Abstract
Because of structural simplicity, low production costs and small atomizing droplets, ultrasonic atomization used in aerospace, medical, energy saving and other fields. With the increasing need to increase improve the atomization efficiency, Researchers have made a lot of efforts. However, the micro-tapered hole group on atomizer has not been deeply studied. In this study, the electrolytic polishing processing is proposed to improve the quality of the micro-tapered holes. First, a mathematical relation of atomization amount and polishing time was established. Second, the relevant parameter were measured using a microscope. Lastly, the mathematical relation was determined and revised through an atomization amount experiment. The experimental results show that when the polishing time is 4 min, the atomization amount reaches the maximum of 4.53 ml/min, which is much higher than that of the untreated atomizer. This study demonstrates that polishing can effectively remove burr in the process of hole machining, which will further improve the atomization efficiency of atomizers.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Ultrasonic atomization is the use of electronic high-frequency oscillation, through the ceramic atomization plate high-frequency resonance, the molecular bonds between liquid water molecules to break up and produce natural floating water mist. The micro conical hole on the metal plate of atomizer is the core position to produce the moving cone Angle effect and realize atomization [1,2,3]. Maehara pioneered the atomizer structure consisting of multiple pinhole sheets and show that the maximum atomization rate is approximately proportional to the number of micro-tapered holes [4]. Toda proposed a plate atomizer with small holes to obtain the smallest piezoelectric atomizer, which was not only convenient to carry but also improved the atomization efficiency [5, 6]. Than Lu and Shen studied an atomizer with a cymbal-type high-power driver [7, 8].
At the same time, researchers have optimized vibration to improve the atomization amount. Maehara further studied the existing atomizer to improve its atomization efficiency [9]. Whereafter, Lam found that a PMN-PT single crystal with ultrahigh voltage electric coefficient could generate higher axial displacement [10]. In recent years, Yan and Pallavi has conducted extensive research on the influence of dynamic cone Angle, frequency and other parameters on the atomization rate of ultrasonic atomizer [11,12,13,14].
At present, micro-tapered holes of metal sheets of ultrasonic atomizer are mostly made by laser thermal processing technology, so the molten metal fragments will gather near micro-tapered holes to form uneven burrs, which will affect the atomization efficiency of the atomizer sheets [15]. In order to solve the influence of burr on atomization effect caused by this process, electrolytic polishing process is proposed to treat metal sheet in this study.
2 Mathematical Relation of Atomization Amount and Polishing Time
2.1 Theoretical Model of Atomization Amount and Polishing Time
Given \(D^{2} + d^{2} + Dd = D_{1}\), where D is the diameter of the large end of a micro-tapered hole, d is the diameter of the small end of a micro-tapered hole. The actual metal sheet mass is the mass of the entire metal sheet without laser processing minus the mass of micro-tapered hole
where ρ is the density of the stainless steel, R is the radius of the metal sheet, and \(\xi\) is the thickness of the metal sheet, and N1 is the number of micro-tapered holes.
Figure 1 shows a structural drawing of the atomization sheet. Given that the radius R of the metal sheet changes minimally in electrolytic polishing, it can be ignored. Hence, According to Faraday’s law of electrolysis and the law of conservation of mass
This paper adds a correction coefficient \(\mu\) when calculating the burr. Given \(D + d = D_{2}\), the following equation is obtained:
where \(\mu\) represents the correction coefficient, h is the burr height, and l is the busbar length. The difference between the volume of the cavity where the atomization sheet reaches the maximum deformation when vibrating after electrolytic polishing and the maximum deformation when vibrating before electrolytic polishing is
For the micro-tapered hole, the atomization volume delivered is as follows [16]:
where Q is the amount of atomization produced by the atomization sheet, f is the vibration frequency, \({\upxi }_{{\text{d}}}\) is the conical diffuser loss coefficient, and \({\upxi }_{{\text{n}}}\) is the nozzle diffuser loss coefficient. Let \(hlD_{2} - h^{\prime} l^{\prime} D_{2}^{\prime } = P\), \(\frac{{\left( {\upxi _{{\text{d}}} -\upxi _{{\text{n}}} } \right)}}{{\left( {{2} +\upxi _{{\text{d}}} +\upxi _{{\text{n}}} } \right)}} = K\). In summary, the expression of the atomization quantity of the atomization sheet can be written as
Given that f, M, I, N1, N2, F, ρ, and \(\mu\) are constants or measurable quantities and the change in K is minimal in a short electrolytic time, the change in K is temporarily ignored. The relationship between electrolytic time t and K is then discussed in the following experiment. Moreover, the value of atomization quantity Q can be calculated by integrating the parameters D, d, and h measured in the experiment into Eq. (6).
3 Electrolytic Polishing Experiment
3.1 The Composition of the Electrolyte
The main factors altering the effect of electrolytic polishing are time, voltage, temperature, and the distance between anode and cathode plates [17, 18]. The traditional electrolytic polishing with strong acid composite solution is easy to lead to excessive corrosion. Since this experiment only requires the removal of burrs at a micron height, the chromic anhydride component is removed, while the amount of phosphoric acid and sulfuric acid is greatly reduced, and polyethylene glycol is added as a thickening agent to replace the traditional process containing chromium anhydride [19, 20]. The electrolyte components used in this experiment are shown in Table 1.
3.2 Experimental Equipment System
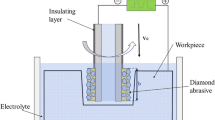
In this experiment, electrolytic polishing was used to remove the uneven burr on the surface of microporous metal sheet. In the test, the atomizing plate is the anode and the lead plate is the cathode. Figure 2 is the experimental device and schematic diagram of electrolytic polishing, respectively. Where, n a is the distance from the anode plate to the liquid surface, and the experimental value is 40 mm; m is the distance between the anode and cathode plates, and the experimental value is 45 mm. According to the above analysis, the experimental control of polishing voltage is 6 V, and electrolyte temperature is 35 °C. The polishing time range is 1–6 min, and the interval time is 1 min in the experiment to conduct the group experiment.
4 Measurement Experiment of Metal Sheet
After the electrolytic polishing experiment, the large hole diameter D, small hole diameter d and burr height h of the metal sheet before and after electrolytic polishing were measured by the ultra-depth of field three-dimensional microscope system, so as to explore the influence of electrolytic polishing on the diameter of the large and small ends of micro-tapered holes and the surface quality of a laser-machined metal sheet.
4.1 Measurement of Related Parameters of Metal Sheet
According to the different electrolytic time, the experiment was divided into seven groups. The first group was atomized sheet without treatment, and the polishing time was 1 min, 2 min to 6 min, and the second to the seventh group successively. When measuring the diameter of large holes of metal sheets, the diameter of 100 holes of each group of metal sheets was measured and counted, and 700 large hole diameters were obtained in 7 groups. Similarly, a total of 700 small hole diameters were measured by measuring the small hole diameters of the above 7 groups of metal sheets. Figure 3a is the measurement diagram of the ultra-depth-of-field 3D microscope. Figure 3b is a schematic of enlarging the burr height of the metal sheet.
4.2 Data Analysis
According to the statistical data of the previous experiments, a box plot based on the statistical data of the previous experiments is drawn in Fig. 4. Figure 4a presents the relationship between the electrolytic polishing time and the diameter of micro-tapered holes of the metal sheet, and Fig. 4b presents the relationship between the electrolytic polishing time and the burr height of micro-tapered holes of the metal sheet.
It can be found that with the increase of polishing time, the diameter of the metal plate increases, but the burr height decreases. Burr height dispersion is also reduced.
5 Experimental Analysis of Atomization Amount
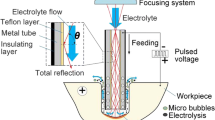
In order to further verify the influence of electrolytic polishing on atomization amount. Figure 5 is the atomization test device. In this experiment, the atomization volume of 7 groups of nebulizing tablets with the operating voltage of 40 V and the polishing time of 1–6 min were measured.
From the atomization experiment, the atomization amount of different polishing time is basically consistent with the theory analyzed above. However, with the in-crease in electrolytic time, the influence of K on Q can no longer be ignored, that is, when t > 4, K cannot be regarded as a constant. The reason is that with the increase in electrolytic polishing time, K will affect the cone angle θ along with the change in t. The conical and nozzle diffuser loss coefficients are then affected gradually, thus altering K. According to the experiment, when t > 4, the correction coefficient should be added to make the correction.
From Eq. (7), the change in atomization amount with polishing time can be obtained, as shown in Fig. 6. The atomization amount basically increases with the increase in electrolytic polishing time, indicating that the theoretical analysis is consistent with the experimental results. When the electrolysis time is 4 min, the atomization amount reaches the maximum value of 4.53 ml/min.
As can be seen from Fig. 6, In the AB region, the presence of an initial passivation film on the surface of the sheet metal prevents the metal from dissolving, resulting in atomized volume. Later, in BC zone, the atomization volume increases with the increase of polishing time. When the polishing time is 4 min, the atomization volume reaches the maximum, reaching 4.53 ml/min. Then the metal sheet enters the CD passivation zone and a new passivation film is formed on the anode surface. Due to the obstruction of the passivation film, the metal dissolution rate is very slow, resulting in the atomization volume beginning to decrease. Therefore, in order to achieve a good polishing effect, the electrolytic time should be controlled within a reasonable range of the over-passivation zone.
6 Conclusions
-
With the increase in polishing time, the diameter of the metal sheet increases, but its burr height decreases. The dispersion of the burr height also decreases. Polishing can dissolve the uneven burr on the surface of the atomization sheet and improve the atomization effect of atomizers. Specifically, when the polishing time is 4 min, the maximum atomization amount reaches 4.53 ml/min.
-
When the polishing time is extremely long, the atomization amount will decrease. When the polishing time t is excessively long, K can no longer be treated as a constant. According to the atomization experiment, K should be corrected when t > 4.
References
Guerra-Bravo E, Lee H-J, Baltazar A, Loh KJ (2021) Vibration analysis of a piezoelectric ultrasonic atomizer to control atomization rate. Appl Sci 11:8350
Jianhui Z, Qiufeng Y, Wanting S (2020) Advances in piezoelectric atomizers. Trans Nanjing Univ Aeronautics Astronautics
Pritchard JN, Hatley RH, Denyer J, Hollen DV (2018) Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Therapeutic Deliv 9:121–136
Maehara N, Ueha S, Mori E (1986) Influence of the vibrating system of a multipinhole-plate ultrasonic nebulizer on its performance. Rev Sci Instrum 57:2870–2876
Toda K, Akimura Y (1994) An ultrasonic atomizing device using coupled-mode vibration. Rev Sci Instrum 65:3276–3278
Toda K, Ishii JIJ (1995) Operation performance of self-oscillation ultrasonic vibrating device for liquid atomization. Jpn J Appl Phys 34:5332
Lu C, Fu C, Yang J, Chen C, Cheng C (2006) 4A-3 traveling wave driven micro-dispenser for CPU cooling application. In: Proceedings of the 2006 IEEE ultrasonics symposium, pp 54–57
Shen S-C, Wang Y-J, Chen Y-Y (2008) Design and fabrication of medical micro-nebulizer. Sens Actuators A 144:135–143
Maehara N, Ueha S, Mori E (1987) Optimum design procedure for multi-pinhole-plate ultrasonic atomizer. Jpn J Appl Phys 26:215
Lam KH, Chan H, Luo H, Yin Q, Yin Z (2005) Piezoelectrically actuated ejector using PMN–PT single crystal. Sens Actuators A 121:197–202
Dupuis E, Momen AM, Patel VK, Shahab S (2018) Multiphysics modeling of mesh piezoelectric atomizers. In: Proceedings of the active and passive smart structures and integrated systems XII, pp 429–437
Sar G, Aydn YN, Bilekli MG, Gl MB (2021) Dynamics of liquid drop on a vibrating micro-perforated plate. Zeitschrift fur Naturforschung A 76
Sharma P, Jackson N (2022) Vibration analysis of MEMS vibrating mesh atomizer. J Micromechanics Microengineering 32
Yan Q, Sun W, Zhang J (2020) Study on the influencing factors of the atomization rate in a piezoceramic vibrating mesh atomizer. Appl Sci 10:2422
Zhang L, Tong H, Li Y (2015) Precision machining of micro tool electrodes in micro EDM for drilling array micro holes. Precis Eng 39:100–106
Akiyoshi O (2004)Flow direction of piezoelectric pump with nozzle/diffuser-elements. Chin J Mech Eng 107–109
Han W, Fang F (2019) Fundamental aspects and recent developments in electropolishing. Int J Mach Tools Manuf 139:1–23
Huang Y, Wang C, Ding F, Yang Y, Li N (2021) Principle, process, and application of metal plasma electrolytic polishing: a review. Int J Adv Manuf Technol
Han W, Fang F (2019) Electropolishing of 316L Stainless Steel using sulfuric acid-free electrolyte. J Manuf Sci Eng 141
Zhou C, Su H, Qian N, Zhang Z, Xu J (2022) Characteristics and function of vapour gaseous envelope fluctuation in plasma electrolytic polishing. Int J Adv Manuf Technol 119:7815–7825
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Huang, WQ., Liang, JL., Zhang, JH., Zhang, F. (2024). Optimization of Ultrasonic Atomization by Electrolytic Polishing. In: Wang, W., Liu, X., Na, Z., Zhang, B. (eds) Communications, Signal Processing, and Systems. CSPS 2023. Lecture Notes in Electrical Engineering, vol 1032. Springer, Singapore. https://doi.org/10.1007/978-981-99-7505-1_28
Download citation
DOI: https://doi.org/10.1007/978-981-99-7505-1_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-7539-6
Online ISBN: 978-981-99-7505-1
eBook Packages: EngineeringEngineering (R0)