Abstract
Environmental contamination caused by the abusage of chemical compounds is a worldwide phenomenon, arising as a result of human activities and population growth. There are various pollutants ranging from the pharmaceutical to the agricultural source. In recent times, there is an increasing demand for pharmaceuticals, which in turn has placed a matter of concern for public sector. Additionally, the usage of unlawful drugs has resulted in the discharge of harmful carcinogens into the water system. The release of these chemicals poses a number of short- and long-term effects on the natural ecosystem, taking into consideration the harmful effects of the chemical pollutants and their unavoidable usage in the modern era. This chapter overlooks some of the majorly employed chemicals (xenobiotics and pesticides), and their fate in the aquatic community, their modes of action, and some of the mitigation strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Scientific, technological, and industrial revolutions have permitted humans to overutilize the resources, thereby creating an imbalance in the natural ecosystem (Sikandar et al. 2013). Enormous amounts of toxic effluents let out as a result of industrial processes have caused widespread contamination of the ecosystem. It was reported that nitrated and halogenated hydrocarbons are few among the major contaminants (Jain et al. 2011). Various fertilizers, insecticides, and herbicides have been employed in agricultural activities. In addition, industry-based synthetic compounds like dyes, pharmaceuticals, hydraulics, pigments, agrochemicals, halogenated compounds, and fire retardants have been extensively used (Reineke and Knackmuss 1988). Due to the inevitable uses in veterinary and anthropoid medications, pharmaceutical wastes have developed as a significant cause of prolonged environmental condition (Gani et al. 2021). The chemicals thus let out into the surrounding environments are believed to possess specific modes of action and thus impart a certain number of hazards on the aquatic flora and fauna, in comparison to the other chemical substances. These chemical compounds persist within the environment making them a potential agent causing health hazards and posing toxic effects on the surrounding niche. Xenobiotics can have a range of impacts, such as immunological reactions, medicine toxicity, and climate change. Xenobiotics are substances that are either nonbiodegradable or only partially biodegradable, which may result in sluggish biotransformation and persistence in the setting for an extended period of time (Dar et al. 2020).
With this point of view, in this chapter, we sought to discuss dormant pollutants (pesticides and xenobiotics), their metabolism, and their harmful effects on aquatic life.
2 Pesticides and Xenobiotics
Pesticides are a collective term used to represent all the compounds including herbicides, insecticides, and fungicides that are applied to regulate pests or undesirable organisms. Water resources contaminated with pesticides are found to affect both humans and the ecosystem. Pesticides have sought to be as probable mutagens, as they are capable of triggering deviations in the DNA. The World Health Organization has estimated that around 1,000,000 human beings have been affected by acute poising as a result of toxicant contact; in addition, annually, a death rate ranging between 0.4% and 1.9% has been recorded. It has been reported that constant exposure to pesticides in the medium and long term resulted in various syndromes and tumors including the nervous system disorder. Accustomed application of agrochemicals such as pesticides, soil conditioners, acidifying agents, chemicals involved in animal husbandry (hormones and antibiotics), and fertilizers is popular. Since the beginning of the industrial era in 1950, the use of pesticides has had a severe impact on the environment niche. It has been extensively employed as a pest control agent wherein monocular cultivation is involved. Few demerits are still involved, despite the development of chemistry in the field of pesticide formulations; majorly, the pesticides perturb the predator-prey interactions, thus causing an imbalance in the biodiversity. Furthermore, the pesticides can cause exceptional health concerns. Though the usage of a few chemical compounds is limited/controlled by the agricultural sector, it is said that agriculture is one of the areas which purposefully discharges chemicals into the surrounding niche leading to the adverse effects (WHO 2020; Warra and Prasad 2020). Of the manufactured compounds, the usage of the pesticides by the agricultural sector has been recorded the highest (Sharma et al. 2019; Laxmi et al. 2019). It is often difficult to distinguish among the pesticide effect and environmental effects on the ecosystem because the industrial effluents are let out into the surroundings to a greater extent accidently or intentionally. On the contrary, significant evidence suggests that the usage of pesticides in the agricultural field has had a considerable impact on the water quality causing major influence and concerns on the environment (FAO 1990; Warra and Prasad 2020). Despite the fact that the amount of pesticide practice is relatively extensive, it is plausible that substantial use of chemicals is associated only with few pesticide products. Of the million tons of pesticides produced, 29.5% insecticides, 47.5% herbicides, 5.5% other pesticides, and 17.5% fungicides are the categories extensively in application (De et al. 2014). Howbeit, the use of pesticides is in a range of low to zero in subsistence and conventional farming in countries like Asia and Africa. However, the environment, water quality, and health hazards are associated with its inappropriate usage.
2.1 Classification of Pesticides
Pesticide is a collective term that describes diverse groups of insecticides, herbicides, rodenticides, garden chemicals, fungicides, and household disinfectants employed to both protect and destroy pests (Mohapatra et al. 2021). Each pesticide groups differ in its physical and chemical properties. On that account, it is preferable to classify them based on their properties. As recommended by Drum (1980), the widely used method of classification of pesticide groups depends on their (a) chemical structure, (b) mode of entry, and (c) mode of action and type of organisms they target (Yadav et al. 2015). Based on their source, chemical pesticides have been classified into four types: pyrethroid pesticides, organophosphate, carbamate, and organochlorine (Yadav and Devi 2017). Another class of pesticides are biopesticides, occurring naturally or are naturally derived from living organisms such as bacteria, plants, and fungi (Mehrotra et al. 2017). Microbial pesticides, biochemical pesticides, and incorporated protectants are the three major groups of biopesticides.
2.2 Pesticide Categories
2.2.1 Chemical Structure of Pesticides
The most general and applicable method of classifying insecticides is based on their chemical description and the chemical composition of the active ingredients. The chemical classification of pesticides delivers the efficacy and physical and chemical properties of special pesticides (Fig. 1).
2.2.2 Organochlorine Pesticides (OCPs)
Chemicals belonging to this group are very stable and persistent in the environment and have the potential of accumulating in the adipose tissue (Lee et al. 2017). It is said that in humans, these chemicals/their metabolites largely work on the central nervous system by altering their electrophysiological properties and altering enzymatic nerve membranes and changing the flow kinetics of K+ and Na+ through the nerve cell membrane and may cause seizures from apnea and acute poisoning death (Zaffar et al. 2016). Based on their structure, the organochlorines are categorized into five categories: (a) hexachlorocyclohexane (HCH), such as lindane, (b) DDT and its analogues including DDT and dichlorodiphenyldichloroethylene (DDE), (c) dichlorodiphenyldichloroethane (DDD), (d) mirex and chlordecone, and (e) cyclodienes including aldrin, dieldrin, endrin, heptachlor, chlordane, and endosulfan (Singh and Singh 2017). Higher concentrations of most of the OCPs generally result in acute toxicity and death under natural circumstances and may be gradually causing chronic illness. The persistent nature of the OCPs and their lipophilic nature might lead to long-term storage in the adipose tissue, following their release into the circulatory system (Kumar et al. 2013). This process takes a longer duration from the initial time of exposure to the onset of effects; it is said that DDT may remain in the human body for 50 years and more.
2.2.2.1 Organophosphate Pesticides
Organophosphate pesticides are ester derivatives of phosphoric acid. These esters work on the central nervous system by blocking acetylcholine. This enzyme is responsible for maintaining the levels of acetyl cholinesterase, which disturbs the nerve impulse by the phosphorylation of the serene OH group in the active site of the enzyme (Lionetto et al. 2011; Laxmi et al. 2020). Headache, dizziness, loss of reactions, nausea, convulsions, cramps, and ultimately coma and death are the intoxication symptoms of organophosphate pesticides.
Atropine is said to be the definitive treatment for organophosphate poisoning, which competes with acetylcholine at the muscarinic receptors. The recommended initial dose for adults is 2–5 mg IV, and for children, a dosage of 0.05 mg/kg IV is recommended. In case the patient does not respond to the initial doses, the dosage is doubled every 3–5 min until the respiratory secretions have been cleared and there are no signs of bronchoconstriction (van Heel and Hachimi-Idrissi 2011). The atropine is given as a continuous infusion or in bolus in patients with severe poisoning for a couple of days until the patient shows any signs of improvement.
2.2.2.2 Carbamate Pesticides
Some organic ester compounds which are the derivatives of dimethyl N-methyl carbamic acid are applied as fungicides, herbicides, nematicides, and insecticides, collectively termed as carbamates. Propoxur, pyridostigmine, molinate, carbaryl, thiobencarb, methiocarb, and disulfiram (Antabuse) are being widely used in dogs and cats (Hassaan and El Nemr 2020). The toxicity of the carbamate compounds depends on their molecular structure, and generally, they have short duration in comparison to that of the organochlorines and organophosphates; organochlorines are said to inhibit acetyl cholinesterase. Carbamates are short-lived, and thus precautions must be taken while administering the atropine (Hernández et al. 2013). Symptoms caused by acute poisoning of carbamate insecticides and organophosphate are often common and severe. The symptoms of poisoning develop in different organs as follows: cough, increased secretions, pulmonary edema, bronchial tree (dyspnea, wheezing,), cardiovascular effects (hypotension and bradycardia), abdominal cramps, gastrointestinal manifestations (diarrhea, vomiting, nausea, and incontinence), glandular stimulation (lacrimation, increased salivation, and sweating), eye problems (miosis, and blurred vision), compromised motor activity (cramps, fasciculation, muscle twitching, weakness, depression of respiratory and circulatory), bladder dysfunction (incontinence and frequency), sympathetic dysfunction (tachycardia, hypertension, and pallor), central nervous system effects (generalized weakness, drowsiness, restlessness, tremor, emotional lability, confusion, slurred speech, Cheyne-Stokes respiration , areflexia, ataxia, convulsion, hypothermia, and coma), nicotinic receptor stimulation (including sympathetic and motor neurons), bronchoconstriction, and cyanosis (Muhammad et al. 2017).
2.2.2.3 Pyrethroid Pesticides
Pyrethroids are natural insecticides that are the derivatives of pyrethrum extracts from the flowering chrysanthemum, commonly called pyrethrin. These chemicals act on the central nervous system causing fluctuations in the dynamics of sodium cation channels on the membrane of the nerve cells, leading to an increased opening time of the sodium channels. In both insects and vertebrates, the sodium cation stream extends across the membrane, and the neuronal hyperexcitation experienced could be the result of all these symptoms put together (Clark and Symington 2011). Since there is an increased demand for the usage of pyrethroids, and also a shortage of the essential oils required for the production of natural organic pyrethrum, researchers have opted for synthetic pyrethroids. Majority of the pyrethroid insecticides exhibit lower toxicity toward mammals and birds while higher rates of toxicity to arthropods, as they require very low doses for the effects to show up (Lengai et al. 2020). When applied directly to water, these are highly toxic to the fishes and act rapidly against chewing insects. Even though majority of the pyrethroid insecticides are absorbed by the insect pests, they are not very effective in penetrating the soil in order to kill the underground pests, as they adhere tightly to the organic matter and soil. Pyrethroids are employed as active substance in the production of several products such as topical mosquito repellents, pet shampoos, human head lice treatments, pet sprays, and insecticide sprays in farms and homes (Hassaan and El Nemr 2020).
2.2.2.4 Pesticides in Water Resources
Water is considered as one of the most predominant natural resources, essential for all the living creatures. The condition of surface water and soil is a matter of concern in many developing countries due to the contaminants reaching the surface water and soil over the past few years.
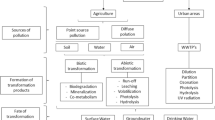
It is mandatory for the pesticide users to clearly understand the cycle of pesticide contamination of both surface and ground water (Fig. 2). In order to reduce the pesticide off-site movement, it is utmost important for the pesticides users to implicate safe practices when introducing pesticides into the surface and ground water. The groundwater contamination by the pesticides is hard to be eliminated due to the pollutant concentration, in comparison to other environments, which is worrisome (Sjerps et al. 2019). The entry of pesticides into the water source is by rundown (flowing) and leaching (filtering), of which both modes are related to the earth’s hydrological cycle. While taking into consideration the utilization of water for surface flow, the pesticides in the municipal wastewater are suitable for hydrological model. Some of the factors affecting the quality of water are usage of active ingredients in the formulation of pesticides, degradable compounds as a result of microbial/photochemical or chemical degradation of the active compounds, adhesives, buffers, preservatives, and emulsifier/wetting agents used as additive mixture along with the active ingredients.
2.2.2.5 Fate and Effects of Pesticides in Aquatic Ecosystems
Employing pesticides in various sectors contaminates the aquatic niche via several ways, namely, leaching, spray drift and, runoff, and this may cause harmful effects on the terrestrial and aquatic life (Van den Brink 2013; Wijngaarden et al. 2005). Fishes are said to be directly affected by the pesticide usage; the small fishes are affected severely in comparison to the larger ones. Indirect toxic effects of pesticides on fishes include decrease in the fish’s food sources (plankton and algae), deterioration in the quality of the aquatic habitat, and changes in their food pattern. The reduced abundance of primary producers as a result of pesticide application significantly decreases the primary and secondary consumers.
Organochlorine pesticides severely affect the primary consumers such as zooplankton. Microcrustaceans are also being affected by the employment of various insecticides. It can be noted that the interferences used in the pesticide formulation might further increase the toxic effects of the pesticides; however, these are not a part of the active ingredients (Singh et al. 2016). For instance, TFM (3-trifluoromethyl-4-nitro phenol), a lamprey pesticide, has found its application in the tributaries of the Great Lakes for years together in Egypt, in order to control the lamprey (Gllderhus and Johnson 1980). The environmental fate of TFM has been studied for years, and reports have stated that the formulation consists of additional potent interferences that have an impact on the hormonal system of fishes, leading to liver diseases.
2.2.2.6 Direct Effects of Pesticides
The aquatic ecosystem consists of diverse groups of organisms such as microorganisms, invertebrates, plants, fishes, and amphibians. Pesticides can impart both direct and indirect effects on these living forms. The physiological action of the particular pesticides within the organisms brings about the direct effects. The biotic community is distinguished by the interactions among the species like predation or competition; indirect effects involve the consequences mediated through these communications.
Mostly, the direct effects of chemicals on any organism are dependent on the concentration or the dosage of the pesticide used. Nevertheless, few factors impact the magnitude and occurrence of the adverse effects.
-
Exposed life stage: The effects of pesticides in different life stages of the organisms vary, for instance, younger invertebrates, fishes, and amphibians are more susceptible to the toxic effects than adult ones (Babalola and Van Wyk 2018).
-
Duration of exposure: Longer duration of exposure to pesticides has potent effects. It can be noted that biomagnification indirectly delays the temporal effects caused by pesticide usage. The pesticide organochlorine biomagnifies along the food web, resulting in higher magnitudes of pesticide concentration/lipid weight within the organisms, at the top of the food web pyramid (Tooker and Pearsons 2021).
-
Additional stressors: Enhanced effect could be visualized when an additional single compound was found in conjunction with the pesticides. Other stressors include food scarcity, predation, UV radiation, and parasitism (Hooper et al. 2013).
-
Population density: Reduction of negative intraspecific interaction, toxicant-induced effects in high-density populations could be seen, although there remains an altered population structure, the toxicant displays delay in development and age-dependent mortality (Liess and Foit 2010).
-
History of the community: Higher sensitivity or acquired tolerance/pollution-induced community tolerance (PICT) may be experienced, in the case of the communities previously being exposed to the toxicants (Schmitt et al. 2005).
2.2.2.7 Indirect Effects of the Pesticide on the Aquatic Community
In the ecosystem, interactions within the species and with the other species and their respective abiotic environment could be observed. These interactions could be altered as a result of the direct effects of the pesticides, causing the secondary or indirect effects on the related species which are not affected directly. Listed ecological relationships may result in the indirect effects through the propagating direct effects, namely, predation, which comprises the parasite-host, predator-prey, and herbivore-plant relationships; competition, which is intraspecific/interspecific; and species-habitat relation, that is, commensalism or mutualism.
As mentioned earlier, the primary producers are usually at a higher risk of being adversely affected by herbicide usage. Thus, a reduction in the population of the primary producers eventually leads to a decrease in the herbivore populations, as a consequence of habitat loss or limitations in the food source. In a study reporting the effects of the herbicide (atrazine) on the freshwater communities, for instance, in artificial ponds, the reproduction rate of the zooplankton (e.g., Daphnia pulex, Simocephalus serrulatus) gradually decreased as a result of the reduction in the phytoplankton biomass. Similarly, it was observed that due to the reduction of periphyton and as a loss of macrophyte habitat (Typha latifolia, Chara sp.), there was diminished biomass of the amphibian tadpole (Rana catesbeiana) (DeNoyelles et al. 1982, 2020). While in the nutrient-dense ecosystems, the indirect effect pronounced on the herbivores as a result of reduction in the food sources is often less common. Effects may be propagated to the subsequent higher tropic levels, when there is a reduction in the herbivore source, for instance, the predators preying on the herbivores. The reduction in the populations of the macroinvertebrates (e.g., Chironomidae spp.) and zooplanktons ensued owing to herbicide-induced reductions and resulted in the loss of food sources like the primary producers and habitat depletion, ultimately leading to the total biomass decrease of bluegill sunfish (Lepomis macrochirus) (DeNoyelles et al. 2020). The ecological chain effect indicates the bottom-up indirect effects due to the primary producers (lowest trophic level) bearing alterations on the higher trophic levels. These indirect effects being observed are a consequence of predatory ecological relationships, that is, elimination of sensitive primary competitors by the pesticides, thereby promoting competitive relationship among the primary consumers making them tolerant. For instance, the herbicide linuron increased the algal bloom Chlamydomonas sp., due to the decreased population of the macrophyte Elodea nuttallii (Van den Brink et al. 1997).
Photosynthesis is considered a significant part of the ecosystem, influencing the water quality. The outcome of the herbicide usage is lowered pH value of water during day time and lowered concentration of dissolved oxygen (DO). As recorded by a study, usage of linuron (50–150 μg/L) resulted in lowered pH of about 40% and 25% and reduced the DO (Cuppen et al. 1997). Acute mortality of the macrophages as a result of herbicide usage further has been said to reduce the concentration of DO and pH (Brock et al. 2000). This decrease in the water quality can have detrimental effects on the sensitive invertebrate species and results in indirect effects as a result of species-habitat relationships. Decreased populations of Copepoda and Cladocera were characterized to lowered DO levels (20%) in comparison to the controls (Thompson et al. 1993).
The usage of insecticides adversely affects the zooplanktons and invertebrate species. The impact of pesticides on the freshwater ecosystems is the reduction of the invertebrate prey. The significantly decreased populations of macroinvertebrates such as dipterans and ephemeropterans (mayflies) and also two zooplankton groups Cyclopidae and Daphniidae, followed by treatment with methyl parathion, resulted in the mean weights of the rainbow trout (Salmo gairdneri) (Crossland 1984). Reduction of Copepoda (Isopoda Cladocera) and few invertebrate communities and the eradication of insecta and amphipoda due to the usage of the insecticide chlorpyrifos resulted in an increase (two to threefold) in the periphyton chlorophyll-a, along with blooms of Oscillatoria sp. (Brock et al. 1992). In a study conducted on chlorpyrifos, herbivorous rotifers, and freshwater bivalve mollusks (Sphaeriidae), an increase was found in their population, as a result of reduced competition for food sources among the sensitive invertebrates (Brock et al. 1992). Identical study was performed to check the exposure effects of pesticide on the increased tolerant species and decreased sensitive species; in this scenario the effects on the ecosystem are ambiguous. As there is observed increased pH and DO concentration, due to the increase in the population of the primary producers, the decomposition of the zooplankton and the dead invertebrates by the bacteria or the fungi decreases the water quality (Brock et al. 2000). A study conducted on the French streams illustrated that a three to fivefold reduction in the invertebrate fauna inhibited the leaf-litter decomposition due to the application of the insecticides. The decomposition of the leaf-litter decomposition describes a significant energy source in the stream ecosystems, and the reduction in the process could negatively affect the river sections up to several kilometers downstream. These alterations put together indirectly affect the flora and fauna of the aquatic ecosystem in the long term (Schäfer et al. 2007).
Lethal concentration 50 (LC50), a common toxicity dosage measurement, is employed since not all animals of a species perish at the same dose (some are more tolerant than others). This is the demonstration of a pesticide that, in a certain time frame, often between 24 and 96 h kills 50% of the test population of animals.
LC50s and hazard ratings ranging from minor to extremely hazardous for frequently used fungicides, insecticides, and herbicides are mentioned in the Table 1. For instance, the pesticide permethrin’s 24 h LC50 for rainbow trout is 12.5 ppb. This indicates the pesticide’s extreme toxicity to trout, as half of the fish exposed to 12.5 ppb of permethrin died within 24 h (Maurya et al. 2019).
A pesticide’s biological availability (bioavailability), bioconcentration, biomagnification, and environmental persistence all affect how much fish and other aquatic creatures are exposed to it. The quantity of pesticide in the environment that is accessible to fish and other wildlife is referred to as bioavailability (Maurya et al. 2019; Adnan et al. 2016). Some insecticides degrade quickly after being used. Some are less available because they cling firmly to stream bottoms or soil particles floating in the water column. Some are less accessible to aquatic life because they swiftly dissolve in water or rapidly volatilize into the air. The accumulation of pesticides at levels higher than those in the water or soil where they were sprayed is known as bioconcentration. Some fish may have concentrations of some pesticides ten million times higher than those found in the water in their bodily tissues and organs (particularly lipids) (Katagi 2010; Adnan and Indulkar 2017).
2.3 Pesticide Metabolism in Aquatic Animals
The chemicals released by industrial effluents, agricultural runoff, petroleum refining, home sewage, etc. affect aquatic life by causing water pollution. The adverse effects in aquatic life are primarily reducing the dissolved oxygen, direct toxicity to animals, and reduced taste of the meat. These pollutants seriously damage the physical and biological process of the animals. Sometimes, they also affect the embryonic stages. Knowledge on pesticide toxicity and metabolism in aquatic environment is essential for better understanding and application of such chemicals. The most commonly used fungicide is copper sulfate (CuSO4) as fungicide in agriculture and paraquat (PQ, 1,1′-dimethyl-4,4′-bipyridinium-dichloride), a common herbicide.
2.3.1 Paraquat
A paraquat is a nonselective, broad-spectrum herbicide that affects the photosynthetic system (PQ, 1,1′-dimethyl-4,4′-bipyridinium-dichloride). PQ is frequently used in agriculture to manage weeds and kills practically all plants after spraying within a few days. PQ easily dissolves in water to its fullest extent. Invading the plant, the positively charged PQ ion impacts the harmful effect by obstructing photosynthesis. Paraquat is a potent electron acceptor, which limits the amount of NADPH 84 formed by competitively inhibiting ferredoxin reduction by removing electrons from photosystem I. PQ ion is converted to a water-soluble free radical during this process and is comparatively stable (Nemcsok and Benedeczky 1995).
2.3.2 Copper Sulfate
Initially, copper sulfate was used as a herbicide to control weeds in wheat fields and as a caustic against wheat mildew. Its use spreads after the discovery of the so-called Bordeaux combination, wherein the initial application took place. Given that metals may form chelates, copper sulfate functions as a fungicide. The electron negativity of the cations has a big impact on this process’ stability of metal binding, as well as that of metal chelates and sulfide. The following metal cations lose relative effectiveness as fungicides: Ag, Hg, Cu, Cd, Cr, Ni, Pb, Co, Zn, Fe, and Ca. The extent of the fungicidal effects is probably determined by the strength of the covalent or coordinational binding of the metal complexes attached to cell walls. When Cu2+ ions penetrate cells, they form complexes with the thiol and amino groups therein, preferentially inhibiting key enzymes and other proteins. This is the basis for the fungicidal activity of copper-containing medications (Nemcsok and Benedeczky 1995).
2.3.3 Diquat
Diquat is a broad-spectrum herbicide that can be used to reduce algae and weeds that are submerged, but it is not particularly effective against weeds that are emerging. Diquat-treated water for livestock consumption, agricultural irrigation, or drinking must be contained for 14 days according to the legislation before use. There are no limitations on fishing; however, for swimming, a waiting period of 1 day should be observed. After 10 days, diquat is seldom ever found in treated water. Even when a herbicide is applied that is not specifically hazardous to fish, fish deaths might nevertheless happen following application. Because of the massive amounts of decaying water weeds destroyed by the herbicide, which break down and lower oxygen levels, fish indirectly suffocate rather than poisoned by the herbicide. To provide fish the flexibility to relocate to untreated, oxygen-rich sections of the pond or lake, treat no more than half (or less) of the lake at a time when applying herbicides. Utilize herbicides in the spring when dissolved oxygen levels are greater and water temperatures are lower than in the summer. At lower temperatures, some herbicides are less harmful. Apply in the early spring when there are fewer weeds to break down and they are tiny and less well-established (Helfrich et al. 2009).
2.4 Bioconcentration of Pesticides
A specific instance of bioaccumulation is bioconcentration. The definition of bioconcentration is the uptake and retention of a chemical from just water. The use of other sources is not taken into account. The majority of laboratory investigations on the absorption by aquatic creatures expose the organisms to water containing the target chemical(s) in solution.
The bioconcentration factor (BcF) quantifies the magnitude of bioconcentration. The BcF is the ratio of a chemical’s concentration in an organism’s tissues to its concentration in solution in the water to which it was exposed at equilibrium. The uptake rate constant or uptake clearance to the release rate constant ratio can also be used to calculate the BcF:
where Ct and Cw represent the chemical’s equilibrium concentrations in tissues and water, respectively; k1 denotes uptake clearance; and k2 denotes release rate constant. Units for uptake clearance are mass of chemical/mass of tissue/mass of time, which translates to time−1 (Spacie and Hamelink 1982). BcF is unitless since the release rate constant likewise has units of time−1.
Pesticides are primarily bioconcentrated toward aquatic species through passive diffusion through the gastrointestinal system, epithelial tissues, and gills. According to Miyamoto et al. (1990), Connell (1988), Barron (1990), and Landrum and Fisher (1999), the physicochemical properties of the chemicals, the surrounding environmental conditions, and the physiological disposition of each organism involved are what essentially influence bioconcentration. Since compounds must first pass a diffusion barrier, like mucus and biological membranes, to reach circulation fluids, the relative solubilities of such molecules in water and n-octanol may act as a stand-in for lipids. Another important factor that could repeat the processes of diffusion and partitioning in tissues that contain lipids is the size of the molecules. The propensity to bioconcentrate is influenced by both lipid solubility and molecular size (Fig. 3).
For a number of chemical class combinations with fish, the classic hydrophobicity model has demonstrated a strong correlation between the n-octanol/water partition coefficient (Kow) or water solubility (WS) and BcF. Equation (2) describes this relationship, where a and b are constants (Mackay 1982; Ellgehausen et al. 1980; Veith et al. 1979; Neely et al. 1974):
The association between physicochemical traits and BcF has been studied for a range of aquatic creatures, as indicated in Table 1. When many aquatic species or chemical classes are taken into consideration, lower values are seen in the coefficient of correlation (r2) between the two approaches (Zaroogian et al. 1985; Axelman et al. 1995; Mailhot 1987; Hawker and Connell 1986). However, the correlation coefficient (r2) is typically greater than 0.7–0.8. A range of urea herbicides were tested on Chlorella fusca, and Manthey et al. (1993) found a moderate association; metabolism and hydrophobicity both played a part in this investigation.
The link between fish BcF and Kow was initially discussed by Mackay (1982). For a chemical involved in passive uptake from water, the biological membrane, which is primarily composed of lipid bilayers, acts as the principal barrier. An example of this is the gill epithelium. Steric characteristics like molecule size and shape can occasionally play a significant role in passive absorption (Barron 1990; Landrum and Fisher 1999). Sometimes a chemical’s isomorphism affects bioconcentration. There are four isomers (α–δ) of hexachlorocyclohexane (HCH), and the lindane-30 isomer is the most common. BCFs in clams vary according to isomer as follows: δ > α > β ≈ γ. However, the order of the elimination procedure was different: γ > α » δ ≈ β (Yamato et al. 1983). Similar results in the mussel Mytilus edulis (Ernst 1979) and blue-green alga Anabaena sp. (Mathur and Saxena 1986) suggested the presence of yet another separate order; these changes imply that the bioconcentration of these isomers varies depending on the species. According to Moore et al. (1977), there were species-specific changes in the absorption of chlordane-24 isomers, and the predominant difference between the isomers in Mysis relicta was due to the enantioselective metabolism of the (or trans)-isomer (Warner and Wong 2006). Additionally, flucythrinate’s enantioselective bioconcentration in oysters has been studied (Schimmel et al. 1983). Endosulfan-27 has two isomers, α (or I) and β (or II), that differ in the structure of the cycloheptyl moiety. According to studies on crayfish Procambarus clarkii (Naqvi and Newton 1990) and the Daphnia magna (DeLorenzo et al. 2002), the β-isomer bioconcentrates more than the α-isomer, most likely because it undergoes less metabolic conversion to the equivalent sulphate. However, it was noted that algae had a higher bioconcentration of the α-isomer (Narayana Rao and Lal 1987; DeLorenzo et al. 2002).
The main factors influencing how quickly a chemical gets bioconcentrated into an organism from water are absorption and removal mechanisms. In aquatic creatures such as mollusks, algae, crustaceans, and insects (other than fish), the pesticide bioconcentration factor and elimination clearance periods (CL50) are provided in Tables 2, 3, 4, and 5. Data on the larvae, nymphs, and inhabitants of the aquatic environment are provided for Insecta members. Exposures to pesticides are carried out utilizing static or flow-through systems. The hydrolytic stability and water solubility of the chemical determine which system is employed. The 287 compounds that the EPI Suite program determined to have log Kow values between 3 and 7 have log BcF values that are usually between 0 and 6 for such xenobiotics (USEPA 2008). BcF levels among studied species greatly vary, even for a single chemical. Log BcF values for the organochlorine pesticides mentioned in Table 2 typically range from 3 to 5, with some species showing variation within a factor of two. Endosulfan, hexachlorobenzene, and DDT showed a higher variance. High log Kow values (5.73 and 6.91) for the latter two pesticides are likely caused by the individual species’ varied lipid levels. The crayfish Procambarus clarkii may more significantly metabolize xenobiotics to their sulfate forms, which results in significantly lower BcF values, by resemblance to the metabolism of D. magna (DeLorenzo et al. 2002). Aldrin has an extremely high hydrophobicity (log Kow = 6.5); however, due to its oxidation to dieldrin, it is rapidly eliminated in ostracods (Kawatski and Schmulbach 1972).
Some pesticides seem to be quite species-specific in their ability to be eliminated. In polychaetes as opposed to bivalves and isopods, the CL50 values for lindane-30 were longer and had a higher bioconcentration (Thybaud and Caquet 1991; Thybaud and Le Bras 1988; Ernst 1979). Pentachlorophenol, DDT, and its metabolite DDE, which are more hydrophobic insecticides, showed significant differences in elimination. Compared to polychaetes and shrimp, which require 2–3 weeks for elimination (Landrum and Dupuis 1990: Ernst 1979), mollusks swiftly eliminated, which has a CL50 of less than 1 day (Tjeerdema and Crosby 1992; Shofer and Tjeerdema 1993). Significant variations in CL50 (by 100 days) were found for the amphipods (Lotufo et al. 2000). Organophosphorus insecticides with intermediate hydrophobicity were reported to have lower BcF values, often between 1 and 2 (Table 3). In comparison to other aquatic animals, the BcF values of chlorpyrifos are 1–2 orders of magnitude higher in the isopods Artemia sp. and Asellus aquaticus (Varo et al. 2000; Montañés et al. 1995). In shrimp and copepods, fenitrothion, diazinon, and malathion were found to have a comparable effect (Kashiwada et al. 1995). In these species, higher bioconcentration rates probably result from less metabolic activity. Organophosphorus insecticides have a relatively quick clearance rate (CL50 values are fewer than 4 days). The log BcF values of the pyrethroid insecticides shown in Table 4 are 2–4, which is nearly an order of magnitude less than what the regression equations in Table 1 would indicate. Toxaphene and hexachlorobenzene are two organochlorine insecticides with log Kow values about 6. Organochlorine insecticides are often believed to be resistant to metabolism, despite the fact that they demonstrate log BcF values of 4–6 in mollusks, which are close to predicted levels. However, these values are higher than those observed for pyrethroid. The terminal residues of the pyrethroids are therefore more hydrophilic than their parent compounds, indicating that they are likely to be metabolized through ester cleavage and hydroxylation.
Other than the pesticides described above, bioconcentration values for pesticides are shown in Table 5. Carbamates, triazines, ionizable acids, ureas, esters, and amides have BcF values less than 1000. In addition to having these characteristics, many pesticides also feature chemical groups that make hydrogen bonding easier and easier to digest. Such groups often produce BcF values below those predicted by the regression equations in Table 1. In comparison, the amphipod Pontoporeia hoyi’s log BCF value of carbaryl (log Kow = 1.85) is 4.3, which is significantly higher than the similar value (2.2) in Mysis relicta. The very sluggish elimination and decreased metabolic activity account for the disparity (Landrum and Dupuis 1990). It may be because of its impact on algal development that the ionizable salicylic acid demonstrated a surprisingly high bioconcentration (log BCF = 3) in green algae (Wang and Lay 1989). These results show that the chemical class being tested and the type of aquatic life have a substantial impact on the BcF values for pesticides.
2.5 Bioaccumulation of Pesticides
Bioaccumulation, or the buildup of a chemical in an organism in relation to its level in the surrounding environment, is a significant environmental hazard. Thus, a common and growing method for determining the chemical state of aquatic ecosystems is to measure chemical concentrations in biota. Aquatic species can take in chemicals by eating contaminated prey or silt, or they can take them directly from the water by using their respiratory organs (such their gills). Therefore, in any kinetic investigation of bioaccumulation, each of these exposure pathways should be considered. A substance that is not yet digested and is dissolved in interstitial water or sediment is the most basic model for bioaccumulation. Bioaccumulation factor values and biosediment accumulation factors of different pesticides were reported in Tables 6 and 7.
While individual pesticides are bad for the ecology, pesticide mixtures increase their toxicity. There is a dearth of information regarding how particular herbicides from these families interact with aquatic life. As a result, it might be challenging to forecast whether or not a given combination of drugs will increase toxicity (Deneer 2000). The degree of bioaccumulation of several pesticides in fish is influenced by water solubility and the polarity of the pesticides. The bioaccumulation of a pesticide chemical in fish is inversely correlated with its water solubility. As the pesticide becomes more soluble in water, the amount of bioaccumulation decreases. As a result, water solubility is a crucial factor in reducing pesticide dynamics in aquatic ecosystems (Haque et al. 1977). Pesticide desorption or elimination rates seem to vary depending on the species. The rate of elimination reactions and rate of absorption determine the quantity of pesticides in a specific species (Matsumura 1977). To identify and map the spread of pesticide residues in the aquatic ecosystem, significant effort has been made. A thorough residue analysis program has revealed numerous harmful effects connected to organochlorine pesticides and metabolites of the organophosphates (Livingston and de La Cruz 1977). Pesticides that enter aquatic environments have the potential to induce undesirable ecological loss in the form of disease and aquatic animal mortality. As a result, aquatic bacteria, vertebrates like fish and water birds, and invertebrates like frogs, muscles, turtles, and prawns all experience deterioration. Because they are a component of natural food chains and other animals rely on them for food, the hazardous compounds present in pesticides have an effect on these aquatic species (Lakhani 2015).
In aquatic environments including estuaries and rivers, pesticides can have an impact on the microorganisms through spills, agricultural runoff, and drift. Both the structure and the function of microbial communities can be harmed by pesticide toxicity. Pesticides can be bioaccumulated in the ecosystem or digested by microorganisms. Different mechanisms of pesticide toxicity in microorganisms exist depending on the chemical and the microbial species exposed to it (DeLorenzo et al. 2001).
D. magna has been proven to be susceptible to pesticide contamination in bioassays in which Parachromis dovii and Daphnia magna were exposed to contaminated water taken from the field (Diepens et al. 2014). Shrimp farming is one of the significant sources of seafood that could be damaged by environmental or accidental exposure to neonicotinoid insecticides like imidacloprid. Neonicotinoids influence how insects’ neural systems function. In a study on adult black tiger shrimp (Penaeus monodon), stress enzyme activity was assessed for both acute and chronic imidacloprid effects in the abdomen, head, gills, and hepatopancreas. This demonstrated an increase in these biomarkers’ activity, and the enzymatic activity was positively linked with imidacloprid accumulation in the tissue. The effects had dose- and time-dependent variations in how various tissues reacted. Based on an elevated response in each of the aforementioned biomarkers during routine monitoring, imidacloprid seems to serve as an ambient chemical stressor for adult black tiger shrimp (Penaeus monodon) (Butcherine et al. 2022). On primary cell cultures of the gonad, mantle, digestive gland tissues, and gill of Unio sp., the cytotoxic effects of a neonicotinoid, a pyrethroid insecticide, acetamiprid, and flumethrin were investigated. This investigation demonstrated that flumethrin was more cytotoxic to all tested cells than acetamiprid (Arslan et al. 2021). Daphnia was shown to have a greater risk quotient for the majority of organophosphate pesticides than fish or algae (Sumon et al. 2018).
The simplest model for bioaccumulation is a nonmetabolized chemical dissolved in soil or interstitial water. When evaluating dissipation from the body of aquatic creatures that have consumed polluted sediments, both gastrointestinal clearance and metabolism of the contaminating substances should be taken into account. In order to evaluate the kU, kM, kPE, and kME values (wherein k is the first-order rate constant; U, uptake; M, metabolism; P, parent molecule; E, elimination), Schuler et al. (2003) employed a three-compartment model to investigate the bioaccumulation of sediment-spiked benzo[α]pyrene in worms, amphipods, and larval midges. With alterations that were equivalent to those in kPE and kME values, only the larval midge demonstrated substantial metabolism (75% after 3 days).
In aquatic species, the elimination of particular compounds can occasionally result in biphasic profiles (Egeler et al. 1997; Muir et al. 1982; Landrum and Scavia 1983; Shaw and Connell 1987; Richter and Nagel 2007). Such profiles are the result of a number of mechanisms, such as decreased metabolite clearance or bound residue formation. Additionally, because stomach contents can leave the body either rapidly or slowly, they might affect how chemical elimination curves seem.
According to research by Bartlett et al. (2004), Hyalella azteca held in a cage above the sediment at an early stage of exposure exhibited higher body residues of tributyltin than those kept in a water-sediment system with added sediment. If all processes involved in uptake and dissipation are considered, the kinetic and fugacity models may be able to satisfactorily describe the bioaccumulation profiles of a chemical in aquatic species (i.e., uptake via gills, ingestion of food or sediment, metabolism, and elimination). Gobas et al. (1991) developed the fugacity model for fish and recommended feeding rates and transport variables that lead to gastrointestinal absorption in the water and lipid phases. Using monitoring data on sediment, crayfish, mussels, caddisfly larvae, and gammarus, the fugacity of PCB congeners in biota (fB) was theoretically estimated for the detritivorous and filter-feeding benthic invertebrates (Morrison et al. 1996).
The biosediment accumulation factor (BASF) values, which were calculated using the connection between fB/f = 0.62×BASF, were found to be 0.5–12.4. The ratio of fB to the fugacity in sediment (fS) ranged from 0.3 to 7.7. The fB/fS values demonstrated a propensity to follow a parabolic relationship with log Kow and statistically outperformed the equilibrium partition strategy in predicting bioaccumulation. The percentage of organic carbon or fat in the meal that was lost during digestion, together with the velocity of food absorption, was the most sensitive factor. Additionally, the fugacity model was utilized to estimate bioaccumulation at various trophic levels (Hendriks et al. 2001) (Table 8). In various aquatic creatures that interact via a food web, this model was only marginally successful in forecasting the absorption process (r2 = 0.39), but it was more successful in predicting elimination (r2 = 0.70). For the chemicals maintaining log Kow values of 2–7, the projected bioaccumulation values were usually consistent with those that had been observed. Thomann and Komlos (1999) successfully implemented a kinetic model to analyze the low BSAF values (0.01–0.1) observed in crayfish exposed to different polycyclic aromatic hydrocarbons (PAH) in a creek (Table 9).
They found that for log Kow values of PAHs above roughly 5–6, the proportional contribution of the food exposure pathway toward water steadily increased, with its magnitude being underlined by alkyl substitution in the ring structures. Aquatic animals have a tendency to bioaccumulate these toxins along the food chain, making compliance with current rules and developing mitigation measures essential.
3 Xenobiotics
To begin with, the term xenobiotic is of Greek origin from the word xenos meaning strange or foreign and bio which means life. These are chemicals that exhibit abnormal structural characteristics (Fetzner 2002). The aberrant presence of any substance for that matter at higher concentrations could also be termed as xenobiotics. At the same time, natural substances, which found its way into animals or humans, could also be defined as xenobiotic. Banjoko (2014) suggested the term xenobiotics as the biological and physiological effects of exogenous substances either synthetic or natural on the cells, tissues, or the organs of organisms. The xenobiotic sources that are caused by humans include industrial, domestic, pharmaceutical, agricultural, and transportation sources (Essumang 2013). Based on the physiological and biological effects of exogenous substances, whether natural or synthetic (drugs, chemicals), on the cells, tissues, or organs of animals, Banjoko (2014) introduced the term “xenobiotic.”
Numerous xenobiotics are possibly hazardous to the organisms which are exposed to them within the environment niche. Nevertheless, the bioavailability of such chemical substances depends on the attribute of the chemical, organism, and the environment. The bioaccumulation of the chemical residue within the organism decides the toxicity of the xenobiotic (Mäenpää 2007). It is said that the long-term effects of the xenobiotics in the environment might be contained up to months to years within the environment.
Advanced technologies to regulate the trace polar compounds have aided in providing new perceptions on the removal of xenobiotics. Initially, in the USA, pharmaceutical products (0.8–2 μg/L) were recorded in the treated wastewater (Garrison et al. 1976). Following which, clofibric acid (1 μg/L) was reported in the UK, which was found in the rivers (Richardson and Bowron 1985). Ibuprofen and naproxen concentrations were identified in the Canadian wastewaters by Rogers. Significant reduction in the population of the Indian and Asian white-backed vultures nesting in the Keoladeo National Park from North-Western India was an impact of the diclofenac (pain killer) accumulation, a pain killer applied in the veterinary sector to treat cattle. Several toiletries and drugs, phthalates, insect repellents, and steroids were reported by the Geological Survey Department of the USA (Embrandiri et al. 2016). Although, there was traces of the xenobiotic concentrations, effects of the chronic exposure were uncertain. Increase in the usage of bulk drugs lately, has been associated as a significant source of environmental pollution consisting of active pharmaceutical compounds in definitive locations (Gunnarsson et al. 2009; Fick et al. 2010). In addition, there is a cause of concern globally on the residues (pharmaceutical) found in the surface water which can pose negative effects on the aquatic organisms. Thereby, there is challenging to develop, an unambiguous strategy to prioritize drugs on which major focus is on the environmental research (Fick et al. 2010).
3.1 Common Xenobiotic Compounds Based on Its Course of Action
Xenobiotics target specified molecular and metabolic pathways in animals and humans in the ecosystem. Howbeit, the xenobiotics when introduced into the environment might impart effects on some pathways in animals, exhibiting identical target biomolecules, cells, tissues, or organs. The present ecotoxicological effects of pharmaceuticals confront basically acute toxicity and are extensively centered on aquatic organisms. The impact of environmental variables such as pH on its toxicity has been rarely or has not been investigated. The majority of the studies have been directed toward the acidic pharmaceuticals that induce various toxicities subjected to speciation at distinct ambient pH. Furthermore, till date, limited research has been conducted on the effects of drug metabolites. Discussed below are the frequently used xenobiotic compounds that are predicted to pose environmental distress.
3.1.1 Analgesics and Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
Extensively used nonsteroidal anti-inflammatory drugs (NSAIDs) are diclofenac, ibuprofen, and naproxen, and their metabolites like the carboxy-ibuprofen and hydroxyl-ibuprofen could be traced in the water sewage and surface. In the USA, the levels of NSAID in the sewage system exceeded to 1 μg/L, and its concentration in the effluents of the conventional sewage plants (biological treatment and mechanical clarification) exceeded to 0.1 μg/L (Gross et al. 2004). The deacylated form of acetylsalicylic acid, which is believed to be the more active form, has been determined in majority of the municipal wastewaters to levels up to 4.1 μg/L, 13 μg/L, or even 59.6 μg/L, respectively (Embrandiri et al. 2016.). Acetaminophen (paracetamol), which is similar to the acetylsalicylic acid, has been recovered from the sewage-treated water. However, it was reported that in 24% of samples from US streams, acetaminophen (up to 10 μg/L, median 0.11 μg/L) was spotted. In addition, in several countries, analgesic codeine (median 0.01 μg/L) was recurrently detected in 7% of samples. Furthermore, diclofenac was also commonly identified in the wastewater and in minute amounts in the surface water. Ibuprofen and their metabolites (0.1–20 μg/L) were detected in all the seawater and sewage samples in Norway (Wiegel et al. 2004). In the stream water samples, ibuprofen (1 μg/L, median 0.2 μg/L) was traced in high concentrations (Kolpin et al. 2002). Besides, many other NSAID compounds have also been traced in the surface, sewage, and drinking water samples.
3.1.2 Mode of Action
Pharmaceutical and xenobiotic substances have an impact on traditional sewage treatment facilities and may hinder biological processes like nitrification. The oxidation of ammonium to nitrite, the initial stage in the nitrification process, is sensitive to the presence of xenobiotic chemicals. Under uncontrolled circumstances, xenobiotics can completely stop the biological nitrogen process by inhibiting the first stages of nitrification (Essumang et al. 2009). It has been determined that many medication combinations are exposed in streams and rivers. Diphenhydramines that are antidiabetic and antihistamine have been proven to significantly disturb the biofilm community, which is crucial to the ecology. In biofilms, which are microbial aggregations, cells that are frequently encased in extracellular polymeric substances (EPS) matrix cling to one another or to a surface. The vital food source for invertebrates, which in turn feed fish and other large animals, is biofilms. Therefore, animals in the stream food web including insects and fish may be affected by diphenylamines’ impacts on biofilm (Rosi-Marshall 2013). Antidepressant use causes some shellfish to begin spawning early, upsetting the aquatic balance. Additionally, it was discovered that fluoxetine and propranolol negatively affected zooplankton and benthic species (Hoffman et al. 2005). The development of biofilms by bacteria is caused by a variety of factors, including cellular recognition of specific or nonspecific attachment sites, nutritional signals, or exposure of planktonic cells to subinhibitory concentrations of antibiotics (Hoffman et al. 2005; Karatan and Watnick 2009). When exposed to tributyltin, female marine snails displayed masculinization (imposex) (TBT). Due to imposex, local populations of the dog whelk (Nucella lapillus), a “species of predatory sea snail,” have declined or gone extinct all across the world, especially in coastal regions all over Europe and the North Sea. Diverse fish species exposed to effluents have been negatively impacted by EDCs (endocrine disruptors), leading to reproductive issues. Similar effects have also been seen in turtles (Cleuvers 2003; Le Page et al. 2011). Most cleaning products contain the broad-spectrum antimicrobial ingredient triclosan (TCS), which works to stop bacterial, fungal, and mildew growth. Triclosan is released into water streams by leaking sewers, sewage overflows, and domestic wastewater. The ongoing use of these antibiotics causes the development of resistant bacteria, which could reduce the effectiveness of crucial antibiotics (Drury et al. 2013).
Xenobiotic substances that are released into surface water may leak into groundwater, although this practice is currently severely prohibited since it could compromise the ecological integrity of aquatic ecosystems. Important biological markers of xenobiotic contamination include some aquatic creatures (Fent et al. 2006). Xenobiotic substances can enter the environment as metabolites or in their original forms. Humans may process xenobiotic chemicals through consumption, excretion, and wastewater disposal (Singh et al. 2016). In typical sewage treatment facilities, some xenobiotic chemicals are nonbiodegradable and discharged with treated runoff, which could contaminate aquatic systems like rivers, lakes, and estuaries (Embrandiri et al. 2016). The most significant and crucial characteristics of xenobiotics are their high production, environmental persistence, and biological impacts. Concerns have been raised around the world due to studies showing an increase in the number of xenobiotic chemicals discovered in aquatic systems (surface water) (Embrandiri et al. 2016). Animals in the food chain, such as fish and insects, are impacted by xenobiotics (Rosi-Marshall 2013).
3.1.3 NSAIDs (Nonsteroidal Anti-inflammatory Drugs)
The NSAIDs (nonsteroidal anti-inflammatory drugs) are usually employed to relieve fever and treat pain and inflammation, and under few circumstances, they are employed in the long-term treatment of rheumatic diseases. NSAIDs act by inhibiting either one isoforms of the cyclooxygenase enzyme (COX-1 and COX-2), either reversibly or irreversibly, which are involved in the synthesis of various prostaglandins from arachidonic acid. The classical NSAIDs inhibit the COX-1 and COX-2 at different degrees, while the new NSAIDs act more specifically on the COX-2, which is an inducible form, responsible for various inflammatory reactions. The selectivity of the drugs is mainly due to the differences in binding site size, while the former NSAIDs work in nonspecific fashion (Szewczuk et al. 2004).
3.1.4 Blood Lipid Regulators
Habitually described pharmaceutical in the observational studies is the clofibric acid, an active metabolite and is commonly employed to regulate blood lipids like etofibrate, etofylline, and clofibrate. These chemicals have been detected in copious amounts in surface waters, wastewaters, and seawaters, specifically at a quite higher concentration in the groundwater (4 μg/L) and drinking water (0.07–0.27 μg/L), respectively. Gemfibrozil and bezafibrate, known as lipid-lowering agents, have been found in extreme concentrations (up to 4.6 and 0.79 μg/L) in surface water and wastewater, respectively (Kolpin et al. 2002). Additionally, auxiliary drugs acting as metabolites of fenofibrate such as fenofibric, gemfibrozil, and clofibric acid have also been traced in surface water and sewage water (Heberer 2002).
Two types of antilipidemic drugs are fibrates and statins, which are employed in lowering the triglycerides (fibrates) and cholesterol concentration (statins and fibrates) in the blood plasma. These drugs are often frequently targeted in the aquatic environment. Statins (inhibitors of cholesterol synthesis) are said to act by the inhibition of 3-hydroxymethylglutaril coenzyme A reductase (HMG-CoA), which is important to convert HMG-CoA to mevalonate. Reports have demonstrated that the statins have exhibited effects on the biosynthesis (in vitro) and on the mandibular organ of lobsters and also on the juvenile hormone synthesis in insects. Alteration in the gene transcription, which encode for the proteins that control the metabolism of lipoprotein, in addition to activating the enzyme lipoprotein lipase, whose main role is controlling the protein metabolism, is one of the major effects of the fibrates. The enzyme lipoprotein lipase has a role to play in the conversion of very-low-density lipoprotein (VLDL) to high-density lipoproteins (HDL), thereby decreasing the concentration of the plasma triglyceride. Moreover, it stimulates the uptake by converting the acetyl CoA derivatives and catabolism by the beta-oxidation pathways. A combination of these processes leads to the reduction of the triglyceride synthesis and fatty acids and thus decreases the VLDL production. Studies have demonstrated that chronic exposure to fibrates caused hepatic damages; this could be due to the mitochondrial oxidative phosphorylation inhibition. At the same time, it was observed that the fibrates in rodents caused massive proliferation of peroxisomes. There was a strong co-relation established between hepatocarcinogenicity and fibrate exposure in rodents, while this wasn’t demonstrated in humans (Cajaraville et al. 2003). These demonstrations enhance the interest to focus on the ecotoxicological impact of therapeutic applications of these drugs.
3.1.5 Beta-Blockers
Various beta-blockers like metoprolol, propranolol, and bisoprolol were found in the wastewater of concentrations 2.2 μg/L, 0.59 μg/L, and 2.9 μg/L, respectively. Few other beta-blockers, betaxolol (0.028 μg/L) and nadolol, in the surface waters were detected at its lowest concentration (Ternes 1998). The presence of metoprolol, bisoprolol, and propranolol was detected in the surface water, in addition to the detection of sotalol in groundwater (Sacher et al. 2001).
They act by inhibiting the beta-adrenergic receptors. Generally, these are employed in the treatment of hypertension and preventing heart attacks in high-risk patients. Some of the functions of the adrenergic system are bronchodilation, vasodilatation of blood vessels, oxygen supply, and heartbeat regulation. Moreover, it is necessary for the metabolism of lipids and carbohydrates in case of starvation. Beta-blockers could selectively hinder one or more β-receptor types based on the requirement. For example, these chemicals are involved in the treatment of hypertension by preventing the cardiac arrests, as the β-2 blocker subtype is not present in the heart.
Unlike the metoprolol which lack the ability to stabilize cell membranes, the beta-blocker 9 propranolol, a beta-1-adrenoceptor antagonist, exhibits those properties (Doggrell 1990). Negative effects of these beta-blockers are majorly disturbed peripheral circulations and bronchoconstriction. These work by passing the blood-brain barrier, to act on the central nervous system, due to their lipophilicity (Heberer 2002). Ractopamine and clenbuterol have the role of β-agonist in mammals; however, they showed different reactions in rainbow trouts. The difference in the functions may be due to the difference in their function and structures and varied affinity with β-blockers and mechanisms triggered by these drugs.
3.1.6 Neuroactive Compounds (Antiepileptics and Antidepressants)
The antiepileptic carbamazepine was most commonly detected at the highest concentration in the wastewater (up to 6.3 μg/L) (Ternes 1998) and in lesser concentrations in other media (Heberer 2002). In all the effluent samples, carbamazepine was traced on the Canadian sewage treatment plant (STP) (2.3 μg/L). In addition, it was also reported to be traced on all the samples of German river Elbe and streams (Wiegel et al. 2004), which exceeded a concentration of 1 μg/L in the surface waters (Ternes 1998; Heberer 2002) and also was detected in the groundwater (Sacher et al. 2001). Carbamazepine was detected at average concentrations of 20.9 ng/mg in the STP. In Germany, diazepam was reported to be present in 8 out of 20 treatments, at lower concentrations (0.04 μg/L) (Ternes 1998), whereas it was recorded at a concentration of 0.66 μg/L (van der Ven et al. 2004) in Belgium. Fluoxetine, an antidepressant, was found in the US streams and Canadian effluent samples at a median concentration of 0.012 μg/L (Kolpin et al. 2002). Besides, primidone (0.6 μg/L), an antiepileptic drug, was reported in the sewage (Heberer 2002) samples.
3.1.7 Mode of Action
The overall neuronal activity will be suppressed by the antiepileptic drugs. This could be brought about either by enhancing the inhibitory effects of the neurotransmitter (GABA) by binding to the site exactly which corresponds to the gamma subunit of the corresponding receptor (member of benzodiazepine family/diazepam). One more way is by blocking the voltage-dependent sodium channels of excitatory neurons (e.g., carbamazepine). Serotonin uptake is inhibited by fluoxetine (antidepressant). Serotonin is a neurotransmitter which interferes with the food intake, sexual behavior, and neuronal and hormonal mechanisms. Norfluoxetine, fluoxetine, desmethylsertraline, and sertraline have been found accumulated from the wild fish samples in the, reflecting on the bioaccumulation potential (Brooks et al. 2005).
3.1.8 Various Other Compounds
The effluents of the surface waters and sewage treatment plants have found to be contaminated by drugs comprising of cotinine and caffeine (a nicotine metabolite). Caffeine was found detectable in higher levels (6.0 μg/L (median 0.1 μg/L (Kolpin et al. 2002); this can act as an anthropogenic marker in aquatic systems because of its ubiquity in the groundwater, seawater, and surface water (Wiegel et al. 2004). In the streams of the USA, the antacids ranitidine and cimetidine were found to occur at the respective concentrations (0.58 and 0.01 μg/L) (Kolpin et al. 2002). In the surface water (0.49 μg/L), groundwater, and municipal wastewater (15 μg/L), iopamidol was found to be detected. Metformin, an antidiabetic compound (5%), was found in the stream water samples, with the estimated levels of 0.11 μg/L (Kolpin et al. 2002). Bronchodilators such as salbutamol and β2-sympathomimetic terbutaline were also observed in the sewage waste waters, however not exceeding 0.2 μg/L concentration (Ternes 1998).
Ranitidine and cimetidine compounds hinder the histamine receptor type 2 in the gastric system, hence inhibiting the antacid (acid secretion) and thus used in the treatment of gastric ulcer. Metformin is an antidiabetic agent; however, the mechanism of action isn’t fully understood. It has been studied that this drug increases the cellular glucose usage, thus inhibiting gluconeogenesis. Metformin is said to act on the insulin receptor by directly stimulating the insulin receptor or indirectly via inhibition of tyrosine phosphatase (Holland et al. 2004).
3.1.9 Effects of Xenobiotics on Ecosystem
It has been recorded that more than 13 million deaths and 24% of the world diseases are a cause of environmental pollution and exposures to various contaminates, which could be indeed avoided. As of today, traceable amounts of pharmaceutical preparations (metabolite/parent drug) are found in both food and water sources (Banjoko 2014). Severe consequences could be predicted as a result of medications for humans and animals, which extends beyond the conventional medical care. Healthcare sectors are one of the significant causes of active pharmaceutical ingredients (API) let out from medication residues and a prime cause of environmental pollution.
3.1.10 Effects on Aquatic Ecosystem
The aquatic organisms could be an important biological indicator of pollution. A comprehensive study was conducted on the occurrence, ultimate fate of the pharmaceuticals in the aquatic environment, and the mechanism of action of various pharmaceuticals and expanded chronic and acute ecotoxicological effects on organisms (Fent et al. 2006).
Pharmaceuticals are said to be the most frequently released effluents into the environment either in their metabolite or the original form. The main pathway in the humans includes ingestion, excretion, and disposal through the wastewater. The largest source of human pharmaceuticals is wastewater.
Wastewaters let out from manufacturers, hospitals, and landfill leachates might contain significant concentrations of pharmaceuticals. The nondegradable pharmaceuticals in the sewage treatment plant (STP) are released into the treated effluents which ultimately results in the contamination of drinking water, groundwater, estuaries, rivers, and lakes. Agricultural sector contamination is a possibility when sewage water is let into the farms and fields. Moreover, the drugs used in the veterinary sector enter the waterways as a course of surface application for agricultural farming purposes, and runoff affects the fish farming. Effluents let out from the pharmaceuticals have high environmental significance and sometimes have high production volume and increased environmental persistence particularly after the long-term exposure. It has been noted that the pharmaceutical concentrations traced in surface waters globally are a matter of concern, mostly with respect to the aquatic flora and fauna. Thereby, its is a huge task in initiating a strategy to prioritize drugs on which majority of the environmental research focus must rely upon. Among the aquatic life, most often shared drug targets with humans are the fishes. Very little information is known regarding the long-term effect of drugs in aquatic organisms. Diclofenac is said to interfere with the organ histology and gene expression of fishes while exposed at a concentration of l μg/L (Cuklev et al. 2012). In India, surface water collected from 27 locations of the rivers in southern India (Tami and Kaveri) exposed the presence of nonsteroidal anti-inflammatory drugs (NSAIDs) such as acetylsalicylic acid, naproxen, ketoprofen, and ibuprofen. This alarming situation imparts direct toxicity in the case of all the consumers of the water (Shanmugam et al. 2014). In a similar scenario, the effluents let out from a treatment plant in Hyderabad, India, was reported to be the reason for the deleterious effects on aquatic organisms. Embryo toxicity assay carried out revealed that smaller concentrations (0.2%) of effluents hindered the growth of tadpole by 40%; however, the growth rate of zebra fish (Danio rerio) was not impeded. Regardless, the study focused on fishes; meanwhile, it also shed light on how the aquatic vertebrates are probably affected as a result of effluent exposures and the substances responsible in causing toxic effects at the threshold dilutions (Shanmugam et al. 2014). Rivers and streams have been identified as sources often exposed to various drug combinations. Significant disruption was caused to the biofilm community, an important part of the ecosystem as a result of usage of antihistamine diphenhydramines and antidiabetics. In addition, the biofilms also serve as a major source of food for the invertebrates that in return are fed by larger animals of the aquatic lives.
Thus, the diphenylamines could affect biofilms and therefore have repercussion for animals in stream food webs like the fishes and insects (Rosi-Marshall 2013). Disruption of the aquatic equilibrium by early activation of spawning in some shellfish might be one of the effects of antidepressant usage.
Moreover, deleterious effects were posed as a result of fluoxetine and propranolol usage on the benthic organisms and zooplanktons (Hoffman et al. 2005). Aspects such as nutritional cues, nonspecific attachment sites, cellular recognition of specific attachment sites, and planktonic cells exposed to antibiotics at the subinhibitory concentrations lead to the formation of biofilms by microbes (Hoffman et al. 2005; Karatan and Watnick 2009). Exposure of female marine snails to tributyltin (TBT) resulted in masculinization (imposex). The decline or extinction of local populations of the dog whelk (Nucella lapillus), a predatory sea snail species in the coastal areas and all over the North Sea and Europe, is due to imposex. One of the best examples of reproductive impairment causing population decline is the DDE (dichlorodiphenyldichloroethylene)-induced eggshell thinning in birds in North America and Europe. Ovotestis in male western gulls is a result of gradual DDT complex (dichlorodiphenyltrichloroethane) exposure. A variety of fishes, exposed to effluents (EDC-endocrine disruptors) have negative effects on their reproductive system. Similar effects have been demonstrated in turtles (Cleuvers 2003; Le Page et al. 2011). Broad-spectrum antimicrobial compounds (Triclosan-TCS) have been active ingredients in the cleaning products for preventing the growth of mildew, bacteria, and fungi. This is reported to enter the domestic wastewater, sewage overflows, water streams, and leaking sewerage, causing adverse effects on the aquatic life forms.
The removal of these contaminants is a severe environmental problem because there are several xenobiotic chemicals present in typical sewage treatment systems, each of which has its own effects. Xenobiotics are persistent in the environment and difficult to break down, for example, trichloroethylene (TCE) and polycyclic aromatic hydrocarbons (PAHs).
Due to these xenobiotics’ unique chemical characteristics, they accumulate in the environment. As a result, these xenobiotics exhibit traits of toxicity and accumulation in the environment and have an impact on both the natural world and human life. Xenobiotic pollutants can have an impact on the climate and human health and are typically found in biological systems, agricultural runoff, and water and wastewater sources (Fatta-Kassinos et al. 2011). Common xenobiotic receptors exist in traditional sewage treatment plants and must be treated with municipal wastewater before being released into aquatic systems. The presence of trace metals, xenobiotic substances, and synthetic organic chemicals such as PAHs, phthalates, and pesticides in water bodies has been documented (Essumang and Ankrah 2010).
Pharmaceutical and xenobiotic substances have an impact on traditional sewage treatment facilities and may hinder biological processes like nitrification. The oxidation of ammonium to nitrite, the initial stage in the nitrification process, is sensitive to the presence of xenobiotic chemicals. Under uncontrolled circumstances, xenobiotics can completely stop the biological nitrogen process by inhibiting the first stages of nitrification (Essumang et al. 2009). It has been determined that many medication combinations are exposed in streams and rivers. Diphenhydramines that are antidiabetic and antihistamine have been proven to significantly disturb the biofilm community, which is crucial to the ecology. In biofilms, which are microbial aggregations, cells that are frequently encased in an extracellular polymeric substance (EPS) matrix cling to one another or to a surface. The vital food source for invertebrates, which in turn feed fish and other large animals, is biofilms. Therefore, animals in the stream food web including insects and fish may be affected by diphenylamines’ impacts on biofilm (Rosi-Marshall 2013). Antidepressant use causes some shellfish to begin spawning early, upsetting the aquatic balance. Additionally, it was discovered that fluoxetine and propranolol negatively affected zooplankton and benthic species (Hoffman et al. 2005). The development of biofilms by bacteria is caused by a variety of factors, including cellular recognition of specific or nonspecific attachment sites, nutritional signals, or exposure of planktonic cells to subinhibitory concentrations of antibiotics (Hoffman et al. 2005; Karatan and Watnick 2009). When exposed to tributyltin, female marine snails displayed masculinization (imposex) (TBT). Due to imposex, local populations of the dog whelk (Nucella lapillus), a species of predatory sea snail, have declined or gone extinct all across the world, especially in coastal regions all over Europe and the North Sea. Diverse fish species exposed to effluents have been negatively impacted by EDCs (endocrine disruptors), leading to reproductive issues. Similar effects have also been seen in turtles (Cleuvers 2003; Le Page et al. 2011). Most cleaning products contain the broad-spectrum antimicrobial ingredient triclosan (TCS), which works to stop bacterial, fungal, and mildew growth. Triclosan is released into water streams by leaking sewers, sewage overflows, and domestic wastewater. The ongoing use of these antibiotics causes the development of resistant bacteria, which could reduce the effectiveness of crucial antibiotics (Drury et al. 2013).
Xenobiotic substances that are released into surface water may leak into groundwater, although this practice is currently severely prohibited since it could compromise the ecological integrity of aquatic ecosystems. Important biological markers of xenobiotic contamination include some aquatic creatures (Fent et al. 2006). Xenobiotic substances can enter the environment as metabolites or in their original forms. Humans may process xenobiotic chemicals through consumption, excretion, and wastewater disposal (Singh et al. 2016). In typical sewage treatment facilities, some xenobiotic chemicals are nonbiodegradable and discharged with treated runoff, which could contaminate aquatic systems like rivers, lakes, and estuaries (Embrandiri et al. 2016). The most significant and crucial characteristics of xenobiotics are their high production, environmental persistence, and biological impacts. Concerns have been raised around the world due to studies showing an increase in the number of xenobiotic chemicals discovered in aquatic systems (surface water) (Embrandiri et al. 2016). Animals in the food chain, such as fish and insects, are impacted by xenobiotics (Rosi-Marshall 2013).
4 Bioconcentration and Bioaccumulation of Xenobiotics
Bioaccumulation of persistent hydrophobic xenobiotics in aquatic species can occur through a variety of methods, including bioconcentration, ingestion, and biomagnification. Even if the subchronic, chronic, or acute consequences are not obvious, bioaccumulation should be considered a hazard criterion in and of itself, because certain harmful effects may not be noticed until later in life.
Bioconcentration refers to the absorption and retention of a substance in an organism solely through respiration from aquatic ecosystems or terrestrial ones. The bioconcentration factor (BCF) is defined as the concentration of a chemical in an organism divided by the concentration of the same chemical in the environment or a component of the environment (e.g., water). The BCF is mostly used to predict the degree of accumulation of an organic contaminant in water by organisms. For terrestrial animals, food is usually the primary source of many xenobiotic compounds, and if the rate of intake is constant, a steady state is eventually formed. Chemicals can enter the organism primarily through three different entryways such as gills, skin, and digestive system. The chemicals are dispersed throughout the various tissues once they have entered the body. The distribution process is influenced by the characteristics of the absorbed compounds, which may have a strong affinity for specific biomolecules like membrane lipids, blood proteins, structural proteins, or storage lipids. As a consequence, they tend to accumulate in organs that are abundant in these biomolecules (Da Cuña et al. 2020; Hou et al. 2017; Grech et al. 2016).
The process by which pesticides enter organisms directly from water through the gills or through epithelial tissues is known as bioconcentration. In the contrary, bioaccumulation includes the effect of dietary assimilation through food consumption or ingestion of bottom sediments. Organic hydrophobic compounds, including PCBs, are predominantly bioaccumulated in the lipid body component of the organisms. The discovery of DDT (dichlorodiphenyltrichloroethane) and methyl mercury residues in fish, fish-eating birds, and other species in the 1960s brought the phenomena of bioaccumulation to the attention of the general public.
Intake of a chemical and its concentration in the organism by all possible means, including contact, respiration, and ingestion, is called bioaccumulation. Bioaccumulation of organic hydrophobic chemicals, such as PCBs, mainly happen in the lipid body fraction of the organisms. The xenobiotic compounds dissolved/suspended in the medium, as well as ingested food and sediment residues, are taken up. Bioaccumulation factor needs to be incorporated.
In the case of a predator, the majority of xenobiotic compounds are consumed through the ingestion of prey that has previously concentrated on a xenobiotic (Fig. 4).
4.1 Biomagnification of Xenobiotics
Biomagnification is the process of transferring xenobiotic chemicals from food to an organism, resulting in larger quantities than the source. The term biomagnification includes the full process of bioconcentration and bioaccumulation. Furthermore, it considers the slow increase in chemical concentration in the tissues of organisms as it moves through the food chain. This is widely assumed to be a common occurrence in marine food webs. Only organic mercury displays biomagnification in studies on metals, while the majority of metals are controlled and eliminated and do not biomagnify.
4.2 Xenobiotic Compounds: Other Effects
High toxicity: Many xenobiotics are harmful to bacteria, lower eukaryotes, and even humans, such as halogenated and aromatic hydrocarbons. They may cause numerous skin issues and impair reproductive capacity at low dosages.
Cancer-causing substances: The majority of xenobiotic chemicals include carcinogens. They experience significant bioaccumulation and biomagnification. The cells become resistant to antibodies as a result of this transmission in the food chain. They have an extremely high risk of developing cancer-like diseases.
High resistivity to the environment: Many xenobiotics are recalcitrant and remain in the environment, causing their concentration to rise with time.
Coral bleaching: When coral is exposed to high quantities of various xenobiotics such as copper, herbicides, and oil, zooxanthellae are lost.
Since high xenobiotic concentrations cause zooxanthellae loss, bleaching from such sources is typically confined and/or transient.
4.3 Marine Life Affected by Xenobiotics
Xenobiotics have a deleterious impact on a number of marine creatures’ metabolic processes, particularly those of developing fish embryos, which results in morphological and functional defects, stunted growth, and eventual death. Fish have also been known to have altered body shape, bodily abnormalities, and delayed hatching, and some even die (Arya et al. 2019). The fact that dyes and paints impede sunlight and obstruct gas exchange makes them xenobiotics even when they are present in minute concentrations (Rápó et al. 2021). The main sources of xenobiotic contamination in marine life are pesticides and herbicides. Organophosphorus, nitrophenols, morpholine, synthetic pyrethroids, and carbamates are just a few examples of the chemicals that are frequently employed in agriculture and daily life. These chemicals later find their way into various water bodies, such as the sea and ocean. Marine life and invertebrates are seriously threatened by insecticides like cypermethrin (Tornero and Hanke 2016). The bioaccumulation of xenobiotics causes the ingested substances to change into a variety of metabolites. In organs such as the liver, gills, and kidneys, metabolic processes occur very vigorously (Gomez et al. 2010). Consequently, typically simpler-to-expel molecules are created, yet there are also known instances in which the metabolic byproducts prove to be more toxic and accumulative than the initial chemicals (La Farre et al. 2008). Excretion is the final stage of interaction of chemicals with the body. In this scenario, elimination occurs predominantly through the gills and through fecal egestion, and the primary routes are identical to absorption (Arnot and Gobas 2006). Additionally, a pseudo-elimination process that involves dilution of chemical substances in developing tissues should be taken into account (Segner 2015). The bioaccumulation phenomenon, which denotes the accumulation of ambient chemicals in living things’ tissues, is caused by all of the abovementioned processes.
4.4 Food-Based Xenobiotic Uptake
The gastrointestinal system easily absorbs the xenobiotics drawn in through the integument and gill surface using similar diffusion and transport mechanisms. Due to prolonged interaction between the food and membranes, lipophilic xenobiotics ingested are easily absorbed. In unionized form, weak acids and bases are absorbed. While the intestinal pH favors the absorption of neutral or weakly basic xenobiotics, the stomach pH favors the diffusion of weak acids.
To fully understand how chemicals enter the body, what happens to them inside, and how they are excreted, it is worthwhile to trace the processes of uptake, distribution, metabolism, and excretion.
Due to the resistivity of xenobiotics, they are highly resistive and complex. There is a need to control the spread of these compounds in the food chain and prevent their further magnification. For the removal and detoxification of toxins from the environment, the microbial bioremediation technique has recently emerged as the best alternative. Synthetic biology is addressing xenobiotic and related compound decontamination and remediation solutions in the environment. It has been discovered that understanding existing metabolic pathways is a prerequisite for removing xenobiotic compounds.
4.5 Xenobiotic Metabolism in Aquatic Animals
Xenobiotics are chemicals which are foreign to an organism’s normal metabolism (Brodie et al. 2002). Most commonly, xenobiotics are referred to carcinogens, drugs and various compounds artificially introduced into environments. Majority of xenobiotics are toxic in nature, and an organism tries to overcome the toxic effect by modifying the chemical structure by set of interconnected reactions called metabolic pathways. These pathways are mediated by a variety of enzymes.
The xenobiotics are ultimately excreted from the body. The excretion can happen in two ways: (1) excretion in unchanged state and (2) metabolized endogenously and then excreted (Johnson et al. 2012). Xenobiotics may be water soluble or lipid soluble. Water-soluble compounds may be eliminated from the body in the unchanged state, whereas the lipophilic or lipid soluble compounds must be metabolized in order to make it more polar and water soluble and can be excreted from the body easily (Schenkman 1999).
Metabolism of xenobiotics can result in (1) activation and (2) detoxification. In case of activation, the metabolism of xenobiotics can result in increasing the toxicity, whereas in the case of detoxification, metabolism results in decreasing toxicity. There are two types of metabolism: Phase I and Phase II.
-
Phase I: This type of metabolism is also called functionalization reaction. The enzymes involved in this phase are responsible for oxidation, reduction, hydration, and hydrolysis, or they introduce functional group like -OH, -COOH, etc. to xenobiotics so that enzymes of Phase II metabolism attach large polar moieties such as glutathione, sulfate, amino acid, etc. The metabolites resulting from Phase I metabolism are more reactive chemically than the parent compound. This metabolism ultimately develops metabolites which is suitable for undergoing Phase II metabolism (Livingstone 1998).
-
Phase II: Alternatively, this type of metabolism is called conjugative reaction. In this metabolism, activated derivatives produced in Phase I metabolism are conjugated with polar moieties like glutathione, amino acids, etc. to produce water-soluble derivatives which can be easily excreted (Livingstone 1998).
4.5.1 Enzymes Involved in Phase I Metabolism
As mentioned earlier, Phase I involves oxidation, reduction, hydrolysis, and hydroxylation. Different enzymes participate in each reaction:
-
1.
Oxidation: Oxidation is performed mainly by three categories, namely, (1) cytochrome P-450 monooxygenase or mixed function oxidase (MFO), (2) microsomal flavin-containing monooxygenase (MFMO), and (3) other oxidative enzymes.
-
(a)
Cytochrome P-450 monooxygenase or Mixed Function Oxidase
The name MFO is coined because it catalyzes reactions in which each of two atoms of O2 is utilized for different purposes in a reaction. These enzymes oxidize two different substrates. The MFO converts the lipophilic substrates (RH in the following equation) into a metabolite which is more hydrophilic than RH (ROH in the following equation) as follows:
$$ \mathrm{NADPH}+{\mathrm{H}}^{+}+{\mathrm{O}}_2=\mathrm{RH}\overset{\mathrm{MFO}}{\to }{\mathrm{NADP}}^{+}+{\mathrm{H}}_2\mathrm{O}+\mathrm{ROH} $$Herein, reaction oxidation of RH and NADPH is performed by each of the atoms of the oxygen. This family of enzymes has the ability to convert a wide variety of substrates (i.e., xenobiotics) such as insecticides, carcinogens, and environmental pollutants to more polar compounds that can be very easily excreted into the environment (Cederbaum 2015).
-
(b)
Microsomal Flavin-Containing Monooxygenase (MFMO)
Flavin-containing monooxygenases (FMOs) and cytochrome P450 are two categories of proteins of microsomal origin. They add molecular oxygen to lipophilic compounds and convert them into water-soluble compound to ensure its rapid excretion. FMOs are responsible for oxygenation of nucleophilic S, N, O, and Se atoms of a wide range of substrates such as thiols, amines, amides, sulfides, etc. (Eswaramoorthy et al. 2006).
-
(c)
Other Oxidative Enzymes
There are many oxidative enzymes like alcohol dehydrogenase, aldehyde dehydrogenase, and aldehyde oxidase involved in degradation of xenobiotics.
-
(a)
-
2.
Reduction: The enzymes which are involved in this mechanism of detoxification of xenobiotics belong to cytochrome P 450 and P 450 reductase. These systems play important role in the metabolism of both endogenous and exogenous compounds including insecticides (Jing et al. 2018). There are many substrates for these enzymes which include epoxides, azo and nitro-compounds, halogenated compounds, and heterocycles.
-
3.
Hydrolysis: It is a reaction involving addition of water as a result of which toxic substances splits into two smaller molecules. During the course of hydrolysis, hydroxyl group is added to one of the fragments, and hydrogen atom is added to another. A broad range of esterases such as carboxyl esterase, amidases, and phosphatases participate in this reaction (Arand et al. 2005). The substrates for these enzymes are amides, esters, hydrazides, and carbonates.
-
4.
Hydration: Hydration is the process of combining with water. The enzymes epoxide hydratase (epoxide hydrase or hydrolase) are mainly involved in hydration. The substrates of this enzyme are epoxides.
4.5.2 Enzymes Involved in Phase II Metabolism
These enzymes facilitate reactions such as glucouronidation, glycosidation, sulfation, etc.
-
1.
Glucouronidation: It is a very important Phase II metabolic pathway. This process involves metabolism of parent compound by an enzyme called UDP- glucuronosyltransferases (also called as UGTs) into negatively charged hydrophilic glucuronides that require efflux transporters for excretion out of the cell. Therefore, removal of xenobiotics via glucouronidation in metabolically active cell requires (1) UGT enzymes for production of glucuronides from the parent compound and (2) efflux transporters for excretion of glucuronides (Yang et al. 2017). Substrates for this reaction include alcohols, carboxylic acids, amides, thiols, sulfonamides, and phenols.
-
2.
Glycosidation: This is a process of addition of sugars to small organic molecules mediated by a superfamily of enzyme called UDP-glycosyltransferases. The enzyme catalyzes the transfer of glucuronic acid to a wide variety of exogenous and endogenous lipophilic substrates (Meech et al. 2019).
-
3.
Sulfation: It is a reaction involving addition of SO3 group. It is mediated by cytosolic enzyme sulfotransferases. The substrates for this enzyme are phenols, alcohols, amines, and thiols.
4.5.2.1 Toxicity of Xenobiotics in Aquatic Ecosystems
A broad range of chemicals are used in many industrial and household activities. It has been observed that these chemicals are known to disturb the normal physiology and endocrinology of living organisms. The xenobiotics are mainly known to cause three major problems: (1) neurophysiological, (2) reproductive, and (3) behavioral. These effects are interrelated i.e., neurophysiological changes cause behavioral changes and behavioral changes affect the reproduction. The effect of xenobiotic on the target organism or community depends on concentration of compound and time of exposure. The toxic effect of xenobiotics can be acute or chronic. In case of acute toxicity, the effect is very rapid, clearly defined, whereas the induction of chronic toxicity requires long exposure to low doses (Zaki and Hamaam 2014).
Xenobiotics are negatively affecting a plethora of metabolic processes in aquatic animals particularly in developing fish embryos, which causes abnormal and retarded growth leading to death, resulting in functional and morphological abnormalities. In addition, several studies recorded the abnormalities like altered body shape, body abnormalities, and delay in hatching in fishes (Arya and Haq 2019). The xenobiotics like dyes and paints restrict the penetration of light and inhibit the gas exchange (Abdelkader et al. 2011). For marine life the pesticides and herbicides are the major sources of xenobiotic pollution. Chemicals like nitrophenols, organophosphorus, morpholine, and synthetic pyrethroids used as agricultural chemicals reached the various waterbodies including the sea. Pesticides like β-cypermethrin causes severe problem to marine life and invertebrates (Zhang et al. 2011).
5 Conclusion
At present, majority of the researchers have focused on the impact of pesticides and xenobiotics on the climate change and their impact on the environment, including the aquatic life forms as well as the human health. Both the chemical forms have adversely affected the climate (salinity and temperature). The increase in temperatures could enhance their metabolism and degradation. In addition, these changes could also facilitate the contaminants to get into the ground and surface waters, thereby affecting the aquatic life forms. These chemical contaminants are transferred into the food webs and chains, thereby affecting all the organisms at the tropic levels as well as the nutrient cycle. In order to mitigate the combined effects of the pollutants on the climate, humans, and aquatic life forms, enhanced awareness to the society must be conveyed regarding the effects of the chemical pollutants and their impact on the environment. The increase in the salinity and temperature, linked to the change in climate, could probably affect the distribution of toxicity of the pollutants employed and increase its persistence in the aquatic ecosystems. Currently, many researchers have been focusing on ways to minimize the pollutant effects on the change in climate and the ecosystems, in addition to making it sustainable for its usage and also enhancing its sustainability toward the environment.
References
Abdelkader E, Nadjia L, Ahmed B (2011) Degradation study of phenazin neutral red from aqueous suspension by paper sludge. J Chem Eng Process Technol 2:109–114
Adnan A, Indulkar ST (2017) Chronic effect of Imidacloprid pesticide, on carcass composition of common carp fingerlings. Int J Agric Sci Res 7(2):343–348
Adnan A, Indulkar ST, Pai R (2016) Sublethal effect of Buprofezin pesticide on carcass composition of Cyprinus carpio communis fingerlings. J Exp Zool India 20(1):269–272
Aly OA, Shehata SA, Farag H (1984) Uptake and accumulation of selected herbicides by the freshwater alga Scenedesmus. Arch Environ Contam Toxicol 13(6):701–705
Anderson RL (1982) Toxicity of fenvalerate and permethrin to several nontarget aquatic invertebrates. Environ Entomol 11(6):1251–1257
Anderson RL, DeFoe DL (1980) Toxicity and bioaccumulation of endrin and methoxychlor in aquatic invertebrates and fish. Environ Pollut Ser A Ecol Biol 22(2):111–121
Arand M, Cronin A, Adamska M, Oesch F (2005) Epoxide hydrolases: structure, function, mechanism, and assay. Methods Enzymol 400:569–588. https://doi.org/10.1016/S0076-6879(05)00032-7
Arnot JA, Gobas FA (2006) A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14(4):257–297
Arslan P, Yurdakok-Dikmen B, Kuzukiran O, Ozeren SC, Filazı A (2021) Effects of acetamiprid and flumethrin on Unio sp. primary cells. Biologia 76(4):1359–1365
Arya P, Haq SA (2019) Effects of xenobiotics and their biodegradation in marine life. In: Smart bioremediation technologies. Academic Press, Cambridge, MA, pp 63–81
Arya M, Patil S, Shruti N (2019) Xenobiotics and their adverse impact on living organisms. J Curr Microbiol 23(7):348–357
Ashauer R, Boxall A, Brown C (2006) Uptake and elimination of chlorpyrifos and pentachlorophenol into the freshwater amphipod Gammarus pulex. Arch Environ Contam Toxicol 51(4):542–548
Axelman J, Broman D, Näf C, Pettersen H (1995) Compound dependence of the relationship log Kow and log BCFL. Environ Sci Pollut Res 2(1):33–36
Babalola OO, Van Wyk JH (2018) Comparative early life stage toxicity of the African clawed frog, Xenopus laevis following exposure to selected herbicide formulations applied to eradicate alien plants in South Africa. Arch Environ Contam Toxicol 75(1):8–16
Banjoko B (2014) Environmental pharmacology—an overview. In: Pharmacology and therapeutics. Intech, Rijeka
Barron MG (1990) Bioconcentration. Will water-borne organic chemicals accumulate in aquatic animals? Environ Sci Technol 24(11):1612–1618
Bartlett AJ, Borgmann U, Dixon DGE, Atchelor SPB, Maguire RJ (2004) Tributyltin uptake and depuration in Hyalella azteca: implications for experimental design. Environ Toxicol Chem 23(2):426–434
Basack SB, Oneto ML, Verrengia Guerrero NR, Kesten EM (1997) Accumulation and elimination of pentachlorophenol in the freshwater bivalve Corbicula fluminea. Bull Environ Contam Toxicol 58(3):497–503
Baturo W, Lagadic L (1996) Benzo [a] pyrene hydroxylase and glutathione S-transferase activities as biomarkers in Lymnaea palustris (Mollusca, Gastropoda) exposed to atrazine and hexachlorobenzene in freshwater mesocosms. Environ Toxicol Chem 15(5):771–781
Bauer I, Weigelt S, Ernst W (1989) Biotransformation of hexachlorobenzene in the blue mussel (Mytilus edulis). Chemosphere 19(10-11):1701–1707
Bedford JW, Zabik MJ (1973) Bioactive compounds in the aquatic environment: uptake and loss of DDT and dieldrin by freshwater mussels. Arch Environ Contam Toxicol 1(2):97–111
Boryslawskyj M, Garrood T, Stanger M, Pearson T, Woodhead D (1988) Role of lipid/water partitioning and membrane composition in the uptake of organochlorine pesticides into a freshwater mussel. Mar Environ Res 24(1–4):57–61
Böttcher T, Schroll R (2007) The fate of isoproturon in a freshwater microcosm with Lemna minor as a model organism. Chemosphere 66(4):684–689
Brock TC, Crum SJH, Van Wijngaarden R, Budde BJ, Tijink J, Zuppelli A, Leeuwangh P (1992) Fate and effects of the insecticide Dursban® 4E in indoor Elodea-dominated and macrophyte-free freshwater model ecosystems: I. Fate and primary effects of the active ingredient chlorpyrifos. Arch Environ Contam Toxicol 23(1):69–84
Brock TCM, Lahr J, Van den Brink PJ (2000) Ecological risks of pesticides in freshwater ecosystems; Part 1: herbicides (No. 88). Alterra, Wageningen
Brodie ED, Ridenhour BJ, Brodie ED (2002) The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution 56(10):2067–2082
Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ (2005) Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem 24(2):464–469
Butcherine P, Kelaher BP, Benkendorff K (2022) Assessment of acetylcholinesterase, catalase, and glutathione S-transferase as biomarkers for imidacloprid exposure in penaeid shrimp. Aquat Toxicol 242:106050
Cajaraville MP, Cancio I, Ibabe A, Orbea A (2003) Peroxisome proliferation as a biomarker in environmental pollution assessment. Microsc Res Tech 61(2):191–202
Canton JH, Greve PA, Slooff W, Van Esch GJ (1975) Toxicity, accumulation and elimination studies of α-hexachlorocyclohexane (α-HCH) with freshwater organisms of different trophic levels. Water Res 9(12):1163–1169
Canton JH, Vanesch GJ, Greve PA, Vanhellemond ABAM (1977) Accumulation and elimination of gamma-hexachlorocyclohexane (gamma-HCH) by the marine algae Chlamydomonas and Dunaliella. Water Res:111–115
Cederbaum AI (2015) Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol 4:60–73. https://doi.org/10.1016/j.redox.2014.11.008
Chaton PF, Ravanel P, Tissut M, Meyran JC (2002) Toxicity and bioaccumulation of fipronil in the nontarget arthropodan fauna associated with subalpine mosquito breeding sites. Ecotoxicol Environ Saf 52(1):8–12
Clark JM, Symington SB (2011) Advances in the mode of action of pyrethroids. Top Curr Chem 314:49–72
Cleuvers M (2003) Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett 142(3):185–194
Connell DW (1988) Bioaccumulation behavior of persistent organic chemicals with aquatic organisms. Rev Environ Contam Toxicol 102:117–154
Crosby DG, Tucker RK (1971) Accumulation of DDT by Daphnia magna. Environ Sci Technol 5(8):714–716
Crossland NO (1984) Fate and biological effects of methyl paration in outdoor ponds and laboratory aquaria: II. Effects. Ecotoxicol Environ Saf 8(5):482–495
Cuklev F, Gunnarsson L, Cvijovic M, Kristiansson E, Rutgersson C, Björlenius B, Larsson DJ (2012) Global hepatic gene expression in rainbow trout exposed to sewage effluents: a comparison of different sewage treatment technologies. Sci Total Environ 427:106–114
Cuppen JG, Van den Brink PJ, Van der Woude H, Zwaardemaker N, Brock TC (1997) Sensitivity of macrophyte-dominated freshwater microcosms to chronic levels of the herbicide linuron. Ecotoxicol Environ Saf 38(1):25–35
Da Cuña RH, Lo Nostro FL, Shimabukuro V, Ondarza PM, Miglioranza KSB (2020) Bioaccumulation and distribution behavior of endosulfan on a cichlid fish: differences between exposure to the active ingredient and a commercial formulation. Environ Toxicol Chem 39(3):604–611
Dar SA, Chatterjee A, Rather MA, Chetia D, Srivastava PP, Gupta S (2020) Identification, functional characterization and expression profiling of cytochrome p450 1A (CYP1A) gene in Labeo rohita against emamectin benzoate. Int J Biol Macromol 158:1268–1278
Day K, Kaushik NK (1987) The adsorption of fenvalerate to laboratory glassware and the alga Chlamydomonas reinhardii, and its effect on uptake of the pesticide by Daphnia galeata mendotae. Aquat Toxicol 10(2-3):131–142
De A, Bose R, Kumar A, Mozumdar S (2014) Targeted delivery of pesticides using biodegradable polymeric nanoparticles. Springer, New Delhi, pp 5–6
DeLorenzo ME, Scott GI, Ross PE (2001) Toxicity of pesticides to aquatic microorganisms: a review. Environ Toxicol Chem 20(1):84–98
DeLorenzo ME, Taylor LA, Lund SA, Pennington PL, Strozier ED, Fulton MH (2002) Toxicity and bioconcentration potential of the agricultural pesticide endosulfan in phytoplankton and zooplankton. Arch Environ Contam Toxicol 42(2):173–181
Deneer JW (2000) Toxicity of mixtures of pesticides in aquatic systems. Pest Manag Sci 56(6):516–520
DeNoyelles F, Kettle WD, Sinn DE (1982) The responses of plankton communities in experimental ponds to atrazine, the most heavily used pesticide in the United States. Ecology 63(5):1285–1293
DeNoyelles F, Dewey SL, Huggins DG, Kettle WD (2020) Aquatic mesocosms in ecological effects testing: detecting direct and indirect effects of pesticides. In: Aquatic mesocosm studies in ecological risk assessment. CRC, Boca Raton, FL, pp 577–603
Derr SK, Zabik MJ (1972) Biologically active compounds in the aquatic environment: The uptake and distribution of [1, 1-dichloro-2, 2-bis (p-chlorophenyl) ethylene], DDE by Chironomus tentans Fabricius (Diptera: Chironomidae). Trans Am Fish Soc 101(2):323–329
Dhanaraj PS, Kumar S, Lal R (1989) Bioconcentration and metabolism of aldrin and phorate by the blue-green algae Anabaena (ARM 310) and Aulosira fertilissima (ARM 68). Agr Ecosyst Environ 25(2–3):187–193
Diepens NJ, Pfennig S, Van den Brink PJ, Gunnarsson JS, Ruepert C, Castillo L (2014) Effect of pesticides used in banana and pineapple plantations on aquatic ecosystems in Costa Rica. J Environ Biol 35(sp. issue):73–84
Doggrell SA (1990) The membrane stabilizing and beta 1-adrenoceptor blocking activity of (+)-and (-)-propranolol on the rat left atria. Gen Pharmacol 21(5):677–680
Donkin P, Widdows J, Evans SV, Staff FJ, Yan T (1997) Effect of neurotoxic pesticides on the feeding rate of marine mussels (Mytilus edulis). Pestic Sci 49(2):196–209
Driscoll SK, McElroy AE (1996) Bioaccumulation and metabolism of benzo [a] pyrene in three species of polychaete worms. Environ Toxicol Chem 15(8):1401–1410
Drum C (1980) Soil chemistry of pesticides. PPG Industries, Pittsburgh, PA
Drury B, Rosi-Marshall E, Kelly JJ (2013) Wastewater treatment effluent reduces the abundance and diversity of benthic bacterial communities in urban and suburban rivers. Appl Environ Microbiol 79(6):1897–1905
Egeler P, Römbke J, Meller M, Knacker T, Franke C, Studinger G, Nagel R (1997) Bioaccumulation of lindane and hexachlorobenzene by tubificid sludgeworms (Oligochaeta) under standardised laboratory conditions. Chemosphere 35(4):835–852
Ellgehausen H, Guth JA, Esser HO (1980) Factors determining the bioaccumulation potential of pesticides in the individual compartments of aquatic food chains. Ecotoxicol Environ Saf 4(2):134–157
Embrandiri A, Kiyasudeen SK, Rupani PF, Ibrahim MH (2016) Environmental xenobiotics and its effects on natural ecosystem. In: Plant responses to xenobiotics. Springer, Singapore, pp 1–18
Ernst W (1979) Factors affecting the evaluation of chemicals in laboratory experiments using marine organisms. Ecotox Environ Saf 3:90–98
Escartín E, Porte C (1996) Bioaccumulation, metabolism, and biochemical effects of the organophosphorus pesticide fenitrothion in Procambarus clarkii. Environ Toxicol Chem 15(6):915–920
Essumang DK (2013) Environmental xenobiotics: PAHs in soil (heavy metals), indoor air and water environment, case studies of Ghana and Denmark
Essumang DK, Ankrah DA (2010) Distribution, levels, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in some water bodies along the coastal belt of Ghana. Sci World J TSW Environ 10(972):985
Essumang DK, Adokoh C, Afriyie J, Mensah E (2009) Source assessment and analysis of polycyclic aromatic hydrocarbon (PAH’s) in the Oblogo waste disposal sites and some water bodies in and around the Accra Metropolis of Ghana
Eswaramoorthy S, Bonanno JB, Burley SK, Swaminathan S (2006) Mechanism of action of a flavin-containing monooxygenase. Proc Natl Acad Sci U S A 103(26):9832–9837. https://doi.org/10.1073/pnas.0602398103
Fahl GM, Kreft L, Altenburger R, Faust M, Boedeker W, Grimme LH (1995) pH-dependent sorption, bioconcentration and algal toxicity of sulfonylurea herbicides. Aquat Toxicol 31(2):175–187
FAO (1990). http://www.fao.org/3/w2598e/w2598e07.htm
Fatta-Kassinos D, Kalavrouziotis IK, Koukoulakis PH, Vasquez MI (2011) The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci Total Environ 409(19):3555–3563
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76(2):122–159
Ferrando MD, Sancho E, Andreu-Moliner E (1996) Accumulation of tetradifon in an algae (Nannochloris oculata) and the cladoceran, Daphnia magna. Bull Environ Contam Toxicol 57(1):139–145
Fetzner S (2002) Biodegradation of xenobiotics. Biotechnology 10:215–246
Feurtet-Mazel A, Grollier T, Grouselle M, Ribeyre F, Boudou A (1996) Experimental study of bioaccumulation and effects of the herbicide isoproturon on freshwater rooted macrophytes (Elodea densa and Ludwigia natans). Chemosphere 32(8):1499–1512
Fick J, Lindberg RH, Tysklind M, Larsson DJ (2010) Predicted critical environmental concentrations for 500 pharmaceuticals. Regul Toxicol Pharmacol 58(3):516–523
Fisher SW, Hwang H, Atanasoff M, Landrum PF (1999) Lethal body residues for pentachlorophenol in zebra mussels (Dreissena polymorpha) under varying conditions of temperature and pH. Ecotoxicol Environ Saf 43(3):274–283
Gani KM, Ali M, Dubey M, Kazmi AA, Kumari S, Bux F (2021) Transport of emerging contaminants from agricultural soil to groundwater. In: Sustainable agriculture reviews, vol 50. Springer, Cham, pp 261–281
Gao J, Garrison AW, Hoehamer C, Mazur CS, Wolfe NL (2000) Uptake and phytotransformation of organophosphorus pesticides by axenically cultivated aquatic plants. J Agric Food Chem 48(12):6114–6120
Garrison AW, Pope JD, Allen FR (1976) GC-MS analysis of organic compounds in domestic wastewater. In: Keith LH (ed) Identification and analysis of organic pollutants in water. Ann Arbor Science, Michigan, pp 517–556
Geyer H, Sheehan P, Kotzias D, Freitag D, Korte F (1982) Prediction of ecotoxicological behaviour of chemicals: relationship between physico-chemical properties and bioaccumulation of organic chemicals in the mussel Mytilus edulis. Chemosphere 11(11):1121–1134
Gllderhus PA, Johnson BGH (1980) Effects of sea lamprey (Petromyzon marinus) control in the Great Lakes on aquatic plants, invertebrates, and amphibians. Can J Fish Aquat Sci 37(11):1895–1905
Glooschenko V, Holdrinet M, Lott JN, Frank R (1979) Bioconcentration of chlordane by the green alga Scenedesmus quadricauda. Bull Environ Contam Toxicol 21(1):515–520
Gobas FA, McNeil EJ, Lovett-Doust L, Haffner GD (1991) Bioconcentration of chlorinated aromatic hydrocarbons in aquatic macrophytes. Environ Sci Technol 25(5):924–929
Gomez CF, Constantine L, Huggett DB (2010) The influence of gill and liver metabolism on the predicted bioconcentration of three pharmaceuticals in fish. Chemosphere 81(10):1189–1195
Govers H, Ruepert C, Aiking H (1984) Quantitative structure-activity relationships for polycyclic aromatic hydrocarbons: correlation between molecular connectivity, physico-chemical properties, bioconcentration and toxicity in Daphnia pulex. Chemosphere 13(2):227–236
Grech A, Quignot N, Brochot C, Dorne J, Bois FY, Beaudouin R (2016, May) Development and application of generic toxicokinetic models in fish to environmental risk assessment of chemicals. In 26. SETAC Europe annual meeting. SETAC
Gregory WW Jr, Reed JK, Priester LE Jr (1969) Accumulation of parathion and DDT by some algae and protozoa. J Protozool 16(1):69–71
Gross B, Montgomery-Brown J, Naumann A, Reinhard M (2004) Occurrence and fate of pharmaceuticals and alkylphenol ethoxylate metabolites in an effluent-dominated river and wetland. Environ Toxicol Chem 23(9):2074–2083
Guanzon NG Jr, Fukuda M, Nakahara H (1996) Accumulation of agricultural pesticides by three freshwater microalgae. Fisheries Sci 62(5):690–697
Gunnarsson L, Kristiansson E, Rutgersson C, Sturve J, Fick J, Förlin L, Larsson DJ (2009) Pharmaceutical industry effluent diluted 1: 500 affects global gene expression, cytochrome P450 1A activity, and plasma phosphate in fish. Environ Toxicol Chem 28(12):2639–2647
Halling-Sørensen B, Nyholm N, Kusk KO, Jacobsson E (2000) Influence of nitrogen status on the bioconcentration of hydrophobic organic compounds to Selenastrum capricornutum. Ecotoxicol Environ Saf 45(1):33–42
Hamer MJ, Goggin UM, Muller K, Maund SJ (1999) Bioavailability of lambda-cyhalothrin to Chironomus riparius in sediment-water and water-only systems. Aquat Ecosyst Health Manage 2(4):403–412
Hansen PD (1979) Experiments on the accumulation of lindane (γ-BHC) by the primary producers Chlorella spec. and Chlorella pyrenoidosa. Arch Environ Contam Toxicol 8(6):721–731
Haque R, Kearney PC, Freed VH (1977) Dynamics of pesticides in aquatic environments. In: Pesticides in aquatic environments. Springer, Boston, MA, pp 39–52
Harkey GA, Klaine SJ (1992) Bioconcentration of trans-chlordane by the midge, Chironomus decorus. Chemosphere 24(12):1911–1919
Hartley DM, Johnston JB (1983) Use of the freshwater clam Corbicula manilensis as a monitor for organochlorine pesticides. Bull Environ Contam Toxicol 31(1):33–40
Hassaan MA, El Nemr A (2020) Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt J Aquat Res 46(3):207–220
Hawker DW, Connell DW (1986) Bioconcentration of lipophilic compounds by some aquatic organisms. Ecotoxicol Environ Saf 11(2):184–197
Heberer T (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131(1–2):5–17
Heisig-Gunkel G, Gunkel G (1982) Distribution of a herbicide (atrazine, s-triazine) in Daphnia pulicaria: a new approach to determination. Arch Hydrobiol 59(4):359–376
Helfrich LA, Weigmann DL, Stinson ER (2009) Pesticides and aquatic animals: a guide to reducing impacts on aquatic systems. Communications and Marketing, College of Agriculture and Life Sciences, Virginia Polytechnic Institute and State University, Publication, Blacksburg, VA, p 24
Hendriks AJ, van der Linde A, Cornelissen G, Sijm DT (2001) The power of size. 1. Rate constants and equilibrium ratios for accumulation of organic substances related to octanol-water partition ratio and species weight. Environ Toxicol Chem 20(7):1399–1420
Hernández AF, Parrón T, Tsatsakis AM, Requena M, Alarcón R, López-Guarnido O (2013) Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology 307:136–145
Hinman ML, Klaine SJ (1992) Uptake and translocation of selected organic pesticides by the rooted aquatic plant Hydrilla verticillata Royle. Environ Sci Technol 26(3):609–613
Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI (2005) Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436(7054):1171–1175
Holland W, Morrison T, Chang Y, Wiernsperger N, Stith BJ (2004) Metformin (Glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem Pharmacol 67(11):2081–2091
Hooper MJ, Ankley GT, Cristol DA, Maryoung LA, Noyes PD, Pinkerton KE (2013) Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ Toxicol Chem 32(1):32–48
Hou R, Liu C, Gao X, Xu Y, Zha J, Wang Z (2017) Accumulation and distribution of organophosphate flame retardants (PFRs) and their di-alkyl phosphates (DAPs) metabolites in different freshwater fish from locations around Beijing, China. Environ Pollut 229:548–556
Ibrahim MS (2007) Persistent organic pollutants in Malaysia. Dev Environ Sci 7:629–655. https://doi.org/10.1016/S1474-8177(07)07014-3
Islam MA, Amin SMN, Brown CL, Juraimi AS, Uddin MK, Arshad A (2021) Determination of median lethal concentration (LC50) for Endosulfan, Heptachlor and Dieldrin Pesticides to African Catfish, Clarias gariepinus and their impact on its behavioral patterns and histopathological responses. Toxics 9(12):340. https://doi.org/10.3390/toxics9120340
Jain PK, Gupta VK, Gaur RK, Lowry M, Jaroli DP, Chauhan UK (2011) Bioremediation of petroleum oil contaminated soil and water. Res J Environ Toxicol 5(1):1
Jing TX, Tan Y, Ding BY, Dou W, Wei DD, Wang JJ (2018) NADPH-Cytochrome P450 Reductase mediates the resistance of Aphis (Toxoptera) citricidus (Kirkaldy) to Abamectin. Front Physiol 9:986
Johnson BT, Saunders CR, Sanders HO, Campbell RS (1971) Biological magnification and degradation of DDT and aldrin by freshwater invertebrates. J Fisheries Board Canada 28(5):705–709
Johnson CH, Patterson AD, Idle JR, Gonzalez FJ (2012) Xenobiotic metabolomics: major impact on the metabolome. Annu Rev Pharmacol Toxicol 52:37–56. https://doi.org/10.1146/annurev-pharmtox-010611-134748
Jonsson CM, Paraiba LC, Mendoza MT, Sabater C, Carrasco JM (2001) Bioconcentration of the insecticide pyridaphenthion by the green algae Chlorella saccharophila. Chemosphere 43(3):321–325
Julin AM, Sanders HO (1977) Toxicity and accumulation of the insecticide imidan in freshwater invertebrates and fishes. Trans Am Fish Soc 106(4):386–392
Kanazawa J (1978) Bioconcentration ratio of diazinon by freshwater fish and snail. Bull Environ Contam Toxicol 20(1):613–617
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73(2):310–347
Kashiwada S, Mochida K, OzoE Y, Nakamura T (1995) Contribution of Zooplankton to disappearance of organophosphorus insecticides in environmental water. J Pestic Sci 20:503–512
Katagi T (2010) Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. In: Whitacre D (ed) Reviews of environmental contamination and toxicology, vol 204. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1440-8_1
Kawatski JA, Schmulbach JC (1972) Uptake and elimination of 14C-aldrin and 14C-dieldrin by the ostracod Chlamydotheca arcuata (Sars). Int J Environ Anal Chem 1(4):283–291
Keil JE, Priester LE (1969) DDT uptake and metabolism by a marine diatom. Bull Environ Contam Toxicol 4(3):169–173
Kent RA, Currie D (1995) Predicting algal sensitivity to a pesticide stress. Environ Toxicol Chem 14(6):983–991
Khan HM, Neudorf S, Khan MAQ (1975) Absorption and elimination of photodieldrin by Daphnia and goldfish. Bull Environ Contam Toxicol 13(5):582–587
Kikuchi R, Yasutaniya T, Takimoto Y, Yamada H, Miyamoto J (1984) Accumulation and metabolism of fenitrothion in three species of algae [concerning ecotoxicology]. J Pestic Sci 9:331
Klosterhaus SL, DiPinto LM, Chandler GT (2003) A comparative assessment of azinphosmethyl bioaccumulation and toxicity in two estuarine meiobenthic harpacticoid copepods. Environ Toxicol Chem 22(12):2960–2968
Knezovich JP, Harrison FL (1988) The bioavailability of sediment-sorbed chlorobenzenes to larvae of the midge, Chironomus decorus. Ecotoxicol Environ Saf 15(2):226–241
Kobayashi K, Nakamura Y, Imada N (1985) Metabolism of an organophosphorus insecticide, fenitrothion, in tiger shrimp Penaeus japonicus. Nippon Suisan Gakkaishi 51(4):599–603
Kobayashi K, Rompas RM, Oshima Y, Imada N (1990) A comparative study on the toxicity, absorption and depuration of fenitrothion and its oxon in Japanese tiger shrimp. Nippon Suisan Gakkaishi 56(6):923–928
Koelmans AA, Jiménez CS (1994) Temperature dependency of chlorobenzene bioaccumulation in phytoplankton. Chemosphere 28(12):2041–2048
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999−2000: a national reconnaissance. Environ Sci Technol 36(6):1202–1211
Kukkonen J, Oikari A (1988) Sulphate conjugation is the main route of pentachlorophenol metabolism in Daphnia magna. Comp Biochem Physiol C Comp Pharmacol 91(2):465–468
Kumar S, Lal R, Bhatnagar P (1988) Uptake of dieldrin, dimethoate and permethrin by cyanobacteria, Anabaena sp. and Aulosira fertilissima. Environ Pollut 54(1):55–61
Kumar S, Sharma AK, Rawat SS, Jain DK, Ghosh S (2013) Use of pesticides in agriculture and livestock animals and its impact on environment of India. Asian J Environ Sci 8(1):51–57
La Farre M, Pérez S, Kantiani L, Barceló D (2008) Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal Chem 27(11):991–1007
Lakhani L (2015) How to reduce impact of pesticides in aquatic environment. Soc Iss Environ Probl 3(9):29–38
Lal S, Lal R, Saxena DM (1987) Bioconcentration and metabolism of DDT, fenitrothion and chlorpyrifos by the blue-green algae Anabaena sp. and Aulosira fertilissima. Environ Pollut 46(3):187–196
Landrum PF, Dupuis WS (1990) Toxicity and toxicokinetics of pentachlorophenol and carbaryl to Pontoporeia hoyi and Mysis relicta. ASTM Spec Tech Publ 1096:278–289
Landrum PF, Fisher SW (1999) Influence of lipids on the bioaccumulation and trophic transfer of organic contaminants in aquatic organisms. In: Lipids in freshwater ecosystems. Springer, New York, NY, pp 203–234
Landrum PF, Scavia D (1983) Influence of sediment on anthracene uptake, depuration, and biotransformation by the amphipod Hyalella azteca. Can J Fish Aquat Sci 40(3):298–305
Laxmi B, Madhavi K, Adnan A, Chamundeswari Devi B, Dhanapal K, Ramana TV (2019) Sub lethal effects of dichlorvos on physiological parameters in fingerlings of Cyprinus carpio. Int J Curr Microbiol App Sci 8(8):372–377
Laxmi B, Madhavi K, Dhanapal K, Adnan A, Sudhakar O, Jesintha N (2020) Biochemical responses of fingerling Cyprinus carpio (Linnaeus, 1758) exposed to sub-lethal concentrations of Dichlorvos. IntJ Curr Microbiol App Sci 9(10):3250–3257
Le Page Y, Vosges M, Servili A, Brion F, Kah O (2011) Neuroendocrine effects of endocrine disruptors in teleost fish. J Toxicol Environ Health Pt B 14(5-7):370–386
Lee YM, Kim KS, Jacobs DR Jr, Lee DH (2017) Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev 18(2):129–139
Legierse KC, Sijm DT, van Leeuwen CJ, Seinen W, Hermens JL (1998) Bioconcentration kinetics of chlorobenzenes and the organophosphorus pesticide chlorthion in the pond snail Lymnaea stagnalis—a comparison with the guppy Poecilia reticulata. Aquat Toxicol 41(4):301–323
Lengai GM, Muthomi JW, Mbega ER (2020) Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci Afr 7:e00239
Leppänen MT, Kukkonen JV (2006) Evaluating the role of desorption in bioavailability of sediment-associated contaminants using oligochaetes, semipermeable membrane devices and Tenax extraction. Environ Pollut 140(1):150–163
Leppänen MT, Landrum PF, Kukkonen JV, Greenberg MS, Burton GA Jr, Robinson SD, Gossiaux DC (2003) Investigating the role of desorption on the bioavailability of sediment-associated 3, 4, 3′, 4′-tetrachlorobiphenyl in benthic invertebrates. Environ Toxicol Chem Int J 22(12):2861–2871
Liess M, Foit K (2010) Intraspecific competition delays recovery of population structure. Aquat Toxicol 97(1):15–22
Lionetto MG, Caricato R, Calisi A, Schettino T (2011) Acetylcholinesterase inhibition as a relevant biomarker in environmental biomonitoring: new insights and perspectives. In: Ecotoxicology around the globe, pp 87–115
Livingston RJ, de La Cruz AA (1977) Review of current literature concerning the acute and chronic effects of pesticides on aquatic organisms. Crit Rev Environ Sci Technol 7(4):325–351
Livingstone DR (1998) The fate of organic xenobiotics in aquatic ecosystems: quantitative and qualitative differences in biotransformation by invertebrates and fish. Comp Biochem Physiol A 120:43–49
Lockhart WL, Billeck BN, de March BG, Muir DC (1983) Uptake and toxicity of organic compounds: studies with an aquatic macrophyte (Lemna minor). In: Aquatic Toxicology and Hazard Assessment: Sixth Symposium. ASTM International, West Conshohocken, PA
Lockhart WL, Metner DA, Billeck BN, Rawn GP, Muir DCG (1984) Bioaccumulation of some forestry pesticides in fish and aquatic plants. In: Garner WY, Harvey J Jr (eds) Chemical and biological controls in forestry. ACS symposium series 238. American Chemical Society, Washington, DC, pp 297–315
Lotufo GR, Landrum PF, Gedeon ML, Tigue EA, Herche LR (2000) Comparative toxicity and toxicokinetics of DDT and its major metabolites in freshwater amphipods. Environ Toxicol Chem 19(2):368–379
Lotufo GR, Farrar JD, Duke BM, Bridges TS (2001a) DDT toxicity and critical body residue in the amphipod Leptocheirus plumulosus in exposures to spiked sediment. Arch Environ Contam Toxicol 41(2):142–150
Lotufo GR, Landrum PF, Gedeon ML (2001b) Toxicity and bioaccumulation of DDT in freshwater amphipods in exposures to spiked sediments. Environ Toxicol Chem 20(4):810–825
Lydy MJ, Hayton WL, Staubus AE, Fisher SW (1994) Bioconcentration of 5, 5′, 6-trichlorobiphenyl and pentachlorophenol in the midge, Chironomus riparius, as measured by a pharmacokinetic model. Arch Environ Contam Toxicol 26(2):251–256
Mackay D (1982) Correlation of bioconcentration factors. Environ Sci Technol 16(5):274–278
Mäenpää K (2007) The toxicity of xenobiotics in an aquatic environment: connecting body residues with adverse effects. Joensuun yliopisto
Mäenpää KA, Sormunen AJ, Kukkonen JV (2003) Bioaccumulation and toxicity of sediment associated herbicides (ioxynil, pendimethalin, and bentazone) in Lumbriculus variegatus (Oligochaeta) and Chironomus riparius (Insecta). Ecotoxicol Environ Saf 56(3):398–410
Mailhot H (1987) Prediction of algal bioaccumulation and uptake rate of nine organic compounds by ten physicochemical properties. Environ Sci Technol 21(10):1009–1013
Mäkelä P, Oikari AO (1990) Uptake and body distribution of chlorinated phenolics in the freshwater mussel, Anodonta anatina L. Ecotoxicol Environ Saf 20(3):354–362
Manthey M, Faust M, Smolka S, Grimme LH (1993) Herbicide bioconcentration in algae: Studies on lipophilicity-sorption-activity relationships (LSAR) with Chlorella fusca. Sci Total Environ 134:453–459
Marquis LY, Comes RD, Yang CP (1981) Absorption and translocation of fluridone and glyphosate in submersed vascular plants. Weed Sci 29(2):229–236
Mason JW, Rowe DR (1976) The accumulation and loss of dieldrin and endrin in the eastern oyster. Arch Environ Contam Toxicol 4(1):349–360
Mathur R, Saxena DM (1986) Effect of hexachlorocyclohexane (HCH) isomers on growth of, and their accumulation in, the blue-green alga, Anabaena Sp.(ARM 310). J Environ Biol 7:239
Matsumura F (1977) Absorption, accumulation, and elimination of pesticides by aquatic organisms. In: Pesticides in aquatic environments. Springer, Boston, MA, pp 77–105
Maund SJ, Hamer MJ, Lane MC, Farrelly E, Rapley JH, Goggin UM, Gentle WE (2002) Partitioning, bioavailability, and toxicity of the pyrethroid insecticide cypermethrin in sediments. Environ Toxicol Chem 21(1):9–15
Maurya PK, Malik DS, Sharma A (2019) Impacts of pesticide application on aquatic environments and fish diversity. In: Kumar V, Kumar R, Singh J, Kumar P (eds) Contaminants in agriculture and environment: health risks and remediation, vol 1. Agro Environ Media, Haridwar, pp 111–128. https://doi.org/10.26832/AESA-2019-CAE-0162-09
McLeese DW, Zitko V, Sergeant DB (1979) Uptake and excretion of fenitrothion by clams and mussels. Bull Environ Contam Toxicol 22(1):800–806
Meech R, Hu DG, McKinnon RA, Mubarokah SN, Haines AZ, Nair PC, Rowland A, Mackenzie PI (2019) The UDP-glycosyltransferase (UGT) superfamily: new members, new functions, and novel paradigms. Physiol Rev 99(2):1153–1222. https://doi.org/10.1152/physrev.00058.2017
Mehrotra S, Kumar S, Zahid M, Garg M (2017) Biopesticides. In: Principles and applications of environmental biotechnology for a sustainable future. Springer, Singapore, pp 273–292
Miyamoto J, Mikami N, Takimoto Y (1990) The fate of pesticides in aquatic ecosystems. Prog Pest Biochem Toxicol 7:123–147
Mohapatra S, Kumar R, Sundaray JK, Patnaik ST, Mishra CSK, Rather MA (2021) Structural damage in liver, gonads, and reduction in spawning performance and alteration in the haematological parameter of Anabas testudineus by glyphosate-a herbicide. Aquacult Res 52(3):1150–1159
Montañés JC, Van Hattum B, Deneer J (1995) Bioconcentration of chlorpyrifos by the freshwater isopod Asellus aquaticus (L.) in outdoor experimental ditches. Environ Pollut 88(2):137–146
Moore R, Toro E, Stanton M, Khan MAQ (1977) Absorption and elimination of 14C-alpha- and gamma-chlordane by a freshwater alga, daphnid, and goldfish. Arch Environ Contam Toxicol 6(1):411–420
Morrison HA, Gobas FA, Lazar R, Haffner GD (1996) Development and verification of a bioaccumulation model for organic contaminants in benthic invertebrates. Environ Sci Technol 30(11):3377–3384
Muhammad G, Rashid I, Firyal S (2017) Practical aspects of treatment of organophosphate and carbamate insecticide poisoning in animals. Matrix Sci Pharma 1(1):10–11
Muir DCG, Grift NP, Townsend BE, Metner DA, Lockhart WL (1982) Comparison of the uptake and bioconcentration of fluridone and terbutryn by rainbow trout and Chironomus tentans in sediment and water systems. Arch Environ Contam Toxicol 11(5):595–602
Naqvi SM, Newton DJ (1990) Bioaccumulation of endosulfan (Thiodan R insecticide) in the tissues of Louisiana crayfish, Procambarus clarkii. J Environ Sci Health Pt B 25(4):511–526
Narayana Rao VS, Lal R (1987) Uptake and metabolism of insecticides by blue-green algae Anabaena and Aulosira fertilissima. Microbios Lett 36(143-144):143–147
Nawaz S, Kirk K (1996) Temperature effects on bioconcentration of DDE by Daphnia. Freshw Biol 35(1):173–178
Nebeker AV, Griffis WL, Wise CM, Hopkins E, Barbitta JA (1989) Survival, reproduction and bioconcentration in invertebrates and fish exposed to hexachlorobenzene. Environ Toxicol Chem 8(7):601–611
Neely WB, Branson DR, Blau GE (1974) Partition coefficient to measure bioconcentration potential of organic chemicals in fish. Environ Sci Technol 8(13):1113–1115
Nemcsok J, Benedeczky I (1995) Pesticide metabolism and the adverse effects of metabolites on fishes. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 59. Elsevier Science, Amsterdam, pp 313–348
Neudorf S, Khan MAQ (1975) Pick-up and metabolism of DDT, dieldrin and photodieldrin by a fresh water alga (Ankistrodesmus amalloides) and a Microcrustacean(Daphnia pulex). Bull Environ Contam Toxicol 13(4):443–450
Nikkilä A, Paulsson M, Almgren K, Blanck H, Kukkonen JV (2001) Atrazine uptake, elimination, and bioconcentration by periphyton communities and Daphnia magna: effects of dissolved organic carbon. Environ Toxicol Chem 20(5):1003–1011
Nikkilä A, Halme A, Kukkonen JV (2003) Toxicokinetics, toxicity and lethal body residues of two chlorophenols in the oligochaete worm, Lumbriculus variegatus, in different sediments. Chemosphere 51(1):35–46
Nuutinen S, Landrum PF, Schuler LJ, Kukkonen JV, Lydy MJ (2003) Toxicokinetics of organic contaminants in Hyalella azteca. Arch Environ Contam Toxicol 44(4):0467–0475
Oliver BG (1987) Biouptake of chlorinated hydrocarbons from laboratory-spiked and field sediments by oligochaete worms. Environ Sci Technol 21(8):785–790
Paris DF, Lewis DL (1976) Accumulation of methoxychlor by microorganisms isolated from aqueous systems. Bull Environ Contam Toxicol 15(1):24–32
Petrocelli SR, Hanks AR, Anderson J (1973) Uptake and accumulation of an organochlorine insecticide (dieldrin) by an estuarine mollusc, Rangia cuneata. Bull Environ Contam Toxicol 10:315
Pillai MK, Mittal PK, Agarwal HC (1980) Bioaccumulation, metabolism & elimination of DDT by the fresh water clam Indonaia caerulea (Lea). Indian J Exp Biol 18(12):1439–1442
Rajendran N, Venugopalan VK (1991) Bioconcentration of endosulfan in different body tissues of estuarine organisms under sublethal exposure. Bull Environ Contam Toxicol 46(1):151
Rápó E, Tonk S (2021) Factors affecting synthetic dye adsorption; desorption studies: a review of results from the last five years (2017–2021). Molecules 26(17):5419
Reineke W, Knackmuss HJ (1988) Microbial degradation of haloaromatics. Annu Rev Microbiol 42(1):263–287
Reinert RE (1972) Accumulation of dieldrin in an alga (Scenedesmus obliquus), Daphnia magna, and the guppy (Poecilia reticulata). J Fisheries Board Canada 29(10):1413–1418
Renberg L, Tarkpea M, Lindén E (1985) The use of the bivalve Mytilus edulis as a test organism for bioconcentration studies: I. Designing a continuous-flow system and its application to some organochlorine compounds. Ecotoxicol Environ Saf 9(2):171–178
Richardson ML, Bowron JM (1985) The fate of pharmaceutical chemicals in the aquatic environment. J Pharm Pharmacol 37(1):1–12
Richter S, Nagel R (2007) Bioconcentration, biomagnification and metabolism of 14C-terbutryn and 14C-benzo [a] pyrene in Gammarus fossarum and Asellus aquaticus. Chemosphere 66(4):603–610
Rosi-Marshall E (2013) Streams stressed by pharmaceutical pollution. https://environmentalchange.nd.edu/news-events/
Roy S, Hänninen O (1994) Pentachlorophenol: uptake/elimination kinetics and metabolism in an aquatic plant, Eichhornia crassipes. Environ Toxicol Chem 13(5):763–773
Sabaliünas D, Lazutka J, Sabaliüniene I, Södergren A (1998) Use of semipermeable membrane devices for studying effects of organic pollutants: comparison of pesticide uptake by semipermeable membrane devices and mussels. Environ Toxicol Chem 17(9):1815–1824
Sacher F, Lange FT, Brauch HJ, Blankenhorn I (2001) Pharmaceuticals in groundwaters: analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J Chromatogr A 938(1-2):199–210
Sathe MC, Shrotri RV, Raghu K, Murthy NBK (2005) Observations on accumulation and depuration of fenthion in various tissues of marine edible clam, Marcia hiantina (Lamarck). J Ecophysiol Occup Health 5(1):33–36
Saxena DM, Lal R, Reddy BVP (1982) DDT uptake and metabolism in Blepharisma intermedium. Acta Protozool 21(2):173
Segner H (2015) In vitro methodologies in ecotoxicological hazard assessment: the case of bioaccumulation testing for fish. Altern Lab Anim 43(2):P14–P16
Schäfer RB, Caquet T, Siimes K, Mueller R, Lagadic L, Liess M (2007) Effects of pesticides on community structure and ecosystem functions in agricultural streams of three biogeographical regions in Europe. Sci Total Environ 382(2-3):272–285
Schauberger CW, Wildman RB (1977) Accumulation of aldrin and dieldrin by blue-green algae and related effects on photosynthetic pigments. Bull Environ Contam Toxicol 17(5):534–541
Schenkman JB (1999) The fate of xenobiotics in the body. In: Arinç E, Schenkman JB, Hodgson E (eds) Molecular and applied aspects of oxidative drug metabolizing enzymes. NATO ASI series, vol 303. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-4855-3_1
Schimmel SC, Patrick JM, Forester J (1977) Uptake and toxicity of toxaphene in several estuarine organisms. Arch Environ Contam Toxicol 5(1):353–367
Schimmel SC, Garnas RL, Patrick JM Jr, Moore JC (1983) Acute toxicity, bioconcentration, and persistence of AC 222,705, benthiocarb, chlorpyrifos, fenvalerate, methyl parathion, and permethrin in the estuarine environment. J Agric Food Chem 31(1):104–113
Schmitt H, Haapakangas H, van Beelen P (2005) Effects of antibiotics on soil microorganisms: time and nutrients influence pollution-induced community tolerance. Soil Biol Biochem 37(10):1882–1892
Schuler LJ, Wheeler M, Bailer AJ, Lydy MJ (2003) Toxicokinetics of sediment-sorbed benzo [a] pyrene and hexachlorobiphenyl using the freshwater invertebrates Hyalella azteca, Chironomus tentans, and Lumbriculus variegatus. Environ Toxicol Chem 22(2):439–449
Schuytema GS, Krawczyk DF, Grlffis WL, Nebeker AV, Robideaux ML, Brownawell BJ, Westall JC (1988) Comparative uptake of hexachlorobenzene by fathead minnows, amphipods and oligochaete worms from water and sediment. Environ Toxicol Chem 7(12):1035–1045
Serrano R, Hernandez F, Pena JB, Dosda V, Canales J (1995) Toxicity and bioconcentration of selected organophosphorus pesticides in Mytilus galloprovincialis and Venus gallina. Arch Environ Contam Toxicol 29(3):284–290
Serrano R, Hernández F, López FJ, Pena JB (1997a) Bioconcentration and depuration of chlorpyrifos in the marine mollusc Mytilus edulis. Arch Environ Contam Toxicol 33(1):47–52
Serrano R, Lopez FJ, Hernandez F, Pena JB (1997b) Bioconcentration of chlorpyrifos, chlorfenvinphos, and methidathion in Mytilus galloprovincialis. Bull Environ Contam Toxicol 59(6):968–975
Shanmugam G, Sampath S, Selvaraj KK, Larsson DG, Ramaswamy BR (2014) Non-steroidal anti-inflammatory drugs in Indian rivers. Environ Sci Pollut Res 21(2):921–931
Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, Handa N, Thukral AK et al (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Applied Sciences 1(11):1–16
Shaw GR, Connell DW (1987) Comparative kinetics for bioaccumulation of polychlorinated biphenyls by the polychaete (Capitella capitata) and fish (Mugil cephalus). Ecotoxicol Environ Saf 13(1):84–91
Shofer SL, Tjeerdema RS (1993) Comparative disposition and biotransformation of pentachlorophenol in the oyster (Crassostrea gigas) and abalone (Haliotis fulgens). Pestic Biochem Physiol 46(2):85–95
Sikandar A, Shehzadi K, Arshad Q, Munir K (2013) Phytoremediation: an analytical technique for the assessment of biodegradation of organic xenobiotic pollutants: a review. Int J Sci Res 4(2):2250–2253
Singh T, Singh DK (2017) Phytoremediation of organochlorine pesticides: Concept, method, and recent developments. Int J Phytoremediation 19(9):834–843
Singh Z, Kaur J, Kaur R, Hundal SS (2016) Toxic effects of organochlorine pesticides: a review. Am J Biol Sci 4(3):11
Sjerps RM, Kooij PJ, van Loon A, Van Wezel AP (2019) Occurrence of pesticides in Dutch drinking water sources. Chemosphere 235:510–518
Södergren A, Svensson B (1973) Uptake and accumulation of DDT and PCB by Ephemera danica (Ephemeroptera) in continuous-flow systems. Bull Environ Contam Toxicol 9:345–350
Spacie A, Hamelink JL (1982) Alternative models for describing the bioconcentration of organics in fish. Environ Toxicol Chem 1(4):309–320
Spehar RL, Tanner DK, Nordling BR (1983) Toxicity of the synthetic pyrethroids, permethrin and AC 222, 705 and their accumulation in early life stages of fathead minnows and snails. Aquat Toxicol 3(2):171–182
Suja S, Williams SE (2018) Determination of lethal concentration (LC 50) of Channa striata. Int J Sci Res 7(8):603–605
Sumon KA, Rashid H, Peeters ET, Bosma RH, Van den Brink PJ (2018) Environmental monitoring and risk assessment of organophosphate pesticides in aquatic ecosystems of north-west Bangladesh. Chemosphere 206:92–100
Szewczuk LM, Forti L, Stivala LA, Penning TM (2004) Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J Biol Chem 279(21):22727–22737
Takimoto Y, Ohshima M, Miyamoto J (1987a) Comparative metabolism of fenitrothion in aquatic organisms: II. Metabolism in the freshwater snails, Cipangopaludina japonica and Physa acuta. Ecotoxicol Environ Saf 13(1):118–125
Takimoto Y, Ohshima M, Miyamoto J (1987b) Comparative metabolism of fenitrothion in aquatic organisms: III. Metabolism in the crustaceans, Daphnia pulex and Palaemon paucidens. Ecotoxicol Environ Saf 13(1):126–134
Tang JX, Siegfried BD (1996) Bioconcentration and uptake of a pyrethroid and organophosphate insecticide by selected aquatic insects. Bull Environ Contam Toxicol 57(6):993–998
Tang J, Hoagland KD, Siegfried BD (1998) Uptake and bioconcentration of atrazine by selected freshwater algae. Environ Toxicol Chem 17(6):1085–1090
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32(11):3245–3260
Thomann RV, Komlos J (1999) Model of biota-sediment accumulation factor for polycyclic aromatic hydrocarbons. Environ Toxicol Chem 18(5):1060–1068
Thompson DG, Holmes SB, Wainio-Keizer K, MacDonald L, Solomon KR (1993) Impact of hexazinone and metsulfuron methyl on the zooplankton community of a boreal forest lake. Environ Toxicol Chem 12(9):1709–1717
Thybaud E, Caquet T (1991) Uptake and elimination of lindane by Lymnaea palustris (Mollusca: Gastropoda): a pharmacokinetic approach. Ecotoxicol Environ Saf 21(3):365–376
Thybaud E, Le Bras S (1988) Absorption and elimination of lindane by Asellus aquaticus (Crustacea, Isopoda). Bull Environ Contam Toxicol 40(5):731–735
Tjeerdema RS, Crosby DG (1992) Disposition and biotransformation of pentachlorophenol in the red abalone (Haliotis rufescens). Xenobiotica 22(6):681–690
Tooker JF, Pearsons KA (2021) Newer characters, same story: neonicotinoid insecticides disrupt food webs through direct and indirect effects. Curr Opin Insect Sci 46:50–56
Tornero V, Hanke G (2016) Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Mar Pollut Bull 112(1–2):17–38
USEPA (2008) Estimation Programs Interface SuiteTM for Microsoft® Windows, v3.20. United States Environmental Protection Agency, Washington, DC, USA
Van den Brink PJ (2013) Assessing aquatic population and community-level risks of pesticides. Environ Toxicol Chem 32(5):972–973
Van den Brink PJ, Hartgers EM, Fettweis U, Crum SJ, Van Donk E, Brock TC (1997) Sensitivity of macrophyte-dominated freshwater microcosms to chronic levels of the herbicide linuron. Ecotoxicol Environ Saf 38(1):13–24
van der Ven K, Van Dongen W, Maes BU, Esmans EL, Blust R, De Coen WM (2004) Determination of diazepam in aquatic samples by capillary liquid chromatography–electrospray tandem mass spectrometry. Chemosphere 57(8):967–973
van Heel W, Hachimi-Idrissi S (2011) Accidental organophosphate insecticide intoxication in children: a reminder. Int J Emerg Med 4(1):1–4
Vance BD, Drummond W (1969) Biological concentration of pesticides by algae. J AWWA 61:360–362
Varo I, Serrano R, Pitarch E, Amat F, Lopez FJ, Navarro JC (2000) Toxicity and bioconcentration of chlorpyrifos in aquatic organisms: Artemia parthenogenetica (Crustacea), Gambusia affinis, and Aphanius iberus (Pisces). Bull Environ Contam Toxicol 65(5):623–630
Veith GD, DeFoe DL, Bergstedt BV (1979) Measuring and estimating the bioconcentration factor of chemicals in fish. J Fisheries Board Canada 36(9):1040–1048
Vonk JA, Kraak MHS (2020) Herbicide exposure and toxicity to aquatic primary producers. In: de Voogt P (ed) Reviews of environmental contamination and toxicology, vol 250, pp 119–171. https://doi.org/10.1007/398_2020_48
Wang WH, Lay JP (1989) Fate and effects of salicylic acid compounds in freshwater systems. Ecotoxicol Environ Saf 17(3):308–316
Wang JS, Simpson KL (1996) Accumulation and depuration of DDTs in the food chain from Artemia to brook trout (Salvelinus fontinalis). Bull Environ Contam Toxicol 56(6):888–895
Warner NA, Wong CS (2006) The freshwater invertebrate Mysis relicta can eliminate chiral organochlorine compounds enantioselectively. Environ Sci Technol 40(13):4158–4164
Warra AA, Prasad MNV (2020) African perspective of chemical usage in agriculture and horticulture—their impact on human health and environment. In: Agrochemicals detection, treatment and remediation. Butterworth-Heinemann, Oxford, pp 401–436
Watanabe S, Watanabe S, Ito K (1985) Accumulation and excretion of herbicides in various tissues of mussel. Food Hyg Saf Sci 26(5):496–499
Wiegel S, Aulinger A, Brockmeyer R, Harms H, Löffler J, Reincke H, Wanke A et al (2004) Pharmaceuticals in the river Elbe and its tributaries. Chemosphere 57(2):107–126
Wijngaarden R, Van PA, Brock T, Brink PJ (2005) Threshold levels for effects of insecticides in freshwater ecosystems: a review. Ecotoxicology 14(3):355–380
Woodburn KB, Hansen SC, Roth GA, Strauss K (2003) The bioconcentration and metabolism of chlorpyrifos by the eastern oyster, Crassostrea virginica. Environ Toxicol Chem 22(2):276–284
World Health Organization (2020) The WHO recommended classification of pesticides by hazard and guidelines to classification 2019. World Health Organization
Yadav IC, Devi NL (2017) Pesticides classification and its impact on human and environment. Environ Sci Eng 6:140–158
Yadav DV, Agarwal HC, Pillai MKK (1978) Uptake, metabolism and excretion of DDT by the fresh water snail, Vivipara heliciformis. Bull Environ Contam Toxicol 15:300–306
Yadav IC, Devi NL, Syed JH, Cheng Z, Li J, Zhang G, Jones KC (2015) Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: a comprehensive review of India. Sci Total Environ 511:123–137
Yamato Y, Kiyonaga M, Watanabe T (1983) Comparative bioaccumulation and elimination of HCH isomers in short-necked clam (Venerupis japonica) and guppy (Poecilia reticulata). Bull Environ Contam Toxicol 31(3):352–359
Yang G, Ge S, Singh R, Basu S, Shatzer K, Zen M, Liu J, Tu Y, Zhang C, Wei J, Shi J, Zhu L, Liu Z, Wang Y, Gao S, Hu M (2017) Glucuronidation: driving factors and their impact on glucuronide disposition. Drug Metab Rev 49(2):105–138. https://doi.org/10.1080/03602532.2017.1293682
You J, Landrum PF, Lydy MJ (2006) Comparison of chemical approaches for assessing bioavailability of sediment-associated contaminants. Environ Sci Technol 40(20):6348–6353
Yuniari SH, Hertika AMS, Leksono AS (2016) Lethal concentration 50 (LC50—96 hours) Nile Tilapia (Oreochromis niloticus) exposed Cypermethrin-based pesticide. JExp Life Sci 6(2):58–62
Yu-yun T, Thumm W, Jobelius-Korte M, Attar A, Freitag D, Kettrup A (1993) Fate of two phenylbenzoylurea insecticides in an algae culture system (Scenedesmus subspicatus). Chemosphere 26(5):955–962
Zaffar H, Irshad U, Pervez A, Naqvi TA (2016) Mode of action, toxicity and biodegradation of organochlorinated pesticides: a mini overview. J Appl Environ Biol Sci 6(8):1–6
Zaki MS, Hamaam AMM (2014) Xenobiotics as stressful factors in aquatic system (in fish). Life Sci J 11(4):188–197
Zaroogian GE, Johnson M, Heltshe JF (1985) Estimation of bioconcentration in marine species using structure-activity models. Environ Toxicol Chem 4(1):3–12
Zhang C, Wang S, Yan Y (2011) Isomerization and biodegradation of beta-cypermethrin by Pseudomonas aeruginosa CH7 with biosurfactant production. Bioresour Technol 102:7139–7146
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
S, A.D. et al. (2023). Pesticide and Xenobiotic Metabolism in Aquatic Organisms. In: Rather, M.A., Amin, A., Hajam, Y.A., Jamwal, A., Ahmad, I. (eds) Xenobiotics in Aquatic Animals. Springer, Singapore. https://doi.org/10.1007/978-981-99-1214-8_1
Download citation
DOI: https://doi.org/10.1007/978-981-99-1214-8_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1213-1
Online ISBN: 978-981-99-1214-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)