Abstract
Cells, mainly stem cells, possess immense potential in regenerative medicine. Stem cells (SCs) can also play a crucial role in treating diseases due to their remarkable ability of self-renewal, proliferation, and differentiation into germ layers or gastrula (i.e., internal layer (ectoderm), middle layer (mesoderm), and outer layer (endoderm)). In this regard, pluripotent stem cells like induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) can differentiate into all three germ layers. However, targeted cellular differentiation is not possible without a suitable induction environment, microenvironment, or substrate. Based on the reports, the group of polymers or biopolymers, bioactive compounds, and biomaterials can provide such a microenvironment or substrate and lead to an increase in the interactions of cell-to-cell and cell-to-substrate, better differentiation, and secretion of growth factors to accelerate tissue regeneration. These materials can synthesize by living organisms such as animals, bacteria, fungi or algae, and plants. In this field, polysaccharides (such as hyaluronic acid, galactans, poly-galactosamine, chitosan, etc.) and proteins (such as silk fibroin, silk sericin, collagen, etc.) are known as natural biomaterials or biopolymers. Moreover, polyphenols, flavonoids, mucilage, pectin, apigenin, quercetin, galangin, curcumin, etc. obtained from medicinal herbs are phytochemicals and bioactive compounds which can be led to an increase in cellular proliferation and differentiation, induction of cellular signaling, promotion of regeneration rate, and immunomodulation. Hence, the present chapter is aimed to study the applications and consequences of bioactive compounds and biomaterials on stem cell proliferation and differentiation, as well as to highlight their role in tissue regeneration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
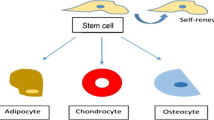
Stem cells are known as precursor biological cells that can be classified into totipotent,Footnote 1 pluripotent,Footnote 2 multipotent,Footnote 3 oligopotent,Footnote 4 and unipotentFootnote 5 stem cells, depending upon the differentiation capacities. These cells possess a high potential for self-renewal and differentiation into other cells, and as a promising resource for medicine, applications can play an important role in regenerative medicine or tissue engineering and cellular therapies (Chagastelles and Nardi 2011). However, applying stem cells to treat diseases and regenerate damaged tissues will be successful when suitable conditions are available to improve cellular interaction control and differentiate into the target cells (Izadyari Aghmiuni et al. 2021). Indeed, the creation of environments for therapeutic cloning that can well control cellular behavior can be the main factor in this regard (Fig. 13.1). Based on the reports, the use of biomaterials or biopolymers and bioactive compounds obtained from medicinal plants is considered a fundamental strategy for this purpose, due to their excellent bioactivity and role in the improvement of cellular biological response as well as in mimicking functional and structural properties of the target tissues (Yu et al. 2019). Such that, recent efforts in the design of biomaterial-based scaffolds, hydrogels, substrates, etc. (Chaudhari et al. 2016; Shafei et al. 2017; Ahmed 2013; Talebian et al. 2019; Zhao et al. 2020; Wang et al. 2018a) have provided significant opportunities to use these materials in regenerative medicine and generate novel substitutes for tissue engineering applications (Ramalingam et al. 2019; Aghmiuni et al. 2020).
There are biomaterials or bioactive materials with different natures which seem to be effective in targeting cell differentiation and tissue regeneration. Medicinal herbs are one of these bioactive materials gaining significant attention among researchers and scientific communities due to the promotion of cell proliferation and controlled differentiation (Izadyari Aghmiuni et al. 2020; Dey et al. 2010). The biopolymers similar to signaling molecules such as glycoproteins, proteoglycans, and glycosaminoglycans which exist in the network of the tissue extracellular matrix (ECM) are other samples from these biomaterials that can promote cell-cell signaling on the engineered substrates to modulate cellular functions (i.e., adhesion, proliferation, differentiation, and morphogenesis). Silk fibers (SF) are one of the biomaterials in this matter that owing to the amino acids of alanine and glycine can mimic the proteoglycans’ behavior in the tissue ECMs (Omenetto and Kaplan 2010). Moreover, hyaluronic acid (HA), as a linear polysaccharide and signaling molecule, can act like glycosaminoglycans of the ECM (Kogan et al. 2006; Volpi et al. 2009; Petrey and de la Motte 2014) and play a crucial role in cell-cell and cell-substrate interactions via communication between cell-surface receptors (Volpi et al. 2009; Lam et al. 2014; Dicker et al. 2014). According to the importance of subject and attention to personalized therapies in tissue engineering or regenerative medicine, the present chapter is aimed to assess the association of bioactive materials and biomaterials with cellular functions (such as cell signaling, proliferation, differentiation, migration, etc.) and their role in increasing effectiveness and safety of therapeutic methods and regenerating damaged tissues.
13.2 Proliferation and Differentiation Potential of Stem Cells
Stem cells are known as cells with extensive biological functions to differentiate into cell types, as well as to enable the growth, healing, or replacement of cells (Fig. 13.2) (Zakrzewski et al. 2019). These cells are found both in embryos (uncontrolled range of differentiation) and adult or somatic cells. Although adult stem cells possess a restricted range of differentiation, however, it is possible to reprogram these cells back to their pluripotent states or improve their capacity of self-renewal (Wu et al. 2018). Therefore, to be useful in therapy and be converted into target cells, as well as avoid teratoma formation, the creation of similar body conditions or imitation of the extracellular microenvironments plays an important role in controlling cell behavior (cellular interaction, proliferation, and differentiation). In this regard, understanding signaling pathways and identifying effective substances to improve these pathways are also the main factors in successful regenerative medicine. In the following, the different materials that can bring us closer to this aim, as well as their role in inducting cell signaling and improving proliferation and differentiation, have been mentioned.
13.3 Herbal Bioactive Compounds to Induce Differentiation of Stem Cells
The tremendous differentiation of stem cells is one of the desirable properties of these cells. However, this property can act as a double-edged sword, which means an increase in the risk of tumorigenicity. Thus, therapeutic strategies which led to the complete and irreversible differentiation of stem cells play an important role in controlling differentiation.
One of these strategies includes the use of biomaterials or bioactive materials to design scaffolds, substrates, or tissue substitutes (Izadyari Aghmiuni and Heidari Keshel 2022; Aghmiuni et al. 2022). This approach can provide suitable physicomechanical properties to differentiate stem cells and lead to a sustained delivery and local of bioactive molecules for tissue regeneration. Indeed, bioactive compounds or biomaterials play the main role in providing the cell differentiation microenvironment, so that they can control cellular functions and interactions via creating an ideal substrate. In this matter, Izadyari Aghmiuni’s research has shown that the phytochemical materials in herbs possess high therapeutic and regenerative effects (Izadyari Aghmiuni et al. 2020). However, their physical forms like gel, mucilage, and the extract or essential oil from different parts of the plants cannot be applied on damaged tissue (such as the bone, skin, cartilage, blood vessels, nerve, cornea, tendon, etc.) directly. This research team showed that quince seed mucilage is one of the bioactive components that can turn an engineered scaffold into a smart biological substrate. They stated that this herbal bioactive material is classified into the group of polysaccharide and composed of xylose(glucuronoxylan) and glucuronic acid; however, swell in water due to the existence of hydrophilic groups (i.e., amino, hydroxyl, amide, and carboxylic acid), unlike some of the polysaccharides such as chitosan which suspended in the acidic solvent. The results of this study indicated that quince seed mucilage-based hybrid scaffolds can create a better porous network compared to chitosan-based scaffolds. Moreover, these scaffolds support the proliferation of dermal fibroblasts and provide high water absorption capacity for the scaffold. Based on this research, the combination of quince seed mucilage and PEG leads to an increase in the transduction of mechanical force-induced signals and improves the biological signals to induce stem cell differentiation (with the same differentiation patterns) into targeted cells such as keratinocytes.
Another study in this regard is Aloe vera-based scaffolds that possess wide applications in biomedical and pharmaceutical sciences. The popularity of these herbal polysaccharides is inducted by their bioactive components (i.e., anthraquinones, anthrones, carbohydrates, proteins, vitamins, etc.) that lead to antimicrobial, anti-inflammatory, and antiviral properties, antioxidant effects, as well as immunomodulatory, neo-angiogenesis, and tissue repair effects (Darzi et al. 2021; Rahman et al. 2017). In this regard, Kallyanashis Paul et al. reported that Aloe vera-based hydrogel could alleviate maternal birth injuries (Paul et al. 2021). Based on this study, Aloe vera-alginate hydrogels can be an immediate treatment by delivering stem cells to regenerate damaged tissue. The results of this research team showed that local injection of hydrogel with/without stem cells can be significantly effective in repairing birth injuries, so that it leads to the improvement of elastin content and smooth muscle. They stated that such hydrogels can be a suitable therapeutic strategy for preventing pelvic organ prolapse or healing birth injuries.
The electrospun mesh of Aloe vera is another sample that can act as a transdermal therapeutic factor. Suganya et al. showed that fibers of poly(caprolactone) containing Aloe vera powder (10 wt%) possess better hydrophilic properties along with higher tensile strength (6.28 MPa) and elastic modulus similar to skin tissue, as well as more desirable cell proliferation, secretion of collagen, and expression of F-actin compared to polycaprolactone-collagen fibers (Suganya et al. 2014a). Based on the reports of Tahmasebi et al., nanofibrous scaffolds of poly(3-hydroxybutyrate-co-3-hydroxy valerate) blended with Aloe vera gel can be also useful for applications of bone tissue engineering (Tahmasebi et al. 2020). The results of this study indicated the higher biocompatibility of the mentioned nanofibrous scaffold when blended with Aloe vera. Moreover, alkaline phosphatase activity, amount of mineralization, and expression of bone-related genes or proteins increase compared to Aloe vera-free nanofibrous scaffold. They stated that Aloe vera gel possesses the osteoinductive potential and can be used for acceleration of bone regeneration as a bio-implant.
According to the reports, the use of Aloe vera, along with other herbal bioactive compounds, can accelerate wound healing processes. In this matter can mention to hybrid nanofibrous scaffolds based on Aloe vera and curcumin were designed by Ezhilarasu et al. to increase their synergistic effects on the proliferation of fibroblasts and antimicrobial activities (Ezhilarasu et al. 2019).
The study of Oryan et al. demonstrates that adipose-derived stem cell-loaded Aloe vera hydrogels can be also effective in the burn wound models (Oryan et al. 2019). The results of this study showed that hydrogels containing Aloe vera and stem cells can significantly increase the rate of burn wound healing, lead to improvement of angiogenesis and reepithelialization, as well as decrease TGF-β1Footnote 6 and interleukin-1β levels. Urtica dioica L. (nettle) is one of the other herbs in this matter that can be led to osteogenic differentiation when blended with biomaterials such as silk fibroin. Zadegan et al. indicated that silk fibroin-nettle nano-fiber possesses better water uptake and cellular attachment as well as higher cellular proliferation than that of silk fibroin nano-fiber. Moreover, nettle-based nano-fibers can express both early and late markers of osteoblast differentiation (Zadegan et al. 2019).
Urtica dioica, along with ZnO nanoparticles, can also provide the synergy effects for the increase of antibacterial activities in the electrospun scaffolds. Ghiyasi et al. stated that incorporation of Urtica dioica and ZnO nanoparticles to poly(caprolactone) scaffolds can be led to an increase in the tensile strength up to 2.54 MPa and improvement of water uptake ability and promotion of fibroblast L929 cell proliferation (Ghiyasi et al. 2021). Another study relates to the induction of periosteal cell differentiation and proliferation by Urtica dioica extract. According to the report of Bing et al., the extract of this herb led to an increase in alkaline phosphatase and calcium nodule levels, as well as induction of osteoblast differentiation and proliferation on the polyclonal lactone scaffolds (Xu and Liu 2018).
Moreover, the study of Hajiali et al. showed that nanofibrous dressings based on sodium alginate-lavender essential oil possess high efficacy for the treatment of UVB-induced skin burns with antibacterial activities (Hajiali et al. 2016). These dressings are also able to decrease and control the inflammatory responses induced in the skin fibroblasts due to UVB exposure. Some reports showed animal fats could also increase the differentiation and proliferation of stem cells. In this regard, the design of emu oil-based electrospun nanofibrous by Pilehvar-Soltanahmadi et al. illustrates that emu oil not only promotes differentiation and proliferation of adipose tissue-derived stem cells into keratinocytes but also can lead to increase in cell adherence and cytoprotection (Pilehvar-Soltanahmadi et al. 2017). Such that, it can be a suitable candidate to fabricate wound dressings and/or bio-engineered substitutes containing stem cells for skin tissue repair.
There are many studies on this matter and some examples are listed in Table 13.1.
Specifically, studies on herbal bioactive compounds have illustrated that herb-derived materials not only can increase the proliferation and differentiation of adult stem cells but also inhibit the proliferation of cancer cells (Saud et al. 2019; Olatunbosun et al. 2012; Kornicka et al. 2017; Potu et al. 2009; Gao et al. 2013). However, studies have shown that the proliferation and/or differentiation ability of stem cells is influenced by the doses of the stimulant compounds. It means that a specific dose of herbal extracts can promote stem cell proliferation and induce its differentiation into the targeted cell. In this regard, we can refer to Zhang’s study on the effects of naringin on human bone mesenchymal stem cell proliferation and osteogenic differentiation (Zhang et al. 2009a). Zhang et al. showed that 1–100 μg/mL concentrations of extract from this citrus lead to an increase in the proliferation of human BM-MSCs and their osteogenic differentiation, while, 200 μg/mL concentration can decrease the growth of these cells. Similar research in this field is related to the study of Yu et al. They showed that naringin in 50 μg/mL concentration can activate the Notch signaling pathway and lead to stimulation of osteogenic differentiation of BMSCs.Footnote 7 However, higher concentrations than 100 μg/mL can suppress the proliferation rate of these cells (Yu et al. 2016).
Zhang et al. also reported that naringin can induce osteogenic activity and differentiation of canine bone marrow stromal cells (Zhang et al. 2021). To this end, they added different concentrations of this bioactive to the mentioned cells. Their results showed that the 10−6 mol/L concentration can promote cell proliferation and be led to an increase in cellular proliferation and calcium nodules, as well as induction of bone marrow stromal cell differentiation into osteoblasts.
Beom Su Kim et al. indicated that the extract of brown algae Laminaria japonica (fucoidan) in 0.1–10 μg/mL concentrations can lead to JNK- and ERKFootnote 8-dependent BMP2Footnote 9–Smad 1/5/8 signaling and induces osteoblast differentiation of human mesenchymal stem cells (Kim et al. 2015). They also found that this osteogenesis bioactive can increase ALP activities and accumulation of calcium and leads to the expression of the osteoblast-specific gene.
Moreover, MaríaSatué et al. reported that some flavonoids lead to the stimulation of MC3T3-E1 cell differentiation into osteoblast and inhibition of osteoclastogenesis in RAW 264.7 cells (Satué et al. 2013). The results of this study illustrated that doses greater than 100 μM of diosmetin, galangin, and chrysin, as well as 500 μM doses of taxifolin, possess a toxic effect on cells. However, quercitrin with safe doses of 200 and 500 μM and taxifolin in safe doses of 100 and 200 μM can induce osteocalcin mRNA and bone sialoprotein expression and lead to higher osteocalcin levels. Fei Li et al. also showed that the echinacoside as phenylethanoid glycosides can promote bioactivities of cell line MC3T3-E1 (i.e., proliferation, differentiation, and mineralization of osteoblastic) (Li et al. 2012). To this end, this bioactive component isolated from Cistanches Herba stems and the amount of the secretion of osteoprotegerin, osteocalcin, and collagen I were evaluated. Based on this study’s results, concentrations between 0.01 and 10 nmol/L can significantly promote cell proliferation, osteocalcin levels, and collagen I content can lead to an increase in the mineralization of osteoblastic. They stated that echinacoside possesses a stimulatory effect on the formation of osteoblastic bone and can potentially be effective against osteoporosis.
Likewise, Hui-HuiXiao et al. observed that vanillic acid isolated from Sambucus williamsii Hance possesses estrogen-like activity in the rat UMR 106 cells that can be due to MAPFootnote 10 kinase (MEK/ERK)-mediated specific estrogen receptor (ER) antagonist signaling pathways (Xiao et al. 2014). The results of this study showed that this phenolic acid stimulates the mentioned cell proliferation and alkaline phosphatase (ALP) activity. Vanillic acid can also increase Runx2,Footnote 11 osteocalcin, and the ratio of osteoprotegerin-receptor activator of nuclear factor kB ligand [i.e., OPG-RANKL] mRNA expression.
Kim’s study is one of the other samples hereof (Kim et al. 2014). Kim et al. illustrated that kirenol extracted from Herba Siegesbeckia can promote osteoblast differentiation via activation of the BMPs and Wnt/β-catenin signaling pathways in MC3T3-E1 cells. Moreover, this natural diterpenoid compound can lead to the promotion of mineralization and ALP activity, as well as the increase in osteopontin, collagen I, and expression of OPG/RANKL. Puerarin and phytoestrogens isolated from Pueraria mirifica are the other bioactive compounds in this field that can lead to an increase in cellular proliferation and the expression of osteoblastic differentiation markers in osteoblast-like UMR106 cells (Tiyasatkulkovit et al. 2012). Such that, Tiyasatkulkovit et al. indicated that puerarin can increase the mRNA expression of ALP, as well as lead to a decrease in the mRNA expression of RANKL and induction of bone gain via increasing osteoblast differentiation in rat osteoblast-like UMR106 cells and suppressing osteoclast functions. They stated that puerarin can induce the differentiation of osteoblast rather than the proliferation of osteoblast in the estrogen receptor-dependent manner; hence, it can be the appropriate option to prevent and treat postmenopausal osteoporosis. Choi et al. stated that honokiol isolated from the bark of Magnolia officinalis not only can stimulate osteoblast MC3T3-E1 cell function (i.e. increase in cell growth, improvement of ATP activity, promotion of collagen synthesis and glutathione content, and release of osteoprotegerin in the cells) but also decrease or inhibit the production of bone-resorbing mediators. They suggested that this phenolic compound can be effective in natural therapies for osteoporosis (Choi 2011).
There are many studies on stem cell differentiation into the progenitor cells of endothelial or vascular, cardiomyocyte, neuronal, osteogenic, neurogenic, etc. that their stimulation factor is bioactive components extracted from herbs. Indeed, these bioactive components can not only promote cellular proliferation and differentiation but also decrease the time required for tissue regeneration. Table 13.2 illustrates some effective herbs in this regard.
13.4 Biomaterials and Their Characteristic to Design Ideal Substrates and Induce Cell Differentiation
The ideal substrates possess different characteristics which originate from their materials and lead to the targeted differentiation of stem cells (Discher et al. 2005).
Regardless of the tissue type, some of the key characteristics of bio- or active material-based substrates when designing and determining the suitability of the scaffolds for regenerative medicine applications include biocompatibility, mechanical properties, biodegradability, the architecture of the scaffold, and manufacturing technology. One of the requirements of a scaffold to regenerate tissue is biocompatible properties; according to Williams, “The biocompatibility of a scaffold or matrix for a tissue engineering product refers to the ability to perform as a substrate that will support the appropriate cellular activity” (Williams 2008). Indeed, the scaffold structure must possess suitable stimuli or recognizable stimuli by cells to colonize scaffolds and regenerate (Parisi et al. 2018). In other words, the cells must first adhere to the scaffold, proliferate, differentiate, and then migrate onto the surface of the scaffold. Finally, differentiated cells should proliferate on the scaffold to create a new matrix (Fig. 13.3).
In this regard, natural polymers are considered one of the main candidates that not only can increase cell biological activities on the scaffold (i.e., adhesion or attachment, spreading, proliferation, differentiation, and migration) but also imitate the structure and function of the native extracellular matrix (ECM) and lead to controlled induction of cell functions (Izadyari Aghmiuni et al. 2020; Izadyari Aghmiuni and Heidari Keshel 2022). Indeed, biomaterials are agents that can provide an ideal microenvironment with suitable physicomechanical and physicochemical properties to mimic the structure of the ECM network and functions of targeted cells and tissues (Izadyari Aghmiuni et al. 2021; Izadyari Aghmiuni and Heidari Keshel 2022). Collagen is one of these biopolymers that is found in most soft and hard tissues such as the blood vessels, nerves, cornea, skin, tendon, cartilage, bone, etc. Given that, this biomaterial leads to maintaining the structural and biological integrity of the native ECM network and can help in the physiological functions of the engineered scaffolds as dynamic material (Barnes et al. 2007; Sell et al. 2010). Moreover, low antigenicity, low cytotoxic and inflammatory response, and biodegradability are other properties of this biomaterial (Farach-Carson et al. 2007; Sell et al. 2007, 2009). The study of Shih et al. indicates that electrospun collagen fibrous mat can promote growth, proliferation, adhesion, motility, and osteogenic differentiation of human MSCsFootnote 12 (Mano et al. 2007).
Many reports have shown that the blends of collagen with other biomaterials can increase the proliferation rate of cells and be used for regeneration templates in different tissues (Aghmiuni et al. 2020; Izadyari Aghmiuni et al. 2020). In this matter, Zhong et al. showed that collagen glycosaminoglycan (GAG)-based blended scaffolds can be led to an increase in the proliferation of dermal fibroblasts (FB) (Zhong et al. 2005). Moreover, the substrates based on chondroitin sulfate-collagen, collagen-nanohydroxyapatite, as well as aggrecan (chondroitin sulfate, dermatan sulfate, keratin sulfate)-collagen can be applied in the regenerating skin, bone, and cartilage, respectively (Choi et al. 2004; Thomas et al. 2007). Based on Chen’s report, scaffolds of collagen/chitosan-PEO (as wound dressing) possessed good in vitro biocompatibility and led to the promotion of 3T3 FBs (Chen et al. 2008). In another study, collagen-chitosan scaffolds also illustrated an increase in the proliferation of smooth muscle cells (SMCs) and endothelial cells (ECs) (Chen et al. 2010). Indeed, mixing collagen with other biopolymers can lead to a decrease in the dimensions of the fibers or an increase in the porosity and tensile strength of the scaffold and consequently promotion of cellular attachment or adhesion on the scaffold surface (Barnes et al. 2007).
Gelatin also is known as an attractive biopolymer for tissue engineering applications due to its biological and biomechanical similarities to collagen (Zhang et al. 2005, 2009b; Heydarkhan-Hagvall et al. 2008). Although fibers of this biopolymer possess higher tensile moduli compared to collagen fibers, however, its gelation at room temperature and dissolution as colloidal-sol at 37 °C or > are major drawbacks of this biomaterial which is often solved via combining with other natural or synthetic polymers (Sell et al. 2010). Given the gelatin’s similarity to collagen, electrospun gelatin-based blended scaffolds have been developed to apply in regenerative medicine (Zhang et al. 2005, 2009b; Heydarkhan-Hagvall et al. 2008; Li et al. 2005, 2006a; Gauthaman et al. 2009; Gupta et al. 2009a, b; Songchotikunpan et al. 2008; Song et al. 2008; Gui-Bo et al. 2010). The electrospun gelatin-polyurethane and poly (ɛ-caprolactone)-gelatin scaffolds are the samples of these substrates that can be used in wound healing and skin regeneration (Chong et al. 2007; Kim et al. 2009). Based on the reports, such scaffolds can lead to better migration of fibroblasts and decrease therapeutic costs. Poly(ɛ-caprolactone)-gelatin scaffolds can also act as a positive factor for supporting neurite outgrowth and nerve differentiation, proliferation, and nerve regeneration (Ghasemi-Mobarakeh et al. 2008). Likewise, conductive nanofibrous scaffold of polyaniline-poly(ɛ-caprolactone)/gelatin can lead to nerve stem cell attachment and proliferation, as well as neurite outgrowth via electrical stimulation of scaffold (Ghasemi-Mobarakeh et al. 2009). Moreover, an electrospun composite based on the synthetic polypeptide-gelatin can lead to the induction of calcium phosphate mineralization and be used as dental biomaterials or a biocompatible substitute for the regeneration of the hard tissue ECM (Ohkawa et al. 2009). Nanohydroxyapatite-gelatin nanofibrous scaffolds are one of the other substrates that are capable of imitation of natural ECM of bone tissue along with effective mineralization, the proliferation of osteoblasts, and successful bone regeneration (Francis et al. 2010).
Indeed, electrospun gelatin or gelatin-based scaffolds can be also used in the regeneration of cardiac tissue. According to the study of Li et al., the combination of gelatin with conductive polymers such as polyaniline leads to the improvement of cardiac myoblast attachment or adhesion, spreading, and migration, as well as an increase in cellular proliferation on the scaffold, modulus, and tensile strength (Li et al. 2006b). Studies have shown that blended gelatin with natural and synthetic polymers can significantly improve mechanical properties and facilitate the excellent proliferation of cardiac myoblasts. In this field, Li et al. indicated that gelatin composites composed of poly(lactic-co-glycolic acid) and α-elastin can be effective in soft tissue engineering applications, such as heart, blood vessels, and lung (Li et al. 2006c). Such composites possess low average diameters; however, upon hydration of the scaffold, the average diameter increases due to the swelling of fibers, without disintegrating the scaffold.
Elastin is one of the main biomaterials in this regard that naturally is found in the wall of many tissues such as vessel walls; hence, the use of fibrous structures of this biopolymer in tissue engineering applications can be favorable (Daamen et al. 2007; Bailey et al. 2003; Deborde et al. 2016). Based on the studies, elastin or elastin-like polypeptides can be effective in the regeneration of cartilage, heart valves, and skin tissues (Neuenschwander and Hoerstrup 2004; Nettles et al. 2010; McHale et al. 2005; Betre et al. 2002). Wang et al. showed that collagen and elastin as the main components of ECM in tissues can improve the proliferation of valve interstitial cells, when used as the engineered 2D or 3D substrates (Wang et al. 2018b). Moreover, such substrates constitute effective tools to engineer 3D tissues and increase endothelial-mesenchymal transition. Chen et al. also illustrated that bilayer collagen-elastin scaffolds play an important role in mimicking the mechanical and biological activities of heart valves (Chen et al. 2013b). Chitosan/γ-poly(glutamic acid) scaffolds modified by albumin, elastin, and poly-l-lysine are another sample of elastin-based scaffold application in cartilage tissue engineering (Kuo et al. 2017). The study of Kuo et al. shows that such a scaffold can provide an effective approach to inhibition of chondrocyte apoptosis and lead to promotion in the growth of chondrocytes, secretion of ECM, and improvement of cartilaginous tissue regeneration. Moreover, the existence of single bond –NH3+ and single bond –COO− in the structure of this scaffold plays an important role in its porous morphology and interconnected network, as well as the mechanical properties of the scaffold.
Chitosan is one of the important polysaccharides in the design of tissue-engineered scaffolds due to the structural similarity to the glycosaminoglycans (GAGs) of ECM of tissues (Huang et al. 2015c). The functional groups of hydroxyl (–OH) and amine (–NH) in this biopolymer can link to other materials to make a new scaffold (He et al. 2011; Du et al. 2016). In this regard, Izadyari Aghmiuni et al. stated that polyethylene glycol-chitosan-poly(ɛ-caprolactone) copolymers containing collagen can be considered as base bio-composites for the design of dermal substrates. Such substrates can imitate dermis properties and lead to the induction of keratinocyte differentiation from stem cells (Izadyari Aghmiuni et al. 2021; Aghmiuni et al. 2020). The design of injectable chitosan-based hydrogels is another example in this field that can be used in cartilage tissue regeneration (Jin et al. 2009). Jin et al. showed that the hydrogels of chitosan-glycolic acid/phloretic acid that are designed via enzymatic crosslinking can increase chondrocyte proliferation and then be degraded by a hydrolytic enzyme such as lysozyme. Likewise, chitosan hydrogels modified by cartilaginous ECM are another type of hydrogel that can be used in cartilage tissue engineering applications (Choi et al. 2014). Mori et al. also stated that spongelike dressings containing chitosan and sericin can treat chronic skin ulcers. Such a substrate possesses suitable mechanical resistance and high hydration, along with cellular proliferation and antioxidant activities on human fibroblast cells (Mori et al. 2016).
Hyaluronic acid (HA) also is a linear polysaccharide and is classified in the group of glycosaminoglycans of native ECMs and can maintain the structural integrity of tissue ECMs when used in the structure of engineered scaffolds (Izadyari Aghmiuni et al. 2021; Kogan et al. 2006; Volpi et al. 2009; Petrey and de la Motte 2014). This biomaterial possesses various biological activities (like modulation of immune cell function, synthesis of proteoglycan, reduction of pro-inflammatory cytokine activities, etc.) and can act as the signaling factor for improvement of cell-to-cell and cell-to-scaffold interactions and acceleration of tissue regeneration (Izadyari Aghmiuni et al. 2021; Lam et al. 2014; Li et al. 2018). Based on the study of Shirzaei Sani et al., HA/elastin-like polypeptide-based hydrogels can act as antimicrobial substrates for tissue engineering applications (Shirzaei Sani et al. 2018). This study illustrated that these hydrogels possess high adhesive strength to attach to the tissue and can support cellular spreading, growth, and proliferation. Izadyari et al. also demonstrated that control of HA content can provide a microporous environment along with higher tensile strength and modulus (Izadyari Aghmiuni et al. 2021). According to the reports, given that hyaluronic acid plays an important role in osmotic balance and regulation of tissue hydration, HA-based substrates can significantly mimic the physical and mechanical properties of ECM (Chan and Tayama 2002).
Silk fibroin (SF) is another biopolymer that can play an important role in cell attachment and spreading when mixed with collagen (Yeo et al. 2008). This biomaterial also possesses high elasticity, resistance to failure (under compression), minimal inflammatory response, slow degradation rate, as well as high biocompatibility, toughness, and strength (Pérez-Rigueiro et al. 2001; Liu et al. 2007; Zhang et al. 2009c). Notably, the existence of hydrophobic domains in the protein’s random coil network of this biomaterial has led to the formation of a β-sheet structure, which is responsible for the elastic properties and tensile strength of SF (Aghmiuni et al. 2020; Zhang et al. 2009c). This property plays a crucial role in the scaffold structure and improves the module and stress strain of scaffolds (Zhang et al. 2010). Moreover, the β-sheet structures of SF can affect the biodegradation rate of the scaffold, so that the biodegradation rate of the scaffold matches with the rate of tissue repair (Izadyari Aghmiuni et al. 2021; Sell et al. 2010; Alessandrino et al. 2008; Bayraktar et al. 2005; Liu et al. 2008; Silva et al. 2008). Hence, silk-based scaffolds can be designed for the ligament, bone, and vascular skin applications (Izadyari Aghmiuni et al. 2020, 2021).
Generally, in our opinion, the features of these biomaterials and other bio- or active materials can provide an exciting opportunity to design engineered hybrid/composite substrates with a high ability to differentiate stem cells and regenerate tissue.
13.5 Future Prospective and Conclusion
Nowadays, the advancement in the field of phyto-sciences, technologies, and the development of studies on herbal extracts have revealed their excellent repairing properties and role in regenerative medicine. Based on the studies, herbal bioactive compounds or active ingredients can play a crucial role in the process of tissue regeneration. Such that, the cellular and molecular researches on herbal extracts indicate that herbs possess positive effects on the promotion, proliferation, and differentiation of types of stem cells. It can be due to the existence of flavonoids; coumarins; glycosides; terpenoids such as mono-terpenoids, sesquiterpenoids, and diterpenoids; as well as anthraquinones, phenolic acids, diarylheptanoids, phenols, and tetrameric stilbene. Hence, if protocols or methods can be created via the use of herbal extracts to proliferate and differentiate stem cells into targeted cells in damaged tissues, it will offer hope to cure many incurable diseases. In this field, knowledge of herbs and the effects of their extracts and therapeutic doses can play a crucial role in the determination of suitable therapeutic methods, such as the design of substrates, encapsulation, gel, etc. Moreover, the studies of tissue engineering demonstrate that a combination of modern and traditional medicine (i.e., use of biomaterials along with herbal bioactive compounds) can provide new developments in the fields of regenerative tissue techniques and stem cell differentiation into targeted tissue cells. This not only can decrease the therapeutic economic burden and healthcare problems but also develop new drugs or methods with easier availability and least/no side effect.
Notes
- 1.
Totipotent is embryonic stem cells of 1–3 days (e.g., zygotes)—differentiation into any cell types.
- 2.
Pluripotent is embryonic stem cells of 5–14 days (e.g., blastula) and induced pluripotent stem cells (iPSCs)—differentiation into cells from any of the three germ layers.
- 3.
Multipotent is adult stem cells—differentiation into a limited range of cell types such as neural stem cells, epithelial stem cells, hematopoietic stem cells, etc.
- 4.
Oligopotent is adult stem cells—differentiation into a limited number of cell types such as neural progenitor cells, myeloid stem cells, lymphoid stem cells, etc.
- 5.
Unipotent is adult stem cells—differentiation into single cell type such as gut cell, erythrocytes, neurons, etc.
- 6.
Transforming growth factor-β1.
- 7.
Bone marrow stromal cells.
- 8.
Extracellular signal-regulated protein.
- 9.
Bone morphogenetic protein.
- 10.
Mitogen-activated protein.
- 11.
Runt-related transcription factor 2.
- 12.
Bone marrow-derived mesenchymal stem cells.
References
Aghmiuni AI, Baei MS, Keshel SH, Khiyavi AA (2020) Design of novel 3D-scaffold as a potential material to induct epidermal-dermal keratinocytes of human-adipose-derived stem cells and promote fibroblast cells proliferation for skin regeneration. Fibers Polym 21:33–44. https://doi.org/10.1007/s12221-020-9402-1
Aghmiuni AI, Ghadi A, Azmoun E, Kalantari N, Mohammadi I, Kordmahaleh HH (2022) Electrospun polymeric substrates for tissue engineering: viewpoints on fabrication, application, and challenges. In: Electrospinning—material technology of the future. InTech, pp 1–26
Agnes Mary S, Giri Dev VR (2015) Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J Textile Inst 106:886–895. https://doi.org/10.1080/00405000.2014.951247
Ahmed LA (2013) Stem cells and cardiac repair: alternative and multifactorial approaches. J Regen Med Tissue Eng 2:10–7243
Alessandrino A, Marelli B, Arosio C, Fare S, Tanzi MC, Freddi G (2008) Electrospun silk fibroin mats for tissue engineering. Eng Life Sci 8:219–225
Asghari F, Rabiei Faradonbeh D, Malekshahi ZV, Nekounam H, Ghaemi B, Yousefpoor Y, Ghanbari H, Faridi-Majidi R (2022) Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr Polym 278:118926. https://doi.org/10.1016/j.carbpol.2021.118926
Azadian Z, Shafiei M, Hosseini S, Kazemi J, Alipour A, Shahsavarani H (2019) Efficient in vitro differentiation of adipose tissue-derived mesenchymal stem cells into the cardiomyocyte using plant-derived natural compounds. Proc Singap Natl Acad Sci 13:47–63. https://doi.org/10.1142/s2591722619400064
Azizsoltani A, Piri K, Behzad S, Soleimani M, Nekouei M, Mahmoudi Z, Kazemi A (2018) Ethyl acetate extract of licorice root (Glycyrrhiza glabra) enhances proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells. Iran J Pharm Res 17:1057–1067
Bailey MT, Pillarisetti S, Xiao H, Vyavahare NR (2003) Role of elastin in pathologic calcification of xenograft heart valves. J Biomed Mater Res A 66:93–102. https://doi.org/10.1002/jbm.a.10543
Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL (2007) Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev 59:1413–1433
Bayraktar O, Malay Ö, Özgarip Y, Batıgün A (2005) Silk fibroin as a novel coating material for controlled release of theophylline. Eur J Pharm Biopharm 60:373–381
Betre H, Setton LA, Meyer DE, Chilkoti A (2002) Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules 3:910–916. https://doi.org/10.1021/bm0255037
Chagastelles PC, Nardi NB (2011) Biology of stem cells: an overview. Kidney Int Suppl 1:63–67. https://doi.org/10.1038/kisup.2011.15
Chan RW, Tayama N (2002) Biomechanical effects of hydration in vocal fold tissues. Otolaryngol Head Neck Surg 126:528–537
Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, Singh SR, Pillai SR (2016) Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci 17:1974. https://doi.org/10.3390/ijms17121974
Chen J-P, Chang G-Y, Chen J-K (2008) Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf A Physicochem Eng Asp 313:183–188
Chen ZG, Wang PW, Wei B, Mo XM, Cui FZ (2010) Electrospun collagen–chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater 6:372–382
Chen JJ, Zhang NF, Mao GX, He XB, Zhan YC, Deng HB, Song DQ, Li DD, Li ZR, Si SY, Qiu Q, Wang Z (2013a) Salidroside stimulates osteoblast differentiation through BMP signaling pathway. Food Chem Toxicol 62:499–505. https://doi.org/10.1016/j.fct.2013.09.019
Chen Q, Bruyneel A, Carr C, Czernuszka J (2013b) Bio-mechanical properties of novel bi-layer collagen-elastin scaffolds for heart valve tissue engineering. Proc Eng 59:247–254. https://doi.org/10.1016/j.proeng.2013.05.118
Choi EM (2011) Honokiol isolated from Magnolia officinalis stimulates osteoblast function and inhibits the release of bone-resorbing mediators. Int Immunopharmacol 11:1541–1545. https://doi.org/10.1016/j.intimp.2011.05.011
Choi JS, Lee SW, Jeong L, Bae S-H, Min BC, Youk JH, Park WH (2004) Effect of organosoluble salts on the nanofibrous structure of electrospun poly (3-hydroxybutyrate-Co-3-hydroxyvalerate). Int J Biol Macromol 34:249–256
Choi B, Kim S, Lin B, Wu BM, Lee M (2014) Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl Mater Interfaces 6:20110–20121. https://doi.org/10.1021/am505723k
Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S, Lim CT (2007) Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater 3:321–330. https://doi.org/10.1016/j.actbio.2007.01.002
Daamen WF, Veerkamp JH, Van Hest JCM, Van Kuppevelt TH (2007) Elastin as a biomaterial for tissue engineering. Biomaterials 28:4378–4398
Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, Xiao Z (2007) Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 14:806–814. https://doi.org/10.1016/j.phymed.2007.04.003
Darzi S, Paul K, Leitan S, Werkmeister JA, Mukherjee S (2021) Immunobiology and application of aloe vera-based scaffolds in tissue engineering. Int J Mol Sci 22:1–19. https://doi.org/10.3390/ijms22041708
Daubney J, Bonner PL, Hargreaves AJ, Dickenson JM (2015) Cardioprotective and cardiotoxic effects of quercetin and two of its in vivo metabolites on differentiated H9c2 cardiomyocytes. Basic Clin Pharmacol Toxicol 116:96–109. https://doi.org/10.1111/bcpt.12319
Deborde C, Simionescu DT, Wright C, Liao J, Sierad LN, Simionescu A (2016) Stabilized collagen and elastin-based scaffolds for mitral valve tissue engineering. Tissue Eng A 22:1241–1251. https://doi.org/10.1089/ten.tea.2016.0032
Dey SK, Banerjee D, Chattapadhyay S, Karmakar KB (2010) Antimicrobial activities of some medicinal plants of West Bengal. Int J Pharm Bio Sci 1:1–10
Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X (2014) Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10:1558–1570. https://doi.org/10.1016/j.actbio.2013.12.019
Discher DE, Janmey P, Wang Y (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143
Don MJ, Lin LC, Chiou WF (2012) Neobavaisoflavone stimulates osteogenesis via p38-mediated up-regulation of transcription factors and osteoid genes expression in MC3T3-E1 cells. Phytomedicine 19:551–561. https://doi.org/10.1016/j.phymed.2012.01.006
Du T, Chen Z, Li H, Tang X, Li Z, Guan J, Liu C, Du Z, Wu J (2016) Modification of collagen-chitosan matrix by the natural crosslinker alginate dialdehyde. Int J Biol Macromol 82:580–588. https://doi.org/10.1016/j.ijbiomac.2015.10.039
Ezhilarasu H, Ramalingam R, Dhand C, Lakshminarayanan R, Sadiq A, Gandhimathi C, Ramakrishna S, Bay BH, Venugopal JR, Srinivasan DK (2019) Biocompatible aloe vera and tetracycline hydrochloride loaded hybrid nanofibrous scaffolds for skin tissue engineering. Int J Mol Sci 20:5174. https://doi.org/10.3390/ijms20205174
Farach-Carson MC, Wagner RC, Kiick KL (2007) Extracellular matrix: structure, function, and applications to tissue engineering. In: Tissue engineering. CRC Press, pp 35–56
Francis L, Venugopal J, Prabhakaran MP, Thavasi V, Marsano E, Ramakrishna S (2010) Simultaneous electrospin-electrosprayed biocomposite nanofibrous scaffolds for bone tissue regeneration. Acta Biomater 6:4100–4109. https://doi.org/10.1016/j.actbio.2010.05.001
Fu SZ, Meng XH, Fan J, Yang LL, Wen QL, Ye SJ, Lin S, Wang BQ, Chen LL, Wu JB, Chen Y, Fan JM, Li Z (2014) Acceleration of dermal wound healing by using electrospun curcumin-loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) fibrous mats. J Biomed Mater Res B Appl Biomater 102:533–542. https://doi.org/10.1002/jbm.b.33032
Gao L-N, An Y, Lei M, Li B, Yang H, Lu H, Chen F-M, Jin Y (2013) The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets. Biomaterials 34:9937–9951
Gauthaman K, Venugopal JR, Yee FC, Peh GSL, Ramakrishna S, Bongso A (2009) Nanofibrous substrates support colony formation and maintain stemness of human embryonic stem cells. J Cell Mol Med 13:3475–3484
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani M-H, Ramakrishna S (2008) Electrospun poly(ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 29:4532–4539. https://doi.org/10.1016/j.biomaterials.2008.08.007
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S (2009) Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng A 15:3605–3619. https://doi.org/10.1089/ten.tea.2008.0689
Ghaseminezhad K, Zare M, Lashkarara S, Yousefzadeh M, Aghazadeh Mohandesi J (2020) Fabrication of Althea officinalis loaded electrospun nanofibrous scaffold for potential application of skin tissue engineering. J Appl Polym Sci 137:48587. https://doi.org/10.1002/app.48587
Ghiyasi Y, Salahi E, Esfahani H (2021) Synergy effect of Urtica dioica and ZnO NPs on microstructure, antibacterial activity and cytotoxicity of electrospun PCL scaffold for wound dressing application. Mater Today Commun 26:102163. https://doi.org/10.1016/j.mtcomm.2021.102163
Godinho JF, Berti FV, Müller D, Rambo CR, Porto LM (2016) Incorporation of aloe vera extracts into nanocellulose during biosynthesis. Cellulose 23:545–555. https://doi.org/10.1007/s10570-015-0844-3
Gong CY, Wu QJ, Wang YJ, Zhang DD, Luo F, Zhao X, Wei YQ, Qian ZY (2013) A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 34:6377–6387. https://doi.org/10.1016/j.biomaterials.2013.05.005
Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R (2004) Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 25:1911–1917. https://doi.org/10.1016/S0142-9612(03)00625-2
Gui-Bo Y, You-Zhu Z, Shu-Dong W, De-Bing S, Zhi-Hui D, Wei-Guo F (2010) Study of the electrospun PLA/silk fibroin-gelatin composite nanofibrous scaffold for tissue engineering. J Biomed Mater Res A 93:158–163
Gupta D, Venugopal J, Prabhakaran MP, Dev VRG, Low S, Choon AT, Ramakrishna S (2009a) Aligned and random nanofibrous substrate for the in vitro culture of schwann cells for neural tissue engineering. Acta Biomater 5:2560–2569
Gupta D, Venugopal J, Mitra S, Dev VRG, Ramakrishna S (2009b) Nanostructured biocomposite substrates by electrospinning and electrospraying for the mineralization of osteoblasts. Biomaterials 30:2085–2094
Hajiali H, Summa M, Russo D, Armirotti A, Brunetti V, Bertorelli R, Athanassiou A, Mele E (2016) Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J Mater Chem B 4:1686–1695
He L, Mu C, Shi J, Zhang Q, Shi B, Lin W (2011) Modification of collagen with a natural cross-linker, procyanidin. Int J Biol Macromol 48:354–359. https://doi.org/10.1016/j.ijbiomac.2010.12.012
Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, Shemin R, Beygui RE, MacLellan WR (2008) Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 29:2907–2914
Huang Q, Gao B, Jie Q, Wei BY, Fan J, Zhang HY, Zhang JK, Li XJ, Shi J, Luo ZJ, Yang L, Liu J (2014) Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone 66:306–314. https://doi.org/10.1016/j.bone.2014.06.010
Huang Q, Shi J, Gao B, Zhang HY, Fan J, Li XJ, Fan JZ, Han YH, Zhang JK, Yang L, Luo ZJ, Liu J (2015a) Gastrodin: an ancient Chinese herbal medicine as a source for anti-osteoporosis agents via reducing reactive oxygen species. Bone 73:132–144. https://doi.org/10.1016/j.bone.2014.12.059
Huang Q, Gao B, Wang L, Zhang HY, Li XJ, Shi J, Wang Z, Zhang JK, Yang L, Luo ZJ, Liu J (2015b) Ophiopogonin D: a new herbal agent against osteoporosis. Bone 74:18–28. https://doi.org/10.1016/j.bone.2015.01.002
Huang R, Li W, Lv X, Lei Z, Bian Y, Deng H, Wang H, Li J, Li X (2015c) Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 53:58–75. https://doi.org/10.1016/j.biomaterials.2015.02.076
Izadyari Aghmiuni A, Heidari Keshel S (2022) The role of the extracellular matrix (ECM) and ECM-like polymeric substrates in health and disease. In: Berhardt LV (ed) Advances in medicine and biology. NOVA Medicine and Health, pp 145–175
Izadyari Aghmiuni A, Heidari Keshel S, Sefat F, Akbarzadeh Khiyavi A (2020) Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and H-ASCs differentiation into keratinocytes. Int J Biol Macromol 142:668–679. https://doi.org/10.1016/j.ijbiomac.2019.10.008
Izadyari Aghmiuni A, Heidari Keshel S, Sefat F, AkbarzadehKhiyavi A (2021) Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater Sci Eng C 120:111752. https://doi.org/10.1016/j.msec.2020.111752
Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, Feijen J (2009) Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 30:2544–2551. https://doi.org/10.1016/j.biomaterials.2009.01.020
Jin G, Prabhakaran MP, Kai D, Annamalai SK, Arunachalam KD, Ramakrishna S (2013) Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 34:724–734. https://doi.org/10.1016/j.biomaterials.2012.10.026
Kant V, Gopal A, Pathak NN, Kumar P, Tandan SK, Kumar D (2014) Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int Immunopharmacol 20:322–330
Kihara T, Ichikawa S, Yonezawa T, Lee JW, Akihisa T, Woo JT, Michi Y, Amagasa T, Yamaguchi A (2011) Acerogenin A, a natural compound isolated from Acer nikoense Maxim, stimulates osteoblast differentiation through bone morphogenetic protein action. Biochem Biophys Res Commun 406:211–217. https://doi.org/10.1016/j.bbrc.2011.02.017
Kim SE, Heo DN, Lee JB, Kim JR, Park SH, Jeon SH, Kwon IK (2009) Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed Mater 4. https://doi.org/10.1088/1748-6041/4/4/044106
Kim MB, Song Y, Hwang JK (2014) Kirenol stimulates osteoblast differentiation through activation of the BMP and Wnt/β-catenin signaling pathways in MC3T3-E1 cells. Fitoterapia 98:59–65. https://doi.org/10.1016/j.fitote.2014.07.013
Kim BS, Kang HJ, Park JY, Lee J (2015) Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp Mol Med 47:1–9. https://doi.org/10.1038/emm.2014.95
Kogan G, Šoltés L, Stern R, Gemeiner P (2006) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29:17–25. https://doi.org/10.1007/s10529-006-9219-z
Kongros K (2012) The effects of seed extract of Mucuna gigantea on the expression of neural markers in mesenchymal stem cells. J Med Plants Res 6:1297–1303. https://doi.org/10.5897/jmpr11.1406
Kornicka K, Kocherova I, Marycz K (2017) The effects of chosen plant extracts and compounds on mesenchymal stem cells—a bridge between molecular nutrition and regenerative medicine-concise review. Phytother Res 31:947–958
Krishnan R, Rajeswari R, Venugopal J, Sundarrajan S, Sridhar R, Shayanti M, Ramakrishna S (2012) Polysaccharide nanofibrous scaffolds as a model for in vitro skin tissue regeneration. J Mater Sci Mater Med 23:1511–1519. https://doi.org/10.1007/s10856-012-4630-6
Kuo YC, Ku HF, Rajesh R (2017) Chitosan/γ-poly(glutamic acid) scaffolds with surface-modified albumin, elastin and poly-L-lysine for cartilage tissue engineering. Mater Sci Eng C 78:265–277. https://doi.org/10.1016/j.msec.2017.04.067
Lam J, Truong NF, Segura T (2014) Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater 10:1571–1580. https://doi.org/10.1016/j.actbio.2013.07.025
Lee YS, Choi EM (2011a) Costunolide stimulates the function of osteoblastic MC3T3-E1 cells. Int Immunopharmacol 11:712–718. https://doi.org/10.1016/j.intimp.2011.01.018
Lee YS, Choi EM (2011b) Apocynin stimulates osteoblast differentiation and inhibits bone-resorbing mediators in MC3T3-E1 cells. Cell Immunol 270:224–229. https://doi.org/10.1016/j.cellimm.2011.05.011
Lee SU, Shin HK, Min YK, Kim SH (2008) Emodin accelerates osteoblast differentiation through phosphatidylinositol 3-kinase activation and bone morphogenetic protein-2 gene expression. Int Immunopharmacol 8:741–747. https://doi.org/10.1016/j.intimp.2008.01.027
Lee SR, Kwak JH, Park DS, Pyo S (2011) Protective effect of kobophenol A on nitric oxide-induced cell apoptosis in human osteoblast-like MG-63 cells: involvement of JNK, NF-κB and AP-1 pathways. Int Immunopharmacol 11:1251–1259. https://doi.org/10.1016/j.intimp.2011.04.004
Lee CH, Huang YL, Liao JF, Chiou WF (2012a) Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur J Pharmacol 676:26–33. https://doi.org/10.1016/j.ejphar.2011.12.001
Lee J, Roh KB, Kim SC, Lee J, Park D (2012b) Soy peptide-induced stem cell proliferation: involvement of ERK and TGF-β1. J Nutr Biochem 23:1341–1351. https://doi.org/10.1016/j.jnutbio.2011.08.003
Leishi L (2005) Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J 118:1987–1993
Leishi L (2007) Icariine stimulates proliferation and differentiation of human osteoblasts by increasing production of bone morphogenetic protein 2. Chin Med J 120:204–210
Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI (2005) Electrospun protein fibers as matrices for tissue engineering. Biomaterials 26:5999–6008
Li J, He A, Zheng J, Han CC (2006a) Gelatin and gelatin-hyaluronic acid nanofibrous membranes produced by electrospinning of their aqueous solutions. Biomacromolecules 7:2243–2247
Li M, Guo Y, Wei Y, MacDiarmid AG, Lelkes PI (2006b) Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 27:2705–2715. https://doi.org/10.1016/j.biomaterials.2005.11.037
Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI (2006c) Elastin blends for tissue engineering scaffolds. J Biomed Mater Res A 79:963–973. https://doi.org/10.1002/jbm.a
Li F, Yang Y, Zhu P, Chen W, Qi D, Shi X, Zhang C, Yang Z, Li P (2012) Echinacoside promotes bone regeneration by increasing OPG/RANKL ratio in MC3T3-E1 cells. Fitoterapia 83:1443–1450. https://doi.org/10.1016/j.fitote.2012.08.008
Li Q, Niu Y, Xing P, Wang C (2018) Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin Med (United Kingdom) 13:1–11. https://doi.org/10.1186/s13020-018-0166-0
Liao MH, Tai YT, Cherng YG, Liu SH, Chang YA, Lin PI, Chen RM (2014) Genistein induces oestrogen receptor-α gene expression in osteoblasts through the activation of mitogen-activated protein kinases/NF-κB/activator protein-1 and promotes cell mineralisation. Br J Nutr 111:55–63. https://doi.org/10.1017/S0007114513002043
Liu H, Ge Z, Wang Y, Toh SL, Sutthikhum V, Goh JCH (2007) Modification of sericin-free silk fibers for ligament tissue engineering application. J Biomed Mater Res B Appl Biomater 82:129–138
Liu L, Liu J, Wang M, Min S, Cai Y, Zhu L, Yao J (2008) Preparation and characterization of nano-hydroxyapatite/silk fibroin porous scaffolds. J Biomater Sci Polym Ed 19:325–338
Machado AK, Cadoná FC, Azzolin VF, Dornelles EB, Barbisan F, Ribeiro EE, Mânica-Cattani MF, Duarte MMMF, Saldanha JRP, da Cruz IBM (2015) Guaraná (Paullinia cupana) improves the proliferation and oxidative metabolism of senescent adipocyte stem cells derived from human lipoaspirates. Food Res Int 67:426–433. https://doi.org/10.1016/j.foodres.2014.11.056
Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP (2007) Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 4:999–1030
McHale MK, Setton LA, Chilkoti A (2005) Cartilaginous tissue repair. Tissue Eng 11:1768–1779
Mendi AH, Gökçınar Yağcı B, Sarac N, Kızıloğlu M, Yılmaz D, Uçkan D (2017) Effect of Ocimum basilicum on mesenchymal stem cell proliferation and differentiation: does the effect change according to niches? Int J Second Metab 4:1–10. https://doi.org/10.21448/ijsm.356244
Merrell JG, Mclaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS, Phil M, Exp C, Author PP (2009) Curcumin loaded poly(ε-caprolactone) nanofibers: diabetic wound dressing with antioxidant and anti-inflammatory properties NIH public access author manuscript. Clin Exp Pharmacol Physiol 36:1149–1156. https://doi.org/10.1111/j.1440-1681.2009.05216.x.Curcumin
Mitra T, Manna PJ, Raja STK, Gnanamani A, Kundu PP (2015) Curcumin loaded nano graphene oxide reinforced fish scale collagen-a 3D scaffold biomaterial for wound healing applications. RSC Adv 5:98653–98665. https://doi.org/10.1039/c5ra15726a
Mo Y, Guo R, Zhang Y, Xue W, Cheng B, Zhang Y (2017) Controlled dual delivery of angiogenin and curcumin by electrospun nanofibers for skin regeneration. Tissue Eng A 23:597–608. https://doi.org/10.1089/ten.tea.2016.0268
Mori M, Rossi S, Ferrari F, Bonferoni MC, Sandri G, Chlapanidas T, Torre ML, Caramella C (2016) Sponge-like dressings based on the association of chitosan and sericin for the treatment of chronic skin ulcers. I. Design of experiments–assisted development. J Pharm Sci 105:1180–1187. https://doi.org/10.1016/j.xphs.2015.11.047
Nettles DL, Chilkoti A, Setton LA (2010) Applications of elastin-like polypeptides in tissue engineering. Adv Drug Deliv Rev 62:1479–1485. https://doi.org/10.1016/j.addr.2010.04.002
Neuenschwander S, Hoerstrup SP (2004) Heart valve tissue engineering. Transpl Immunol 12:359–365. https://doi.org/10.1016/j.trim.2003.12.010
Nguyen VC, Nguyen VB, Hsieh MF (2013) Curcumin-loaded chitosan/gelatin composite sponge for wound healing application. Int J Polym Sci 2013. https://doi.org/10.1155/2013/106570
Ohkawa K, Hayashi S, Kameyama N, Yamamoto H, Yamaguchi M, Kimoto S, Kurata S, Shinji H (2009) Synthesis of collagen-like sequential polypeptides containing O-phospho-L-hydroxyproline and preparation of electrospun composite fibers for possible dental application. Macromol Biosci 9:79–92. https://doi.org/10.1002/mabi.200800122
Olatunbosun LO, Caxton-Martin A, Jimoh OR, Biliaminu SA, Ghazal OK (2012) Proliferative effect of aqueous extracts of Parquetina nigrescens on haemopoietic multipotent stem cells in irradiated guinea pigs. J Appl Pharm Sci 2:108–111
Omenetto FG, Kaplan DL (2010) New opportunities for an ancient material. Science (New York, N.Y.) 329:528–531. https://doi.org/10.1126/science.1188936
Oryan A, Alemzadeh E, Mohammadi AA, Moshiri A (2019) Healing potential of injectable aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res 377:215–227. https://doi.org/10.1007/s00441-019-03015-9
Parisi L, Toffoli A, Ghiacci G, Macaluso GM (2018) Tailoring the interface of biomaterials to design effective scaffolds. J Funct Biomater 9:1–31. https://doi.org/10.3390/jfb9030050
Paul K, Darzi S, Del Borgo MP, Cousins FL, Werkmeister JA, Gargett CE, Mukherjee S (2021) Vaginal delivery of tissue engineered endometrial mesenchymal stem/stromal cells in an aloe vera-alginate hydrogel alleviates maternal simulated birth injury. Appl Mater Today 22:100890. https://doi.org/10.1016/j.apmt.2020.100890
Pérez-Rigueiro J, Elices M, Llorca J, Viney C (2001) Tensile properties of silkworm silk obtained by forced silking. J Appl Polym Sci 82:1928–1935
Petrey AC, de la Motte CA (2014) Hyaluronan, a crucial regulator of inflammation. Front Immunol 5:1–14. https://doi.org/10.3389/fimmu.2014.00101
Pilehvar-Soltanahmadi Y, Nouri M, Martino MM, Fattahi A, Alizadeh E, Darabi M, Rahmati-Yamchi M, Zarghami N (2017) Cytoprotection, proliferation and epidermal differentiation of adipose tissue-derived stem cells on emu oil based electrospun nanofibrous mat. Exp Cell Res 357:192–201. https://doi.org/10.1016/j.yexcr.2017.05.015
Potu BK, Bhat KMR, Rao MS, Nampurath GK, Chamallamudi MR, Nayak SR, Muttigi MS (2009) Petroleum ether extract of Cissus quadrangularis (Linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics 64:993–998
Rahman S, Carter P, Bhattarai N (2017) Aloe vera for tissue engineering applications. J Funct Biomater 8:6
Ramalingam V, Raja S, Sundaramahalingam S, Rajaram R (2019) Chemical fabrication of graphene oxide nanosheets attenuates biofilm formation of human clinical pathogens. Bioorg Chem 83:326–335
Ranjbar-Mohammadi M, Rabbani S, Bahrami SH, Joghataei MT, Moayer F (2016) Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(ε-caprolactone) electrospun nanofibers. Mater Sci Eng C 69:1183–1191. https://doi.org/10.1016/j.msec.2016.08.032
Rezaei Moghadam M, Jafarinasab M-R, Yousefi Z, Sanjari Moghaddam A, Memarzadeh H, Rezaei Kanavi M (2020) Aloe vera gel-derived eye drops for alkaline corneal injury in a rabbit model. J Ophthal Vis Res. https://doi.org/10.18502/jovr.v15i1.5932
Salehi M, Farzamfar S, Bastami F, Tajerian R (2016) Fabrication and characterization of electrospun PLLA/collagen nanofibrous scaffold coated with chitosan to sustain release of aloe vera gel for skin tissue engineering. Biomed Eng Appl Basis Commun 28:1650035. https://doi.org/10.4015/S1016237216500356
Satué M, Arriero MDM, Monjo M, Ramis JM (2013) Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem Pharmacol 86:1476–1486. https://doi.org/10.1016/j.bcp.2013.09.009
Saud B, Malla R, Shrestha K (2019) A review on the effect of plant extract on mesenchymal stem cell proliferation and differentiation. Stem Cells Int 2019. https://doi.org/10.1155/2019/7513404
Sell S, Barnes C, Smith M, McClure M, Madurantakam P, Grant J, Mcmanus M, Bowlin G (2007) Extracellular matrix regenerated: tissue engineering via electrospun biomimetic nanofibers. Polym Int 56:1349–1360
Sell SA, McClure MJ, Garg K, Wolfe PS, Bowlin GL (2009) Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv Drug Deliv Rev 61:1007–1019
Sell SA, Wolfe PS, Garg K, McCool JM, Rodriguez IA, Bowlin GL (2010) The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers 2:522–553. https://doi.org/10.3390/polym2040522
Shafei S, Foroughi J, Stevens L, Wong CS, Zabihi O, Naebe M (2017) Electroactive nanostructured scaffold produced by controlled deposition of PPy on electrospun PCL fibres. Res Chem Intermed 43:1235–1251
Shanmugavel S, Reddy VJ, Ramakrishna S, Lakshmi B, Dev VG (2014) Precipitation of hydroxyapatite on electrospun polycaprolactone/aloe vera/silk fibroin nanofibrous scaffolds for bone tissue engineering. J Biomater Appl 29:46–58. https://doi.org/10.1177/0885328213513934
Sharmila G, Muthukumaran C, Kirthika S, Keerthana S, Kumar NM, Jeyanthi J (2020) Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int J Biol Macromol 156:430–437. https://doi.org/10.1016/j.ijbiomac.2020.04.059
Shirzaei Sani E, Portillo-Lara R, Spencer A, Yu W, Geilich BM, Noshadi I, Webster TJ, Annabi N (2018) Engineering adhesive and antimicrobial hyaluronic acid/elastin-like polypeptide hybrid hydrogels for tissue engineering applications. ACS Biomater Sci Eng 4:2528–2540. https://doi.org/10.1021/acsbiomaterials.8b00408
Silva SS, Maniglio D, Motta A, Mano JF, Reis RL, Migliaresi C (2008) Genipin-modified silk-fibroin nanometric nets. Macromol Biosci 8:766–774
Song J-H, Kim H-E, Kim H-W (2008) Production of electrospun gelatin nanofiber by water-based co-solvent approach. J Mater Sci Mater Med 19:95–102
Songchotikunpan P, Tattiyakul J, Supaphol P (2008) Extraction and electrospinning of gelatin from fish skin. Int J Biol Macromol 42:247–255
Srivastava S, Bankar R, Roy P (2013) Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine 20:683–690. https://doi.org/10.1016/j.phymed.2013.03.001
Suganya S, Venugopal J, Agnes Mary S, Ramakrishna S, Lakshmi BS, Giri Dev VR (2014a) Aloe vera incorporated biomimetic nanofibrous scaffold: a regenerative approach for skin tissue engineering. Iran Polym J 23:237–248. https://doi.org/10.1007/s13726-013-0219-2
Suganya S, Venugopal J, Ramakrishna S, Lakshmi BS, Dev VRG (2014b) Aloe vera/silk fibroin/hydroxyapatite incorporated electrospun nanofibrous scaffold for enhanced osteogenesis. J Biomater Tissue Eng 4:9–19. https://doi.org/10.1166/jbt.2014.1139
Suganya S, Venugopal J, Ramakrishna S, Lakshmi BS, Giri Dev VR (2014c) Herbally derived polymeric nanofibrous scaffolds for bone tissue regeneration. J Appl Polym Sci 131. https://doi.org/10.1002/app.39835
Suh KS, Choi EM, Lee YS, Kim YS (2013) Protective effect of albiflorin against oxidative-stress-mediated toxicity in osteoblast-like MC3T3-E1 cells. Fitoterapia 89:33–41. https://doi.org/10.1016/j.fitote.2013.05.016
Sun H, Li L, Zhang A, Zhang N, Lv H, Sun W, Wang X (2013) Protective effects of sweroside on human MG-63 cells and rat osteoblasts. Fitoterapia 84:174–179. https://doi.org/10.1016/j.fitote.2012.11.010
Tahmasebi A, Shapouri Moghadam A, Enderami SE, Islami M, Kaabi M, Saburi E, Daei Farshchi A, Soleimanifar F, Mansouri V (2020) Aloe vera-derived gel-blended PHBV nanofibrous scaffold for bone tissue engineering. ASAIO J 66:966–973. https://doi.org/10.1097/MAT.0000000000001094
Talebian S, Mehrali M, Taebnia N, Pennisi CP, Kadumudi FB, Foroughi J, Hasany M, Nikkhah M, Akbari M, Orive G (2019) Self-healing hydrogels: the next paradigm shift in tissue engineering? Adv Sci 6:1801664
Tang CH, Yang RS, Chien MY, Chen CC, Fu WM (2008) Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and ERK-dependent pathway in osteoblasts. Eur J Pharmacol 579:40–49. https://doi.org/10.1016/j.ejphar.2007.10.013
Tang DZ, Hou W, Zhou Q, Zhang M, Holz J, Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, Chen D, Wang YJ (2010) Osthole stimulates osteoblast differentiation and bone formation by activation of β-catenin-BMP signaling. J Bone Miner Res 25:1234–1245. https://doi.org/10.1002/jbmr.21
Tang DZ, Yang F, Yang Z, Huang J, Shi Q, Chen D, Wang YJ (2011) Psoralen stimulates osteoblast differentiation through activation of BMP signaling. Biochem Biophys Res Commun 405:256–261. https://doi.org/10.1016/j.bbrc.2011.01.021
Thomas V, Dean DR, Jose MV, Mathew B, Chowdhury S, Vohra YK (2007) Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite. Biomacromolecules 8:631–637
Tiyasatkulkovit W, Charoenphandhu N, Wongdee K, Thongbunchoo J, Krishnamra N, Malaivijitnond S (2012) Upregulation of osteoblastic differentiation marker mRNA expression in osteoblast-like UMR106 cells by puerarin and phytoestrogens from Pueraria mirifica. Phytomedicine 19:1147–1155. https://doi.org/10.1016/j.phymed.2012.07.010
Venkata V, Reddy S, Kuppusamy G, Talluri SV, Mannemala SS, Kollipara R, Wadhwani AD, Mulukutla S, Rama K, Raju S, Malayandi R (2016) Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2016.05.038
Volpi N, Schiller J, Stern R, Soltes L (2009) Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem 16:1718–1745. https://doi.org/10.2174/092986709788186138
Wang Y, Wang WL, Xie WL, Li LZ, Sun J, Sun WJ, Gong HY (2013) Puerarin stimulates proliferation and differentiation and protects against cell death in human osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt activation. Phytomedicine 20:787–796. https://doi.org/10.1016/j.phymed.2013.03.005
Wang Y, Adokoh CK, Narain R (2018a) Recent development and biomedical applications of self-healing hydrogels. Expert Opin Drug Deliv 15:77–91
Wang X, Ali MS, Lacerda CMR (2018b) A three-dimensional collagen-elastin scaffold for heart valve tissue engineering. Bioengineering 5. https://doi.org/10.3390/bioengineering5030069
Williams DF (2008) On the mechanisms of biocompatibility. Biomaterials 29:2941–2953
Wu C, Chen L, Huang Y, Huang Y, Parolini O, Zhong Q, Tian X, Deng L (2018) Comparison of the proliferation and differentiation potential of human urine-, placenta decidua basalis-, and bone marrow-derived stem cells. Stem Cells Int 2018:1–11. https://doi.org/10.1155/2018/7131532
Xiao HH, Gao QG, Zhang Y, Wong KC, Dai Y, Yao XS, Wong MS (2014) Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J Steroid Biochem Mol Biol 144:382–391. https://doi.org/10.1016/j.jsbmb.2014.08.002
Xu B, Liu Y (2018) Urtica extracts induce periosteal cell proliferation and differentiation: tissue-engineered bone construction and ultrastructural changes. Chin J Tissue Eng Res 22:1805–1810. https://doi.org/10.3969/j.issn.2095-4344.0196
Xu D, Xu L, Zhou C, Lee WYW, Wu T, Cui L, Li G (2014) Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int J Biochem Cell Biol 51:1–9. https://doi.org/10.1016/j.biocel.2014.03.005
Yeo I-S, Oh J-E, Jeong L, Lee TS, Lee SJ, Park WH, Min B-M (2008) Collagen-based biomimetic nanofibrous scaffolds: preparation and characterization of collagen/silk fibroin bicomponent nanofibrous structures. Biomacromolecules 9:1106–1116
Yoon HY, Yun SI, Kim BY, Jin Q, Woo ER, Jeong SY, Chung YS (2011) Poncirin promotes osteoblast differentiation but inhibits adipocyte differentiation in mesenchymal stem cells. Eur J Pharmacol 664:54–59. https://doi.org/10.1016/j.ejphar.2011.04.047
Yu GY, Zheng GZ, Chang B, Hu QX, Lin FX, Liu DZ, Wu CC, Du SX, Li XD (2016) Naringin stimulates osteogenic differentiation of rat bone marrow stromal cells via activation of the notch signaling pathway. Stem Cells Int. https://doi.org/10.1155/2016/7130653
Yu Q, Chang J, Wu C (2019) Silicate bioceramics: from soft tissue regeneration to tumor therapy. J Mater Chem B 7:5449–5460
Zadegan S, Nourmohammadi J, Vahidi B, Haghighipour N (2019) An investigation into osteogenic differentiation effects of silk fibroin-nettle (Urtica dioica L.) nanofibers. Int J Biol Macromol 133:795–803. https://doi.org/10.1016/j.ijbiomac.2019.04.165
Zahedi E, Esmaeili A, Eslahi N, Shokrgozar MA, Simchi A (2019) Fabrication and characterization of core-shell electrospun fibrous mats containing medicinal herbs for wound healing and skin tissue engineering. Mar Drugs 17:27. https://doi.org/10.3390/md17010027
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10:68. https://doi.org/10.1186/s13287-019-1165-5
Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang Z-M (2005) Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res 72B:156–165. https://doi.org/10.1002/jbm.b.30128
Zhang P, Dai KR, Yan SG, Yan WQ, Zhang C, Chen DQ, Xu B, Xu ZW (2009a) Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol 607:1–5. https://doi.org/10.1016/j.ejphar.2009.01.035
Zhang S, Huang Y, Yang X, Mei F, Ma Q, Chen G, Ryu S, Deng X (2009b) Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J Biomed Mater Res A 90A:671–679. https://doi.org/10.1002/jbm.a.32136
Zhang Q, Yan S, Li M (2009c) Silk fibroin based porous materials. Materials 2:2276–2295
Zhang Y, Yang H, Shao H, Hu X (2010) Antheraea pernyi silk fiber: a potential resource for artificially biospinning spider dragline silk. J Biomed Biotechnol 2010:1–8. https://doi.org/10.1155/2010/683962
Zhang JK, Yang L, Meng GL, Fan J, Chen JZ, He QZ, Chen S, Fan JZ, Luo ZJ, Liu J (2012) Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur J Pharmacol 689:31–37. https://doi.org/10.1016/j.ejphar.2012.05.045
Zhang J, Xu DP, Shang J, Liang XJ, Zhang XB (2021) Effects of naringin on the proliferation and osteogenic differentiation of canine bone marrow stromal cells in vitro. J Hard Tissue Biol 30:73–78. https://doi.org/10.2485/jhtb.30.73
Zhao Z, Vizetto-Duarte C, Moay ZK, Setyawati MI, Rakshit M, Kathawala MH, Ng KW (2020) Composite hydrogels in three-dimensional in vitro models. Front Bioeng Biotechnol 8:611
Zhong S, Teo WE, Zhu X, Beuerman R, Ramakrishna S, Yung LYL (2005) Formation of collagen-glycosaminoglycan blended nanofibrous scaffolds and their biological properties. Biomacromolecules 6:2998–3004
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Aghmiuni, A.I., Ghadi, A., Asadi, M., Khiyavi, A.A. (2023). Application of Bioactive Compounds and Biomaterials in Promoting Cell Differentiation, Proliferation, and Tissue Regeneration. In: Sheikh, F.A., Majeed, S., Beigh, M.A. (eds) Interaction of Nanomaterials With Living Cells. Springer, Singapore. https://doi.org/10.1007/978-981-99-2119-5_13
Download citation
DOI: https://doi.org/10.1007/978-981-99-2119-5_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2118-8
Online ISBN: 978-981-99-2119-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)