Abstract
The dissolved fractions of polycyclic aromatic hydrocarbons (PAHs) in wastewater have many adverse effects on humans, animals, and water species. The objective of this study is to study the removal of PAHs in produced water (PW) using oil palm leaves activated carbon (OPLAC). The OPLAC was applied for the degradation of naphthalene (NAP), one of the commonly found PAH, in aquatic solution and PW at various pH (3–9), dosage (100–3000 mg/L) and contact time (5–90 min). The parameters were optimized using response surface methodology (RSM). The optimum parameters were as follows: pH of 3, dosage of 2197 mg/L and contact time of 72 min giving removal efficiency of 92.48% in aquatic solution. The results were verified in the laboratory. The removal efficiency of NAP from PW sample was 70.5% at optimized parameters. It was closer to the experimental results with 19% difference.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Oil is one of the world’s primary energy sources, and its production is a critical issue. While oil is being produced, some negative environmental effects occur. One of the most significant sources of negative effects is produced water (PW), which is produced as wastewater during the oil production process [1]. The water is highly contaminated by polycyclic aromatic hydrocarbons (PAHs), salinity, heavy metals, radionuclides, and oilfield chemicals [2]. Numerous investigations have established that PAHs are the primary cause of PW’s acute and chronic toxicity [3]. The PAHs include two or more fused aromatic rings of carbon and hydrogen atoms and have been classified as priority pollutants by the U.S. Environmental Protection Agency (USEPA) because of their prevalence and cancer-causing potential [4]. Therefore, it is essential to investigate economical and ecotechnological options for reducing PAHs from PW.
Adsorption is a widely used and efficient method for treating wastewater [5]. However, adsorption is quite material-dependent in terms of its effectiveness and cost. For the process of wastewater treatment, activated carbon (AC) is a renowned adsorbent [6]. Nowadays, attempts are made to replace commercially available AC with AC produced from biomass waste due to the necessity for inexpensive adsorbents [7]. The current study focused on the removal of PAHs in PW by using an AC synthesized by locally available oil palm leaves waste.
Malaysia is the leading exporter of palm oil on the global market, and the country alone produces almost 53 million tonnes of oil palm waste per year, with that quantity rising at a rate of 5% each year [8]. The utilization of oil palm waste as a precursor material for AC has been researched over the years. In wastewater, it has been utilized to adsorb a variety of organic and inorganic contaminants [9, 10]. However, very few research have documented the synthesis of AC utilizing waste oil palm leaves, which are widely accessible, and their use for PAHs adsorption in wastewater is also unexplored, particularly in PW.
This study focused on the application of AC using oil palm leaves waste in Malaysia for the treatment of PAHs in PW. Naphthalene (NAP) was chosen as representative PAH compound. It is one of the commonly found (USEPA priority) PAHs. The response surface methodology (RSM) was used to model the adsorption processes and optimize the parameters [11]. The removal of NAP, as the current study’s target pollutants cannot only offer helpful information for NAP treatment but also be viewed as the main research approach for handling complex PAHs. To meet the objectives of sustainable development through waste management and water purification, this study will contribute to extending the application of largely available oil palm leaves waste, developing green materials, and treating abundantly found PAHs.
2 Materials and Methods
2.1 Materials
The zinc chloride (ZnCl2) and Sigma-Aldrich Supelco analytical solid standard of NAP (C10H8, mol. weight = 128.17 g/mol) was purchased from Avantis Laboratory Supply. The oil palm leaves waste was taken from FELCRA Berhad, Perak in Malaysia. The PW sample was collected from an oil and gas company operating in Southeast Asia
2.2 Characterization of PW
The NAP in PW was quantified using high performance liquid chromatography (HPLC). An inertsil ODS-P HPLC column (5 μm, 250 × 4.6 mm) and an ultraviolet (UV, 254 nm) detector were used. The injection volume was 20 μL and column temperature was 40 ℃. The quantified concentration of NAP in PW was 195 ± 5 μg/L. The finding was consistent with past studies, in which a number of studies observed PAHs of PW in the range of 100–1000 μg/L [3].
2.3 Oil Palm Leaves AC
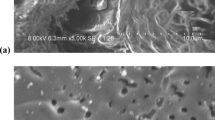
The oil palm leaves AC was synthesized as our previous reported method [12]. Briefly, the leaves waste was chemically activated by ZnCl2 (1:1) and pyrolyzed at 800 ℃ using tube furnace. The obtained AC was named oil palm leaves AC (OPLAC) and stored for further use.
2.4 Adsorption Batch Experiments
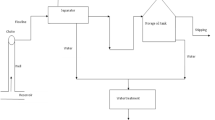
The impact of initial pH of wastewater (3–9), dosage of OPLAC (100–3000 mg/L) and contact time (5–90 min) was analyzed through batch testing. The batch experiments were conducted on a hot plate with magnetic stirrer in a beaker. The aqueous solution was prepared at initial concentration of NAP as 200 μg/L, similar as obtained by the analysis of PW. The samples were placed for stirring (220 rpm at 25 ± 5 ℃). Following the completion of the stirring period, samples were removed, filtered using a 0.45 m syringe filter, and then analyzed using HPLC to determine the final amount of NAP.
2.5 Design of Experiments Using RSM and Application of OPLAC in PW
The RSM-based polynomial central composite design (CCD) in design expert (DE) software was used for the design of adsorption batch experiments. Adsorptive removal efficiency of NAP was taken as outputs. Initial pH of wastewater (3–9), dosage of OPLAC (100–3000 mg/L) and contact time (5–90 min) were taken as inputs. The design gave a total of 25 experimental runs (Table 1).
The experimental data collected through batch tests is represented in Table 1. Analysis of variance (ANOVA) was used to assess the suitability of the developed model. ANOVA also helps to assess the statistical significance of the constant regression coefficients. The obtained optimized parameters were confirmed in the lab. The optimized parameters were used to evaluate the removal efficiency of NAP in PW.
3 Results and Discussion
3.1 Batch Experiments Results
Figure 1 (a) shows the results of the percentage removal of NAP in wastewater using OPLAC, with a change in the initial pH value (3–9). The results showed that percentage removal of NAP in wastewater reduced from 72% to 65% (±2%) with an increase in pH from 3 to 6. A further increase in pH, from 7 to 9, slightly increased the NAP (68 ± 2%) removal. Hence, pH 3 was considered as optimum value for the removal of NAP using OPLAC. Similar results have been observed for removal of PAHs in wastewater, where pH of 2–3 was found as suitable pH for the adsorption of NAP using AC [13, 14]. Figure 1 (b) shows the plot between the removal efficiency of NAP and OPLAC dosage. The removal efficiency of PAHs increased with increasing dosage of OPLAC from 100 mg/L to 3000 mg/L due to increased amounts of available binding sites. The maximum adsorptive removal of NAP was 70.62 ± 2% around dosage of 3000 mg/L. The change in NAP removal efficiency at various contact times (5–90 min) is represented in Figure 1 (c). The removal of NAP considerably increased when the contact time increased from 0 to 5 min followed by a slower adsorption rate. Increasing the contact time further did not improve the removal capacity considerably. Hence, a contact time of 45 min was the optimal time for the removal of NAP. The maximum removal efficiency of NAP was 65.17 ± 2% at this contact time. Similar adsorption trend has been reported in other studies for PAHs removal [15].
3.2 Modelling of RSM
A total 25 experimental runs were conducted as obtained by RSM in DOE software. Table 1 lists the performed runs and their experimental results. The findings of ANOVA show the validity of the used design as well as the relationships and relevance of variables. Table 2 shows that all parameters pH, dosage, and contact time significantly influenced the adsorption process. The Model F-value of 27.59 implied that the model was significant for adsorption of NAP onto OPLAC. There was only a 0.01% chance that an F-value this large could occur due to noise. A p-values less than 0.05 indicated that model terms were significant. The Predicted R2 of 0.8514 was in closer agreement with the Adjusted R2 of 0.9394. Adeq. Precision (AP) ratio should be greater than 4. Here ratio of 16.915 indicated an adequate signal for NAP.
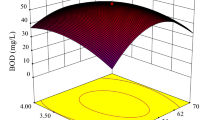
3.3 3D Plots Describing Impact of Operating Variables on NAP Adsorptions
3D plots with various peak values and curves show how independent variables and their interactions affect the removal of NAP. In 3D plots, two independent variables were deliberately changed while the other independent variable was kept constant. Fig. 2 (a–c) indicate the 3-D plots on effects of pH, dosage and contact on NAP removal efficiency (%). Higher removal efficiency of NAP was observed at pH of 3 and 9 showing that increase in H+ and OH- ions increased the electrostatic interactions between OPLAC and NAP. Increasing the dosage of OPLAC and increasing contact time until 45 min increased the removal efficiencies due to abundant available adsorption sites on OPLAC. Further increase in contact time did not increase the removal efficiencies due to saturation of OPLAC. It can be seen that the wastewater pH did not significantly affect the removal efficiencies of NAP. Whereas dosage and contact time greatly affect the removal efficiencies.
To assess the adequacy of the selected models, two types of plots were chosen: predicted vs experimental values plot and normal plot of residual. Figures 2 (d) shows that model’s anticipated and experimental responses were related. The plots revealed a good match between actual and experimental values. Furthermore, as shown in Figure 2 (e), the distribution of all data points was near to the 45° straight line.
3.4 Optimization and Application of OPLAC in PW
After ANOVA model validation, the RSM-based method of numerical optimization is frequently employed to identify the optimal values for each independent variable. The NAP removal efficiencies predicted by numerical optimization was 92.48%, using OPLAC. This removal efficiency weas estimated at the wastewater pH of 3, dosage of OPLAC 2197 mg/L and contact time of 72 min. Additional tests were carried out utilizing the suggested optimal values of independent parameters to validate the optimal conditions and the complete model. The model’s validity was confirmed by a small divergence between experimental and predicted outcomes under optimal conditions (Table 3).
The OPLAC was utilized to extract NAP from PW sample to confirm the applicability of the OPLAC. The PW sample was mixed with OPLAC at optimized parameters viz., pH (3), dosage (2197 mg/L) and contact time (72 min). Table 3 shows the effectiveness of NAP removal from PW sample. The removal efficiency of NAP from PW sample was closer to the experimental result with a 19% difference. It is evident that OPLAC was able to remove 70.5% NAP in PW. The lower removal of PAHs from PW compared to aqueous solution may be correlated to the presence of numerous other pollutants in PW [16].
4 Conclusion
The oil palm leaves AC (OPLAC) was efficiently applied for the removal of NAP in wastewater. The removal process was optimized by RSM, and the model was validated by ANOVA analysis. The model was significant (p-value <0.05) to predict the adsorption process. The NAP removal efficiency predicted by numerical optimization was 92.48% at pH of 3, dosage of OPLAC 2197 mg/L and contact time of 72 min. The results were verified in the laboratory experiments. The removal efficiency of NAP from PW sample was closer to the experimental results (70.5%) with a 19% difference at optimized parameters.
References
Al-Ghouti MA, Al-Kaabi MA, Ashfaq MY, Da’na DA (2019) Produced water characteristics, treatment and reuse: a review. J Water Process Eng 28:222–239. https://doi.org/10.1016/j.jwpe.2019.02.001
Lu J, Wang X, Shan B, Li X, Wang W (2006) Analysis of chemical compositions contributable to chemical oxygen demand (COD) of oilfield produced water. Chemosphere 62:322–331. https://doi.org/10.1016/j.chemosphere.2005.04.033
Haneef T, et al (2020) Removal of polycyclic aromatic hydrocarbons (PAHs) from produced water by Ferrate (VI) oxidation. Water 12:1–17. https://doi.org/10.3390/w12113132
Malakahmad A, Law MX, Ng KW, Manan TSA (2016) The fate and toxicity assessment of polycyclic aromatic hydrocarbons (PAHs) in water streams of Malaysia. Procedia Eng 148:806–811. https://doi.org/10.1016/j.proeng.2016.06.572
Khurshid H, Mustafa MRU, Isa MH (2022) A comprehensive insight on adsorption of polyaromatic hydrocarbons, chemical oxygen demand, pharmaceuticals, and chemical dyes in wastewaters using biowaste carbonaceous adsorbents. Adsorpt Sci Technol (2022). https://doi.org/10.1155/2022/9410266
Khurshid H, Mustafa MRU, Isa MH (2022) Adsorption of chromium, copper, lead and mercury ions from aqueous solution using bio and nano adsorbents: a review of recent trends in the application of AC, BC, nZVI and MXene. Environ Res 212:113138. https://doi.org/10.1016/j.envres.2022.113138
Zhou Y, Zhang L, Cheng Z (2015) Removal of organic pollutants from aqueous solution using agricultural wastes: a review. J Mol Liq 212:739–762. https://doi.org/10.1016/j.molliq.2015.10.023
Mohammed MAA, et al (2011) Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew Sustain Energy Rev 15:1258–1270. https://doi.org/10.1016/j.rser.2010.10.003
Sahu JN, Karri RR, Jayakumar NS (2021) Improvement in phenol adsorption capacity on eco-friendly biosorbent derived from waste palm-oil shells using optimized parametric modelling of isotherms and kinetics by differential evolution. Ind Crops Prod 164:113333. https://doi.org/10.1016/j.indcrop.2021.113333
Chew TL, Husni H (2019) Oil palm frond for the adsorption of Janus Green dye. Mater Today Proc 16:1766–1771. https://doi.org/10.1016/j.matpr.2019.06.047
Alhothali A, Khurshid H, Mustafa MRU, Moria KM, Rashid U, Bamasag OO (2022) Evaluation of contemporary computational techniques to optimize adsorption process for simultaneous removal of COD and TOC in wastewater. Adsorpt Sci Technol (2022). https://doi.org/10.1155/2022/7874826
Khurshid H, Mustafa MRU, Isa MH (2022) Modified activated carbon synthesized from oil palm leaves waste as a novel green adsorbent for chemical oxygen demand in produced water. Sustainability 14. https://doi.org/10.3390/su14041986
Balati A, Shahbazi A, Amini MM, Hashemi SH (2015) Adsorption of polycyclic aromatic hydrocarbons from wastewater by using silica-based organic–inorganic nanohybrid material. J Water Reuse Desalin 5:50–63. https://doi.org/10.2166/wrd.2014.013
Gupta H (2015) Removal of phenanthrene from water using activated carbon developed from orange rind. Int J Sci Res Environ Sci 3:248–255. https://doi.org/10.12983/ijsres-2015-p0248-0255
Dai Y, Niu J, Yin L, Xu J, Xi Y (2011) Sorption of polycyclic aromatic hydrocarbons on electrospun nanofibrous membranes: sorption kinetics and mechanism. J Hazard Mater 192:1409–1417. https://doi.org/10.1016/j.jhazmat.2011.06.055
Godlewska P, Siatecka A, Kończak M, Oleszczuk P (2019) Adsorption capacity of phenanthrene and pyrene to engineered carbon-based adsorbents produced from sewage sludge or sewage sludge-biomass mixture in various gaseous conditions. Bioresour Technol 280:421–429. https://doi.org/10.1016/j.biortech.2019.02.021
Acknowledgements
The authors acknowledge the financial support provided under FRGS grant with cost center 0153AB-L71 and YUTP grant with cost center 015LCO-190. The oil palm leaves waste provided for this study by FELCRA Berhad, Perak, Malaysia, is also acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 Institute of Technology PETRONAS Sdn Bhd
About this paper
Cite this paper
Ul Mustafa, M.R., Khurshid, H., Ho, YC., Isa, M.H. (2024). Removal of Naphthalene from Produced Water Using Oil Palm Leaves Waste Activated Carbon. In: Mohammed, B.S., Min, T.H., Sutanto, M.H., Joewono, T.B., As’ad, S. (eds) Proceedings of the International Conference on Emerging Smart Cities (ICESC2022). ICESC 2022. Lecture Notes in Civil Engineering, vol 324. Springer, Singapore. https://doi.org/10.1007/978-981-99-1111-0_14
Download citation
DOI: https://doi.org/10.1007/978-981-99-1111-0_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1110-3
Online ISBN: 978-981-99-1111-0
eBook Packages: EngineeringEngineering (R0)