Abstract
Two-day rhythms, referred to as circa“bi”dian rhythms, were first reported in humans. In insects, a circabidian rhythm has been reported in the flight activity of the cool-weather mosquito Culiseta incidens under constant darkness. In both humans and mosquitoes, the appearance of the circabidian rhythm is labile under constant conditions, and the rhythm does not continue for a long time. In contrast, the black chafer Holotrichia parallela exhibited a rigid 2-day circabidian rhythm under both field and laboratory conditions. Three characteristics of the biological rhythms, free-running, entrainment to the zeitgeber, and temperature compensation of the period, were observed in the circabidian rhythm in H. parallela. Phase responses to light pulses suggest that the circadian clock mechanism is involved in the circabidian rhythm. The results of the brain surgery experiments imply that the optic lobe-pars intercerebralis axis in the brain is involved in the circabidian rhythm of H. parallela. Molecular phylogeny and behavioral observations suggest that after separation into Pedinotrichia, including H. parallela and Holotrichia picea, and Nigrotrichia, the circabidian rhythm probably appeared once in the ancestral species of Pedinotrichia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
It is widely considered that most organisms on Earth have circadian clocks with a period close to Earth’s rotation cycle. The circadian clock is a physiological mechanism that measures approximately 24 h to drive circadian rhythms in behavior and physiology (Dunlap et al. 2004; Patke et al. 2020; Chap. 2). Organisms use rhythms to prepare in advance for daily changes in the physical environment, such as temperature, humidity, and illumination, and the resulting changes in the biological environment, such as food availability and predation risk (DeCoursey et al. 1997, 2000).
Periodicities with an integral multiple of ~24 h have been reported, although this period seems irrelevant to environmental cycles. A circaseptan rhythm with a period of approximately 7 days appears in unicellular marine algae: the growth rate of Acetabularia mediterranea (Chlorophyta) and the glow intensity of Gonyaulax polyedra (Dinoflagellata) (Cornelissen et al. 1986; Schweiger et al. 1986). In both cases, the amplitude of the diurnal rhythm changed over a period of approximately 7 days. However, their periodicity is mostly unclear, and it is debatable whether endogenous circaseptan rhythms actually exist (Piccione et al. 2004).
Another integral multiple of ~24 h is the 2-day periodicity (Table 7.1). When humans were isolated in a cave or underground bunker, a 2-day periodicity was observed in the sleep-wakefulness rhythm under constant dim light conditions, although the period of the body temperature rhythm was approximately 25 h (Aschoff et al. 1967; Colin et al. 1968). Endogenous 2-day periodicity is called circa“bi”dian rhythm, and in humans, different patterns were observed. For example, sleep and wakefulness times were approximately twice as long as normal; an extremely lengthened activity time interrupted by naps appeared with a relatively small increase in sleep time (Honma and Honma 1988; Wever 1979). These human circabidian rhythms were often unstable and subsequently returned to their circadian rhythm or became obscure. Circabidian rhythms are considered to result from internal desynchronization, in which uncoupling of multiple oscillators, such as an oscillator for activity and an oscillator for body temperature, occurs under constant conditions (Aschoff et al. 1967).
There are a few reports of biological rhythms with 2-day periodicity under natural conditions. In mollusks, shell pattern formation of the hard clam Mercenaria mercenaria and the tropical scallop Comptopallium radula exhibits 2-day periodicity (Table 7.1, Pannella and Macclintock 1968; Thébault et al. 2006). In M. mercenaria, daily shell layer growth commonly occurs in one thick increment, followed by a relatively thin one. Juvenile C. radula forms one stria every 2 days under natural daily environmental conditions. In the Pacific Ocean, some meteorological and oceanographic parameters have displayed 2-day variations (Kenyon 1996). For sea-level atmospheric pressure and wind velocity, the amplitude of the 2-day variations was larger than that of the diurnal variations. This may act as a zeitgeber to shell growth rhythm in these bivalves (Thébault et al. 2006). However, it remains unknown whether the 2-day periodicity is endogenous in these species.
Some insects also exhibit a 2-day periodicity or circabidian rhythms (Yoshioka and Yamasaki 1983; Kawasaki et al. 2017; Shiga et al. 2022), although any 2-day variation in the physical parameters of the terrestrial environment has rarely been reported. This chapter introduces the insect circabidian rhythm and discusses its mechanisms.
2 Circabidian Rhythm in the Mosquito Culiseta incidens
In insects, the circabidian rhythm was first reported in the flight activity of the cool-weather mosquito Culiseta incidens under constant darkness (DD) (Table 7.1, Clopton 1984). Adult C. incidens exhibits a circadian rhythm with activity in the subjective night of DD. Many individuals, but not all, displayed radical changes in the free-running period (τ) calculated from activity onset (τon, Fig. 7.1a, b). Circabidian rhythmicity is occasionally found in prolonged DD, as a highly variable phenomenon (Clopton 1984, 1985). τon doubled abruptly, with little or no prior period lengthening (Fig. 7.1b). As τoff, calculated from the activity offset, and τon change differently in many individuals, it has been considered that a circadian pacemaker controlling flight activities consists of two mutually coupled oscillators, E and M, which predominantly control the evening (τon) and morning (τoff) flight rhythms, respectively, as in the common house mosquito Culex pipiens (Jones 1982; Clopton 1984, 1985). The circabidian rhythm can be explained by the period of the E-oscillator lengthening to where it synchronizes with the M oscillator in two consecutive cycles of M: one cycle of the E mode (Clopton 1984). Clopton (1984) proposed that E and M oscillators may behave similarly to the human activity and temperature oscillators mentioned above (see Sect. 7.1). In contrast to the human model, circadian components were not observed during the circabidian period of C. incidens. After the τ of the E oscillator lengthens to a period longer than 24 h, E predominates over the M oscillator (Clopton 1984).
Representative flight activity rhythm of the mosquito Culiseta incidens. (a, b) Double-plotted actogram of the flight activities under constant darkness. Previous light and dark schedule bar is shown in (a). τon and τoff indicate free-running of the dusk (E) and dawn (M) activity rhythm, respectively (refer to the main text). (b) Under prolonged constant darkness, circabidian rhythm with the periodicity of 46.16 h occasionally occurs. Adapted from Clopton (1984), with permission from Springer
3 Circabidian Rhythm in Holotrichia Species
In both C. incidens and humans, the appearance of the circabidian rhythm is labile under constant conditions, and the rhythm does not continue for a long time (Fig. 7.1b). In contrast, some Holotrichia species (Insecta: Coleoptera: Scarabaeidae) exhibited a rigid 2-day periodicity under both field and laboratory conditions (Table 7.1). Yoshioka and Yamasaki (1983) originally reported that H. parallela populations appear on the ground every 2 days. Furthermore, measurements of pheromone titers in the pheromone glands of field-collected females suggest a 2-day periodicity in H. parallela (Leal et al. 1993). The genus Holotrichia includes serious pests of agricultural crops, such as H. loochooana loochooana of sugar canes and H. parallela of potatoes and glass roots in East Asia. Their behavior has been examined in different aspects of pest control, but their chronobiology has been unknown until recently.
3.1 Three Characteristics of Biological Rhythms in the Circabidian Rhythm of the Ground Emergence Activity
Under laboratory conditions, field-collected H. parallela, individually placed in a plastic container with soil, exhibited an approximately 2-day emergence rhythm on the ground. Under a 12 h light/12 h dark cycle (LD12:12) at 25 °C, male and female beetles appeared on the ground during the dark phase every 2 days (Fig. 7.2a). On the ground, beetles feed and walk around and remain underground for the rest of the time. Under LD12:12, the period of the ground emergence rhythm was 48.0 h in both males and females. Under DD, their emergence rhythm continued with a free-running period of slightly less than 48 h (Fig. 7.2a, Kawasaki et al. 2017). Thus, H. parallela exhibited a clear endogenous circabidian rhythm with a period of approximately 48 h under DD, and this rhythm was entrained to two cycles of LD 12:12 (Fig. 7.2a).
Circabidian activity rhythm in Holotrichia species. (a) Representative actograms and chi-square periodogram of H. parallela under 12 h:12 h light: dark (LD) cycles and constant darkness (DD). (b) Average and SD of free-running periods under DD at different temperatures in H. parallela. Three calculated Q10 values are shown in the graph. (c) Representative actograms and chi-square periodogram of H. picea under LD cycles and DD. (c1) and (c3), female adults; c2, sex undetermined. (a) Adapted from Kawasaki et al. (2017); (b) Issei Nakagawa and Sakiko Shiga (unpublished); (c) Adapted from Shiga et al. (2022), with permission from Zoological Science
The circabidian rhythm might be due to developmental or physiological processes specific to H. parallela, such as gonadal development or the digestive system independent of the clock. Yoshioka and Yamasaki (1983) reported that H. parallela females deposit eggs in the daytime just before the night of adult emergence on the ground. A half-day feeding may require a long digestive time of 1.5 days. These physiological processes may suppress the behavioral output from the circadian clock every other day to produce a 2-day rhythmicity. If this is the case, the period of behavioral rhythm may become 24 h at higher temperatures because these physiological functions are temperature-dependent. Activity rhythms were recorded under DD conditions at different temperatures. Free-running periods did not differ at a range from 20.0 to 30.0 °C. The free-running period was 47.5 ± 0.5 h (mean ± S.D., N = 11) at 20 °C, 47.7 ± 0.2 h (N = 11) at 25 °C, and 47.4 ± 0.2 h (N = 11) at 30 °C. The average calculated temperature coefficient Q10 was 1.00 (Nakagawa and Shiga, unpublished Fig. 7.2b). This contradicts the hypothesis that the mechanism underlying 2-day rhythmicity involves some temperature-dependent physiological or developmental processes but suggests that the timekeeping mechanism in the circabidian rhythm solely involves a temperature-independent biological clock in H. parallela. This circabidian rhythm is also found in another species, H. picea, with some variation (Fig 7.2c, Shiga et al. 2022). Details are discussed in a later section (Sect. 7.4).
3.2 Phase Response of the Circabidian Rhythm to Light Pulses
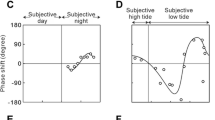
There are two possibilities for the clock mechanism underlying circabidian rhythm: the circabidian rhythm is driven by the circadian clock or by the circabidian clock (Fig. 7.3a). Circabidian behavior may be driven by every two cycles of the circadian clock. In this case, a mechanism doubling the circadian clock cycle must be present in the brain to produce a 2-day period. Alternatively, circabidian behavior might be driven by the cycle of the circabidian clock. One way to estimate the cycle of the clock that drives a rhythm is to examine the phase responses of the rhythm to zeitgeber stimuli. It is well known that a phase advance or delay of a rhythm occurs in response to a zeitgeber stimulus depending on the clock phase, which is a unique characteristic of oscillator-type clocks (Pittendrigh 1960; Chap. 3). Phase-response curves have been drawn for different biological rhythms. In the circadian clock, the clock phases are divided into subjective day and night periods. Circadian rhythms driven by the circadian clock exhibit little or no response to light pulses during the subjective day but a delay in the first half and an advance in the last half of the subjective night (Okada et al. 1991). Because the H. parallela circabidian rhythm entrains to two LD 12:12 cycles, light must function as a zeitgeber. If the circabidian rhythm is driven by “a circabidian clock,” phase delay and phase advance would occur once in a circabidian cycle. However, if the circabidian rhythm is driven by “a circadian clock,” phase delay and advance would occur twice in the circabidian cycle (Fig. 7.3b). Based on this assumption, Kawasaki et al. (2017) examined the phase responses to light pulses. After the light pulse was applied, the onset of the activity phase was advanced, delayed, or unchanged depending on the light pulse phase (Fig. 7.3c1, c2). Although the shape of the response curve was not very clear, two sets of the less-responsive period (circabidian time, CbT 0–12, CbT 24–36) and more-responsive period (delay or advance CbT 12–24, CbT 36–48) appeared in one circabidian cycle (Fig. 7.3d). This suggests that the circabidian cycle is composed of two cycles of the circadian clock. Occasionally, circadian-like activity rhythms appeared after the light pulse (6.2%, N = 65, Fig. 7.3c3, Kawasaki et al. 2017). This also suggested the presence of an oscillator with a period of approximately 24 h in H. parallela.
Phase response of the circabidian rhythm to light pulses in Holotrichia parallela. (a) Two clock models underlying the circabidian rhythm. (b) Putative phase responses of the 24-h clock (upper) and of 48-h clock (lower). Advanced and delayed phase shift values are plotted as positive and negative values on the ordinate. (c) Representative actograms with light pulses (in yellow) twice. Phase changes are indicated by red arrows. (d) Phase responses to 3-h light pulses emitted at different circabidian times under constant darkness. (c and d) Adapted from Kawasaki et al. (2017)
3.3 The Optic Lobe-Pars Intercerebralis Axis in the Brain Is Involved in Circabidian Rhythm of H. parallela
To discuss circadian clock involvement in the circabidian rhythm, brain regions necessary for the rhythm were examined. In insect brains, circadian clock cells are localized in the optic lobe of flies and cockroaches (King and Sehgal 2020; Shiga and Numata 2009; Reischig and Stengl 2003). Another brain region known to be involved in circadian rhythm output is a region called the pars intercerebralis (PI), where neurosecretory cells are concentrated. In Drosophila melanogaster, cells in the PI are connected to the circadian clock cells in the optic lobe through a polysynaptic circuit (Cavanaugh et al. 2014). Neuropeptides expressed in PI cells are required for locomotor activity rhythms (Cavanaugh et al. 2014; King and Sehgal 2020). The optic lobes and PI were examined to determine whether they are necessary for H. parallela circabidian rhythms. Adult beetles collected in the field were kept in the laboratory and subjected to removal of the optic lobes or PI (Watanabe and Shiga 2020).
3.3.1 Roles of the Optic Lobe
In H. parallela, the brain with bilateral optic lobes is located posteriorly in the head (Fig. 7.4). To remove the optic lobes, small cuticular openings were made just medial to the compound eyes, and the optic lobes were removed (Fig. 7.4 left). Most intact and sham-operated control beetles showed a clear circabidian rhythm, although some stayed underground, probably because of winter dormancy in the late season of the experiment (Watanabe and Shiga 2020, Fig. 7.5a, b). When the bilateral optic lobes were removed, approximately half of the beetles exhibited arrhythmicity, and the other half never appeared on the ground. None of the beetles exhibited circabidian rhythms (Fig. 7.5a, b1). After recording, beetles were dug up and their survival was confirmed. All beetles underground were active, similar to those in the intact and sham-operated groups. This suggests that the optic lobe itself, or the connection between the compound eye and midbrain, is involved in the formation of the circabidian rhythm and probably in emergence behavior on the ground. Clock protein PERIOD-immunoreactive cells have been found in the optic lobe of the beetle Pachymorpha sexguttata (Frisch et al. 1996). In H. parallela, a putative clock cell neuropeptide, pigment-dispersing factor, was observed in approximately 100 somata at the anterior base of the optic lobe medulla with medial fiber projections (Fig. 7.4 right, Hamanaka et al. 2022). Based on these findings, it is possible that circadian clock cells are located in the optic lobe of H. parallela and may be involved in the circabidian rhythm.
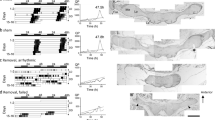
Dorsal view of the head and brain of Holotrichia parallela. For optic lobe removal, bilateral small windows were opened (left). Distribution of pigment-dispersing factor (PDF) immunoreactive neurons and pars lateralis neurons (right). La lamina, Lo lobula, Lop lobula plate, Me medulla. Left, photo courtesy of Kohei Watanabe; right, redrawn from Hamanaka and Shiga et al. (2022)
Effects of brain surgery on the circabidian rhythm in Holotrichia parallela. (a) Representative actograms under 12 h:12 h light/dark (LD) cycles and constant darkness (DD). Chi-square periodogram is shown for activities in DD. (b) Summary of effects of optic lobe (OL) removal (b1 and b2) and pars intercerebralis (PI) removal (b3). (c) The average number and SE of paraldehyde fuchsin-stained PI cells left. Arrhythmic beetles in the PI removal group had significantly reduced cell numbers compared to those in the control groups (P < 0.05, Tukey test). Adapted from Watanabe and Shiga (2020), with permission from Springer
Questions such as the following, are both optic lobes (possibly bilateral clock systems) necessary for the circabidian rhythm and is it possible that an interaction between the two circadian clocks doubles the cycle of the 24-h clock?, still require exploration. If the two circadian clocks on the left and right sides are able to inhibit output signals from the contralateral clock to the premotor circuitry every 24 h and after the inhibition is released they become refractory to contralateral inhibition for 24 h, it might be possible to produce a 48-h cycle. This is called internal masking, in which the pacemaker output appears to be internally masked (Page 1989). Page (1989) introduced the circabidian rhythm of C. incidens and cockroaches as an example of internal masking.
For internal masking, unilateral optic lobe removal was performed. However, circabidian rhythm remained in all individuals (Fig. 7.5b2), suggesting that the bilateral organization of the optic lobes, possibly containing the circadian clock, is not a prerequisite for circabidian rhythm. Interestingly, about one-fifth of circabidian beetles showed “day switching” (Fig. 7.5a, b2). In day switching, beetles maintained a 2-day periodicity but changed their appearance night from odd to even days or vice versa by emerging on or skipping two serial nights (Kawasaki et al. 2017). Day switching has occasionally been observed in the field in H. parallela (Kawasaki et al. 2017). It occurs after heavy rain, suggesting some physiological mechanisms to suppress emergence on the ground under adverse conditions. The appearance of day switching by a single optic lobe may indicate that bilateral coupling between two optic lobes is necessary to maintain a regular 2-day rhythm, and weakening connections or decoupling between the right and the left circadian clock in the optic lobe may cause day switching. In the cockroach Rhyparobia (Leucophaea) maderae and the cricket Gryllus bimaculatus, the circadian clocks in left and right optic lobes possess neural communication to allow synchronization (Page 1989; Tomioka 1993). This indicates that coupling between the bilateral optic lobe circadian clocks is responsible for the 24-h clock oscillation, and in H. parallela, decoupling of bilateral clocks or a solitary clock may weaken 24-h oscillation to cause an irregular pattern of the circabidian rhythm, that is, day switching. Clock coupling between the two optic lobes may be necessary for robustness of the 48-h rhythmic cycle.
In the unilateral optic lobe removal, another 40% of circabidian beetles did not go underground and remained aboveground throughout the day (Fig. 7.5b2). Even beetles restricted above ground exhibited circabidian rhythms. Although underground behavior has not been shown, observation of their activities throughout the day on the ground raised the possibility that they are inactive underground and the total (above and below the ground) locomotor activity is circabidian.
3.3.2 Roles of the Pars Intercerebralis
In the PI of H. parallela, approximately 100 cells were positive for paraldehyde fuchsin, which stains a certain type of neurosecretory cell (Watanabe and Shiga 2020). Brain surgery experiments targeting the PI cells were performed, similar to optic lobe removal (Fig. 7.4). The surgery caused a reduction in paraldehyde fuchsin-stained cells in the PI (Watanabe and Shiga 2020). After this surgery, approximately one-fourth of the beetles showed arrhythmicity (Fig. 7.5b3). The number of paraldehyde fuchsin-stained PI cells in the arrhythmic group was significantly reduced compared with that in the intact and sham-operated groups (Fig. 7.5c). Arrhythmic beetles walked and ate leaves randomly throughout the day, and their activity levels were higher than those of the control groups and PI-removed circabidian beetles (Watanabe and Shiga 2020). These results suggest that some paraldehyde fuchsin-stained PI cells are necessary for circabidian rhythm and the suppression or regulation of locomotor activity.
The results of these surgical experiments suggest that cells of the optic lobe and part of the PI are necessary for circabidian rhythms. The neural circuit of circadian clock cells in the optic lobe to the PI might be evolutionarily conserved for biological timing mechanisms in insects and may be involved in the generation of circabidian rhythms in H. parallela.
3.4 Two-Day Rhythm of H. parallela in the Field
Field observations by Kawasaki et al. (2017) showed male and female adults of H. parallela mostly appeared above ground a few hours after sunset from June to October and visited trees such as the Chinese elm Ulmus parvifolia. H. parallela remained on the tree throughout the night, and their appearance and disappearance were mostly synchronized with sunset and sunrise, respectively (Kawasaki et al. 2017). At sunrise, they dug into the soil in an area of 15-m semidiameter around the tree where they stayed at night. A mark and recapture study showed that beetles repeatedly appeared on the same tree approximately every 2 nights (Kawasaki et al. 2017). However, the periodicity was not very rigid, and individuals often switched appearance days. Figure 7.6 shows individual plots of beetle appearance over 40 days in the field. Male no. 34 emerged on the same tree on days 10, 12, 14, 16, and 18 (every 2 days). In contrast, female no. 2 appeared on even days until day 8, but from day 9, she switched appearance day to odd days until day 29. From day 29, she appeared every day for 3 days and returned to an even-day appearance with 2-day periodicity (Fig. 7.6). Although a large amount of precipitation causes day switching in many beetles, day switching was sometimes observed without rain (Kawasaki et al. 2017).
Representative individual plots of Holotrichia parallela appearance in the field. The horizontal axis indicates the number of days from the first appearance. Orange cells and blue cells indicate an even and odd numbers of days, respectively, counted from the first appearance. Adapted from Kawasaki et al. (2017)
The occurrence of day switching also supports the idea that the circabidian clock drives circabidian rhythm. If the circabidian rhythm was created by a hypothetical circabidian clock (Fig. 7.3a right), a phase shift of a half-cycle (∼24 h) of the clock would have had to occur to switch the appearance days. For an oscillator-type clock, it is difficult to make a half-period phase shift at one time (Benstaali et al. 2001), and it usually requires a transient period to complete a full shift. If day switching was adaptive (e.g., facultative avoidance of aversive conditions or increased population size), the beetles would have had to develop a mechanism to shift the appearance day without transients. If the circabidian rhythm is driven by the circadian clock system (Fig. 7.3a, left), an immediate switch in the appearance day may be possible. Circabidian output might be activated or suppressed every two circadian oscillations by unknown mechanisms, such as counting two circadian cycles. If so, some environmental stimuli may provide an input signal to the cycle counting mechanism to produce an output after one or three circadian oscillations, thus resulting in day switching.
Phase responses to the light pulse, involvement of the optic lobe-PI axis, and day-switching characteristics suggest that the circabidian rhythm in H. parallela is driven by a ~ 24-h circadian clock. If this is the case, a novel function for the circadian clock that creates an integral multiple rhythm can be proposed. Circabidian rhythms may be produced by the release of an output signal from the circadian clock every two cycles to produce a 2-day rhythm. In future experiments, molecular and neuronal bases of the involvement of the circadian clock in circabidian rhythm should be elucidated.
4 Origin of the Circabidian Rhythm in the Genus Holotrichia
To elucidate the ancestral state of the circabidian rhythm in an evolutionary context, the activity rhythms of related species were examined (Shiga et al. 2022). Holotrichia (Coleoptera, Scarabaeidae, Melolonthinae, Rhizotrogina) is a large genus that includes heterogeneous species groups inhabiting Southeast and East Asia (Ward et al. 2002; Anitha et al. 2006; Matsumoto 2016). In addition to H. parallela, Holotrichia picea also exhibited a circabidian rhythm (Fig. 7.2c). In H. picea, three types of rhythms, including the regular circabidian pattern, circabidian patterns with day switching, and a circadian activity-like pattern, were observed under laboratory conditions. In the day-switching pattern, H. picea switched appearance from odd to even days, or vice versa, as did H. parallela (Fig. 7.2c2). In the circadian-like activity patterns, major whole-night activity and minor dusk activity appeared alternately (Fig. 7.2c3). The switching and circadian-like behavioral patterns in H. picea also support the idea that circabidian rhythms in Holotrichia species are driven by the circadian clock mechanism. Holotrichia kiotonensis, Holotrichia convexopyga, and Holotrichia loochooana loochooana exhibit a 24-h circadian rhythm (Shiga et al. 2022).
Two distinct clades were recognized in the phylogenetic trees – constructed using histone H3, cytochrome c oxidase subunit 1, and 16S ribosomal RNA – of the Holotrichia species (Shiga et al. 2022). This phylogenetic separation was in accordance with the subgeneric classification based on external morphology by Matsumoto (2015a, 2015b, 2016) and behavioral rhythms (Fig. 7.7a, b, Shiga et al. 2022). One clade included Nigrotrichia group members, H. kiotonensis, H. convexopyga, and H. loochooana loochooana, showing circadian rhythms, while the other clade included Pedinotrichia group members, H. parallela and H. picea, showing circabidian rhythms (Fig. 7.7). This suggests that after separation into Nigrotrichia and Pedinotrichia groups, the circabidian rhythm probably appeared once in the ancestral species of Pedinotrichia.
Molecular phylogeny and behavioral analysis in Holotrichia species inhabiting Japan. (a) Histone H3, cytochrome c oxidase subunit 1, and 16S ribosomal RNA phylogenetic trees revealed two distinct clades: one clade (Nigrotrichia) including Holotrichia kiotonensis, Holotrichia convexopyga, and Holotrichia loochooana loochooana exhibiting a circadian rhythm, and the other clade (Pedinotrichia) including H. parallela and Holotrichia picea exhibiting circabidian rhythm. (b) Dorsal and lateral views of Holotrichia species (males). Adapted from Shiga et al. (2022), with permission from Zoological Science
5 Mechanism for Doubling the Circadian Clock Cycle
The occurrence of circadian-like activity patterns and day-switching patterns in H. parallela and H. picea suggests circadian clock involvement in the circabidian behavioral rhythm (Figs. 7.2c2, c3, 7.3c3, and 7.5a). The phase-response patterns to light pulses (Fig. 7.3d) and the necessity of the optic lobes in H. parallela (Fig. 7.5b1) also support this hypothesis. If this is the case, there must be some mechanism doubling the circadian clock cycle to achieve a 2-day periodicity. If H. parallela and H. picea possess circadian clock cells with a conventional transcriptional-translational negative feedback loop of clock genes, such as in D. melanogaster (Chap. 4), clock cycle doubling might occur in the clock cells intracellularly or intercellularly (Fig. 7.8).
Hypothetical mechanisms for doubling the circadian clock cycle. (a) In an intracellular mechanism, the first transcriptional-translational feedback loop produces conventional 24-h oscillation, and the second loop oscillates every two cycles of the first loop. Ovals indicate different types of clock proteins. (b) In intercellular mechanisms, neuronal circuitry containing flip-flop neurons or counter neurons may double the circadian clock cycle
Kinases may play an important role in intracellular mechanisms. In cultured mammalian cells, perturbation of phosphorylation by casein kinase 1ε (CK1ε) or CK1δ changes the period of clock gene expression rhythm from circadian (24 h) to circabidian (48 h) (Isojima et al. 2009). Kinases may be involved in the rate control of circadian oscillations. In clock cells of the circabidian species, some negative feedback loops (here, we say the first loop) of clock genes may exhibit a 24-h rhythm in their expression, and another loop (the second loop) may produce a 48-h rhythm by certain phosphorylation processes activated every two cycles of the first loop (Fig. 7.8a). In a single clock cell, clock-controlled genes for output signals that are under the control of the second loop may be able to produce a 2-day periodicity. The blind cavefish Phreatichthys andruzzii, which lives in perpetual darkness, does not show a clear circadian rhythm, and the clock gene expression of their cultured cells exhibits circabidian oscillation (Cavallari et al. 2011). These results suggested that circadian clock genes may be able to oscillate in a double period under certain circumstances.
Another possibility is that a 2-day periodicity is produced in the neuron network (Fig. 7.8b). Two-day periodicity can be produced by alternate suppression and activation of circadian clock cell output. Flip-flops and counters are sequential circuits with alternating on and off outputs. In moth olfactory processing systems, flip-flopping interneurons have been reported, and the flip-flop signal is thought to underlie locomotion occurring during pheromone-triggered orientation behavior (Olberg 1983; Namiki and Kanzaki 2016). This type of neuron switches back and forth between long-lasting high- and low-firing rates in response to repeated stimuli (Olberg 1983). A similar type of flip-flop interneuron might be incorporated into the clock network in H. parallela and H. picea. These neurons receive daily input from 24-h clock cells to alternate turning on and off of the postsynaptic neurons to produce 2-day cycles (Fig. 7.8b left). Another candidate is a network that contains counter neurons. Counter neurons count the number of circadian cycles; they do not activate (turn off) postsynaptic neurons when counting one cycle from the circadian clock but do (turn on) when signals for 2 days accumulate in them (Fig. 7.8b right). With this counter circuit, it is possible to produce other integral multiples of 24 h, such as 3- or 7-day rhythms.
When female bedbugs Cimex lectularius were fed on a 7-day cycle during juvenile development and allowed to feed and mate every 7 days after eclosion, the lysozyme-like activity gradually increased for the antibacterial immune response in the blood lymph in anticipation of mating in the next 7 days (Siva-Jothy et al. 2019). The male bedbug traumatically inseminates a freshly fed female, so it makes sense for the female to increase lysozyme activity prior to mating in order to prepare an immune response to bacteria entering the female’s body during insemination. This report suggests that bedbugs learn the 7-day cycle of feeding and mating to produce a 7-day cycle of immune activity. This may involve some mechanism to learn an integral multiple of days and might support the presence of the clock-counter system.
6 Concluding Remarks
Several physiological data suggest the involvement of the circadian clock in circabidian rhythm. However, molecular evidence is missing. In the near future, oscillation patterns of circadian clock genes should be clarified, and their function in circabidian rhythm should be examined. Furthermore, the mechanism underlying clock cycle multiplication is fascinating. Using neuroanatomy and molecular genetics, it should be clarified whether the doubling of the circadian clock cycle occurs intracellularly or intercellularly in the brain.
Another important question is the ecological significance of the circabidian rhythm. In general, a reduction in the number of appearance days is unfavorable for feeding and mating opportunities. If predation pressure is high, it may make sense to reduce the number of emergence days. However, H. parallela remains on tree leaves during the night, and no active predators are known against H. parallela and H. picea. Therefore, it is unclear why these species exhibit circabidian rhythms. It is possible that some radical environmental changes have caused unfavorable conditions in a restricted region or era, and this may have benefited beetles in the Pedinotrichia group to emerge every 2 days, resulting in the appearance of the circabidian rhythm. However, if a few individuals return to the circadian rhythm when unfavorable environmental conditions disappear, beetles with circabidian rhythms may have lower reproductive fitness than those with circadian rhythms. Subsequently, the circabidian rhythm is lost. However, this rhythm has persisted in certain species, suggesting that the circabidian rhythm may have advantages that we are unaware of.
References
Anitha V, Rodgers DJ, Wightman J, Ward A (2006) Distribution and abundance of white grubs (Coleoptera: Scarabaeidae) on groundnut in southern India. Crop Prot 25:732–740. https://doi.org/10.1016/j.cropro.2005.10.001
Aschoff J, Gerecke U, Wever R (1967) Desynchronization of human circadian rhythms. Jpn J Physiol 17:450–457. https://doi.org/10.2170/jjphysiol.17.450
Benstaali C, Mailloux A, Bogdan A, Auzéby A, Touitou Y (2001) Circadian rhythms of body temperature and motor activity in rodents their relationships with the light-dark cycle. Life Sci 68:2645–2656. https://doi.org/10.1016/s0024-3205(01)01081-5
Cavallari N, Frigato E, Vallone D, Fröhlich N, Lopez-Olmeda JF, Foà S et al (2011) A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol 9:e1001142. https://doi.org/10.1371/journal.pbio.1001142
Cavanaugh DJ, Geratowski JD, Wooltorton JRA, Spaethling JM, Hector CE, Zheng X et al (2014) Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell 157:689–701. https://doi.org/10.1016/j.cell.2014.02.024
Clopton JR (1984) Mosquito circadian and circa-bi-dian flight rhythms: a two oscillator model. J Comp Physiol A 155:1–12. https://doi.org/10.1007/BF00610925
Clopton JR (1985) Circa-bi-dian rhythmicity in the flight activity of the mosquito Culiseta incidens. Comp Biochem Physiol 80:469–475. https://doi.org/10.1016/0300-9629(85)90399-8
Colin J, Timbal J, Boutelier C, Houdas Y, Siffre M (1968) Rhythm of the rectal temperature during a 6-month free-running experiment. J Appl Physiol 25:170–176. https://doi.org/10.1152/jappl.1968.25.2.170
Cornelissen G, Broda H, Halberg F (1986) Does Gonyaulax polyedra measure a week? Cell Biophys 8:69–85. https://doi.org/10.1007/BF02788461
DeCoursey PJ, Krulas JR, Mele G, Holley DC (1997) Circadian performance of suprachiasmatic nuclei (SCN)-lesioned antelope ground squirrels in a desert enclosure. Physiol Behav 62:1099–1108. https://doi.org/10.1016/S0031-9384(97)00263-1
DeCoursey PJ, Walker JK, Smith SA (2000) A circadian pacemaker in free-living chipmunks: essential for survival? J Comp Physiol A 186:169–180. https://doi.org/10.1007/s003590050017
Dunlap JC, Loros JJ, Decoursey PJ (2004) Chronobiology: biological timekeeping. Sinauer, Massachusetts
Frisch B, Fleissner G, Fleissner G, Brandes C, Hall JC (1996) Staining in the brain of Pachymorpha sexguttata mediated by an antibody against a Drosophila clock-gene product: labeling of cells with possible importance for the beetle’s circadian rhythms. Cell Tissue Res 286:411–429. https://doi.org/10.1007/s004410050711
Hamanaka Y, Lu Z, Shiga S (2022) Morphology and synaptic connections of pigment-dispersing factor-immunoreactive neurons projecting to the lateral protocerebrum in the large black chafer, Holotrichia parallela. J Comp Neurol 530:2994–3010. https://doi.org/10.1002/cne.253
Honma K, Honma S (1988) Circabidian rhythm: its appearance and disappearance in association with a bright light pulse. Experientia 44:981–983. https://doi.org/10.1007/BF01939893
Isojima Y, Nakajima M, Ukai H, Fujishima H, Yamada RG, Masumoto K et al (2009) CKIε/δ-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci U S A 106:15744–15749. https://doi.org/10.1073/pnas.0908733106
Jones M (1982) Coupled oscillators controlling circadian flight activity in the mosquito Culex pipiens quinquefasciatus. Physiol Entomol 7:281–289. https://doi.org/10.1111/j.1365-3032.1982.tb00301.x
Kawasaki Y, Nishimura H, Shiga S (2017) Plausible link between circa‘bi’dian activity rhythms and circadian clock systems in the large black chafer Holotrichia parallela. J Exp Biol 220:4024–4034. https://doi.org/10.1242/jeb.163253
Kenyon KE (1996) Bi-daily variation of meteorological properties at sea level across the Pacific along 35°N. Atmos Res 43:31–46. https://doi.org/10.1016/S0169-8095(96)00003-8
King AN, Sehgal A (2020) Molecular and circuit mechanisms mediating circadian clock output in the Drosophila brain. Eur J Neurosci 51:268–281. https://doi.org/10.1111/ejn.14092
Leal WS, Sawada M, Matsuyama S, Kuwahara Y, Hasegawa M (1993) Unusual periodicity of sex pheromone production in the large black chafer Holotrichia parallela. J Chem Ecol 19:1381–1391. https://doi.org/10.1007/BF00984883
Matsumoto T (2015a) Separation of Amphitrichia from Holotrichia (Scarabaeidae, Melolonthinae, Melolonthini) with description of a new species and new records of two known species. Kogane 17:11–17. (in Japanese) https://iss.ndl.go.jp/books/R100000002-I000000162723-00
Matsumoto T (2015b) Taxonomic notes on the genus Eotrichia Medvedev (Scarabaeidae, Melolonthinae, Melolonthini) with description of a new species and new records of two known species. Kogane 17:19–24. (in Japanese) https://iss.ndl.go.jp/books/R000000004-I026803277-00
Matsumoto T (2016) Three new genera of the subtribe Rhizotrogina (Scarabaeidae, Melolonthinae, Melolonthini). Kogane 18:5–14. (in Japanese) http://id.ndl.go.jp/bib/000000162723
Namiki S, Kanzaki R (2016) The neurobiological basis of orientation in insects: insights from the silkmoth mating dance. Curr Opin Insect Sci 15:16–26. https://doi.org/10.1016/j.cois.2016.02.009
Okada Y, Tomioka K, Chiba Y (1991) Circadian phase-response curves for light in nymphal and adult crickets, Gryllus bimaculatus. J Insect Physiol 37:583–590. https://doi.org/10.1016/0022-1910(91)90035-X
Olberg RM (1983) Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J Comp Physiol 152:297–307. https://doi.org/10.1007/BF00606236
Page T (1989) Masking in invertebrates. Chronobiol Int 6:13–11. https://doi.org/10.3109/07420528909059137
Pannella G, Macclintock C (1968) Biological and environmental rhythms reflected in molluscan shell growth. J Paleontol 42(S2):64–80. https://doi.org/10.1017/S0022336000061655
Patke A, Young MW, Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21:67–84. https://doi.org/10.1038/s41580-019-0179-2
Piccione G, Caola G, Refinetti R (2004) Feeble weekly rhythmicity in hematological, cardiovascular, and thermal parameters in the horse. Chronobiol Int 21:571–589. https://doi.org/10.1081/CBI-200026447
Pittendrigh CS (1960) Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol 25:159–184. https://doi.org/10.1101/SQB.1960.025.01.015
Reischig T, Stengl M (2003) Ectopic transplantation of the accessory medulla restores circadian locomotor rhythms in arrhythmic cockroaches (Leucophaea maderae). J Exp Biol 206:1877–1886. https://doi.org/10.1242/jeb.00373
Schweiger HG, Berger S, Kretschmer B, Mörler H, Halberg E, Sothern RB et al (1986) Evidence for a circaseptan and a circasemiseptan growth response to light/dark cycle shifts in nucleated and enucleated Acetabularia cells, respectively. Proc Natl Acad Sci U S A 83:8619–8623. https://doi.org/10.1073/pnas.83.22.8619
Shiga S, Numata H (2009) Roles of PER immunoreactive neurons in circadian rhythms and photoperiodism in the blow fly, Protophormia terraenovae. J Exp Biol 212:867–877. https://doi.org/10.1242/jeb.027003
Shiga S, Omura Y, Kawasaki Y, Watanabe K (2022) Phylogenetic separation of Holotrichia species (Insecta, Coleoptera, Scarabaeidae) exhibiting circadian rhythm and circa‘bi’dian rhythm. Zool Sci 39:227–235. https://doi.org/10.2108/zs210091
Siva-Jothy MT, Zhong W, Naylor R, Heaton L, Hentley W, Harney E (2019) Female bed bugs (Cimex lectularius L) anticipate the immunological consequences of traumatic insemination via feeding cues. Proc Natl Acad Sci U S A 116:14682–14687. https://doi.org/10.1073/pnas.1904539116
Thébault T, Chauvaud L, Clavier J, Fichez R, Morize E (2006) Evidence of a 2-day periodicity of striae formation in the tropical scallop Comptopallium radula using calcein marking. Mar Biol 149:247–267. https://doi.org/10.1007/s00227-005-0198-8
Tomioka K (1993) Analysis of coupling between optic lobe circadian pacemakers in the cricket Gryllus bimaculatus. J Comp Physiol A 172:401–408. https://doi.org/10.1007/BF00213522
Ward S, Moore C, Anitha V, Wightman J, Rogers DJ (2002) Identification of the sex pheromone of Holotrichia reynaudi. J Chem Ecol 28:515–522. https://doi.org/10.1023/a:1014535910946
Watanabe K, Shiga S (2020) The optic lobe-pars intercerebralis axis is involved in circa‘bi’dian rhythm of the large black chafer Holotrichia parallela. J Comp Physiol A 206(6):819–829. https://doi.org/10.1007/s00359-020-01440-8
Wever R (1979) The circadian system of man. Springer, New York
Yoshioka K, Yamasaki Y (1983) Ecology of Lachnosterna morosa Waterhouse. I Behavior of the time of appearance on the ground and oviposition of adult insects. Jpn J Appl Entomol Zool 27:52–54. (in Japanese). https://doi.org/10.1303/jjaez.27.52
Acknowledgment
The author thanks Mr. Issei Nakagawa and Mr. Kohei Watanabe at Osaka University for providing the data and photo.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shiga, S. (2023). Circabidian Rhythm. In: Numata, H., Tomioka, K. (eds) Insect Chronobiology. Entomology Monographs. Springer, Singapore. https://doi.org/10.1007/978-981-99-0726-7_7
Download citation
DOI: https://doi.org/10.1007/978-981-99-0726-7_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0725-0
Online ISBN: 978-981-99-0726-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)