Abstract
Microalgae and cyanobacteria produced abundant high-valued bioproducts in small arable land in a short time. The bioproducts range from their biomass for food, feed, and biofuels to extractable fine bioproducts. The growing market and techno-economical aspect support the viability of this biomass production. Microalgae and Cyanobacteria are also highly diverse thus progression of its current usage in biomass production served as a challenge of its own but also an opportunity. In this book chapter, progression in cultivating and screening of technologies on bioprocess engineering of microalgae and cyanobacteria will be discussed with their high-demands on food, feed, and energy industry. This chapter further discusses the advance and manufacturer of different valuable bioproducts through technologies and production platforms for Microalgae and Cyanobacteria.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Microalgae and cyanobacteria consist of a broad spectrum of photoautotrophic microorganisms which grow through photosynthesis. The conversion of chemical energy from solar as a unicellular form made them known as the oldest life form/thallophytes (primitive plants) known (Abreu et al. 2022). As primitive plants, they exhibit an absence of roots, stems, and leaves and possess chlorophyll-a as the main photosynthetic pigment for energy conversion. This characteristic enables them to adapt toward the predominant environmental circumstances, hence allowing said lifeforms to flourish for a long period of time (Kumar et al. 2020). As for the types of the cells, cyanobacteria—as the oldest of the two—have prokaryotic cells which are characterized by the lack of membrane-bound organelles usually present around plastids, mitochondria, nuclei, Golgi bodies, and flagella. Meanwhile, microalgae are eukaryotic cells which have these organelles that control the function of cells. Microalgae are mainly eukaryotes grouped into many classes defined accordingly to their pigmentation, life cycle, and basic cellular structure; with green algae (Chlorophyta), red algae (Rhodophyta), and diatoms (Bacillariophyta) posing as the three most important classes in microalgae. Both microalgae and cyanobacteria can be either autotrophic (inorganic compounds such as CO2, salts, and light as prerequisites) or heterotrophic (with an external source of organic compounds and nutrients due to their non-photosynthetic nature). Several photosynthetic algae or cyanobacteria are mixotrophic (capable to perform both photosynthesis and acquire exogenous organic nutrients). Autotrophs rely on photosynthesis for their survival, as they convert solar irradiance and absorbed CO2 by chloroplast into adenosine triphosphate (ATP) and O2. Both ATP and O2 are usable energy packages at the cellular level, which are then used in respiration to produce energy to support their growth (Farhan et al. 2017).
Recently there are numerous studies related to techniques and large-scale production of microalgal and cyanobacterial biomass (Ugoala et al. 2012). In general, there are two types of cultivation techniques, namely open pond system and closed photobioreactor system. Both systems have advantages and disadvantages, therefore preference for the being used system depends on the characteristics of targeted products (Milledge 2011). Difficulties in controlling the contamination and predation in open pond systems frequently occur. On the contrary, photobioreactor systems enable to control of nutrients for growth and operating parameter such as pH, temperature, dissolved CO2, and contamination/predation. Unfortunately, photobioreactor systems need a high investment cost and are quite specific to strains of microalgal and cyanobacterial physiologies which being cultivated (Acién Fernández et al. 2013). Therefore, a consideration of the production to facilitate an optimum production for specific microalga or cyanobacterium is quite important. The harvesting process is conducted for dewatering the algal and cyanobacterial biomass. Methods for this purpose are flocculation, centrifugation, and filtration. A favorable dewatering process for harvesting must apply to a wide range of microalgal and cyanobacterial strains. This aims to concentrate biomass recovery and cost-effective production. The important matter to consider for mass biomass production is combining cultivation and dewatering processes at the possibly lowest cost while maximizing the microalgal and or cyanobacterial biomass production. Bioprocess engineering in microalgal and cyanobacterial biomass production provides various downstream products for commercial purposes. Microalgal and cyanobacterial biomass contains an abundant bioactive compound that useful being used in many important industries such as pharmaceuticals (for the manufacture of antioxidants, antibiotics, immunomodulators, etc.) (Mobin and Alam 2017; Kholssi et al. 2021). Meanwhile, for human consumption, microalgae biomass can be extracted to obtain its high protein contents, vitamins, and polysaccharides (Catone et al. 2021; Hernández et al. 2015). Some microalgae and cyanobacteria are known to contain high lipids which are then extracted (oil press, solvent extraction, supercritical fluid extraction and ultrasound) and converted (trans-esterified) into biofuels (Castro et al. 2021; Felix et al. 2019). Furthermore, the residue of lipid-extracted microalgal and cyanobacterial biomass can be converted into other forms of biofuels, such as biomethane, bioethanol, and biohydrogen (Felix et al. 2019; Nitsos et al. 2020).
Microalgae and cyanobacteria also display a capability to overcome emerging environmental issues, for example, the greenhouse effect and water (industrial, domestic, and agricultural) pollution (Gil-izquierdo et al. 2021; Liu et al. 2021). These microorganisms can sequester CO2 from flue gas for their photosynthetic activity and reduce aquatic nutrients efficiently from wastewater at minimal cost (Song et al. 2019). Some species of microalgae and cyanobacteria show the capability to fix nitrogen and absorb phosphorus as well as heavy metals from wastewater (Gonçalves et al. 2017; Singh and Ahluwalia 2013; Satya et al. 2017, 2021a, b; Vijayaraghavan and Balasubramanian 2015). Those facts demonstrated that microalgae and cyanobacteria can provide a promising solution to address emerging environmental problems and concomitantly generate many valuable consumer products. Conceptually, the production of microalgal and cyanobacterial biomass can be generated from the carbon recycling process (Fig. 1).
Conceptual diagram for producing microalgae and cyanobacteria biomass adapted from Chisti (2007)
This chapter book discusses the different cultivation, harvesting, and processing methods of microalgae and cyanobacteria for producing several bioproducts. These materials involve biofuels, fine biochemicals, and food/functional food. The prospect of microalgae and cyanobacteria overcoming emerging environmental problems is also delivered in this chapter book.
2 Microalgae Cultivation
2.1 Open System
An open system for cultivating microalgae and cyanobacteria is generally found as an open pond in a variety of shapes and sizes. There are some advantages and disadvantages related to the implementation of this cultivation system. Examples of open pond types are raceway pond with paddle wheels (Fig. 2a), open tanks (Fig. 2b), shallow big ponds (Fig. 2c), circular ponds, etc. The place where the pond is located becomes a determining factor for choosing the proper type of pond the selected strain of microalgal or cyanobacterial being used and the availability of light for photosynthesis. The open pond is a function of the local climate; therefore, the chosen location will affect the achievement of cultivation. Several key growth parameters (solar irradiance, temperature, pH, and concentration of dissolved oxygen) are limiting the performance of the open pond (Ashokkumar et al. 2014). Another critical problem for an open pond system is the occurrence of predation due to the higher risk of contamination. Only several microalgal or cyanobacterial can be grown successfully in an open pond even in severe environmental conditions (such as Dunaliella in high salinity, Arthrospira in high alkalinity and Chlorella in high aquatic nutrients). Dunaliella salina was cultivated for producing carotenoids, this compound in nature protects this microalga against the high intense sunlight during grown in open pond. Other reports mentioned that Chlorella sp achieved a decent photosynthetic activity in raceway system, while Muriellopsis sp. was cultured for producing lutein in an open tank equipped with a paddle wheel (Blanco et al. 2007). These biomasses can be used as food colorant, feed additives in aquaculture, and poultry. Cost for cultivating microalga and cyanobacterium is an important factor for considering a choice between using an open system and photobioreactor. The investment and maintenance costs in open pond construction is less than photobioreactor, even so the biomass productivity in open pond is lower than photobioreactor and biomass quality in open pond is more variable compared to photobioreactor (Ugoala et al. 2012; Debeni Devi et al. 2022). Consequently, for providing the needs of bulk requirement of biomass (e.g., for producing biofuel), an open pond form is preferable.
2.2 Photobioreactor System
Photobioreactor provides better control on most operational parameter compared to open pond system, therefore extensive research on designing photobioreactors for cultivating microalgae and cyanobacteria is a must (Huang et al. 2017; Duan and Shi 2014). Higher biomass productivity can be achieved in a controlled environment which is the advantage of using this system. Careful considerations, however, are needed since the performance of a bioreactor is assessed from its productivity. These considerations stem from difficulties in comparing the productivity among photobioreactors because the difference in microalgae strains and scales of the photobioreactors used. In the principle, photobioreactor types are differentiated into tubular and plate shapes. The tubular reactors are considered to be more suitable for outdoor cultivation. It is because of the use of transparent material for configuring the tubes, which exhibits large illuminated surfaces. Various configurations can be made depending on the specification of the system and its purposes. Generally, those tubing configurations may be found in straight-line forms and or coiled forms (Johnson et al. 2018; Nwoba et al. 2019). Geometry of the photobioreactor also determines its performance, as tubular reactors can be configured in inclined, horizontal, or vertical planes. The vertical design enables better mass transfer and requires lower energy supply. In the case of the horizontal photobioreactor design, a larger area is required than vertical design. In terms of scalability, horizontal design is more preferred (Sirohi et al. 2022; Xia et al. 2013).
Tubular photobioreactors for culturing microalgae and cyanobacteria can be configured as vertical, horizontal, and helical forms. Another configuration is flat-plate photobioreactors which are characterized by narrow lightpath and therefore able to maintain a higher cell concentration up to an order magnitude than another configuration. Moreover, this configuration type of photobioreactor is favorable since it allows a lower power energy consumption and high mass transfer capacity. It also allows a reduction in oxygenic accumulation, providing no dark volume and high photosynthetic efficiency. A proper photobioreactor design is needed to obtain the maximum biomass cell production. The flat-plate photobioreactor for culturing microalgae and cyanobacteria may be constructed in the form of glass, thick transparent polyvinyl chloride materials, V-shaped, and inclined. The translucent material gives a maximum light penetration meanwhile other materials for designing are inexpensive and easy to construct. Figure 3 describes the basic diagram of a tubular photobioreactor (3a) and a real view of a tubular photobioreactor system on a pilot scale.
Schematic basic diagram of a tubular photobioreactor (3a) and a real scene of tubular photobioreactor at a pilot scale. After Fernandez et al. (2014)
The two existing cultivation systems present their own advantages and disadvantages with few similarities in operation as seen in Table 1. Optimization of the two systems can be done by either change of several operative conditions or even combining the two systems. The goal of combining the two systems is to gain higher biomass yield with few design parameters. Important design parameters for tubular photobioreactors are mixing, gas hold-up, bubble diameter, and intensity of light and dark cycles. Meanwhile, important design parameters for flat-plate photobioreactors are mixing, gas hold-up, bubble diameter, light-to-dark cycle efficiency, and illuminated surface-to-volume ratio (Cui et al. 2021). The factors affecting missing in flat-plate photobioreactors are much more than that in tubular reactors. In flat-plate photobioreactors, aside from resident time distribution (RTD) and circulation time which are present in a tubular reactor’s mixing factor, shear rate played a bigger role as sheer stress affects cells, nutrients, temperature, and eventually toxic levels of dissolved oxygens and carbon demands with novel system dealing with the particular problem showed significant improvement of biomass production up to 61% (Yaqoubnejad et al. 2021). There are also significantly more hardware designs needed in developing a tubular reactor. These harder designs in addition to sparger designs are methods of mixing and pumping. However, the tubular reactor offered much more sparger designs (orifice, ring, foam types) to allow some enhancement methods. These enhancement methods are the incorporation of static mixers, static kinetics mixers, helical mixers, or swirl flow for a tangential inlet (Sirohi et al. 2022; Sung et al. 2022). Flat-plate photobioreactors provide easier control in massive microalgae biomass production but its maintenance and large light areas are their major bottleneck. For outdoor cultivation, solar-irradiation filtration technologies should be considered for garnering multiple advantages in addition to combining the existing systems (Wu et al. 2023; Huang et al. 2023).
3 Harvesting of Microalgae
3.1 Flocculation
Flocculation is the first stage in the bulk harvesting process. This stage aims to aggregate the microalgae and cyanobacteria cells to increase the effective particle size for harvest. This method is usually applied as the initial step in harvesting (dewatering) which significantly facilitates the next processing steps. Their effectiveness depends on their ionic charge. Microalgal and cyanobacterial cells pose a negative charge, therefore, repulsed themselves from aggregating into suspension. The surface charge of microalgal and cyanobacterial cells can be neutralized by adding chemicals such as flocculating agents (flocculants). These cationic compounds coagulate the suspended microalgal and cyanobacterial cells without affecting the composition and harming the cells and not poisoning the product as can be seen in Fig. 4. The typical flocculants are multivalent salts such as FeCl3, Al2(SO4)3, and Fe2(SO4)3. Cationic polymers (those are polyelectrolytes) also can be used as flocculants by physically linking cells together, thus advancing little to no disruptions on the cells. Key polymer characteristics involved are charge, molecular weight, and concentration. The preference for polymer types depends on the properties of microalgae or cyanobacteria cultures such as charge in suspension, pH, and biomass concentration (Wu et al. 2012). Harvesting using Fe [III] flocs induced with pH improved efficiency up to 80% (Knuckey et al. 2006). Flocculation using FeCl3 can be suppressed by exopolymers released by Aphanotece halophytica therefore extra addition of this flocculant is needed (Chen et al. 2009). Chlorella was found better to be flocculated using cationic polyelectrolytes, while anionic polyelectrolytes gave no flocculation.
The use of organic flocculants is advantageous due to their stability by being less sensitive to pH, allowing them for a wide range of applications and in most cases needing a lower dose of flocculant depending on the presence of ions in mediums. Brackish and saline waters, for example, needed more chemical flocculants due to the presence of competing cations (Abbaslou et al. 2020). Aside from cationic ions, natural flocculants can also be used such as in the case of Oscillatoria, Spirulina, Chlorella, and Synechocystis flocculated using chitosan. The dose of given flocculants depends on algal or cyanobacterial species. For example, dose of 40 mg/L chitosan is effective to flocculate Tetraselmis chui, Thalassiosira pesudonana, and Isochrysis sp, while Chaetoceros muellaris required 150 mg/L of chitosan (Divakaran and Sivasankara Pillai 2002). Auto-flocculation can also naturally occur by interrupting the CO2 supply in culture microalgal or cyanobacterial broth. This fact attributes to elevated pH through photosynthetic CO2 consumption related to salt precipitations containing calcium, magnesium, phosphate, and carbonate. The positive charge of calcium phosphate is prone to react with the negative charge of microalgal or cyanobacterial cells then leads to flocculation (Sukenik and Shelef 1984).
3.2 Centrifugation
The most preferred method for harvesting microalgal and cyanobacterial cells is centrifugation. Centrifugation is conducted by using centripetal acceleration to separate the microalgal growth medium into sections depending on their densities which correlate with their growth. Mature cells will be obtained in the lower part of the centrifugation vessel. The separated mature cells of microalgae or cyanobacteria biomass (supernatant) can later be obtained by simply draining the medium solution from the saturated supernatant at the bottom of the centrifuge cells. This method is reasonable for microalgae harvesting with one drawback: the shear forces during the spinning can disrupt cells. That prevents the faster centrifugation speed which would generate higher separation capability. There are several key parameters to look for in applying centrifugation for large-scale harvesting of microalgae. These factors are concentration which determines the rate of rotation needed to separate; energy consumption which correlates with the amount of energy needed to power the rotation; the cost which is how much of a percentage the whole harvesting process would require; operation mode which determines the size of the operation; concentrating method which determines whether or not there needs to be a prior concentrating method such as cooling or addition of binding agents; and last but not least is the reliability of the centrifugation method to harvest the biomass. Centrifugation is the most efficient method for microalgal and cyanobacterial harvesting (95–100% with 88–100% cell viability) compared to drum filtration and dissolved air floatation. Centrifugation on a laboratory scale is suitable when cell concentration is about 30 mg/L. The centrifugation method, however, is not cost-effective when implemented on a large scale. This is because of its high-power consumption. Other limitations are its higher gravitational and shear stress frequently damage the cell structure (Ashraf et al. 2020; Shao et al. 2015).
3.3 Filtration
The filtration method is preferable in harvesting microalgae and cyanobacteria compared to other harvesting methods. Filtration methods consisted of several types such as dead-end filtration, microfiltration, ultra-filtration, pressure filtration, vacuum filtration, and tangential filtration. In the principle, filtration is implemented by passing through the culture broth through filters then biomass will accumulate on the filter membrane while the medium passes through the filter continuously until the filter contains a cake of algal or cyanobacterial biomass (Mohan and Sivasubramanian 2010). To further suitably concentrate the biomass, filter presses under pressure or vacuum are added into the system for microalgae or cyanobacterium with larger sizes for example Arthrospira (Spirulina) platensis, but not for smaller sizes like Chlorella and Dunaliella. Tangential flow filtration and pressure filtration are considered energy-efficient methods for separating microalgal or cyanobacterial biomass from its medium culture. This fact is suggested by the amount of output and input of the feedstock (Shao et al. 2015). Back mixing becomes a drawback to the use of dead-end filtration; however, this simple filtration method can be combined with centrifugation for improving the separation process. In general, the filtration method is frequently associated with wide-ranging running costs and concealed pre-concentration requirements (Senatore et al. 2022; Morais et al. 2020).
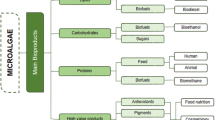
4 Bioproducts from Microalgae
4.1 Biodiesel
Generation of biodiesel is a process of breaking down vegetable oils or animal fats that contain triglycerides which comprise of three fatty acid chains linked with a glycerol molecule. In the process of generating biodiesel, glycerol substitutes with methanol which forms fatty acid methyl ester more commonly known as biodiesel. A phase separation method was then implemented to separate glycerol (as a by-product) from biodiesel. The process is denoted as transesterification which is a process of replacing methanol for glycerol in a chemical reaction with an acid or alkali catalyst. The encounters of replacing conventional diesel with biodiesel are: (1) biodiesel feedstock must be sufficient at a commercial scale, (2) must have a lower price than conventional fossil fuel, and (3) meet standard specifications of fuel quality. Those reasons are met by the microalgal and cyanobacterial biomasses for biofuel since it can provide raw material at a cheaper yet faster biomass productivity rate reaching up to 50 times magnitudes than ordinary terrestrial plants with adequate lipid contents fraction for biodiesel. Their lipids are mostly neutral lipids with a lower level of unsaturated grade. According to Chisti (2007) Botryococcos braunii, Nannochloropsis sp, and Schizochytrium sp. respectively contain lipids ranging between 25–75%, 31–68%, and 50–77%, respectively.
Many methods for algal/cyanobacterial lipid extraction are known, but the most commonly practiced methods of algal/cyanobacterial lipid extraction are by the use of an expeller, supercritical fluid extraction (SFE), solvent extraction (liquid–liquid extraction), and various ultrasound techniques. In the method of expeller/oil press, the biomass of microalgae must be dried for effective extraction, cell breaking was then conducted by pressure which squeezed the oil out. In addition to that, this method was only capable to extract 75% of oil with a longer extraction time. Solvent extraction is excellent to extract from microalgae and cyanobacteria, faster and simpler than the SFE method (Jacob-Lopes and Franco 2013).
In the solvent extraction method, organic solvents (benzene, cyclohexane, hexane, acetone, or chloroform) are added to algal/cyanobacterial paste. The solvent’s function is to destroy algal/cyanobacterial cell walls which allows the extraction process to ensue. The extraction will result in the formation of a lipid layer on top of the aqueous medium since their higher solubility in organic solvents than in water (medium). Then the solvent extract can be distilled to separate the oil from the solvent. The solvent can later be reused. Among the reusable solvent in this process, Hexane is the most efficient solvent due to its lower cost and higher extraction capacity. Lipid extraction also can be conducted in two steps using ethanol then followed secondly with hexane. This procedure is aimed to purify extracted lipids with a yield recovery of 80%. Temperatures can also improve this extraction method. Some studies reported that fatty acids were always nearly extractable at 100 °C compared to ambient temperature mainly saturated acids (16:0, 18:0), but polyunsaturated fatty acids (18:2;18:3) or PUFA resulted in lower yield with hot propanol-water (3:1 v/v). However, fatty acids content varied with microalgal strains, and the solvents for extraction (such as chloroform, and methanol) were also hazardous and destructive to the environment and human health (Slade and Bauen 2013).
Supercritical extraction (SFE) ruptures the microalgal cells through high pressures and temperatures. This method is extremely time efficient and is commonly used. The implemented high pressures and temperatures did not give any effect on the yield of the extracted compounds but affected the rate of extraction. It was observed in lipid extraction from Nannochloropsis sp for obtaining PUFA using SFE at 45 and 55 °C, 400–700 bar. A higher yield result was found when SFE was used in Spirulina platensis for obtaining PUFA compared to extraction using solvent (Amorim et al. 2020; Bleakly and Hayes 2017). Ultrasound method for lipid extraction from microalgae and cyanobacteria is also promising. This method treats microalgae and cyanobacteria with high-intensity of ultrasonic waves which form minute cavitation bubbles around cells. The shockwaves resulting from collapsing bubbles will shatter and rupture the cells which then released desired compounds into solution. Fatty acids and pigment extraction from Scenedesmus obliquus using ultrasound showed over 90% without any changes or breakdown in the product (related to time storage), while almost complete extraction of lipid was achieved in Chaetoceros gracilis. Ultrasonic can increase the rate of extraction of oil content in microalgae at a laboratory scale, further study on its feasibility for commercial production in this method is needed (Vandamme et al. 2013; Kumar et al. 2017).
4.2 Bioethanol
Bioethanol is commonly produced through a biochemical process (fermentation) and thermochemical process (gasification) of biomass sources. The conventional biomass sources (sugar cane, corn, and bit) have a general acute problem that is high value for food utilization and the requirement on land to be cultivated. Therefore, this problem is a constraint for expanding biofuel production. Alternatively, microalgal and cyanobacterial biomass as feedstock for the fermentation process in bioethanol production eludes those problems. These biomasses contain carbohydrates and proteins (Table 2) which enable being used as carbon sources in the fermentation process.
Other microorganisms such as fungi, bacteria, and yeast (Saccharomyces saravesei) are ordinarily used for fermenting the microalgal and cyanobacterial carbohydrates under anaerobic conditions for the production of bioethanol. Theoretically, the maximum yield is 0.51 kg ethanol and 0.49 kg CO2 per kg of glucose. The produced bioethanol then can be purified for producing biofuel while produced CO2 can be recycled for cultivating microalgae as a growth nutrient source. In the second stage, the remaining biomass after fermentation can be used as feedstock for the anaerobic digestion process resulting in methane (CH4) gas which can later be converted into electrical power (Demirbas 2010, 2011).
Hon-Nami (2006) mentioned that Chlamydomonas periglanulata can be fermented for producing ethanol, butanediol, acetic acid, and CO2. They also reported that the recovery of H2 and carbon was attained by 139 and 105%. Some advantages of microalgal and cyanobacterial fermentation for producing bioethanol are the less requirement for energy consumption and more simplicity of the process compared to the conventional biodiesel production system. Even so, the production of bioethanol from microalgal and cyanobacterial biomass still needs further research for commercialization.
4.3 Biomethane
The methane fermentation technology on microalgae Chroococcus sp. and Tetraselmis sp. Chlorella sp. (Koutra et al. 2018) and cyanobacteria (Aphanizomenon ovalisporum and Anabaena planktonica) biomass are perspective since they can produce economical by-products. One of these by-products is biogas (Catone et al. 2021). Biogas consists of a mixture of 55–75% of CH4 and 25–45% of CO2 during microbial anaerobic digestion. The CH4 can later be converted into electricity and fuel gas. As for the residual biomass that is left after the process, they can be processed into biofertilizer. Therefore, these processes yield support renewable and sustainable agricultural production systems by improving efficient practices and lowering microalgal/cyanobacterial production costs. Microalgae and cyanobacteria are considered to have no lignin and lower cellulose ingredient, thus processes of turning their biomass would be considered faster than conventional biomass sources. This character gives excellent conversion efficiency and stability for the anaerobic digestion process. In the anaerobic digestion method, the biogas production from microalgal/cyanobacterial biomass is determined by its organic loadings, temperature, pH, and retention time in the bioreactor. In the principle, long solid retention and high organic loading can significantly affect CH4 yield. This method can be performed in either mesophilic or thermophilic conditions. Integration between microalgae and cyanobacteria cultivation in a wastewater treatment pond and harvested its biomass anaerobically digested for producing biogas can offer good potentials in overcoming environmental problems (water pollution) and commercializing biogas production from microalgal and cyanobacterial biomass (Ramos-Suárez et al. 2014; Tijani et al. 2015).
4.4 Fine Biochemicals
Carbohydrate content in microalgae and cyanobacteria biomass has the potential in Acetone-Butanol fermentation (fermented using bacteria such as Clostridium sp) for producing biobutanol with bio acetone as a by-product. These fine biochemicals are valuable organic solvents. Biobutanol belongs to renewable transportation fuel, while bio acetone is utilized as a multi-purpose solvent such as a cleaning agent, an extraction solvent, and other laboratory works. Applying biobutanol as fuel for vehicles is reported to not require any engine modification as it can be directly blended in higher concentrations with gasoline compared to other biofuels. Blending biobutanol with gasoline is aimed to lower vapor pressure. Production of butanol with Neochloris aquatica CL-M1 was done by using wastewater medium to yield 0.89 g/(L.h) of butanol with 96.2% efficient removal of NH3-N. This process’ success in yielding butanol meant circular usage for extraction is possible by using the produced butanol. Meanwhile, on the genus of Dunaliella (D.tertiolectra, D.primoelectra, D.parva, D.bardawil, and D.salina) fermented by Clostridium pasteurianum was found to yield four different kinds of organic solvents. These four organic substances namely propanediol, acetic acid, ethanol, and n-butanol were present as a mixture with a concentration of 14–16 g/L. However further study still needs to be conducted related to the mechanism of the process (Veza et al. 2021; Nakas et al. 1983).
4.5 Food and Functional Foods
4.5.1 Omega 3 Oil
Naturally, microalgae and cyanobacteria contain omega-3 fatty acids that can be purified into high-value added bioproducts such as food supplement. The sources of omega-3 fatty acid in microalgae and cyanobacteria biomass are eicosapentanoic acid (EPA) and docosahexaenoic acid (DHA). These compounds are widely found in fish oil, but due to its low supply, unpalatable taste, and inadequate oxidative stability of fish oil made it not a convenient source for omega-3 fatty acids. Microalgae and cyanobacteria are self-producing omega-3 fatty acids, therefore processing on microalga biomass is simpler than fish biomass. The use of EPA is widely known in human health purposes for curing heart and inflammatory diseases (asthma, arthritis, migraine, and psoriasis). According to Hu et al. (2008) outdoor cultivation of Pavlova viridis gave lower total fatty acid but higher EPA than of indoor cultivation system, hence concluded that outdoor cultivation is more favorable in producing EPA. Nannochloropsis sp. also show similar result according to Cheng-Wu et al. (2001). Production yields of EPA are also determined by the season. It was found that the production of EPA is higher as 35% in summer than in winter. Temperature and irradiance were not significantly impacting on yield of EPA (4% of dry biomass) produced by Nannochloropsis sp. in an outdoor tubular reactor, and this report showed the potential of this eustigmatophyte as an alternative source of EPA (Chini Zittelli et al. 1999). Vazhappily and Chen (1998) reported that the highest EPA proportion (% of total fatty acids) was produced by Monodus subterraneus UTEX 151 (34.2%), followed by Chlorella minutissima UTEX 2341 (31.3%) and Phaeodactylum tricornutum UTEX 642 (21.4%). But further studies are still needed to ensure the feasibility of this EPA production system. In terms of DHA, this compound is also useful to fight against cancer, AIDS, and heart diseases by reducing cholesterol, boosting immune system, and detoxification of the body. Production of DHA depends on the cultivated species of both microalgae and cyanobacteria. Marine species of both microalgae and cyanobacteria have significantly higher DHA (mainly consist of saturated or monosaturated fatty acids) content than freshwater species (Patil et al. 2007). Marine microalga, Schizochytrium mangrove, contains DHA in the range of 33–39% of total fatty acids (Jiang et al. 2004), whileVazhappily and Chen (1998) reported that the highest DHA proportion (% of total fatty acids) was obtained in Crypthecodinium cohnii UTEX L1649 (19.9%), followed by Amphidinium carterae UTEX LB 1002 (17.0%) and Thraustochytrium aureum ATCC 28211 (16.1%). Another report by Patil et al. (Patil et al. 2007) mentioned that Isochrysis galbana contained a significant amount of DHA with a specific productivity of around 0.16 g/(L.d). The amount of CO2, light intensity, and operation modes (batch and continuous) significantly affect the productivity of DHA (which was found of 1.29 mg/(L.d) under optimized conditions in the cultivation of microalga Pavlova lutheri (Carvalho and Malcata 2005).
4.5.2 Chlorophyll-a
In microalgae and cyanobacteria, there are mainly two main types of chlorophyll being produced. They consist of chlorophyll a and chlorophyll b. The presence of chlorophyll as a photosynthetic pigment is found in all photoautotrophic organisms. Chlorophyll has been widely used as a medicinal drug due to its ability to stimulate liver function recovery and increase bile secretion. It also possesses the ability to repair damaged cells, increase the amount of hemoglobin in blood and encourage rapid cell growth. Chlorophyll has also been reported to have various properties (antimutagenic, anticarcinogenic, and antioxidant). Traditionally, chlorophyll has been used in the food industry as a natural pigment due to the increasing consumer demands for natural foods. Its use as a natural pigment possesses the same properties as listed previously. Chlorophyll is best extracted by the use of the supercritical fluid extraction (SFE) method. High-performance liquid chromatography was found to be the most accurate and sensitive technique to fractionate and quantify chlorophyll along with its derivatives (Silva and Sant’Anna 2016).
Microalgae (Chlorella sp, Scenedesmus sp) and cyanobacteria (Spirulina sp.) seem to be promising alternative sources for chlorophyll. The growth stage of a particular algal species was found to be highly linked with the amount of chlorophyll extracted. Microalgae extracted during the stationary growth phase were shown to have a substantially higher amount of chlorophyll as compared to the same species extracted during the logarithmic phase. Chlorophyll a has been recently revealed to be a key compound in the treatment of ulcers which makes it vital in the postoperative treatment of rectal surgery on humans. During the removal of large areas of tissue, recovery can be difficult and the area near said removed tissue tends to be painful. With the application of chlorophyll, the stimulation of cells in the host and the consequent acceleration in tissue formation increases the rate of recovery, in many cases up to 25%. Moreover, the use of chlorophyll was also found to eliminate foul odor emanating from the wound after a few administrations. Chlorophyll's non-toxic nature, antibacterial properties, and ability to deodorize make it a prominent product in treating oral sepsis (Stirbet et al. 2018). In conclusion, a downstream process needs to be developed to purify chlorophyll a and b from microalgae and cyanobacteria.
4.5.3 Phycocyanin
Phycocyanin is a colorant present in cyanobacteria and red microalgae. When purified, phycocyanin shows a brilliant blue color in the solution. Phycocyanin is composed of two different subunits α and β combined into one. Its presence in nature exists as monomers, trimers, or hexamers; small quantities of oligomers have been found as well. In general, phycocyanin includes C-phycocyanin and allophycocyanin. Both possess different maximum absorption peaks, which is at 620 and 650 nm, respectively. Studies have suggested that both C-phycocyanin (C-PC), and allophycocyanin each have their own ratio of absorbance to indicate their purity; with C-PC possessing a ratio of 620 and 280 nm, and allophycocyanin possessing a ratio of 650 and 280 nm. Phycocyanin shows numerous special bioactivities which are gradually recognized to have potential as raw materials for healthy food products. Studies have indicated that phycocyanin also has various bioactivities such as anti-neoplasm, antioxidant, and anti-inflammatory effects. Several reports have also suggested that C-PC has anticancer bioactivity.
The high content of protein in Arthrospira (Spirulina) sp. (~70% in dry cell weight) poses as a valid reason to consider cyanobacteria as an alternate source of protein, which is vital due to its presence at several cell locations as enzymes, structural component, linked carbohydrate, among others under various forms. Phycobiliproteins correspond to nearly 60% of all the soluble protein in cyanobacteria among the protein pool, while about 20% corresponds to C-PC. Pigment purity within the cultivation of microalgae is of utmost importance, especially when it's used as a fluorescent marker in biomedical research where the presence of impurities can severely impair the quality of the extract. Extract of C-PC obtained from A. platensis which was grown in a nitrogen-reduced medium indicated higher levels of purity (0.80) followed by the extract cultivated within a control group (0.55) and lastly within a nitrogen-free medium (0.21). Several studies have demonstrated crude extraction of C-PC (with varying levels of purity between 0.19 and 1.4) extracted from A. platensis. What is considered to be food grade C-PC has a purity of 0.7 (with a commercial value of ~US$ 0.13 per mg), while 3.9 is considered as reactive grade C-PC (value varies ~US$ 5 per mg) and a purity level of greater than 4.0 as analytical grade C-PC (value can be as high as US$ 15 per mg. All in all, C-PC obtained from A. plantesis grown within a nitrogen-reduced medium presents a very promising use within the food industry (Qiang et al. 2021; Pagels et al. 2019).
4.5.4 Carotene
Carotenoids (Carotene) are known as one major class of photosynthetic pigments. In microalgae, there are generally three known pigments which are chlorophyll, phycocyanin, and carotenoids. Carotenoids, like chlorophyll, are water-soluble. They consisted of terpenoid pigments derived from a 40-carbon chained polyene. This distinctive molecular structure is associated with their chemical properties which allowed electron transfers induced by light-absorption which is essential in photosynthetic activities. This pigment may be complemented by cyclic groups and oxygen functional groups. The oxygenated derivatives are particularly known as xanthophylls as there is the presence of hydroxyl groups (e.g. lutein), oxy groups (e.g., canthaxanthin), or both combinations (e.g. astaxanthin). However, carotenoids are usually typed as two, namely primary and secondary carotenoids. Primary carotenoids come from their structural and functional components in the cellular photosynthetic apparatus (i.e., xanthophylls). Meanwhile, secondary carotenoids consisted of those produced at a large level after exposure to specific environmental stimuli. Relatively, xanthophyl are hydrophobic therefore, they are typically found linked on the membranes or noncovalently bound to specific proteins (Srivastava et al. 2022; Begum et al. 2016).
There are more than 400 variants of carotenoids within nature. Among them, β-carotene is considered to be the most prominent. Moreover, some carotenoids contain provitamin A and possess a broad range of biological functions and actions, most notably in relation to human health (Pisal and Lele 2005). Researchers have reported the benefits of β-carotene for the human body as the human body converts β-carotene to vitamin A via the body tissue. The necessity of vitamin A in the immunity of the human body is to prevent cataract, night blindness, and skin diseases. In the context of multivitamin preparations, β-carotene is often used as pro-vitamin A (retinol) and as an ingredient in the formulation of healthy foods. Alternative use of β-carotene within the context of food production, is as a food colorant to improve the appearance of margarine, cheese, fruit juices, confectionary, and other food products to increase appeal toward customers, such as the case of the β-carotene cultivated from Dunaliella. β-carotene has also been reported to decrease the hazard of several degenerative diseases such as cancer (Nethravathy et al. 2019). Studies have found that β-carotene from Dunaliella sp. contains 40% 9-cis and 50% all-trans stereoisomers which play a crucial role in lowering incidence of several varities of cancer and other degenerative diseases. Furthermore, an investigation of the antioxidant properties of β-carotene was found allowing it to help mediate the harmful effects of free radicals thus preventing life-threatening diseases such as arthritis, coronary heart diseases, premature aging, and various forms of cancer. Another study has also shown that β-carotene has the ability to stimulate the immune system, potentially preventing various kinds of life-threatening diseases. In addition, it can also reduce the cognitive impairment linked with Alzheimer's which is caused by persistent oxidative stress within the brain (Nethravathy et al. 2019; Murthy et al. 2005).
4.6 Microalgae for Phytoremediation
Phytoremediation is a process of remediating environmental contaminants or excess nutrients through the use of plants. Phytoremediation usually focuses on controlling Total Phosphorus (TP), Total Nitrogen (TN), and their related constituents such as \({\text{PO}}_{4}^{3 - } ,\;{\text{NO}}_{3}^{ - } ,\;{\text{NH}}_{4}^{ + }\), and much more which can be seen as Total Dissolved Salts (TDS). Conventionally, complex plant systems available in the environment especially in aquatic environments are used to remediate environmental contaminants. Phytoremediation often uses available plants with little value in the market such as common reeds, water lilies, and pteridophytes (Pandey 2012; Wang et al. 2022; Riggio et al. 2015). The downside of the conventional route is the process of developing enough biomasses to effectively remediate an area required years of acclimation and cultivation in the environment. In the wake of sustainable development and circular economy, phytoremediation using microalgae and cyanobacteria fits the criteria. In developing a sustainable business, the technology applied should not further factor into the depletion of arable lands and not compete with the existing Food-Energy-Water nexus (Olabi et al. 2023). The adaptive capability and structural simplicity of microalgal and cyanobacterial cells make them a perfect phytoremediation agent. Owing to their adaptive capability, microalgae can live in numerous conditions depending on the strains and their evolutionary pathways in combating extreme conditions. A class of microalgae is even equipped with an additional protective layer called frustules to accommodate their cells to live in extreme heat or sudden temperature changes (Kooistra et al. 2007; Kim et al. 2017). This adaptability of microalgae and cyanobacterial cells also resulted from their cellular simplicity which allowed for an optimum growth rate attained in a shorter period than complex plants.
In determining the process for microalgae or cyanobacteria phytoremediation, there are three main factors to consider. These factors are interconnected and serve as important determinants in the growth of algae and thus the success of phytoremediation. These considering factors are Environmental, Biological, and Operational factors (Nie et al. 2020). The connections between them go as follows: Environmental conditions provide biological activities, biological activities ensure the optimum operating conditions are chosen, and the chosen operating conditions are chosen to sustain the environmental conditions (Fig. 5). Nine different genera of Chlorophyta phylum: Asterarcys, Chlorella, Chloroccoum, Chlorosarcinopsis, Coelastrella, Desmodesmus, Micratinium, Parachlorella, and Scenedemus are often considered the native to a freshwater environment involving aquaculture (Couto et al. 2022). Their removal capacity has been studied in Galicia and continental Spain (without Coalastrella, Asterarcys or Parachlorella genera as they are not usually present in their freshwater streams) and showed removal efficiencies of 99, 92, and 49% for ammonium, nitrite, and nitrate, respectively, on aquaculture-derived effluents in a raceway pond with microalgae biomass production around 30–40 mg/L on the 7th day.
Aside from Chlorophyta, Bacillariophyta (Diatoms), Cyanophyta (blue-green algae), and Chrysophytae (golden algae) are the most abundant microalgae classes in aquatic ecosystems (Vieira et al. 2020). In a present study of treating municipal wastewater with three native microalgae species (Navicula veneta -Diatom-, Chlorella vulgaris -Chlorophyta-, and Nostoc muscorum-Cyanophyta-), the Navicula veneta treatment was found to produce reusable effluent with high-rate removal of COD, TP, and TN by 95.75%, 99.8%, and 96.96%, respectively (Sisman-Aydin 2022).
Microalgae phytoremediation does not require large areas with easier controllability as the internet of things (IoT) can be incorporated into monitoring its growth and even the environment in a working system (Peter et al. 2021; Abdul-Hadi et al. 2013). Recent research also suggested a phytoremediation system that included energy production. This was done by the construction of microbial fuel cells (MFCs) where microalgae functioned as biocathode (Mathuriya et al. 2016). The energy conversion efficiency of microalgae MCFs showed a maximum output of up to 9% while other photosynthetic plants were at 4.6–6% (Shukla and Kumar 2018). Generally, the external resistance of this system is at 1000 Ω with pollutant removal focusing on Chemical Oxygen Demand (COD). There seems to be a relationship between working volume and the type of wastewater playing a role in the maximum power density as seen in recently published data regarding wastewater treatment and maximum bioelectricity in Table 3 (Sharma et al. 2022). This type of phytoremediation is regarded as a complete recycling machine (Greenman et al. 2019) with the potential for high-yield hydrogen gas production (Logan et al. 2008). Further improvement in phytoremediation using microalgae and cyanobacteria cells was also done by the introduction of immobilized systems in wastewater treatment to improve retention time in optimizing nutrient capture (Han et al. 2022; Shen et al. 2017).
Phytoremediation of microalgae usually utilized the use of an open pond cultivation system. That, however, resulted in lower biomass yield which is why the development of tubular and flat-panel photobioreactors in bioremediation is of interest (Luo et al. 2017). The system would require flue gas submersions into the photobioreactor allowing for the capture of CO2 from the environment-utilizing the sequestration or CO2 fixing pathways which have been done in a consortium with Clostridium sp., E.coli, and Saccharomyces cerevisiae (Hu et al. 2019). Sequestration of CO2 for the valorization of waste mitigation has also been analyzed for massive algal biomass production which showed positive outcomes with the integration of (Ma et al. 2022; Yadav et al. 2019). In addition to sequestration, microalgae are also reportedly able to interact with known emerging contaminants such as heavy metals, antibiotics, and microplastics which all resulted in oxidative stresses that either boost or decrease their lipid content or antioxidant levels to adapt to the surrounding (Satya et al. 2023). These positive outcomes are overall improved cradle-to-gate approach of microalgae biorefineries to renew the current economic model, sustainable recycling of water and supporting food security as well as energy with their biorefineries, and helping economic growth in tropical countries while improving or saving their environment (Hosseinizand et al. 2017; Wu et al. 2018; Hossain et al. 2019).
5 Conclusion
Production of microalgae and cyanobacteria biomass is the new key to a sustainable future. The biomass generation is higher than that of conventional plants yet requires smaller land to cultivate with their applicable closed cultivation system using photobioreactors. The photobioreactors also ensure the quality of the biomass and biorefineries are safe for even consumption levels. There are many potentials in the biomass of microalgae. As bioproducts (bioethanol, biomethane, and biodiesel), microalgae and cyanobacterial biomass have unique properties which are their higher lipid levels with a faster rate of growth than conventional biomass sources. As they are simple cells, the cost of separating the required biomass from contaminants (stems, leaves, etc.) is relatively cheaper. The same can be said in applying microalgae as a living cell in phytoremediation, replacing the conventional phytoremediators which needed a longer time to acclimate to the conditions. The water conditions coming from microalgae phytoremediation have also been shown to be within the permissible limit of water reuse. The cradle-to-gate approach of using microalgae and cyanobacteria products also showed higher interest in the availability of omega-3 oils and colorants (Phycocyanins) which are in high demand for food industries and the new sustainable industries that follow.
References
Abbaslou H, Hadifard H, Ghanizadeh AR (2020) Effect of cations and anions on flocculation of dispersive clayey soils. Heliyon 6:e03462. https://doi.org/10.1016/J.HELIYON.2020.E03462
Abdul-Hadi A, Mansor S, Pradhan B, Tan CK (2013) Seasonal variability of chlorophyll-a and oceanographic conditions in Sabah waters in relation to Asian monsoon—A remote sensing study. Environ Monit Assess 185:3977–3991. https://doi.org/10.1007/s10661-012-2843-2
Abreu AP, Morais RC, Teixeira JA, Nunes J (2022) A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew Sustain Energy Rev 159:112247. https://doi.org/10.1016/J.RSER.2022.112247
Acién Fernández FG, Fernández Sevilla JM, Molina Grima E (2013) Photobioreactors for the production of microalgae. Rev Environ Sci Biotechnol 12:131–151. https://doi.org/10.1007/s11157-012-9307-6
Amorim ML, Alimentos BB, Soares J, Leite MDO (2020) Microalgae proteins: production, separation, isolation, quantification, and application in food and feed microalgae proteins: production, separation, isolation, quantification and application in food and feed. https://doi.org/10.1080/10408398.2020.1768046
Ashokkumar V, Rengasamy R, Deepalakshmi S et al (2014) Mass cultivation of microalgae and extraction of total hydrocarbons: a kinetic and thermodynamic study. Fuel 119:308–312. https://doi.org/10.1016/j.fuel.2013.11.062
Ashraf O, Chang C, Yi H et al (2020) Energy conversion and management: X prospects of industry 5.0 in algae: customization of production and new advance technology for clean bioenergy generation. Energy Convers Manag X:100048. https://doi.org/10.1016/j.ecmx.2020.100048
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press
Begum H, Yusoff FMD, Banerjee S et al (2016) Availability and utilization of pigments from microalgae. Crit Rev Food Sci Nutr 56:2209–2222. https://doi.org/10.1080/10408398.2013.764841
Blanco AM, Moreno J, Del Campo JA et al (2007) Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl Microbiol Biotechnol 73:1259–1266. https://doi.org/10.1007/s00253-006-0598-9
Bleakly S, Hayes M (2017) Review algal proteins: extraction, application, and challenges concerning production 1–34. https://doi.org/10.3390/foods6050033
Carvalho AP, Malcata FX (2005) Optimization of ω-3 fatty acid production by microalgae: crossover effects of CO2 and light intensity under batch and continuous cultivation modes. Mar Biotechnol 7:381–388. https://doi.org/10.1007/s10126-004-4047-4
Castro JDS, Assis LR De, Calijuri ML (2021) Technologies for improving microalgae biomass production coupled to effluent treatment: a life cycle approach 57. https://doi.org/10.1016/j.algal.2021.102346
Catone CM, Ripa M, Geremia E, Ulgiati S (2021) Bio-products from algae-based biorefinery on wastewater: a review. J Environ Manag 293:112792. https://doi.org/10.1016/j.jenvman.2021.112792
Chen L, Li P, Liu Z, Jiao Q (2009) The released polysaccharide of the cyanobacterium Aphanothece halophytica inhibits flocculation of the alga with ferric chloride. J Appl Phycol 21:327–331. https://doi.org/10.1007/s10811-008-9371-z
Cheng-Wu Z, Zmora O, Kopel R, Richmond A (2001) An industrial-size flat plate glass reactor for mass production of nannochloropsis sp. (Eustigmatophyceae). Aquaculture 195:35–49. https://doi.org/10.1016/S0044-8486(00)00533-0
Chini Zittelli G, Lavista F, Bastianini A et al (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. In: Osinga R, Tramper J, Burgess JG, Wijffels RHBT-P in IM (eds) Marine bioprocess engineering. Elsevier, pp 299–312
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Couto AT, Cardador M, Santorio S et al (2022) Cultivable microalgae diversity from a freshwater aquaculture filtering system and its potential for polishing aquaculture-derived water streams. J Appl Microbiol 132:1543–1556. https://doi.org/10.1111/jam.15300
Cui X, Yang J, Cui M et al (2021) Comparative experiments of two novel tubular photobioreactors with an inner aerated tube for microalgal cultivation: enhanced mass transfer and improved biomass yield. Algal Res 58. https://doi.org/10.1016/j.algal.2021.102364
da Silva Ferreira V, Sant’Anna C (2016) Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J Microbiol Biotechnol 33:20. https://doi.org/10.1007/s11274-016-2181-6
Debeni Devi N, Chaudhuri A, Goud VV (2022) Algae biofilm as a renewable resource for production of biofuel and value-added products: a review. Sustain Energy Technol Assess 53:102749. https://doi.org/10.1016/j.seta.2022.102749
Demirbas A (2010) Use of algae as biofuel sources. Energy Convers Manag 51:2738–2749. https://doi.org/10.1016/j.enconman.2010.06.010
Demirbas MF (2011) Biofuels from algae for sustainable development. Appl Energy 88:3473–3480. https://doi.org/10.1016/j.apenergy.2011.01.059
Divakaran R, Sivasankara Pillai VN (2002) Flocculation of algae using chitosan. J Appl Phycol 14:419–422. https://doi.org/10.1023/A:1022137023257
Duan Y, Shi F (2014) Bioreactor design for algal growth as a sustainable energy source. Elsevier B.V.
Farhan A, Udaiyappan M, Abu H, Sobri M (2017) A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J Water Process Eng 20:8–21. https://doi.org/10.1016/j.jwpe.2017.09.006
Felix C, Ubando A, Madrazo C et al (2019) Investigation of direct biodiesel production from wet microalgae using definitive screening design. Energy Procedia 158:1149–1154. https://doi.org/10.1016/j.egypro.2019.01.296
Fernández I, Acién FG, Berenguel M, Guzmán JL (2014) First principles model of a tubular photobioreactor for microalgal production. Ind Eng Chem Res 53:11121–11136. https://doi.org/10.1021/ie501438r
Gil-izquierdo A, Pedreño MA, Montoro-garcía S et al (2021) Science of the total environment a sustainable approach by using microalgae to minimize the eutrophication process of Mar Menor lagoon. Sci Total Environ 758:143613. https://doi.org/10.1016/j.scitotenv.2020.143613
Gonçalves AL, Pires JCM, Simões M (2017) A review on the use of microalgal consortia for wastewater treatment. Algal Res 24:403–415. https://doi.org/10.1016/j.algal.2016.11.008
Greenman J, Gajda I, Ieropoulos I (2019) Microbial fuel cells (MFC) and microalgae; photo microbial fuel cell (PMFC) as complete recycling machines. Sustain Energy Fuels 3:2546–2560. https://doi.org/10.1039/C9SE00354A
Han M, Zhang C, Li F, Ho SH (2022) Data-driven analysis on immobilized microalgae system: new upgrading trends for microalgal wastewater treatment. Sci Total Environ 852
Hernández D, Riaño B, Coca M, García-González MC (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J 262:939–945. https://doi.org/10.1016/j.cej.2014.10.049
Hon-Nami K (2006) A unique feature of hydrogen recovery in endogenous starch-to-alcohol fermentation of the marine microalga, Chlamydomonas perigranulata. Appl Biochem Biotechnol 131:808–828. https://doi.org/10.1385/ABAB:131:1:808
Hossain N, Zaini J, Indra Mahlia TM (2019) Life cycle assessment, energy balance and sensitivity analysis of bioethanol production from microalgae in a tropical country. Renew Sustain Energy Rev 115:109371. https://doi.org/10.1016/j.rser.2019.109371
Hosseinizand H, Lim CJ, Webb E, Sokhansanj S (2017) Economic analysis of drying microalgae Chlorella in a conveyor belt dryer with recycled heat from a power plant. Appl Therm Eng 124:525–532. https://doi.org/10.1016/J.APPLTHERMALENG.2017.06.047
Hu C, Li M, Li J et al (2008) Variation of lipid and fatty acid compositions of the marine microalga Pavlova viridis (Prymnesiophyceae) under laboratory and outdoor culture conditions. World J Microbiol Biotechnol 24:1209–1214. https://doi.org/10.1007/s11274-007-9595-0
Hu G, Li Y, Ye C et al (2019) Engineering microorganisms for enhanced CO2 sequestration. Trends Biotechnol 37:532–547
Huang J, Wang J, Huang Z et al (2023) Photothermal technique-enabled ambient production of microalgae biodiesel: Mechanism and life cycle assessment. Bioresour Technol 369:128390. https://doi.org/10.1016/j.biortech.2022.128390
Huang Q, Jiang F, Wang L, Yang C (2017) Design of photobioreactors for mass cultivation of photosynthetic organisms 3:318–329
Jacob-Lopes E, Franco TT (2013) From oil refinery to microalgal biorefinery. J CO2 Utiliz 2:1–7. https://doi.org/10.1016/j.jcou.2013.06.001
Jiang Y, Fan K-W, Tsz-Yeung Wong R, Chen F (2004) Fatty acid composition and squalene content of the marine microalga schizochytrium mangrovei. J Agric Food Chem 52:1196–1200. https://doi.org/10.1021/jf035004c
Johnson TJ, Anderson GA, Gibbons WR (2018) Photobioreactor cultivation strategies for microalgae and cyanobacteria photobioreactor cultivation strategies for microalgae and cyanobacteria. https://doi.org/10.1002/btpr.2628
Kholssi R, Vogelei P, Marks EAN et al (2021) Biocatalysis and agricultural biotechnology biotechnological uses of microalgae: a review on the state of the art and challenges for the circular economy. Biocatal Agric Biotechnol 36:102114. https://doi.org/10.1016/j.bcab.2021.102114
Kim M, Gwak Y, Jung W, Jin ES (2017) Identification and characterization of an isoform antifreeze protein from the antarctic marine diatom, chaetoceros neogracile and suggestion of the core region. Mar Drugs 15. https://doi.org/10.3390/md15100318
Knuckey RM, Brown MR, Robert R, Frampton DMF (2006) Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquacult Eng 35. https://doi.org/10.1016/j.aquaeng.2006.04.001
Kooistra WHCF, Gersonde R, Medlin LK, Mann DG (2007) The origin and evolution of the diatoms: their adaptation to a planktonic existence. Evol Primary Prod Sea 207–249. https://doi.org/10.1016/B978-012370518-1/50012-6
Koutra E, Economou CN, Tsafrakidou P, Kornaros M (2018) Bio-based products from microalgae cultivated in digestates. Trends Biotechnol 36:819–833. https://doi.org/10.1016/j.tibtech.2018.02.015
Kumar P, Prajapati SK, Malik A, Vijay VK (2017) Cultivation of native algal consortium in semi-continuous pilot scale raceway pond for greywater treatment coupled with potential methane production. Biochem Pharmacol. https://doi.org/10.1016/j.jece.2017.10.044
Kumar G, Shekh A, Jakhu S et al (2020) Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Front Bioeng Biotechnol 8. https://doi.org/10.3389/fbioe.2020.00914
Liu R, Li S, Tu Y, Hao X (2021) Capabilities and mechanisms of microalgae on removing micropollutants from wastewater: a review. J Environ Manag 285:112149. https://doi.org/10.1016/j.jenvman.2021.112149
Logan BE, Call D, Cheng S et al (2008) Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ Sci Technol 42:8630–8640. https://doi.org/10.1021/ES801553Z
Luo Y, Le-Clech P, Henderson RK (2017) Simultaneous microalgae cultivation and wastewater treatment in submerged membrane photobioreactors: a review. Algal Res 24:425–437. https://doi.org/10.1016/j.algal.2016.10.026
Ma Z, Cheah WY, Ng IS et al (2022) Microalgae-based biotechnological sequestration of carbon dioxide for net zero emissions. Trends Biotechnol. https://doi.org/10.1016/J.TIBTECH.2022.09.002
Mathuriya AS, Yakhmi J v. (2016) Microbial fuel cells—Applications for generation of electrical power and beyond. Crit Rev Microbiol 42:127–143. https://doi.org/10.3109/1040841X.2014.905513
Milledge JJ (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biotechnol 10:31–41. https://doi.org/10.1007/s11157-010-9214-7
Mobin S, Alam F (2017) Some promising microalgal species for commercial applications: a review. Energy Procedia 110:510–517. https://doi.org/10.1016/j.egypro.2017.03.177
Morais WG, Gorgich M, Corrêa PS et al (2020) Microalgae for biotechnological applications: Cultivation, harvesting and biomass processing. Aquaculture 528:735562. https://doi.org/10.1016/j.aquaculture.2020.735562
Murthy KNC, Vanitha A, Rajesha J et al (2005) In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci 76:1381–1390. https://doi.org/10.1016/j.lfs.2004.10.015
Mohan N, Sivasubramanian V (2010) Mass cultivation of chroococcus turgidus and Oscillatoria sp. and effective harvesting of biomass by low-cost methods. Nat Prec. https://doi.org/10.1038/npre.2010.4331.1
Nakas JP, Schaedle M, Parkinson CM et al (1983) System development for linked-fermentation production of solvents from Algal biomass. Appl Environ Microbiol 46:1017–1023. https://doi.org/10.1128/aem.46.5.1017-1023.1983
Nethravathy MU, Mehar JG, Mudliar SN, Shekh AY (2019) Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability 00:1–16. https://doi.org/10.1111/1541-4337.12500
Nie X, Mubashar M, Zhang S et al (2020) Current progress, challenges and perspectives in microalgae-based nutrient removal for aquaculture waste: a comprehensive review. J Clean Prod 277:124209. https://doi.org/10.1016/J.JCLEPRO.2020.124209
Nitsos C, Filali R, Taidi B, Lemaire J (2020) Current and novel approaches to downstream processing of microalgae: a review. Biotechnol Adv 45:107650. https://doi.org/10.1016/j.biotechadv.2020.107650
Nwoba EG, Parlevliet DA, Laird DW et al (2019) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:101433. https://doi.org/10.1016/j.algal.2019.101433
Olabi AG, Shehata N, Sayed ET et al (2023) Role of microalgae in achieving sustainable development goals and circular economy. Sci Total Environ 854. https://doi.org/10.1016/J.SCITOTENV.2022.158689
Pagels F, Guedes AC, Amaro HM et al (2019) Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnol Adv 37:422–443. https://doi.org/10.1016/j.biotechadv.2019.02.010
Pandey VC (2012) Phytoremediation of heavy metals from fly ash pond by Azolla caroliniana. Ecotoxicol Environ Saf 82:8–12. https://doi.org/10.1016/J.ECOENV.2012.05.002
Patil V, Källqvist T, Olsen E et al (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult Int 15:1–9. https://doi.org/10.1007/s10499-006-9060-3
Peter AP, Khoo KS, Chew KW et al (2021) Microalgae for biofuels, wastewater treatment and environmental monitoring. Environ Chem Lett 19:2891–2904
Pisal DS, Lele SS (2005) Carotenoid production from microalga, Dunaliella salina. Indian J Biotechnol 4:476–483
Qiang X, Wang L, Niu J et al (2021) Phycobiliprotein as fluorescent probe and photosensitizer: a systematic review. Int J Biol Macromol 193:1910–1917. https://doi.org/10.1016/j.ijbiomac.2021.11.022
Ramos-Suárez JL, Cuadra FG, Acién FG, Carreras N (2014) Benefits of combining anaerobic digestion and amino acid extraction from microalgae. Chem Eng J 258:1–9. https://doi.org/10.1016/j.cej.2014.07.086
Riggio VA, Comino E, Rosso M (2015) Above ground part of common reed to enhance anaerobic co-digestion of farm biomasses: potential, monitoring and efficiency. Ecol Eng 79:35–41. https://doi.org/10.1016/J.ECOLENG.2015.01.015
Satya A, Harimawan A, Haryani GS (2017) Non-linear isotherm models, cadmium kinetics, and biosorption thermodynamics of dried biomass of native aphanothece sp. in a batch system 49:617–638. https://doi.org/10.5614/j.eng.technol.sci.2017.49.5.5
Satya A, Harimawan A, Haryani GS et al (2021a) Integrated treatment of submerged membrane and adsorption using dried Aphanothece sp for removing cadmium from synthetic wastewater. J Water Process Eng 41:102022. https://doi.org/10.1016/j.jwpe.2021.102022
Satya A, Harimawan A, Sri Haryani G et al (2021b) Fixed-bed adsorption performance and empirical modelingof cadmium removal using adsorbent prepared from the cyanobacterium Aphanothece sp cultivar. Environ Technol Innov 21. https://doi.org/10.1016/j.eti.2020.101194
Satya ADM, Cheah WY, Yazdi SK et al (2023) Progress on microalgae cultivation in wastewater for bioremediation and circular bioeconomy. Environ Res 218:114948. https://doi.org/10.1016/j.envres.2022.114948
Senatore V, Oliva G, Buonerba A et al (2022) An integrated algal membrane photobioreactor as a green-transition technology for the carbon capture and utilization. J Environ Chem Eng 10:107344. https://doi.org/10.1016/j.jece.2022.107344
Shao P, Darcovich K, McCracken T et al (2015) Algae-dewatering using rotary drum vacuum filters: process modeling, simulation and techno-economics. Chem Eng J 268:67–75. https://doi.org/10.1016/j.cej.2015.01.029
Sharma M, Salama ES, Zhang P et al (2022) Microalgae-assisted microbial fuel cells for electricity generation coupled with wastewater treatment: Biotechnological perspective. J Water Process Eng 49:102966. https://doi.org/10.1016/J.JWPE.2022.102966
Shen Y, Li H, Zhu W et al (2017) Microalgal-biochar immobilized complex: a novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour Technol 244:1031–1038. https://doi.org/10.1016/J.BIORTECH.2017.08.085
Shukla M, Kumar S (2018) Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew Sustain Energy Rev 82:402–414
Singh UB, Ahluwalia AS (2013) Microalgae: a promising tool for carbon sequestration. Mitig Adapt Strateg Glob Chang 18:73–95. https://doi.org/10.1007/s11027-012-9393-3
Sirohi R, Kumar Pandey A, Ranganathan P et al (2022) Design and applications of photobioreactors-a review. Bioresour Technol 349:126858. https://doi.org/10.1016/j.biortech.2022.126858
Sisman-Aydin G (2022) Comparative study on phycoremediation performance of three native microalgae for primary-treated municipal wastewater. Environ Technol Innov 28. https://doi.org/10.1016/j.eti.2022.102932
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38. https://doi.org/10.1016/j.biombioe.2012.12.019
Song C, Xie M, Qiu Y et al (2019) Integration of CO2 absorption with biological transformation via using rich ammonia solution as a nutrient source for microalgae cultivation. Energy 179:618–627. https://doi.org/10.1016/j.energy.2019.05.039
Srivastava A, Kalwani M, Chakdar H et al (2022) Biosynthesis and biotechnological interventions for commercial production of microalgal pigments: a review. Bioresour Technol 352:127071. https://doi.org/10.1016/j.biortech.2022.127071
Stirbet A, Lazár D, Papageorgiou GC, Govindjee (2018) Chlorophyll a fluorescence in cyanobacteria: relation to photosynthesis
Sukenik A, Shelef G (1984) Algal autoflocculation–verification and proposed mechanism. Biotechnol Bioeng 26:142–147. https://doi.org/10.1002/BIT.260260206
Sung YJ, Yoon HK, Lee JS et al (2022) Novel 3D-printed buoyant structures for improvement in flue gas CO2-derived microalgal biomass production by enhancing anti-biofouling on vertical polymeric photobioreactor. J Clean Prod 366. https://doi.org/10.1016/j.jclepro.2022.133030
Tijani H, Abdullah N, Yuzir A (2015) Integration of microalgae biomass in biomethanation systems. Renew Sustain Energy Rev 52:1610–1622. https://doi.org/10.1016/j.rser.2015.07.179
Ugoala E, Ndukwe G, Mustapha K, Ayo R (2012) Constraints to large scale algae biomass production and utilization. J Algal Biomass Utln 3:14–32
Vandamme D, Foubert I, Muylaert K (2013) Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol 31:233–239. https://doi.org/10.1016/j.tibtech.2012.12.005
Vazhappilly R, Chen F (1998) Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J Am Oil Chem Soc 75:393–397. https://doi.org/10.1007/s11746-998-0057-0
Veza I, Muhamad Said MF, Latiff ZA (2021) Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 144:105919. https://doi.org/10.1016/j.biombioe.2020.105919
Vieira MV, Pastrana LM, Fuciños P (2020) Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar Drugs 18
Vijayaraghavan K, Balasubramanian R (2015) Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J Environ Manag 160:283–296. https://doi.org/10.1016/j.jenvman.2015.06.030
Wang X, Jain A, Chen B et al (2022) Differential efficacy of water lily cultivars in phytoremediation of eutrophic water contaminated with phosphorus and nitrogen. Plant Physiol Biochem 171:139–146. https://doi.org/10.1016/J.PLAPHY.2021.12.001
Wu Z, Zhu Y, Huang W et al (2012) Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium. Bioresour Technol 110:496–502. https://doi.org/10.1016/j.biortech.2012.01.101
Wu W, Lin KH, Chang JS (2018) Economic and life-cycle greenhouse gas optimization of microalgae-to-biofuels chains. Bioresour Technol 267:550–559. https://doi.org/10.1016/j.biortech.2018.07.08
Wu W, Tan L, Chang H et al (2023) Advancements on process regulation for microalgae-based carbon neutrality and biodiesel production. Renew Sustain Energy Rev 171. https://doi.org/10.1016/j.rser.2022.112969
Xia L, Ge H, Zhou X et al (2013) Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus obtusus XJ-15. Bioresour Technol 144:261–267. https://doi.org/10.1016/j.biortech.2013.06.112
Yaashikaa PR, Keerthana Devi M, Senthil Kumar P et al (2022) A review on pretreatment methods, photobioreactor design and metabolic engineering approaches of algal biomass for enhanced biohydrogen production. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2022.10.092
Yadav G, Dash SK, Sen R (2019) A biorefinery for valorization of industrial waste-water and flue gas by microalgae for waste mitigation, carbon-dioxide sequestration and algal biomass production. Sci Total Environ 688:129–135. https://doi.org/10.1016/j.scitotenv.2019.06.024
Yaqoubnejad P, Rad HA, Taghavijeloudar M (2021) Development a novel hexagonal airlift flat plate photobioreactor for the improvement of microalgae growth that simultaneously enhance CO2 bio-fixation and wastewater treatment. J Environ Manag 298:113482. https://doi.org/10.1016/J.JENVMAN.2021.113482
Acknowledgements
The authors would like to acknowledge PKR-Biomassa and Biorefinery UNPAD-BRIN for funding the publication of this chapter book.
Author information
Authors and Affiliations
Contributions
Awalina Satya, Azalea Dyah Maysarah Satya, and Tjandra Chrismadha were the main contributors (data curation, writing original draft); others were supporting contributors: Nofdianto Nofdianto (review), Gunawan Gunawan (review), Ika Atman Satya (review), Souvia Rahimah (review), Efri Mardawati (review), Sara Kazemi Yazdi (review), and Pau-Loke Show (review).
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Satya, A. et al. (2024). The Production of Microalgae and Cyanobacteria Biomass and Their Valuable Bioproducts. In: Lubis, M.A.R., et al. Biomass Conversion and Sustainable Biorefinery. Green Energy and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-7769-7_7
Download citation
DOI: https://doi.org/10.1007/978-981-99-7769-7_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-7768-0
Online ISBN: 978-981-99-7769-7
eBook Packages: EnergyEnergy (R0)