Abstract
Biosensors are sensitive, selective, and rapid bioanalytical applications for diagnosis in cell and tissue engineering technologies. Compared to the enzyme-linked immunosorbent assay performed by standard methods, biosensors provide many advantages. In particular, biosensors designed through a combination of chemical, biological, and physical methods provide a good strategy for monitoring microbiophysiological signals in real time and in situ. This chapter summarizes the latest developments in innovative biosensor applications for different technologies of biological interest, essentially cell and tissue engineering. The latest studies and innovative approaches to biosensors for the diagnosis and bioimaging of tissue disease modes due to central nervous system, cardiovascular system, and endocrine system disorders are discussed. In addition, various biochip approaches such as cell/tissue-based biosensors, flexible biosensors, and paper-based biochips are mentioned. Finally, we discuss the diagnostic challenges current biosensors face and highlight the future prospects of biosensors for cell/tissue engineering applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Biosensors are known as the next generation sensing technology that encompasses a variety of technologies and knowledge from many disciplines, including biology, chemistry, and physics. Combining interdisciplinary technologies, biosensors offer a functional analytical platform to analyze the need in environmental, food, public health, microbiology, and biomedical sciences. Biosensors first detect results through optical, chemical, or electrical components and then use receivers, transducers, and imaging systems to convert this detection into a measurable signal [6]. Thanks to components, biosensors can measure signals at very low levels. Thus, they provide fast results with high stability and high sensitivity from a small number or quantity of human samples, without the need for specialized personnel. The first biosensor to be developed for measured glucose with a method based on electrochemical techniques using an electrode containing immobilized glucose oxidase [11] enzyme. With the advances in manufacturing techniques [50] and innovative approaches including nanotechnology, electrochemistry, and photolithography [51], biosensor applications have made incredible progress. Nowadays, the parts of biosensors that act as receptors have been given various functions to detect toxic substances, proteins, cell, and tissue behavior [59, 60]. Especially in clinical studies, biosensors developed to detect molecules are widely used to detect genes, proteins, or cytokines in patient samples. Molecule-labeled biosensors utilize a variety of specific biochemical reactions mediated by DNA and ion channels to detect enzymes, receptors, antigens, and antibodies [7]. In particular, the detection of substances released as a result of cellular reaction in microfluidic biochips is possible by immobilizing antibodies or aptamers specific to the cells used as biomarkers to the biosensor [39, 61]. The main advantage of molecular-based biosensors is that the conjugation of biomarkers with highly selective biomolecules allows the identification of a range of analytes with high sensitivity and selectivity [15]. In order to analyze the targeted molecules, genomic probes with specificity such as antibodies, nucleic acids, enzymes, etc., are integrated onto the surface of the sensor by physical adsorption and chemical grafting methods. Subsequently, the change of piezoelectric, calorimetric, optical, or electrochemical signals from the transducer components is converted into output in the form of electrical signals [12]. In order to detect interleukin-2 (IL-2), Arya and co-workers used 4-fluoro-3-nitrophenyl (FNP) as a coupling agent to bind the IL-2 antibody to a gold electrode surface. Thus, in the developed biosensor, IL-2 was detected by measuring the anti-IL-2-fixed cyclic voltammetry (CV) results resulting from the interactions between antigen and antibody on the gold electrode surface [2]. Furthermore, Chen et al. developed a biosensor consisting of aptamer-based porous silica nanoparticles (MSN) that can provide controlled release [8]. In this sensor, they conjugated the FITC fluorescent dye encapsulated in MSN to DNA by click-chemistry method. After detection of thrombin with DNA aptamers on the MSN, the encapsulated FITC fluorescent dye is revealed from the pores of the MSN, indicating that biosensors can also be controlled depending on a stimulus. Another technique exploits the physical properties of material surfaces to recognize different molecules.
Cell- or tissue-based biosensors, like molecule-based biosensors, offer significant innovations for the detection of cell-associated analytes in situ over the last decade [22, 36, 45]. Such biosensors provide information about the responses of cells and tissues by measuring phenotypic outcomes in cells [18]. Essentially, cell-based biosensors consist of three components: viable cells, bioactive components, and transducers. Thanks to the bioactivity of the components present as substrates, biomarkers released from cells grown on the biosensors can be measured, or cellular polarity resulting from the charge exchange of the cells.
After cells are treated with pharmaceutical or biochemical agents, the changes induced by the agents in the cells can be recorded through physiological parameters such as change in cellular polarity, change in cell membrane permeability, or ligand expression [36]. Molecule-based detection biosensor technologies perform a high selectivity for the analyte molecule to be detected compared to cell-based detection biosensors. However, the short lifetime and the cost of the isolation process of the molecules that will identify the biomarker of interest, such as antibodies, limit the applications of such biosensors. Therefore, cell-based biosensors offer an innovative approach to the diagnosis of diseases with rapid analysis. Biosensor systems are advanced from the cell level to the tissue level to provide more precise results for disease diagnosis. Multicellular cultures and organoids are often used in tissue-based biosensors as they are considered biomimetic structures due to their resemblance to natural tissues. Instead of multicellular cultures or organoids, complex 3D tissue-like structures that provide the functions and properties of natural tissues are being developed by tissue engineering. In recent developments, the production of 3D structures with biological effects in vitro has contributed significantly to tissue-based biosensors, offering potential biosensor strategies to predict, monitor, and diagnose the effects of pharmaceutical agents. The innovative tissue-based biosensors, the so-called organ-on-a-chip platform, is attracting intense interest as the 3D structures developed to mimic the properties of biological tissues. Integrated with a mobile control system, the structures can behave as physiological mechanisms such as blood flow. Another study by Bavli et al., an innovative biosensor, was developed using particle-type oxygen sensors and liver organoid [5]. With this sensor, it is possible to monitor the change in mitochondrial function after the administration of drugs, providing a liver-like response and exciting those working in this field.
In this book chapter, we review the current biosensor progress in the biomedical field, including biosensor fabrication technologies in different biological materials, biosensor types, and their applications in various fields, in order to highlight how and for what purpose biosensors are used in the field of tissue engineering. Thus, we hope to contribute to the knowledge of scientists working in this field to understand the shortcomings and working mechanisms of current biosensors to enable them to realize advanced biomedical applications.
2 Current Technological Approaches for Development of Biosensors
Various sensors such as biochips, paper-based biosensors, nanoparticles, and labeled or label-free biosensors including flexible biosensors are being developed. In recent years, different methods such as computerized numerical control (CNC), photolithography, and casting have been used to develop various biosensors. Biosensors are often produced not only by one of the mentioned methods, but by combining two or more methods. This shows that existing techniques perform a multifunctional activity to design complex or simple sensor mechanisms.
Photolithography is a favored technique for modeling proteins and cell structures in biosensor applications and tissue engineering studies. Photolithography is the method of transferring different patterns on a mask to a glass or silicon surface using UV light [23]. In biosensor design, photolithography allows microelectrodes of different shapes and sizes to be patterned on the surface of biochips using UV light. This enables the fabrication of transducer structures in very small sizes [40].
The developed biochips require model cells to create biomimetic structures. Factors affecting cell binding and cell behavior include pattern shape, size, surface modification, and surface topology. In developed enzyme sensors, DNA sensors, immune sensors, and several biomarkers are modeled on biochips with interlocking electrode arrays [13, 43].
3 Biosensor Development in Tissue Engineering
Tissue engineering, which works in line with engineering sciences and biological principles, carries out studies to develop various tissue structures to restore, repair, or maintain the impaired functions of damaged cells and tissues [31]. In tissue engineering techniques, cell-to-cell and cell-to-material interactions must coexist for optimal physical and cellular signaling to occur. Accordingly, it is required to identify and monitor cellular responses, cellular signals, cell functionality, and behavior. As seen in recent developments, biosensors are widely used in tissue engineering applications. In particular, it has become common to develop tissue engineering systems in microfluidic platforms where biosensors developed to monitor cellular behavior and specific biomolecules in tissues are integrated. These miniaturized systems provide critical information for rapid and real-time prediction of physiological response through electrical, optical, and electrochemical systems [24].
3.1 Biosensors for Cell-Based Applications
3.1.1 Biosensors for Monitoring Cell Polarity Change
Biosensors based on measuring polarity change are used to monitor biochemical reactions occurring at the cellular level. In a study, a polarity-sensitive biosensor that can fluoresce was developed based on the detection of cell apoptosis with images of viable cells [28]. This biosensor was applied to investigate changes in neuronal degeneration process in vivo and in vitro.
3.1.2 Biosensors Developed for Monitoring Cell Behaviors Such as Metabolization, Proliferation of Stem Cells
Biosensors developed for monitoring cellular changes are used in the fields of cellular signal detection, cell behavior, drug toxicity, and disease modeling. Various biosensors have been developed for the transmission of cellular signals such as measurement of cellular metabolic activities, and monitoring the change of charge potential in the cell membrane. Tissue engineering is critical for the evaluation of electrophysiological properties of cardiomyocytes and neuronal cells. Microelectrode arrays (MEAs) have been developed to detect the electrophysiological properties of living cells as a result of their reactions. Chowdhury et al. developed a method that combines MEA with optical mapping to record action potential and contact electrograms simultaneously. Because they thought that the cellular action potential and contact electrogram are linked and should change during arrhythmogenesis [10]. In addition, biomechanical measurements associated with cardiomyocytes to investigate the pathophysiological electro-mechanical coupling were previously performed using different devices [29]. In another study, microbead-based sensors based on fluorescence irradiation were developed to detect hepatocyte growth factor and transforming growth factor-β1 [48]. Various sensors have also been developed to measure signaling molecules released from cells such as nitric oxide (NO) and hydrogen peroxide (H2O2) [47, 55]. NO is an important messenger molecule in biological systems and H2O2 plays an active role in cellular communication, cell migration, and immunity formation. In Fig. 1, Au nanoclusters and a poly(toluidine blue) modified electrode were combined to detect H2O2 and nitric oxide, respectively using microfluidic technology. Similarly, Visser and co-workers preferred inkjet methods to print carbon MEAs on materials that can mimic the extracellular matrix consisting of gelatin, PDMS, and various types of hydrogels to produce soft MEAs [52].
Detection of H2O2 by Au nanoparticles combined microfluidic droplet sensor. Reprinted with permission from [47]. Copyright (2018) ACS publishing
3.2 Strategies of Biosensors to Detect Cell-Released Analytes
3.2.1 Label-Free Biosensors
The determination of the number of proliferating cells, protein quantification, or cellular migration is often preferable to monitoring cellular behavior in a given culture medium. Traditionally, the detection of cell viability and cell number can usually be performed by counting cells under a microscope, measuring DNA content, or detecting viability, such as the MTT assay. However, these techniques are labor-intensive and lengthy procedures. Noninvasive and label-free detection methods, on the other hand, offer advantages in tissue engineering applications and stem cell technology, such as the ability to stain, fix, and fluoresce as desired.
3.2.2 Surface Plasmon Resonance (SPR)-Based Biosensors
Biosensors based on SPR have recently been revealed as a multipurpose biosensor providing the advantages of small sample volumes, live cell analysis, and high throughput. In addition, SPR biosensors enable label-free, real-time analysis with increased sensitivity to the change in refractive index of the analyzed structures [27].
In recent studies, an SPR-based biosensing device has been developed that offers an innovative approach to analyze the osteogenic change of mesenchymal stem cells [30]. Moreover, the SPR-based biosensor has been used to monitor cardiac troponin T and fatty acid binding protein 3, two biomarkers used clinically for the assessment of cardiotoxicity [1].
Furthermore, Fathi and co-workers designed an SPR detection system in Fig. 2 that responds accurately and rapidly to SPR signals by analyzing vascular endothelial-cadherin expression, which can detect endothelial differentiation early [19].
Schematic illustration of SPR biosensor for detecting vascular endothelial-cadherin expression. Reprinted with permission from [19]. Copyright (2017) ELSEVIER publishing
3.3 Biosensor Applications in Various Diseases
3.3.1 Biosensor Applications in Neural Diseases
Biosensors enable the diagnosis of diseases by detecting various signals from tissue. Traditional methods of diagnosing neurological diseases are time-consuming and inconvenient to use. This is because a clinician is needed to check the symptoms of the diseases and in most of diseases, such as Parkinson's disease (PD), there is a risk that about 40% of people may be underestimated in the early stages [44]. Neurological studies are one of the research areas where cell-based biosensors are proving to be important. With MEA technology, it is possible to monitor neuronal circuits, physiological system and abnormalities, and detect malfunctions [46]. The MEA technique offers advantages such as multisite recording, long-term culture, and non-invasive monitoring of the electrophysiological activity of neuronal cells for high-throughput screening [9]. Furthermore, Lourenco and co-workers developed a new multimodal technique for metabolic, electrical, and hemodynamic measurements together with neuron cell activity [38].
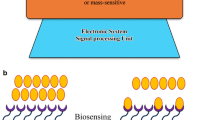
Based on the MEA technique, the scientists fabricated an innovative microelectrode arrays in Fig. 3 that combined neuronal network development under the guidance of neurite outgrowth ITO-PEM microfluidic system [37]. The biochip in Fig. 3 included 3 different functional layers [37]. It offers a good alternative for on-chip organ development, tissue engineering, drug discovery, disease modeling, and biomaterial testing.
Schematic illustration of the design of a biochip by using the MEA method. a Schematic illustrating the design of the biochip. b The process used to fabricate the biochip. Reprinted with permission from [37]. Copyright (2018) ACS publications
Biochips integrated into biosensors are exciting and make important contributions to related fields of study. In particular, biomimetic chip and biosensor integrated systems make it possible to monitor the electrophysiological properties of cells, early-stage differentiation, stem cell proliferation, neural network formation, and stimulation response. Even micro-sized and ultra-flexible electrocorticography arrays using glassy carbon electrodes have been used to monitor brain activity [53]. For instance, in another study, Xie et al. developed a nanoelectronic probe with microscale pores to solve problems such as mechanical incompatibility and instability of conventional metal and silicon microprobes used for brain recording [57]. Thus, they developed a device with 3D microscale pores decorated with wire-shaped nanoelectrodes. This probe with micropores supports integration with the brain by providing a neuron/probe interface. This makes it possible to record action potentials from the somatosensory cortex in the brain. Live cell-based devices have been used to monitor endothelial barrier function and molecules released from cells [34]. Li and co-workers fabricated a reversible electrode for rapid diagnosis of Alzheimer’s disease using graphene oxide nano structures with magnetic properties (Fig. 4) [33]. They integrated an Alzheimer's disease’s biomarker of Amyloid-beta peptide 1–42 (Aβ42), onto a nitrogen-doped graphene (MNG) with magnetic properties. Furthermore, another biosensor that rapidly detects PD was developed by Yang et al. (Fig. 5) [58]. They formed a monolayer (SAM) by attaching a DNA aptamer and an SH-spacer onto the substrate molecule. When the biosensor is treated with PD biomarker (a-synuclein), the DNA aptamer performs a specific attaching to the a-synuclein protein. Subsequently, the changing signals based on optical analysis resulting from the capturing of a-synuclein can be easily identificated under a microscope. When the developed biosensor is compared to conventional Western blot or ELISA methods, these sensors appear to be novel, easily applicable and rapid strategies to facilitate the diagnosis of neurologically based diseases.
Schematic illustration of Aβ42-immobilized graphene-based biosensors for diagnosis of Alzheimer’s disease. Reprinted with permission from [33]. Copyright (2016) Springer Nature Limited.
Schematic illustration of the diagnosis of a PD using PD biomarker (α-synuclein) with a biosensor. Reprinted with permission from [58]. Copyright (2020) Royal Society of Chemistry
3.3.2 Biosensor Applications in Cardiac Diseases
It has been clearly demonstrated that the monitoring and measurement of electrical signals generated in physiological mechanisms in the body is one of the main functions of biosensors. Remarkably, bioelectric activity is often monitored to control the function of cardiac tissues. Bioelectric activity can be generated by cardiomyocytes, which induce changes in the action potential in the membrane of the capillary cells. Thus, due to the alteration of the action potential, cardiac cells can induce a synchronized pumping behavior through organized electrical propagation [16]. Dutta et al. conducted a study showing that the difference in oxygen level measured in tissues significantly interrupts the regular routine of the action potential [17]. Consequently, continued monitoring of electrocardiogram (ECG) data has emerged as a traditional method to detect the cardiac rhythm signal in the diagnosis of cap-related diseases. Lee and co-workers designed a small-size wearable flexible biosensor to easily and continuously monitor heart rhythm signals [32]. With the developed cardiac biosensor, changes in patients’ heart rhythm signals can be monitored directly on their smartphones (Fig. 6). Feiner and colleagues also produced a degradable electronic scaffold as a heart patch. With this flexible product, the spontaneous contraction-relaxation signals of heart cells can be detected. It also provides an external electrical stimulation to control the rhythm of irregularly contracting heart cells [21]. This work, a chip containing a biosensor, also contributes to heart cells that mimic heart-related diseases.
Schematic images of a flexible, soft cardiac biosensor capable of electrocardiogram (ECG) waveforms and measuring real-time heart rate. Reprinted with permission from [32]. Copyright (2018) Springer Nature Limited.
Liu and co-workers developed a Pt nanostructure array integrated on Au electrode as shown in Fig. 7 [35]. The developed on-chip biosensor makes a significant contribution to explain the effect on the electrophysiological behavior of the heart in hypoxic state.
Schematic illustrations of a heart-on-a-chip with Au electrode and PDMS channels. Reprinted with permission from [35]. Copyright (2020) ACS publications
3.3.3 Biosensor Applications in Cancer Diseases Detection
In the last decade, cancer research has gained great momentum. Traditionally, cancer research has focused on the development of effective therapeutic methods to treat cancer diseases. But in some cases, patients are often diagnosed with cancer at the last stage. Early diagnosis is vital in cancer treatment. For this reason, the most effective treatment period for patients diagnosed at the last stage is over [20]. Therefore, how to diagnose cancer diseases quickly and accurately has recently become a popular topic for cancer research. Various biomimetic cancer models have been designed to investigate the formation, mutation, and metastasis mechanisms of cancer cells. In another study, Kamei et al. produced a microfluidic chip that creates a cancer model by designing a chip combining heart and liver cancer cells [26]. With this model, the effects of drugs in the bloodstream on the migration and metabolism of liver cancer cells can be easily monitored. Biosensors developed for early-stage detection offer a fast and easy strategy for producing biosensors that can diagnose versatile cancers. For instance, Pan et al. designed a chip labeled with two different biomarkers, vascular endothelial growth factor (VEGF) and prostate-specific antigen (PSA), to detect prostate cancer and its tumor cells (Fig. 8) [42]. These biomarkers were designed by immobilizing gold nanorods (GNR) on a silicon chip. Then, biomarkers secreted from cancer cells in the circulatory system were specifically captured by the labeled chip. The binding was proven by the absorbance value obtained from UV-Vis spectrophotometer after one hour of incubation.
Schematic representation of a prostate cancer biosensor that captures VEGF and PSA. Reprinted with permission from [42]. Copyright (2017) Ivy Spring International Publisher
In another study, Hu et al. designed a different type of visible signal-generating biosensor that could enable detection. Detection of low-expressed extracellular vesicle (EV)-associated RNA in the early stage of cancer is very difficult. To overcome this challenge, they developed a chip that integrates nanoparticles to capture and detect EV-associated RNA. The cationic polymer nanoparticles triggered the binding of glypican-1 mRNA, a pancreatic cancer biomarker, to EV in serum, producing multiple signal outputs after 30 min of incubation. This technique is an innovative approach to detect pancreatic cancer patients even at an early stage. These studies show that biosensors integrating genomic probes play a critical role in the rapid and early stage diagnosis of cancer diseases (Fig. 9) [25].
Schematic illustration of the biochip by linking neutravidin with a tether lipid-polymer hybrid nanoparticle (LPHN) and loading extracellular vesicle (EV) on an Au layer. Reprinted with permission from [25]. Copyright (2017) Springer Nature Limited.
3.4 Biosensor Applications in Bioimaging Technologies
Apart from in vitro applications for disease detection, biosensors are used to improve sensing capability in bioimaging applications as they have a fast and sensitive labeling function. Related to this concept, nanomaterials, which are candidates for bioimaging due to their small size, high surface area, and modifiable properties, are used in biosensor design for various purposes [56]. Various types of nanomaterials, including polymers, silicas, polymers, and carbon dots have been fabricated for different aims, suitable for diagnostic machines such as computer topography imaging (CT imaging), magnetic resonance imaging (MRI), fluorescence microscope images [56]. In a study, they synthesized porous silica nanoparticles encapsulated with a dye that fluoresces in the near infrared (NIR) as a breast cancer cell targeting agent with LS277 [41]. Compared to the case where only LS277 is delivered, the mesoporous silica nanoparticle delivers images with five times more resolution than that used in LS277 alone. Furthermore, Bao et al. used an NIR triggering technique to develop carbon dot-containing nanoparticles [3]. By synthesizing carbon dots with urea, DMSO, citric acid an N, S-doped carbon dot with NIR fluorescence can be produced and rapidly removed from organs such as kidney or liver 24 h after intravenous injection. Interestingly, NIR fluorescence images after injection showed that S, N-doped carbon dots accumulated considerably in tumor tissues after application. This result indicates that S, N-doped carbon dots activate tumor labeling of a compound. Bao and co-workers developed magnetic iron oxide nanoparticles for MRI imaging and Dong et al. developed gold nanoparticles for bioimaging in CT techniques [4, 14]. Both results from their studies confirmed that the nanomaterials function as contrast agents for use in CT and MRI imaging. By adjusting the size of gold nanoparticles or magnetic iron oxide, the image contrast of the nanoparticles in diverse tissues and organs can be enhanced (Fig. 10). For instance, magnetic iron oxide in 4 nm sizes can enhance T1 image contrasts in MR images. The same is true for CT imaging when the size of gold nanoparticles is 4 nm [4, 14]. Moreover, by decorating the surface of nanoparticles with chemical structures such as folic acid, they can act as tumor contrast agents [4]. Therefore, various types of nanoparticles used in the design of biosensors have potential use as bioimaging agents.
a Schematic illustration of iron oxide nanoparticles as T1 MRI contrast agents and possible integration with CT and PET. Reprinted with permission from 64. Copyright (2018) Royal Society of Chemistry. b The TEM images of gold nanoparticles with different particle sizes. The images indicate that controlling the size of nanoparticles effect enhances the image contrast at various organs. Reprinted with permission from [14]. Copyright (2019) Springer Nature Limited.
4 Challenges and Future Perspectives
Next-generation biosensors are promising techniques that provide sensitive, selective, and rapid disease diagnosis for various applications in the field of tissue engineering. Compared to conventional ELISA assays, the developed biosensors offer a good solution for real-time acquisition of physiological signals by combining chemical, physical, and biological technologies. Biosensor systems have made significant progress in the last decade, but there is still a need to improve some of their functions. The biggest challenges of biosensors are their long-term stability and scale-up process. Stability is still a major challenge, especially during conversion to commercial products. Biosensors are mostly developed as prototypes in a research laboratory. For this reason, scale-up technology is very important in the commercialization phase. This is because it requires rapid mass production of good quality biosensors from the laboratory level to the industrial level. It is very difficult and costly to scale up the results obtained in the research laboratory to a commercially viable level on a large scale. Furthermore, the time from the industrial production level to the retail level to reach the user is often longer than expected. To overcome this challenge, some biosensor chips are developed by immobilizing proteins, growth factors, and antigens that can cause a short expiry date.
In general, biosensors suffer from the problem of stability in order to be able to successfully perform the sensor task again in the case of long-term storage. Furthermore, biosensors are advanced systems that allow even extremely weak signals to be recorded from small amounts of samples in a low-noise environment. But in a real clinical setting, there is a complex matrix that cannot be predicted in advance. Therefore, background noise can increase, which can reduce the accuracy of the results. Furthermore, when biosensors are working, they often need to be connected to controllers, imagers, and other equipments. This can make biosensors less desirable to the user as they require complex operation and have user-unfriendly interfaces.
In order to solve the aforementioned problems, techniques with higher sensitivity and faster response should be developed through interdisciplinary studies during the production phase of biosensors. For instance, the development of various nanostructures such as nanorods, nanocages, and nanostars within nanotechnological developments may provide the opportunity to increase the signal and sensitivity of the samples [54]. In addition, cell-based biosensors that can be used in vitro have continuous and real-time monitoring functions, while lightweight biosensors in small sizes, such as paper-based biosensors, offer great convenience for direct observation of results. Lightweight biosensors are more suitable for industrial production and are more prone to the feasibility of scale-up from the prototype stage developed in the laboratory to the industrial stage. Consequently, the current progression of sensors developed for tissue engineering applications is toward the evolution of small-sized, flexible, and relatively lightweight biosensors. To this end, technologies are being developed to facilitate the integration of biosensors into wearable devices. For instance, tear-based biosensors have been preferred in the development of biosensors integrated into contact lenses. By using tears as a sample, glucose levels can be measured, enabling continuous monitoring of diabetes [49].
Thus, by using body fluids such as saliva, sweat, and tears through wearable biosensors, information about the physiological system can be obtained directly. Furthermore, wearable biosensors integrated with smartphone applications offer an innovative perspective that can be used for real-time continuous monitoring, diagnosis, or disease prognosis. We hope that this book chapter will be informative for scientists producing new technologies in the fields of biology, chemistry, and physics on how to design disease-related biosensors for applications in tissue engineering. Thus, we hope that new potential applications and technologies can be discovered for the active use of biosensors developed through academic research in clinical applications.
References
Andersson H, Steel D, Asp J, Dahlenborg K, Jonsson M, Jeppsson A et al (2010) Assaying cardiac biomarkers for toxicity testing using biosensing and cardiomyocytes derived from human embryonic stem cells. J Biotechnol 150(1):175–181. https://doi.org/10.1016/j.jbiotec.2010.06.023
Arya SK, Park MK (2014) 4-Fluoro-3-nitrophenyl grafted gold electrode based platform for label free electrochemical detection of interleukin-2 protein. Biosens Bioelectron 61:260–265. https://doi.org/10.1016/j.bios.2014.05.029
Bao X, Yuan Y, Chen J, Zhang B, Li D, Zhou D et al (2018) In vivo theranostics with near-infrared-emitting carbon dots-highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci Appl 7:91. https://doi.org/10.1038/s41377-018-0090-1
Bao Y, Sherwood JA, Sun Z (2018) Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J Mater Chem C 6(6):1280–1290. https://doi.org/10.1039/C7TC05854C
Bavli D, Prill S, Ezra E, Levy G, Cohen M, Vinken M et al (2016) Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc Natl Acad Sci U S A 113(16):E2231-2240. https://doi.org/10.1073/pnas.1522556113
Bhalla N, Jolly P, Formisano N, Estrela P (2016) Introduction to biosensors. Essays Biochem 60(1):1–8. https://doi.org/10.1042/ebc20150001
Bousse L (1996) Whole cell biosensors. Sens Actuators B Chem 34(1):270–275. https://doi.org/10.1016/S0925-4005(96)01906-5
Chen Z, Sun M, Luo F, Xu K, Lin Z, Zhang L (2018) Stimulus-response click chemistry based aptamer-functionalized mesoporous silica nanoparticles for fluorescence detection of thrombin. Talanta 178:563–568. https://doi.org/10.1016/j.talanta.2017.09.043
Chiappalone M, Vato A, Tedesco MB, Marcoli M, Davide F, Martinoia S (2003) Networks of neurons coupled to microelectrode arrays: a neuronal sensory system for pharmacological applications. Biosens Bioelectron 18(5–6):627–634. https://doi.org/10.1016/s0956-5663(03)00041-1
Chowdhury RA, Tzortzis KN, Dupont E, Selvadurai S, Perbellini F, Cantwell CD et al (2018) Concurrent micro- to macro-cardiac electrophysiology in myocyte cultures and human heart slices. Sci Rep 8(1):6947. https://doi.org/10.1038/s41598-018-25170-9
Clark LC Jr, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 102:29–45. https://doi.org/10.1111/j.1749-6632.1962.tb13623.x
Contreras-Naranjo JE, Aguilar O (2019) Suppressing non-specific binding of proteins onto electrode surfaces in the development of electrochemical immunosensors. Biosensors (Basel) 9(1). https://doi.org/10.3390/bios9010015
Diakoumakos CD, Douvas A, Raptis I, Kakabakos S, Dimotikalli D, Terzoudi G, Argitis P (2002) Dilute aqueous base developable resists for environmentally friendly and biocompatible processes. Microelectron Eng 61–62:819–827. https://doi.org/10.1016/S0167-9317(02)00451-3
Dong YC, Hajfathalian M, Maidment PSN, Hsu JC, Naha PC, Si-Mohamed S et al (2019) Effect of gold nanoparticle size on their properties as contrast agents for computed tomography. Sci Rep 9(1):14912. https://doi.org/10.1038/s41598-019-50332-8
Du H, Strohsahl CM, Camera J, Miller BL, Krauss TD (2005) Sensitivity and specificity of metal surface-immobilized “molecular beacon” biosensors. J Am Chem Soc 127(21):7932–7940. https://doi.org/10.1021/ja042482a
Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT (1998) Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273(19):11619–11624. https://doi.org/10.1074/jbc.273.19.11619
Dutta S, Mincholé A, Quinn TA, Rodriguez B (2017) Electrophysiological properties of computational human ventricular cell action potential models under acute ischemic conditions. Prog Biophys Mol Biol 129:40–52. https://doi.org/10.1016/j.pbiomolbio.2017.02.007
Fang Y, Ferrie AM, Fontaine NH, Mauro J, Balakrishnan J (2006) Resonant waveguide grating biosensor for living cell sensing. Biophys J 91(5):1925–1940. https://doi.org/10.1529/biophysj.105.077818
Fathi F, Rezabakhsh A, Rahbarghazi R, Rashidi MR (2017) Early-stage detection of VE-cadherin during endothelial differentiation of human mesenchymal stem cells using SPR biosensor. Biosens Bioelectron 96:358–366. https://doi.org/10.1016/j.bios.2017.05.018
Fayanju OM, Jeffe DB, Elmore L, Ksiazek DN, Margenthaler JA (2013) Patient and process factors associated with late-stage breast cancer diagnosis in safety-net patients: a pilot prospective study. Ann Surg Oncol 20(3):723–732. https://doi.org/10.1245/s10434-012-2558-1
Feiner R, Fleischer S, Shapira A, Kalish O, Dvir T (2018) Multifunctional degradable electronic scaffolds for cardiac tissue engineering. J Control Release 281:189–195. https://doi.org/10.1016/j.jconrel.2018.05.023
Gui Q, Lawson T, Shan S, Yan L, Liu Y (2017) The application of whole cell-based biosensors for use in environmental analysis and in medical diagnostics. Sensors (Basel) 17(7). https://doi.org/10.3390/s17071623
Hammarback JA, Palm SL, Furcht LT, Letourneau PC (1985) Guidance of neurite outgrowth by pathways of substratum-adsorbed laminin. J Neurosci Res 13(1–2):213–220. https://doi.org/10.1002/jnr.490130115
Hasan A, Nurunnabi M, Morshed M, Paul A, Polini A, Kuila T et al (2014) Recent advances in application of biosensors in tissue engineering. Biomed Res Int 2014:307519. https://doi.org/10.1155/2014/307519
Hu J, Sheng Y, Kwak KJ, Shi J, Yu B, Lee LJ (2017) A signal-amplifiable biochip quantifies extracellular vesicle-associated RNAs for early cancer detection. Nat Commun 8(1):1683. https://doi.org/10.1038/s41467-017-01942-1
Kamei K-I, Kato Y, Hirai Y, Ito S, Satoh J, Oka A et al (2017) Integrated heart/cancer on a chip to reproduce the side effects of anti-cancer drugs in vitro. RSC Adv 7(58):36777–36786. https://doi.org/10.1039/C7RA07716E
Kato K, Ishimuro T, Arima Y, Hirata I, Iwata H (2007) High-throughput immunophenotyping by surface plasmon resonance imaging. Anal Chem 79(22):8616–8623. https://doi.org/10.1021/ac071548s
Kim YE, Chen J, Chan JR, Langen R (2010) Engineering a polarity-sensitive biosensor for time-lapse imaging of apoptotic processes and degeneration. Nat Methods 7(1):67–73. https://doi.org/10.1038/nmeth.1405
Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML et al (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111(7):943–955. https://doi.org/10.1016/s0092-8674(02)01226-6
Kuo YC, Ho JH, Yen TJ, Chen HF, Lee OK (2011) Development of a surface plasmon resonance biosensor for real-time detection of osteogenic differentiation in live mesenchymal stem cells. Plos One 6(7):e22382. https://doi.org/10.1371/journal.pone.0022382
Langer R, Vacanti JP (1993) Tissue engineering. Science 260(5110):920–926. https://doi.org/10.1126/science.8493529
Lee SP, Ha G, Wright DE, Ma Y, Sen-Gupta E, Haubrich NR et al (2018) Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. NPJ Digit Med 1:2. https://doi.org/10.1038/s41746-017-0009-x
Li SS, Lin CW, Wei KC, Huang CY, Hsu PH, Liu HL et al (2016) Non-invasive screening for early Alzheimer’s disease diagnosis by a sensitively immunomagnetic biosensor. Sci Rep 6:25155. https://doi.org/10.1038/srep25155
Li X, Soler M, Özdemir CI, Belushkin A, Yesilköy F, Altug H (2017) Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab Chip 17(13):2208–2217. https://doi.org/10.1039/c7lc00277g
Liu H, Bolonduro OA, Hu N, Ju J, Rao AA, Duffy BM et al (2020) Heart-on-a-chip model with integrated extra- and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett 20(4):2585–2593. https://doi.org/10.1021/acs.nanolett.0c00076
Liu Q, Wu C, Cai H, Hu N, Zhou J, Wang P (2014) Cell-based biosensors and their application in biomedicine. Chem Rev 114(12):6423–6461. https://doi.org/10.1021/cr2003129
Liu YC, Lee IC, Lei KF (2018) Toward the development of an artificial brain on a micropatterned and material-regulated biochip by guiding and promoting the differentiation and neurite outgrowth of neural stem/progenitor cells. ACS Appl Mater Interfaces 10(6):5269–5277. https://doi.org/10.1021/acsami.7b17863
Lourenço CF, Ledo A, Gerhardt GA, Laranjinha J, Barbosa RM (2017) Neurometabolic and electrophysiological changes during cortical spreading depolarization: multimodal approach based on a lactate-glucose dual microbiosensor arrays. Sci Rep 7(1):6764. https://doi.org/10.1038/s41598-017-07119-6
Ma C, Fan R, Ahmad H, Shi Q, Comin-Anduix B, Chodon T et al (2011) A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nat Med 17(6):738–743. https://doi.org/10.1038/nm.2375
Mir M, Dondapati SK, Duarte MV, Chatzichristidi M, Misiakos K, Petrou P et al (2010) Electrochemical biosensor microarray functionalized by means of biomolecule friendly photolithography. Biosens Bioelectron 25(9):2115–2121. https://doi.org/10.1016/j.bios.2010.02.012
Palantavida S, Tang R, Sudlow GP, Akers WJ, Achilefu S, Sokolov I (2014) Ultrabright NIR fluorescent mesoporous silica nanoparticles. J Mater Chem B 2(20):3107–3114. https://doi.org/10.1039/c4tb00287c
Pan LH, Pang ST, Fang PY, Chuang CK, Yang HW (2017) Label-free biochips for accurate detection of prostate cancer in the clinic: dual biomarkers and circulating tumor cells. Theranostics 7(17):4289–4300. https://doi.org/10.7150/thno.21092
Petrou PS, Chatzichristidi M, Douvas AM, Argitis P, Misiakos K, Kakabakos SE (2007) A biomolecule friendly photolithographic process for fabrication of protein microarrays on polymeric films coated on silicon chips. Biosens Bioelectron 22(9–10):1994–2002. https://doi.org/10.1016/j.bios.2006.08.036
Raknim P, Lan KC (2016) Gait monitoring for early neurological disorder detection using sensors in a smartphone: validation and a case study of parkinsonism. Telemed J E Health 22(1):75–81. https://doi.org/10.1089/tmj.2015.0005
Raut N, O’Connor G, Pasini P, Daunert S (2012) Engineered cells as biosensing systems in biomedical analysis. Anal Bioanal Chem 402(10):3147–3159. https://doi.org/10.1007/s00216-012-5756-6
Seymour JP, Wu F, Wise KD, Yoon E (2017) State-of-the-art MEMS and microsystem tools for brain research. Microsyst Nanoeng 3:16066. https://doi.org/10.1038/micronano.2016.66
Shen R, Liu P, Zhang Y, Yu Z, Chen X, Zhou L et al (2018) Sensitive detection of single-cell secreted H(2)O(2) by integrating a microfluidic droplet sensor and au nanoclusters. Anal Chem 90(7):4478–4484. https://doi.org/10.1021/acs.analchem.7b04798
Son KJ, Gheibi P, Stybayeva G, Rahimian A, Revzin A (2017) Detecting cell-secreted growth factors in microfluidic devices using bead-based biosensors. Microsyst Nanoeng 3. https://doi.org/10.1038/micronano.2017.25
Tseng RC, Chen, C. C., Hsu, S. M., & Chuang, H. S. (2018). Contact-Lens Biosensors. Sensors (Basel), 18(8). doi:https://doi.org/10.3390/s18082651
Turner AP (2013) Biosensors: sense and sensibility. Chem Soc Rev 42(8):3184–3196. https://doi.org/10.1039/c3cs35528d
Vigneshvar S, Sudhakumari CC, Senthilkumaran B, Prakash H (2016) Recent advances in biosensor technology for potential applications—An overview. Front Bioeng Biotechnol 4:11. https://doi.org/10.3389/fbioe.2016.00011
Visser CW, Kamperman T, Karbaat LP, Lohse D, Karperien M (2018) In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio)materials. Sci Adv 4(1):eaao1175. https://doi.org/10.1126/sciadv.aao1175
Vomero M, Castagnola E, Ciarpella F, Maggiolini E, Goshi N, Zucchini E et al (2017) Highly stable glassy carbon interfaces for long-term neural stimulation and low-noise recording of brain activity. Sci Rep 7:40332. https://doi.org/10.1038/srep40332
Wang X, Lu X, Chen J (2014) Development of biosensor technologies for analysis of environmental contaminants. Trends Environ Anal Chem 2:25–32
Wang Y, Hu S (2006) A novel nitric oxide biosensor based on electropolymerization poly(toluidine blue) film electrode and its application to nitric oxide released in liver homogenate. Biosens Bioelectron 22(1):10–17. https://doi.org/10.1016/j.bios.2005.11.012
Wolfbeis OS (2015) An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev 44(14):4743–4768. https://doi.org/10.1039/c4cs00392f
Xie C, Liu J, Fu TM, Dai X, Zhou W, Lieber CM (2015) Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat Mater 14(12):1286–1292. https://doi.org/10.1038/nmat4427
Yang X, Li H, Zhao X, Liao W, Zhang CX, Yang Z (2020) A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem Commun (Camb) 56(40):5441–5444. https://doi.org/10.1039/d0cc01025a
Zhang W, Chen C-T, Yang D, Dong G, Jia S, Zhao B et al (2016) Optical biosensors based on nitrogen‐doped graphene functionalized with magnetic nanoparticles. Adv Mater Interfaces 3
Zhang W, Li X, Zou R, Wu H, Shi H, Yu S, Liu Y (2015) Multifunctional glucose biosensors from Fe3O4 nanoparticles modified chitosan/graphene nanocomposites. Sci Rep 5:11129. https://doi.org/10.1038/srep11129
Zhu H, Stybayeva G, Macal M, Ramanculov E, George MD, Dandekar S, Revzin A (2008) A microdevice for multiplexed detection of T-cell-secreted cytokines. Lab Chip 8(12):2197–2205. https://doi.org/10.1039/b810244a
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Akcay, G., Celik, C., Ildız, N., Ocsoy, I. (2024). Functional Biosensors in Cell and Tissue Fabrication for Smart Life-Sciences Applications. In: Mandal, A.K., Ghorai, S., Husen, A. (eds) Functionalized Smart Nanomaterials for Point-of-Care Testing. Smart Nanomaterials Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-5787-3_13
Download citation
DOI: https://doi.org/10.1007/978-981-99-5787-3_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5786-6
Online ISBN: 978-981-99-5787-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)