Abstract
Municipal landfilling is the most common waste management practice to eliminate urban solid waste, but a critical problem coupled with landfills is the generation of toxic landfill leachates. These landfill leachates contain toxic heavy metals and recalcitrant compounds and must be treated before being released into the water bodies. This chapter recapitulates wide-ranging studies on various leachate treatment technologies such as physicochemical, biological (aerobic and anaerobic), and combination of physicochemical and biological processes. It is comprehended that individual physicochemical and biological leachate treatment process is incapable to attain strict discharge standards for direct release into the water bodies, whereas a combination of physicochemical and biological treatment process can accomplish acceptable treatment efficiencies of both ammoniacal nitrogen and chemical oxygen demand removal. In this regard, a decrease in the toxicity of treated leachates will aid in evaluating the efficiency of a leachate treatment technology. Currently, the practice of up-flow anaerobic sludge blanket reactor (UASB) coupled with reverse osmosis (RO) membrane (UASB-RO) has shown to be a requisite means for accomplishing toxic-free treated leachate.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Landfill leachates
- Leachate treatment technology
- Physicochemical process
- Biological process
- Ammoniacal nitrogen
- Chemical oxygen demand
7.1 Introduction
The increase in municipal solid waste (MSW) generation has resulted in a significant problem to the society due to the increased environmental risk during landfilling (Bai and Sutanto 2002; Chen et al. 2010; Luo et al. 2019a). Municipal landfilling is a widely used, inexpensive method for the management of MSW when compared to previously adopted technologies such as composting and incineration (Renou et al. 2008; Luo et al. 2017). It is claimed that around 95% of the total global MSW generated is disposed off in landfills (Gao et al. 2014). Disposal of MSW over the land usually originates risk, due to the disposing of hazardous wastes which pose a significant risk to the environment. Although currently MSW disposal is carried out in highly engineered modern landfill facilities, the generation of landfill leachate (LL) is still a major problem for modern landfills, due to the significant risk of contaminating soil, surface, and groundwater (Kjeldsen et al. 2002; Yan et al. 2015; Luo et al. 2019b). The composition of leachate is highly dependent on the age of the landfill, as the leachate parameters (Ammoniacal nitrogen, BOD, BOD/COD ratio) significantly changes as the landfill tends to stabilize (Kjeldsen et al. 2002; Kulikowska and Klimiuk 2008). Various physicochemical and biological methods, as well as combination of both, have been adopted in order to fulfill the stringent discharge standards in different parts of the world (Wiszniowski et al. 2006; Silva et al. 2017; Torretta et al. 2017). Physicochemical methods have been used for the removal of refractory organics as well as a refining step for the biologically treated leachate (Kurniawan et al. 2006; Chys et al. 2015).
Biological aerobic and anaerobic methods are considered to be simple, reliable, and highly cost-effective and are used for the treatment of bulk leachate containing a high concentration of organics (Miao et al. 2019). In the aerobic biological treatment, microorganisms result in the breakdown of organic compounds to carbon dioxide and sludge and to biogas (carbon dioxide and methane) under anaerobic conditions. Furthermore, combination of physicochemical and biological treatments can accomplish acceptable treatment efficiencies of contaminants such as ammoniacal nitrogen, heavy metals, refractory organics, BOD, and COD (Hassan et al. 2017; Pastore et al. 2018; Gomes et al. 2019). Several studies, exercising the use of physicochemical, biological, and combined treatments for the treatment of landfill leachate have been studied worldwide in the last decades. However, not many endeavors have been made to acquire a detailed overview of all the treatment methods in terms of optimum conditions for the maximum removal of ammoniacal nitrogen and COD for the landfill leachate. This chapter covers the state-of-art treatment technologies, technical applicability, and efficiency of all available physicochemical, biological, and combined treatments for the LL treatment. This chapter shall significantly contribute to the future sustainable treatment of landfill leachate.
7.2 Landfill Leachate Treatment
Treatment of LL in past has been carried out using three major groups: (1) physicochemical processes; (2) biological processes (aerobic and anaerobic), and (3) a combination of physicochemical and biological processes (Wiszniowski et al. 2006; Torretta et al. 2017; Renou et al. 2008).
7.2.1 Physicochemical Processes for LL Treatment
Physicochemical treatments are considered to be the most appropriate for the removal of refractory compounds from the leachate. Various physicochemical processes are employed in the treatment of LL for the removal of COD, BOD, NH3-N, and/or heavy metals. In recent years, various studies have been carried out worldwide on the performance of different physicochemical methods for the treatment of SL. Various physicochemical methods and their technical applicability, and performance are discussed below. The combined effect of different processes and their advantages and limitations are also discussed.
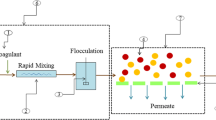
7.2.1.1 Coagulation-Flocculation
Coagulation-Flocculation is a two-step process employed for the removal of organic compounds present in LL that are usually nonbiodegradable (Amokrane et al. 1997; Diamadopoulos 1994; Urase et al. 1997). It is an ion-dependent process where the colloidal particles are destabilized by the addition of a coagulant. The colloidal particles present in the leachate are negatively charged and thus remain suspended. Upon the addition of a positively charged coagulant, these suspended particles get attracted to the coagulant, forming clusters and this process is called coagulation (Fig. 7.1). In order to enlarge the size of those particles, coagulation is usually followed by flocculation where there is the formation of bulky floccules thus settling occurs more rapidly as represented in Fig. 7.2 (Cheng et al. 1994). This process can be employed for heavy metal removal from LL and usually involves pH adjustment as the first step which is followed by the addition of a coagulant such as alum or ferric salts and then flocculating agent (Tatsi et al. 2003). Examples of coagulation-flocculation process include the removal of heavy metals using FeCl3 and the removal rate was found to be more at pH 9.0 than pH 4.0 which was the actual pH of that leachate. Thus, it demonstrates that the removal of heavy metals by precipitation is more effective in basic conditions than the actual pH condition (Urase et al. 1997). A comparative study between the effectiveness of alum and FeCl3 was carried out and the results showed that ferric chloride gave a higher removal of organic compounds (55%) than alum (43%) thus proving that ferric chloride is more efficient than alum in the coagulation process (Amokrane et al. 1997).
Overall, the coagulation-flocculation process is effective for the removal of heavy metals and organic compounds from the LL. Lime can also be used as a coagulant for better COD removal. High cost due to the consumption of chemicals, generation of sludge as a result of the settlement of suspended particles, and sensitivity to pH are the drawbacks associated with this technique. pH, velocity gradient, and settling time have to be noted carefully since they play key roles in the settlement of colloidal particles (Kurniawan et al. 2006).
7.2.1.2 Ammonium Stripping
It is the most extensively used technique for the removal of ammoniacal nitrogen (NH3-N) from LL (Calli et al. 2005; Cheung et al. 1997; Diamadopoulos 1994; Marttinen et al. 2002; Ozturk et al. 2003). Here, the leachate containing NH3-N is allowed to interact with the air phase in a counter-current flow in a stripping tower where the NH3-N from the leachate is transported into the air and is then adsorbed into a strong acid like H2SO4. It can also be directly fluxed into the ambient air (Bonmatí and Flotats 2003). Ammonium stripping is considered as the most effective technique for NH3-N removal.
In a case study, nanofiltration and ammonium stripping were applied for the treatment of a YL from Finland (Bonmatí and Flotats 2003). When nanofiltration alone was applied, only 50% of NH3-N and 66% of COD were removed whose initial concentrations were 220 mg/L and 920 mg/L, respectively. But at pH 11, 89% of NH3-N and 21% of COD were removed by the application of ammonium stripping with the same initial concentrations and these results obtained agree with those obtained in another study where about 85% of NH3-N was removed by ammonium stripping from anaerobically pretreated leachate from Turkey (Oyaderi landfill) whose initial concentration was 1025 mg/L (Calli et al. 2005).
Thus, ammonium stripping is an efficient technique for the removal of ammoniacal nitrogen from LL and this can be followed by biological treatment for better COD removal. To enhance the removal of NH3-N, the pH of the leachate can be adjusted to basic conditions before the treatment. Being able to meet the ammoniacal nitrogen discharge standard using ammonium stripping alone is another advantage of this process (Bae et al. 1997). It is also more economical when compared to other techniques like reverse osmosis and nanofiltration (Kurniawan et al. 2006). Despite all the advantages, this technique has a few drawbacks. The major problem associated with this technique is the release of ammonia gas into the air and its impact on the environment. Thus, there is a requirement for additional treatment of the gas with acids like hydrochloric acid or sulphuric acid which in turn increases the operational cost of the treatment process. Other limitations include the requirement for pH adjustment of treated effluent before discharge since the operation occurs in basic conditions, CaCO3 scaling of stripping tower during pH adjustment using lime and the difficulty in removal of lesser concentration of NH3-N (<100 mg/L) (Li and Zhao 1999; Tanaka and Matsumura 2002).
7.2.1.3 Chemical Precipitation
Chemical precipitation has been used for the exclusion of heavy metals, NH3-N, and nonbiodegradable organics from LL because of its simplicity and cost-effectiveness (Calli et al. 2005; Cecen and Gursoy 2000; Li et al. 1999; Ozturk et al. 2003). During the process of chemical precipitation, the ions dissolved in the mixture get converted into insoluble ions by chemical reactions. Mostly, metal precipitates in the form of hydroxide from the solution. Depending on the target removal magnesium ammonium phosphate (MAP), known as Struvite (for NH3-N) or lime (heavy metals) is used as the precipitant (Kurniawan et al. 2006).
Chemical precipitation using struvite was applied for NH3-N removal from anaerobically pretreated leachate from the Oyaderi landfill (Turkey) (Ozturk et al. 2003). Here, ammonia that was present in the leachate got converted into a nitrogen fertilizer such as urea (Eq. 7.1). Around 50% and 90% of COD and NH3-N have been removed whose initial concentrations were 4024 mg/L and 2240 mg/L, respectively. These results indicate that struvite can efficiently remove NH3-N from leachate than organic compounds. This is in agreement with the results of another study where struvite was used to decrease the ammoniacal nitrogen concentration in the leachate (Calli et al. 2005). Around 98% of NH3-N was precipitated at pH 7.5 along with 20% of COD removal.
7.pKs = 12.6 (25 °C)
The main advantage of using struvite as the precipitant is that if there is no presence of heavy metals in the leachate, the sludge produced from this process can be used as a fertilizer. However, biological treatment has to be carried out to lower the level of COD of the leachate (Li and Zhao 2001). In the case of the uptake of heavy metals like copper, nickel, manganese, lead, and iron, lime was found to be an effective precipitant (Cecen and Gursoy 2000). Besides the use of lime, adjusting the pH to basic conditions enhanced the level of metal precipitation. The limitations of this method include the requirement of high dose precipitant, sensitivity to pH and sludge generation, and further disposal of it (Kurniawan et al. 2006).
7.2.1.4 Membrane Filtration Tecnologies
7.2.1.4.1 Microfiltration (MF)
It is employed in the removal of colloidal particles whose size ranges from 0.05 to 1.0μm by a cross-flow at low pressure. Since the rate of retention was not significant and only 25–35% of the COD reduction was achieved, it is usually employed as a pretreatment for other membrane processes or in combination with chemical treatments (Fig. 7.3) (Abbas et al. 2009).
7.2.1.4.2 Ultrafiltration (UF)
In this process of selective fractionation using pressure of up to 10 bar, suspended solids and solutes weighing more than 1000 Da are concentrated. The infiltrate has salts and solutes of low molecular weight. Depending on the type of material used as a membrane, UF can be beneficial in the removal of molecules of macro size as represented in Fig. 7.3. It can be used employed to fractionate organic compounds and so can assess the molecular weight of those compounds present in the leachate. In addition, by analyzing the permeates of the membrane, knowledge about the nature and toxicity of the compound can be gained. It has also been stated that UF can be an effective pretreatment process for reverse osmosis. It can remove larger molecules that result to foul the membranes used in reverse osmosis. In combination with biological treatment, UF has been employed and successful treatment of leachate has been achieved (Abbas et al. 2009).
7.2.1.4.3 Nanofiltration (NF)
Nanofiltration has exceptional properties that ultrafiltration and reverse osmosis membranes do not have and thus have been employed in the treatment of LL (Linde and Jönsson 1995; Ozturk et al. 2003; Urase et al. 1997). Through the electrostatic interactions between the ions and the membranes, particles of molecular mass greater than 300 Da along with inorganic substances are removed in this process. The surface charges on the membrane reject the charged solutes smaller than the membrane pores along with bigger neutral solutes and salts which makes the process significant (Fig. 7.3) (Kurniawan et al. 2006).
The use of NTR-7250 for the heavy metal removal achieved 99% of removal whose original metal concentrations were 0.69 mg/L and 0.23 mg/L of Cr3+ and Cu2+, respectively (Urase et al. 1997).
Nanofiltration was used for the treatment of anaerobically pretreated leachate (Oyaderi landfill) (Ozturk et al. 2003). Around 90% and 70% of COD and NH3-N have been removed whose initial COD concentrations were 3000 mg/L and 920 mg/L, respectively. The total operational cost was US$ 0.8/m3 which made the process more efficient.
Thus, NF has established a reasonable treatment performance in the reduction of organic load with COD concentrations ranging from 900 to 3000 mg/L. Due to the presence of negatively charged groups in its membrane, NF can also effectively remove heavy metals. Monovalent and divalent ions dissolved in the solution can also be separated by the application of NF, the materials dissolved in the solution can be separated into monovalent and divalent ions as represented in Fig. 7.3. Unlike RO, NF has a looser structure that enables higher fluxes and lowers operating pressure during the treatment process.
7.2.1.4.4 Reverse Osmosis (RO)
Reverse osmosis is another physicochemical method applied for the treatment of SL that has higher flexes and can operate over a wide temperature and pH range. In RO application, the metal concentration is reduced by controlling the flow of metal cation-containing solvent into the membrane (Jenkins et al. 2003). Organic compounds, dissolved solids, and suspended/colloidal particles can be removed by the application of RO with a 98–99% rejection rate of organic and inorganic contaminants (Fig. 7.3) (Kurniawan et al. 2006).
Removal of dioxins like polychlorinated biphenyls (PCB), polychlorinated dibenzofurans (PCDF), and polychlorinated dibenzo-p-dioxins (PDPD) by the application of RO was studied and results showed complete removal of dioxins whose initial concentration was 2.35 mg/L. COD was completely removed and 98% removal of NH3-N was also removed whose initial concentrations were 97.4 mg/L and 33.7 mg/L, respectively (Ushikoshi et al. 2002).
The application of RO membrane during the treatment of YL from a landfill in South Korea, resulted in 96–97% COD and NH3-N removal whose initial concentrations were 1500 mg/L and 1400 mg/L (Ahn et al. 2002). The results prove that RO can be used to enhance the treatment efficiency by the removal of nonbiodegradable organic compounds from LL.
A comparative study between RO and NF for the treatment of SL revealed that RO (99%) was more efficient than UF (52%) in the removal of COD with an initial concentration of 1780 mg/L (Peters 1998a, b). However, due to varying compositions, a combination of biological treatment and RO has to be carried out for the effective treatment of leachate.
There are various factors that govern the efficiency of the RO. The characteristic of the membrane used greatly affects the treatment performance on the basis of the removal of organic compounds and ammoniacal nitrogen. Charge, porosity, hydrophilicity, thickness, roughness, and the material used are the factors that affect the water passage through the membrane. Higher removal of organic compounds and ammoniacal nitrogen can be obtained by the use of membranes made of cellulose acetate or polyamide which can also work in a wide range of temperatures (5–35 °C) when compared to membranes composed of PVC.
It may be because of the high permeability and hydrophilicity of polyamide when compared to other materials like polyethylene-terephthalate and polysulphone. Other factors that have to be considered while choosing a membrane are the characteristics, pH, temperature of the leachate, nature, and concentration of components present in it (Alvarez-Vazquez et al. 2004).
Overall, RO is an efficient technique for the removal of both COD and ammoniacal nitrogen from LL. However, the main disadvantage of RO is membrane fouling, where, suspended or dissolved substances get undesirably deposited on the outer surface of the membrane (Choo and Lee 1996). Another limitation of RO is those small molecules that pass through the membrane have low retention time and also there is high energy consumption. It is also reported that about 60–80% cost of RO treatment accounts for energy consumption (Peters 1998b). Therefore, during the selection of treatment, affordability has to be considered to justify it as the solution.
7.2.1.5 Activated Carbon Adsorption (ACA)
Of all the treatment technologies discussed, adsorption is the most broadly employed method for the removal of toxic contaminants from LL (Abdul Aziz et al. 2004; Babel and Kurniawan 2004, 2003; Fettig 1999; Heavey 2003; Imai et al. 1998; Morawe et al. 1995; Wasay et al. 1999). In general, during adsorption, by means of mass transfer, a substance is transferred from the liquid phase to the surface of the solid and becomes bound by physical and/or chemical interactions. Significant attention has been given in the past few years to adsorption using granular activated carbon (GAC) and/or powdered activated carbon (PAC) in the removal of contaminants from wastewater due to its large surface area, high adsorption capacity, inherent physical properties, microporous structure, and surface reactivity (Kurniawan et al. 2006).
In a study, carried out in 1995, GAC was used for the removal of the level of COD and the results showed that 91% of COD was removed (Initial concentration: 940 mg/L). It was also found that along with film diffusion, the rate of adsorption and the internal surface diffusion on the solid surface of the adsorbent greatly affected the kinetic rate of adsorption (Morawe et al. 1995).
GAC, granular activated alumina (GAA), and/or ferric chloride were used separately for the treatment of LL and of the three adsorbents examined, GAC was most effective in the heavy metal removal such as cadmium, chromium, manganese, lead, and zinc. About 80–96% of heavy metal at a pH range of 6–7.7 was removed with 2 g/L of GAC. It was reported that GAC adsorption was represented by Freundlich isotherm (Wasay et al. 1999).
A relative study for NH3-N removal from leachate with an initial ammoniacal nitrogen concentration of 1909 mg/L using GAC and/or lime was carried out (Abdul Aziz et al. 2004). About 40% removal was achieved using 42 g/L of GAC, whereas, with 52 g/L of lime, only 19% NH3-N was removed under the same concentration. Even though lime was less effective, it was more cost-effective when compared to GAC for the removal of NH3-N.
By varying the concentration from 0.2 to 10 g/L of PAC for the treatment of SL, it was found that 95% of COD was removed using 6 g/L of PAC whose initial concentration was 5690 mg/L (Diamadopoulos 1994). Here, Freundlich isotherm was applicable for adsorption and thus indicating the occurrence of multilayer adsorption on the surface of PAC (Imai et al. 1998).
Other than GAC and PAC, other locally available nonconventional materials such as industrial by-products or agricultural waste can be chemically altered and used as low-cost adsorbents (Babel and Kurniawan 2003; Kurniawan and Babel 2015). By converting waste into activated carbon which can be used in the treatment of wastewater there is an increase in the economic value. It also helps the industry in reducing the cost of waste disposal and also acts as a budget-friendly substitute for commercially available high-cost adsorbents (Babel and Kurniawan 2004; Heavey 2003). Coconut shell (Kurniawan and Lo 2009) and zeolite (Kargi and Pamukoglu 2004) are examples of low-cost adsorbents that are employed for the removal of COD from LL.
Overall, the usage of activated carbon (GAC or PAC) as adsorbent is a virtuous technique for the removal of nonbiodegradable compounds from LL. However, this cannot be applied for NH3-N removal since it is less efficient when compared to other techniques. With concentrations ranging from 940–7000 mg/L, around 90% COD removal was achieved. In spite of this, recurrent regeneration of activated carbon and high cost of GAC act as limiting factors for the treatment of LL.
7.2.2 Biological Processes for LL Treatment
Biological treatment also known as bioremediation of municipal LL involves the use of microorganisms such as bacteria, fungi, and some protozoa to degrade the organics present in the LL. Biological treatment is one of the most economical, efficient, and reliable method for the removal of BOD as the microorganism uses the organic matter present in the leachate as their carbon source and converts them into simpler, less toxic substances (Di Iaconi et al. 2006). Due to their growth, adaptability to the given environment, and pliability, municipal LL treatment depend on microbes to degrade the organic compounds and produce clear effluent water by utilizing the ability of microorganisms to challenge the source of larger issues like degrading odor (Luo et al. 2019). They offer major advantages over alternative treatment strategies. The biological treatment process can be broadly classified into aerobic and anaerobic based on the requirement of oxygen by the microorganism used for the treatment (Renou et al. 2008).
7.2.2.1 Aerobic Biological Treatment Processes
The treatment of municipal LL under aerobic condition involves the use of aerobic microorganism that converts the complex organic substance into H2O and CO2 in the presence of O2. Aerobes use oxygen to oxidize the substrate, that is, the organic portion of the leachate to obtain the energy required for their metabolism. This process is known as cellular respiration in which oxygen acts as a terminal electron acceptor (Mohd-Salleh et al. 2020).
7.2.2.1.1 Aerated Lagoon (AL)
Aerated lagoons also known as aerated pond is an economical and simple leachate treatment system wherein the leachate is added to a basin and artificial aeration is provided to promote biological oxidation. It can be used for in situ treatment of the LL. In a study, it was found that the leachate with a low COD and high ammonium concentration of 1241 mg/L treated with four connected aerated lagoons lead to 75% and 80% removal of COD and Ammonium concentration, respectively (Mehmood et al. 2009). When a two-staged anaerobic/facultative lagoon system is used for leachate with COD of 5050 mg/L and 1670 mg/L of TN, removal efficiencies equal to COD 40%, BOD 64%, NH4+-N 77%, NO3−-N 63%, TN 77%, P 42%, SO42– 44%, Mn 44%, and Fe 30% was achieved (Frascari et al. 2004). In an aerated lagoon about 80–88% phenol can be removed (Orupõld et al. 2000).
The process of lagooning is not a satisfactory operation for leachate treatment as it is unable to meet the environmental regulations. One of the significant limitations of the process is that it is temperature-dependent which may hinder microbial activity.
7.2.2.1.2 Activated Sludge Process (ASP)
Activated sludge is a concoction of bacterial biomass which can utilize the organic matters present in the leachate and convert them into H2O, CO2, and minerals along with the generation of new microbial biomass via the aerobic respiration process (Fig. 7.4) (Luo et al. 2014).
Activated sludge process can be adapted for the co-treatment of municipal LL and sewage. When an anaerobically pretreated municipal LL with COD 270–1000 mg/L and NH4+-N 53–270 mg/L is subjected to an activated sludge process with the addition of plastic carrier material in a laboratory-scale reactor with temperatures varying from 5–10 °C, the COD reduced to 150–500 mg/L, less than 7 mg/L BOD, and less than 13 mg/L NH4+-N (Hoilijoki et al. 2000). In a research, the treatment efficiency of aerobic granular sludge and activated sludge systems for a young LL were compared and it was found that 99% of partial nitrification took place in granular sludge and 77 ± 10% in activated sludge system. COD removal was also more efficient in granular sludge when compared to activated sludge. The removal of phosphorus was the same in both cases (Renou et al. 2008). Researches also show that the nitrification process during the activated sludge process can be improved with the mixing of powdered activated carbon into the activated sludge reactors (Özgür Akta 2001).
Although the activated sludge system is effective in eliminating the organic load and ammonia content in the municipal LL, this method has several downsides like generation of a huge quantity of sludge leading to investments in sludge disposal, high energy consumption, longer aeration time and inhibition of microbial growth due to high ammoniacal nitrogen concentration (Renou et al. 2008; Torretta et al. 2017). Hence, more efficient technologies should be developed for the removal of COD and nitrogen.
7.2.2.1.3 Sequencing Batch Reactor (SBR)
Sequencing batch reactor also known as a sequential batch reactor is a type of activated sludge system. The SBR is an array of tanks that operates based on a fill-and-draw mechanism. The SBR is operated in four steps, that is, the tanks are filled, aerated for a specific period, contents can settle, and finally the supernatant is decanted (Liu et al. 2005). The municipal LL can be treated through concurrent oxidation of organic matter and nitrification.
Researches show that the rate of removal of COD is reduced with an upsurge in the influent ammonium concentration when the leachate is treated with granular sludge SBR (Wei et al. 2012). In order to meliorate the performance of SBR for leachate treatment, ultrasonic pretreatment is carried out due to which 90% COD and 70% ammonia are removed (Neczaj et al. 2005). To completely utilize the organic load present in the leachate (COD/TN ratio = 1–4, NH4+-N = 1000 ± 50 mg/L), a modified SBR that operates at anaerobic-aerobic-anoxic mode has been developed. When this was operated for 70 days, efficient nitrogen removal up to 10 mg/L was achieved at a C/N ratio of 4 (Miao et al. 2015). When coagulation process, fenton system and SBR were combined, COD was removed up to 97.3% (100 mg/L), and 99% (3 mg/L) ammonia was removed (Li et al. 2009). When a PAC-SBR was operated with optimum conditions like 1 L/min of aeration and 5.5 h of contact time, 64.1% COD, 71.2% NH4+-N, and color has been removed (Aziz et al. 2011).
Therefore, when compared to other leachate treatment technologies SBR process is more flexible for municipal LL treatment because of its high degree of variability in terms of both quality and quantity (Kennedy and Lentz 2000).
7.2.2.1.4 Rotating Biological Contractor (RBC)
Rotating biological contractor, also known as rotating biological filters, is an alternate to the traditional activated sludge process. They are made up of rotating discs that are fixed on a horizontal shaft (Fig. 7.5). A mechanical motor or a compressed air drive is used for the continuous rotation of the shaft. These are partially or completely submerged (Cortez et al. 2008), and it rotates as the wastewater flows through it. A biofilm is formed on the surface of the rotating disc by using biofilm-forming microorganism which oxidizes the organic matter present in the wastewater. The revolution of the disc facilitates the transfer of O2 to maintain the biomass under aerobic conditions. It also offers turbulence in the mixed liquor surface and simplifies the elimination of extra solid from the media (Patwardhan 2003; Rodgers and Zhan 2004).
A study investigated the feasibility of applying RBC system and anaerobic system for the treatment of municipal LL (Castillo et al. 2007) reported that about 65% removal of COD for an influent leachate 2500–9000 mg/L can be achieved. Aresearch which studied the efficiency of removal of nitrate from a mature landfill with nitrate load of 530 mg/L using the RBC system reported that nearly 100% nitrogen-nitrate removal could be achieved (Cortez et al. 2011). When the same was investigated for the removal of nitrite in the leachate, it was observed that the RBC system is insensitive to high nitrite load (up to 100 mg/L of NO2−-N) which resulted in only a temporary decrease in the removal efficiency (Cema et al. 2007).
The RBC system is suitable for the remediation of leachate with low organic content (Kurniawan et al. 2010). However, it is inefficient in treating the high-strength leachate because of the clogging caused due to biomass deposition. The efficiency of the RBC system can be improved by coupling it with other technologies like biological treatment or physical-chemical processes (Castillo et al. 2007).
7.2.2.1.5 Trickling Filter (TF)
A trickling filter, also called as biofilter or biological filter is an aerobic treatment method which consists of a bed of rock or other coarse material through which the wastewater is trickled or sprayed that enables the microorganism present in the wastewater to attach themselves to the bed of rocks in the form of biofilm (Matthews et al. 2009). When the microorganism encounters wastewater, it utilizes the organic matter and converts it to CO2 and water. This process is facilitated by diffusing or forcing air through the bed (Torretta et al. 2017). A study reported that in a laboratory-constructed pilot-scale crushed brick trickling filter with loading rates of 100–130 mg/L/day at 25 °C and 50 mg/L/day at temperatures 5–10 °C was able to 90% of nitrification (Jokela et al. 2002). Another study which used bench-scale TF and SBR processes for leachate treatment found that there was a decrease in suspended solids (73.17%), turbidity (71.96%), COD (49%), BOD (76.69%), and NH4+-N (59.50%) in TF process. This technology can be applied only for the treatment of mature leachates and not for young leachates due to high organic load in them (Aluko and Sridhar 2013). A different study that used pilot-scale submerged aerobic biofilter for co-treatment of sewage and municipal LL reported 98% BOD, 80% COD, 90% suspended solids, and 90% NH4+-N removal (Ferraz et al. 2014).
7.2.2.1.6 Moving-Bed Biofilm Reactor (MBBR)
The moving-bed biofilm reactor system is a category of attached growth system that consists of an aeration tank in which the sludge is collected onto recyclable plastic carriers. These carriers have a huge internal surface area in which a biofilm can grow. The system is supplied with aeration to keep the carriers with biomass in motion to have enough interaction between the wastewater and aerobic microorganisms. The biomass consumes the organic matter in the wastewater and produces new biomass along with water and carbon dioxide. The excess sludge will come out from the carrier, flow with the treated water, and removed in the final separator as represented in Fig. 7.6.
A research investigated the execution of MBBR system for the removal of COD and ammonium from a municipal LL with an organic loading rate of 4.08–15.70 × 103 mg/L/day COD. It reported that 92–95% of COD and 97% of ammonium were removed (Chen et al. 2008). When granular activated carbon MBBR system was used to treat a LL, 85–90% ammonia and 60–81% of COD were removed (Loukidou and Zouboulis 2001). In a study, the membrane bioreactor and MBBR process were combined to treat the LL. It resulted in 95% oxidation of total nitrogen with effluent ammonium nitrogen concentration less than 50 mg/L (Canziani et al. 2006).
The advantages of MBBR process over traditional activated sludge process is that MBBR system has higher biomass concentration due to the large surface area provided by the carriers, they are less sensitive to toxic compounds, less sludge settling time, and removal of high ammonia concentration in a single process.
7.2.2.1.7 Fluidized-Bed Biofilm Reactor (FBBR)
In a fluidized-bed biofilm reactor, the reactor is packed with beads in which the microorganisms attach and grow as a biofilm on the surface. The fluidization of the beads in the column can be achieved by the recirculation of wastewater or sparging air from the bottom of the column (Fig. 7.7). When the air is sparged, the beads start to flow, and the microorganisms oxidize the organic matter when it encounters the wastewater (Bello et al. 2017). Depending on the treatment process the reactor can be run in a single or double column system. A study which evaluated the reliability and commercial viability of LL treatment using integrated liquid-solid circulating FBBR reported 85% removal of COD with loading rate of 2150 mg/L/day, 80% removal of nitrogen with loading rate of 700 mg/L/day, and 70% removal of phosphorus at a loading rate of 14 mg/L/day, respectively (Eldyasti et al. 2010). Another study showed that, in the treatment of acid mine drainage using high rate FBBR, the LL can be used as an inexpensive soluble carbon source for sulfate-reducing bacteria (Sahinkaya et al. 2013). In FBBR process, the biomass grown of the expanded bed can be easily harvested as there is no filtration of solids that are from the passing flow (Torretta et al. 2017). Hence, it is considered as a feasible system for the treatment of municipal LL.
7.2.2.1.8 Membrane Bioreactor (MBR)
Membrane bioreactor is a wastewater treatment technology which integrates the biological treatment process like conventional activated sludge system with a semipermeable membrane like microfiltration (Alvarez-Vazquez et al. 2004). The biological process helps in the oxidation of organic matter and the semipermeable membrane separates the biosolids/microorganisms from the treated effluent. Based on the position of the membrane, the membrane bioreactor can be classified into submerged membrane and outer membrane as represented in Fig. 7.8 (Xue et al. 2015).
The mature LL is characterized by a small BOD/COD ratio contributing to the low biodegradability of the leachate (Saleem et al. 2018b) and hence MBR has a great potential in treating the mature LL. The MBR could remove 90% of BOD and ammonia and 75% of COD when compared to conventional treatment technologies, regardless of the landfill age (Ahmed and Lan 2012). When a lab-scale submerged pre-anoxic and post-aerobic bioreactor configuration like the dynamic membrane bioreactor is used for municipal LL treatment, it was observed that 98% of ammoniacal nitrogen and 90% of total nitrogen could be achieved (Saleem et al. 2018a). The combination of the MBR system with electrochemical oxidation is considered feasible for the treatment of LL as it is efficient in the reduction of several parameters such as COD (85%), TN (94%), and color (99%) with organic loading rates of 1900 and 2700 mg/L/day COD (Feki et al. 2009). In a study when MBR alone is used for the treatment of mature LL with an organic load rate of 1200 mg/L/day COD resulted in 63, 35, 98, and 52% removal of COD, TOC, NH4+-N, and phosphorous (Zolfaghari et al. 2016). MBR is known as a versatile biological technology for the treatment of LL as it can be used to treat both young and mature LL.
7.2.2.1.9 Constructed Wetlands
Constructed wetlands are artificially constructed systems that include natural geological, chemical, and biological processes in the ecosystem for the remediation of wastewater. The constructed wetlands are mainly made of three components: an impervious layer which prevents the infiltration of wastewater into the groundwater, a grit layer where the biological treatment and denitrification occurs, and the above-ground vegetation layer that contains the plant species (Kivaisi 2001). For a municipal LL treatment, constructed wetlands have been developed in a lab-scale, pilot-scale, and full-scale system with greater removal efficiencies (Nivala et al. 2007). The toxic contaminants like phenol, bisphenol A (BPA), and 4-tert-butylphenol (4-t-BP) present in synthetic young and mature can be removed using a lab-scale vertical flow CWs. The percentage removal of phenolic compounds is in the following order: phenol (88–100%) > 4-t-BP (18–100%) ≥ BPA (9–99%) (Dan et al. 2017).
A removal efficiency of 72% NH4+-N and 46% TN can be obtained by using pilot-scale vertical flow CWs planted with Canna indica (Camaño Silvestrini et al. 2019). When a pilot-scale sub-surface flow CW system planted with Cyperus haspan was used, it was able to remove 39–86.6% of turbidity, 63.5–86.6% of color, 39.2–91.8% of COD, 60.8–78.7% of BOD, 29.8–53.8% of NH4+-N, and 33.8–67.0% of TN (Akinbile et al. 2012). A research reported that full-scale hybrid CW system can remove >90% of PPCPs, EDCs, ARGs, and antibiotic-resistant genes from mature land (Yi et al. 2017). The same researchers also employed a full-scale tropical CW system for the removal of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from the leachate and observed that around 61% of total PFASs and 50–96% of individual PFASs can be removed (Yin et al. 2017).
CWs can be low-cost, easy handling, and maintaining technology for the remediation of municipal LL, especially in developing countries but these are not being extensively applied in developed countries due to its poor performance in the winters and large area requirement (Kivaisi 2001).
7.2.2.1.10 Myco-Remediation
Myco-remediation is a fungal-based form of bioremediation that uses fungi and their extracellular enzymes for the degradation or sequestration of contaminants in wastewater. Myco-remediation is beneficial for municipal LL treatment because the fungal mycelium is capable of secreting extracellular enzymes and acids that can degrade both non-stabilized organic matters like cellulose, hemicellulose, and lignin in young LL and stable refractory organic matters like humic and fulvic acids in mature LL (Ghosh and Thakur 2017; Zavarzina et al. 2004). Some fungi also act as hyper accumulators which are capable of absorbing and concentrating heavy metals on their fruiting bodies. The white-rot fungus Dichomitus squalens could grow on mature LL and consume the organic matter present in it as its carbon source. A study showed that the treatment of landfill leachate with the fungus Aliivibrio fischeri resulted in 60% removal of DOC and COD along with a reduction in the toxicity levels (Kalčíková et al. 2014). For a 50% diluted leachate with COD = 4500–45,000 mg /L and NH4+-N = 640–4990 mg/L, about 79% and 68% of COD removal was attained when two strains of white-rot fungi Trametes trogii and Phanerochaete chrysosporium was used. Another selected stain Bjerkandera adusta along with glucose and cellulose as co-substrates was used to treat a mature LL in which, 63% of COD was removed with glucose as co-substrate and 54% COD was removed with cellulose as co-substrate (Bardi et al. 2017). Hence, myco-remediation is an efficient technology for the treatment of municipal LL and its application should be further studied.
7.2.2.1.11 Phytoremediation
Phytoremediation is a type of bioremediation process which uses plants and its associated microorganisms to remediate contaminated wastewater (Lavagnolo et al. 2016). The different types of phytoremediation process include phytoextraction, phytostabilization, phytodegradation, phytostimulation, phytovolatilization, and rhizofiltration. These processes have the ability to degrade and detoxify the potentially harmful compounds present in the municipal LL (Kim and Owens 2010). Irrigation of willow and short-rotation coppice are some of the popular phytoremediation techniques used for the treatment of LL (Aronsson et al. 2010; Justin et al. 2010). When, 126 kg/ha/year of N, 6.7 kg/ha/year of P, and 707 kg/ha/year of TOC were supplied to willows grown on clay, about 93.8% of N, 99.8% of P, and 92.5% of TOC retention could be attained. Due to the irrigation properties of compounds present in LL, it had the ability to significantly improve the biomass production of Salix and Populus plants (Justin et al. 2010).
Phytoremediation is considered as a cost-effective, less harmful, and environmentally acceptable method of leachate treatment when compared to other traditional biological treatment processes. Furthermore, researches and studies need to be established to completely understand the mechanisms of phytoremediation for municipal LL so that effective and efficient remediation models could be developed (Justin et al. 2010).
7.2.2.2 Anaerobic Biological Treatment Processes
Anaerobic treatment is a type of biological treatment which employs anaerobic microorganisms to degrade the organic load in the wastewater (Kurniawan et al. 2010). The anaerobes consume the organic contaminants in the wastewater as their carbon source and convert them into carbon dioxide and methane gas as well as produce new biomass in the absence of oxygen (Smaoui et al. 2017). Anaerobic treatment occurs in four stages. The first stage is called hydrolysis in which the complex organic substances are hydrolyzed to simpler compounds by hydrolytic bacteria, the second stage is acidogenesis during which the simpler organic compounds are converted to acids which are then converted to acetic acid in the acetogenesis stage (Kurniawan et al. 2010). Finally, the acetic acids through the action of methanogenic bacteria are converted to CH4 and CO2 (Begum et al. 2018). When compared to aerobic process, anaerobic process is more beneficial due to lower sludge production, reduction of malodours, reduction in volatile content of sludge, and its ability to degrade recalcitrant compounds (Kheradmand et al. 2010; Smaoui et al. 2017).
7.2.2.2.1 Anaerobic Filter (AF)
An anaerobic filter is a fixed-bed biological reactor that consists of an array of filtration chamber. When the wastewater flows in the chamber, the particles greater than the size of pores in the filter get trapped and are used up by the microorganism that is grown on the filter membrane. To prevent the washout of the biomass, the anaerobic filter is usually operated in an up-flow mode with a hydraulic retention time (HTR) of 12–36 h. In a study, anaerobic filter made up of reticulated polyurethane foam was used to study the feasibility of treating alkaline sulfate-rich leachate. It reported that around 90% and 73% of COD could be removed from the influent leachate with a loading rate of 0.76 and 4.58 kg m3/day COD, respectively (Wang and Banks 2007). Sulfate removal (88%) was also achieved when AF was used for the treatment of partially SL with COD 3750 mg/L and BOD/COD = 0.3 and from a relatively new LL with COD 14,000 mg/L and BOD/COD = 0.7, about 90% removal of COD could be achieved at room temperature with HRT of 24–96 h (Torretta et al. 2017). Due to its high COD removal efficiencies at shorter HRTs for high organic loading rates, it is considered as a suitable technology for the treatment of highly polluted wastewater.
7.2.2.2.2 Up-Flow Anaerobic Sludge Blanket (UASB) Reactor
Up-flow anaerobic sludge blanket reactor or UASB reactor is a type of anaerobic digester in which a layer or sheet of granular sludge is suspended in the reservoir. As the wastewater drifts upwards, the anaerobes utilize the organic matter as their carbon source and convert them into CH4 and CO2 as represented in Fig. 7.9. The up flow of the wastewater coupled with the effect of gravity, suspends the sludge blanket with the help of flocculants.
When a sequential batch UASB reactor was used for the treatment of municipal LL at a loading rate of 0.6–19.7 g/L/day COD, 71–92% of removal efficiency was achieved whereas when a continuous flow USAB reactor was used, around 77–91% of COD was removed (Kennedy and Lentz 2000). At mesophilic conditions, 82.4% COD could be removed from influent leachate with COD 70,390–75,480 mg/L and loading rate of 12.5 kg m3day1 (Ye et al. 2011). A study investigated the co-digestion of LL along with septage using UASB reactor and reported that 68.2%, 73.4%, 44.3%, 47.8%, 53.7%, and 44.4% of total COD, soluble COD, total solids, volatile solids, total VFA, total phosphorus, NH4+-N, carbohydrate, and protein, respectively, could be removed at a hydraulic retention time of 1.5 days (Lin et al. 2000).
UASB reactor is mostly used along with other technologies for the treatment of LL (Wu et al. 2015). For example, 98% of COD and 99.6% of NH4+-N can be removed by using a two-stage sequential UASB/aerobic completely stirred tank reactor (Aǧdaǧ and Sponza 2005). When a two-stage UASB-SBR system was used, around 96.7% of COD removal and 620 99.7% of NH4+-N removal at a low temperature of 14.9–10.9 °C was achieved (Sun et al. 2010). In order to remove COD, BOD, chloride, and NH4+, the efficiency of the hybrid system UASB reactor-RO was also investigated (Bohdziewicz and Kwarciak 2008). One of the major disadvantages of UASB reactor is that it is sensitive to the toxic substances present in the LL.
7.2.2.2.3 Anaerobic Ammonium Oxidation (ANAMMOX)
Anaerobic ammonium oxidation, also known as Anammox, is a part of the N2 cycle in which the anammox bacteria under anaerobic condition converts ammonium and nitrogen dioxide into nitrogen gas and water (Shalini and Joseph 2012; Wang et al. 2016). The bacteria achieve the degradation by using ammonium as its electron donor and nitrite as its electron acceptor (Eqs. 7.2, 7.3, 7.4, and 7.5) (Gao et al. 2015; Renou et al. 2008).
When continuous flow nitration and anammox process were applied for the treatment of a mature LL, nearly 94% of total nitrogen and 62% COD removal was reported when the influent ammonia was 1330 mg/L and COD was 2250 mg/L (Wang et al. 2016). Another treatment process that used two-stage anammox system with a sequencing biofilm batch reactor reported that 95% of total nitrogen was removed with an influent ammonia concentration of 3000 ± 100 mg/L at 35 °C for 107 days (Miao et al. 2016). A novel combined process which consists of a partial nitritation reactor, an anammox reactor, and two underground soil infiltration systems was used for the treatment of leachate with high 643 ammonium and organic matter concentration. About 97% NH4+-N, 87% TN, and 89% COD 644 removal was reported with influent leachate compositions of 1430–2720 mg/L NH4+-N, 1524–2912 mg/L TN, and 1165–2599 mg/L COD (Liang and Liu 2008). Combined partial nitritation and anammox process can be used to achieve high nitrogen removal up to 93 ± 1% and 81 ± 1.2% at nitrogen loading rates of 4.2 kg/m3/day TN and 8.3 kg/m3/day kg/m3/day, respectively (Nhat et al. 2014).
Anammox is an efficient technology for the remediation of municipal LL with high ammonia concentrations as this does not require an organic carbon source for nitrification (Wu et al. 2018). It also produces less sludge, no aeration, and decreased carbon dioxide emission. Hence, Anammox is preferred for the treatment of leachates which contain nonbiodegradable COD and high nitrogen content. Moreover, in detail research on the complete Anammox process and its optimum conditions are required to develop new Anammox reactors for the treatment of LL.
7.2.3 Combined Treatment Processes for LL Treatment
7.2.3.1 Combination of Two or More Physicochemical Treatments
Research was carried out to study the effect of coagulation-flocculation and Fenton oxidation in combination with GAC adsorption on SL treatment (Zamora et al. 2000). The outcome showed that pretreatment with Fenton oxidation enhanced the GAC adsorption thus leading to improved COD removal at pH 4.0. This is because of the oxidation by-products formed by the conversion of organic compounds that had smaller molecules which made them enter the micropores of GAC easily.
Photooxidation was carried out for the treatment of SL by UV-vis irradiation at the wavelength of 313 nm and by that, about 31% of COD was removed. But while using coagulation and photooxidation in combination, 64% of COD removal was achieved at the same concentration (Wang et al. 2002). This implies that the treatment can be effective when a combined technique is used rather than using individual processes.
About 48% of COD was removed while using coagulation alone for the treatment of SL from a landfill in South Korea (Yoon et al. 1998). But it was increased to 73% when a coagulation-Fenton treatment was used. This indicates that the addition of the coagulation process improved the Fenton oxidation process thus helping in the increased removal of organic compounds.
Ozonation-coagulation combination treatment was used for the removal of organic pollutants present in SL (Monje-Ramirez and de Velásquez 2004). Pretreatment using Fe (III) as a coagulant was found to be effective. The initial COD concentration was 5000 mg/L and about 78% of it was removed at pH 4–5 using a two-step treatment using ozonation.
NF and PAC adsorption were used in combination for the treatment of leachate that has been pretreated biologically and the results showed that combined treatments removed 97% COD whose initial concentration was 1450 mg/L (Meier et al. 2002). This recommends that combined treatments provide better treatment results than separate processes.
An integration of biologically activated carbon and UF where the cross-flow filtration unit is integrated with the adsorption of organic matter was used for the treatment of YL from the landfill in the USA. With a COD initial concentration of 3050 mg/L, 97% of COD was removed. Permeate flux deterioration was mitigated by the addition of PAC due to membrane fouling (Pirbazari et al. 1996).
Coagulation, flocculation, and Fenton oxidation were employed in a sequence for the removal of colloidal particles from the leachate. Using 0.8 mg/L FeCl3 at pH 8.5, around 90% of COD was removed whose initial concentration was 7400 mg/L (Zamora et al. 2000).
Overall, using different physicochemical techniques in combination improves the removal of recalcitrant compounds and enhances the treatment efficiency. However, the use of each technique and the order of operation have to be analyzed to justify the combined treatment technology.
7.2.3.2 Combination of Physicochemical and Biological Treatment
RO and Activated Sludge (AS) combination was adopted for the treatment of YL where COD and NH3-N were almost completely removed having initial concentrations of 6440 and 1153 mg/L, respectively, which suggests that using a physicochemical and biological treatment in combination was effective in the removal of organic compounds as well as ammoniacal nitrogen (Baumgarten and Seyfried 1996).
For the treatment of SL with higher ammonia concentration, a combination of GAC and nitrification was carried out and about 93% of ammoniacal nitrogen and 55% of COD were removed whose initial concentrations were 830 and 2450 mg/L which shows that the GAC-nitrification combination is not successful in the removal of organic load from the leachate (Horan et al. 1997).
Up-flow anaerobic sludge blanket (UASB) reactors and reverse osmosis were used in combination for the treatment of SL from a landfill in the Netherlands. The pretreatment of leachate was done using UASB reactor and the effluent has been discharged to surface water since all of the recalcitrant compounds contributing to COD and ammoniacal nitrogen have been completely removed (Kurniawan et al. 2006).
With a combination of GAC adsorption and aerobic treatment, a comparative study was carried out which showed that about 65% of COD and 97% of NH3-N were removed whose initial concentrations were 1980 and 130 mg/L, respectively. The treatment process was capable of meeting the discharge standards and thus released into surface water (Schwarzenbeck et al. 2004).
A dual-step process for the treatment of YL from Turkey was carried out using UASB and precipitation using struvite with the stoichiometric ratio of Mg: NH4: PO4 = 1:1:1 (Altinbaş et al. 2002). Around 83% of COD and 85% of ammoniacal nitrogen were removed at pH 9.2 whose initial concentrations were 8900 mg/L and 2240 mg/L, respectively, with a total treatment cost of US$ 0.9/m3.
A three-step process constituting aerobic pretreatment, adsorption using GAC, and coagulation was explored for SL treatment from Germany and about 92% of COD was removed whose initial concentration was 1400 mg/L. Due to chemical consumption, the treatment cost was higher (US$ 2.3/m3) than other processes.
Due to the synergistic effect of two individual processes, combined treatment technology was found to be more effective and efficient than individual process and also helps in overcoming their limitations. Thus, combined treatment is undeniably effective in improving the quality of the wastewater with lower operational cost and minimal residue generation.
7.2.4 Miscellaneous Treatment Technologies
7.2.4.1 Ion Exchange
It is a reversible exchange between solid and liquid phases where there is no permanent change in the solid’s structure. This technique is successful in efficiently eliminating the traces of metal contaminants to meet more stringent discharge standards in certain countries. However, the leachate has to be subjected to biological treatment before ion exchange. Though the treatment of LL using ion exchange has not been extensively studied, it has got a fair interest in Germany for the removal of humic -ontaining nonbiodegradable compounds from leachate (Fettig 1999).
The results of the study on the treatment of SL using ion exchange resins like Amberlite XAD-8, XAD-4, and Amberlite IR-120 and/or granulated activated carbon adsorption was evaluated and it was shown that among all the adsorbents GAC was able to remove 93% COD followed by 53% and 46% removal of Amberlite XAD-8 and XAD-4, respectively (Kurniawan et al. 2006). Amberlite IR-120 showed the least removal (31%) at the initial COD concentration of 5108 mg/L for all adsorbents. There is a competition with the heavy metals in leachate for the binding site and the effect of this competition made GAC more effective in COD removal than other synthetic resins.
A study comparing the effect of ion exchange and ozonation on the removal of ammonium from LL was carried out (Lin and Wu 1996). Ozonation can convert nitrite into nitrate, but it is not very effective in converting ammonia into nitrate. On the other hand, ion exchange is capable of reducing the concentrations of both nitrate and ammonium ions to the preferred level and it was stated that at the pH range of 7–9, 500 bed volume (BV) of ammonia was removed whose initial concentration was 20 mg/L using ion exchange alone. In the case of ozonation, only 250 BV of ammonia was removed at the expense of 0.29 mg of NH4+/mg of ozone at the same pH range (Lin and Wu 1996).
Other than the removal of ammonia and organic compounds, adsorption using kaolinite was also been used for the removal of heavy metals like Ni (II) and Cd (II) from leachate (Majone et al. 1998). Ninety-nine percent of Ni (II) and 90% of Cd (II) were removed whose initial concentrations were 0.94 mg/L and 0.002 mg/L, respectively, and it was found that the two metals were removed when came in contact with kaolinite. The ion exchange technique is effective in the removal of heavy metals based on the type of the prevailing contaminant and the resin that has been employed for ion exchange. Ion exchange can achieve excellent metal removal from effluent after a proper aerobic pretreatment. However, the main limitation is the requirement of suitable pretreatment processes like the removal of suspended solids present in the leachate prior to ion exchange.
7.2.4.2 Electrochemical Treatment
Treatment using an electrochemical technique like electrodialysis has been employed in environmental protection. In Brazil, electro degradation by the application of a flow electrochemical reactor of SL was investigated and the highest COD and NH3-N removal of 73% and 49%, respectively, were obtained using a flow rate of 2000 L/h for 180 min and a current density of 1160 A/m2. Thus, electro-degradation was found to be an alternate for the treatment of LL. However, this technology has not been widely explored for the treatment of leachate because of its high cost and energy consumption (Moraes and Bertazzoli 2005).
7.3 Conclusion
Municipal landfill leachate poses a substantial risk to the environment due to the occurrence of toxic substances such as refractory organic compounds, ammonia nitrogen, and heavy metals. To meet stringent discharge standards for release into water bodies, various individual and/or combined sustainable technologies have been recommended and evaluated for their efficiency in leachate treatment. Depending on various factors like location of the landfill, composition of the leachate, and the concentration of various components (COD, BOD, and NH3-N), different physicochemical, biological, and combined treatment technologies have been adopted. This comprehensive chapter summarizes the recent advancement in understanding the role of treatment technologies in leachate treatment. It was concluded that though various physicochemical and biological technologies have been used for the removal of heavy metals, refractory organic and inorganic compounds from landfill leachate, a combination of technologies has been found to be more effective than individual techniques. Combining two or more physicochemical processes or physicochemical and biological treatments is necessary for more effective removal of toxic contaminants from leachate. Typically, a pretreatment of leachate by physicochemical processes best complements the biological treatment. Of all the physicochemical and biological processes discussed, chemical precipitation, membrane filtration, adsorption, and up-flow anaerobic sludge blanket (UASB) reactor are most commonly used and applied worldwide for the removal of refractory organic compounds present in the landfill leachate. Over 95% of COD removal is achieved through both nanofiltration and activated carbon with an initial concentration ranging from 5000–17,000 mg/L and about 98% of NH3-N with an initial concentration varying from 3300–5618 mg/L was removed through chemical precipitation using struvite. A combined physicochemical and biological treatment is required for the effectual removal of both COD and NH3-N from landfill leachate.
References
Abbas AA, Jingsong G, Ping LZ, Ya PY, Al-Rekabi WS (2009) Review on landfill leachate treatments. Am J Appl Sci 6(4):672–684. https://doi.org/10.3844/ajas.2009.672.684
Abdul Aziz H, Adlan MN, Zahari MSM, Alias S (2004) Removal of ammoniacal nitrogen (N-NH3) from municipal solid waste leachate by using activated carbon and limestone. Waste Manag Res 22:371–375. https://doi.org/10.1177/0734242X04047661
Aǧdaǧ ON, Sponza DT (2005) Anaerobic/aerobic treatment of municipal landfill leachate in sequential two-stage up-flow anaerobic sludge blanket reactor (UASB)/completely stirred tank reactor (CSTR) systems. Process Biochem 40:895–902. https://doi.org/10.1016/j.procbio.2004.02.021
Ahmed FN, Lan CQ (2012) Treatment of landfill leachate using membrane bioreactors: a review. Desalination 287:41–54. https://doi.org/10.1016/j.desal.2011.12.012
Ahn WY, Kang MS, Yim SK, Choi KH (2002) Advanced landfill leachate treatment using an integrated membrane process. Desalination 149:109–114. https://doi.org/10.1016/S0011-164(02)00740-3
Akinbile CO, Yusoff MS, Ahmad Zuki AZ (2012) Landfill leachate treatment using sub-surface flow constructed wetland by Cyperus haspan. Waste Manag 32:1387–1393. https://doi.org/10.1016/j.wasman.2012.03.002
Altinbaş M, Yangin C, Ozturk I (2002) Struvite precipitation from anaerobically treated municipal and landfill wastewaters. Water Sci Technol 46:271–278. https://doi.org/10.2166/wst.2002.0257
Aluko OO, Sridhar MKC (2013) Evaluation of leachate treatment by trickling filter and sequencing batch reactor processes in Ibadan, Nigeria. Waste Manag Res 31:700–705. https://doi.org/10.1177/0734242X13485867
Alvarez-Vazquez H, Jefferson B, Judd SJ (2004) Membrane bioreactors vs conventional biological treatment of landfill leachate: a brief review. J Chem Technol Biotechnol 79:1043–1049. https://doi.org/10.1002/jctb.1072
Amokrane A, Comel C, Veron J (1997) Landfill leachates pretreatment by coagulation-flocculation. Water Res 31:2775–2782. https://doi.org/10.1016/S0043-1354(97)00147-4
Aronsson P, Dahlin T, Dimitriou I (2010) Treatment of landfill leachate by irrigation of willow coppice - plant response and treatment efficiency. Environ Pollut 158:795–804. https://doi.org/10.1016/j.envpol.2009.10.003
Aziz SQ, Aziz HA, Yusoff MS, Bashir MJK (2011) Landfill leachate treatment using powdered activated carbon augmented sequencing batch reactor (SBR) process: optimization by response surface methodology. J Hazard Mater 189:404–413. https://doi.org/10.1016/j.jhazmat.2011.02.052
Babel S, Kurniawan TA (2003) A research study on Cr(VI) removal from contaminated wastewater using natural zeolite. J Ion Exch 14:289–292. https://doi.org/10.5182/jaie.14.supplement_289
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54:951–967. https://doi.org/10.1016/j.chemosphere.2003.10.001
Bae JH, Kim SK, Chang HS (1997) Treatment of landfill leachates: ammonia removal via nitrification and denitrification and further COD reduction via Fenton’s treatment followed by activated sludge. Water Sci Technol 36:341–348. https://doi.org/10.1016/S0273-1223(97)00736-1
Bai R, Sutanto M (2002) The practice and challenges of solid waste management in Singapore. Waste Manag 22:557–567. https://doi.org/10.1016/S0956-053X(02)00014-4
Bardi A, Yuan Q, Siracusa G, Chicca I, Islam M, Spennati F, Tigini V, Di Gregorio S, Levin DB, Petroni G, Munz G (2017) Effect of cellulose as co-substrate on old landfill leachate treatment using white-rot fungi. Bioresour Technol 241:1067–1076. https://doi.org/10.1016/j.biortech.2017.06.046
Baumgarten G, Seyfried CF (1996) Experiences and new developments in biological pretreatment and physical post-treatment of landfill leachate. Water Sci Technol 34:445–453. https://doi.org/10.1016/S0273-1223(96)00777-9
Begum S, Anupoju GR, Sridhar S, Bhargava SK, Jegatheesan V, Eshtiaghi N (2018) Evaluation of single and two stage anaerobic digestion of landfill leachate: effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour Technol 251:364–373. https://doi.org/10.1016/j.biortech.2017.12.069
Bello MM, Abdul Raman AA, Purushothaman M (2017) Applications of fluidized bed reactors in wastewater treatment–a review of the major design and operational parameters. J Clean Prod 141:1492–1514. https://doi.org/10.1016/j.jclepro.2016.09.148
Bohdziewicz J, Kwarciak A (2008) The application of hybrid system UASB reactor-RO in landfill leachate treatment. Desalination 222:128–134. https://doi.org/10.1016/j.desal.2007.01.137
Bonmatí A, Flotats X (2003) Air stripping of ammonia from pig slurry: characterisation and feasibility as a pre-or post-treatment to mesophilic anaerobic digestion. Waste Manag 23:261–272. https://doi.org/10.1016/S0956-053X(02)00144-7
Calli B, Mertoglu B, Inanc B (2005) Landfill leachate management in Istanbul: applications and alternatives. Chemosphere 59:819–829. https://doi.org/10.1016/j.chemosphere.2004.10.064
Camaño Silvestrini NE, Maine MA, Hadad HR, Nocetti E, Campagnoli MA (2019) Effect of feeding strategy on the performance of a pilot scale vertical flow wetland for the treatment of landfill leachate. Sci Total Environ 648:542–549. https://doi.org/10.1016/j.scitotenv.2018.08.132
Canziani R, Emondi V, Garavaglia M, Malpei F, Pasinetti E, Buttiglieri G (2006) Effect of oxygen concentration on biological nitrification and microbial kinetics in a cross-flow membrane bioreactor (MBR) and moving-bed biofilm reactor (MBBR) treating old landfill leachate. J Membr Sci 286:202–212. https://doi.org/10.1016/j.memsci.2006.09.044
Castillo E, Vergara M, Moreno Y (2007) Landfill leachate treatment using a rotating biological contactor and an upward-flow anaerobic sludge bed reactor. Waste Manag 27:720–726. https://doi.org/10.1016/j.wasman.2006.08.003
Cecen F, Gursoy G (2000) Characterization of landfill leachates and studies on heavy metal removal. J Environ Monit 2:436–442. https://doi.org/10.1039/b004729p
Cema G, Wiszniowski J, Zabczyński S, Zabłocka-Godlewska E, Raszka A, Surmacz-Górska J (2007) Biological nitrogen removal from landfill leachate by deammonification assisted by heterotrophic denitrification in a rotating biological contactor (RBC). Water Sci Technol 55:35–42. https://doi.org/10.2166/wst.2007.239
Chen S, Sun D, Chung JS (2008) Simultaneous removal of COD and ammonium from landfill leachate using an anaerobic-aerobic moving-bed biofilm reactor system. Waste Manag 28:339–346. https://doi.org/10.1016/j.wasman.2007.01.004
Chen X, Geng Y, Fujita T (2010) An overview of municipal solid waste management in China. Waste Manag 30:716–724. https://doi.org/10.1016/j.wasman.2009.10.011
Cheng RC, Liang S, Wang HC, Beuhler MD (1994) Enhanced coagulation for arsenic removal. J Am Water Works Assoc 86:79–90. https://doi.org/10.1002/j.1551-8833.1994.tb06248.x
Cheung KC, Chu LM, Wong MH (1997) Ammonia stripping as a pretreatment for landfill leachate. Water Air Soil Pollut 94:209–220. https://doi.org/10.1023/A:1026413031434
Choo KH, Lee CH (1996) Membrane fouling mechanisms in the membrane-coupled anaerobic bioreactor. Water Res 30:1771–1780. https://doi.org/10.1016/0043-1354(96)00053-X
Chys M, Oloibiri VA, Audenaert WTM et al (2015) Ozonation of biologically treated landfill leachate: efficiency and insights in organic conversions. Chem Eng J 277:104–111. https://doi.org/10.1016/j.cej.2015.04.099
Cortez S, Teixeira P, Oliveira R, Mota M (2008) Rotating biological contactors: a review on main factors affecting performance. Rev Environ Sci Biotechnol 7:155–172. https://doi.org/10.1007/s11157-008-9127-x
Cortez S, Teixeira P, Oliveira R, Mota M (2011) Mature landfill leachate treatment by denitrification and ozonation. Process Biochem 46:148–153. https://doi.org/10.1016/j.procbio.2010.07.033
Dan DA, Fujii D, Soda S, Machimura T, Ike M (2017) Removal of phenol, bisphenol a, and 4-tert-butylphenol from synthetic landfill leachate by vertical flow constructed wetlands. Sci Total Environ 578:566–576. https://doi.org/10.1016/j.scitotenv.2016.10.232
Di Iaconi C, Ramadori R, Lopez A (2006) Combined biological and chemical degradation for treating a mature municipal landfill leachate. Biochem Eng J 31:118–124. https://doi.org/10.1016/j.bej.2006.06.002
Diamadopoulos E (1994) Characterization and treatment of recirculation-stabilized leachate. Water Res 28:2439–2445. https://doi.org/10.1016/0043-1354(94)90062-0
Eldyasti A, Chowdhury N, Nakhla G, Zhu J (2010) Biological nutrient removal from leachate using a pilot liquid-solid circulating fluidized bed bioreactor (LSCFB). J Hazard Mater 181:289–297. https://doi.org/10.1016/j.jhazmat.2010.05.010
Feki F, Aloui F, Feki M, Sayadi S (2009) Electrochemical oxidation post-treatment of landfill leachates treated with membrane bioreactor. Chemosphere 75:256–260. https://doi.org/10.1016/j.chemosphere.2008.12.013
Ferraz FM, Povinelli J, Pozzi E, Vieira EM, Trofino JC (2014) Co-treatment of landfill leachate and domestic wastewater using a submerged aerobic biofilter. J Environ Manag 141:9–15. https://doi.org/10.1016/j.jenvman.2014.03.022
Fettig J (1999) Removal of humic substances by adsorption/ion exchange. Water Sci Technol 40:173–182. https://doi.org/10.1016/S0273-1223(99)00654-X
Frascari D, Bronzini F, Giordano G, Tedioli G, Nocentini M (2004) Long-term characterization, lagoon treatment and migration potential of landfill leachate: a case study in an active Italian landfill. Chemosphere 54:335–343. https://doi.org/10.1016/j.chemosphere.2003.08.013
Gao J, Oloibiri V, Chys M et al (2014) The present status of landfill leachate treatment and its development trend from a technological point of view. Rev Environ Sci Biotechnol 14:93–122. https://doi.org/10.1007/s11157-014-9349-z
Gao JL, Oloibiri V, Chys M, De Wandel S, Decostere B, Audenaert W, He YL, Van Hulle SWH (2015) Integration of autotrophic nitrogen removal, ozonation and activated carbon filtration for treatment of landfill leachate. Chem Eng J 275:281–287. https://doi.org/10.1016/j.cej.2015.04.012
Ghosh P, Thakur IS (2017) Treatment of landfill leachate using fungi: an efficient and cost-effective strategy. In: Satyanarayana T, Deshmukh S, Johri B (eds) Developments in fungal biology and applied mycology. Springer, Singapore, pp 341–357. https://doi.org/10.1007/978-981-10-4768-8_18
Gomes AI, Santos SGS, Silva TFCV et al (2019) Treatment train for mature landfill leachates: optimization studies. Sci Total Environ 673:470–479. https://doi.org/10.1016/j.scitotenv.2019.04.027
Hassan M, Wang X, Wang F et al (2017) Coupling ARB-based biological and photochemical (UV/TiO2 and UV/S2O82−) techniques to deal with sanitary landfill leachate. Waste Manag 63:292–298. https://doi.org/10.1016/j.wasman.2016.09.003
Heavey M (2003) Low-cost treatment of landfill leachate using peat. Waste Manag 23:447–454. https://doi.org/10.1016/S0956-053X(03)00064-3
Hoilijoki TH, Kettunen RH, Rintala JA (2000) Nitrification of anaerobically pretreated municipal landfill leachate at low temperature. Water Res 34:1435–1446. https://doi.org/10.1016/S0043-1354(99)00278-X
Horan NJ, Gohar H, Hill B (1997) Application of a granular activated carbon-biological fluidised bed for the treatment of landfill leachates containing high concentrations of ammonia. Water Sci Technol 36:369–375. https://doi.org/10.1016/S0273-1223(97)00410-1
Imai A, Onuma K, Inamori Y, Sudo R (1998) Effects of pre-ozonation in refractory leachate treatment by the biological activated carbon fluidized bed process. Environ Technol 19:213–221. https://doi.org/10.1080/09593331908616673
Jenkins BM, Mannapperuma JD, Bakker RR (2003) Biomass leachate treatment by reverse osmosis. Fuel Process Technol 81:223–246. https://doi.org/10.1016/S0378-3820(03)00010-9
Jokela JPY, Kettunen RH, Sormunen KM, Rintala JA (2002) Biological nitrogen removal from municipal landfill leachate: low-cost nitrification in biofilters and laboratory scale in-situ denitrification. Water Res 36:4079–4087. https://doi.org/10.1016/S0043-1354(02)00129-X
Justin MZ, Pajk N, Zupanc V, Zupančič M (2010) Phytoremediation of landfill leachate and compost wastewater by irrigation of Populus and Salix: biomass and growth response. Waste Manag 30:1032–1042. https://doi.org/10.1016/j.wasman.2010.02.013
Kalčíková G, Babič J, Pavko A, Gotvajn AŽ (2014) Fungal and enzymatic treatment of mature municipal landfill leachate. Waste Manag 34:798–803. https://doi.org/10.1016/j.wasman.2013.12.017
Kargi F, Pamukoglu MY (2004) Adsorbent supplemented biological treatment of pre-treated landfill leachate by fed-batch operation. Bioresour Technol 94:285–291. https://doi.org/10.1016/j.biortech.2004.01.003
Kennedy KJ, Lentz EM (2000) Treatment of landfill leachate using sequencing batch and continuous flow upflow anaerobic sludge blanket (UASB) reactors. Water Res 34:3640–3656. https://doi.org/10.1016/S0043-1354(00)00114-7
Kheradmand S, Karimi-Jashni A, Sartaj M (2010) Treatment of municipal landfill leachate using a combined anaerobic digester and activated sludge system. Waste Manag 30:1025–1031. https://doi.org/10.1016/j.wasman.2010.01.021
Kim KR, Owens G (2010) Potential for enhanced phytoremediation of landfills using biosolids–a review. J Environ Manag 91:791–797. https://doi.org/10.1016/j.jenvman.2009.10.017
Kivaisi AK (2001) The potential for constructed wetlands for wastewater treatment and reuse in developing countries: a review. Ecol Eng 16:545–560. https://doi.org/10.1016/S0925-8574(00)00113-0
Kjeldsen P, Barlaz MA, Rooker AP et al (2002) Critical reviews in environmental science and technology present and long-term composition of MSW landfill leachate: a review present and long-term composition of MSW landfill. Crit Rev Environ Sci Technol 32:37–41. https://doi.org/10.1080/10643380290813462
Kulikowska D, Klimiuk E (2008) The effect of landfill age on municipal leachate composition. Bioresour Technol 99:5981–5985. https://doi.org/10.1016/j.biortech.2007.10.015
Kurniawan TA, Babel S (2015) A research study on Cr(VI) removal from contaminated wastewater using low-cost adsorbents and commercial activated carbon. In: Proceedings of the 2nd international conference on energy technology towards a clean environment (RCETE), vol. 2
Kurniawan TA, Lo WH (2009) Removal of refractory compounds from stabilized landfill leachate using an integrated H2O2 oxidation and granular activated carbon (GAC) adsorption treatment. Water Res 43:4079–4091. https://doi.org/10.1016/j.watres.2009.06.060
Kurniawan TA, Lo WH, Chan GYS (2006) Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J Hazard Mater 129:80–100. https://doi.org/10.1016/j.jhazmat.2005.08.010
Kurniawan TA, Lo W, Chan G, Sillanpää MET (2010) Biological processes for treatment of landfill leachate. J Environ Monit 12:2032–2047. https://doi.org/10.1039/c0em00076k
Lavagnolo MC, Malagoli M, Garbo F, Pivato A, Cossu R (2016) Lab-scale phytotreatment of old landfill leachate using different energy crops. Waste Manag 55:265–275. https://doi.org/10.1016/j.wasman.2016.06.016
Li XZ, Zhao QL (1999) Inhibition of microbial activity of activated sludge by ammonia in leachate. Environ Int 25:961–968. https://doi.org/10.1016/S0160-4120(99)00068-9
Li XZ, Zhao QL (2001) Efficiency of biological treatment affected by high strength of ammonium-nitrogen in leachate and chemical precipitation of ammonium-nitrogen as pretreatment. Chemosphere 44:37–43. https://doi.org/10.1016/S0045-6535(00)00382-9
Li XZ, Zhao QL, Hao XD (1999) Ammonium removal from landfill leachate by chemical precipitation. Waste Manag 19:409–415. https://doi.org/10.1016/S0956-053X(99)00148-8
Li HS, Zhou SQ, Sun YB, Feng P (2009) Advanced treatment of landfill leachate by a new combination process in a full-scale plant. J Hazard Mater 172:408–415. https://doi.org/10.1016/j.jhazmat.2009.07.034
Liang Z, Liu J (2008) Landfill leachate treatment with a novel process: anaerobic ammonium oxidation (Anammox) combined with soil infiltration system. J Hazard Mater 151:202–212. https://doi.org/10.1016/j.jhazmat.2007.05.068
Lin SH, Wu CL (1996) Removal of nitrogenous compounds from aqueous solution by ozonation and ion exchange. Water Res 30:1851–1857. https://doi.org/10.1016/0043-1354(95)00329-0
Lin CY, Chang FY, Chang CH (2000) Co-digestion of leachate with septage using a UASB reactor. Bioresour Technol 73:175–178. https://doi.org/10.1016/S0960-8524(99)00166-2
Linde K, Jönsson AS (1995) Nanofiltration of salt solutions and landfill leachate. Desalination 103:223–232. https://doi.org/10.1016/0011-9164(95)00075-5
Liu Y, Wang ZW, Tay JH (2005) A unified theory for upscaling aerobic granular sludge sequencing batch reactors. Biotechnol Adv 23:335–344. https://doi.org/10.1016/j.biotechadv.2005.04.001
Loukidou MX, Zouboulis AI (2001) Comparison of two biological treatment processes using attached-growth biomass for sanitary landfill leachate treatment. Environ Pollut 111:273–281. https://doi.org/10.1016/S0269-7491(00)00069-5
Luo HW, Chen JJ, Sheng GP, Su JH, Wei SQ, Yu HQ (2014) Experimental and theoretical approaches for the surface interaction between copper and activated sludge microorganisms at molecular scale. Sci Rep 4:1–7. https://doi.org/10.1038/srep07078
Luo H, Wu Y, Zhao A et al (2017) Hydrothermally synthesized porous materials from municipal solid waste incineration bottom ash and their interfacial interactions with chloroaromatic compounds. J Clean Prod 162:411–419. https://doi.org/10.1016/j.jclepro.2017.06.082
Luo H, Zeng Y, Cheng Y, He D, Pan X (2019) Recent advances in municipal landfill leachate: a review focusing on its characteristics, treatment, and toxicity assessment. Sci Total Environ 703:135468. https://doi.org/10.1016/j.scitotenv.2019.135468
Luo H, Cheng Y, He D, Yang EH (2019a) Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci Total Environ 668:90–103. https://doi.org/10.1016/j.scitotenv.2019.03.004
Luo H, He D, Zhu W et al (2019b) Humic acid-induced formation of tobermorite upon hydrothermal treatment with municipal solid waste incineration bottom ash and its application for efficient removal of Cu (II) ions. Waste Manag 84:83–90. https://doi.org/10.1016/j.wasman.2018.11.037
Majone M, Papini MP, Rolle E (1998) Influence of metal speciation in landfill leachates on kaolinite sorption. Water Res 32:882–890. https://doi.org/10.1016/S0043-1354(97)00288-1
Marttinen SK, Kettunen RH, Sormunen KM, Soimasuo RM, Rintala JA (2002) Screening of physical-chemical methods for removal of organic material, nitrogen and toxicity from low strength landfill leachates. Chemosphere 46:851–858. https://doi.org/10.1016/S0045-6535(01)00150-3
Matthews R, Winson M, Scullion J (2009) Treating landfill leachate using passive aeration trickling filters; effects of leachate characteristics and temperature on rates and process dynamics. Sci Total Environ 407:2557–2564. https://doi.org/10.1016/j.scitotenv.2009.01.034
Mehmood MK, Adetutu E, Nedwell DB, Ball AS (2009) In situ microbial treatment of landfill leachate using aerated lagoons. Bioresour Technol 100:2741–2744. https://doi.org/10.1016/j.biortech.2008.11.031
Meier J, Melin T, Eilers LH (2002) Nanofiltration and adsorption on powdered adsorbent as process combination for the treatment of severely contaminated waste water. Desalination 146:361–366. https://doi.org/10.1016/S0011-9164(02)00513-1
Miao L, Wang S, Li B, Cao T, Xue T, Peng Y (2015) Advanced nitrogen removal via nitrite using stored polymers in a modified sequencing batch reactor treating landfill leachate. Bioresour Technol 192:354–360. https://doi.org/10.1016/j.biortech.2015.05.013
Miao L, Wang S, Cao T, Peng Y, Zhang M, Liu Z (2016) Advanced nitrogen removal from landfill leachate via Anammox system based on sequencing biofilm batch reactor (SBBR): effective protection of biofilm. Bioresour Technol 220:8–16. https://doi.org/10.1016/j.biortech.2016.06.131
Miao L, Yang G, Tao T, Peng Y (2019) Recent advances in nitrogen removal from landfill leachate using biological treatments–a review. J Environ Manag 235:178–185. https://doi.org/10.1016/j.jenvman.2019.01.057
Mohd-Salleh SNA, Shaylinda MZN, Othman N, Azizan MO, Yashni G, Afnizan WMW (2020) Sustainability analysis on landfilling and evaluation of characteristics in landfill leachate: a case study. IOP Conf Ser Mater Sci Eng 736:072002. https://doi.org/10.1088/1757-899X/736/7/072002
Monje-Ramirez I, de Velásquez MTO (2004) Removal and transformation of recalcitrant organic matter from stabilized saline landfill leachates by coagulation-ozonation coupling processes. Water Res 38:2359–2367. https://doi.org/10.1016/j.watres.2004.02.011
Moraes PB, Bertazzoli R (2005) Electrodegradation of landfill leachate in a flow electrochemical reactor. Chemosphere 58:41–46. https://doi.org/10.1016/j.chemosphere.2004.09.026
Morawe B, Ramteke DS, Vogelpohl A (1995) Activated carbon column performance studies of biologically treated landfill leachate. Chem Eng Process Process Intensif 34:299–303. https://doi.org/10.1016/0255-2701(94)04017-6
Neczaj E, Okoniewska E, Kacprzak M (2005) Treatment of landfill leachate by sequencing batch reactor. Desalination 185:357–362. https://doi.org/10.1016/j.desal.2005.04.044
Nhat PT, Biec HN, Tuyet Mai NT, Thanh BX, Dan NP (2014) Application of a partial nitritation and anammox system for the old landfill leachate treatment. Int Biodeterior Biodegrad 95:144–150. https://doi.org/10.1016/j.ibiod.2014.05.025
Nivala J, Hoos MB, Cross C, Wallace S, Parkin G (2007) Treatment of landfill leachate using an aerated, horizontal subsurface-flow constructed wetland. Sci Total Environ 380:19–27. https://doi.org/10.1016/j.scitotenv.2006.12.030
Orupõld K, Tenno T, Henrysson T (2000) Biological lagooning of phenols-containing oil shale ash heaps leachate. Water Res 34:4389–4396. https://doi.org/10.1016/S0043-1354(00)00210-4
Özgür Akta FÇ (2001) Addition of activated carbon to batch activated sludge reactors in the treatment of landfill leachate and domestic wastewater. J Chem Technol Biotechnol 76:793–802. https://doi.org/10.1002/jctb.450
Ozturk I, Altinbas M, Koyuncu I, Arikan O, Gomec-Yangin C (2003) Advanced physico-chemical treatment experiences on young municipal landfill leachates. Waste Manag 23:441–446. https://doi.org/10.1016/S0956-053X(03)00061-8
Pastore C, Barca E, Del Moro G et al (2018) Comparison of different types of landfill leachate treatments by employment of nontarget screening to identify residual refractory organics and principal component analysis. Sci Total Environ 635:984–994. https://doi.org/10.1016/j.scitotenv.2018.04.135
Patwardhan AW (2003) Rotating biological contactors: a review. Ind Eng Chem Res 42:2035–2051. https://doi.org/10.1021/ie0200104
Peters TA (1998a) Purification of landfill leachate with membrane filtration. Filtr Sep 35:33–36. https://doi.org/10.1016/S0015-1882(97)83113-8
Peters TA (1998b) Purification of landfill leachate with reverse osmosis and nanofiltration. Desalination 119:289–293. https://doi.org/10.1016/S0011-9164(98)00171-4
Pirbazari M, Ravindran V, Badriyha BN, Kim SH (1996) Hybrid membrane filtration process for leachate treatment. Water Res 30:2691–2706. https://doi.org/10.1016/S0043-1354(96)00183-2
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150:468–493. https://doi.org/10.1016/j.jhazmat.2007.09.077
Rodgers M, Zhan X (2004) Moving-medium biofilm reactors. Rev Environ Sci Biotechnol 2:213–224. https://doi.org/10.1023/B:RESB.0000040467.78748.1e
Sahinkaya E, Dursun N, Ozkaya B, Kaksonen AH (2013) Use of landfill leachate as a carbon source in a sulfidogenic fluidized-bed reactor for the treatment of synthetic acid mine drainage. Miner Eng 48:56–60. https://doi.org/10.1016/j.mineng.2012.10.019
Saleem M, Cristina M, Campanaro S, Squartini A (2018a) Dynamic membrane bioreactor (DMBR) for the treatment of land fill leachate; bioreactor’s performance and metagenomic insights into microbial community evolution. Environ Pollut 243:326–335. https://doi.org/10.1016/j.envpol.2018.08.090
Saleem M, Spagni A, Alibardi L, Bertucco A, Cristina M (2018b) Assessment of dynamic membrane filtration for biological treatment of old land fill leachate. J Environ Manag 213:27–35. https://doi.org/10.1016/j.jenvman.2018.02.057
Schwarzenbeck N, Leonhard K, Wilderer PA (2004) Treatment of landfill leachate–high tech or low tech? A case study. Water Sci Technol 48:277–284. https://doi.org/10.2166/wst.2004.0860
Shalini SS, Joseph K (2012) Nitrogen management in landfill leachate: application of SHARON, ANAMMOX and combined SHARON–ANAMMOX process. Waste Manag 32:2385–2400. https://doi.org/10.1016/j.wasman.2012.06.006
Silva TFCV, Soares PA, Manenti DR et al (2017) An innovative multistage treatment system for sanitary landfill leachate depuration: studies at pilot-scale. Sci Total Environ 576:99–117. https://doi.org/10.1016/j.scitotenv.2016.10.058
Smaoui Y, Mlaik N, Bouzid J, Sayadi S (2017) Improvement of anaerobic digestion of landfill leachate by using coagulation-flocculation, Fenton’s oxidation and air stripping pretreatments. Environ Prog Sustain 37:1–9. https://doi.org/10.1002/ep.12781
Sun H, Yang Q, Peng Y, Shi X, Wang S, Zhang S (2010) Advanced landfill leachate treatment using a two-stage UASB-SBR system at low temperature. J Environ Sci 22:481–485. https://doi.org/10.1016/S1001-0742(09)60133-9
Tanaka J, Matsumura M (2002) Kinetic studies of removal of ammonia from seawater by ozonation. J Chem Technol Biotechnol 77:649–656. https://doi.org/10.1002/jctb.624
Tatsi AA, Zouboulis AI, Matis KA, Samaras P (2003) Coagulation-flocculation pretreatment of sanitary landfill leachates. Chemosphere 53:737–744. https://doi.org/10.1016/S0045-6535(03)00513-7
Torretta V, Ferronato N, Katsoyiannis IA et al (2017) Novel and conventional technologies for landfill leachates treatment: a review. Sustainability 9(1):9. https://doi.org/10.3390/su9010009
Urase T, Salequzzaman M, Kobayashi S, Matsuo T, Yamamoto K, Suzuki N (1997) Effect of high concentration of organic and inorganic matters in landfill leachate on the treatment of heavy metals in very low concentration level. Water Sci Technol 36:349–356. https://doi.org/10.1016/S0273-1223(97)00723-3
Ushikoshi K, Kobayashi T, Uematsu K, Toji A, Kojima D, Matsumoto K (2002) Leachate treatment by the reverse osmosis system. Desalination 150:121–129. https://doi.org/10.1016/S0011-9164(02)00937-2
Wang Z, Banks CJ (2007) Treatment of a high-strength sulphate-rich alkaline leachate using an anaerobic filter. Waste Manag 27:359–366. https://doi.org/10.1016/j.wasman.2006.01.028
Wang ZP, Zhang Z, Lin YJ, Deng NS, Tao T, Zhuo K (2002) Landfill leachate treatment by a coagulation-photooxidation process. J Hazard Mater 95:153–159. https://doi.org/10.1016/S0304-3894(02)00116-4
Wang Z, Peng Y, Miao L, Cao T, Zhang F, Wang S, Han J (2016) Continuous-flow combined process of nitritation and ANAMMOX for treatment of landfill leachate. Bioresour Technol 214:515–519. https://doi.org/10.1016/j.biortech.2016.04.118
Wasay SA, Barrington S, Tokunaga S (1999) Efficiency of GAC for treatment of leachate from soil washing process. Water Air Soil Pollut 116:449–460. https://doi.org/10.1023/A:1005115820429
Wei Y, Ji M, Li R, Qin F (2012) Organic and nitrogen removal from landfill leachate in aerobic granular sludge sequencing batch reactors. Waste Manag 32:448–455. https://doi.org/10.1016/j.wasman.2011.10.008
Wiszniowski J, Robert D, Surmacz-Gorska J et al (2006) Landfill leachate treatment methods: a review. Environ Chem Lett 4:51–61. https://doi.org/10.1007/s10311-005-0016-z
Wu L, Peng Y, Shi X, Peng C, Zhang J (2015) Advanced nitrogen removal via nitrite from municipal landfill leachate using a two-stage UASB–A/O system. Soil Biol Biochem 23:1225–1230. https://doi.org/10.1016/j.cjche.2015.05.014
Wu L, Li Z, Zhao C, Liang D, Peng Y (2018) A novel partial-denitrification strategy for post-anammox to effectively remove nitrogen from land fill leachate. Sci Total Environ 633:745–751. https://doi.org/10.1016/j.scitotenv.2018.03.213
Xue Y, Zhao H, Ge L, Chen Z, Dang Y, Sun D (2015) Comparison of the performance of waste leachate treatment in submerged and recirculated membrane bioreactors. Int Biodeterior Biodegradation 102:73–80. https://doi.org/10.1016/j.ibiod.2015.01.005
Yan H, Cousins IT, Zhang C, Zhou Q (2015) Perfluoroalkyl acids in municipal landfill leachates from China: occurrence, fate during leachate treatment and potential impact on groundwater. Sci Total Environ 524–525:23–31. https://doi.org/10.1016/j.scitotenv.2015.03.111
Ye J, Mu Y, Cheng X, Sun D (2011) Treatment of fresh leachate with high-strength organics and calcium from municipal solid waste incineration plant using UASB reactor. Bioresour Technol 102:5498–5503. https://doi.org/10.1016/j.biortech.2011.01.001
Yi X, Han N, Yin T, He Y, Gin KY (2017) Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res 121:46–60. https://doi.org/10.1016/j.watres.2017.05.008
Yin T, Chen H, Reinhard M, Yi X, He Y, Yew-hoong K (2017) Perfluoroalkyl and polyfluoroalkyl substances removal in a full-scale tropical constructed wetland system treating landfill leachate. Water Res 125:418–426. https://doi.org/10.1016/j.watres.2017.08.071
Yoon J, Cho S, Cho Y, Kim S (1998) The characteristics of coagulation of Fenton reaction in the removal of landfill leachate organics. Water Sci Technol 38:209–214. https://doi.org/10.1016/S0273-1223(98)00481-8
Zamora RMR, Moreno AD, De Velásquez MTO, Ramírez IM (2000) Treatment of landfill leachates by comparing advanced oxidation and coagulation-flocculation processes coupled with activated carbon adsorption. Water Sci Technol 41:231–235. https://doi.org/10.2166/wst.2000.0033
Zavarzina AG, Leontievsky AA, Golovleva LA, Trofimov SY (2004) Biotransformation of soil humic acids by blue laccase of Panus tigrinus 8/18: an in vitro study. Soil Biol Biochem 36:359–369. https://doi.org/10.1016/j.soilbio.2003.10.010
Zolfaghari M, Jardak K, Drogui P, Kaur S (2016) Land fill leachate treatment by sequential membrane bioreactor and electro-oxidation processes. J Environ Manag 184:318–326. https://doi.org/10.1016/j.jenvman.2016.10.010
Acknowledgment
The authors are highly thankful to “The Department of Science and Technology, Ministry of Science and Technology, Government of India,” for funding the project under the Scheme “Scheme for Young Scientists and Technologists (SYST)-Science for Equity and Empowerment Division (SEED)”; Project No: SP/YO/2019/1360(G). DST-INSPIRE Fellow Maseed Uddin [DST/INSPIRE Fellowship/2019/IF190897] acknowledges the Department of Science and Technology, Ministry of Science and Technology, Government of India for providing the fellowship and financial support to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Uddin, M., Lakshmi Priya, B.S., Rajarubini, R., Venkatesan, S.K., Ganesan, S., Kandasamy, R. (2023). The Role of Wastewater Treatment Technologies in Municipal Landfill Leachate Treatment. In: Samuel Jacob, B., Ramani, K., Vinoth Kumar, V. (eds) Applied Biotechnology for Emerging Pollutants Remediation and Energy Conversion. Springer, Singapore. https://doi.org/10.1007/978-981-99-1179-0_7
Download citation
DOI: https://doi.org/10.1007/978-981-99-1179-0_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1178-3
Online ISBN: 978-981-99-1179-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)