Abstract
The large black chafer Holotrichia parallela exhibits ~ 48-h circa’bi’dian rhythm. Although circabidian rhythm is suggested to involve the circadian clock, no physiological studies have been conducted to verify this involvement. We examined the effects of optic lobe or pars intercerebralis removal on the circabidian rhythm. After removing both optic lobes, all beetles lost their circabidian rhythms (N = 25), but all beetles exhibited circabidian rhythm after removing unilateral optic lobe (N = 18). However, 22% of the latter group exhibited day switching. After removal of the pars intercerebralis, 26.3% beetles showed arrhythmic patterns (N = 19). The number of paraldehyde fuchsin-stained pars intercerebralis cells in the arrhythmic group was significantly reduced compared to in the intact and sham-operated groups. The activity in the pars intercerebralis-removed beetles was significantly higher than that in the control groups. The results show that the optic lobe and at least part of the pars intercerebralis are necessary for circabidian rhythm, and bilateral optic lobes are necessary to maintain regularity of the two-day rhythm in H. parallela. This suggests that a neural circuit of circadian clock cells in the optic lobe to pars lateralis might be evolutionally conserved and used also for the generation of circabidian rhythm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most organisms have endogenous biological clocks entrained by various regular environmental cues, such as solar, tidal, and lunar cycles (Numata and Helm 2014). Endogenous circadian rhythms are driven by the 24-h circadian clock (Dunlap et al. 1999). Surprisingly, the large black chafer Holotrichia parallela (Coleoptera: Scarabaeidae; previously Lachnosterna morosa) appears every two nights in the field (Yoshioka and Yamazaki 1983). Adult beetles emerge from the soil around sunset to feed and mate on deciduous trees, and burrow back into the soil at sunrise. However, they typically remain within the soil the following day (Kawasaki et al. 2017). This two-day rhythm is also clearly observed under 12 h light and 12 h dark (12L:12D) conditions in the laboratory; moreover, this activity rhythm continues with a period of about 48 h even under constant darkness and is called “circa‘bi’dian rhythm” (Kawasaki et al. 2017). Physical cues on a two-day cycle are not known in the environment; thus, the biological significance and mechanism of this beetle’s rhythm are unknown. The phase responses of the rhythm to light pulses suggest that a driving oscillator for circabidian rhythm uses the 24-h circadian clock, and there may be a mechanism by which two cycles of the circadian clock are counted to produce a circabidian rhythm (Kawasaki et al. 2017).
If circabidian rhythm disappears after elimination of the circadian clock, this would support the idea that the mechanism underlying circabidian rhythm employs the circadian clock. In this study, we focus on the circadian clock region of the brain. In Drosophila melanogaster, circadian clock cells containing the clock protein PERIOD and driving locomotor activity rhythm are present near the accessory medulla of the optic lobe (OL) (Helfrich-Förster 1995; Grima et al. 2004; Stoleru et al. 2005). OLs are known to be the location of the circadian clock in many other species as well (Helfrich-Förster 2005). In Anthia sexguttata (Coleoptera: Scarabaeidae), disappearance of the retinal potential rhythm after removal of the OLs showed that the circadian clock is present in the OL (Fleissner 1982). PERIOD-immunoreactive cells also exist near the accessory medulla in Pachnoda marginata (Coleoptera: Scarabaeidae; Závodská et al. 2003). These studies strongly suggest that circadian clock cells are located in the OLs of scarabs.
Another area of the brain that controls circadian rhythm is the pars intercerebralis (PI). Ablation of the PI caused arrhythmicity in circadian locomotor rhythm in the cockroaches Rhypharobia maderae and Periplaneta americana, and in the house cricket Acheta domesticus (Nishiitsutsuji-Uwo et al. 1967; Cymborowski 1973). Currently, the PI is not considered the clock itself, but as an output route of circadian rhythm. In D. melanogaster, PI cells are connected from the clock cells, including cells in the OL, through a polysynaptic circuit (Cavanaugh et al. 2014). Neuropeptides have been identified as circadian output molecules that are specifically expressed by PI neurons and required for locomotor activity rhythms (Cavanaugh et al. 2014; King and Sehgal 2020). The importance of the PI in the output of the activity rhythm has also been shown in the mangrove cricket, Apteronemobius asahinai. Their activity rhythm has a circatidal component (about 12.4 h) entraining to tidal cycles, and a circadian component entraining to light–dark cycles (Satoh et al. 2008). Removal of the OL or knockdown of circadian clock genes eliminated the circadian component but did not affect the circatidal component, suggesting that the circadian and circatidal clocks are separate and located in different regions of the brain (Takekata et al. 2012, 2014a, b). Further, removal experiments have suggested that the PI determines which clock should dominate over the other to control behavioral rhythm (Takekata et al. 2018). Therefore, it is possible that the PI modifies the clock output to drive the two-day rhythm of H. parallela.
In this study, we hypothesized that a neural circuit of circadian clock cells between the OL and PI may generate circabidian rhythm in H. parallela. To test this, we surgically removed the OL and PI regions of adult H. parallela to observe the effects on circabidian rhythm. Circabidian rhythm disappeared after bilateral removal of the OL and partial removal of the PI cells also caused arrhythmicity in some individuals.
Materials and methods
Insects
Adults of H. parallela (Motschulsky 1854) were collected on riverbeds of the Yamato River (34° 35′ 14″ N, 135° 30′ 16″ E) and Ina River (34° 78′ 62″ N, 135° 42′ 61″ E) in Osaka, Japan between July and September of 2017, 2018, and 2019. Collected beetles were kept in the laboratory being exposed to natural photoperiod through the window at room temperature 13–26 °C until use. Only male beetles were collected using H. parallela sex pheromone and used for the experiments (Leal et al. 1992; Kawasaki et al. 2017).
Surgical removal of the optic lobes and pars intercerebralis
Before surgery, beetles were anesthetized with CO2 for 1 min, then promptly mounted in a sealing compound (Apiezon; M&I Materials, Manchester, UK) with the dorsal surface of the head exposed. For surgery, a plastic container with a lid (11 cm diameter; 4.5 cm depth) was used as a CO2 chamber. A hole (1 cm2) was made in the side wall of the container to introduce CO2 using a silicon tube, and a second hole (1 cm2) was made in the lid for viewing. The beetle was set in the CO2 chamber with the dorsal surface of the head exposed under the stereoscope through the lid hole. CO2 was continuously introduced to the chamber during operation (0.05 MPa).
To remove the OLs, a small area of cuticle was cut out bilaterally or unilaterally on the dorsum of the head just medial to the compound eyes using a scalpel, and the OL was removed using forceps under a stereoscopic microscope. The cuticle holes were dusted lightly with a few pieces of penicillin powder (023-07731; Wako., Tokyo, Japan) to prevent microbial infection, and the piece of cut cuticle was replaced and sealed on using dental wax (melting point, 59.3 °C; GC Corp., Tokyo, Japan). Sham operations were performed for bilateral or unilateral OL removal in which all procedures were the same except for OL removal. Intact beetles (without operation) were also included as controls.
PI removal was performed in the same manner as in the OL removal regarding anesthetization, cuticle incision, and cuticle closing. To remove the PI, a hole was cut in the cuticle at the mid dorsal region of the head. A region between the bilateral mushroom body calyces was removed to about 100 µm depth using a tungsten needle. In the sham operation group, the dorsal region of one mushroom body calyx was scraped off to about 100 µm depth from the brain surface.
After surgery, each beetle was placed individually in a container (7.5 cm diameter; 4.5 cm depth) with wet cotton at 13–26 °C under a natural photoperiod to recover for 3 days. Then, beetles were placed in a recording chamber. Bilateral OL removal was performed between August and December 2017. Unilateral OL removal was performed between July and October in 2018 and 2019. PI removal was conducted mainly between August and October in 2018 and between July and September in 2019.
Activity recording
An activity recording method was adopted from Kawasaki et al. (2017). Beetles were individually kept in cylindrical polystyrene containers (11.5 cm height; 7 cm diameter) with transparent glass plate lids. Two-thirds of the container were filled with leaf mold (Dorcus Owners Shop, Osaka, Japan) as soil and the side wall of each container was covered with aluminum foil to prevent light exposure to beetles under the soil. The light source was a fluorescent lamp (Panasonic FL15W; Panasonic, Osaka, Japan) with intensity of 1.35 W/m2. During the recording period, a leaf of the Japanese cherry Prunus yedoensis “Somei-yoshino” was provided as food every 3 days, and approximately 20–25 mL of water was sprayed on the soil surface when it dried.
Beetle activity on the soil surface was recorded under 12L:12D photoperiod for 6 days and subsequent constant darkness (DD) for 10 days at 25 ± 1 °C. For recording, a color image of the soil surface was captured every 1 min with a web camera (DC-NCR13U; Hanwha Japan, Tokyo, Japan) using LiveCapture3 freeware (https://lc3.daddysoffice.com/). The total pixel value in a fixed region covering one container area was calculated, and the pixel value difference between two serial images with 1-min interval was assessed for 6 min by a self-written program (Kawasaki et al. 2017). If the difference value was above a certain noise level at least three times during 6 min, the beetle was assigned “active” status (score of one). If the difference was detected in fewer than three, the beetle was assigned “inactive” status (score of zero).
The activity score of one or zero for each 6 min was plotted on an actogram, and the assessment was repeated every 6 min until the end of the 16 days. Total activity per 2 days, as well as average activity per 2 days (one cycle), during DD was calculated for each beetle. Presence of rhythmicity in the beetles’ activity was determined for the 10 days of DD using a Chi square periodogram analysis (Enright 1965; Sokolove and Bushell 1978), except for beetles with a day switching pattern.
Hematoxylin and eosin (HE) staining
After the activity recording, paraffin sections of the head were made for histological examination of the OL removal. The head of the beetle was fixed with aqueous Bouin’s fixative at 25 ± 4 °C overnight. The head was then washed in 70% ethanol, serially dehydrated in ethanol, incubated in xylene for about two weeks, and paraffin embedded. This long incubation in xylene was effective for paraffin penetration through the hard cuticle. After sectioning and deparaffinization, sections were stained with Mayer’s Hematoxylin solution (8650; Sakura Finetek Japan Co., Tokyo. Japan) for 5 min and eosin solution (8659; Sakura Finetek Japan Co., Tokyo. Japan) for 3 min.
Paraldehyde fuchsin (PAF) staining
To identify neurosecretory cells in the PI, we performed PAF staining. The staining method was adopted from Panov (1980) and Takekata (2018) with some modifications. Incubation in PAF solution was performed at 26 ± 2 °C for 30 min. The number of PAF-stained cells were counted per brain in a series of paraffin sections. We carefully compared adjacent sections and the same cell was excluded from counting.
Imaging of stained sections
Stained sections were photographed using a digital microscope camera (BX51 DP72; Olympus, Tokyo, Japan). We modified the contrast and/or brightness of the images to show the neuropil structures and cells clearly. Because of the large size of the brain, the photographs in Figs. 1, 3 were montaged.
Effect of bilateral removal of the optic lobe (OL) on locomotor activity rhythm in adult Holotrichia parallela beetles. Representatives of aboveground appearance rhythm presented by a double-plotted actogram (left), associated periodogram analyses (center), and a photomicrograph of the OLs and central brain (right). Beetles were kept under LD 12:12 h (LD) for the first 6 days and under constant darkness (DD) for the subsequent 10 days. a, b In the intact (a) and sham-operated control (b), typical two-day rhythmicity with aboveground activities every other night is observed under LD, and the rhythmicity continues under DD as a circabidian rhythm. The lamina (La), medulla (Me), and lobula (Lo) are observed in the OL, and calyces (Ca) of the mushroom body in the posterior region of the protocerebrum. A notch was artificially made in the right optic lobe in b during the sectioning process (an asterisk). c After the removal of the bilateral OLs, this beetle showed an arrhythmic pattern. The lamina, medulla, and lobula were completely removed. d An example in which the medulla and lobula remained (black arrow heads) after the removal operation. A circabidian rhythm was observed in the last 4 days. All pictures are in the same scale. Scale bar, 500 μm
Results
For the three experiments conducted (bilateral optic lobe removal, unilateral optic lobe removal, and pars intercerebralis removal), further details of experimental procedures and behavior of each beetle are provided in Supporting Table 1.
Effect of OL removal on the appearance rhythm
In the intact group, most beetles (80%, N = 15) showed a clear circabidian activity rhythm under the DD period (Fig. 1a). The free-running period (τ) of intact individuals was 47.8 ± 0.5 h (mean ± SD, N = 12). The other intact beetles (20%) did not appear on the surface during the observation period, but it was confirmed after recording that they were all alive but seemed to be in a dormant state. These individuals were classified as having a “no appearance” pattern. Images in Fig. 1 show representative brain sections after behavioral recording. In intact individuals, the retina, three neuropil structures of the lamina, medulla, and lobula were found in the OL. In the central brain, calyces of the mushroom bodies were observed in the posterior dorsal protocerebrum.
In sham-operated beetles, the same brain structures—including the OL—were found, as in the intact beetles (Fig. 1b). The majority of sham beetles (86%, N = 14) exhibited a circabidian rhythm (τ = 47.7 ± 0.5 h, N = 12, Fig. 1b) and the proportion was not significantly different from that of the intact group (p > 0.05, Tukey’s multiple comparison test for proportions, Fig. 2a). The remaining sham beetles showed the no appearance pattern.
Effects of optic lobe (OL) removal on circabidian rhythm under constant darkness in adult Holotrichia parallela beetles. a Proportions of beetles showing no appearance, an arrhythmic and circabidian pattern are shown. The OL removal group includes only beetles for which the OLs were completely removed. A significant difference is detected in proportions of circabidian rhythm pattern with different letters (p < 0.01, Tukey’s multiple comparison test for proportions). b The effect of OL removal on activity level. The mean activity and SE are shown. There were no significant differences in activity levels between the experimental groups (p > 0.05, Tukey’s test)
In beetles with bilateral OLs removal, the OLs (including the lamina, medulla, and lobula) were verified to have been completely removed in 25 out of 27 individuals, but neuropil structures of the central brain were found as in the intact or sham operation groups (Fig. 1c). Twelve out of 25 beetles exhibited an arrhythmic pattern while eating and walking at random (Fig. 1c). Some of these beetles did not burrow into the soil and repeat activities and pauses on the soil surface, while others did not emerge from the soil until the middle of observation period. Thirteen of the 25 beetles showed no appearance pattern. The proportion of no appearance pattern increased significantly after bilateral OL removal compared to the sham groups (p < 0.05, Tukey’s multiple comparison test for proportions) but did not differ from the intact group (p > 0.05). In contrast to the control groups, no appearance beetles after OL removal were all vigorous and ate leaves when dug up after observation. In histological examination, no morphological differences were observed between beetles showing no appearance pattern and those showing an arrhythmic pattern. After complete bilateral removal of the OLs, no beetles showed circabidian rhythm (Fig. 2a). The average activity level in 2 days during DD (maximum five cycles in 10 days) was not significantly different among intact, sham, and OL removal groups (Fig. 2b).
Of the remaining two beetles of which OL was incompletely removed, one did not appear in the first 8 days but showed circabidian-like activity in the last 8 days and did not burrow back into the soil. The histological examination of this beetle showed that a small region of the lobula remained. The other did not appear on the surface in the first 12 days but showed a complete circabidian rhythm only for the last 4 days. In this beetle, the medulla and lobula remained in the OL (Fig. 1d).
We performed unilateral removal of the OL to determine whether both the left and right OL are necessary for the two-day rhythm. We recorded activities in intact and sham-operated beetles for unilateral removal experiment. All control beetles exhibited circabidian rhythm (intact N = 14, sham N = 13, Figs. 3a, b, 4). Circabidian rhythm was also observed for all beetles in the unilateral OL removal group (N = 18), but within this group three different patterns were apparent (Fig. 3c−e). Thirty-nine percent of the beetles burrowed back into the soil during the rest period, as in the intact and sham groups (Fig. 3c), while 39% stayed on the surface during the rest period (Fig. 3d) and 22% changed the day of appearance (Figs. 3e, 4). One beetle appeared on the 2nd, 4th and 6th day (even days) during the first six LD cycles; however, at the first DD cycle, it rested for whole 2 days and appeared on the 9th day (an odd day) followed by odd day appearance for the last 6 days (Fig. 3e). Another beetle changed appearance day after unilateral OL removal to appear in two continuous nights of LD conditions and temporarily behaved as though exhibiting a circadian rhythm (not shown). These patterns were defined as “day switching.” Although periodogram analysis for the total constant dark period was not applicable in one beetle for less numbers of days, we categorized this beetle into the circabidian pattern because clear two-day rhythmicity was observed in the actogram before and after switching by eye inspection. In the histological examination of the unilateral removal group, the OL was completely removed in either side. However, no noticeable difference was found in the brain structure among three patterns after unilateral OL removal (Fig. 3).
Effect of unilateral removal of the optic lobe (OL) on locomotor activity rhythm in adult Holotrichia parallela beetles. Representatives of appearance rhythm on the ground presented by a double-plotted actogram (left), associated periodogram analyses (center), and photomicrographs of the OLs and central brain (right). a, b In the intact (a) and sham-operated controls (b), typical two-day rhythmicity and intact brain structures were observed. c–e Three representatives after unilateral OL removal. All beetles showed circabidian rhythm, but they were classified into regular circabidian (c), circabidian with no burrowing into the soil (d), and circabidian with day switching pattern (e). The lamina, medulla, and lobula of these beetles were completely unilaterally removed. All pictures are in the same scale. Scale bar, 500 μm
Effect of PI removal on the appearance rhythm
Many PAF-stained cells were distributed in the PI, a posterior dorsal region between the mushroom body calyces (Fig. 5). These cells were located in a depth of 100–130 μm from the brain surface. Cell diameters were 17.28 ± 2.23 μm (mean ± SD, n cells = 286, N = 3).
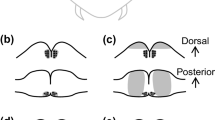
Distribution of paraldehyde fuchsin (PAF)-stained pars intercerebralis cells in adult Holotrichia parallela beetles. a–c Representative photomicrographs of PAF-stained cells in the brain. a, b In the horizontal section, PAF-stained cells (black arrow heads) were widely distributed from the midline of the brain to the medial edge of the mushroom body calyx. c In a frontal section, stained cells were found from the posterior dorsal surface to a depth of 100–130 μm. d Brain diagrams showing PAF cells. Scale bars, 100 µm. b and c are in the same scale. A., anterior; D., dorsal
In the intact (N = 15) and sham groups (N = 12), all beetles showed a clear circabidian activity rhythm under DD through the experimental period (Fig. 6a, b, 7a). We examined PAF-stained cells in the PI after behavioral recording. There were 101.88 ± 22.77 (mean ± SD, N = 8) PAF-stained PI cells in the intact and 88.67 ± 15.73 (N = 6) cells in the sham group (Fig. 7b).
Effect of pars intercerebralis (PI) removal on locomotor activity rhythm in adult beetle Holotrichia parallela. Representatives of appearance rhythm (left), associated periodogram analyses (center), and photomicrographs of the paraldehyde fuchsin (PAF)-stained PI cells (black arrow heads) in the dorsal view in a–e or another periodogram analysis in f (right). a, b In the intact (a) and sham (b) groups, typical two-day rhythmicity with activity on the surface was observed, and PI cells were stained. c–f Activity rhythms in beetles with the PI removed. c Circabidian rhythm pattern similar to the control beetles. Many PAF-stained PI cells remained. d A beetle showing a high activity level with rhythmicity. e A beetle showing an arrhythmic pattern. Less PI cells were found. f Another beetle showing arrhythmic pattern during DD. Left periodogram is from DD (arrhythmic) and right periodogram from the first 8 days (6LD and 2DD cycles, rhythmic). Circadian periodicity was detected in the first half period. All pictures are in the same scale. Scale bar, 50 μm
Effects of pars intercerebralis (PI) removal on rhythmicity and activity levels in adult Holotrichia parallela beetles. a Proportions of beetles showing arrhythmic and circabidian rhythm patterns. In the PI removal group, 26.3% showed arrhythmic pattern. There were no significant differences in the proportions of beetles with circabidian rhythm among the three groups (p > 0.05, Tukey’s multiple comparison test for proportions). b The mean number and SE of paraldehyde fuchsin-stained PI cells remaining. Arrhythmic beetles in the PI removal group had significantly reduced cell numbers compared to the control groups (p < 0.05, Tukey test). c The average activity level per 2 days for arrhythmic beetles and rhythmic beetles in the PI removal group were significantly higher than that in the other control groups, and in the PI removal group the activity amounts of arrhythmic beetles were higher than those of rhythmic beetles (p < 0.05, Steel–Dwass test)
After removal of the PI (N = 19), 73.7% of beetles showed circabidian rhythm, while the remaining 26.3% showed an arrhythmic pattern (Fig. 6c–f, 7a). However, there were no significant differences in the proportion of circabidian rhythm patterns among the intact, sham, and PI removal groups (Fig. 7a). Histological examination revealed that PAF-stained cells partly remained in the PI removal group (Fig. 6c–e). In the PI removal group, 68.5 ± 23.36 cells remained in beetles showing circabidian rhythm (N = 14), and 39.6 ± 34.3 cells in arrhythmic beetles (N = 5). The number of PAF-stained cells in PI-removed arrhythmic beetles was significantly reduced compared to the control groups (Fig. 7b, p < 0.05, Tukey’s test).
In beetles showing the circabidian pattern (N = 14) after PI removal, 64.3% showed a regular circabidian rhythm. However, beetles sometimes appeared briefly on the surface during the rest period, which was not often observed in the control groups (Fig. 6c). The other 35.7% of beetles showed relatively high activity levels (> 200 activities per two days, Fig. 6d, 7c). Although their actogram was partly similar to the arrhythmic pattern, the periodogram analysis detected significant rhythmicity. Arrhythmic beetles moved and ate randomly through the day and their activity levels were significantly higher compared to the control and PI removed-circabidian groups (Fig. 6e, f, 7c). In one beetle, the actogram seemed to contain a 24-h rhythm component; periodogram analysis of the first 8 days (6 LD cycles and 2 DD cycles) of recording detected circadian periodicity of 24.1 h (Fig. 6f, right periodogram). However, this beetle was grouped in the arrhythmic pattern because a significant peak of the periodogram was not detected during DD (Fig. 6f, left periodogram).
During LD cycles, intact and sham-operated beetles emerged on the surface at lights-off (Fig. 6a, b). Among 14 beetles showing circabidian patterns in the PI removal group, 5 beetles appeared before lights-off (Fig. 6c, sTable 1). Two beetles exhibiting circabidian rhythm changed activity phase dramatically such that they seemed diurnal during the LD period (Fig. 6d, sTable 1). The beetle in Fig. 6f was arrhythmic in DD but its behavior in LD also appeared to be diurnal.
PI-removed arrhythmic beetles showed higher activity levels than PI-removed beetles with circabidian rhythm, intact, and sham-operated beetles (Fig. 7c, p < 0.05, Steel–Dwass test). When we compared the activities and the number of remaining PAF-stained PI cells, no significant correlation was detected (R2 = 0.103).
Discussion
Role of the OL in circabidian rhythm
All beetles with complete bilateral OL removal lost circabidian rhythm, indicating that the OLs are necessary for circabidian rhythm. No significant differences in total activities per cycle were detected among the intact, sham, and OL removal groups; therefore, brain surgery caused no significant damage to the locomotor activity itself. Maintenance of circabidian rhythmicity in beetles with unilateral OL removal suggests that circabidian rhythm can be produced with either left or right OL, although both OLs seem to be necessary for the regularity of the two-day cycle.
How does the OL contribute to circabidian rhythm? The most conceivable idea is that a circadian clock located in the OL provides time information, producing circabidian rhythm. In many insect species, it is reported that circadian clocks controlling activity are located near the accessory medulla of the OL (Helfrich-Förster 2005). In the Goldsmith beetle Pachnoda marginata from the same family as H. parallela (Scarabaeidae), circadian clock protein PERIOD-immunoreactive cells exist near the accessory medulla (Závodská et al. 2003), suggesting that H. parallela also have circadian clock cells in the OL. To confirm this hypothesis, clock cell location and oscillation cycle of period expression in the brain of H. parallela should be examined. In a previous study of H. parallela, the phase responses of the circabidian rhythm to light pulses suggest that a circabidian cycle is composed of two cycles of the circadian oscillator. In addition, a few beetles temporarily showed circadian-like activity (Kawasaki et al. 2017). Our results therefore support the idea of Kawasaki et al. (2017) that the circadian clock is involved in producing circabidian rhythm.
After the bilateral removal of OLs, the proportion of beetles with no appearance patterns (those which remained within the soil for the entire period) increased (Fig. 2a). However, these beetles were very active if purposely removed from the soil and placed on the surface. This contrasts with no appearance beetles in sham and intact groups, which remained inactive even when placed on the surface. We suppose that factors causing no appearance are different between the control (intact and sham) and OL removal groups. In the intact and sham groups, 20% and 15% showed no appearance, respectively, and might remained in a dormant state. This may be related to the period when we conducted the experiment (conducted in 2017 including November and December). Kawasaki et al. (2017) reported that H. parallela are most active in June and July, and very few are found in the field in September or later. The late season might have caused these beetles to become dormant for overwintering and thus remain within the soil during the experiment recording period.
The no appearance pattern could be caused by the suppression of burrowing and emergence behavior; many beetles did not burrow into the soil after bilateral or unilateral OL removal. These results suggest that OLs might be involved in vertical locomotion. According to Tokuda et al. (2010), the white grub beetle Dasylepida ishigakiensis shows a standby behavior before emerging from the soil at sunset. The beetle stays just below the surface and uses changes in luminance to decide when to emerge. The timing of this behavior suggests that it is under the control of a circadian clock (Tokuda et al. 2010). Standby behavior is also observed in intact H. parallela (Kawasaki and Shiga, unpublished). Upon the loss of the OL (a potential clock site), the standby behavior might not be initiated, resulting in a lack of emergence. It is also possible that interruptions in light signal pathways from the compound eyes caused by the absence of the OL gave the beetle no information on when to emerge or burrow. It is reported in D. ishigakiensis that an environmental time cue and illumination are required for burrowing (Tokuda et al. 2010). H. parallela move away from trees to burrow underground at sunrise (Kawasaki et al. 2017). These reports suggest the importance of time or light information for vertical locomotion. By removing the OLs, loss of timing signals from the circadian clock or light signal pathways to the brain may keep the beetles aboveground or underground throughout the day. Future studies should record beetle activities underground after OL removal. In this study, the individuals that did appear aboveground were found to have lost their circabidian rhythm.
After unilateral OL removal, 22% of beetles showed day-switching behavior in which the day of appearance switched from even to odd days counted from the first observed active day. In the field, day switching was observed in 9.1% (N = 142) of marked H. parallela beetles (Kawasaki et al. 2017). This was observed after heavy rain, suggesting that there is some physiological mechanism inducing day switching to adjust the day of appearance to occur with favorable conditions. Single optic lobe-driven day-switching may implicate weakening connections or decoupling between right and left OL circadian clock as a mechanism. In species, such as the cockroach R. maderae (previously Leucophaea maderae) and the cricket Gryllus bimaculatus, clock cells in the left and right OLs possess neural communication to allow synchronization (Page 1983; Tomioka 1993). The coupling between both optic lobe circadian clocks can be responsible for the 24-h oscillation, and decoupling of bilateral clocks or a solitary clock may weaken 24-h oscillation to cause irregular circabidian rhythm, that is, day switching. It is possible that clock coupling between the two OLs is necessary for the robustness of the 48-h rhythmic cycle. When only one OL is present, the clock coupling between the two OLs is lost, and subsequent weakening of the circadian oscillation may cause counting a day to be skipped, resulting in day switching. Such mutual communication to synchronize bilateral clock systems might be related to the regularity of the two-day cycle.
Roles of the PI in circabidian rhythm
In the PI removal group, 26.3% of beetles lost their circabidian rhythm and the number of PAF-stained PI cells in these beetles was significantly reduced compared to those of the control groups. In addition, about a half of beetles including all arrhythmic beetles showed a significant increase in activity compared to all control groups. These results suggest that a part of the PAF-stained PI cells is necessary for circabidian rhythm and suppression or regulation of the activity level. In D. melanogaster, polysynaptic inputs from circadian clock cells to PI neurons are reported as a circadian output pathway to the locomotor activity rhythm (Cavanaugh et al. 2014), suggesting that the removed PI cells may also include cells in charge of conveying the clock output to the locomotory system in H. parallela.
The PI is known as a neurosecretory center in which different kinds of cells are located in a cluster (Shiga 2003; Cavanaugh et al. 2014). A part of the PI cells is probably involved in circabidian rhythm in H. parallela, and it is possible that only beetles that receive serious damage to these cells lose this rhythm. In D. melanogaster, the expression of neuropeptides diuretic hormone 44 (DH44) and SIFamide (SIFa) in different PI cells are important for rest:activity rhythm (Cavanaugh et al. 2014). The effects of the selective activation or ablation of just six DH44-expressing PI cells especially cause arrhythmicity. Importantly, it was reported that the clock cell function is unaltered following PI cell ablation, indicating that the PI cells come after clock cells in the pathway (Cavanaugh et al. 2014). In addition, certain types of PI cells, such as DH44 or SIFamide cells, might be responsible for the output of circabidian rhythm in H. parallela.
Increased activity was also reported after removal of the PI in the cockroaches R. maderae and P. americana and the cricket A. domesticus (Nishiitsutsuji-Uwo et al. 1967; Cymborowski 1973). These insects lost their circadian rhythms and showed an arrhythmic pattern with a high activity level. In contrast, in another study on P. americana, PI-removed cockroaches lost their rhythmic locomotor activity without an increase in activity, suggesting that the activity suppressor and neurons responsible for rhythmicity exist independently in P. americana (Matsui et al. 2009). In H. parallela, some PI-removed beetles showed arrhythmic pattern with high activity, but others showed an increase in activity while maintaining their circabidian rhythm (Fig. 7c). The latter can be caused by the removal of only the activity suppressor, which is not involved in the output route of activity rhythm in accordance with Matsui et al. (2009) about P. americana. Since there was no relationship between activity and the number of remaining PAF-stained PI cells, not all PAF-stained PI cells are activity related.
Kawasaki et al. (2017) suggested that circabidian rhythm could be generated by the circadian clock alongside a counter mechanism in H. parallela. One behavioral output signal might be produced every two circadian clock cycles. Although we have thus far no idea about how two circadian clock cycles are counted, some type of clock-counter mechanism is located in neural circuits involving the clock cells and behavioral command neurons. In the circuits, clock cells oscillate with a 24-h frequency, but neurons responsible for behavioral output must exhibit command every 48-h to promote activity. We hypothesize that there is some mechanism doubling clock cycles, located in the circuit between the clock cells and behavioral command neurons. We call such a doubling mechanism “a counter.” Because our results suggest that the PI is partially important for circabidian rhythm output, it would be of interest to examine electrical activity or gene expression rhythm in PI cells. Neurons responsible for the counter might be present in the PI. In future, it will be necessary to identify clock cells and PI cells associated with circabidian rhythm to locate the clock-counter circuit.
References
Cavanaugh DJ, Geratowski JD, Wooltorton JRA, Spaethling JM, Hector CE, Zheng X, Johnson EC, Eberwine JH, Sehgal A (2014) Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell 157:689–701
Cymborowski B (1973) Control of the circadian rhythm of locomotor activity in the house cricket. J Insect Physiol 19:1423–1440
Dunlap JC, Loros JJ, Liu Y, Crosthwaite SK (1999) Eukaryotic circadian systems: cycles in common. Genes Cells 4:1–10
Enright JT (1965) The search for rhythmicity in biological time-series. J Theor Biol 8:426–468
Fleissner G (1982) Isolation of an insect circadian clock. J Comp Physiol 149:311–316
Grima B, Chélot E, Xia R, Rouyer F (2004) Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431:869–873
Helfrich-Förster C (1995) The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA 92:612–616
Helfrich-Förster C, Stengl M, Homberg U (2005) Organization of endogenous clocks in insects. Biochem Soc T 33:957–961
Kawasaki Y, Nishimura H, Shiga S (2017) Plausible link between circa‘bi’dian activity rhythms and circadian clock systems in the large black chafer Holotrichia parallela. J Exp Biol 220:4024–4034
King AN, Sehgal A (2020) Molecular and circuit mechanisms mediating circadian clock output in the Drosophila brain. Eur J Neurosci 51:268–281
Leal WS, Matsuyama S, Kuwahara Y, Wakamura S, Hasegawa M (1992) An amino acid derivative as the sex pheromone of a scarab beetle. Naturwissenschaften 79:184–185
Matsui T, Matsumoto T, Ichihara N, Sakai T, Satake H, Watari Y, Takeda M (2009) The pars intercerebralis as a modulator of locomotor rhythms and feeding in the American cockroach, Periplaneta americana. Physiol Behav 96:548–556
Nishiitsutsuji-Uwo J, Petropulos SF, Pittendrigh CS (1967) Central nervous system control of circadian rhythmicity in the cockroach. I. Role of the pars intercerebralis. Biol Bull 133:679–696
Numata H, Helm B (2014) Annual, lunar, and tidal clocks: patterns and mechanisms of nature’s enigmatic rhythms. Springer, Berlin, Germany
Page TL (1983) Effects of optic-tract regeneration on internal coupling in the circadian system of the cockroach. J Comp Physiol 153:353–363
Satoh A, Yoshioka E, Numata H (2008) Circatidal activity rhythm in the mangrove cricket Apteronemobius asahinai. Biol Lett 4:233–236
Shiga S (2003) Anatomy and functions of brain neurosecretory cells in Diptera. Microsc Res Tech 62:114–131
Sokolove PG, Bushell WN (1978) The Chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol 72:131–160
Stoleru D, Peng Y, Nawathean P, Rosbash M (2005) A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438:238–242
Takekata H, Matsuura Y, Goto SG, Satoh A, Numata H (2012) RNAi of the circadian clock gene period disrupts the circadian rhythm but not the circatidal rhythm in the mangrove cricket. Biol Lett 8:488–491
Takekata H, Numata H, Shiga S (2014a) The circatidal rhythm persists without the optic lobe in the mangrove cricket Apteronemobius asahinai. J Biol Rhythms 29:28–37
Takekata H, Numata H, Shiga S, Goto SG (2014b) Silencing the circadian clock gene Clock using RNAi reveals dissociation of the circatidal clock from the circadian clock in the mangrove cricket. J Insect Physiol 68:16–22
Takekata H, Numata H, Shiga S (2018) Effects of pars intercerebralis removal on circatidal rhythm in the mangrove cricket, Apteronemobius asahinai. J Comp Physiol A 204:801–810
Tokuda M, Tanaka S, Maeno K, Harano KI, Wakamura S, Yasui H, Fukaya M (2010) A two-step mechanism controls the timing of behaviour leading to emergence from soil in adult males of the scarab beetle Dasylepida ishigakiensis. Physiol Entomol 35:231–239
Tomioka K (1993) Analysis of coupling between optic lobe circadian pacemakers in the cricket Gryllus bimaculatus. J Comp Physiol A 172:401–408
Yoshioka K, Yamasaki Y (1983) Ecology of Lachnosterna morosa Waterhouse. I. Behaviour of the time of appearance on the ground and oviposition of adult beetle. Jpn J Appl Entomol Zool 27:52–54
Závodská R, Sauman I, Sehnal F (2003) Distribution of PER protein, pigment-dispersing hormone, prothoracicotropic hormone, and eclosion hormone in the cephalic nervous system of insects. J Biol Rhythms 18:106–120
Acknowledgements
The authors would like to thank Dr. Tetsuro Shinada of Osaka City University for the technical advice and use of equipment for pheromone synthesis and Dr. Lauren Des Marteaux at Osaka City University for reading the manuscript. This study was supported by the Yamada Science Foundation to S.S. The authors would like to thank Editage (www.editage.com) for English language editing.
Funding
This funding was provided by Japan Society for the Promotion of Science (Grant No. 19K22425)
Author information
Authors and Affiliations
Contributions
KW conducted all experiments and wrote the manuscript. SS made the experimental design and wrote the manuscript. All the authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Watanabe, K., Shiga, S. The optic lobe–pars intercerebralis axis is involved in circa’bi’dian rhythm of the large black chafer Holotrichia parallela. J Comp Physiol A 206, 819–829 (2020). https://doi.org/10.1007/s00359-020-01440-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-020-01440-8