Abstract
Over the past 20 years, the development of biosensors has significantly benefited from nanotechnology. The creation of nanomaterials (NMs), their use in novel biosensing and diagnostic applications, and their thorough characterization for in vitro and in vivo applications are a few of these. The introduction of numerous new signal transduction technologies has been made possible by using nanomaterials to create biosensors, which has increased their sensitivity and performance. Mechanical devices based on nanobiosensor architecture, optical resonators, functionalized nanoparticles, nanowires, nanotubes, and nanofibers have all been utilized. Remarkably, the potential medical applications of nanomaterials, including gold nanoparticles, carbon nanotubes, magnetic nanoparticles, and quantum dots, have been extensively researched. Therefore, we cover many advanced nanomaterials that have been widely applied in recent years and discuss how they were fabricated. The main objective of this chapter is to offer an overview of recently created advanced nanomaterial-based biosensors used to diagnose a range of diseases as well as therapeutic purposes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
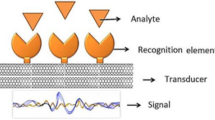
As per IUPAC, the biosensor is defined as “a self-contained integrated device, which is capable of providing specific quantitative or semi-quantitative analytical information using a biological recognition element that is in direct spatial contact with a transducer element.” The sensor comprises a transducer, a readout system, and a detection system known as a receptor (Fig. 2.1). In biosensors, the acceptor is a biological component that is variously fixed to the converter. The method by which the bioreceptor layer (biological component) is mounted on the transducer significantly impacts biosensors’ success. Selectivity is a crucial sensor property that shows how well the system can separate the analyte from other components in the sample. Sensitivity is the next critical feature. High sensitivity indicates that a considerable change in the output signal from the sensor can be seen even with slight differences in analyte concentration. Such a sensor has a high bit resolution. Another crucial aspect that is included in the definition of accuracy is the repeatability (measurability) of the measurement results (Ge et al. 2014; Sangadevan and Periasamy 2014; Vashist et al. 2012; Huang et al. 2021; Pandit et al. 2016). The development of biosensors is increasingly dependent on nanotechnology. Biosensors are becoming more sensitive and effective, thanks to nanomaterials’ use in their production. By combining sensors, fluidics, and signal-processing circuits, nanotechnology will make it possible to further miniaturize bioanalytical systems. This will allow for the large-scale integration of various biological reactions on a more compact scale (Vo-Dinh et al. 2001).
Due to their substantial specific surface area and high free surface energy, nanoparticles play a significant role in the surface adsorption of biomolecules. Materials with 1 and 100 nm diameters are commonly referred to as nanomaterials. Due to their small size, these particles have interesting physicochemical characteristics (Wang et al. 2014; Bai and Jiang 2013; Gholipour et al. 2020). Going nano means a matter can be altered at the molecular and atomic levels in addition to its reduced size. The developing ability to manipulate the patterns of matter on the nanoscale scale will fundamentally alter the ability to sense and detect the condition of biological systems and live organisms in the case of biosensing (Alivisatos 2004). It is anticipated that such a drastic transformation will make it possible for living cells to detect several signals simultaneously and at the single molecular level. Going nano will help to enhance sensitivity and lower the detection limit, reduce the volume of analyte reagent, and shorten the detection time at the systems level. Biosensors can be reduced in size so that they can be inserted at any desired position within the body.
2.2 Various Detection Methods
These methods can be generally categorized into mechanical, optical, electromagnetic, electrical, thermal, magnetic, and electrochemical processes.
2.2.1 Mechanical Detection
Mechanical-based transducers typically use mechanical deformations or mechanical waves (or acoustic waves) as their sensing mechanisms. In order to use this technique, the underlying transducer is frequently a mechanical structure in the form of a cantilever beam, a double-clamped beam, or a disc. The surface of the transducer is functionalized by immobilizing a layer of a sensitive element (e.g., antibodies or enzymes) on it for target binding. Ex vivo applications are more suited for this kind of detection (Stenger et al. 2001).
A conventional method of detection in the case of a cantilever beam involves measuring the cantilever deflection brought on by the surface stresses produced as a result of molecule binding. Intermolecular forces like electrostatic contact or van der Waals force of attraction are the leading causes of surface stress. The cantilever will deflect due to the generation of differential stress (Berger et al. 1997). Two methods are frequently used to measure cantilever deflection. In the first, the cantilever deflection is measured using a four-segment photodetector, while a laser beam is focused on the free end of the cantilever. The second method uses electrical equipment to measure the cantilever deflection using resistive or capacitive circuitry (Porter et al. 2003).
Advantages of mechanical detection included the ability to perform protein recognition with extremely high sensitivity and the ability to identify mismatches in oligonucleotide hybridization without labeling. Additionally, this technique works with various analyte species in gaseous or aqueous forms. The method faces some limitations as well, such as the fact that if the molecule binding events are exothermic, the heat created may make it difficult to detect them since differential thermal stress might cause the cantilever to deflect (Mertens et al. 2003). Another problem is that the molecular structures are nonlinear and viscoelastic, which might make them impossible to utilize.
Changes in acoustic properties, such as the resonant frequency, attenuation, and phase of wave propagation, are the typical approach of detection in the circumstances of a double-clamped beam or a disc structure. A molecule binding event serves as mass loading in this mode of detection, where mechanical structures behave like oscillators. This mass loading frequently causes a shift in the resonant frequency, an increase in amplitude attenuation, or a delay in the phase of wave propagation. However, the complexity of detecting the type and uniformity of the bound species makes this type of mechanical detection challenging, making it less precise in biological sensing (Rao and Zhang 2006).
2.2.2 Optical and Electromagnetic Detection
Optical detection is one of the most commonly utilized mechanisms for biosensing. This technique may be integrated into a wide range of spectroscopic techniques, which include luminescence, absorption, polarization, and fluorescence. This detection technology allows for the measurement of a target analyte’s amplitude, energy, polarization, decay time, and phase, among other spectrochemical parameters. The most prominent optical technology is undoubtedly fluorescence-based detection. In this method, fluorescent markers that produce light at particular wavelengths are utilized as detection labels for the target analytes. The presence of the targets or the binding of the targets to the probes is determined by measurements of fluorescence intensity (Vo-Dinh and Cullum 2000).
Another common approach of optical detection is the evanescent wave-based sensing approach. By total internal reflection, an optical waveguide is utilized in this technique to restrict the light passing through it. Evanescent wave-based sensors are ideal for measuring molecular interactions in situ and in real time since they are susceptible to the detection of biological and chemical species at lower levels (Liu and Tan 1999). The theory of surface-enhanced Raman spectroscopy (SERS) serves as the foundation for a widely used electromagnetic detection technique. Because of surface plasmon resonance, molecules positioned near to a rough metal surface with silver or gold nanostructures (such as nanoparticles or nanowires) can exhibit surface-enhanced Raman scattering.
2.2.3 Electrical Detection
Electrical detection offers several desired characteristics as an underlying transducer because of its simplicity of use, label-free detecting capability, portability, and downsizing, even if it has not been as commonly employed as the mechanical or optical detection methods. When a biological binding event takes place at the electrode surfaces, conductometric and potentiometric techniques, two popular types of electrical detection, primarily rely on the measurement of changes in conductance (or impedance) and potential. Changes in the electrical resistance or impedance between two electrodes are detected by conductometric sensors (Chen et al. 2004). In this instance, the variations in resistance or impedance are brought on by metabolite excretion in the vicinity of the electrode surfaces or in the surrounding medium or by molecular interactions between nucleotides, proteins, antigens, and antibodies. This method of detection is appealing because, unlike electrochemical detection, it does not call for a particular reference electrode. Potentiometric sensors quantify the potential changes between electrodes. Ion-sensitive field-effect transistors or chemical field-effect transistors are used in the most popular potentiometric sensor design. Due to the inherent molecular charges on the DNA, potentiometric sensors have been employed to perform label-free detection of DNA hybridization by detecting the field effect in silicon (Fritz et al. 2002).
2.2.4 Electrochemical Detection
The electrical responses produced by the electrochemical reactions of the target redox species catalyzed by the enzymatic-sensitive elements are frequently measured by biosensors that use an electrochemical technique as the underlying transducer. Typically, these biosensors are set up in a three-electrode arrangement: a working electrode, a counter electrode, and a reference electrode. Amperometric, voltammetric, and impedimetric modes of operation are the most frequently utilized for biological detections. The electrical current produced by the exchange of electrons between the electrodes and ionic species in response to electrode polarization at a constant potential is measured by amperometric biosensors. Voltammetric biosensors evaluate the current-potential relationships (sometimes called voltammograms) brought on by a redox process. Upon cyclic excitations of the working electrode at a predetermined frequency range, impedimetric biosensors monitor changes in the complex impedance of an electrochemical process. The detection of glucose, lactose, urea, lactate, and DNA hybridization has all been done using electrochemical-based biosensors (Zhu and Snyder 2003; Zhang 2009).
2.3 Development in Nanotechnology-Based Biosensors
However, biosensors based on nanotechnology are a more comprehensive word that can be seen from three perspectives. Type A nanotechnology-based biosensors are those that contain nanomaterials in their structural design, according to the first point of view. These might include various nanomaterials that have been coated with bioreceptors and vary in size, shape, and construction materials. These nanoparticles increase the surface area of these biosensors and increase their sensitivity. Type B is concerned with biosensors that include nanostructured structures and are made using self-assembly or nanofabrication techniques. In this type, electrodes are typically nanoscale in size and coupled to a processor that is larger, like a computer. The last type (type C) indicates the biosensors in nanoscale, which means a device for sensing usage with at least one dimension below 100 nm. This type will be a highly packed and complex device than integrate sensing and processing in one device (Liu et al. 2009).
In the last 10 years, the fields of in vitro diagnostics, imaging, and therapies have seen the extensive application of NMs. They have made it possible to diagnose diseases at a very early stage and to simultaneously multiplex detect a large number of disease biomarkers (Cheng et al. 2011). They have also made it possible to investigate the detection of extremely low concentrations of the target analytes and have produced ultrasensitive, quick, and economical assays that only need a small sample.
The capacity to detect single molecules can also be achieved by using nanostructure (nanopores, nanowires, nanopillars, and nanogaps)-based devices, which utilize gold nanoparticles (GNPs) that have been labeled with brief DNA segments. In the past 10 years, a number of intriguing NMs have been applied to diagnostics and biosensors, including carbon nanotubes (CNTs), graphene, quantum dots (QDs), nanoparticles (NPs), and nanocomposites. The NMs with improved performance and functionality, like QDs and NPs, make effective imaging agents (Kairemo et al. 2008). Nanotechnology advancements will be very helpful in moving from late-stage diagnosis (including expensive and socially demanding therapy) to early-stage diagnosis (relatively less costly and less invasive). Due in large part to the multibillion-dollar glucose monitoring market, the significant application has nearly invariably been glucose sensing.
2.4 Nanotechnology-Based Biosensors
2.4.1 Thin Films
Due to inherent features related to their nanoscale dimensions, nanostructured thin films have made it possible to create electrochemical sensors and biosensors with high detection powers. Additionally, a wide variety of organic and inorganic components can be used for the growth of films. Additionally, the use of suitable materials such as natural polymers can help to explain the potential for improving the detection limit in biosensing devices. The purpose of using these materials is to maintain the structural integrity of biomolecules while combining a high level of detection with their ability to function as biocatalysts (Pandit et al. 2016).
2.4.2 Nanomaterial-Based Biosensors
In order to exploit certain nanomaterials in better biological signaling and transduction pathways, their electrical and mechanical properties have been investigated. Nanotubes, nanowires, nanorods, nanoparticles, and thin films comprised of crystalline solids are some of these often used materials. Colloidal nanoparticles can be employed for immunosensing and immunolabeling applications by conjugating with antibodies. Additionally, metal-based nanoparticles are excellent materials for electronic and optical applications, and by taking advantage of their optoelectronic characteristics, they can be effectively used for the detection of nucleic acid sequences. Nanomaterials like metal nanoparticles, oxide nanoparticles, magnetic nanomaterials, carbon materials, quantum dots, and metallophthalocyanines are now used due to significant advancements in the field of nanotechnology to enhance the electrochemical signals of biocatalytic events that take place at the electrode/electrolyte interface. For use in biosensors to detect biological molecules, functional nanoparticles that bind to biological molecules (such as peptides, proteins, and nucleic acids) have been produced (Pandit et al. 2016).
2.4.2.1 Carbon Nanotubes (CNTs)
CNTs have been one of the most widely employed NMs in the last 10 years for biosensors, diagnostics, tissue engineering, cell tracking and labeling, and medication and biomolecule delivery. Carbon nanotubes are a perfect material for use in chemical and biological sensing due to their nano-dimensions, graphitic surface chemistry, and electrical characteristics. Biosensors have utilized both single-walled nanotubes (SWNT) and multiwalled carbon nanotubes (MWNT). They are single-, double-, or multiwalled CNTs, which are hollow cylindrical tubes made of one, two, or more concentric graphitic layers and capped by fullerene spheres. For the susceptible detection of analytes in healthcare, industry, environmental monitoring, and food quality analysis, CNT-based biosensors and diagnostics have been used (Li et al. 2008; Muguruma et al. 2008). They have primarily been employed in electrochemical sensing, principally for the detection of glucose, but also for the detection of fructose, galactose, neurotransmitters, neurochemicals, amino acids, immunoglobulin, albumin, streptavidin, insulin, human chorionic gonadotropin, C-reactive protein, cancer biomarkers, cells, microorganisms, DNA, and other biomolecules (Davis et al. 2003). One instance involved coating glucose oxidase onto the surface of single-walled nanotubes (SWNT), which prevented a significant loss of enzyme function (Azamian et al. 2002). Similar to this, it was shown that horseradish peroxidase (HRP) adsorbed on a carbon nanotube microelectrode could transmit electrons straight to the electrode and still function as a catalytic enzyme for H2O2 (Zhao et al. 2002).
2.4.2.2 Graphene
Graphene, an atomically thin layer of sp2-hybridized carbon, is another NM that has recently been used extensively for diagnostics and biosensors due to its captivating and exciting properties, such as high mechanical strength, high thermal conductivity, high elasticity, tunable optical properties, tunable bandgap, very high room temperature electron mobility, and demonstration of the room temperature quantum Hall effect. It is a translucent material with extremely little environmental impact and minimal production costs. For the detection of a variety of analytes, including glucose, cytochrome c, NADH, hemoglobin, cholesterol, ascorbic acid, dopamine, uric acid, hydrogen peroxide, HRP, catechol, DNA, heavy metal ions, and gases, it has been widely used in electrochemical, impedance, fluorescence, and electrochemiluminescence biosensors (Bai et al. 2020).
2.4.2.3 Quantum Dots
Nanoscale semiconductor crystals called quantum dots (QDs) shine or fluoresce when activated by a light source like a laser. The fluorescence of QDs is incredibly persistent, and they have a high level of resistance to deterioration. QDs are effective tagging agents because they can be selectively attached to biological components like cells, proteins, and nucleic acids. Any wavelength of light, from infrared to ultraviolet to visible, can be built into QDs. This makes it possible to employ a lot of different colors, which makes it possible to run multiplexed assays. The potential for a wide range of applications, including diagnostic tools, has been created by the development of a novel coating for inorganic particles at the nanoscale that may be able to disguise QDs as proteins. This technique enables particles to function as probes that can enter into cells and illuminate specific proteins therein (Jaiswal et al. 2004). Due to their distinct photoluminescent characteristics and possible uses, quantum dots have been the focus of extensive research (Lu et al. 2003). In cellular imaging (Kaul et al. 2003), immunoassays (Medintz et al. 2005), DNA hybridization (Hoshino et al. 2005), biosensors, and optical barcoding (Han et al. 2001), for instance, quantum dots, have been employed successfully.
Fluorescence resonance energy transfer (FRET) and the dynamic course of signal transduction in living cells have both been utilized to analyze the interaction between protein molecules using quantum dots (Jares-Erijman and Jovin 2003). In comparison with conventional fluorescent dyes, these synthesized quantum dots have a number of advantages, including improved stability, increased fluorescence intensity, and a variety of colors that may be achieved by varying the size of the quantum dots (Medintz et al. 2005). They have also been used to identify cancer target areas in vivo.
2.4.2.4 Chitosan
Due to its outstanding biocompatibility, complete biodegradability, and nontoxic makeup, chitosan is one of the most favorable NMs for the assimilation of biological components in medical devices. Natural metabolites that result from the breakdown of chitosan are completely safe. Because it is transparent, optical sensors can use it. Due to the porous nature of the chitosan films and their high ion permeability, it is also suitable for electrochemical sensors. The amine groups of chitosan make it easier for biomolecules to attach to it covalently and for polymers or NPs to form nanocomposites, and its pH-dependent solubility makes it easier for stable films to form at neutral and basic pH levels. But in order to make it more soluble in water and other common solvents, it needs to undergo chemical alteration, such as carboxymethylation. It has seen considerable use in lab-on-a-chip devices, biosensors, diagnostics, and other biomedical or bioanalytical applications (Sashiwa and Aiba 2004; Koev et al. 2010).
2.4.2.5 Dendrimers
The three-dimensional macromolecules known as dendrimers are hyperbranched, monodispersed, star-shaped, nanometer-scale and have a very high density of surface functional groups. They consist of three distinct components: the core, the inside dendron, and the outside surface containing terminal functional groups. They are widely used in a variety of biosensors and diagnostics, including those based on electrochemistry, fluorescence, surface-enhanced Raman scattering, impedimetry, and surface plasmon resonance. This is mainly because they improve analytical sensitivity, stability, and reproducibility while lowering nonspecific interactions. They have also been applied to various bioanalytical processes, including gene transfection, medication delivery, and catalysis (Satija et al. 2011; Shen and Shi 2010).
2.4.2.6 Nanoparticles
Due to their distinct optical and other features, NPs have also been widely exploited in various bioanalytical applications, particularly for the creation of biosensors, diagnostics, imaging, drug administration, and treatment. The attachment of molecules to their surface causes them to change color. For a variety of bioanalytical applications, the ability of nanoparticles to change their properties by altering their size or shape has been used (Parveen et al. 2012).
2.4.2.6.1 Gold Nanoparticles
By electrochemical stabilization, gold nanoparticles can also be deposited on the electrode. To finish preparing the enzymatic electrode, the electrode is submerged in a bath containing par benzoquinone, chitosan, glucose oxidase, and ionic liquid. By varying the solution concentration, the working potential, and the precipitation duration in this process, nanoparticle size and morphology can be adjusted.
The effective early-stage detection and photothermal treatment of cancer and other disorders are made possible by the remarkable plasmon absorption and scattering capabilities of GNPs. They have been employed as nanocarriers for the delivery of medicines, DNA, and genes for the treatment of cancer and other disorders due to their preferred accumulation at the tumor sites (Boisselier and Astruc 2009). Because they can be found using a number of analytical methods, including optical absorption, fluorescence, Raman scattering, atomic and magnetic force, and electrical conductivity, gold nanoparticles make excellent labels for sensors. The most popular nanoparticles for transferring electrons between the active core of redox proteins and the electrode surface are gold nanoparticles. The electron transfer rate constant between the enzyme glucose oxidase and the substrate in glucose detection is increased seven times by using gold nanoparticles on the electrode surface. This asymmetric biosensor for glucose responds in less than 5 s, and when compared to the same correction performed on a smooth gold electrode (without the inclusion of gold nanoparticles), the sensitivity is increased 2.8 times and the detection limit is increased by 20 times (Yang and Cui 2008).
A layer-by-layer (LBL) method may be used to assemble GNPs and methylene blue (MB) into films over a glassy carbon electrode (GCE) that had been adapted for the detection of human chorionic gonadotrophin (HCG) (He et al. 2008). Due to the enormous specific surface area and excellent biocompatibility of GNPs, HRP can be adsorbed onto a GNP layer for the detection of H2O2 without losing any biological activity (Gao et al. 2007). The ZrO2/Au film electrode surface may firmly adsorb organophosphate pesticides (OPs), offering a quantitative method for OP detection that is effective. Wang and Li (2008) detailed the synthesis of ZrO2/Au nanocomposite films using a sol-gel process and electroless plating.
2.4.2.6.2 Silver Nanoparticles
Similar to how gold nanoparticles are utilized, silver nanoparticles are also used to alter the surface of electrode. The high electrocatalytic reactivity and bioactivity of biomolecules are preserved by biocompatibility of silver nanoparticles. Hepatitis B antibodies have been fixed on platinum electrodes using silver nanoparticles as a substrate. Sulfur atoms stabilizing them on silver nanoparticles cause oligonucleotide receptors to assemble on the surface of their monolayer electrode. High levels of sensitivity, selectivity, and stability are displayed by the sensors made using this technique (Qian et al. 2020). Some electrochemical reactions can also be catalyzed by silver nanoparticles. These nanoparticles have been utilized to create enzymatic sensors for glucose and hydrogen peroxide. Cysteine has been detected and measured using a glass carbon electrode enhanced with silver nanoparticles and doped with mercury (Kianfar Farshad et al. 2015).
2.4.2.6.3 Platinum and Copper Nanomaterials
Due to their appropriate catalytic characteristics, platinum nanoparticles are used in electrochemical studies. High-sensitivity and low-detection sensors are created as a result of the catalytic role that platinum nanoparticles play in redox processes and the hybridization of DNA strands. To create sensors for the detection of NO, nitrite, and uric acid, Pt-Fe(III) nanoparticles have also been placed on the electrode surface in addition to platinum nanoparticles. Due to the potential for asymmetric technique detection at the constant potential for analyzing carbohydrates and amino acids, the copper electrode is of great importance. In addition to the previously indicated benefit, using electrodes enhanced with copper nanoparticles in electrochemical sensors speeds up the reaction because of the catalytic capabilities of the nanoparticles. With great sensitivity and constant potential in phosphate buffer solution with pH = 8, all 20 amino acids may be identified on the surface of the electrode coated with copper nanoparticles. Nanoparticles of other metals, such as palladium, nickel, and iridium, have also been utilized as catalysts in electroanalytical systems in addition to gold, silver, platinum, and copper nanoparticles (Özbek et al. 2021; Sivasankar et al. 2018).
2.4.2.6.4 Magnetic Nanoparticles
Due to their unique magnetic properties, magnetic nanoparticles (MNP) have been extensively researched for usage in applications like hyperthermia (Kim et al. 2005), MRI contrast agent (Lee et al. 2006), tissue repair (Ito et al. 2005), immunoassay (Sincai et al. 2005), drug/gene delivery (Morishita et al. 2005), cell separation (Guedes et al. 2005), GMR sensor (Rife et al. 2003), etc. They can typically be manufactured as single domain or superparamagnetic ferrites (MeO·Fe2O3, where Me = Ni, Co, Mg, Zn, Mn, etc.), greigite (Fe3S4), maghemite (g-Fe2O3), and other forms of ferrites (Fe3O4). When exposed to a magnetic field, antibodies that have been marked with magnetic nanoparticles emit magnetic signals. As loose antibodies spread in all directions and do not provide a net magnetic signal, antibodies bound to targets can be distinguished from those that are not. A novel type of magnetic dextran microsphere (MDMS) was synthesized by Zhang et al. (2008a) by suspending cross-linking iron nanoparticles and dextran. Then, a GCE modified with MDMS was used to immobilize HRP. An amperometric H2O2 biosensor was created using the immobilized HRP-modified electrode and hydroquinone (HQ) as the mediator. A magnetic chitosan microsphere (MCMS) was created by Lai et al. (2008) utilizing carbon-coated MNPs and chitosan. With the help of glutaraldehyde cross-linking, hemoglobin (Hb) was successfully immobilized on the surface of MCMS-modified GCE.
In fact, numerous techniques have been created for detecting and counting specific micron-scale magnetic particles. The Maxwell bridge, frequency-dependent magnetometer, superconducting quantum interference device (SQUID), and magnetoresistance techniques are all used for direct detection of magnetic particle labels. The force-amplified biological sensor (FABS), a microcantilever-based device, and magnetic relaxation switches (MRS) are examples of indirect detection. A highly accurate, linear, and hysteresis-low giant magnetoresistance-spin valve (GMR-SV) biosensing device was recently created using photolithography (Park et al. 2008). For its detection and analysis, even a single drop of human blood or nanoparticles in distilled water provided enough signal.
2.4.2.7 Protein Chips
At the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, protein chips based on protein-binding silica nanoparticles are being developed (Stuttgart, Germany). Many different capture proteins can be arranged on the surface of a silica nanoparticle with a diameter of under 100 nm. The chips can be examined using cutting-edge mass spectrometry (MS)-matrix-assisted laser desorption/ionization (MALDI)-time-of-flight (TOF) MS after coming into contact with a sample. It is possible to identify bound proteins directly by knowing their masses (Hsu and Huang 2004).
2.4.2.8 Silicon Nanowires
Cui et al. announced the development of sensitive, real-time electrically based sensors for chemical and biological species using boron-doped silicon nanowires (SiNWs). The pH-dependent conductance of the SiNWs that had been functionalized with amine and oxide may be explained in terms of the change in surface charge during protonation and deprotonation. Streptavidin was detected using biotin-modified SiNWs down to at least a picomolar concentration range. Additionally, antigen-functionalized SiNWs demonstrated real-time concentration-dependent detection and reversible antibody binding. These semiconductor nanowires’ small size and capacity to detect a wide spectrum of chemical and biological species in real time without labels can be used in array-based screening and in vivo diagnostics (Cui et al. 2001).
In order to identify the presence of hazardous substances inside individual cells, Cullum et al. employed optical fibers with a distal-end diameter of less than 1 μm that were coated with antibodies. They were able to gauge the amount of benzo(a)pyrene tetrol (BPT) in both rat liver epithelial cells and human mammary cancer cells (Cullum et al. 2000).
2.4.2.9 Nanobarcodes
Nanobarcodes are metallic barcodes that are submicron in size and have stripes created by successive electrochemical deposition of metal ions. By using traditional light microscopy, it is possible to identify the striped patterns due to the varying reflectivities of neighboring stripes (Walton et al. 2002). As shown by DNA and protein bioassays, this readout process does not obstruct the use of fluorescence for the detection of analytes bound to particles by affinity capture. SNP mapping and multiplexed assays for protein diagnostics can both be done using nanobarcodes. These particles are valuable for bioanalytical analyses because their surfaces can be chemically altered. Larger numbers of striping patterns and more accurate identification will result from improvements in production and identification procedures.
2.4.2.10 Biomimic-Based Biosensors or Molecular Self Assembly
Molecular self-assembly is a crucial link between physics, chemistry, and biology that mirrors natural systems. Utilizing molecular self-assembly, new devices, materials, and architectures can be produced for biosensors (Boozer et al. 2003). Thin lipid films and liposomes are the self-assembled structures gaining the most attention in terms of biosensors. Lipid films and liposomes are made of phospholipids or other amphiphiles, just like a cell membrane is. They can spontaneously assemble structures due to their hydrophilic/hydrophobic properties. The supported bilayer lipid membrane (BLM) offers a suitable setting for embedding proteins, receptors, membrane/tissue fragments, and whole cells in a well-defined orientation and under nondenaturing circumstances. Because of this, BLMs are particularly appealing for use in biosensors. The ion channel switch biosensor described by Cornell et al. was a successful biomimetically built device based on BLMs (Cornell et al. 1997). Two gramicidin molecules were used to create ion channels in the membrane: one in the lower layer linked to a gold electrode and the other in the upper layer tethered to biological receptors like antibodies or nucleotides (Cornell et al. 2001). The supporting substrate for immobilizing the biorecognition molecules is commonly liposomes. Additionally, visual, sound wave, and electrochemical signals are amplified using liposomes (Baeumner et al. 2003).
2.4.2.11 Nanofabrication
Utilizing techniques developed exclusively for micromachining and integrated circuit manufacture, nanofabrication produces things with a nanoscale resolution. The four fundamental operations of photolithography, thin film growth/deposition, etching, and bonding are frequently used in combination during nanofabrication processes. By using impedimetric measurements, nanofabricated electrodes enable the detection of affinity binding of biomolecular structures (such as DNA or antigens). For instance, measuring the double-layer impedance might be used to keep track of the immobilization of glucose oxidase. Based on an ultrathin platinum film on an oxidized silicon base, a sensitive conductometric immunosensor has been shown (Pak et al. 2001). Researchers from IBM have also described using a microarray of cantilevers to simultaneously detect several unlabeled biomolecules at nanomolar concentrations in a matter of minutes (McKendry et al. 2002). This array allowed for numerous binding experiments to be run simultaneously and was capable of detecting femtomoles of DNA on the cantilever at a concentration of 75 nM in solution.
A force-amplified biological sensor (FABS) was created by the Naval Research Laboratory in the USA and is capable of identifying biological species like cells, proteins, poisons, and DNA at concentrations as low as 10−18 M (Baselt et al. 1996).
2.4.2.12 Ion Channel Switch (ICS) Biosensor Technology
The ion channel switch (ICSk), an advanced biosensor developed by Ambri Ltd. (Chatswood, Australia), is based on a synthetic membrane that self-assembles and functions like a biological switch to detect the presence of particular chemicals by igniting an electrical current (Wright and Harding 2000). By delivering precise and quantitative test results in an immediate time frame, the system reduces the time of emergency diagnoses from hours down to minutes.
2.4.2.13 Electronic-Based Nanobiosensors
The Biodetect™ technology detects the binding of a target DNA molecule to sensors on a microchip electronically. The target molecules connect two wires that are electrically isolated by forming a bridge. The chemical development of the attached target molecules into conductive DNA wires, which are metalized and visible by electron microscopy, produces a strong, clear signal. By analyzing the resistance or other electrical characteristics of the sensor, the bridges, which may be seen using fluorescence imaging techniques, can be quickly found. These DNA lines function like an on/off switch to “turn on” a sensor. Multiple sensors are present on each chip, and each sensor can be individually addressed using capture probes for various target DNA molecules from the same or other organisms (Jain 2005).
2.4.2.14 Viral Nanosensor
As a nanosensor for therapeutically important viruses, magnetic nanobeads have been constructed in response to HSV and adenovirus. The nanobeads have a dextran-coated supramagnetic iron oxide core (Perez et al. 2003). As a partner in the binding of antiviral antibodies, protein G is attached. Using a bifunctional linker, anti-HSV antibodies are directly attached to nanobeads in order to prevent protein G and medium components from interacting in an unintended way. As few as five virus particles can be captured by a magnetic field, and because it is less expensive, quicker, and detects fewer artifacts in a 10 mL blood sample than PCR-based detection methods, this system is more sensitive than ELISA-based approaches.
2.4.2.15 PEBBLE-Based Nanosensors
The nanosensors known as probes encapsulated by biologically localized embedding (PEBBLE) are spherical sensors with a size range of 20–200 nm that are made of sensor molecules trapped in a chemically inert matrix (Sumner et al. 2002). These sensors are immune to protein interference and can image ions and chemicals both inside and outside of cells in real time. The early identification of cancer is another application for PEBBLE. Additionally, PEBBLE nanosensors exhibit very quick reaction times, no protein interference, and excellent reversibility and resilience to leaching and photobleaching. They exhibit strong oxygen detecting ability in human plasma, unaffected by light scattering and autofluorescence (Cao et al. 2004).
2.4.2.16 Optical Biosensors
Many commercially available biosensors use the optical characteristics of lasers to track and measure interactions between biomolecules that take place on specifically designed surfaces or biochips. The most well-known instance of this technology is surface plasmon resonance. An optical-electrical phenomena called surface plasmon resonance (SPR) involves the interaction of light with the electrons of a metal. Researchers can profile and describe biomolecular interactions in parallel with the aid of the next-generation microarray-based SPR devices. There is an excellent demand for tiny optical sensors that can selectively detect low amounts of environmental and biological chemicals. There is currently no optical sensor that can identify the aforementioned species at naturally occurring concentrations without using amplification methods (Haes and Duyne 2004).
2.4.2.17 Laser Biosensors
A laser nanosensor works by firing laser light into a fiber, creating an evanescent field at the tip of fibers, and then using that field to excite target molecules attached to antibody molecules. The optical signal (such as fluorescence) coming from the analyte molecules or from the analyte-bioreceptor reaction is detected using a photometric detection device (Vo-Dinh 2005). Laser nanosensors can be used for in vivo analysis of proteins and biomarkers in individual living cells.
2.5 Applications of Nanobiosensors
Enhanced detection sensitivity and specificity are two benefits that have considerable promise in applications, including detecting DNA, RNA, proteins, glucose, pesticides, and other small molecules from clinical samples, food industry samples, and environmental sensing. The scopes of nanobiosensor are shown in Fig. 2.2.
2.5.1 Detection of Glucose
The glucose biosensor is frequently employed as a clinical diabetes indication. The performance of glucose sensors is greatly influenced by nanoscale materials as GNPs, CNTs, magnetic nanoparticles, platinum nanoparticles, quantum dots, etc. Fibrous morphology and wrapping polydiallyl dimethylammonium chloride (PDDA) over multiwalled carbon nanotubes (MWCNTs) cause a significant loading of glucose oxidase into the electrospun matrix (Manesh et al. 2008).
2.5.2 Detection of DNA and Protein
As optical biosensors, single-stranded DNA-CNT probes may be utilized to identify particular categories of DNA oligonucleotides (Cao et al. 2008). Single-stranded DNA probes can be efficiently immobilized on MWNTs/ZnO/chitosan composite film-modified glass carbon electrode (GCE) to distinguish between various DNA sequences (Zhang et al. 2008b). The bionanocomposite layer of MWNT in chitosan placed on a screen-printed carbon electrode (SPCE) is used to create a biosensor for the detection of deep DNA damage (Galandova et al. 2008). By utilizing the interactions between chemical substances and DNA, GNPs can also be utilized to identify DNA sequences. GNPs functionalized with alkanethiol-capped LNA/DNA chimaeras in a tail-to-tail hybridization mode might also perform well for single-stranded DNA (McKenzie et al. 2007), and these probes exhibit amazing discriminating between a complementary target and one that contains a single base mismatch. The first nanowire field-effect transistor-based biosensor, described by Maki et al. (2008), offers straightforward and susceptible electronic DNA methylation detection without the need for time-consuming bisulfite treatment or PCR amplification.
2.5.3 Detection of Other Molecules
Zhang et al. (2007) created a method for the detection of Escherichia coli (E. coli) utilizing modified bismuth nanofilm and flow injection analysis (FIA). A biochip sensor system made of two titanium (Ti) contact pads and a 150-nm-wide Ti nanowell device on a LiNbO3 substrate was created by Seo et al. (2008). A colorimetric assay was created by Medley et al. (2008) for the quick identification of sick cells. The selectivity and affinity of aptamers and the spectroscopic benefits of GNPs are combined in this test using aptamer-conjugated GNPs. While nontarget samples did not change color, samples with sick cells showed a clear color shift. One of the initial insults in diabetes has been theorized to be mitochondrial oxidative stress (MOS). According to some evidence, the antioxidant response element (ARE) and MOS are both more responsive to high blood sugar than each other (Prow et al. 2008). According to Li et al. (2007), an electrochemical aptamer biosensor for the detection of adenosine based on impedance spectroscopy measurement provides a label-free and reusable platform for making the detection of smaller molecules easy and effective.
2.5.4 In Immunohistochemistry
Using immunostaining of cytokeratin in skin basal carcinoma as an example, nanocrystal (NC)-antibody conjugates prepared using the method described can be utilized for the targeted detection of antigens in paraffin-embedded formaldehyde-fixed cancer tissue specimens. Thus, NC-Ab conjugates could be used as extremely sensitive, photostable markers for immune histochemical detection (Sukhanova et al. 2004).
2.5.5 Detection of Disease Biomarkers
The discovery of disease biomarkers, particularly in body fluid, is of great interest and is being researched extensively. Biomarker detection uses genomics, proteomics, and related technologies. Clinically significant prostate-specific antigen (PSA) concentrations can be found using microcantilever technology, even in the presence of other proteins. Due to the lack of labeling and the ability to complete the procedure in a single reaction, the technique is possibly more efficient and less complicated than other diagnostic techniques. False positives, which are frequently brought on by the nonspecific binding of other proteins, are less likely to occur. A biobarcode assay can detect PSA at a concentration of 30 attomolars since it allows for significant amplification (Nam et al. 2004).
As a biomarker for Alzheimer’s disease, the concentration of amyloid h-derived diffusible ligands (ADDLs) in the cerebrospinal fluid (CSF) was determined using the nanoparticle-based biobarcode assay (Kaul et al. 2003). While commercial enzyme-linked immunoassays (ELISA) can only identify ADDLs in brain tissue where the biomarker is most concentrated, this test can detect as few as 50 molecules of Aβ in the CSF of patients with Alzheimer’s disease (Georganopoulou et al. 2005).
2.5.6 Detection of Single Nucleotide Polymorphism
A microarray-based method for multiplex single nucleotide polymorphism (SNP) genotyping in the entire human genomic DNA is made possible by nanosphere’s ClearRead™ nanoparticle technology without the use of target amplification (Bao et al. 2005). The probe, a very sensitive gold nanoparticle, generates a strong signal precisely showing the presence of a particular target SNP, while one section detects any DNA changes.
2.5.7 Detection of Disease Genes
The identification of mutant genes linked to human disease can be done with DNA-based electrochemical sensors since they are sensitive, selective, and inexpensive. Electrochemical amplifications using nanoparticles are one of the innovations in this field (Drummond et al. 2003).
2.5.8 Detection of Microorganisms
2.5.8.1 Bacteria
Rapid and accurate pathogenic bacterial identification is crucial for medical diagnostics and bioterrorism defenses. Most standard diagnostic techniques have limitations, such as a lack of ultrasensitivity or a delay in results. Within 20 min, a single bacterium can be found using a bioconjugated nanoparticle-based bioassay for in situ pathogen quantification (Zhao et al. 2004). The nanoparticle is easily integrated into a biorecognition molecule, such as an antibody, and offers an extraordinarily strong fluorescent signal for bioanalysis. Through interaction and recognition between the antibody and the antigen, the antibody-conjugated nanoparticles can quickly and accurately identify a range of bacteria, including Escherichia coli O157:H7. By utilizing a 384-well microplate format, this technology can be applied to several bacterial samples with high throughput. The quick detection of mecA gene in methicillin-resistant Staphylococcus aureus genomic DNA samples has been carried out using the nanomaterial-based colorimetric assay, which enhances detection sensitivity by over four orders of magnitude when compared to a previously published absorbance-based technique (Storhoff et al. 2004). A change in the surface stress on the silicon nitride cantilever surface in situ upon bacterial adhesion allows for the detection of a small number of Salmonella enterica bacteria.
2.5.8.2 Viruses
Plaque tests, immunological assays, transmission electron microscopy, and PCR-based viral nucleic acid testing are examples of well-developed techniques for viral investigation. These techniques frequently call for a considerable degree of sample manipulation, which is problematic for infectious materials, and have not been successful in achieving quick detection at the single virus level. Nanowire field-effect transistors provide highly selective direct, real-time electrical detection of single virus particles (Patolsky et al. 2004). The presence of influenza A caused discrete conductance changes indicative of binding and unbinding, but not paramyxovirus or adenovirus, according to tests using nanowire arrays modified with anti-influenza A antibodies. The presence of 100 or more distinct viruses might be simultaneously screened by larger arrays of repeatable nanowire devices. Ultimately, research using nanowire devices modified with antibodies specific for either influenza or adenovirus has demonstrated the selective parallel detection of numerous viruses.
2.5.9 Cancer Diagnosis
Quantum dots (QD) bioconjugates are highly stable and luminous due to recent advancements. By enabling the imaging of cancer cells in living animals, these bioconjugates provide alternative opportunities for investigating genes, proteins, and therapeutic targets in single cells, tissue specimens, and even living organisms (Gao and Nie 2003). Magnetic microparticle probes with antibodies that mainly bind a target of interest, such as prostate-specific antigen (PSA) in the case of prostate cancer, have been used to develop a method for detecting protein analytes. For the immunofluorescent tagging of breast cancer markers, QDs covered with a polyacrylate cap and covalently coupled to antibodies or streptavidin have been employed (Wu et al. 2003). A method for investigating extravasation in vivo has been developed using QDs and emission spectrum scanning multiphoton imaging. Mice were intravenously injected with QD-labeled tumor cells, and their extravasation into lung tissue was monitored. In vivo, the behavior of tumor cells that had been QD-labeled was identical to that of unlabeled cells. Based on circulating cancer cells in the blood, magnetic nanoparticle-based assays are being developed to screen, diagnose, stage, and track cancer (Voura et al. 2004).
2.6 Challenges and Future Trends
2.6.1 Challenges
Applications of nanomaterials in biosensors have opened up new possibilities for creating a new era of biosensor technologies in recent years. Nanomaterial-based biosensors are progressing toward single-molecule biosensors and high throughput biosensor arrays, and they can enhance the mechanical, electrochemical, optical, and magnetic features of biosensors (Kerman et al. 2008). But they encounter numerous challenges, just like any newly developing field. The construction of single-molecule multifunctional nanocomposites, nanofilms, and nanoelectrodes requires the utilization of biomolecules, which have unique structures and functionalities, and nanomaterials. This presents a significant difficulty. Additionally, the mechanism governing the interaction of proteins with nanomaterials is still not fully elucidated. Another challenging task is figuring out how to innovate new, multifunctional, or homogeneous nanofilms or how to alter electrodes using these rules and principles of an optimal biosystem. Other significant challenges for the currently available techniques include processing, characterization, interface issues, the availability of high quality nanomaterials, tailoring of nanomaterials, and the mechanisms governing the behavior of these nanoscale composites on the surface of electrodes. For instance, it is very difficult to align nanomaterials like CNTs in a polymer matrix in the same direction. Another major difficulty is figuring out how to improve the signal-to-noise ratio as well as signal transduction and amplification. Due to their inability to meet the quality control requirements set by certifying organizations like the FDA, items based on nanotechnology are still only permitted in research and development environments. Since the current market price of nanomaterials is too high for any practical commercial application, there is an urgent need to create cost-effective and reproducible manufacturing procedures to lower the cost of producing nanomaterials. Nevertheless, these difficulties would soon be effectively solved because of ongoing technological advancements.
2.6.2 Future Trends
It is expected that nanotechnology-based biosensors will continue to advance and increase their application in many fields of the life sciences in the future years, particularly in the areas of biomedical diagnosis and drug delivery. These biosensors are anticipated to have some ideal characteristics for drug delivery applications, including high sensitivity, high specificity, quick response and action, low detection limit (so that a timely screening of clinically significant proteins and cancer markers is feasible), continuous and long-term monitoring capability, carrier of personalized medicine for site-specific and rate-controlled delivery, passive operation, and high specificity (possess the ability to communicate wirelessly with external monitoring devices). Some nanosensors are already developed, and they hold promise for detecting bioterrorism chemicals that are currently undetectable by molecular diagnostic methods. Nanodiagnostics will soon shorten the time spent waiting for test results. For instance, individuals with sexually transmitted diseases may provide a urine sample when they first visit an outpatient clinic or the office of the doctor, and the findings may be ready when they arrive for their appointment. The patient would have less time to worry, and the process would be less expensive if they were given the prescription right away.
The downsizing of biochip technology to the nano-range will continue to influence diagnostic trends in the future. Additionally, there is a growing desire to create diagnostic tools from the smallest building blocks. It remains to be seen if interest and use of nanomechanical detection will sustain over the long term. Another trend is to avoid fluorescent labeling since miniaturization weakens the signal. However, there have been advancements that make fluorescence with nanoparticles possible.
2.7 Conclusion
The creation of biosensors is being revolutionized by nanotechnology. More and more innovative biosensors are being created using nanomaterials and nanofabrication methods. The study of the many nanoeffects, which are peculiar to nanomaterials and are actually their most alluring feature (e.g., quantum size effect, micro size effect, surface effect, macro-quantum tunnel effect), is sadly receiving very little attention. For usage in biosensors, novel nanomaterials and nanostructures must be researched. Nanotechnology-based biosensors should ideally be built into tiny biochips with integrated electronics for handling and analyzing samples. By offering devices that are compact, portable, simple to use, inexpensive, disposable, and extremely flexible diagnostic devices, functionality will be considerably improved.
Aggregated biochips can be created by decreasing the size of the biosensor and the requirement for extremely small sample sizes that come from the usage of nanostructures. These systems are crucial for clinical diagnostic applications because they contain all the components required for sample presentation, sensing, and data processing. The use of biochip technology, which has a small sample size requirement, obviates the need for medical diagnostic laboratories, which use expensive equipment and waste time and money, and allows for the simultaneous analysis of multiple analytes in a single sample, enabling accurate and rapid diagnosis. These technology and tools will benefit patients by being less intrusive, making them more comfortable, improving the accuracy of sensing results, and improving the site, amount, and pace of controllable drug delivery.
References
Alivisatos P (2004) The use of nanocrystals in biological detection. Nat Biotechnol 22(1):47–52
Azamian BR, Davis JJ, Coleman KS, Bagshaw C, Green MLH (2002) Bioelectrochemical single walled carbon nanotubes. J Am Chem Soc 124(43):12664–12665
Baeumner AJ, Cohen RN, Miksic V, Min J (2003) RNA biosensor for the rapid detection of viable Escherichia coli in drinking water. Biosens Bioelectron 18:405–413
Bai J, Jiang X (2013) A facile one-pot synthesis of copper sulfide decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal Chem 85:8095–8101
Bai Y, Xu T, Zhang X (2020) Graphene-based biosensors for detection of biomarkers. Micromachines (Basel) 11(1):60
Bao YP, Huber M, Wei TF, Marla SS, Storhoff JJ, Muller UR (2005) SNP identification in unamplified human genomic DNA with gold nanoparticle probes. Nucleic Acids Res 33(2):e15
Baselt DR, Lee GU, Colton RJ (1996) Biosensor based on force microscope technology. J Vac Sci Technol B 14(2):789–793
Berger R, Delamarche E, Lang HP, Gerber C, Gimzewski JK, Meyer E et al (1997) Surface stress in the self-assembly of alkanethiols on gold. Science 276:2021–2023
Boisselier E, Astruc D (2009) Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 38:1759–1782
Boozer C, Yu Q, Chen S, Lee C, Homola J, Yee SS et al (2003) Surface functionalization for self-referencing surface plasmon resonance (SPR) biosensors by multi-step self-assembly. Sensors Actuators B Chem 90:22–30
Cao Y, Lee Koo YE, Kopelman R (2004) Poly(decyl methacrylate)-based fluorescent PEBBLE swarm nanosensors for measuring dissolved oxygen in biosamples. Analyst 129:745–750
Cao C, Kim JH, Yoon D, Hwang ES, Kim YJ, Baik S (2008) Optical detection of DNA hybridization using absorption spectra of single-walled carbon nanotubes. Mater Chem Phys 112:738–741
Chen RJ, Choi HC, Dai H (2004) An investigation of the mechanism of electronic sensing of protein adsorption on carbon nanotube devices. J Am Chem Soc 126:1563–1568
Cheng Y, Zhao L, Li Y, Xu T (2011) Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev 40:2673–2703
Cornell BA, Braach-Maksvytis VLB, King LG, Osman PDJ, Raguse B, Wieczorek L et al (1997) A biosensor that uses ion-channel switches. Nature 387:580–583
Cornell BA, Krishna G, Osman PD, Pace RD, Wieczorek L (2001) Tethered bilayer lipid membranes as a support for membrane-active peptides. Biochem Soc Trans 29:613
Cui Y, Wei Q, Park H, Lieber CM (2001) Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 293:1289–1292
Cullum B, Griffin G, Miller G, Vo-Dinh T (2000) Intracellular measurements in mammary carcinoma cells using fiberoptic nanosensors. Anal Biochem 277:25–32
Davis JJ, Coleman KS, Azamian BR, Bagshaw CB, Green MLH (2003) Chemical and biochemical sensing with modified single walled carbon nanotubes. Chem Eur J 9:3732–3739
Drummond TG, Hill MG, Barton JK (2003) Electrochemical DNA sensors. Nat Biotechnol 21:1192–1199
Fritz J, Cooper EB, Gaudet S, Sorger PK, Manalis SR (2002) Electronic detection of DNA by its intrinsic molecular charge. Proc Natl Acad Sci U S A 99:14142–14146
Galandova J, Ziyatdinova G, Labuda J (2008) Disposable electrochemical biosensor with multiwalled carbon nanotubes - chitosan composite layer for the detection of deep DNA damage. Anal Sci 24:711–716
Gao X, Nie S (2003) Molecular profiling of single cells and tissue specimens with quantum dots. Trends Biotechnol 21:371–373
Gao F, Yuan R, Chai Y, Chen S, Cao S, Tang M (2007) Amperometric hydrogen peroxide biosensor based on the immobilization of HRP on nano-Au/Thi/poly (p-aminobenzene sulfonic acid)-modified glassy carbon electrode. J Biochem Biophys Methods 70:407–413
Ge X, Asiri AM, Du D, Wen W, Wang S, Lin Y (2014) Nanomaterial-enhanced paper-based biosensors. TrAC Trends Anal Chem 58:31–39
Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL et al (2005) Nanoparticle based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc Natl Acad Sci U S A 102:2273–2276
Gholipour G, Zhang C, Mousavi AA (2020) Numerical analysis of axially loaded RC columns subjected to the combination of impact and blast loads. Eng Struct 219:110924
Guedes MHA, Sadeghiani N, Peixoto DLG, Coelho JP, Barbosa LS, Azevedo RB et al (2005) Effects of AC magnetic field and carboxymethyldextran-coated magnetite nanoparticles on mice peritoneal cells. J Magn Magn Mater 293:283–286
Haes AJ, Duyne RP (2004) Preliminary studies and potential applications of localized surface plasmon resonance spectroscopy in medical diagnostics. Expert Rev Mol Diagn 4:527–537
Han M, Gao X, Su JZ, Nie S (2001) Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol 19:631–635
He X, Yuan R, Chai Y, Shi Y (2008) A sensitive amperometric immunosensor for carcinoembryonic antigen detection with porous nanogold film and nano-Au/chitosan composite as immobilization matrix. J Biochem Biophys Methods 70:823–829
Hoshino A, Fujioka K, Manabe N, Yamaya SI, Goto Y, Yasuhara M et al (2005) Simultaneous multicolor detection system of the single-molecular microbial antigen with total internal reflection fluorescence microscopy. Microbiol Immunol 49:461–470
Hsu HY, Huang YY (2004) RCA combined nanoparticle-based optical detection technique for protein microarray: a novel approach. Biosens Bioelectron 20:123–126
Huang X, Zhu Y, Kianfar E (2021) Nano biosensors: properties, applications and electrochemical techniques. J Mater Res Technol 12:1649–1672
Ito A, Ino K, Kobayashi T, Honda H (2005) The effect of RGD peptide-conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials 26:6185–6193
Jain KK (2005) Nanotechnology in clinical laboratory diagnostics. Clin Chim Acta 358(1–2):37–54
Jaiswal JK, Goldman ER, Mattoussi H, Simon SM (2004) Use of quantum dots for live cell imaging. Nat Methods 1:73–78
Jares-Erijman EA, Jovin TM (2003) FRET imaging. Nat Biotechnol 21:1387–1395
Kairemo K, Erba P, Bergström K, Pauwels EKJ (2008) Nanoparticles in cancer. Curr Radiopharm 1:30–36
Kaul Z, Yaguchi T, Kaul SC, Hirano T, Wadhwa R, Taira K (2003) Mortalin imaging in normal and cancer cells with quantum dot immuno-conjugates. Cell Res 13:503–507
Kerman K, Saito M, Tamiya E, Yamamura S, Takamura Y (2008) Nanomaterial-based electrochemical biosensors for medical applications. TrAC Trend Anal Chem 27:585–592
Kianfar Farshad K, Reza MMS, Ehsan K (2015) Synthesis of spiro pyran by using silica-bonded N-propyldiethylenetriamine as recyclable basic catalyst. Indian J Sci Technol 8(11):68669
Kim DH, Lee SH, Kim KN, Kim KM, Shim IB, Lee YK (2005) Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application. J Magn Magn Mater 293:287–292
Koev ST, Dykstra PH, Luo X, Rubloff GW, Bentley WE, Payne GF et al (2010) Chitosan: an integrative biomaterial for lab-on-a-chip devices. Lab Chip 10:3026–3042
Lai GS, Zhang HL, Han DY (2008) A novel hydrogen peroxide biosensor based on haemoglobin immobilized on magnetic chitosan microspheres modified electrode. Sens Actuators B Chem 129:497–503
Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S (2006) Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc 128:7383–7389
Li B, Du Y, Wei H, Dong S (2007) Reusable, label-free electrochemical aptasensor for sensitive detection of small molecules. Chem Commun 36:3780–3782
Li G, Xu H, Huang W, Wang Y, Wu Y, Parajuli R (2008) A pyrrole quinoline quinone glucose dehydrogenase biosensor based on screen-printed carbon paste electrodes modified by carbon nanotubes. Meas Sci Technol 19:065203
Liu X, Tan W (1999) A fiber-optic evanescent wave DNA biosensor based on novel molecular beacons. Anal Chem 71:5054–5059
Liu Y, Zhu Y, Zeng Y, Xu F (2009) An effective amperometric biosensor based on gold nanoelectrode arrays. Nanoscale Res Lett 4(3):210–215
Lu W, Ji Z, Pfeiffer L, West KW, Rimberg AJ (2003) Real-time detection of electron tunneling in a quantum dot. Nature 423:422–425
Maki WC, Mishra NN, Cameron EG, Filanoski B, Rastogi SK, Maki GK (2008) Nanowire-transistor based ultra-sensitive DNA methylation detection. Biosens Bioelectron 23:780–787
Manesh KM, Kim HT, Santhosh P, Gopalan AI, Lee KP (2008) A novel glucose biosensor based on immobilization of glucose oxidase into multiwall carbon nanotubes-polyelectrolyte-loaded electrospun nanofibrous membrane. Biosens Bioelectron 23:771–779
McKendry R, Zhang J, Arntz Y, Strunz T, Hegner M, Lang HP et al (2002) Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc Natl Acad Sci U S A 99:9783–9788
McKenzie F, Faulds K, Graham D (2007) Sequence-specific DNA detection using high-affinity LNA-functionalized gold nanoparticles. Small 3:1866–1868
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4:435–446
Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan W (2008) Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem 80:1067–1072
Mertens J, Finota E, Thundat T, Fabrea A, Nadal MH, Eyraud V et al (2003) Effects of temperature and pressure on microcantilever resonance response. Ultramicroscopy 97:119–126
Morishita N, Nakagami H, Morishita R, Takeda SI, Mishima F, Terazono B et al (2005) Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem Biophys Res Commun 334:1121–1126
Muguruma H, Shibayama Y, Matsui Y (2008) An amperometric biosensor based on a composite of single-walled carbon nanotubes, plasma-polymerized thin film, and an enzyme. Biosens Bioelectron 23:827–832
Nam JM, Stoeva SI, Mirkin CA (2004) Bio-bar-code-based DNA detection with PCR-like sensitivity. J Am Chem Soc 126:5932–5933
Özbek MA, Yaşar A, Çete S, Er E, Erk N (2021) A novel biosensor based on graphene/platinum nanoparticles/Nafion composites for determination of glucose. J Solid State Electrochem 25:1601–1610
Pak SC, Penrose W, Hesketh PJ (2001) An ultrathin platinum film sensor to measure biomolecular binding. Biosens Bioelectron 16:371–379
Pandit S, Dasgupta D, Dewan N, Prince A (2016) Nanotechnology based biosensors and its application. Pharma Innov 5(6):18–25
Park SH, Soh KS, Hwang DG, Rhee JR, Lee SS (2008) Detection of magnetic nanoparticles and Fe-haemoglobin inside red blood cells by using a highly sensitive spin valve device. J Magnetics 13:30–33
Parveen S, Misra R, Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 8:147–166
Patolsky F, Zheng G, Hayden O et al (2004) Electrical detection of single viruses. Proc Natl Acad Sci U S A 101:14017–14022
Perez JM, Simeone FJ, Saeki Y, Josephson L, Weissleder R (2003) Viral-induced self-assembly of magnetic nanoparticles allows the detection of viral particles in biological media. J Am Chem Soc 125:10192–10193
Porter TL, Eastman MP, Macomber C, Delinger WG, Zhine R (2003) An embedded polymer piezoresistive microcantilever sensor. Ultramicroscopy 97:365–369
Prow TW, Bhutto I, Grebe R, Uno K, Merges C, McLeod DS et al (2008) Nanoparticle-delivered biosensor for reactive oxygen species in diabetes. Vis Res 48:478–485
Qian J, Feng S, Li Y, Tao T, Han J, Chen Q et al (2020) Single-shot absolute 3D shape measurement with deep-learning-based color fringe projection profilometry. Opt Lett 45(7):1842–1845
Rao LR, Zhang G (2006) Enhancing the sensitivity of SAW sensors with nanostructures. Curr Nanosci 2(4):311–318
Rife JC, Miller MM, Sheehan PE, Tamanaha CR, Tondra M, Whitman LJ (2003) Design and performance of GMR sensors for the detection of magnetic microbeads in biosensors. Sens Actuators A Phys 107:209–218
Sangadevan S, Periasamy M (2014) Recent trends in biosensors and their application. Rev Adv Mater Sci 36:62–69
Sashiwa H, Aiba SI (2004) Chemically modified chitin and chitosan as biomaterials. Prog Polym Sci 29:887–908
Satija J, Sai VVR, Mukherji S (2011) Dendrimers in biosensors: concepts and applications. J Mater Chem 21:14367–14386
Seo S, Dobozi King M, Young RF, Kish LB, Cheng M (2008) Patterning a nanowell sensor biochip for specific and rapid detection of bacteria. Microelectron Eng 85:1484–1489
Shen M, Shi X (2010) Dendrimer-based organic/inorganic hybrid nanoparticles in biomedical applications. Nanoscale 2:1596–1610
Sincai M, Ganga D, Ganga M, Argherie D, Bica D (2005) Antitumor effect of magnetite nanoparticles in cat mammary adenocarcinoma. J Magn Magn Mater 293:438–441
Sivasankar K, Rani KK, Wang SF, Devasenathipathy R, Lin CH (2018) Copper nanoparticle and nitrogen doped graphite oxide based biosensor for the sensitive determination of glucose. Nanomaterials (Basel) 8(6):429
Stenger DA, Gross GW, Keefer EW, Shaffer KM, Andreadis JD, Ma W et al (2001) Detection of physiologically active compounds using cell-based biosensors. Trends Biotechnol 19(8):304–309
Storhoff JJ, Lucas AD, Garimella V, Bao YP, Mqller UR (2004) Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat Biotechnol 22:883–887
Sukhanova A, Devy J, Venteo L et al (2004) Biocompatible fluorescent nanocrystals for immunolabeling of membrane proteins and cells. Anal Biochem 324:60–67
Sumner JP, Aylott JW, Monson E, Kopelman R (2002) A fluorescent PEBBLE nanosensor for intracellular free zinc. Analyst 127:11–16
Vashist SK, Venkatesh AG, Mitsakakis K, Czilwik G, Roth G, Stetten FV et al (2012) Nanotechnology-based biosensor and diagnostics: technology push versus industrial/healthcare requirements. BioNano Sci 2:115–126
Vo-Dinh T (2005) Optical nanosensors for detecting proteins and biomarkers in individual living cells. Methods Mol Biol 300:383–402
Vo-Dinh T, Cullum B (2000) Biosensors and biochips: advances in biological and medical diagnostics. Fresenius J Anal Chem 366:540–551
Vo-Dinh T, Cullum BM, Stokes DL (2001) Nanosensors and biochips: frontiers in biomolecular diagnostics. Sens Actuators B Chem 74:2–11
Voura EB, Jaiswal JK, Mattoussi H, Simon SM (2004) Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med 10:993–998
Walton ID, Norton SM, Balasingham A et al (2002) Particles for multiplexed analysis in solution: detection and identification of striped metallic particles using optical microscopy. Anal Chem 74:2240–2247
Wang M, Li Z (2008) Nano-composite ZrO2/Au film electrode for voltammetric detection of parathion. Sens Actuators B Chem 133:607–612
Wang H, Li S, Si Y, Zhang N, Sun Z, Wu H et al (2014) Platinum nanocatalysts loaded on graphene oxide dispersed carbon nanotubes with greatly enhanced peroxidase-like catalysis and electrocatalysis activities. Nanoscale 6:8107–8116
Wright LS, Harding H (2000) Detection of DNA via an ion channel switch biosensor. Anal Biochem 282:70
Wu X, Liu H, Liu J et al (2003) Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 21:41–46
Yang D, Cui D (2008) Advances and prospects of gold nanorods. Chem Asian J 3:2010–2022
Zhang G (2009) Nanotechnology-based biosensors in drug delivery. In: de Villiers MM, Aramwit P, Kwon GS (eds) Nanotechnology in drug delivery. Biotechnology: pharmaceutical aspects, vol X. Springer, New York, pp 163–189
Zhang W, Tang H, Geng P, Wang Q, Jin L, Wu Z (2007) Amperometric method for rapid detection of Escherichia coli by flow injection analysis using a bismuth nano-film modified glassy carbon electrode. Electrochem Commun 9:833–838
Zhang HL, Lai GS, Han DY, Yu AM (2008a) An amperometric hydrogen peroxide biosensor based on immobilization of horseradish peroxidase on an electrode modified with magnetic dextran microspheres. Anal Bioanal Chem 390:971–977
Zhang W, Yang T, Huang DM, Jiao K (2008b) Electrochemical sensing of DNA immobilization and hybridization based on carbon nanotubes/nano zinc oxide/chitosan composite film. Chin Chem Lett 19:589–591
Zhao Y-D, Zhang W-D, Chen H, Luo Q-M, Li SFY (2002) Direct electrochemistry of horseradish peroxidase at carbon nanotube powder microelectrode. Sens Actuators B Chem 87:168–172
Zhao X, Hilliard LR, Mechery SJ et al (2004) A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Natl Acad Sci U S A 101:15027–15032
Zhu H, Snyder M (2003) Protein chip technology. Curr Opin Chem Biol 7(1):55–63
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Patel, A., Patel, N.C., Patel, J.K., Amin, S. (2023). Nanotechnology-Based Biosensors in Medicine. In: Sheikh, F.A., Majeed, S., Beigh, M.A. (eds) Interaction of Nanomaterials With Living Cells. Springer, Singapore. https://doi.org/10.1007/978-981-99-2119-5_2
Download citation
DOI: https://doi.org/10.1007/978-981-99-2119-5_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2118-8
Online ISBN: 978-981-99-2119-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)