Abstract
Municipal solid waste leachate, a kind of wastewater, can severely damage the environment and contaminate the groundwater because of its high organic matter and toxic heavy metal concentrations. Due to its complex composition, this wastewater must be properly treated prior to being discharged into the environment. In recent decades, several biological approaches (e.g., bioremediation, phytoremediation, and bioreactors) and physicochemical processes (e.g., coagulation/flocculation, air stripping, and advanced oxidation processes) have proven effective at removing the organic load and the toxicity of this effluent. Physicochemical treatments have been applied as pretreatment or post-treatment steps for biological processes, but these methods do not always provide satisfactory results and can cause secondary pollution in some cases. In addition, owing to the high concentrations of organic matter, ammonia, and trace metals in landfill leachate, combined approaches to leachate treatment have been reported to be efficient. This article highlights the advantages and drawbacks of these approaches to the treatment of leachate by providing an updated overview of the various methods that have been successfully applied in this field. Further studies should focus on improving landfill leachate treatment to maximize removal performance.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, large quantities of municipal waste have been generated, mainly due to increasing urbanization as well as industrial activities and agricultural practices. The disposal of waste without adequate treatment can cause serious pollution problems. One of the major issues associated with the continuous discharge of municipal waste is the production of effluents known as landfill leachate (LFL) (Pan et al. 2017). This wastewater, which is generated by the decomposition of organic waste and rainfall percolation through this waste material (Khanzada and Övez 2017), is a complex liquid that contains excessive concentrations of biodegradable and nonbiodegradable compounds, including dissolved organic matter, ammonium, trace metals, and microorganisms (Wang et al. 2018a, b). The characteristics of LFL depend on several factors, such as waste age and composition, landfill age and climatic conditions, and landfill design. The chemical composition of the leachate determines its relative treatability, which is evaluated as a function of landfill age and/or based on the biodegradabilityof the leachate (defined via its biological oxygen demand/chemical oxygen demand ratio, BOD5/COD) (Kamaruddin et al. 2015). Recently, numerous methods have been proposed for LFL treatment, including biological, physical, chemical, and physicochemical techniques (Kamaruddin et al. 2015; Galvão et al. 2020). Generally, biological treatments are preferred due to their reliability, simplicity, and high cost-effectiveness (Wang et al. 2018a, b). However, some compounds, such as ammonia, are difficult to biodegrade because of their toxicity to microorganisms when they are present at high concentrations (Shalini and Joseph 2012; Wang et al. 2018a, b). Several studies have confirmed that physicochemical processes represent attractive pretreatment methods for reducing effluent toxicity. Nevertheless, most landfill leachate treatments have their advantages and drawbacks (Kamaruddin et al. 2015). More recently, technology that permits effective LFL treatment through the combination of two or more physicochemical treatments, or with a combination of physicochemical and biological treatments, has been developed (Ai et al. 2017; Hu et al. 2016). Such technology seems to be a cost-effective and a promising approach that can be used to improve the effectiveness of landfill leachate treatment. For instance, different types of physicochemical and electrochemical methods, membrane filtration techniques, and advanced oxidative processes (AOPs) have been integrated with biological processes (Amor et al. 2019; Di Maria and Sisani 2017). The present review paper looks at the trends in LFL treatment, including the physicochemical, biological, and combined treatments that have been especially well described in the literature during the last decade.
Physico-chemical and biochemical characteristics of LFL
In light of the information gathered from the literature, leachates can be categorized into three types: young, intermediate, and old. Generally, young leachate (< 5 years old) is characterized by high concentrations of BOD and COD, moderately high levels of ammoniacal nitrogen, a high BOD5/COD ratio (> 0.6), and a pH of around 4 (Cesaro et al. 2015). Intermediate or medium leachate ( 5–10 years old) is characterized by the presence of substantial loads of recalcitrant COD, volatile fatty acids, and a pH of > 7 (Djelal et al. 2015). In contrast, a low BOD5/COD ratio (< 0.1) and a slightly basic pH value imply that the LFL is old or mature (> 10 years old). Furthermore, considerable amounts of humic and fulvic acids and NH3–N are produced at this stage due to anaerobic decomposition (Ai et al. 2017), indicating low biodegradability as a result of the release of high concentrations of ammonia, nitrogen, and recalcitrant macromolecular organic molecules. Moreover, some kinds of organic matter interact with heavy metals, meaning that both of these substances are major problematic factors in landfill leachate treatment.
Physicochemical processes overview
Physical treatment involves the application of a physical phenomenon to enhance leachate quality. For instance, the sedimentation process involves the settling of solids by gravitational forces, leading to a short residence time in the sedimentation tank. This method is crucial for floc formation. In aeration, another type of physical treatment, oxygen is used as the oxidation agent in a leachate lagoon to enhance the removal of BOD5 in the pretreatment, as proven by Kamaruddin et al. (2015). In contrast, chemical treatment is based on the application of chemical additives to improve the leachate quality. These processes can be categorized into destructive and nondestructive processes based on the separation or degradation of organic pollutants. Nondestructive processes include coagulation–flocculation, adsorption, and membrane processes that transfer pollutants from a liquid to a solid phase as sludge (Taoufik et al. 2018). Destructive processes are commonly known as advanced oxidation processes (AOPs) due to the production of hydroxyl radicals (•OH), which are nonselective and highly reactive; they attack most organic molecules. Generally, the physicochemical process is carried out as a pretreatment or at the final stage of the leachate treatment process. Moreover, physicochemical processes can be applied along with biological treatments to improve treatment performance when the biological process is inhibited by the presence of biorefractory compounds in wastewater (Ai et al. 2017; Wang et al. 2018a, b).
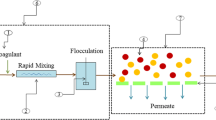
Coagulation–flocculation
Among the various physicochemical technologies, coagulation–flocculation is a relatively simple and controllable technique that is widely used in the pretreatment of old and stabilized landfill leachates prior to either a biological or another physicochemical process. Several reports on the use of coagulation–flocculation for LFL treatment are available (Chaouki et al. 2017). The coagulation performance mainly depends on the coagulant and/or flocculent rate and type and the experimental conditions (pH, time, and temperature). This process has been successfully employed for the removal of nonbiodegradable organic compounds, suspended solids, colloidal particles, turbidity, color, and heavy metals with a high efficiency that depends on the contaminant and coagulant/flocculant type (Mohd-Salleh et al. 2019). Colloidal particles can be destabilized by the addition of a coagulant. Coagulation is usually followed by a flocculation treatment to increase the particle size and transfer unstable particles into bulky floccules so that they can settle more easily. Nevertheless, the coagulation process has mainly been investigated as a final polishing treatment stage for stabilized or biologically pretreated landfill leachate (Abood et al. 2014). During coagulation–flocculation, trace metal salts such as aluminum sulfate, ferrous sulfate, ferric chloride, and ferric chlorosulfate are added to generate high-valence cations within the solution, thereby reducing the zeta potential (Mohd-Salleh et al. 2019). In general, salts of the ferric ion are superior to those of aluminum, mainly because ferric ion salts are insoluble across a wider pH range (Ghafari et al. 2009). A coagulation process using FeCl3 was applied by Taoufik et al. (2018) as a pretreatment process for LFL with the aim of removing COD and turbidity. Response surface methodology (RSM) with Box–Behnken design indicated that the addition of 7.2 g L−1 of FeCl3 and 0.2 mL L−1 of flocculant increased the COD removal rate by 45%. Additionally, Chaouki et al. (2017) compared different coagulants [lime (Ca(OH)2, ferric chloride (FeCl3), and alum Al2(SO4)3] in terms of their effectiveness at decolorizing LFL from the city of Casablanca (Morocco). The process efficiency was measured in terms of chemical oxygen demand (COD), trace metals, color, and turbidity. Lime treatment led to significant decreases in COD, discoloration, and turbidity (66.25%, 98%, and 80%, respectively). FeCl3 treatment led to reductions in COD (62.5%), color (82%), and turbidity (92.5%). On the other hand, the application of Al2(SO4)3 removed only about 11% of the COD and 6% of the turbidity. Regarding the analysis of trace metals, treatment with lime induced a notable reduction in Cr (86%). FeCl3 treatment showed high affinities for Cd, Cu, Ni, Cr, and Zn. Alum was found to be very effective for removing Ni, Pb, and Cr (95%, 94%, and 93% of those metals, respectively). Recently, Mohd-Salleh et al. (2018) studied the effects of various operating conditions (such as coagulant dosage and pH) on the efficiency of the treatment of LFL with polyaluminum chloride (PAC). They found that satisfactory removal of suspended solids, COD, Fe, and Cr (reductions of 95%, 53%, 97%, and 79%, respectively) was achieved at pH 7 with PAC at 3750 mg L−1.
Recently, Djeffal et al. (2021) reported the effectiveness of three coagulants (ferric chloride, aluminum sulfate, and ordinary alum) and two types of agitation (mechanical and ultrasound) as coagulation treatments for landfill leachate. A significant reduction in turbidity (99.4%) was obtained with a coagulant dosage of 15%, 250 rpm as the stirring speed, and a reaction time of about 15 min for ferric chloride. Furthermore, bacteriological analyses proved that fecal coliforms, total germs, and streptococci were absent. Additionally, the LFL was improved by using ultrasound waves with a frequency of 37 kHz and a power of 30 W. In the experiments, maximum values of 0.19 NTU and 100 mg O2/L were achieved for simultaneous turbidity and BOD5 removal, respectively, using ferric chloride. These outcomes were in agreement with the finding of Chaouki et al. (2021), who used ferric chloride for the removal of pollutants from landfill leachate. The experimental results demonstrated that the coagulation process reduced the color by 80%, the turbidity by 90%, the COD by 50%, and BOD5 by 99% when 12 g Fe3+/L was applied as the optimum dose. However, most studies have mainly focused on the application of aluminum sulfate, ferrous sulfate, ferric chloride, and polyaluminum chloride as coagulants during coagulation–flocculation processes (Yao et al. 2017). Nevertheless, the application of these chemicals has several drawbacks, such as harmful effects on human health, the production of large sludge volumes, and relatively high costs (Patale and Pandya 2012). In the same vein, composites or hybrid materials represent another type of coagulant that has shown good performance in wastewater treatment. Al-Sahari et al. (2020) discussed the preparation and application of hybrid materials in a coagulation–flocculation process. They proved that hybrid materials could be combinations of different structures, such as inorganic–organic, organic–organic, inorganic–inorganic, inorganic–biopolymer, and organic–natural polymer structures. The use of such hybrid materials can reduce treatment times. The wastewater treatment can be carried out by adding one chemical product to one tank instead of the two separate tanks used in the conventional coagulation flocculation process. PAC–FeCl3 and PAC–chitosan are among the main hybrid materials applied as coagulants. Therefore, there has been increasing interest in the use of natural coagulants as natural coagulant aids. The experiments of Nithya and Abirami (2018) confirmed the positive coagulation properties of chitosan and pine bark in terms of removing trace metals and reducing the turbidity of LFL. They also reported that the addition of natural coagulants was most efficient under acidic and neutral conditions. At the optimum pH, 6, the highest turbidity removal rate (91.3%) was recorded with a chitosan dosage of 0.6 g mL−1. At pH 7, the best turbidity removal rate, 85.2%, was achieved with a pine bark dosage of 4 g mL−1. Furthermore, outcomes proved the efficiency of natural coagulants in the removal of toxic trace metals such as As, Cu, Cr, Pb, Hg, and Ni. In the same vein, Rasool et al. (2016) demonstrated the performance of Ocimum basilicum L. as a bio-based coagulant for the pretreatment of LFL. According to Zainol et al. (2018), 8000 mg of red earth effectively removed 90% of the turbidity, 46.7% of the NH3–N, and 53.9% of the COD when the pH was < 2. This highlights the advantage of red earth as a natural coagulant for the remediation of LFL. Additionally, Cheng et al. (2020) reported the efficiency of guar gum as a green coagulant when it was used to remove organic matter from landfill leachate (22.57%) under optimum conditions—a guar gum dosage of 44.39 mg/L, pH 8.56 (natural pH of leachate), and a mixing speed of 79.27 rpm. They proved that guar gum coagulation has high overall potential as a LFL treatment. In 2021, Righetto et al. tested the efficency of tannin-based materials as natural coagulants for treating landfill leachate. The optimal dosage (11.1 mL/L) and pH (7.3) were determined using response surface methodology (RSM). About 47% of the TP, 15% of the TOC, 20% of the NH3‒N, and 4% of the TN were removed. Furthermore, significant removal of the metals Fe, Ti, Cr, Al, Ba, and V was achieved, while lower percentages of other metals were also removed. Similar results were reported by Banch et al. (2019), who proved that a tannin-based natural coagulant could successfully remove organic matter and heavy metals from stabilized LFL.
On the other hand, the application of electrocoagulation (EC) for improving the biodegradability and treatability of LFL has attracted great interest (Kallel et al. 2016). One important factor to consider in electrocoagulation treatment is the type of electrode applied in the electrochemical process. Aluminium and iron electrodes are the preferred electrodes owing to their effectiveness, abundance, and low price (Fu et al. 2021; Guo et al. 2021). Bouhezila et al. (2011) showed that the removal rates of COD, TN, color, and turbidity were 70%, 24%, 56%, and 60% with Al electrodes and 68%, 15%, 28%, and 16% with Fe electrodes, respectively. They also calculated that the electrical energy consumption and operating cost were 0.022 kWh L−1 and 0.54 USD m−3 (for treated leachate), respectively, with Al electrodes, and 0.019 kWh L−1 and 0.47 USD m−3, respectively, with Fe electrodes. Zailani et al. (2018) evaluated the performance of an aluminum electrode in removing COD, ammonia, turbidity, color, and suspended solids (SS) from Simpang Renggan landfill leachate. Based on the experiments performed, the optimum conditions were a current density of 200 A/m2 with a reaction time of 20 min at an optimum pH value of 4. The overall abatement rates of COD, ammonia, color, turbidity, and SS were 60%, 37%, 94%, 88%, and 89%, respectively. Thus, research findings indicate that EC is an efficient treatment for landfill leachate. Recently, De Oliveira et al. (2021) studied the removal of heavy metals and coliforms from LFL by EC using electrodes made from steel swarf (SfE). Abatement rates of detected heavy metals were 51% for Cr, 59% for As, 71% for Cd, 72% for Zn, 92% for Ba, 95% for Ni, and > 99% for Pb. The microbial load of coliforms was reduced to less than 1 CFU/ml after treatment with SfE (i.e., a reduction of approximately 100%). EC using SfE can be applied as an effective alternative landfill leachate treatment. Khoramipour et al. 2021 demonstrated that the hybrid process of sonoelectrocoagulation is an applicable and effective process for LFL treatment. Central composite design (CCD) and response surface methodology (RSM) were applied to optimize the important factors (pH, reaction time, and direct electrical current). A COD removal rate of 86% was attained under optimal experimental conditions (pH 5.7, reaction time: 80 min, direct current: 1.9 A). Sonoelectrocoagulation was found to modify the microscopic structure and elemental composition of the sludge.
Chemical precipitation
Chemical precipitation has been used as a pretreatment to remove nonbiodegradable organic compounds, NH3–N, and trace metals from LFL. During this process, dissolved ions in the solution are converted to the insoluble solid phase via chemical reactions. In general, the metal precipitate from the solution takes the form of the hydroxide. Considering the aim of removing either trace metals or NH3–N, lime or struvite (magnesium ammonium phosphate, MAP) is usually employed as the precipitant (Reynier et al. 2015). Jaafarzadeh Haghighifard et al. (2016) studied the removal of ammonium from mature LFL using struvite and found that 87% of the NH4 was removed at pH 8.5. More recently, Dogan et al. (2018) used struvite as a precipitant for the removal of NH4+ from LFL. Struvite precipitation was optimized through modeling based on response surface methodology and central composite design. The results revealed that the maximum ammonium removal efficiency was 99.8% at a molar rate of 1.2 for Mg/N and 1.27 for N/P at pH 9.2. Consequently, the ammonium concentration in LFL can be significantly reduced through struvite precipitation, but the high cost of the chemicals needed remains the main disadvantage of this technology.
Activated carbon
Adsorption is the most widely applied technology for removing recalcitrant organic compounds from LFL. Activated carbon (AC) is known to be one of the most efficient and practical adsorbents for removing various types of organic and inorganic contaminants such as ammonium nitrogen and trace metals (Shehzad et al. 2015). Granular activated carbon (GAC) and powdered activated carbon (PAC) are two types of activated carbon, which is commonly applied in leachate treatment because of its high area to volume ratio, large porous specific surface area, thermostability, and adsorption kinetics (Gao et al. 2015). The adsorption of organic compounds in leachate collected from Jeram Sanitary Landfill (Malaysia) onto powder AC was studied by Erabee et al. (2018). Those authors explored three methods of modifying the AC to improve its adsorption capacity: treating it with nitric acid (HNO3) or potassium permanganate (KMnO4) and heating it at 600 °C. The removal rates of TSS, NH3–N, Zn, Cu, and S2− reached 91%, 99%, 86%, 100%, and 57%, respectively, after 120 min of contact time when AC-KMnO4 was applied. Mohammad-pajooh (2018) investigated the removal using activated carbon (AC) of organic compounds from biologically pretreated leachate collected from a German landfill. Outcomes showed that 71% COD removal was achieved after 8 h of contact time with an AC concentration of 20 g L−1. Mahdavi et al. (2018) studied the effectiveness of synthesized activated carbon for organic matter and trace metal removal. Their findings revealed that an adsorbent dosage of 15 g L−1 led to the removal of 84.7% of the COD at pH 4. Research by Rohers et al. (2021) showed the performance of an activated carbon column in the treatment of LFL. Reductions of up to 74% of the COD, 47% of the BOD5, 93% of the color, and 90% of the ammonia were achieved, along with an increase from 0.3 to 0.9 in the BOD5/COD ratio. Moreover, the AC adsorption process resulted in the removal of up to 60% of the heavy metal content. The production of activated carbon (AC) from agricultural by-products is a research field that has attracted great interest because of its potential in the disposal of agroresidues and for environmental applications (Reshadi et al. 2020). In this context, Chávez et al. (2019) evaluated the potential of AC produced from coffee waste in the treatment of landfill leachate. Additionally, they reported the efficiency of the adsorption process in reducing organic matter in LFL (97%) when using 3 g/L of adsorbent. Despite the numerous advantages offered by activated carbon, this adsorption method involves high activated carbon consumption and regeneration costs, which in turn result in increased treatment costs, limiting its application (Foo and Hameed 2009; Chys et al. 2014).
Other natural materials such as zeolite and clays are effective adsorbents—comparable to AC—for landfill leachate treatment (Augusto et al. 2019). The application of natural minerals such as zeolites as adsorbents has been proposed due to their high adsorption and ion exchange capacities and their low cost (Aziz et al. 2020). Zeolites are composed of crystalline hydrated aluminosilicates; their structures include pores filled with water. Researchers have found that the transferable cations (Ca2+, K+, and Mg2+) in the physical structure of zeolite are easily replaceable. Clay minerals are a large family of adsorbents that have great potential because of their relatively low cost and environmentally friendly nature as well as their high specific surface area, chemical and mechanical stability, and ionic exchange capacity (Luo et al. 2019a, b). According to the literature, chemical agents have been applied to modify clays to enhance their capacity to adsorb pollutants. However, this may entail a risk to the environment because these chemical modifiers can eventually be released from the clay, which may then necessitate costly additional treatment (Costa et al. 2019). In 2021, Mosanefi et al. (2021) investigated the effect of natural zeolite on ammonium ion removal from LFL. The effects of different variables [pH, contact time (CT), and zeolite concentration (ZC)] in a batch system were studied to optimize ammonium removal from LFL. First, the effect of pH was studied in the pH range 5–9. Then, at the optimal pH (7), the effect of the ZC was tested in the ZC range 10–200 g/L. The results indicated that increasing the ZC from 10 to 80 g/L increased the amount of ammonium ion removed. Meanwhile, increasing the ZC from 80 to 200 g/L decreased the removal efficiency. Finally, the outcomes of the experiments indicated that the optimal conditions for ammonium ion removal from LFL (44.49%) were a pH of 7, a ZC of 80 g/L, and a CT of 30 min. This research indicated that clinoptilolite zeolite (CZ) could be used as an inexpensive and efficient adsorbent for removing ammonium ions from LFL.
Air stripping
Air stripping or ammonia stripping has gained great attention as a viable alternative for reducing the NH3 content of leachates (Degermenci and Yildiz 2012). This physical process is efficient at pH 11; at this pH, all NH3 forms turn to gas and will then react with acid solution to give stable ammonium salts, which can be used as mineral fertilizer. Yuan et al. (2016) reported that a removal efficiency of ammonia from LFL of 95% was successfully achieved at the laboratory and pilot scales at ambient temperature using a continuous-flow rotating packed bed (RPB) as an air stripper. Therefore, more intense efforts to improve the design of the ammonia stripping process are underway. Leite et al. (2018) removed around 97% of the COD after 100 days of treatment using an ammonia nitrogen stripping process in an open horizontal flow reactor. They proved that the ammonia removal performance was directly proportional to the applied superficial load, and that the carbonaceous material removal was proportional to the organic matter in the influent. Nevertheless, the main disadvantage of this process is the low efficiency of organic matter degradation and the release of NH3 into the atmosphere, as this can be an environmental pollutant. In 2021, Khoi et al. (2021) designed an air stripper for the removal of ammonia from LFL. The effects of different parameters on the ammonia stripping efficiency were tested, such as the pH, hydraulic loading rate (HLR), gas/liquid (G/L) ratio, and recirculation of liquid. The outcomes showed that increasing the pH from 9 to 12 significantly increased the ammonia removal efficiency (which was up to 99.0% after 3 h), irrespective of the change in G/L or HLR. At a HLR of 57.6 or 172.8 m3/m2 day, increasing the G/L ratio enhanced the removal efficiency, with the highest value (56%) achieved at a HLR of 172.8 m3/m2 day, pH 12, and a G/L of 728. The study proved the high performance of air stripping as a pretreatment process for ammonia removal from landfill leachate.
To meet stringent effluent discharge standards, advanced oxidation processes (AOPs), including TiO2 photocatalysis, ozonation, and Fenton’s reaction, have widely been applied in advanced treatments of landfill leachate (Betancourt-Buitrago et al. 2019), as discussed in the next section (Table 1).
Advanced oxidation processes
Destructive processes are commonly known as advanced oxidation processes (AOPs). The main mechanism for AOPs is the production of hydroxyl radicals (•OH). These radicals are able to oxidize numerous complex organics. They react with carbon–carbon double bonds efficiently and attack aromatic nuclei, which are prevalent features of refractory organic compounds (Azadi et al. 2017).
Fenton’s reaction
Among AOPs, the Fenton process is widely employed for the pretreatment, post-treatment, or full treatment of leachate because of its ability to oxidize refractory organic molecules and its moderate cost, and because it is simple to use (Jain et al. 2018). This process utilizes a mixture of ferrous ion (Fe2+) and hydrogen peroxide (H2O2). Significantly, most studies in the literature on the removal of contaminants by Fenton reaction processes have demonstrated that the parameters that most strongly influence the Fenton process are the ferrous ion and peroxide concentrations, the initial amount of the pollutant, its reaction time, and the pH and temperature. Smaoui et al. (2018) tested the use of Fenton’s oxidation for LFL treatment. Experiments involving H2O2 concentrations within the range 0–4 g/L were performed at pH 3 with 1 g/L of Fe0. The overall removal efficiencies of COD and BOD5 were 48% and 30%, respectively. The authors demonstrated that increasing the H2O2 concentration to 3 g/L H2O2 had a positive effect on COD removal, but that increasing the H2O2 concentration further did not improve the COD removal efficiency. They explained this behavior by invoking the phenomenon of oxidation saturation. Maslahati Roudi et al. (2018) proved that the maximum COD removal rate (100%) was achieved using the optimum operational conditions of 781.25 mg L−1 Fe2+, pH 3, and a reaction time of 28.03 min. Recently, Makhatova et al. (2020) tested the applicability of the Fenton process for the removal of toxic compounds from LFL. They proved that the most favorable concentrations of H2O2 and ferric ion for carbon removal were 6660 mg L−1 and 400 ppm, respectively. As a result, the removal rates of total organic carbon, total inorganic carbon, total nitrogen, and color were 88.7%, 100%, 96.5%, and 98.2%, respectively. Durai et al. (2020) proved that the Fenton process provided good performance in the removal of toxic compounds from LFL. Under the optimum conditions for the Fenton process (pH 3, 29.12 mM H2O2, 14.44 mM FeSO4), the maximum simultaneous COD and TOC removal rates (97.83% and 74.24%, respectively) were attained. In the same vein, electro-Fenton (EF) technology is widely applied as a LFL treatment. In the EF process, H2O2 is produced in situ from the reduction of oxygen gas at the cathode. For instance, in the presence of Fe2+ as a metal catalyst, hydroxyl radicals are generated by the decomposition of H2O2 (Jain et al. 2018). The EF process can be used in two phases or configurations. In the first one, inert anodes with high catalytic activity are employed, and the catalyst is added to the electrochemical cell. In the second one, the dissolution of sacrificial electrodes provides the required metal catalyst, and only the H2O2 is added to the cell. Mahtab et al. (2021) studied the efficiency of the Fenton process in the treatment of landfill leachate. This process was optimized using response surface methodology (RSM) coupled with central composite design (CCD). Several variables were optimized to study the COD removal response. The highest COD removal rate, 61%, was obtained at a pH of 3.1, a reaction time of 36 min, an Fe2+ dosage of 0.04 mol L−1, and an H2O2 dosage of 0.075 mol L−1.
Various authors have argued that, compared with other AOPs, the Fenton process presents advantages such as high performance, easy implementation, and no energy requirement for H2O2 activation (Singa et al. 2018; Maslahati Roudi et al. 2018). Major disadvantages of this method include the high amount of iron sludge produced at the end of the experiment, the risk from storing hydrogen peroxide, and the need to adjust the pH to the acidic range. However, no report has focused on the costs of this process. Cassano et al. (2011) reported the operating costs of the photo-Fenton process for the treatment of municipal landfill leachate with an initial COD of 2.8–3.6 g O2/L. They demonstrated that the operating costs were around 0.72–1.59 €/m 3 for a final COD of 160 mg O2/L.
TiO2 photocatalysis
Because of their small size, high surface area, crystal form, and high reactivity, nanomaterials have proven to be a promising alternative for the purification and the treatment of pollutants to convert them to nonhazardous materials (Nasrollahzadeh et al. 2016). In fact, over the past few decades, nanotechnology has gained widespread attention, and various nanomaterials have been developed for treating and decontaminating water based on adsorption and photocatalytic and antibacterial activity (Pant et al. 2017).
TiO2 is used mainly due to its chemical stability, its nontoxicity, its insolubility in water, its hydrophobicity, and its high efficiency in the removal of contaminants from wastewater. In an oxidation–reduction reaction, TiO2 produces highly active radicals (the hydroxyl radical and other radicals, such as OH, HO2, and HO3), resulting in the degradation of compounds adsorbed on its surface. TiO2 converts photon energy into chemical energy, which leads to activation sites on TiO2 and the degradation of compounds (Pavithra and Shanthakumar 2017). A number of researchers have applied TiO2 for LFL treatment or pretreatment to enhance the biodegradability of refractory organic compounds in landfill leachate, and for LFL post-treatment to ensure that effluent discharge standards are met (Betancourt-Buitrago et al. 2019). Mokhtarani et al. (2016) investigated TiO2 photocatalysis for LFL treatment. As a result of their experiments, maximum simultaneous COD and color removal rates of 58% and 36%, respectively, were attained in the presence of 48.8 g m−2 of immobilized TiO2 and by applying 21.5 h of radiation with a light intensity of 7.5 mW Cm−2 at pH 5.7. Indeed, Desai et al. (2020) used composite central design (CCD) based on response surface methodology (RSM) and ANN models to demonstrate the efficiency of TiO2 for the removal of COD from landfill leachate using a photoreactor in natural sunlight. The constructed photoreactor was found to be efficient at capturing solar photons from sunlight, and the maximum removal rate of COD was found to be 57.97% at pH 2 and 53.16% at pH 3 with 1 g/L of TiO2 and 150 min of irradiation. Carard et al. (2021) studied the efficiency of the TiO2 photocatalytic process for LFL treatment. To define the optimum conditions, three factors (TiO2 concentration, pH, and air flow) were analyzed using central composite rotatable design (CCRD). The significant conditions were a TiO2 concentration of 0.012 g, a pH of 3.3, and air flow of 9.0 L/min−1. As a result, the COD removal rate was around 30% and the increase in biodegradability ratio (BOD/COD) was 0.59.
However, TiO2 photocatalysis poses a serious disadvantage. Only UVA irradiation can be used for catalyst photoactivation. Only 3–5% of the solar radiation that reaches the surface of the Earth is UVA, so it would be great to extend the absorbance of TiO2 into the visible region (Ahmari et al. 2018). In the same vein, several investigations have established that doping TiO2 with metal ions is one of the most effective methods of improving the photoelectrochemical properties of TiO2 irradiated with UV and sunlight (Ahmari et al. 2018).
Zhou et al. (2017) studied the efficiency of the photocatalytic treatment of LFL using Cu/N-codoped TiO2. Under optimal operating conditions, they achieved removal rates of COD and TOC of 67% and 82.5%, respectively. Azadi et al. (2017) tested TiO2 and tungsten-doped TiO2 (W-doped TiO2) NPs for the removal of toxic compounds from LFL. They found that the best reaction time was 34 h and that increasing the tungsten content to an optimal value led to an increase in process performance. Increasing the W content further caused some reactive sites on the surface to become blocked to photocatalytic activity and thus negatively affected the photocatalytic treatment. The authors pointed out that W-doped TiO2 was a more effective catalyst than TiO2 and that COD degradation reached 46% under optimal experimental conditions (pH 6.63 and a contact time of 34 h).
Recently, Azadi and coworkers (2020) investigated the treatment of LFL using a photocatalytic process that employs tungsten (W)/carbon (C)-codoped titanium dioxide (TiO2) nanoparticles under visible light irradiation. The researchers designed a cascade photoreactor with immobilized W/C-codoped TiO2 nanoparticles. The effects of operating factors (for example, the leachate recirculation flow rate, coating surface density, and light intensity) on the COD removal efficiency was investigated. The experimental results showed that the leachate COD was reduced by 84% after 40 h of treatment under the optimum conditions of 40 W light intensity, 10.59 g m−2 coating surface density, and 1 L min−1 leachate flow rate.
Ozonation
Ozonation is another advanced oxidation process based on the infusion of ozone into water. Ozone (O3) is one of the most powerful oxidants (Rathnayake and Herath 2018). Ozonation is an appropriate LFL treatment as it involves the formation of very reactive oxygen species that can degrade a wide range of organic and inorganic pollutants and all microorganisms (Ramdhani et al. 2018). Ozone is produced by subjecting oxygen (O2) to energy (a high electric voltage or UV radiation). Depending on the types of pollutants present and the experimental conditions, ozone oxidation is most efficient at higher pH values, as a high pH leads to increased production of hydroxyl radicals. Furthermore, to enhance treatment performance, the ozone is combined with light irradiation, hydrogen peroxide, or iron complexes that act as catalysts (Yao et al. 2017). The principal limitation of this process is its relatively high cost, the large amount of energy it requires, and the potential fire hazard from and toxicity associated with ozone generation (Betancourt-Buitrago et al. 2019). Leszczyński and Jolanta Walery (2018) used ozone for the removal of organic compounds from LFL. COD was reduced by 46%. The best conditions for the H2O2/O3 process were an H2O2/O3 ratio of 0.8 and an ozone dose of 0.6 g O3 dm−3. Kwarciak-Kozłowska (2018) showed that the application of ozone as a pretreatment of LFL was more effective at alkaline pH (8.5). It was found that the TOC removal efficiency was 37% (346 mg dm−3) after 60 min of ozonation. More recently, Hoffmann et al. (2020) assessed the efficiency of ozonation in the treatment of raw LFL. Runs were carried out using a batch system. The initial pH was adjusted to 7 and 10, and the contact times between the gas and the leachate were 20, 40, 60, 80, and 100 min. The outcomes showed that ozonation presented high removal efficiencies of COD, color, and UV abs when the pH was 7. However, the turbidity degradation was higher when the pH was 10. The greatest removal of color (~ 90%) and UV abs (~ 70%) occurred with 40 min of reaction time. Therefore, ozonation can be considered a pretreatment method for leachate because of its great capacity for organic compound removal (Table 2).
Supercritical water oxidation (ScWO)
Another method that can be used in landfill leachate treatment is supercritical water oxidation. This advanced oxidation process takes advantage of the fascinating properties of supercritical water as a reaction medium at temperatures and pressures higher than those corresponding to the critical point of water (374 °C and 22.1 MPa). Water is totally miscible with oxygen and organic compounds, so it is possible to achieve rapid oxidation reactions in a single-phase medium at high temperature without mass transfer limitations. The main oxidants used in ScWO are pure oxygen and hydrogen peroxide. ScWO has been considered as a clean energy process. The heat released by the oxidation reaction could be converted to heat and shaft work, assuring a self-sustaining reaction and generating excess shaft power to drive both the high-pressure pump and the air compressor (Scandelai et al. 2020). ScWO has been successfully used to treat different wastewaters characterized by a high COD and a high heavy metal concentration. Marulanda Cardona et al. (2017) performed an experimental study of the supercritical water oxidation of landfill leachate in a batch reactor in the temperature range 400–500 °C for reaction times of 15–30 min and with the oxygen excess (OE) ranging from 100% to 300%. Total organic carbon (TOC) and total nitrogen (TN) were the main responses considered, and the effects of the studied variables were analyzed using an analysis of variance (ANOVA). The results proved that it is possible to achieve simultaneous TOC and TN removal from leachate wastewater by ScWO treatment at 400 °C with 100% OE, with residence times longer than 30 min, and without using a catalyst, whether using a batch or a continuous process. Weijin et al. (2018) tested the application of ScWO using a batch-type reactor in the treatment of landfill leachate. Different catalysts such as NaOH, KOH, K2CO3, and Na2CO3 were used. The effects of temperature (380–500 °C) and retention time (5–25 min) on the hydrogen mole fraction, hydrogen yield, carbon gasification rate, COD, TOC, and TN removal efficiency were studied. The outcomes showed that the gaseous products mostly contained hydrogen, methane, carbon dioxide, and carbon monoxide when there was no catalyst. However, hydrogen and methane were the main gaseous products when NaOH, KOH, K2CO3, or Na2CO3 was added. The temperature had a positive effect on landfill leachate gasification in the absence of a catalyst. At 500 °C, the hydrogen mole fraction, hydrogen yield, and carbon gasification ratio were 55.6%, 107.15 mol kg−1, and 71.96%, respectively. Furthermore, the TOC, COD, and TN abatement rates increased with increasing temperature. In the presence of a catalyst, the maximum hydrogen mole fraction of 74.40% and the maximum hydrogen yield of 70.05 mol kg−1 were obtained using 5 wt% NaOH at 450 °C and 28 MPa for 15 min. Recently, CC Martins and coworkers (2020) demonstrated that temperature was the most influential factor in the ScWO of landfill leachate. As a result, significant removal rates of true color (87%), total dissolved solids (94%), nitrate (70%), total phosphorus (96%), and COD (57%) were achieved.
In other studies, researchers found that the addition of other materials to the ScWO enhanced LFL treatment efficiency (Gong et al. 2018). In 2018, Scandelai et al. (2018) tested combined ozonation and ScWO processes for landfill leachate degradation. The combination of ozonation for 30 min and supercritical water oxidation (O3-30′/ScWO) was found to produce the most efficient degradation of the leachate. Under these conditions, high removal efficiencies of color 97%, COD (92%), TOC (79%), nitrite (78%), nitrate (84%), and dissolved (96%) and suspended (94%) solids were reached. Moreover, the proposed technology produced a significant decrease (68%) in electrical conductivity (EC). Thus, O3/ScWO may have high potential as a LFL treatment. More recently, Scandelai et al. (2020) demonstrated the great potential of the application of ScWO in combination with zeolite to landfill leachate. Thus, ScWO was performed at a pressure of 23 MPa and at 600 or 700 °C. As a result, ScWO (600 °C) reduced 100% of the nitrite (NO2–N), 98% of the nitrate (NO3–N), 90% of the ammoniacal nitrogen (NH3–N), 81% of the TOC, and 74% of the COD, proving that this technology is a promising alternative for leachate treatment. However, the final concentrations of NH3–N and COD slightly exceeded the limits (20 and 200 mg L−1, respectively) defined by Brazilian discharge standards. Thus, these findings suggest that ScWO requires further development for its application to be feasible.
Electro-oxidation
According to the literature, electro-oxidation (EO) has been proven highly capable and efficient when applied to reduce several pollutants in landfill leachate. However, the performance of electro-oxidation depends on the experimental conditions used and the nature of the electrode materials employed. Various electrode materials have been applied in the electro-oxidation of different kinds of wastewaters. Recently, graphite carbon electrodes, Ti/PbO2 anode/stainless steel cathode, boron-doped diamond (BDD) electrodes, carbon anode/stainless steel cathode, and Ti/RuO2–IrO2 anode/stainless steel cathode have been employed in the treatment of landfill leachate using EO (Pierpaoli et al. 2021; Du et al. 2021). Yan et al. (2021) studied the electro-oxidation process for LFL treatment. Under the optimal conditions (a reaction time of 3 h, a current density of 32.89 mA cm−2, a liquid circulation velocity of 0.46 cm s−1, and a specific electrode area of 65.1 m2 m−3), COD and TOC removal efficiencies of 68% and 40.6%, respectively, were achieved. According to research by Pasalari and coworkers (2021), electro-oxidation produced interesting results under the optimum conditions of a current density of 10–40 mA/cm2, an electrode gap of 0.5–2 cm, and a contact time of 15–60 min.
Membrane filtration technology
Over the past two decades, membranes have become increasingly popular in industrial technologies where reliable and repeatable purification or concentration is required (Dolar et al. 2016). Membrane filtration is a very suitable process for removing and reducing different pollutants such as organic and inorganic matter, microorganisms, and trace metals (Ramaswami et al. 2018). According to the literature, there are four types of membrane methods: nanofiltration, ultrafiltration, microfiltration, and reverse osmosis (Anand and Singh 2014; Rathnayake and Herath 2018).
Reverse osmosis
RO is generally implemented as a complement to other treatments, but in some cases it is the only treatment applied to mature leachate. In RO, any solvent that contains metal cations is passed through a membrane in such a way that the metal concentrations are removed. RO can be used for the removal of trace metals, suspended/colloidal materials, and dissolved solids from landfill leachate (Ramaswami et al. 2018). Talalaj (2015) pointed out the high removal rates of different pollutants from intermediate leachate with the use of reverse osmosis. The average reductions in COD, NH4+–N, total inorganic nitrogen, CN−, Fe, and Cl− were 97%, 98.7%, 99%, 93%, 97.6%, and 98%, respectively. Fatima et al. (2017) tested the performance of a treatment that applied a membrane process based on reverse osmosis to Indian LFL. The outcomes showed that RO is a promising method of removing BOD, COD, and TDS, as it yielded removal rates of 99.7%, 98%, and 94%, respectively. Additionally, the reduction rates of various trace metals ranged between 76% and 99.99%. However, information about the cost of this treatment is scarce. To fill this gap, Almeida et al. (2020) proposed a study to estimate the capital expenses (CAPEX), the operational expenses (OPEX), the specific total treatment cost, and the total costs per m3 of the treated leachate and the RO treatment of leachate. The CAPEX for this full-scale RO was estimated at MUS$ 1.413, and the OPEX ranged between US$ 0.132 and US$ 0.265 m3 per year. The cost of this leachate treatment has been estimated at US$ 8.58 m3.
In general, the RO process has demonstrated high rejection rates of both the organic and inorganic contaminants dissolved in the leachate. The major disadvantage of this technology is membrane fouling, which influences treatment effectiveness and concentrates production, which is difficult to manage.
Nanofiltration
Compared to RO, nanofiltration (NF) produces high-quality permeates and is performed at low pressure, resulting in lower operating costs (Mohammad et al. 2015). NF is effective in the treatment of leachate with high salt concentrations (Madsen and Søgaard 2014). In this treatment, the membrane rejects most of the trace metals because they are multivalent cations, but allows monovalent cations, which are considered relatively harmless (Silva 2018). In general, NF has led to greater COD and trace metal removal and ion separation than OR and UF. Due to negatively charged groups on the membrane, NF allows the separation of organic substances from different kinds of salts. A study done by Istirokhatuna et al. (2018) reported that the COD, TSS, and TDS were reduced by 96%, 100%, and 62%, respectively, using a NF process. In the same vein, Silva et al. (2018) demonstrated that NF technology can be considered as an efficient polishing step when applying the membrane bioreactor (MBR) process in LFL treatment. Nevertheless, NF cannot operate under higher fluxes.
Ultrafiltration
Ultrafiltration (UF) is widely used as pretreatment before a final stage involving RO in membrane bioreactors or as a pretreatment at a final stage of RO (Rathnayake and Herath 2018). In addition, UF is applied to remove particles, microorganisms, and a certain amount of dissolved organic matter. As it is a simple and easy-to-operate process, UF has been increasingly used in water treatment and the separation and purification of different proteins. Abuayyash et al. (2018) investigated the feasibility of using UF as an extra enhanced treatment stage during biological treatment. They showed that UF is highly efficient at removing TSS, nitrate and phosphate, Al, and Zn (for which the removal rates were 100%, 98%, 95%, 100%, and 82%), respectively. However, the ultrafiltration porosity limits the passage of suspended and large dissolved compounds through the membrane.
Microfiltration
A microfiltration (MF) membrane is used to remove high molecular mass compounds (particle size range: 0.02–10 µm) from old landfill leachates (Ameen et al. 2011). The major advantages of the MF process are its low operational costs, a decreased number of operations, high separation efficiency and enhancement effluent quality. On the other hand, the major problem associated with the MF process is membrane fouling, which is generally caused by specific physical and chemical interactions between the contaminants in the wastewater and the membrane (Nakamura et al. 2012; Xiong et al. 2016).
Pertile et al. (2018) evaluated the application of MF to a tertiary treatment system for landfill leachate from the Rincão das Flores landfill (Brazil) and reported that this process removed 43% of the organic matter and 63% of the BOD5.
Biological treatment
Biological processes are mainly based on the use of microorganisms to break down the organic matter present in LFL. Such processes can be categorized according to the type of microorganisms applied in the treatment into aerobic and anaerobic processes.
These methods have drawn increasing attention due to their good performance, reliability, simplicity, and high cost-effectiveness. In addition, these processes provide many advantages in terms of biodegradable matter and nitrogen compound removal (Wang et al. 2018a, b). Biological treatments make use of microorganisms; the capabilities of various bacteria, fungi, and algae in the treatment of LFL have been reported. However, there are some drawbacks of using these methods. For example, some contaminants such as NH4+–N are difficult to biodegrade because of their toxicity.
Aerobic treatment
Different factors such as the types of microorganisms and organic compounds present in LFL affect the performance of aerobic treatments. The removal of organic and other compounds from LFL is achieved by applying a variety of microorganisms (Wang et al. 2018a, b). They degrade the complex compounds into simple products and extra biomass. Studies in this field have focused on the degradation of organic compounds and toxic heavy metals (Hashemi et al. 2017; Mohd et al. 2015). Aerobic biodegradation has been tested as an approach for the removal of organic compounds and different toxic pollutants from LFL. Sequencing batch reactor (SBR) technology is the predominant process applied to landfill leachate. This configuration has a simple structure, flexibility, and a high capacity for removing nutrients from wastewater. Chakraborty et al. (2015) used a SBR to process leachate with an hydraulic retention time (HRT) of 2.5 days and an organic loading rate (OLR) of 1.7 kg COD m−3 day−1. Phosphate was reduced by 29–67% and 54–85% of the COD was removed. Furthermore, the ammonia removal rate varied in the range 13–35%, whereas the sulfate removal rate was in the range 7–66%. Hashemi et al. (2017) tested the performance of a SBR in the treatment of leachate. The average removal efficiencies for COD, total nitrogen, phosphorus, and BOD5 were 92.45%, 73.6%, 66.5%, and 96%, respectively. In general, numerous studies have proved that algae and fungi are more effective than bacteria in degrading organic matter and different pollutants in landfill leachate (Reis et al. 2017). Spina et al. (2018) compared different autochthonous fungi in terms of their ability to decolorize the landfill leachate. Five autochthonous fungi—Penicillium brevicompactum, Pseudallescheria boydii, P. boydii, Phanerochaete sanguinea, and Flammulina velutipes—were selected for landfill leachate treatment. Regarding color degradation, they established that the most efficient species were P. spadiceum and P. boydii, which removed 60% of the color. Razarinah et al. (2015) immobilized the fungus Trametes menziesii on Ecomat for leachate treatment. Their findings suggested that this fungus removed 89.14% of the BOD5 and 2.11% of the COD. Furthermore, the biotreatability of organic compounds using microorganisms, namely algae, has received great attention due to their efficiency when applied in wastewater treatment (Gonçalves et al. 2017). Paskuliakova et al. (2018) demonstrated the possible application of microalgae in LFL treatment. Their work indicated that Chlamydomonas sp. was able to grow on 10% permeate leachate. They reported that 93% of the ammonia nitrogen and 54% of the nitrate were removed after 70 days of treatment. Recently, El Ouaer et al. (2020) noted that a pure culture of Chlorella sp. was able to remove notable percentages of the COD (60%), NH4+–N (100%), and salinity (10%) from leachate.
Likewise, Cherni et al. (2020) highlighted the performance of a Lactococcus lactis and Kluyveromyces marxianus coculture isolated from kefir grains in the bioremediation of LFL. This research indicated that coculture with 1% inoculum led to the most efficient degradation of different pollutants. The overall removal rates of COD, NH4+–N, and salinity were 75.8%, 85.9%, and 75.13%, respectively. Furthermore, the bioremediation process removed up to 75% of the Ni and Cd and 73.45%, 68.53%, and 58.17% of the Cu, Pb, and Fe, respectively. However, the main disadvantages of this process are the production of a large volume of sludge and the high capital costs of aeration equipment. Moreover, it is very difficult to achieve the required removal efficiencies of the different compounds using aerobic processes since these processes can be used as pretreatment or post-treatment steps to enhance the efficiency of the main treatment process used.
To alleviate this problem, biogranulation is used as innovative treatment of landfill leachate. The granular sludge observed in biological reactors is a microbial consortium with a regular outer shape and a high density. The granules are dense and have a strong microbial structure compared to flocs. Granular biomass has a relatively high settling velocity, which allows for the application of a high hydraulic load to a reactor without incurring biomass washout. The granules are microecosystems consisting of different layers with diverse microorganisms (Gomez-Gallegos et al. 2021). Wang et al. (2018a, b) used a full-scale internal circulation (IC) reactor to treat landfill leachate from an incineration plant. Findings showed that the IC reactor achieved excellent treatment performance under high OLRs of 21.06–25.16 kg COD/(m3 day). The COD removal efficiency and biogas yield were, respectively, 89.4–93.4% and 0.42–0.50 m3/kg COD. The formation of extracellular polymeric substances (EPS) was related to sludge granulation. Furthermore, protein was the dominant component of the sludge EPS, and its content increased remarkably from 21.6 to 99.7 mg/g volatile suspended solids (VSS). The sludge zeta potential and hydrophobicity were positively correlated with the protein/polysaccharide ratio in the EPS, and they increased from − 26.2 mV to − 10.6 mV and from 30.35% to 78.67%, respectively. These conditions were beneficial to microbial aggregation.
Anaerobic treatment
Anaerobic digestion, which is carried out by anaerobic bacteria, has been proven to be an effective LFL treatment and to remove organic compounds because of the low energy requirements of these bacteria, their low sludge production, and their generation of methane with a high energy content. However, these bacteria can be inhibited by toxic pollutants such as total ammoniacal nitrogen (TAN), sulfides, and heavy metals (Pirsaheb et al. 2017; Begum et al. 2018). The main advantages of anaerobic processes are minimal sludge formation, the production of biogas, low nutrient demands, and a low energy input.
Begum et al. (2018) examined the initial organic load (IOL) and pH in terms of the COD for the production of added-value products during single-stage and two-stage anaerobic digestion. Results showed that at an optimal IOL of 48 g L−1, butyric acid was dominant at pH 5.5–6.0 and 10–11, whereas acetic acid dominated at pH 5.5. The level of volatile fatty acids (VFA) depended on the IOL and ranged between 0.26 and 0.36 g VFA g−1 of COD. Moreover, methane was produced during single-stage and two-stage AD, varying from 0.21 to 0.34 L of CH4 per g of COD removed and from 0.2 to 0.32 L CH4 per g of COD removed, respectively. In addition, those authors reported that the decrease in COD was 21% greater following two-stage AD than with single-stage AD. The anaerobic baffled reactor (ABR) is one of the most favorable anaerobic treatment reactors, and is used to treat, for example, palm oil mill effluent, textile dye wastewater, and landfill leachate. Moreover, the ABR presents many advantages, such as high strength under organic and hydraulic shock loading, significant removal of microbial products, low sludge production, and low energy consumption (Aris et al. 2017; Aqaneghad et al. 2018). In 2021, Cirik and coworkers (2021) demonstrated the performance of an ABR in LFL treatment. In their research, the effect of the level of dilution (5%, 10%, 20%, or 50%) on LFL pollutant removal was investigated. The maximum removal was noted when the dilution was 20%. The outcomes indicated that the dissolved organic carbon (DOC), color, COD, total nitrogen (TN), nitrate (NO3−), and ammonium (NH4+) removal efficiencies were approximately 61%, 17%, 81%, 15%, 1%, and 5%, respectively. Based on information gathered from the literature, ammonia is the main pollutant in landfill leachate. Anaerobic ammonium oxidation (anammox) is a new biological denitrification process that exhibits a high denitrification capacity and low energy consumption (Zhang et al. 2021). Zhang et al. 2021 tested the efficiency of the anammox process in the treatment of LFL. Satisfactory effluent quality was obtained, 10.3 mg/L TN, which was indicative of a nitrogen removal efficiency of 86.2%. Furthermore, the mass balance showed that 67.2% of the nitrate generated by the anammox process could be reduced to nitrite and reused in situ. The results from microbiological analyses showed that anammox bacteria genes and the nitrate reductase/nitrite reductase ratio of anammox bacteria were highly detected. Moreover, Ca. Brocadia triumphed among several groups of anammox bacteria, increasing from 1.2% to 3.6% at the end of the experiment. Numerous studies performed over the past few years have demonstrated that biological processes are highly effective in the treatment of young LFL (< 5 years old) and are widely used for the reduction of biodegradable compounds. On the other hand, older LFL (> 10 years old) presents many technical problems and challenges that can be attributed mainly to the characteristics of this leachate, such as its low biodegradability, high concentrations of NH3–N and COD, and the existence of toxic organic and inorganic compounds (Ding et al. 2018; Smaoui et al. 2019). To overcome these difficulties, more reliable and suitable technology should be investigated. As reported in the literature, no single method seems to provide efficient landfill leachate treatment. Hence, combined processes have emerged as a promising approach to removing different contaminants from LFL (Grosser et al. 2019).
Combined treatments
The treatment of leachate is very complicated due to its complex composition, with significant variations in both volumetric flow and also chemical composition. (Ding et al. 2018; Donneys-Victoria et al. 2018; Smaoui et al. 2019). However, combining individual treatment processes has been shown to be more effective, and this approach has emerged as a favorable choice for treating landfill leachate (De Almeida et al. 2017). Combined treatments have the ability to synergize the advantages of each of the processes included in the treatment, and they have proven to be more effective and economical for mature landfill leachate treatment (Ai et al. 2017). The combination of an adsorption pretreatment with a biological treatment has already been implemented at many landfill leachate sites (Septiariva et al. 2019).
In most cases, activated carbon adsorption have been found to be more suitable not only for the removal of organic compounds and color from LFL but also as a refining step prior to biological treatment. Research by Septiariva et al. (2019) investigated the effectiveness of ozonation and a biological process as pretreatment methods for stabilized landfill leachate. Ozonation had a significant effect on the leachate treatment, with the COD removal rate rising from 50.8% to 75%. Thus, ozonation can be applied as a post-treatment with biological processes in order to enhance the organic matter degradation. In a study by Er et al. (2018), lightweight aggregates were applied at a level of 24% w/v as an adsorbent for contaminant removal, and biological treatment with B. panacihumi strain ZB1 was tested as a means of improving the final effluent quality. The results showed that more than 70% of the ammonia–nitrogen was removed from the leachate wastewater. Several authors have reported that electrocoagulation (EC) is a promising alternative that can be coupled with other processes to improve the effectiveness of landfill leachate treatment. The principle of EC is to generate coagulants in situ using an electric potential difference. These coagulants can then agglomerate colloidal suspensions and form insoluble metal hydroxides onto which organic matter can be adsorbed (Dia et al. 2016). In fact, Donneys-Victoria et al. (2018) studied the color, turbidity, chloride, and COD removal efficiencies achieved when iron electrodissolution and flocculation processes were applied to LFL. The findings showed that 85%, 96%, and 76% of the COD, color, and turbidity, respectively, were removed after 150 min at pH 8.5 using 0.225 g L−1 of hydrogen peroxide. Luo et al. (2019) proved the feasibility of combining chemical flocculation and microelectrolysis-Fenton (MEF) processes using RSM for the effective treatment of mature sanitary landfill leachate. Under optimum conditions (pH 3.2; H2O2 concentration: 3.57 g L−1; Fe–C dosage: 104.52 g L−1), the combined process provided satisfactory results in terms of COD and humic acids (HA), with removal rates of 90.27% and 93.79%, respectively. PAC coagulation removed protein, while the MEF process effectively destroyed organic recalcitrant pollutants, especially humic and fulvic substances. De Oliveira et al. (2019) tested the treatment of landfill leachate through coagulation/flocculation (alum) combined with electrochemical techniques. The optimum conditions for coagulation/flocculation were 20 mL L−1 Al2 (SO4)3 (50 g L−1) at pH 6. Color, turbidity, and ammoniacal nitrogen were completely eliminated from the treated leachate by the end of the treatment. Moreover, Ding et al. (2018) investigated the feasibility and practicability of anodic oxidation integrated with an electrocoagulation process using a dual-anode system for the disposal of biologically pretreated landfill leachate. The obtained results revealed that 78% of the COD and 99.7% of the ammonia were removed with an energy consumption of 25.6 kW h m−3 after 5 days, which implies that this process could be effective in large-scale industrial applications. Recently, Elleuch and coworkers (2020) investigated a practical, low-cost approach for the removal of toxic compounds from LFL using a combination of biological pretreatment with kefir grains and a Ag-doped TiO2 photocatalytic process for the simultaneous removal of different pollutants from LFL. The overall removal efficiencies of TOC, COD, NH4+–N, and PO43− were 98%, 96%, 85%, and 93%, respectively. In this context, Cherni et al. (2020) proved the efficiency of a combined LFL treatment process consisting of a TiO2/Ag photocatalytic pretreatment and a biological process that used Candida tropicalis. The abatement rates of COD, NH4 +–N, TOC, Fe, Zn, and Cu were 90%, 75%, 84.61%, 50%, 63.8%, and 83%, respectively, while 95% of the Pb and Cd was removed. More recently, El Mrabet et al. (2021) studied a landfill leachate treatment that used a combination of the Fenton process and adsorption onto natural local bentonite clay. First, the conditions used in the Fenton process were optimized. The optimal conditions were found to be 2000 mg L–1 of Fe2+ and 2500 mg L–1 of H2O2 at pH 3, which removed 92% of the color and 73% of the COD from the leachate. After that, the natural bentonite clay was used as an adsorbent for the pretreated leachate. The effects of several factors (effluent pH, contact time, adsorbent concentration, and temperature) on the adsorption efficiency were investigated. Pseudo-second-order and Freundlich models were the most important models applied to the adsorption. The combination of the Fenton process with adsorption (bentonite concentration: 3 g L–1; pH 5; reaction time: 5 h; T = 35 °C) removed 84% of the total COD and 98% of the color. Thus, the combination process is an ideal alternative method for leachate treatment. Talalaj et al. (2021) tested the effectiveness of the combination of a sequencing batch reactor (SBR) and reverse osmosis (RO) when it was used to treat young and stabilized landfill leachate. The biological pretreatment showed good removal efficiency (> 98% for both leachates) for ammoniacal nitrogen. The removal efficiencies of the SBR for Fe (29%), Cl− (0.2%), TOC (− 5.3%), and BOD (64%) in young leachate were lower than those in stabilized leachate. The pretreated leachate was directed to a RO system that presented better operational parameters during the filtration of the stabilized leachate: the average permeate flux was higher by 3.3 L m−2 s 10−6 and the filtration time was shorter by 110 min compared to those obtained during the filtration of the young leachate. These differences could be due to the formation of complexes between the iron and natural organic matter that may precipitate on the membrane surface or inside the pores. Consequently, the combined process presented high removal efficiencies (up to 80%) for all analyzed parameters. Table 3 summarizes some selected combined treatment processes with high removal rates of leachate pollutants.
Landfill leachate and soil properties
In the absence of LFL contaminants, leachate migration is highly dependent on the physical and chemical properties of the soil, which control the movement and the storage of water and solutes. Soil chemical properties (pH, adsorption, and ionic exchange) influence solute transport, the soil-leachate interaction mechanism of which are controlled by the quantity and type of colloids (clay and humus) present (Rodríguez-Eugenio et al. 2018). Soil physical properties (horizon sequence, texture, and structure) affect the hydraulic and hydrologic characteristics of the soil profile, such as the permeability, volumetric water content, and field capacity (also referred to as the drained upper limit, DUL) (Mavimbela and van Rensburg 2015; Mengistu et al. 2018). Therefore, leaching could modify the soil properties, so it is necessary to study the mechanism soil-leachate (Nayak et al. 2007). Accordingly, several studies around the world have focused on the physical and chemical characteristics of leachate-soil mixture (Ozcoban et al. 2006; Narayana 2009). In 2013, Harun and coworkers (2013) studied the effect of leachate on sandy clay soil properties. They found that the soil cohesion was reduced from 156 to 55.44 kN/m2 when the fraction of landfill leachate in the soil was increased from 0 to 20%. According to the literature, the optimum moisture content (OMC) and the maximum dry density (MDD) are the principal indicators of soil compaction. Nayak et al. (2007) tested the effect of leachate on the MDD and OMC of lateritic soil. The obtained results revealed that the OMC was 26% and the MDD was reduced to 14.3 kN/m2. Table 4 presents a summary of the effect of leachate on soil properties.
Conclusion
In recent decades, increasing industrial activity and population growth have contributed to an increase in municipal solid waste. Landfill leachate generated by the decomposition of organic wastes and rainfall percolation through those wastes is currently one of the main challenges in landfill management. Thus, there has been increasing interest worldwide in realizing suitable treatments for landfill leachate. Over the years, various landfill leachate treatment technologies have been developed, including physicochemical methods, membrane technologies, and biological processes. As summarized in this paper, conventional treatment is not sufficient to reduce the high concentrations of pollutants in leachate. Hence, a biological process using a consortium of microorganisms, resulting in a novel short treatment pathway, was reviewed. Furthermore, combined processes such as a physical/chemical process with a biological process have proven to be more effective, and these combined processes have emerged as the favored choice of landfill operators. The principal objective of waste management is to generate a universal treatment approach that is inexpensive, effective, and sustainable in the community. Solid waste management and treatment technologies should be based on the waste management strategy of reduce, reuse, and recycle. Adopting of this hierarchy will preserve the environment by channeling energy and resources to the most efficient and environmentally freindly treatment processes.
References
Abood AR, Bao J, Du J, Zheng D, Luo Y (2014) Non-biodegradable landfill leachate treatment by combined process of agitation, coagulation, SBR and filtration. Waste Manag 34:439–447
Abuayyash A, Sayara T, Kanan A, Qurie M (2018) Characterization and Treatment of Al-Menya Landfill leachate using Biological and Physical Methods. Environ Sci: An Indian J 14(2):161
Ahmari H, Heris S Z, Khayyat MH (2018) The effect of titanium dioxide nanoparticles and UV irradiation on photocatalytic degradation of lmidaclopride. Environ Technol 39(4):536–547
Ai J, Wu X, Wang Y, Zhang D, Zhang H (2017) Treatment of landfill leachate with combined biological and chemical processes: changes in the dissolved organic matter and functional groups. Environ Technol 40:1–7
Al-Sahari M, Al-Gheethi AAS, Mohamed RMSR (2020) Natural coagulates for wastewater treatment; a review for application and mechanism. In: Al-Gheethi AAS, Mohamed RMSR, Noman EA, Kassim AHM (eds) Prospects of fresh market wastes management in developing countries. Springer, Cham, pp 17–31
Amor C, Rodriguez-Chueca J, Fernandes JL, Dominguez JR, Lucas MS, Peres JA (2019) Winery wastewater treatment by sulphate radical based-advanced oxidation processes (SR-AOP): thermally vs UV-assisted persulphate activation. Process Saf Environ Prot 122:94–101
Ameen ES, Muyibi SA, Abdulkarim M I (2011) Microfiltration of pretreated sanitary landfill leachate. The Environmentalist 31(3):208–215
Anand A, Singh S (2014) Membrane technique for leachate treatment: a literature review. Int J Environ Res Dev 4:33–36
Aqanaghad M, Mousavi G, Ghanbari R (2018) Anaerobic baffled reactor and hybrid anaerobic baffled reactor performances evaluation in municipal wastewater treatment. Iran J Health Saf Environ 5(3):1027–1034
Aris MAM, Chelliapan S, Din MFD, Anuar AN, Shahperi R, Selvam SB, Yuzir A (2017) Effect of organic loading rate (OLR) on the performance of modified anaerobic baffled reactor (MABR) supported by slanted baffles. Desalination Water Treat 79:56–63
Augusto PA, Castelo-Grande T, Merchan L, Estevez AM, Quintero X, Barbosa D (2019) Landfill leachate treatment by sorption in magnetic particles: preliminary study. Sci Total Environ 648:636–668
Azadi S, Karimi-Jashni A, Javadpour S (2017) Photocatalytic treatment of landfill leachate using W-doped TiO2 nanoparticles. J Environ Eng 143:04017049
Azadi S, Karimi-Jashni A, Javadpour S, Amiri H (2020) Photocatalytic treatment of landfill leachate using cascade photoreactor with immobilized WC-codoped TiO2 nanoparticles. J Water Process Eng 36:101307
Aziz HA, Ramli SF (2018) Recent development in sanitary landfilling and landfill leachate treatment in Malaysia. Int J Environ Eng 9:201–229
Aziz HA, Noor AFM, Keat TW, Alazaiza MYD, Abd A (2020) Heat activated zeolite for the reduction of ammoniacal nitrogen, colour, and COD in landfill leachate. Int J Environ Res 14:463–478. https://doi.org/10.1007/s41742-020-00270-5
Banch TJ, Hanafiah MM, Alkarkhi AF, Abu Amr SS (2019) Factorial design and optimization of landfill leachate treatment using tannin-based natural coagulant. Polymers 11(8):1349
Begum S, Anupoju GR, Sridhar S, Bhargava SK, Jegatheesan V, Eshtiaghi N (2018) Evaluation of single and two stage anaerobic digestion of landfill leachate: effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Biores Technol 251:364–373
Betancourt-Buitrago LA, Hernandez-Ramirez A, Colina-Marquez JA, Bustillo-Lecompte CF, Rehmann L, Machuca-Martinez F (2019) Recent developments in the photocatalytic treatment of cyanide wastewater: an approach to remediation and recovery of metals. Processes 7:225
Bogacki J, Marcinowski P, El-Khozondar B (2019) Treatment of landfill leachates with combined acidification/coagulation and the Fe0/H2O2 process. Water 11:194
Bourechech Z, Abdelmalek F, Ghezzar MR, Addou A (2018) Treatment of leachate from municipal solid waste of Mostaganem district in Algeria: decision support for advising a process treatment. Waste Manag Res 36:68–78
Bouhezila F, Hariti M, Lounici H, Mameri N (2011) Treatment of the OUED SMAR town landfill leachate by an electrochemical reactor. Desalination 280(1-3):347–353
Carard RF, Schiavon GJ, Castro TM, Medeiros FV, Paula GD, Landgraf ACM, Arantes EJ (2021) Photocatalytic ozonation performance in landfill leachate treatment. Anais da Academia Brasileira de Ciências 93
Cassano D, Zapata A, Brunetti G, Del Moro G, Di laconi C, Oller I, Mascolo G (2011) Comparaison of several combined/ integrated biological-AOPs setups for the treatment of municipal landfill leachate: minimization of operating costs and effluent toxicity Chem Eng J 172(1):250–257
Cesaro A, Russo L, Belgiorno V (2015) Combined anaerobic/aerobic treatment of OFMSW: performance evaluation using mass balances. Chem Eng J 267:16–24
Chakraborty S, Ranjan K, Verma M, Iqbal J, Naresh R (2015) Assessing the feasibility of co-treatment of landfill leachate and municipal wastewater in sequencing batch reactor (SBR). In: Proceedings of International Conference on Sustainable Energy and Built Environment, pp 574–578
Chaouki Z, Hadri M, Nawdali M, Benzina M, Zaitan H (2021) Treatment of a landfill leachate from Casablanca city by a coagulation-flocculation and adsorption process using a palm bark powder (PBP). Sci Afr 12:e00721
Chaouki Z, El Mrabet I, Khalil F, Ijjaali M, Rafqah S, Anouar S, Zaitan H (2017) Use of coagulation-flocculation process for the treatment of the landfill leachates of Casablanca city (Morocco). J Mater Environ Sci 8:2781–2791
Chávez RP, Pizarro ECC, Galiano YL (2019) Landfill leachate treatment using activated carbon obtained from coffee waste. Eng Sanit Ambient 24:833–842
Chemlal R, Azzouz L, Kernani R, Abdi N, Lounici H, Grib H, Drouiche N (2014) Combination of advanced oxidation and biological processes for the landfill leachate treatment. Ecol Eng 73:281–289
Cheng SY, Show PL, Juan JC, Ling TC, Lau BF, Lai SH, Ng EP (2020) Sustainable landfill leachate treatment: optimize use of guar gum as natural coagulant and floc characterization. Environ Res 188:109737
Cherni Y, Botta C, Kasmi M, Franciosa I, Cocolin L, Chatti A, Trabelsi I (2020) Mixed culture of Lactococcus lactis and Kluyveromyces marxianus isolated from Kefir grains for pollutants load removal from Jebel Chakir leachate. Water Environ Res 92(12):2041–2048
Chys M, Declerck W, Audenaert WTM, Van Hulle SWH (2014) UV/H2O2, O3 and (photo-)Fenton as treatment prior to granular activated carbon filtration of biologically stabilized landfill leachate. J Chem Technol Biotechnol 90:525–533
Cirik K, Goçer S, Duyar A, Kozak M (2021) Treatment of landfill leachate by anaerobic baffled reactor (ABR). Environ Res Technol 4(2):134–139
Comninellis C, Kapalka A, Malato S, Parsons SA, Poulios I, Mantzavinos D (2008) Advanced oxidation processes for water treatment: advances and trends for R&D. J Chem Technol Biotechnol 83:769–776
Costa AM, Alfaia RGDSM, Campos JC (2019) Landfill leachate treatment in Brazil—an overview. J Environ Manag 232:110–116
De Almeida R, Oroski FA, Campos JC (2017) Treatment of landfill leachate by a combined process of coagulation flocculation and nanofiltration. In: Proc Sardinia 2017/16th Int Waste Management and Landfill Symp, Cagliari, Italy, 2–6 Oct 2017
De Almeida R, Bila DM, Quintaes BR, Campos JC (2020) Cost estimation of landfill leachate treatment by reverse osmosis in a Brazilian landfill. Waste Manage Res 38(10):1087–1092
De Oliveira MS, da Silva LF, Barbosa AD, Romualdo LL, Sadoyama G, Andrade LS (2019) Landfill leachate treatment by combining coagulation and advanced electrochemical oxidation techniques. Chem Electro Chem 6:1427–1433
De Oliveira MT, Torres IMS, Ruggeri H, Scalize P, Albuquerque A, Gil EDS (2021) Application of electrocoagulation with a new steel-swarf-based electrode for the removal of heavy metals and total coliforms from sanitary landfill leachate. Appl Sci 11(11):5009
Değermenci N, Ata ON, Yildız E (2012) Ammonia removal by air stripping in a semi-batch jet loop reactor. J Ind Eng Chem 18:399–404
Di Maria F, Sisani F (2017) A life cycle assessment of conventional technologies for landfill leachate treatment. Environ Technol innovat 8:411–422
Desai NN, Soraganvi VS, Madabhavi VK (2020) Solar photocatalytic degradation of organic contaminants in landfill leachate using TiO2 nanoparticles by RSM and ANN. Nat Environ Pollut Technol 19(2):651–662
Dia O, Drogui P, Buelna G, Dubé R, Ben Salah I (2016) Coupling biofiltration process and electrocoagulation using magnesium-based anode for the treatment of landfill leachate. J Environ Manag 181:477–483
Ding J, Wei L, Huang H, Zhao Q, Hou W, Kabutey FT, Dionysiou D (2018) Tertiary treatment of landfill leachate by an integrated electro-oxidation/electro-coagulation/electro-reduction process, performance and mechanism. J Hazard Mater 351:90–97
Djeffal K, Bouranene S, Fievet P, Déon S, Gheid A (2021) Treatment of controlled discharge leachate by coagulation-flocculation: influence of operational conditions. Sep Sci Technol 56(1):168–183
Djelal H, Lelievre Y, Ricordel C (2015) Combination of electro-coagulation and biological treatment by bioaugmentation for landfill leachate. Desalin Water Treat 54:2986–2993
Dogan S, Aygun A, Argun ME, Esmeray E (2018) Optimization of struvite precipitation for landfill leachate treatment. Uludağ Univ J Fac Eng 23:65–76
Dolar D, Košutic K, Strmecky T (2016) Hybrid processes for treatment of landfill leachate, coagulation/UF/NF-RO and adsorption/UF/NF-RO. Sep Purif Technol 168:39–46
Donneys-Victoria D, Marriaga-Cabrales N, Camargo-Amado RJ, Machuca-Martínez F, Peralta-Hernandez JM, Martínez-Huitle CA (2018) Treatment of landfill leachate by a combined process, iron electrodissolution, iron oxidation by H2O2 and chemical flocculation. Sustain Environ Res 28:12–19
Du X, Li Z, Xiao M, Mo Z, Wang Z, Li X, Yang Y (2021) An electro-oxidation reactor for treatment of nanofiltration concentrate towards zero liquid discharge. Sci Total Environ 783:146990
Durai NJ, Gopalakrishna GVT, Padmanaban VC, Selvaraju N (2020) Oxidative removal of stabilized landfill leachate by Fenton's process modeling, optimization and analysis of degraded products. RSC Advances 10(7):3916–3925
El Mrabet I, Ihssane B, Valdés H, Zaitan H (2021) Optimization of Fenton process operating conditions for the treatment of the landfill leachate of Fez city (Morocco). Int J Environ Sci Technol 12:920–924
Er XY, Seow TW, Lim CK, Ibrahim Z, Chan NW (2018) Landfill leachate management by using combined adsorption and biological treatment. AIP Conf Proc 2016:020041
Elleuch L, Messaoud M, Djebali K, Attafi M, Cherni Y, Kasmi M, Elaoud A, Trabelsi I, Chatti A (2020) A new insight into highly contaminated landfill leachate treatment using Kefir grains pre-treatment combined with Ag-doped TiO2 photocatalytic process. J Hazard Mater 382:12119
El Ouaer M, Turki N, Kallel A, Halaoui M, Trabelsi I, Hassen A (2020) Recovery of landfill leachate as culture medium for two microalgae: Chlorella sp. and Scenedesmus sp. Environ Dev Sustain 22(3):2651–2671
Erabee IK, Ahsan A, Jose B, Aziz MMA, Ng AWM, Idrus S, Daud NNN (2018) Adsorptive treatment of landfill leachate using activated carbon modified with three different methods. KSCE J Civ Eng 22:1083–1095
Fatima S, Jehangir A, Bhat G (2017) Treatment of Landfill leachate using Reverse Osmosis and its potential for reuse. ID 214704296
Fernandes A, Santos D, Pacheco MJ, Ciríaco L, Lopes A (2016) Electrochemical oxidation of humic acid and sanitary landfill leachate, influence of anode material, chloride concentration and current density. Sci Total Environ 541:282–291
Foo KY, Hameed BH (2009) An overview of landfill leachate treatment via activated carbon adsorption process. J Hazard Mater 171:54–60
Fu S, Jia H, Meng X, Guo Z, Wang J (2021) Fe-C micro-electrolysis-electrocoagulation based on BFDA in the pre-treatment of landfill leachate: enhanced mechanism and electrode decay monitoring. Sci Total Environ 781:146797
Galvão N, de Souza JB, de Sousa Vidal CM (2020) Landfill leachate treatment by electrocoagulation: effects of current density and electrolysis time. J Environ Chem Eng 8(5):104368
Gao JL, Oloibiri V, Chys M, De Wandel S, Decostere B, Audenaert W, Van Hulle SWH (2015) Integration of autotrophic nitrogen removal, ozonation and activated carbon filtration for treatment of landfill leachate. Chem Eng J 275:281–287
Ghafari S, Aziz HA, Isa MH, Zinatizadeh AA (2009) Application of response surface methodology (RSM) to optimize coagulation–flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 163:650–656
Gomez-Gallegos MA, Reyes-Mazzoco R, Flores-Cervantes DX, Jarayathne A, Goonetilleke A, Bandala ER, Sanchez-Salas JL (2021) Role of organic matter, nitrogen and phosphorous on granulation and settling velocity in wastewater treatment. J Water Process Eng 40:101967
Goncalves AL, Pires JC, Simoes M (2017) A review on the use of microalgal consortia for wastewater treatment. Algal Res 24:403–415
Gong Y, Guo Y, Sheehan JD, Chen Z, Wang S (2018) Oxidative degradation of landfill leachate by catalysis of CeMnOx/TiO2 in supercritical water: mechanism and kinetic study. Chem Eng J 331:578–586
Goswami D, Choudhury BN (2013) Chemical characteristics of leachate contaminated lateritic soil. Int J Innov Res Sci Eng Technol 2(4):999–1005
Grosser A, Neczaj E, Madela M, Celary P (2019) Ultrasound-assisted treatment of landfill leachate in a sequencing batch reactor. Water 11:516
Guo Z, Zhang Y, Jia H, Guo J, Meng X, Wang J (2021) Electrochemical methods for landfill leachate treatment: a review on electrocoagulation and electrooxidation. Sci Total Environ 150529
Harun NS, Ali ZR, Rahim AS, Lihan T, Idris RMW (2013) Effects of leachate on geotechnical characteristics of sandy clay soil. In AIP Conference Proceedings 1571(1):530–536
Hashemi H, Zad TJ, Derakhshan Z, Ebrahimi AA (2017) Determination of sequencing batch reactor (SBR) performance in treatment of composting plant leachate. Health Scope 6:e13356
Hoffmann LT, Jorge MCB, Amaral AGD, Bongiovani MC, Schneider RM (2020) Ozonation as a pre-treatment of landfill leachate. Revista Ambiente Água 15:1
Hu L, Zeng G, Chen G, Dong H, Liu Y, Wan J, Yu Z (2016) Treatment of landfill leachate using immobilized Phanerochaete chrysosporium loaded with nitrogen doped TiO2 nanoparticles. J Hazard Mater 301:106–118
Inglezakis VJ, Amzebek A, Kuspangaliyeva B, Sarbassov Y, Balbayeva G, Yerkinova A, Poulopoulos SG (2018) Treatment of municipal solid waste landfill leachate by use of combined biological, physical and photochemical processes. Desalin Water Treat 112:218–231
Ismail S, Tawfik A (2016) Performance of passive aerated immobilized biomass reactor coupled with Fenton process for treatment of landfill leachate. Int Biodeterior Biodegrad 111:22–30
Istirokhatun T, Amalia DA, Oktiawan W, Rezagama A, Budihardjo MA, Nofiana N, Susanto H (2018) Removal of refractory compounds from landfill leachate by using nanofiltration. Jurnal Teknologi 80:2–3
Jaafarzadeh Haghighifard NA, Jorfi S, Ahmadi M, Mirali S, Kujlu R (2016) Treatment of mature landfill leachate by chemical precipitation and Fenton advanced oxidation process. Environ Health Eng Manag J 3:35–40
Jain B, Singh AK, Kim H, Lichtfouse E, Sharma VK (2018) Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ Chem Lett 16:947–967
Kallel A, Attour A, Trabelsi I (2017) Electro-coagulation treatment of raw and autoclaved landfill leachate with aluminium electrodes: case study of Djebel Chakir (Tunisia). Arab J Geosci 10(4):85
Kamala SS, Tey LH, Sim YL (2018) Combined chemical, physical and biological treatment using Chlorella vulgaris sp. on landfill leachate. AIP Conf Proc 2026:020006
Kamaruddin MA, Yusoff MS, Aziz HA, Hung YT (2015) Sustainable treatment of landfill leachate. Appl Water Sci 5:113–126
Karami MA, Amin MM, Bina B (2018) Treatment of compost leachate by ferro-sonication process. Effect of some operational variables. Int J Environ Health Eng 7:6
Khanzada ZT, Övez S (2017) Microalgae as a sustainable biological system for improving leachate quality. Energy 140:757–765
Khoi TT, Thuy TTT, Nga NT, Huy NN, Thuy NT (2021) Air stripping for ammonia removal from landfill leachate in Vietnam: effect of operation parameters. TNU J Sci Technol 226(06):73–81
Khoramipour S, Mehralipour J, Hosseini M (2021) Optimisation of ultrasonic-electrocoagulation process efficiency in the landfill leachate treatment: a novel advanced oxidation process. Int J Environ Anal Chem 2021:1–19
Kwarciak-Kozłowska A (2018) Pretreatment of stabilized landfill leachate using ozone. J Ecol Eng 19:186–193
Leite VD, Paredes JM, de Sousa TA, Lopes WS, de Sousa JT (2018) Ammoniacal nitrogen stripping from landfill leachate at open horizontal flow reactors. Water Environ Res 90:387–394
Leszczyński J, Maria JW (2018) The removal of organic compounds from landfill leachate using ozone-based advanced oxidation processes. E3S Web Conf 45:00046
Luo K, Pang Y, Li X, Chen F, Liao X, Lei M, Song Y (2019a) Landfill leachate treatment by coagulation/flocculation combined with microelectrolysis-Fenton processes. Environ Technol 40:1862–1870
Luo H, Cheng Y, He D, Yang EH (2019b) Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci Total Environ 668:90–103
Luo K, Pang Y, Li X, Chen F, Liao X, Lei M, Song Y (2019) Landfill leachate treatment by coagulation/ flocculation combined with microelectrolysis-Fenton processes. Environ Technol 40(14):1862-1870
Madsen HT, Søgaard EG (2014) Applicability and modelling of nanofiltration and reverse osmosis for remediation of groundwater polluted with pesticides and pesticide transformation products. Sep Purif Technol 125:111–119
Mahdavi AR, Ghoresyhi AA, Rahimpour A, Younesi H, Pirzadeh K (2018) COD removal from landfill leachate using a high-performance and low-cost activated carbon synthesized from walnut shell. Chem Eng Commun 205:1193–1206
Mahtab MS, Islam DT, Farooqi IH (2021) Optimization of the process variables for landfill leachate treatment using Fenton based advanced oxidation technique. Eng Sci Technol 24(2):428–435
Makhatova A, Mazhit B, Sarbassov Y, Meiramkulova K, Inglezakis VJ, Poulopoulos SG (2020) Effective photochemical treatment of a municipal solid waste landfill leachate. PLoS ONE 15(9):e0239433
Martins CC, J Scandelai A P, Cardozo-Filho L, Tavares C R (2020) Supercritical water oxidation treatment of humic acid as a model organic compound of landfill leachate. Can J Chem Eng 98(4):868-878
Marulanda Cardona VF, Marulanda Buitrago PA, Alvarado Acosta DH (2017) Landfill leachate treatment by batch supercritical water oxidation. Cienc Ing Neogranad 27(2):5–26
Maslahati Roudi A, Chelliapan S, Wan Mohtar W, Kamyab H (2018) Prediction and optimization of the Fenton process for the treatment of landfill leachate using an artificial neural network. Water 10:595
Mavimbela SSW, Van Rensburg L (2015) Evaluating models for predicting hydraulic characteristics of layered soil. 9(1):301–336
Mengistu AG, Van Rensburg LD, Mavimbela SS (2018) Shallow groundwater effect on evaporation and soil temperature in two windblown sands (Eutric Cambisol and Chromic Luvidol) in South Africa. Geoderma Regional 15:e00190
Mohajeri S, Hamidi AA, Isa MH, Zahed MA (2019) Landfill leachate treatment through electro-Fenton oxidation. Pollution 5:199–209
Mohammad AW, Teow YH, Ang WL, Chung YT, Oatley-Radcliffe DL, Hilal N (2015) Nanofiltration membranes review: recent advances and future prospects. Desalination 356:226–254
Mohammad-pajooh E, Turcios AE, Cuff G, Weichgrebe D, Rosenwinkel KH, Vedenyapina MD, Sharifullina LR (2018) Removal of inert COD and trace metals from stabilized landfill leachate by granular activated carbon (GAC) adsorption. J Environ Manag 228:189–196
Mohd-Salleh SNA, Mohd-Zin NS, Othman N, Mohd-Amdan NS, Mohd-Shahli F (2018) Dosage and pH optimization on stabilized landfill leachate via coagulation-flocculation process. MATEC Web Conf 250:06007
Mohd-Salleh SNA, Mohd-Zin NS, Othman N (2019) A review of wastewater treatment using natural material and its potential as aid and composite coagulant. Sains Malaysiana 48:155–164
Mokhtarani N, Khodabakhshi S, Ayati B (2016) Optimization of photocatalytic post-treatment of composting leachate using UV/TiO2. Desalin Water Treat 57:22232–22243
Mosanefi S, Alavi N, Eslami A, Saadani M, Ghavami A (2021) Ammonium removal from landfill fresh leachate using zeolite as adsorbent. J Mater Cycles Waste Manag 2021(4)
Müller GT, Giacobbo A, dos Santos Chiaramonte EA, Rodrigues MAS, Meneguzzi A, Bernardes AM (2015) The effect of sanitary landfill leachate aging on the biological treatment and assessment of photoelectrooxidation as a pre-treatment process. Waste Manag 36:177–183
Nakamura K, Hirayama W, Nittami T, Matsumoto K (2012) Simultaneous determination of pore size and surface charge density of microfiltration membranes by streaming potential measurement. J Chem Eng Japan 45:1203230348
Nasrollahzadeh M, Atarod M, Jaleh B, Gandomirouzbahani M (2016) In situ green synthesis of Ag nanoparticles on graphene oxide/TiO2 nanocomposite and their catalytic activity for the reduction of 4-nitrophenol, Congo red and methylene blue. Ceram Int 42:8587–8596
Narayana T (2009) Municipal solid waste managment in India: from waste disposal to recovery of resources? Waste Manage 29:1163–1166
Nayak S, Sunil BM, Shrihari S (2007) Hydraulic and compaction characteristics of leachate-contaminated lateritic soil. Eng Geol 94 (3-4):137–144
Nithya M, Abirami M (2018) The leachate treatment by using natural coagulants (pine bark and chitosan). Int J Eng Res Technol 5:2711–2714
Oloibiri V, Ufomba I, Chys M, Audenaert WT, Demeestere K, Van Hulle SW (2015) A comparative study on the efficiency of ozonation and coagulation–flocculation as pretreatment to activated carbon adsorption of biologically stabilized landfill leachate. Waste Manag 43:335–342
Ozcoban MS, Tufekci N, Tutus S, Sahin U, Celik S O (2006) Leachate removal rate and the effect of leachate on the hydraulic conductivity of natural (undisturbed) clay. J scient Ind Res 65:264–269
Pan H, Lei H, Liu X, Wei H, Liu S (2017) Assessment on the leakage hazard of landfill leachate using three-dimensional excitation-emission fluorescence and parallel factor analysis method. Waste Manag 67:214–221
Pant B, Park M, Kim HY, Park SJ (2017) CdS-TiO2 NPs decorated carbonized eggshell membrane for effective removal of organic pollutants: a novel strategy to use a waste material for environmental remediation. J Alloy Compd 699:73–78
Pasalari H, Esrafili A, Rezaee A, Gholami M, Farzadkia M (2021) Electrochemical oxidation pretreatment for enhanced methane potential from landfill leachate in anaerobic co-digestion process: performance, Gompertz model, and energy assessment. Chem Eng J 422:130046
Paskuliakova A, McGowan T, Tonry S, Touzet N (2018) Microalgal bioremediation of nitrogenous compounds in landfill leachate—the importance of micronutrient balance in the treatment of leachates of variable composition. Algal Res 32:162–171
Patale V, Pandya J (2012) Mucilage extracts of Coccinia indica fruit as coagulant-flocculent for turbid water treatment. Asian J Plant Sci Res 2:442–445
Pavithra S, Shanthakumar S (2017) Removal of COD, BOD and color from municipal solid waste leachate using silica and iron nano particles—a comparative study. Glob NEST J 19:122–130
Payandeh PE, Mehrdadi N, Dadgar P (2017) Study of biological methods in landfill leachate treatment. Open J Ecol 7:568
Pertile C, Zanini M, Baldasso C, Andrade MZ, Tessaro IC (2018) Evaluation of membrane microfiltration fouling in landfill leachate treatment. Matéria (Rio de Janeiro) 23(1)
Pierpaoli M, Szopińska M, Wilk BK, Sobaszek M, Łuczkiewicz A, Bogdanowicz R, Fudala-Książek S (2021) Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes. J Hazard Mater 403:123606
Pirsaheb M, Hossini H, Secula MS, Parvaneh M, Ashraf GM (2017) Application of high rate integrated anaerobic-aerobic/biogranular activated carbon sequencing batch reactor (IAnA-BioGACSBR) for treating strong municipal landfill leachate. Sci Rep 7:3109
Poblete R, Oller I, Maldonado MI, Luna Y, Cortes E (2017) Cost estimation of COD and color removal from landfill leachate using combined coffee-waste based activated carbon with advanced oxidation processes. J Environ Chem Eng 5:114–121
Ramaswami S, Behrendt J, Otterpohl R (2018) Comparison of NF-RO and RO-NF for the treatment of mature landfill leachates: a guide for landfill operators. Membranes 8:17
Ramdhani MY, Sururi MR, Ainun S (2018) Leachate treatment from Sarimukti landfill using ozone with sludge from water treatment plant as a catalyst. MATEC Web Conf 147:04006
Rasool MA, Tavakoli B, Chaibakhsh N, Pendashteh AR, Mirroshandel AS (2016) Use of a plant-based coagulant in coagulation–ozonation combined treatment of leachate from a waste dumping site. Ecol Eng 90:431–437
Rathnayake WAPP, Herath GBB (2018) A review of leachate treatment techniques. In: 9th Int Conf on Sustainable Built Environment, Kandy, Sri Lanka, 13–14 Dec 2010
Razarinah WARW, Zalina MN, Abdullah N (2015) Utilization of the white-rot fungus, Trametes menziesii for landfill leachate treatment. Sains Malays 44:309–316
Rebolledo LP, Arana VA, Trilleras J, Barros GE, González-Solano AJ, Maury-Ardila H (2019) Efficiency of combined processes coagulation/solar photo Fenton in the treatment of landfill leachate. Water 11:1351
Reis BG, Silveira AL, Teixeira LPT, Okuma AA, Lange LC, Amaral MCS (2017) Organic compounds removal and toxicity reduction of landfill leachate by commercial bakers’ yeast and conventional bacteria based membrane bioreactor integrated with nanofiltration. Waste Manag 70:170–180
Reshadi MAM, Bazargan A, McKay G (2020) A review of the application of adsorbents for landfill leachate treatment: focus on magnetic adsorption. Sci Total Environ 731:138863
Reynier N, Coudert L, Blais J, Mercier G, Besner S (2015) Treatment of contaminated soil leachate by precipitation, adsorption and ion exchange. J Environ Chem Eng 3:977–985
Righetto I, Al-Juboori RA, Kaljunen JU, Mikola A (2021) Multipurpose treatment of landfill leachate using natural coagulants—pretreatment for nutrient recovery and removal of heavy metals and micropollutants. J Environ Chem Eng 9(3):105213
Rodriguez-Eugenio N, Mclaughlin M, Pennock D (2018) Soil pollution: a hidden reality. FAO
Rohers F, Dalsasso RL, Nadaleti WC, Matias MS, de Castilhos Júnior AB (2021) Physical–chemical pre-treatment of sanitary landfill raw leachate by direct ascending filtration. Chemosphere 285:131362
Scandelai APJ, Cardozo Filho L, Martins DCC, de Souza Freitas TKF, Garcia JC, Tavares CRG (2018) Combined processes of ozonation and supercritical water oxidation for landfill leachate degradation. Waste Manag 77:466–476
Scandelai APJ, Sloboda Rigobello E, Oliveira BLCD, Tavares CRG (2019) Identification of organic compounds in landfill leachate treated by advanced oxidation processes. Environ Technol 40:730–741
Scandelai APJ, Zotesso JP, Jegatheesan V, Cardozo-Filho L, Tavares CRG (2020) Intensification of supercritical water oxidation (ScWO) process for landfill leachate treatment through ion exchange with zeolite. Waste Manag 101:259–267
Septiariva IY, Padmi T, Damanhuri E, Helmy Q (2019) A study on municipal leachate treatment through a combination of biological processes and ozonation. MATEC Web Conf 276:06030
Shalini SS, Joseph K (2012) Nitrogen management in landfill leachate: application of SHARON, ANAMMOX and combined SHARON–ANAMMOX process. Waste Manag 32:2385–2400
Shehzad A, Bashir MJ, Sethupathi S, Lim JW (2015) An overview of heavily polluted landfill leachate treatment using food waste as an alternative and renewable source of activated carbon. Process Saf Environ Prot 98:309–318
Singa PK, Isa MH, Ho YC, Lim JW (2018) Treatment of hazardous waste landfill leachate using Fenton oxidation process. In E3S Web of conferences 34: 02034. EDP Sciences
Smaoui Y, Mlaik N, Bouzid J, Sayadi S (2018) Improvement of anaerobic digestion of landfill leachate by using coagulation-flocculation, Fenton’s oxidation and air stripping pretreatments. Environ Prog Sustain Energy 37:1041–1049
Smaoui Y, Bouzid J, Sayadi S, Smaoui Y, Bouzid J, Sayadi S (2019) Combination of air stripping and biological processes for landfill leachate treatment. Environ Eng Res 25:80–87
Spina F, Tigini V, Romagnolo A, Varese G (2018) Bioremediation of landfill leachate with fungi: autochthonous vs allochthonous strains. Life 8:27
Sunil BM, Shrihari S, Nayak S (2008) Soil-leachate interaction and their effects on hydraulic conductivity and compaction characteristics. The 12th international conference of IACMAG, Gao India
Talalaj IA (2015) Removal of organic and inorganic compounds from landfill leachate using reverse osmosis. Int J Environ Sci Technol 12(9):2791–2800
Tałałaj IA, Biedka P, Bartkowska I (2019) Treatment of landfill leachates with biological pretreatments and reverse osmosis. Environ Chem Lett 17:1–17
Tałałaj IA, Bartkowska I, Biedka P (2021) Treatment of young and stabilized landfill leachate by integrated sequencing batch reactor (SBR) and reverse osmosis (RO) process. Environ Nanotechnol Monit Manag 16:100502
Tang X, Zheng H, Teng H, Sun Y, Guo J, Xie W, Chen W (2016) Chemical coagulation process for the removal of heavy metals from water: a review. Desalin Water Treat 57:1733–1748
Taoufik M, Elmoubarki R, Moufti A, Elhalil A, Farnane M, Machrouhi A, Machrouhi M, Abdennouri SQ, Barka N (2018) Treatment of landfill leachate by coagulation-flocculation with FeCl3: process optimization using Box–Behnken design. J Mater Environ Sci 9:2458–2467
Torretta V, Ferronato N, Katsoyiannis I, Tolkou A, Airoldi M (2016) Novel and conventional technologies for landfill leachates treatment: a review. Sustainability 9:9
Wang K, Li L, Tan F, Wu D (2018a) Treatment of landfill leachate using activated sludge technology: a review. Archaea 2018:1–10
Wang T, Huang Z, Ruan W, Zhao M, Shao Y, Miao H (2018b) Insights into sludge granulation during anaerobic treatment of high-strength leachate via a full-scale IC reactor with external circulation system. J Environ Sci 64:227–234
Weijin G, Binbin L, Qingyu W, Zuohua H, Liang Z (2018) Supercritical water gasification of landfill leachate for hydrogen production in the presence and absence of alkali catalyst. Waste Manag 73:439–446
Xiong B, Zydney AL, Kumar M (2016) Fouling of microfiltration membranes by flowback and produced waters from the Marcellus shale gas play. Water Res 99:162–170
Yan C, Cheng Z, Quan X, Feng C, Cheng G (2021) Electrochemical pretreatment of landfill leachate RO concentrate with multi-channel mesh electrodes. Environ Chem 2:603–613
Yao W, Wang J, Wang P, Wang X, Yu S, Zou Y, Wang X (2017) Synergistic coagulation of GO and secondary adsorption of heavy metal ions on Ca/Al layered double hydroxides. Environ Poll 229:827–836
Yi EX, Wee ST, Lim CK, Ibrahim Z, Chan NW (2018) Combined adsorption and biological treatment for landfill leachate management. J Adv Res Fluid Mech Therm Sci 50:26–31
Yuan MH, Chen YH, Tsai JY, Chang CY (2016) Ammonia removal from ammonia-rich wastewater by air stripping using a rotating packed bed. Process Saf Environ Prot 102:777–785
Zailani LM, Amdan NM, Zin NSM (2018) Removal efficiency of electrocoagulation treatment using aluminium electrode for stabilized leachate. IOP Conf Ser Earth Environ Sci 140(1):012049
Zainol NA, Pin LB, Rashid NA, Ghani AA, Zailani SN, Rani ALA (2018) Treatment of landfill leachate by coagulation-flocculation process using red earth as coagulant. AIP Conf Proc 2030:020043
Zhang D, Vahala R, Wang Y, Smets BF (2016) Microbes in biological processes for municipal landfill leachate treatment: community, function and interaction. Int Biodeterior Biodegrad 113:88–96
Zhang J, Wu X, Qiu D, Mao J, Zhang H (2017) Pilot-scale in situ treatment of landfill leachate using combined coagulation–flocculation, hydrolysis acidification, SBR and electro-Fenton oxidation. Environ Technol 40:2191–2200
Zhang F, Peng Y, Liu Y, Zhao L (2021) Improving stability of mainstream Anammox in an innovative two-stage process for advanced nitrogen removal from mature landfill leachate. Bioresour Technol 340:125617
Zhou X, Zhou S, Feng X (2017) Optimization of the photoelectrocatalytic oxidation of landfill leachate using copper and nitrate co-doped TiO2 (Ti) by response surface methodology. PLoS ONE 12(7):e0171234
Zou X, Chen C, Wang C, Zhang Q, Yu Z, Wu H, Zhang TC (2021) Combining electrochemical nitrate reduction and anammox for treatment of nitrate-rich wastewater: a short review. Sci Total Environ 800:149645
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Responsible Editor: Eric van Hullebusch.
Rights and permissions
About this article
Cite this article
Cherni, Y., Elleuch, L., Messaoud, M. et al. Recent technologies for leachate treatment: a review. Euro-Mediterr J Environ Integr 6, 79 (2021). https://doi.org/10.1007/s41207-021-00286-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-021-00286-z