Abstract
A novel composite asymmetric chitosan/CM-chitosan membrane (C-P-C) was prepared, the top-layer was chitosan (CS), the intermediate was PVA, and the substrate was carboxymethyl chitosan (CM-CS). C-P-C membrane had capability in mechanical strength, light transparence, vapor permeability, and wound skin joining. The CS and CM-CS in C-P-C membrane were selected by series independent experiments, respectively. CS (MW 90,000 Da) had the highest antibacterial activity for E.coli. CM-CS had biocompatibility, no cytotoxicity, and had the activity of promoting growth of human skin fibroblast and inhibiting the growth of keloid fibroblast. The normal skin fibroblast can growth on the CM-CS surface of C-P-C, and have no conglomeration in higher cell density, and the keloid fibroblast could not growth on CM-CS surface of C-P-C. The animal experiment demonstrated that wound, covered with the C-P-C membrane, was hemostatic, healing quickly and had histocompatibility. The results indicated that the C-P-C membrane could be used as dressing of skin repair, and had the potential in promoting wound healing and inhibiting the keloid formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Larger area skin damnification, created by hurt, ulcer and other disease, need to be skin grafted or covered by dressing to prevent the running off of tissue fluid or body fluid and to prevent the losing of electrolyte and protein, another important function of the dressing is inhibiting the invader and promote the recover of the wound. There were some artificial skins and dressings had been used in clinic, such as hogskin [1], collagen film [2], collagen sponge and polyurethane film [3]. The animal skin had no histocompatibility with human skin, the collagen dressing had shortage in mechanical strength and had antigenicity [4], polyurethane film induced the empyema and infection of the wound [5]. The research of new innovation wound dressing is the hotspot in the field of biomaterials and skin repair.
Chitosan (CS) had been used in wound repair [6, 7], and the film hand some characteristics such as biodegradation, biocompatibility and antibacterial [8]. The water insolubility of chitosan was a limitation to the effectiveness as dressing. In our previous reports, Carboxymethyl chitosan (CM-CS) was a water-soluble chitosan-derivative, had no cytotoxicity, could promote the growth of human skin fibroblast, and had potential in the wound repair [9, 10]. The single membrane of CS, CM-CS and the composite membrane of CS and CM-CS had been reported [11], the ductility, stability and multifunction had not the suitable to usage on clinic. Coencen (1993) present a new concept of dressing with three layers of the membrane, the substrate of the dressing touch the wound skin and has a function of promote the tissue repair, the intermediate layer of dressing can absorb the extravasate of the wound, and the surface layer of dressing antibacteria and eliminate the empyema of the wound. Thereafter, the idealized dressing had no more advanced report. CS and CM-CS were thought to be close to Coencen’ concept in the physicochemical properties and biofunctions according to our previous studies [9, 10, 12] and many other reports [13].
In this paper, CS, CM-CS was prepared, respectively. The optimal molecular weight (MW) CS, CM-CS and PVA (poly-vinyl alcohol) were used to make the composite asymmetric membrane (C-P-C), the physicochemical properties, biocompatibility and wound repair as dressing were described.
Materials and methods
Materials
Chitosan was made from crab shell, MW 5 × 105 Da, degree of deacetylation (DDa) 80.0%; normal skin fibroblast and keloid skin fibroblast: plasma colt explant culture, fresh tissue specimens were obtained from Fangzhi Hospital of Qingdao (Qinadao, China); Escherichia coli ATCC 25922 (E.coli), Monilia candida ATCC 14053 were kindly presented gifts by Clinical Laboratory of Qingdao University Hospital; mouse: wistar, 250–350 g, ♂ and ♀ each half; PVA-124, DMEM culture media, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were Sigma products (Sigma Co. ST. Louis, U.S.A).
Membrane preparation
Different MW CS
Chitosan (5 g, 100 mesh powder) was dissolved in 95 mL of 5% aqueous acetic acid, incubated at 50 °C for different hours, respectively, then centrifugated (5,000g) for 20 min. The supernate was added 4 N aqueous NaOH to pH 7–9. The sediment was filtered and sequentially rinsed in water and ethanol, and dried at 50 °C.
CM-CS synthesis
CM-chitosan was prepared from chitosan by the method of our previous report [9].
Membrane preparation
The membranes were prepared by a cast method [14]. The bubble-free solution of chitosan (3% w/v, 2 mL) was spread on a plastic dish (ø = 40 mm) and dried at 50 °C for over night, PVA solution (3%, w/v,) in the same amount was added on the chitosan film to dry at the same condition, then CM-CS solution was added and dry. The membrane was separated from the dish, soaked in ethyl alcohol (70%, v/v), and then dried at 50 °C. The thickness and swelling degree of the membrane were measured by a thickness gages (SM-1201, Japan) before and after the membranes were soaked in distilled water for 24 h.
Physicochemical characteristics
FTIR
The infrared spectra was determined by 2 mg CS in 100 mg KBr pellet, with FT/IR-430 Fourier Transform Infrared Spectrometer [9]. The data analysis was Jwstda-32 file, windows 95/NT.
Vapor permeability
Sodium chloride solution 20 mL (0.9%, w/w) was added in a glass bottle (50 mL, caliber 37 mm), closed with C-P-C membrane, and weighted it (w1), after incubated at 37 ± 0.5 °C for 24 h, weighted it (w2), the vapor permeability calculation was: Vapor permeability = (w2 − w1)/(A × d); here: A— effective area of the C-P-C membrane; d— days, (n = 5).
Water absorption
C-P-C membrane (0.5 g) was soaked in PBS solution (pH = 7.2) at 37 ± 0.5 °C for 24h, then take out from the solution, the liquid on C-P-C was observed with filter paper and weighted (w1), then drying it at 50 °C to constant weight (w2), calculated the water absorption: Water absorption = [(w 1 − w 2)/w 2] × 100%, (n = 5).
Cell culture
The fibroblasts were inoculated at a 96-well tissue culture plate with 4–6 × 103 cells per well. The sample concentrations were 0–250 μg CM-CS per mL culture media. The cell numbers were determined using the MTT method [15]. C-P-C membranes were casted in the cell culture flask, then used in cell culture.
Germiculture
A representation colony was picked off with a wire loop and placed in YPD culture medium (peptone 10 g, ferment 10 g, glucose 10 g, distilled water 1,000 mL, pH 5.5) to shake and cultivated at 37 °C (HZQ-F160 All-Temperature Vibrating Incubator Harbin Donglian Electronic& Technology Development Co. LTD China). The turbidity of the medium was measured at 610 nm [16].
Flat culture
The bacteria suspension 1 mL (cell density, 1 × 106/mL) was cast on the surface of YDP culture medium in petri dish, the film samples were cut into disks with diameter 1.3cm and sterilized, then were put onto the culture medium, incubated at 37 °C for 48 h [17].
Animal experiment
Skin with size 1.0 cm × 1.0 cm were cut into wound on the double side of back spine of wistar mice, each side 2 wounds, one side was control, another side was treated with C-P-C membrane, histo-specimens were collected and observed [18].
Results and disscusion
The degradation products of chitosan were shown in Table 1, the range of the MW of the products was from 5.5 × 104 Da to 15.5 × 104 Da, the DDa of the products was 85% ± 0.5. The FTIR spectrum (Fig. 1) shows that the structure had no obviously different between the samples, all of the chitosan products had different MW with the similar DDa. The optimal MW of chitosan in the activity of antibiosis with E.coli was 9 × 104 Da (see in Fig. 2), at the concentration of chitosan 0.05%(w/v), the result was same to the report of Liu et al (2001). The chitosan (CS) product with MW 9 × 104 Da was selected to make C-P-C membrane.
Chitosan was water insoluble, it was reacted with chloroacetic acid to get the water-soluble product—CM-chitosan (CM-CS), 1H NMR spectrum of CM-CS show in our previous report [10]. The total substitute degree (SD) of carboxymethyl group (CM) on the residue of glucosamine in chitosan molecule was 1.13, the CM groups were distributed in 2,3,6 position on residue of glucosamine, and the SD were 0.13, 0.24 and 0.76, respectively. The most CM group was on the 6 position of residue of glucosamine in chitosan. In the culture of normal skin fibroblast and keloid fibroblast with CM-CS, CM-CS promoted the growth of normal skin fibroblast, and inhibited the growth of keloid fibroblast; the result was shown in Fig. 3. The mechanism that CM-CS inhibited the secretion of collagen I to result in the falling down of the ratio of collagen I/III in keloid fibroblast was described in our previous report [10].
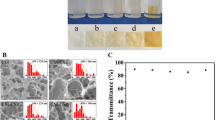
The caste method was used to make CS film, CM-CS film, PVA film and C-P-C membrane, respectively. The top layer of C-P-C membrane was CS, the intermediate layer was PVA, and the substrate was CM-CS. The C-P-C membrane was a composite-asymmetric-membrane, and was according with Coencen’s idea [12] in the hierarchy. The SEM picture of C-P-C was shown in Fig. 4, the CS layer was uniform and compact in the quality (Fig. 4B), CM-CS layer was spongy and porous (Fig. 4A). C-P-C membrane had stronger mechanical strength, higher thickness and lower light transparence than each single film. The water solubility of C-P-C was lower than CM-CS film and higher than CS and PVA film. The vapor permeability of the films were CM-CS > CS > C-P-C > PVA, the results seen in Table 2. The CM-CS film had a lot of porous, the vapor permeability was the highest in all of the film, C-P-C membrane had higher vapor permeability than PVA film, this reason was due to the alternation of the structure of PVA layer in the process of the membrane preparation to influence by CS and CM-CS, the PVA-CS layer and PVA-CM-CS layer had more porous than single PVA film to result in the higher vapor permeability. Ideal wound dressing would have ability of removing the secretion and having no dehydration, the vapor permeability is about 2,500 g m−2 d−1 [7]. According to the results in table 2, C-P-C membrane was very close to the ideal wound dressing in the properties.
E.coli and Monilia candida did no growth on C-P-C membrane. The results show that C-P-C membrane had the function of anti-bacteria; the function was thought to be from CS.
The normal skin fibroblast could growth on the surface of CM-CS side of C-P-C membrane, there no cytotoxicity was found in higher cell density, but the keloid fibroblast could not growth on the CM-CS surface of the membrane (see in Fig. 5), the result show that C-P-C membrane had the potential in benefit of skin repair and inhibition of keloid formation during process of wound healing.
C-P-C could adhere on the surface of the wound skin of the mouse, had good bio-compatibility, and had function of blockading the wound and hemostasia, could promote the wound healing, the results was shown in Table 3.
Conclusion
CS had higher activity in antibacterial; CM-CS had the function of promoting the growth of normal skin fibroblast and inhibiting the growth of keloid fibroblast. C-P-C film made from CS, CM-CS and PVA had mechanical strength, transparence, water absorption and vapor permeability, had activity of antibacterial. C-P-C membrane supported the growth of normal skin fibroblast, inhibited the growth of keloid fibroblast. The C-P-C membrane could be adhered on the wound surface, and promote the wound recovery. C-P-C membrane had good potential to be a wound healing biomaterial.
References
T. T. NGUYN, D. A. GILPIN and N. A. MEYER et al Ann. Surg. 223 (1996) 14
C.H. LEE, A. SINGLA and Y. LEE, Int. J. Pharm. 211 (2001) 1
A. De CONINCK, J.-P. DRAYE and A. Van STRUBARQ et al J. Dermatol. Sci. 13 (1996) 202
A. SIONKOUSKA, Polym. Degrad. Stab. 68 (2000) 147
A. J. SINGER, MUZHAR. MOHAMMAD and HENRY. C. Jr THODE et al Burns 26 (2000) 388
G. C. RITTHIDEJ, T. PHAECHAMUD and T. KOIZUMI, Int. J. Pharm. 232 (2002) 11
F.W.U. LONG MI, S.-S. SHYU and Y.-B. WU et al Biomaterials 22 (2001) 165
E.. KHORA and L. Y. LIMB, Biomaterials 24 (2003) 2339
X. G.CHEN and H. J. PARK, Carbohydr. Polym. 53(4) (2003) 355
X. G. CHEN, Z. WANG and W. S. LIU et al Biomaterials 23 (2002) 4609
M. ZHANG, X. H. LI, Y. D. GONG and N. M. ZHAO et al Biomaterials 23 (2002) 2641
J. M. F. H. COENEN, M. F. JONKMAN, H. J. KLASEN, J. H. De GROOT, and A. J. PENNINGS, in “Late Results of a Triple-Layer Artificial Skin”, edited by K. C. JUDKINS (European Burn Association 5th Congress, Brighton, England, 1993)
F. W. U. LONG MI, Y. B. WU and S. S. SHYU et al J. Memb. Sci. 212 (2003) 237
Y. M. SUN, W. F. HUANG and C. C. CHANG, J. Memb. Sci. 157 (1999) 159
K. ABE and N. MATSUKI, Neurosci. Res. 38 (2000) 325
X. F. LIU, Y.L. GUAN and D. Z. YANG et al J. Appl. Polymer Sci. 79 (2001) 1324
S. MIEHLKE and D. Y.GRAHAM, Int. J. Antimicrob. Agents 8 (1997) 171
R.. MATSUI, K.-i. OSAKI and J. KONISHI et al Biomaterials 17 (1996) 989
Acknowledgements
The authors are indebted to the financial support from NSFC (30670566), ISTCP (2006DFA3350) and the NSF of Shandong Province (CY2006C110).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pang, H.T., Chen, X.G., Ji, Q.X. et al. Preparation and function of composite asymmetric chitosan/CM-chitosan membrane. J Mater Sci: Mater Med 19, 1413–1417 (2008). https://doi.org/10.1007/s10856-007-3168-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3168-5