Abstract
The skin is the largest organ of the body, composed of the epidermis, dermis, and subcutaneous tissue, each with unique functions. The development of ex-vivo human skin models for chemical testing is a current challenge in skin research. While 3D printing technology has been used to develop bioprinted skin, few studies have included 3D printed sebaceous glands, making it challenging to create a fully functional skin model. The ideal biomaterial for skin bioprinting should have mechanical properties similar to those of native skin, support high cell viability, have adequate biodegradation rate, provide a suitable microenvironment for skin cell functionality, and be highly biocompatible. Natural biomaterials are commonly used in skin bioprinting, but they lack stable mechanical properties and have low gelation levels. Synthetic materials have controllable mechanical and chemical properties, but low biocompatibility and biodegradability. Composite natural and synthetic biomaterials can help balance the biological and mechanical features and provide more stable bioink. The development of bioprinted skin models will help to advance skin research and provide a customizable approach to the development of skin tissue. In summary, both skin bioprinting and organoid technology have revolutionized the field of tissue engineering and modeling. Skin bioprinting has shown promising results in the fabrication of skin substitutes for wound healing and has the potential to transform the cosmetic industry. Organoids have broad applications in disease modeling, drug testing, and the development of treatment strategies for various genetic and infectious diseases. While animal models remain the gold standard, organoids provide a closer recapitulating system of human organs and have the advantage of being easily cultured, genetically modified, and cryopreserved while maintaining their phenotype. Overall, these technologies offer new possibilities for research, dermatopathology, wound healing, and drug and vaccine development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Part 1: Introduction
As the largest organ of the body, skin weighs in at 16% of an adult’s total weight. It is comprised of three layers: epidermis, dermis, and subcutaneous (hypodermal) tissue. The epidermis is the outermost layer, consisting primarily of keratinocytes (KCs), without blood vessels. The epidermis can be further segregated into five layers. Beginning with the innermost layer, these are the stratum basale followed by the stratum spinosum, the stratum granulosum, the stratum lucidum, and the stratum corneum on the uppermost outer part (Kanitakis 2002). These epidermal sub-layers harbor a number of cells such as KCs, melanocytes (MCs), Langerhans cells, and Merkel cells.
Following the epidermis is the dermis, a significantly thicker section residing in the middle of the skin. Much like the epidermis, the dermis can also be subcategorized, divided into papillary dermis and reticular dermis. The papillary layer lies below the epidermis and contains dermal papillae that project into the epidermis. These papillae help anchor the two layers together. In comparison, the reticular layer is deeper within the dermis and contains fibroblasts (FB), collagen, and elastin fibers, which give skin its strength and elasticity. Both layers are inundated with blood vessels and nerves, which serve as conduits for nutrition and sensation. In addition, the dermis contains important sub-structures, which provide functionality to the skin including sweat glands, hair follicles (HF), and sebaceous glands (Marques et al. 2017).
The final layer of the skin is the subcutaneous tissue or hypodermis. This layer is composed of adipocytes (ACs) surrounded by collagen. Despite its simple makeup, the hypodermal layer has many important protective roles, including thermal equilibrium, providing padding to protect deeper tissues from blunt trauma, and buoyancy. In addition, the subcutaneous tissue can be reabsorbed as an energy reserve and in times of need can function as an endocrine system. Together, these three layers protect internal tissues from external forces, providing a physical barrier to micro-organisms/external materials. It prevents the loss of fluid, allows temperature regulation, acts as a moderator for the immune system, and allows each individual to sense the external world (Marques et al. 2017).
2.1.1 Skin Appendages
Internal structures within the skin include HFs, sebaceous glands, and sweat glands. While it is readily acknowledged that these are important for fully functional skin, bioengineered models of these appendages are scarce. This review is focused on the use of 3D printing as a technology for furthering the development of bioprinted skin. To date, very few studies have included 3D-printed sebaceous glands. Instead, the field has focused on the development of appropriate organization (including the three layers discussed above) and the addition of hair follicles/sweat glands. HFs reside in the dermal layer of the skin. These structures consist of hair papillae, hair matrix, root sheath, and hair bulges. The base of the HF is the papilla, a large structure of primarily connective tissue with a capillary loop. The papilla acts as a control center for the HF, determining many characteristics of the hair, including length, hardness, and the overall growth cycle of the follicle. Surrounding this is a root sheath, a dual-layered covering containing bother internal and external sheaths. The outmost sheath contains the hair bulge. This is also the point of insertion of the arrector pili muscle (Buffoli et al. 2014). The hair bulge houses several types of stem cells with superior clonogenicity and proliferative capacity, which supplies the entire HF with new cells and assists in healing any epidermal injuries. HFs are complex but vital sub-structures within the skin, which have been shown to aid antibacterial abilities and inhibit scar formation (Weng et al. 2021), making them of extreme interest to those developing bioengineered skin.
The second structure of popular interest is sweat glands, particularly eccrine sweat glands. These are found across all skin surfaces but are especially prevalent on the palms and soles of the feet. Playing an important role in the regulation of body temperature, eccrine sweat glands contain a coiled secretory tubule, which is connected to the exterior of the epidermis via a long duct. These glands are activated via changes in temperature or emotion, resulting in the excretion of sweat, complete with proteolytic enzymes and interleukin-1. These exuded factors are believed to play a part in the overall barrier function of skin (Lee et al. 2009). In addition, sweat contains urea, lactic acid, and creatine, which contribute to the inhibition of bacterial growth.
Alongside the structural subcomponents of skin, there is a nonstructural feature that is of extreme interest to those trying to engineer replicates: color. Skin color, if primarily developed through MCs, located in the basal layer of the epidermis. These cells produce melanin/melanosomes, which, when deposited into the extracellular matrix (ECM), result in pigmentation. Possibly more important than the visual pigmenting, these melanosomes are utilized by KCs to aid in protection from ultraviolet (UV) damage. Studies have shown that the MCs and KCs have two-way communication, while the MCs provide protections, growth factors from KCs aid in the proliferation, differentiation, and migration of MCs (Weng et al. 2021). Besides HFs and sweat glands, skin color is one of the important skin parameters. Skin color is mainly related to MCs, which are located in. This makes the development of appropriate pigmentation in bioengineered skin vital not only from a cosmetic point, but also through ensuring adequate UV protection.
2.2 Part 2: Reconstructed Human Epidermis (RHE) and Full-Thickness Skin (FTS) Models
There are several in vitro skin models commercially available, the two most common types being full-thickness skin (FTS) models and reconstructed human epidermis (RHE). FTS models are typically defined as an epidermal and dermal layer, where FBs are seeded onto a scaffold and KCs are seeded on top. RHE models differ in that there is no base dermal component, rather only KCs embedded on a scaffold (Camarena et al. 2020; Catarino et al. 2018).
2.2.1 Reconstructed Human Epidermis (RHE)
The first attempt of in vitro model of RHE was carried out by a group of researchers in France, culturing human KCs on a dermal equivalent with an air–liquid interface to recapitulate a functional epidermis (Asselineau et al. 1986). A primary question posed in this work was whether any epidermis obtained in vitro could be considered as “normal,” able to recapitulate native in vivo epidermal functions with a focus on the epidermis’ role as a barrier. While still an ongoing question in the skin community, 4 years later, in 1990 Rosdy and Clauss successfully obtained a terminal epidermal differentiation of human KCs grown onto inert filters via air–liquid interface in a chemically defined medium (Rosdy and Clauss 1990). RHEs, based on these early studies, mimic solely the epidermis and typically consist of normal human KCs. Fabrication of a RHE begins with KCs that are first expanded in culture, then seeded onto a scaffold, and finally cultured using an air–liquid interface to promote differentiation and maturation.
Since their development in 1986, RHEs have become a useful tool for researchers, especially those interested in toxicology. As an incomplete model of FTS, RHEs have been developed and validated as in vitro skin models. Importantly, these have been validated as alternatives for conventional animal models, according to criteria/guidelines outlined by the Organization for Economic Co-Operation and Development (OECD). These regulations remain the standard for studies aiming to improve upon in vitro skin models. In 2010, Liao et al. developed a new RHE model utilizing the OECD guidelines. The epithelium, developed at Industrial Technology Research Institute (ITRI), and name EPiTRI (epithelium-ITRI), was validated using an OECD-approved skin irritation test (SIT). Briefly, EPiTRI was tested with 20 reference chemicals with known Irritant Index and the results showed an accuracy of irritation response of 96%, that comparable to animal and in vitro reference models, meeting the OECD criteria for screening irritating chemicals in vitro (Liao et al. 2021). There are currently seven RHE models that are considered validated reference methods for in vitro skin irritation testing viz. EPiSkin™ (VRM), EpiDerm™ SIT (EP-200) (VRM), SkinEthic RHE™, LabCyte EPI-Model24 SIT, epiCS®, Skin+®, and KeraSkin™ SIT (OECD 2021). All seven models adhere to the performance standards in OECD TG439. OECD TG431 includes five of these as validated for corrosion testing—EPiSkin™ (SM), EpiDerm™ SCT (EPI-200), epiCS®, and LabCyte EPI-Model24 SCT (OECD n.d.).
Despite these validated RHE models, ongoing research still shows that RHEs are incomplete models of FTS. Catarino et al. (2018) compared novel RHE models to FTS, monitoring their responses when subjected to OECD skin corrosion assays. The results of the study showed higher cell viability of the FTS model compared to the RHE model. This indicates that the FTS maintained an improved barrier function, following the exposure to the substances test on the corrosion assays (including 2-phenylethyl bromide, benzylacetone, lactic acid, and octanoic acid), compared to the single-layer RHE. In addition, the RHE models were found to be significantly more permeable than ex vivo human skin, while their FTS counterparts were been found to have enhanced barrier function (Catarino et al. 2018). This study emphasizes the need for continued development of truly physiologically relevant skin models for in vitro use, which better mimic the in vivo situation for the toxicological detection of substances (Catarino et al. 2018), while RHE models are the only commercial models verified to be used in irritation and corrosion tests (Catarino et al. 2018). It is important to note that without the representation of a dermal layer, the use of RHE models may not fully represent the human skin response in irritation and corrosion tests as the interaction between the epidermal and dermal layers has been shown to affect skin homeostasis.
2.2.2 Full-Thickness Skin (FTS)
FTS models differ from RHEs by including a second layer that mimics the dermal layer in human skin. Typically, the reconstructed dermis is formed using proteins that are found in the human ECM. Collagen type 1 and human FBs are commonly used and provide the basal layer that is then embedded with normal human KCs to form the top epidermal layer (Catarino et al. 2018). Mok et al. demonstrated the formation of a reconstructed human skin equivalent (RSE) with a self-assembled dermal layer. This model consisted of dermal and epidermal layers, making use of the FB ability to secrete their own ECM. The model was developed according to OECD TG439 and was evaluated for toxicity. During 4 weeks of culture, primary dermal FBs formed a dermal FB sheet by secreting ECM. Human KCs were subsequently embedded into this dermal FB sheet. This model was able to closely mimic native human skin structure with a stratified epidermis (Mok et al. 2022).
2.2.3 Future Developments of FTS and RHE Models
While models of FTS and RHEs exist, they are by no means perfect substitutions for in vivo skin. As such, there is continued research improving and redesigning these systems. This is not solely focused on the development of individual cell layers, but also the refinement of the scaffolding material used to provide structure to the models. A recent study by Camarena et al. (2020) demonstrated that FTS and RHE models can be created using novel electrospun scaffolds. They used synthetic polymers instead of animal protein-based materials to create electrospun polymer mats that served as a base for seeding FBs and KCs. PET, PBT, and N6/6 are among the tested synthetic polymers that could be used in place of the typical scaffold materials (i.e., polycarbonate filters or collagen) (Camarena et al. 2020). The ability to alter the growth matrix for these systems may lead to exciting new developments in altering cellular interactions and development to create truly physiological in vitro skin models.
2.3 Part 3: Bioprinting of Skin Constructs
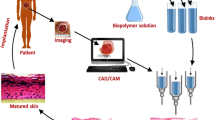
The models discussed above were created through conventional cell seeding methods, and while they have created a solid foundation for skin models in the laboratory, they can be time-consuming and inimitable to develop in large numbers. The shift from hand-crafted to high-speed fabrication of tissues and organs became closer to reality with development of the first bioprinter by Dr. Thomas Boland. Developed through the modification a standard HP inkjet printer to place layer of cells on top of one another in the early 2000s, this marked an important step toward rapid manufacturing of cellularized constructs (Thayer et al. 2018). The rapid advancement of printing technologies and computer-aided design (CAD) has transformed bioprinting into a premium manufacturing platform. Capable of generating custom tissues with defined deposition of living cells, biomaterials, and growth factors at micro- and macro-scales, bioprinting is characterized by both its high customizability and repeatability in the generation of new tissues (Murphy and Atala 2014). These traits have remained true as 3D bioprinting has expanded to include a variety of different printing methods such as laser-assisted bioprinting, inkjet-based bioprinting, pressure-assisted bioprinting, and electrohydrodynamic jetting (Ng et al. 2016; Yan et al. 2018).
3D bioprinting holds several advantages over traditional methods of tissue engineering in skin construct preparation: (1) Computer scanning/imaging technology can be utilized to allow for rapid development of custom skin models matching the shape and depth of the wound surface; (2) the availability of multiple bioinks combined with the ability to deposit them independently within structures can provide skin similar morphology and physiology; (3) the development of in situ printing at the wound surface can be used in clinical treatment; and (4) 3D bioprinting allows for the generation of large, porous constructs providing cell support, gas, and nutrition exchange (Weng et al. 2021). Constant advancement of bioprinting techniques and biomaterials continues to expand this list, with recent studies aiming to add fabrication of complex vasculature and the skin appendages as HFs and sebaceous glands to the 3D printing repertoire.
2.3.1 Biomaterials for Skin Bioinks
The composition of specific bioinks plays a key role in skin bioprinting. The ideal biomaterial should retain mechanical properties similar to those of native skin, support high cell viability and adequate biodegradation rate, a suitable microenvironment for skin cell functionality, good adaptability to printing, and high biocompatibility. The commonly used biomaterials can be divided into natural and synthetic materials (Boland et al. 2003).
Among natural biomaterials, the leading position in skin bioprinting belongs to collagen, an essential ECM component of skin. Other substances present in skin ECM are also used including gelatin, hyaluronic acid, and acellular dermal matrix. Biomaterials sourced from outside the skin are used as well, among them fibrin, agarose, alginate, chitosan, and silk fibroin. Natural biomaterials show high biocompatibility but lack stable mechanical properties and have low gelation levels, making them difficult to handle during and after printing. In contrast, synthetic materials such as polylactic acid (PLA), polycaprolactone (PCL), polyethylene glycol (PEG), and gelatin methacrylate (GelMA) have controllable, and highly reproducible, mechanical, and chemical properties, but low biocompatibility and biodegradability (Yan et al. 2018). Choosing the “right” biomaterial for skin bioprinting is an amorphous challenge, complicated by the many criteria defining “right,” and the need to balance both biological and mechanical features. The development of composite natural and synthetic biomaterial can help address the different biological requirements while improving the stability of the bioink. Structural materials such as collagen, alginate, and chitosan can aid in cell adhesion, proliferation, and differentiation post-printing. In contrast, fugitive and support materials, not containing cells but acting as sacrificial materials, can be rapidly dissolved to create voids and channels within 3D structures (fugitive materials) or to improve physical strength and integrity of bioink (support materials), providing material transport and appropriate internal architecture for a print. Examples include polyurethanes (PUs), PCL, and PLGA. Functional materials are also included in composite bioinks, with molecules such as heparins and GAGs used to stimulate cell behavior and development through signaling and binding with growth factors (Manita et al. 2021). With a field composed of so many uniquely diverse options, the use of bioinks can assist in driving appropriate architecture within skin constructs while also being tuned to aid in appropriate cell maturation in bioengineered skin models.
2.3.2 Advances in Skin Bioprinting
In 2009, Lee et al. successfully bioprinted a multi-layered skin substitute using human skin FBs and KCs using freeform fabrication on collagen matrix (Lee et al. 2009). The authors observed cell proliferation in both planar and nonplanar surfaces in their in vitro model and suggested the feasibility of using 3D printing as an on-demand skin graft fabrication method. Later, in 2010, Binder et al. validated the potential of in situ 3D bioprinting for wound healing. In the study, they used human FBs and KCs in a fibrin and collagen matrix directly in full-thickness wounds on immunodeficient mice using inkjet printing approach. The authors observed decreased contraction and better wound healing compared to controls, untreated allogeneic implant, and hydrogel matrix (Binder et al. 2010).
In 2013, Michael et al. placed FBs and KCs on top of a stabilizing matrix (MatriDerm®) using laser-assisted bioprinting (LAB) in the fabrication of skin for the first time. Maturation of the fabricated constructs was monitored in vitro, with samples maintained at an air–liquid interface, and in vivo, where samples were implanted in the dorsal skin fold chamber of nude mice. The results of this study showed that LAB fabricated skin was able to integrate post-implantation, forming a multi-layered differentiating epidermis in vivo. This epidermis demonstrated basal keratinocyte proliferation, primarily in supra-basal layers, typical of native skin. Interestingly, the in vitro constructs also exhibited the formation of a multi-layered epidermis; however, a less matured version with the basal proliferating keratinocytes was present in all (Michael et al. 2013). This study not only highlighted the enormous impact culture conditions can have on the formation of skin constructs, which replicate native tissue, but also showed the value in utilizing bioprinting to accurately layer cells, positioning them for the appropriate development of multi-layered, functional epidermis.
One of the strengths of bioprinting is the ability to combine technologies. A hybrid 3D cell printing system was developed by Kim et al. (2017), allowing the use of extrusion and inkjet modules at the same time. The extrusion module was used to develop a collagen-based construct embedded with a PCL mesh, designed to prevent the contraction of collagen during tissue maturation. The inkjet system was used simultaneously to distribute KCs uniformly across the surface, developing an epidermal layer on top of the engineered dermis (Kim et al. 2017).
3D printing can not only be combined through multi-printing modalities, but also with other clinical technologies. This was the case in the development of BioMask, combining 3D printing with computed tomography (CT) data to develop custom, patient-specific, models. In short, CT images were used to develop placement patterns for both cellularized hydrogels and a wound dressing material. These were then fabricated using an extrusion printer. The final model (BioMask) contained a porous PU layer, a KC-laden hydrogel layer, and a FB-laden hydrogel layer. The printed construct was then implanted on a mouse and monitored for skin regeneration. Histological assays showed that BioMasks aided in the regeneration of multi-layered skin tissue, consisting of both epidermis and dermis, in complex wounds (Seol et al. 2018).
While BioMask focused on smaller, complex wounds, others have pursued larger wounds with the goal of not only developing patient-specific prints but also being able to print these constructs directly into the patient. In 2019, Albanna et al. described a novel, mobile skin bioprinting system, meant for the treatment of extensive wounds through in situ printing. Using integrated wound imaging technology, the group scanned a wound and then delivered either dermal FBs or epidermal KCs directly to the injury (Albanna et al. 2019). This replicated the layered skin structure without the use of secondary support materials or the need to transfer the print from a build plate to the wound site, acting as proof-of-concept in the validation of a mobile, patient-specific in situ bioprinter. The following year, another group was able to use bioprinted skin to recreate an epidermal barrier in a full-thickness wound model, complete with normal, non-scarring, collage remodeling (Jorgensen et al. 2020). The results of these studies, taken in combination, highlight a just a few of the very exciting opportunities that 3D bioprinting could exploit to develop new and complete models of skin for both the bench and the clinic.
Bioprinting has shown many strengths in differentiation itself as a rapid manufacturing technique. However, in biology, it is not the process that reigns supreme—it is the final product. In moving forward with a new manufacturing technique, it is important to compart the fabricated constructs not only to the goal (FTS) but also to prior models, to understand alterations and where researchers might expect differences from previously obtained results. In 2018, a group of researchers from the Singapore Centre for 3D bioprinting did just this, comparing 3D-bioprinted pigmented skin constructs with pigmented skin constructs fabricated using a conventional manual casting approach. The group completed an in-depth characterization of these models, concluding that the 3D-printed pigmented models more closely resembled the native skin control. This was true not only for the development of macro-architecture (dermal and epidermal layers) but also for micro-architectures, including the development of a continuous basement membrane, which was not present in the manually cast samples. The group concluded that the 3D-printed constructs were an improvement over conventionally manufactured pigmented skin models with potential for toxicology testing and furthering fundamental cell biology research on the bench (Ng et al. 2018).
2.3.3 Bioprinting Limitations and New Prospective
The introduction of 3D-bioprinted skin has enabled the development of customizable skin constructs for patients, printed either onto a conventional build plate or directly onto wound sites. However, while the strengths of bioprinting have been highlighted above, it is important to consider the limitations of the techniques as well. These include significant lead time required to develop enough cells to print (3–4 weeks/m2 according to Cubo et al. 2016), highly trained and specialized personnel to run the printers and develop the 3D models, and high production costs (driven by the price of cell expansion, bioink development, and bioprinter costs). Despite these drawbacks, there is a continually growing demand for artificial skin. These facts together point the skin regeneration field in the direction of automation, standardization, and overall system reduction for both cost and production time. Addressing these points will allow researchers to truly bring RHE and FTS models into the clinic in a meaningful way. New trends in skin bioprinting are focused on these areas while still pressing forward to replicate in vivo skin through the incorporation of stem cells in the skin substitute to develop microvasculature (Abaci et al. 2016) and sweat glands (Yao et al. 2020) as well as combining bioprinting techniques to fabricate skin appendages during the printing process, streamlining the fabrication of physiological skin (Abaci et al. 2018).
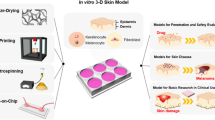
2.4 Part 4: Micro-Tissue Equivalents (Organoids) Models
This review has primarily focused on the development of flat models of skin, developed for both research and clinical purposes. When removing the clinical aspect, researchers aim for the closest recapitulating system, which, as with many organs, is an animal model. Animal models remain the gold standard for replicating the functional and cellular interactions of human tissues. They can be used to predict the development of diseases and the efficacy of treatments. They do have deficiencies driven differences in species biology or sensitivity. In addition, animal models can be very expensive in both monetary value and personnel time, which can lead to lower throughput than may be ideal (Hartung 2008; Shanks et al. 2009). In response to this, 3D organoid cultures have emerged. While these systems do not replicate the macro-structure of organs, they exquisitely mimic the micro-structure and functionality of human organs (Li and Izpisua Belmonte 2019).
Organoids are 3D cell structures made up of = cell mixtures appropriate to the organ being modeled, which better mimic cell–cell and cell–matrix interactions compared to 2D cultures (Bates et al. 2000, p. 200). The 3D microenvironment allows to mimic cellular heterogeneity observed in vivo in different contexts, developing both structural and functional similarities for their in vivo counterparts (Weiswald et al. 2015). Studies have shown that organoids can provide excellent platforms for scientific and clinical applications, recapitulating human physiology and positioning themselves as a contender to replace current models in biological/biomedical research (Bell et al. 1981). When compared to 2D culture, organoids can be cultured for longer time periods, easily cryopreserved, and genetically modified while maintaining their phenotype (Clevers 2016; Drost and Clevers 2018). These features have allowed the use of organoids in various research applications. This has included utilizing organoids as a platform to gain new understanding of organ-specific physiology and to investigate disease-specific modeling in comparison with cell lines. When compared to animal models, organoid culture is advantageous with its high throughput and reduces cost, of particular interest to groups interested in screening large numbers of novel drugs (Weiswald et al. 2015). Organoids can be formed from various stem cells including adipose-derived mesenchymal stromal cells AD-MSCs), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and patient-derived tumor tissue cells, making them applicable to a wide range of systems (Clevers 2016).
While many cell types can be used in fabricating organoids, cancer cells are the most widely used today, due to their easy fabrication in vitro, which is done by embedding cancer cells in a specific ECM, alongside medium niche factors, and additional cells (Ruiz-Garcia et al. 2020; Dominijanni et al. 2020). In 2015, Skardal et al. successfully created liver-based cell organoids in a rotating wall vessel bioreactor. In addition, they were able to combine the manufactured organoids with colon carcinoma cells, developing liver tumor organoids, which acted as in vitro models of liver metastasis. The authors documented that the in vitro 3D liver tumor organoid model replicated tumor responses to current and newly discovered drugs (Skardal et al. 2015). Mazzocchi et al. (2019) created hydrogel-based models to create lung cancer organoids using a single-cell source, pleural effusion aspirate, from multiple lung cancer patients. The authors observed that the cells isolated from the patient, assembled into anatomically relevant structures when seeded into organoids, exhibited behavior specific to lung cancers (Mazzocchi et al. 2019). This application of patient-specific organoids was expanded upon by Forsythe et al. (2022). The group used patient tumor organoids (PTOs) to model rare malignancy “Merkel cell carcinomas” in patient-specific trials. The models were exposed to chemotherapy or immunotherapy agents and monitored for viability after exposure. The authors observed 66% response to chemotherapy in 4/6 specimens with cisplatin and doxorubicin, while immunotherapy was not effective in the immune PTO (iPTO) sets, indicating that these systems could be used to screen for the ideal patient treatment plan (Forsythe et al. 2022). Recently, a group of researchers from Wake Forest developed a novel immune-enhanced tumor organoid (iTO) system to study factors affecting immune checkpoint blockade response (Shelkey et al. 2022).
Unfortunately, these models do not fully mimic human biology. One of the major barriers preventing this is the lack of skin appendages, such as HFs and sweat glands. In addition, the minimalistic approach to skin models has neglected the addition of skin-related cells including dermal fat, sensory cells, and neurons. These deficiencies highlight areas of improvement, which could be targeted to further understand skin through in vitro models (Lee and Koehler 2021). Pushing forward with these 3D models is key, as, 2D cell culture models are less likely to reflect physiological responses than their 3D counterparts (Sun et al. 2006). Many researchers are working on this front, as showcased by the recent development of a skin organoid model that not only uses an air–liquid interface but also included stromal cells, which acted as a source of vital growth factors. Others have focused on the inclusion of new cell types, developing immunocompetent and tumor skin models through the addition of macrophages, T-lymphocytes, melanoma cells, and epithelial carcinoma cells (Gaviria Agudelo and Restrepo 2022).
2.4.1 Organoid Models of Disease
In addition to their application in understanding organ development and drug testing, 3D organoids have applications in disease modeling. Organoids have been used to establish disease models in several tissues. In lung, Wang et al. (2019) used small-cell cancer organoids to investigate the antitumor effect of an irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. The study reported various driver gene mutations in lung cancer (Wang et al. 2019). In 2020, Dieterich et al. developed organoid modeling celiac disease using patient-specific cells and reported dissimilarities in phenotypes between the study groups (Dieterich et al. 2020). Dijkstra et al. in 2021 developed a gastroenteropancreatic neuroendocrine carcinoma model, fabricating organoids from stomach/colon cells used to test drugs for this difficult-to-treat tumor (Dijkstra et al. 2021).
Viral infections can also be studied with organoids. This was showcased through the development of human gastric organoids, which modeled the viral infection of Helicobacter pylori in the stomach (Pompaiah and Bartfeld 2017). Zika virus was modeled in brain organoids. (Sutarjono 2019) and then used to test a variety of chemicals mitigating the hypomorphic effect of zika virus (Xu et al. 2016). Multiple intestinal infections have been modeled including norovirus and rotavirus. These have been successfully cultured in human intestinal organoid models (Ettayebi et al. 2016; Finkbeiner et al. 2012). Major intestinal bacterial pathogens, Salmonella typhi and Clostridium difficile, have likewise been cultured in intestinal organoids (Engevik et al. 2015; Heo et al. 2018).
During the recent global pandemic, organoids proved a valuable research tool for those combating SARS-CoV-2. SARS-CoV-2 was shown to be able to infect and propagate in multiple organ systems including primary human liver–gut organoids PSC-derived blood vessel and kidney through experiments done with organoids (Lamers et al. 2020; Monteil et al. 2020). Skin organoids, fabricated from human-induced pluripotent stem cells (hiPSC), were also utilized with the virus, acting as a pathophysiological model of the infection (Ma et al. 2022). In addition, organoids were able to verify COVID-19 pathogenesis, leading researchers to the discovery of mechanism through which SARS-CoV-2 enters host cells. The angiotensin-converting enzyme 2 was proven to not only assist in initial COVID-19 infection but also in transference to tissues beyond the lungs (Hoffmann et al. 2020).
Human organoids are able to reproduce host–pathogen interactions in vitro. In vitro skin model engineering with optimized interaction with the microbiome may help to understand skin microbial ecology and host-related disease mechanisms. In comparison with their 2D counterparts, organoids have been shown to mimic organ pathologies, acting as effective models for human translational studies. This allows them to be used as development platforms for treatment strategies, applying scientific discoveries to a wider range of human diseases. The miniaturized models allow researchers to recreate complex systems for high-throughput studies. This holds true for skin, where it is important to model not just the cellular makeup, but also the ecology of the skin, complete with bacteria, fungi, and viruses. This can be considered analogous to the microbiome in our gut, which plays an essential role in protecting against pathogens (Belkaid and Segre 2014; Scharschmidt and Fischbach 2013). Disruption of this balance leads to inflammation (Costello et al. 2009; Dekio et al. 2005). In 2018, a model of HF induction was developed using cells derived from interfollicular epidermis (IFE) and HFs in canines (Wiener et al. 2018). Wang and coworkers in 2021 developed a method for the establishment and expansion of human primary epidermal organoids for testing antifungal drugs under chemically defined conditions (Wang et al. 2021). Jung et al. optimized the skin organoid platform using air–liquid interface (ALI) to model atopic dermatitis by Staphylococcus aureus (SA) colonization and infection and observed a disrupted skin barrier and increased production of inflammatory cytokines (Jung et al. 2022). These studies help showcase how organoid technology can be used to understand mutations and potential therapeutic strategies for clinical management of genetic diseases.
Alternatively, approaches for studying genetic diseases have also been used with organoid models. Schwank et al. (2013) fabricated the first gene-corrected intestinal organoids from patients with cystic fibrosis. Biopsies were taken and then processed with CRISPR-Cas9 technology to alter the homozygous CFTR F508 deletion (Schwank et al. 2013). Later studies used patient-derived cells, their homozygous mutations corrected by CRISPR, to develop iPSCs. The resulting gene-corrected iPSC-derived organoids were able to into airway epithelium with normal CFTR expression and function (Firth et al. 2015). Similar technology has been used to assess contractile function in engineered heart tissue. Yang et al. (2018) used iPSC-derived organoids to model abnormal contractile functions in patient-specific organoids from those suffering from familial cardiomyopathy. The myosin heavy-chain 7 mutation (E848G) was modeled effectively and the researchers showed that gene correction was possible in dystrophin mutations, showcasing proof of concept for gene application in the treatment of tissue replacement therapy (Yang et al. 2018).
2.5 Part 5: Chemical Irritation, Corrosion, and Sensitization Testing Using Skin Organoids
Skin is the first barrier of the body and the main target for disruptive and hazardous agents of different origin. Exposure to different substances can lead to either reversible (irritation) or non-reversible (corrosion) skin damages. As a result of exposure, humans may experience contact dermatitis—acute inflammation in the skin as both allergic and non-allergic reactions. Recent analysis showed that allergic contact dermatitis (ACD) accounts for 20% among all cases of dermatoses, and rising level of spreading ACD demands new reliable tests to identify new hazardous agents. The OECD formulated main guidelines and requirements for skin irritation/corrosion testing in vitro. The guidelines determined a panel of well-known chemicals as standards for validation of the models and approved tests for analysis. The validated tests include a viability assay, the evaluation of barrier integrity, and the examination morphology of each skin model after the exposure. Based on these guidelines, researchers are developing new assays applicable specifically in vitro. Thus, Saito et al. described the epidermal sensitization assay based on the microarray analysis of the expression of five genes related to cellular stress response (Saito et al. 2013), and Pfuhler et al. presented the Comet assay-based genotoxicity analysis on the reconstructed human epidermis (Pfuhler et al. 2021).
These in vitro models are poised to compete with in vivo models, not only for clinical studies, but also for the multitude of nonclinical trials, which rely on animal models to predict the effects of drugs, cosmetics, and chemicals. This is of particular importance considering not only the scientific, but also the ethical ramifications of using animals that may not precisely replicate the human condition. Organoid technology is closing the gap between 2D cell culture and the in vivo animal models, as an alternative, accurate in vitro model, and has proven its worth in developmental biology and personalized medicine. At present, organoid technology holds great potential for biomedical applications including disease modeling, drug screening, biobanks, regenerative therapy, genetic screening, and personalized medicine (Kim et al. 2020; Xu et al. 2018). The demand for a rapid, large-scale model suitable for in vitro toxicity and efficacy has also been growing from the pharmaceutical and cosmetic industries, further driving the commercialization of organoid technology.
2.6 Part 6: Summary and Conclusions
Skin tissue engineering is a powerful and highly versatile technology that can be applied for skin development research, dermatopathology, wound healing, and development of new topical drugs and vaccines. Original in vitro models, consisting of single-cell-type cultures, are now being replaced with organoids representing different human skin functions and broaden their scope in the industrial and clinical application. New trends in the fabrication of skin constructs have included the incorporation of stem cells along with pre-fabrication of skin appendages to generate self-supporting, highly functional systems. Parallel advancement of in situ and in vitro 3D skin bioprinting has resulted in promising technologies for on-site treatment of excessive wounds and the formulation patch-on-demand services for clinical applications. The rapid development of in vitro skin models is poised to transform the cosmetic industry, as 3D skin models facilitate the examination of cosmetic products and topical drug for efficiency and toxic influence, with their improvements in cost-efficacy and case-specific relevancy compared with animal models. Skin disease models, as showcased in this review, have a high utility not only for investigative pathology, but also as powerful tool for drug and vaccine development.
References
Abaci HE, Guo Z, Coffman A, Gillette B, Lee WH, Sia SK, Christiano AM (2016) Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv Healthc Mater 5(14):1800–1807. https://doi.org/10.1002/adhm.201500936
Abaci HE, Coffman A, Doucet Y, Chen J, Jacków J, Wang E et al (2018) Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun 9(1):1–11. https://doi.org/10.1038/s41467-018-07579-y
Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, Zhao W, Tan J, El-Amin IB, Dice DD, Marco J, Green J, Xu T, Skardal A, Holmes JH, Jackson JD, Atala A, Yoo JJ (2019) In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep 9(1):1856. https://doi.org/10.1038/s41598-018-38366-w
Asselineau D, Bernard BA, Bailly C, Darmon M, Pruniéras M (1986) Human epidermis reconstructed by culture: is it “Normal”? J Investig Dermatol 86(2):181–186. https://doi.org/10.1111/1523-1747.ep12284237
Bates RC, Edwards NS, Yates JD (2000) Spheroids and cell survival. Crit Rev Oncol Hematol 36(2–3):61–74. https://doi.org/10.1016/s1040-8428(00)00077-9
Belkaid Y, Segre JA (2014) Dialogue between skin microbiota and immunity. Science 346(6212):954–959. https://doi.org/10.1126/science.1260144
Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T (1981) Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 211(4486):1052–1054. https://doi.org/10.1126/science.7008197
Binder KW, Zhao W, Aboushwareb T, Dice D, Atala A, Yoo JJ (2010) In situ bioprinting of the skin for burns. J Am Coll Surg 211(3):S76. https://doi.org/10.1016/j.jamcollsurg.2010.06.198
Boland T, Mironov V, Gutowska A, Roth EA, Markwald RR (2003) Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat Rec A Discov Mol Cell Evol Biol 272(2):497–502. https://doi.org/10.1002/ar.a.10059
Buffoli B, Rinaldi F, Labanca M, Sorbellini E, Trink A, Guanziroli E, Rezzani R, Rodella LF (2014) The human hair: from anatomy to physiology. Int J Dermatol 53(3):331–341. https://doi.org/10.1111/ijd.12362
Camarena DEM, Matsuyama LSAS, Maria-Engler SS, Catalani LH (2020) Development of epidermal equivalent from electrospun synthetic polymers for in vitro irritation/corrosion testing. Nano 10(12):2528. https://doi.org/10.3390/nano10122528
Catarino CM, do Nascimento Pedrosa T, Pennacchi PC, de Assis SR, Gimenes F, Consolaro MEL, de Moraes Barros SB, Maria-Engler SS (2018) Skin corrosion test: a comparison between reconstructed human epidermis and full thickness skin models. Eur J Pharm Biopharm 125:51–57. https://doi.org/10.1016/j.ejpb.2018.01.002
Clevers H (2016) Modeling development and disease with organoids. Cell 165(7):1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R (2009) Bacterial community variation in human body habitats across space and time. Science 326(5960):1694–1697. https://doi.org/10.1126/science.1177486
Cubo N, Garcia M, Del Canizo JF, Velasco D, Jorcano JL (2016) 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 9(1):015006. https://doi.org/10.1088/1758-5090/9/1/015006
Dekio I, Hayashi H, Sakamoto M, Kitahara M, Nishikawa T, Suematsu M, Benno Y (2005) Detection of potentially novel bacterial components of the human skin microbiota using culture-independent molecular profiling. J Med Microbiol 54(Pt 12):1231–1238. https://doi.org/10.1099/jmm.0.46075-0
Dieterich W, Neurath MF, Zopf Y (2020) Intestinal ex vivo organoid culture reveals altered programmed crypt stem cells in patients with celiac disease. Sci Rep 10(1):3535. https://doi.org/10.1038/s41598-020-60521-5
Dijkstra KK, van den Berg JG, Weeber F, van de Haar J, Velds A, Kaing S, Peters DDGC, Eskens FALM, de Groot DJA, Tesselaar MET, Voest EE (2021) Patient-derived organoid models of human neuroendocrine carcinoma. Front Endocrinol 12:627819. https://doi.org/10.3389/fendo.2021.627819
Dominijanni A, Mazzocchi A, Shelkey E, Forsythe S, Devarsetty M, Soker S (2020) Bioengineered tumor organoids. Curr Opin Biomed Eng 13:168–173. https://doi.org/10.1016/j.cobme.2020.03.005
Drost J, Clevers H (2018) Organoids in cancer research. Nat Rev Cancer 18(7):407–418. https://doi.org/10.1038/s41568-018-0007-6
Engevik MA, Engevik KA, Yacyshyn MB, Wang J, Hassett DJ, Darien B, Yacyshyn BR, Worrell RT (2015) Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol 308(6):G497–G509. https://doi.org/10.1152/ajpgi.00090.2014
Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL, Estes MK (2016) Replication of human noroviruses in stem cell-derived human enteroids. Science 353(6306):1387–1393. https://doi.org/10.1126/science.aaf5211
Finkbeiner SR, Zeng XL, Utama B, Atmar RL, Shroyer NF, Estes MK (2012) Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio 3(4):e00159–e00112. https://doi.org/10.1128/mBio.00159-12
Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH, Verma IM (2015) Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12(9):1385–1390. https://doi.org/10.1016/j.celrep.2015.07.062
Forsythe SD, Erali RA, Laney P, Sivakumar H, Li W, Skardal A, Soker S, Votanopoulos KI (2022) Application of immune enhanced organoids in modeling personalized Merkel cell carcinoma research. Sci Rep 12(1):13865. https://doi.org/10.1038/s41598-022-17921-6
Gaviria Agudelo C, Restrepo LM (2022) Human skin cancer: an overview of animal, ex vivo, and in vitro models. Curr Derm Rep 11:168–177. https://doi.org/10.1007/s13671-022-00361-w
Hartung T (2008) Thoughts on limitations of animal models. Parkinsonism Relat Disord 14(Suppl 2):S81–S83. https://doi.org/10.1016/j.parkreldis.2008.04.003
Heo I, Dutta D, Schaefer DA, Iakobachvili N, Artegiani B, Sachs N, Boonekamp KE, Bowden G, Hendrickx APA, Willems RJL, Peters PJ, Riggs MW, O’Connor R, Clevers H (2018) Modelling cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol 3(7):814–823. https://doi.org/10.1038/s41564-018-0177-8
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Jorgensen AM, Varkey M, Gorkun A, Clouse C, Xu L, Chou Z, Murphy SV, Molnar J, Lee SJ, Yoo JJ, Soker S, Atala A (2020) Bioprinted skin recapitulates Normal collagen remodeling in full-thickness wounds. Tissue Eng Part A 26(9–10):512–526. https://doi.org/10.1089/ten.TEA.2019.0319
Jung SY, You HJ, Kim MJ, Ko G, Lee S, Kang KS (2022) Wnt-activating human skin organoid model of atopic dermatitis induced by Staphylococcus aureus and its protective effects by Cutibacterium acnes. iScience 25(10):105150. https://doi.org/10.1016/j.isci.2022.105150
Kanitakis J (2002) Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol 12(4):390–399. Quiz 400–401
Kim BS, Lee JS, Gao G, Cho DW (2017) Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 9(2):025034. https://doi.org/10.1088/1758-5090/aa71c8
Kim J, Kong JS, Han W, Kim BS, Cho DW (2020) 3D cell printing of tissue/organ-mimicking constructs for therapeutic and drug testing applications. Int J Mol Sci 21(20). https://doi.org/10.3390/ijms21207757
Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ et al (2020) SARS-CoV-2 productively infects human gut enterocytes. Science 369(6499):50–54. https://doi.org/10.1126/science.abc1669
Lee J, Koehler KR (2021) Skin organoids: a new human model for developmental and translational research. Exp Dermatol 30(4):613–620. https://doi.org/10.1111/exd.14292
Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, Park JK, Yoo SS (2009) Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 30(8):1587–1595. https://doi.org/10.1016/j.biomaterials.2008.12.009
Li M, Izpisua Belmonte JC (2019) Organoids—preclinical models of human disease. N Engl J Med 380(6):569–579. https://doi.org/10.1056/NEJMra1806175
Liao CC, Wu CY, Lin MH, Hsieh FK, Hsu LT, Chang SY, Chen KJ, Huang HT, Hsu HC, Lin CH, Lin PJ, Lai HM, Kojima H, Todo H, Lin SJ, Li JH, Chen W (2021) Validation study of a new reconstructed human epidermis model EPiTRI for in vitro skin irritation test according to OECD guidelines. Toxicol in Vitro 75:105197. https://doi.org/10.1016/j.tiv.2021.105197
Ma J, Liu J, Gao D, Li X, Zhang Q, Lv L, Wang Y, Li J, Zhu Y, Wu Z, Hu H, Li Y, Ma L, Liu Q, Hu Z, Zhang S, Zhou Y, Wang M, Leng L (2022) Establishment of human pluripotent stem cell-derived skin organoids enabled pathophysiological model of SARS-CoV-2 infection. Adv Sci 9(7):2270041. https://doi.org/10.1002/advs.202270041
Manita PG, Garcia-Orue I, Santos-Vizcaino E, Hernandez RM, Igartua M (2021) 3D bioprinting of functional skin substitutes: from current achievements to future goals. Pharmaceuticals 14(4):362. https://doi.org/10.3390/ph14040362
Marques AP, Reis R, Pirraco RP, Cerqueria M (eds) (2017) Skin tissue models. Academic, Cambridge
Mazzocchi A, Devarasetty M, Herberg S, Petty WJ, Marini F, Miller L, Kucera G, Dukes DK, Ruiz J, Skardal A, Soker S (2019) Pleural effusion aspirate for use in 3D lung cancer modeling and chemotherapy screening. ACS Biomater Sci Eng 5(4):1937–1943. https://doi.org/10.1021/acsbiomaterials.8b01356
Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K (2013) Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One 8(3):e57741. https://doi.org/10.1371/journal.pone.0057741
Mok BR, Shon SJ, Kim AR, Simard-Bisson C, Martel I, Germain L, Kim DH, Shin JU (2022) Structural and functional validation of a full-thickness self-assembled skin equivalent for disease modeling. Pharmaceutics 14(6):1211. https://doi.org/10.3390/pharmaceutics14061211
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, del Pozo CH, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181(4):905–913.e7. https://doi.org/10.1016/j.cell.2020.04.004
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785. https://doi.org/10.1038/nbt.2958
Ng WL, Wang S, Yeong WY, Naing MW (2016) Skin bioprinting: impending reality or fantasy? Trends Biotechnol 34(9):689–699. https://doi.org/10.1016/j.tibtech.2016.04.006
Ng WL, Qi JTZ, Yeong WY, Naing MW (2018) Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 10(2):025005. https://doi.org/10.1088/1758-5090/aa9e1e
OECD(2021) Test guideline no. 439: in vitro skin irritation: Reconstructed human epidermis test methods
OECD (n.d.) Test no. 431: in vitro skin corrosion: reconstructed human epidermis (RHE) test method. https://www.oecd.org/publications/test-no-431-in-vitro-skin-corrosion-reconstructed-human-epidermis-rhe-test-method-9789264242753-en.htm
Pfuhler S, Pirow R, Downs TR, Haase A, Hewitt N, Luch A et al (2021) Validation of the 3D reconstructed human skin comet assay, an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis 36(1):19–35. https://doi.org/10.1093/mutage/geaa009
Pompaiah M, Bartfeld S (2017) Gastric organoids: an emerging model system to study helicobacter pylori pathogenesis. Curr Top Microbiol Immunol 400:149–168. https://doi.org/10.1007/978-3-319-50520-6_7
Rosdy M, Clauss LC (1990) Terminal epidermal differentiation of human keratinocytes grown in chemically defined medium on inert filter substrates at the air-liquid interface. J Invest Dermatol 95(4):409–414. https://doi.org/10.1111/1523-1747.ep12555510
Ruiz-Garcia H, Alvarado-Estrada K, Schiapparelli P, Quinones-Hinojosa A, Trifiletti DM (2020) Engineering three-dimensional tumor models to study glioma cancer stem cells and tumor microenvironment. Front Cell Neurosci 14:558381. https://doi.org/10.3389/fncel.2020.558381
Saito K, Nukada Y, Takenouchi O, Miyazawa M, Sakaguchi H, Nishiyama N (2013) Development of a new in vitro skin sensitization assay (Epidermal sensitization assay; EpiSensA) using reconstructed human epidermis. Toxicol in Vitro 27(8):2213–2224. https://doi.org/10.1016/j.tiv.2013.08.007
Scharschmidt TC, Fischbach MA (2013) What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov Today Dis Mech 10(3–4):e83–e89. https://doi.org/10.1016/j.ddmec.2012.12.003
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EES, Beekman JM, Clevers H (2013) Functional repair of CFTR by CRISPR/Cas 9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13(6):653–658. https://doi.org/10.1016/j.stem.2013.11.002
Seol YJ, Lee H, Copus JS, Kang HW, Cho DW, Atala A, Lee SJ, Yoo JJ (2018) 3D bioprinted bio mask for facial skin reconstruction. Bioprinting 10:e00028. https://doi.org/10.1016/j.bprint.2018.e00028
Shanks N, Greek R, Greek J (2009) Are animal models predictive for humans? Philos Ethics Humanit Med 4:2. https://doi.org/10.1186/1747-5341-4-2
Shelkey E, Oommen D, Stirling ER, Soto-Pantoja DR, Cook KL, Lu Y, Votanopoulos KI, Soker S (2022) Immuno-reactive cancer organoid model to assess effects of the microbiome on cancer immunotherapy. Sci Rep 12(1):9983. https://doi.org/10.1038/s41598-022-13930-7
Skardal A, Devarasetty M, Rodman C, Atala A, Soker S (2015) Liver-tumor hybrid organoids for modeling tumor growth and drug response in vitro. Ann Biomed Eng 43(10):2361–2373. https://doi.org/10.1007/s10439-015-1298-3
Sun T, Jackson S, Haycock JW, MacNeil S (2006) Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol 122(3):372–381. https://doi.org/10.1016/j.jbiotec.2005.12.021
Sutarjono B (2019) Can we better understand how Zika leads to microcephaly? A systematic review of the effects of the Zika virus on human brain organoids. J Infect Dis 219(5):734–745. https://doi.org/10.1093/infdis/jiy572
Thayer PS, Orrhult LS, Martínez H (2018) Bioprinting of cartilage and skin tissue analogs utilizing a novel passive mixing unit technique for bioink Precellularization. J Vis Exp 131:56372. https://doi.org/10.3791/56372
Wang Y, Jiang T, Qin Z, Jiang J, Wang Q, Yang S, Rivard C, Gao G, Ng TL, Tu MM, Yu H, Ji H, Zhou C, Ren S, Zhang J, Bunn P, Doebele RC, Camidge DR, Hirsch FR (2019) HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 30(3):447–455. https://doi.org/10.1093/annonc/mdy542
Wang X, Wang S, Guo B, Su Y, Tan Z, Chang M, Diao J, Zhao Y, Wang Y (2021) Human primary epidermal organoids enable modeling of dermatophyte infections. Cell Death Dis 12(1):35. https://doi.org/10.1038/s41419-020-03330-y
Weiswald LB, Bellet D, Dangles-Marie V (2015) Spherical cancer models in tumor biology. Neoplasia 17(1):1–15. https://doi.org/10.1016/j.neo.2014.12.004
Weng T, Zhang W, Xia Y, Wu P, Yang M, Jin R, Xia S, Wang J, You C, Han C, Wang X (2021) 3D bioprinting for skin tissue engineering: current status and perspectives. J Tissue Eng 12:20417314211028576. https://doi.org/10.1177/20417314211028574
Wiener DJ, Basak O, Asra P, Boonekamp KE, Kretzschmar K, Papaspyropoulos A, Clevers H (2018) Establishment and characterization of a canine keratinocyte organoid culture system. Vet Dermatol 29(5):375–e126. https://doi.org/10.1111/vde.12541
Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W et al (2016) Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22(10):1101–1107. https://doi.org/10.1038/nm.4184
Xu H, Jiao Y, Qin S, Zhao W, Chu Q, Wu K (2018) Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp Hematol Oncol 7:30. https://doi.org/10.1186/s40164-018-0122-9
Yan WC, Davoodi P, Vijayavenkataraman S, Tian Y, Ng WC, Fuh JYH, Robinson KS, Wang C-H (2018) 3D bioprinting of skin tissue: from pre-processing to final product evaluation. Adv Drug Deliv Rev 132:270–295. https://doi.org/10.1016/j.addr.2018.07.016
Yang KC, Breitbart A, De Lange WJ, Hofsteen P, Futakuchi-Tsuchida A, Xu J, Schopf C, Razumova MV, Jiao A, Boucek R, Pabon L, Reinecke H, Kim DH, Ralphe JC, Regnier M, Murry CE (2018) Novel adult-onset systolic cardiomyopathy due to MYH7 E848G mutation in patient-derived induced pluripotent stem cells. JACC Basic Transl Sci 3(6):728–740. https://doi.org/10.1016/j.jacbts.2018.08.008
Yao B, Wang R, Wang Y, Zhang Y, Hu T, Song W et al (2020) Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration. Sci Adv 6(10):eaaz1094. https://doi.org/10.1126/sciadv.aaz1094
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gorkun, A. et al. (2023). Fabrication of Ready-to-Use Ex Vivo Human Skin Models for Chemical Testing: Current Status and Challenges. In: Pant, A.B., Dwivedi, A., Ray, R.S., Tripathi, A., Upadhyay, A.K., Poojan, S. (eds) Skin 3-D Models and Cosmetics Toxicity. Springer, Singapore. https://doi.org/10.1007/978-981-99-2804-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-99-2804-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2803-3
Online ISBN: 978-981-99-2804-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)