receptor protein on transplanted neural Stem/Progenitor survival and axonal regeneration after complete transection of rat spinal cord. Cell Transplant 21:1177–1197. https://doi.org/10.3727/096368911X612503
Denkbaş EB, Seyyal M, Pişkin E (2000) Implantable 5-fluorouracil loaded chitosan scaffolds prepared by wet spinning. J Membr Sci 172:33–38. https://doi.org/10.1016/S0376-7388(00)00314-8
Lin H-Y, Yeh C-T (2010a) Controlled release of pentoxifylline from porous chitosan-pectin scaffolds. Drug Deliv 17:313–321. https://doi.org/10.3109/10717541003713733
Lin H-Y, Yeh C-T (2010b) Alginate-crosslinked chitosan scaffolds as pentoxifylline delivery carriers. J Mater Sci Mater Med 21:1611–1620. https://doi.org/10.1007/s10856-010-4028-2
Prabaharan M, Jayakumar R (2009) Chitosan-graft-β-cyclodextrin scaffolds with controlled drug release capability for tissue engineering applications. Int J Biol Macromol 44:320–325. https://doi.org/10.1016/j.ijbiomac.2009.01.005
Shi PJ, Li YB, Zhang L, Zuo Y, Wu M, Wang HN (2007) Fabrication of n-HA/CS Porous Scaffold Carrying Ampicillin-Loaded Microspheres. Mater Sci Forum 544–545:941–944. https://doi.org/10.4028/www.scientific.net/MSF.544-545.941
Duarte ARC, Mano JF, Reis RL (2009) Preparation of chitosan scaffolds loaded with dexamethasone for tissue engineering applications using supercritical fluid technology. Eur Polym J 45:141–148. https://doi.org/10.1016/j.eurpolymj.2008.10.004
Teng S-H, Lee E-J, Wang P, Jun S-H, Han C-M, Kim H-E (2009) Functionally gradient chitosan/hydroxyapatite composite scaffolds for controlled drug release. J Biomed Mater Res B Appl Biomater 90B:275–282. https://doi.org/10.1002/jbm.b.31283
Noel SP, Courtney HS, Bumgardner JD, Haggard WO (2010) Chitosan sponges to locally deliver amikacin and vancomycin: a pilot in vitro evaluation. Clin Orthop Relat Res 468:2074–2080. https://doi.org/10.1007/s11999-010-1324-6
Thacharodi D, Panduranga Rao K (1996) Collagen-chitosan composite membranes controlled transdermal delivery of nifedipine and propranolol hydrochloride. Int J Pharm 134:239–241. https://doi.org/10.1016/0378-5173(96)04453-5
Li F, Liu WG, Yao KD (2002) Preparation of oxidized glucose-crosslinked N-alkylated chitosan membrane and in vitro studies of pH-sensitive drug delivery behaviour. Biomaterials 23:343–347. https://doi.org/10.1016/S0142-9612(01)00111-9
Thacharodi D, Rao KP (1993) Release of nifedipine through crosslinked chitosan membranes. Int J Pharm 96:33–39. https://doi.org/10.1016/0378-5173(93)90209-X
Thacharodi D, Panduranga Rao K (1995) Collagen-chitosan composite membranes for controlled release of propranolol hydrochloride. Int J Pharm 120:115–118. https://doi.org/10.1016/0378-5173(94)00423-3
Jayakumar R, Prabaharan M, Nair SV, Tokura S, Tamura H, Selvamurugan N (2010) Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog Mater Sci 55:675–709. https://doi.org/10.1016/j.pmatsci.2010.03.001
Tığlı RS, Akman AC, Gümüşderelıoğlu M, Nohutçu RM (2009) In vitro release of dexamethasone or bFGF from Chitosan/Hydroxyapatite scaffolds. J Biomater Sci Polym Ed 20:1899–1914. https://doi.org/10.1163/156856208X399945
Ho Y-C, Mi F-L, Sung H-W, Kuo P-L (2009) Heparin-functionalized chitosan–alginate scaffolds for controlled release of growth factor. Int J Pharm 376:69–75. https://doi.org/10.1016/j.ijpharm.2009.04.048
Reves BT, Bumgardner JD, Cole JA, Yang Y, Haggard WO (2009) Lyophilization to improve drug delivery for chitosan-calcium phosphate bone scaffold construct: A preliminary investigation. J Biomed Mater Res B Appl Biomater 90B:1–10. https://doi.org/10.1002/jbm.b.31390
De la Riva B, Sánchez E, Hernández A, Reyes R, Tamimi F, López-Cabarcos E, Delgado A, Évora C (2010) Local controlled release of VEGF and PDGF from a combined brushite–chitosan system enhances bone regeneration. J Controlled Release 143:45–52. https://doi.org/10.1016/j.jconrel.2009.11.026
Buyens K, De Smedt SC, Braeckmans K, Demeester J, Peeters L, van Grunsven LA, de Mollerat du Jeu X, Sawant R, Torchilin V, Farkasova K, Ogris M, Sanders NN, (2012) Liposome based systems for systemic siRNA delivery: Stability in blood sets the requirements for optimal carrier design. J Controlled Release 158:362–370. https://doi.org/10.1016/j.jconrel.2011.10.009
Mao H-Q, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW (2001) Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Controlled Release 70:399–421. https://doi.org/10.1016/S0168-3659(00)00361-8
Chan P, Kurisawa M, Chung JE, Yang Y-Y (2007) Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials 28:540–549. https://doi.org/10.1016/j.biomaterials.2006.08.046
Kim TH, Nah JW, Cho M-H, Park TG, Cho CS (2006) Receptor-Mediated gene delivery into antigen presenting cells using Mannosylated Chitosan/DNA nanoparticles. J Nanosci Nanotechnol 6:2796–2803. https://doi.org/10.1166/jnn.2006.434
Gao S, Chen J, Xu X, Ding Z, Yang Y-H, Hua Z, Zhang J (2003) Galactosylated low molecular weight chitosan as DNA carrier for hepatocyte-targeting. Int J Pharm 255:57–68. https://doi.org/10.1016/S0378-5173(03)00082-6
Park IK, Kim TH, Park YH, Shin BA, Choi ES, Chowdhury EH, Akaike T, Cho CS (2001) Galactosylated chitosan-graft-poly(ethylene glycol) as hepatocyte-targeting DNA carrier. J Controlled Release 76:349–362. https://doi.org/10.1016/S0168-3659(01)00448-5
Kean T, Roth S, Thanou M (2005) Trimethylated chitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency. J Controlled Release 103:643–653. https://doi.org/10.1016/j.jconrel.2005.01.001
Thanou M, Florea BI, Geldof M, Junginger HE, Borchard G (2002) Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines. Biomaterials 23:153–159. https://doi.org/10.1016/S0142-9612(01)00090-4
Strand SP, Issa MM, Christensen BE, Vårum KM, Artursson P (2008) Tailoring of chitosans for gene delivery: Novel Self-Branched glycosylated chitosan oligomers with improved functional properties. Biomacromol 9:3268–3276. https://doi.org/10.1021/bm800832u
Lee SJ, Huh MS, Lee SY, Min S, Lee S, Koo H, Chu J-U, Lee KE, Jeon H, Choi Y, Choi K, Byun Y, Jeong SY, Park K, Kim K, Kwon IC (2012) Tumor-Homing Poly-siRNA/Glycol Chitosan Self-Cross-Linked nanoparticles for systemic siRNA delivery in cancer treatment. Angew Chem Int Ed 51:7203–7207. https://doi.org/10.1002/anie.201201390
Kang SH, Revuri V, Lee S-J, Cho S, Park I-K, Cho KJ, Bae WK, Lee Y (2017) Oral siRNA delivery to treat colorectal liver metastases. ACS Nano 11:10417–10429. https://doi.org/10.1021/acsnano.7b05547
Singh R, Chacharkar MP, Mathur AK (2008) Chitin membrane for wound dressing application—preparation, characterisation and toxicological evaluation. Int Wound J 5:665–673. https://doi.org/10.1111/j.1742-481X.2008.00482.x
Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF (2004) Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J Biomed Mater Res B Appl Biomater 69B:216–222. https://doi.org/10.1002/jbm.b.30000
Santos TC, Höring B, Reise K, Marques AP, Silva SS, Oliveira JM, Mano JF, Castro AG, Reis RL, van Griensven M (2013) In vivo performance of Chitosan/Soy-Based membranes as Wound-Dressing devices for acute skin wounds. Tissue Eng Part A 19:860–869. https://doi.org/10.1089/ten.tea.2011.0651
Pang HT, Chen XG, Ji QX, Zhong DY (2008) Preparation and function of composite asymmetric chitosan/CM-chitosan membrane. J Mater Sci Mater Med 19:1413–1417. https://doi.org/10.1007/s10856-007-3168-5
Mi F-L, Wu Y-B, Shyu S-S, Chao A-C, Lai J-Y, Su C-C (2003) Asymmetric chitosan membranes prepared by dry/wet phase separation: a new type of wound dressing for controlled antibacterial release. J Membr Sci 212:237–254. https://doi.org/10.1016/S0376-7388(02)00505-7
Meng X, Tian F, Yang J, He C-N, Xing N, Li F (2010) Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J Mater Sci Mater Med 21:1751–1759. https://doi.org/10.1007/s10856-010-3996-6
Zhu X, Chian KS, Chan-Park MBE, Lee ST (2005) Effect of argon-plasma treatment on proliferation of human-skin–derived fibroblast on chitosan membrane in vitro. J Biomed Mater Res A 73A:264–274. https://doi.org/10.1002/jbm.a.30211
de Queiroz AAA, Ferraz HG, Abraham GA, del Mar FM, Bravo AL, Román JS (2003) Development of new hydroactive dressings based on chitosan membranes: Characterization and in vivo behavior. J Biomed Mater Res A 64A:147–154. https://doi.org/10.1002/jbm.a.10265
Mi F-L, Shyu S-S, Wu Y-B, Lee S-T, Shyong J-Y, Huang R-N (2001) Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 22:165–173. https://doi.org/10.1016/S0142-9612(00)00167-8
Wang T, Zhu X-K, Xue X-T, Wu D-Y (2012) Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr Polym 88:75–83. https://doi.org/10.1016/j.carbpol.2011.11.069
Lee YM, Kim SS, Park MH, Song KW, Sung YK, Kang IK (2000) β-Chitin-based wound dressing containing silver sulfurdiazine. J Mater Sci Mater Med 11:817–823. https://doi.org/10.1023/A:1008961730929
Sudheesh Kumar PT, Lakshmanan V-K, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG, Nair SV, Jayakumar R (2012) Flexible and microporous chitosan Hydrogel/Nano ZnO composite bandages for wound dressing. In vitro and in vivo evaluation. ACS Appl Mater Interfaces 4:2618–2629. https://doi.org/10.1021/am300292v
Derfus AM, Chan WCW, Bhatia SN (2004) Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett 4:11–18. https://doi.org/10.1021/nl0347334
Higuchi Y, Oka M, Kawakami S, Hashida M (2008) Mannosylated semiconductor quantum dots for the labeling of macrophages. J Controlled Release 125:131–136. https://doi.org/10.1016/j.jconrel.2007.10.007
Lin S-Y, Chan H-Y, Shen F-H, Chen M-H, Wang Y-J, Yu C-K (2007) Chitosan prevents the development of AOM-induced aberrant crypt foci in mice and suppressed the proliferation of AGS cells by inhibiting DNA synthesis. J Cell Biochem 100:1573–1580. https://doi.org/10.1002/jcb.21152
Gibot L, Chabaud S, Bouhout S, Bolduc S, Auger FA, Moulin VJ (2015) Anticancer properties of chitosan on human melanoma are cell line dependent. Int J Biol Macromol 72:370–379. https://doi.org/10.1016/j.ijbiomac.2014.08.033
Solairaj D, Rameshthangam P, Arunachalam G (2017) Anticancer activity of silver and copper embedded chitin nanocomposites against human breast cancer (MCF-7) cells. Int J Biol Macromol 105:608–619. https://doi.org/10.1016/j.ijbiomac.2017.07.078
Singh A, Dutta PK, Kumar H, Kureel AK, Rai AK (2018) Synthesis of chitin-glucan-aldehyde-quercetin conjugate and evaluation of anticancer and antioxidant activities. Carbohydr Polym 193:99–107. https://doi.org/10.1016/j.carbpol.2018.03.092
Arunraj TR, Sanoj Rejinold N, Ashwin Kumar N, Jayakumar R (2014) Bio-responsive chitin-poly(l-lactic acid) composite nanogels for liver cancer. Colloids Surf B Biointerfaces 113:394–402. https://doi.org/10.1016/j.colsurfb.2013.09.023
Pirzadeh-Naeeni S, Mozdianfard MR, Shojaosadati SA, Khorasani AC, Saleh T (2020) A comparative study on schizophyllan and chitin nanoparticles for ellagic acid delivery in treating breast cancer. Int J Biol Macromol 144:380–388. https://doi.org/10.1016/j.ijbiomac.2019.12.079
Mattaveewong T, Wongkrasant P, Chanchai S, Pichyangkura R, Chatsudthipong V, Muanprasat C (2016) Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-κB and mTOR signaling. Carbohydr Polym 145:30–36. https://doi.org/10.1016/j.carbpol.2016.02.077
Wimardhani YS, Suniarti DF, Freisleben HJ, Wanandi SI, Siregar NC, Ikeda M-A (2014) Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J Oral Sci 56:119–126. https://doi.org/10.2334/josnusd.56.119
Jiang Z, Han B, Li H, Yang Y, Liu W (2015) Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr Polym 129:1–8. https://doi.org/10.1016/j.carbpol.2015.04.040
Gao J, Zhao Y, Wang C, Ji H, Yu J, Liu C, Liu A (2020) A novel synthetic chitosan selenate (CS) induces apoptosis in A549 lung cancer cells via the Fas/FasL pathway. Int J Biol Macromol 158:689–697. https://doi.org/10.1016/j.ijbiomac.2020.05.016
Hasanzade Z, Raissi H (2019) Assessment of the chitosan-functionalized graphene oxide as a carrier for loading thioguanine, an antitumor drug and effect of urea on adsorption process: Combination of DFT computational and molecular dynamics simulation studies. J Biomol Struct Dyn 37:2487–2497. https://doi.org/10.1080/07391102.2018.1496140
Lee J-Y, Termsarasab U, Lee MY, Kim D-H, Lee SY, Kim JS, Cho H-J, Kim D-D (2017) Chemosensitizing indomethacin-conjugated chitosan oligosaccharide nanoparticles for tumor-targeted drug delivery. Acta Biomater 57:262–273. https://doi.org/10.1016/j.actbio.2017.05.012
Li P-W, Wang G, Yang Z-M, Duan W, Peng Z, Kong L-X, Wang Q-H (2016) Development of drug-loaded chitosan–vanillin nanoparticles and its cytotoxicity against HT-29 cells. Drug Deliv 23:30–35. https://doi.org/10.3109/10717544.2014.900590
Fiorillo L (2019) Chlorhexidine Gel Use in the Oral District: A Systematic Review. Gels 5:31. https://doi.org/10.3390/gels5020031
Husain S, Al-Samadani KH, Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Qasim SB (2017) Chitosan biomaterials for current and potential dental applications. Materials 10:602. https://doi.org/10.3390/ma10060602
Ortiz C, Boyce MC (2008) Bioinspired structural materials. Science 319:1053–1054. https://doi.org/10.1126/science.1154295
Zeza B, Wohlfahrt C, Pilloni A (2017) Chitosan brush for professional removal of plaque in mild peri-implantitis. Minerva Stomatol 66:163–168. https://doi.org/10.23736/s0026-4970.17.04040-7
Pippi R, Santoro M, Cafolla A (2017) The Use of a Chitosan-Derived hemostatic agent for postextraction bleeding control in patients on antiplatelet treatment. J Oral Maxillofac Surg 75:1118–1123. https://doi.org/10.1016/j.joms.2017.01.005
Lope-Lopez J, Jan-Pallí E, González-Navarro B, Jané-Salas E, Estrugo-Devesa A, Milani M (2015) Efficacy of chlorhexidine, dexpanthenol, allantoin and chitosan gel in comparison with bicarbonate oral rinse in controlling post-interventional inflammation, pain and cicatrization in subjects undergoing dental surgery. Curr Med Res Opin 31:2179–2183. https://doi.org/10.1185/03007995.2015.1108909
Uysal T, Akkurt MD, Amasyali M, Ozcan S, Yagci A, Basak F, Sagdic D (2011) Does a chitosan-containing dentifrice prevent demineralization around orthodontic brackets? Angle Orthod 81:319–325. https://doi.org/10.2319/062910-359.1
Mohire NC, Yadav AV (2010) Chitosan-based polyherbal toothpaste: As novel oral hygiene product. Indian J Dent Res 21:380. https://doi.org/10.4103/0970-9290.70808
Hayes M, Carney B, Slater J, Brück W (2008) Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan ndash; Part A: extraction methods. Biotechnol J 3:871–877. https://doi.org/10.1002/biot.200700197
Barriada JL, Herrero R, Prada-Rodríguez D, de Vicente MES (2007) Waste spider crab shell and derived chitin as low-cost materials for cadmium and lead removal. J Chem Technol Biotechnol 82:39–46. https://doi.org/10.1002/jctb.1633
Irawan C, Nata IF, Putra MD, Marisa R, Asnia M, Arifin YF (2018) Biopolymer of Chitosan from Fish Scales as Natural Coagulant for Iron–Contaminated Groundwater Treatment. J Rekayasa Kim Lingkung 13:93–99. https://doi.org/10.23955/rkl.v13i2.10601
Kosyakov VN, Yakovlev NG, Veleshko IE (2002) Application of chitin-containing fiber material “Mycoton” for actinide absorption. J Nucl Sci Technol 39:508–511. https://doi.org/10.1080/00223131.2002.10875518
Acknowledgements
The vice chancellor of West Bengal University of Animal and Fishery Sciences, in Kolkata, India, is gratefully acknowledged by the authors. The authors also acknowledge the kind support of the ICAR National Professor project of the Indian Council of Agricultural Research, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Roy, S., Chaudhuri, S., Mukherjee, P., Nandi, S.K. (2024). Biomedical Applications of Chitin, Chitosan, Their Derivatives, and Processing By-Products from Fish Waste. In: Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A. (eds) Fish Waste to Valuable Products. Sustainable Materials and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-8593-7_12
Download citation
DOI: https://doi.org/10.1007/978-981-99-8593-7_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8592-0
Online ISBN: 978-981-99-8593-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)