Abstract
Although revascularization surgery in patients with moyamoya disease improves cerebral hemodynamics and symptoms, its benefits are reduced by perioperative complications through either transient or permanent neurological deficits. Apart from hyperperfusion syndrome, perioperative complications associated with revascularization surgery have been described in the literature and our clinical experience. Typical perioperative complications include ischemic stroke caused by hemodynamic insufficiency, which characteristically occurs in the advanced stages of moyamoya disease. Moreover, the progression of the main cerebral artery occlusion induces cerebral ischemia far from the surgery site. Intracranial hemorrhage associated with postoperative hyperperfusion is the most severe adverse effect after direct anastomosis. Other known and critical postoperative complications include skin necrosis, anastomotic site aneurysm, and arteriovenous shunt formation. Understanding the various complications and their risk factors could contribute toward lowering the perioperative complication rate in revascularization surgery, as well as improving the long-term outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
- Moyamoya disease
- Revascularization
- Complications
- Cerebral infarction
- Intracranial hemorrhage
- Skin trouble
1 Background
Moyamoya disease is a progressive steno-occlusive cerebrovascular disease that is characterized by collateral vascular networks that resemble “a puff of smoke” (moyamoya vessels) at the brain base [1, 2]. Various revascularization procedures can improve cerebral hemodynamics and decrease the ischemic stroke risk. However, hemodynamic compromises and fragile collateral arteries increase the risk of postoperative neurological morbidity. Furthermore, revascularization-specific surgical procedures for moyamoya disease are associated with rare but critical complications. It is important to understand the various perioperative complications and the underlying mechanisms in order to improve patient management and maximize the benefit of revascularization surgery in stroke prevention.
2 Frequency and Pathophysiology of Perioperative Complications
2.1 Classification
Perioperative complications result from a combination of surgical procedures and hemodynamic insufficiency, as well as the fragility of collateral arteries at the brain base. Based on the main underlying mechanisms, complications were characterized as disease- or procedure-related complications. Typical disease-related complications are perioperative ischemic/hemorrhagic stroke, while typical procedure-related complications include skin trouble, hyperperfusion, graft spasm/occlusion, and postoperative intracranial hematoma.
3 Perioperative Stroke
Perioperative stroke is generally indicative of either infarction or intracranial hemorrhage (ICH), intraventricular hemorrhage, and subarachnoid hemorrhage, which develop intraoperatively or within 4 postoperative weeks. Hyperperfusion is mostly observed in adult moyamoya disease and can cause intracerebral hemorrhage or seizures. Hyperperfusion diagnosis largely depends on the definition and frequency of radiological studies.
3.1 Ischemic Complications
In anesthetic management, intraoperative hypocapnia induces a critical decrease in cerebral blood flow (CBF). Moreover, crying is known to induce hyperventilation and stroke during the perioperative period in children [3, 4]. Furthermore, hemodynamic compromise is aggravated by blood loss, decreased circulating volume, and low blood pressure [5, 6].
Previous study reported angiographic outcome of direct/combined bypass as excellent in 57.5% (95% CI; 13.2–100%), good in 35.8% (95% CI; 12.5–70.3%), and poor in 6.7% (95%CI; 0–17.7%), respectively [7]. In indirect bypass, angiographic outcome was excellent in 37.9% (95% CI; 16.4–59.4%), good in 38.0% (95% CI; 23.5–50.8%), and poor in 24.1% (95%CI; 5.8–42.8%). Complication rate was reported as 5.4% (ischemic; 4.1%, hemorrhagic 1.3%) in combined direct/indirect surgery, whereas it was 5.5% (ischemic 5.2%, hemorrhagic; 0.27%) in indirect surgery. In a single university study, the reported postoperative stroke frequency is 4.7% per surgery (95% confidence interval [CI], 2.5–7.0) in direct/combined bypass [7]. Compared with indirect procedures, surgery involving direct anastomosis is technically demanding. However, performing direct anastomosis in both pediatric and adult patients may not increase ischemic complications. In adult patients, there was no significant difference in postoperative stroke between direct/combined bypass (7.6%) and indirect bypass (5.1%). Furthermore, in pediatric patients, perioperative stroke was significantly more frequent in indirect bypass (6.0%) than in direct/combined bypass (2.5%) (odds ratio [OR], 2.36; 95% CI, 1.48–3.76). Additionally, ischemic complications in indirect surgery have been reported in pediatric patients (7.1%) [8]. In combined direct/indirect surgery, postoperative stroke was more frequent (8.5%) in adults than in pediatric patients (1.1%) (OR, 8.29; 95% CI, 1.87–36.79) [7]. However, there is a need for special caution in children aged under 3 years and in surgery within 6 weeks of the most recent stroke [8].
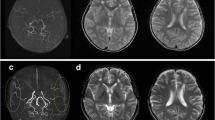
Postoperative cerebral infarction can occur remotely to craniotomy and in the contralateral hemisphere [9, 10]. Progressive occlusion in the main cerebral arteries following revascularization surgery could cause remote cerebral infarction [5, 11]. Since collateral vessel development (i.e., moyamoya vessels) is generally unremarkable in adults and moyamoya syndrome, the acute progression of main cerebral artery occlusion could induce severe consequences [12]. Specifically, in a combined direct/indirect procedure, acute occlusive changes in major cerebral arteries have been reported in approximately half of all postoperative ischemic strokes [7]. Out of 358 procedures, rapid progression (<1 postoperative month) has been observed in the anterior cerebral artery (n = 3), middle cerebral artery (n = 1), and posterior cerebral artery (n = 2)(Fig. 19.1 a-c) [7]. TIA for several postoperative months could be the underlying thromboembolic mechanism when radiological examination reveals the normalization of regional CBF.
Postoperative ischemic stroke in a 31-year-old patient with moyamoya syndrome after combined direct/indirect anastomosis. The bilateral posterior cerebral artery became diminutive after right revascularization surgery (a; preoperative MRA, b; postoperative MRA). Cerebral infarction appeared in the right temporo-occipital region (c)
Risk factors for ischemic stroke include anemia, preoperative frequent TIA, history of minor completed stroke, PCA involvement, and diabetes [6, 13, 14]. Fatal stroke can occur during the postoperative period [9]. A history of cerebral infarction is considered a risk factor for adverse outcomes. Furthermore, spontaneous/iatrogenic intracranial hemorrhage can increase intracranial pressure at a critical level, which causes severe low perfusion in the cerebral tissue and subsequently fatal stroke.
Moreover, ischemic stroke can occur during diagnostic procedures. The acetazolamide test for determining the perfusion reserve could result in critical adverse effects. In patients with severe hemodynamic compromise, the acetazolamide test further reduces CBF in the affected area (steal phenomenon). Therefore, especially in pediatric patients, this test should be avoided when there is a significant reduction in the resting-state CBF.
4 Hemorrhagic Complications
The frequency of postoperative ICH is 1.7–3.0% in revascularization surgery involving direct anastomosis [7, 9, 15]. Previous studies have reported postoperative hemorrhage at 4 hours and 10 days postoperatively [9]. The most frequent ICH onset timing was reported to be within 7 days or within 24 hours postoperatively [15, 16]. Hemorrhage can either occur at the subcortical lesion beneath the anastomosed cortex or as a subarachnoid hemorrhage. A previous study reported that half of the postoperative hemorrhage cases required hematoma evacuation. Untreated cerebral aneurysms can bleed during the postoperative acute phase. Moreover, ICH can occur far from the surgical site [15, 17]. Remote cerebral hemorrhage could involve postoperative hyperperfusion beyond the revascularization area, which is remarkable both in the medial frontal lobe and caudate head [18].
There is an association of postoperative ICH with older age [15]. Furthermore, hemorrhagic presentation at onset and increased blood pressure from the pre- to the postoperative stage are significantly associated with postoperative ICH [15].
4.1 Postoperative Hyperperfusion
Hyperperfusion is a frequent complication mostly observed in adult moyamoya disease that can cause intracerebral hemorrhage or seizures. Recent studies have reported frequent postoperative hyperperfusion [19, 20]. Details regarding hyperperfusion syndrome have been described in part IV, Chap. 16.
5 Procedure-Related Complications
Revascularization surgery involves the several steps of the indirect procedure (inverting the dura matter, suturing the temporalis muscle to the dura, and placing the pericranial flap), as well as harvesting of the scalp arteries. Skin necrosis, temporal muscle swelling, and subdural hematoma are the three most critical procedure-related complications.
-
(a)
Subdural hematoma
An acute subdural hematoma is the most critical complication after direct/combined and indirect surgery. In adults, brain atrophy and the preoperative administration of antiplatelet medication may increase the subdural hematoma risk. Previous studies have reported chronic subdural hematoma [21]; however, it is a rather rare complication of revascularization for moyamoya disease.
-
(b)
Temporal muscle swelling
Temporal muscle swelling causes brain compression [22, 23]. Increased intracranial pressure resulting from the inserted extracranial tissue can cause headache and ischemic brain injury (Fig. 19.2a–d). Temporal muscle swelling is observed when the drainage vein is sacrificed during extracranial work. Moreover, bleeding from the muscle undersurface is critical due to acute subdural hematoma.
-
(c)
Wound break down due to skin necrosis
Postoperative MRI shows temporal muscle swelling in the left frontal region (a). Postoperative magnetic resonance angiography revealed patency of the left superficial temporal artery––middle cerebral artery bypass (b). Moderate reduction of the regional cerebral blood flow was observed on 123I- N-isopropyl-p-[123I]iodoamphetamine/single-photon emission computed tomography (c). Ischemic brain damage was observed along with cortical laminar on diffusion-weighted imaging (d)
Wound complications have been reported in 2%, 17.6%, and 21.4% of cases of revascularization surgery [17, 24]. They are more common when the superficial temporal artery (STA) is peripherally harvested and/or both the frontal and parietal branches of the STA are harvested [24]. Diabetes may increase the risk of skin trouble [24]. Major skin complications have been reported in 21.4% of 98 revascularization surgeries [24]; however, the risk of skin trouble largely depends on the skin incision design and the harvesting procedures. Mild wound ulceration commonly occurs at the hemicoronal incision corner (Fig. 19.3). Mild wound ulceration can be treated using hydroxyproline, antibiotic ointment, and prostaglandin E1 ointment. Severe skin erosion can progress to osteomyelitis in the bone flap. Treatment for severe skin erosion and infection requires bone flap removal and necrotic skin tissue debridement. Once the wound infection subsides, tissue expansion in the midline area can be performed for scalp flap advancement. Cranioplasty using an artificial bone flap was performed after 3–6 months later with scalp defect reconstruction.
The cranial bone is protected from tissue layers, including the skin, subcutaneous tissue, galea, temporal muscle, and pericranium. Since revascularization surgery uses part of the scalp tissue and muscle, the skin layer becomes prone to being atrophic. The edge of the titanium fixation plate can penetrate the skin, which requires it to be removed.
-
(d)
Acute bypass spasm/occlusion
During the direct bypass of the STA-middle cerebral artery (MCA) anastomosis, thrombosis at the anastomosis site is more frequent than that in bypass performed for main cerebral artery occlusion due to arteriosclerosis [25]. The occlusion could be associated with increased flow velocity and endothelial damage. Katsuta et al. reported the phenomenon of the reversible occlusion of the STA-MCA bypass during mouth opening in 5 out of 15 procedures and termed it as a big bite ischemic phenomenon [26]. During mouth opening, the stretched temporalis muscle may compress the STA against the bone window edge.
-
(e)
Acute brain swelling
Acute brain swelling has been reported after dural opening. Acute brain swelling is associated with hypercapnia; child surgery; small craniotomy; and prone position, including revascularization in the posterior cerebral artery territory. Upon the occurrence of acute brain swelling, measures for reducing intracranial pressure, including normalizing PaCO2, dripping mannitol, and elevating head position should be promptly taken.
-
(f)
Aneurysm at the anastomosis site
Hemodynamic stress may cause postoperative aneurysmal formation after several decades [27, 28]. The reported bleeding timing from an anastomotic aneurysm was 6, 8, 14, 20, and 27 postoperative years [27,28,29,30].
-
(g)
Other rare complications
Previous studies have reported other rare complications. De novo formation of arteriovenous malformation has been reported after moyamoya disease diagnosis [31, 32]. Arteriovenous fistula (AVF) has been reported at the STA-MCA anastomosis site [33]. Dural and pial AVF within the prior operative field has been reported in routine 8-month postoperative angiography. Here, AVF spontaneously disappeared without treatment upon a 2-year surveillance cerebral angiogram [34]. Moreover, severe cerebral vasospasm and delayed cerebral infarction have been reported in a 7-year-old girl after intraventricular hemorrhage [35]. A patient with moyamoya disease at 30 years old, after successful pial synangiosis when she was 6 years old, presented with central retinal artery occlusion that caused unilateral blindness [36].
6 Perioperative Management to Prevent Complications
Hemodynamic assessment is performed to evaluate the perioperative risk through nuclear medicine examinations and noninvasive MR. [123I]- p-iodoamphetamine/single-photon emission computed tomography (SPECT) or 15O-gas positron emission tomography (PET) are often preferred. Cerebrovascular insufficiency can be evaluated by administering 10 mg/kg acetazolamide on SPECT or by measuring the oxygen-extracting fraction/cerebral blood volume on PET. For critical hemodynamic insufficiency, 500–1000 ml/day intravenous drip for hydration is preoperatively administered (1 to 3 preoperative days). After general anesthesia induction, PaCO2 was strictly maintained above 35–40 mmHg throughout the surgery. Subsequently, both colloids and crystalloids (25% albumin and/or 6% hydroxyethyl starch) were administered for 3 postoperative days. In addition to volume supplementation, fluid administration (1000 ml/day in pediatric patients and 1500 mL/day in adult patients) is continued for 7 postoperative days. Postoperative CBF measurements are performed to detect hyperperfusion on SPECT. Colloid and crystalloid administration is discontinued in the case of abnormal focal increases in the CBF [19]. Systolic blood pressure is maintained within 120–130 mmHg. To avoid temporal muscle bleeding, antiplatelet agents are discontinued between 7 preoperative days and 3 postoperative days. It remains unclear whether preoperative antiplatelet therapy reduces the postoperative stroke rate [16, 37].
7 Discussion
Revascularization surgery has an approximate complication rate of 5% [7, 9]. Poor cerebrovascular reserve and ischemic attack episodes are hallmark signs and symptoms for surgical indication. However, recent and frequent ischemic attacks increase the risk of postoperative ischemic complications [9, 38]. In children, surgery should be delayed for approximately 6 weeks after the last ischemic infarction [8].
Postoperative hemorrhage mainly occurs in patients with a previous history of intracranial hemorrhage [15]. Age is positively correlated with the postoperative hemorrhage risk [15]. Although an RCT reported the benefit of direct revascularization for preventing recurrent hemorrhage, the surgical revascularization effect can be diminished by postoperative hemorrhagic complications. This demonstrates the need for detecting the early signs of critical hyperperfusion in surgical and remote areas.
The preference for surgical procedures is based on the technical feasibility and complication rate. The procedure involving direct anastomosis allows an immediate increase in the regional CBF and is associated with good neovascularization. However, compared with indirect anastomosis, the direct procedure is considered to have a greater risk of postoperative complications. Nevertheless, the direct/combined procedure has a lower stroke rate than the indirect procedure in pediatric patients [7]. This is counterintuitive since direct anastomosis is a difficult and time-consuming procedure. It could be attributed to the immediate increase in CBF when hemodynamic conditions are unstable. The immediate increase in the blood flow from direct bypass could compensate for the detrimental effects of anemia, crying-induced hypocapnia, hypotension, and circulation volume loss. Contrastingly, the indirect procedure alone may critically decrease the CBF in the acute period.
Additionally, an excessive increase in intracranial pressure can cause critical ischemia in patients with moyamoya disease. The standard encephaloduroarteriosynangiosis procedure is performed with relatively small craniotomy, which can cause brain protrusion from the craniotomy site. Temporal muscle swelling may also aggravate the increased intracranial pressure [22]. Therefore, patients with severe hemodynamic compromise can postoperatively develop global ischemia [39].
Previous studies have mainly focused on hemodynamic stroke originating from hypocapnia, circulating volume loss, anemia, and hypotension [8, 40, 41] as the main causes of perioperative stroke. The importance of acute occlusive changes remote to the superficial area has been emphasized [5, 11]. This complication may be associated with immediate blood flow alterations. Propofol has been recently shown to increase cerebral perfusion pressure during general anesthesia, [42] which suggests that improved anesthetic management may have decreased the incidence of postoperative stroke.
Skin trouble causes distress to both patients and surgeons. Harvesting of double branches is a routine procedure in conventional combined direct/indirect bypass. STA harvesting is often performed as extensively as possible, particularly in direct STA-ACA anastomosis. After 30 years of experience, the improvement of the harvesting technique has reduced the rate of skin trouble. However, direct STA-ACA anastomosis is not indiscriminately performed because of high frequency of skin trouble.
Temporal muscle swelling complicates combined direct/indirect bypass procedure, in which the muscle bulk is inserted under the bone flap. To reduce this complication, we have attempted splitting the muscle in half and using an indirect procedure. However, this muscle splitting technique was also associated with remarkable swelling of the muscle. A sufficient craniotomy size with an arterialized pedicle and preserved venous drainage is necessary for safe indirect procedures.
Detecting bleeding-prone arterial lesions is essential for prognosis. Revascularization surgery is not always effective for eliminating aneurysms in the perforating artery. Angiographical examination is necessary for patients with a history of intracranial hemorrhage. De novo arteriovenous shunt is occasionally observed as a consequence of acute stroke or revascularization surgery. In clinical settings, excision as primary treatment is difficult, given the stroke-prone brain. Furthermore, subsequent surgery is considered more difficult due to neovascularization.
References
Kodama N, Suzuki J. Moyamoya disease associated with aneurysm. J Neurosurg. 1978;48(4):565–9.
Suzuki J, Kodama N. Moyamoya disease--a review. Stroke. 1983;14(1):104–9.
Sakamoto T, et al. Postoperative neurological deterioration following the revascularization surgery in children with moyamoya disease. J Neurosurg Anesthesiol. 1998;10(1):37–41.
Nomura S, et al. Perioperative management protocols for children with moyamoya disease. Childs Nerv Syst. 2001;17(4–5):270–4.
Kuroda S, et al. Frontal lobe infarction due to hemodynamic change after surgical revascularization in moyamoya disease--two case reports. Neurol Med Chir (Tokyo). 2000;40(6):315–20.
Sato K, Shirane R, Yoshimoto T. Perioperative factors related to the development of ischemic complications in patients with moyamoya disease. Childs Nerv Syst. 1997;13(2):68–72.
Kazumata K, et al. The frequency of postoperative stroke in moyamoya disease following combined revascularization: a single-university series and systematic review. J Neurosurg. 2014;121(2):432–40.
Kim SH, et al. Risk factors for postoperative ischemic complications in patients with moyamoya disease. J Neurosurg. 2005;103(5 Suppl):433–8.
Guzman R, et al. Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg. 2009;111(5):927–35.
Sussman ES, et al. Contralateral acute vascular occlusion following revascularization surgery for moyamoya disease. J Neurosurg. 2018;131(6):1702–8.
Huang AP, Tu YK. Progressive PCA steno-occlusive changes after revascularization for moyamoya disease: a neglected phenomenon. Neurosurgery. 2010;67(6):E1865–6. author reply E1866
Hosoda Y, Ikeda E, Hirose S. Histopathological studies on spontaneous occlusion of the circle of Willis (cerebrovascular moyamoya disease). Clin Neurol Neurosurg. 1997;99(Suppl 2):S203–8.
Wei W, et al. Risk factors for postoperative stroke in adults patients with moyamoya disease: a systematic review with meta-analysis. BMC Neurol. 2019;19(1):98.
Iwama T, Hashimoto N, Yonekawa Y. The relevance of hemodynamic factors to perioperative ischemic complications in childhood moyamoya disease. Neurosurgery. 1996;38(6):1120–5. discussion 1125-6
Tokairin K, et al. Postoperative Intracerebral hemorrhage after combined revascularization surgery in Moyamoya disease: profiles and clinical associations. World Neurosurg. 2018;120:e593–600.
Schubert, G.A., et al., Perfusion characteristics of Moyamoya disease: an anatomically and clinically oriented analysis and comparison. (1524–4628 (Electronic)).
Mesiwala AH, et al. Long-term outcome of superficial temporal artery-middle cerebral artery bypass for patients with moyamoya disease in the US. Neurosurg Focus. 2008;24(2):E15.
Kazumata K, et al. Topographic changes in cerebral blood flow and reduced white matter integrity in the first 2 weeks following revascularization surgery in adult moyamoya disease. J Neurosurg. 2017;127(2):260–9.
Uchino H, et al. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke. 2012;43(10):2610–6.
Fujimura M, et al. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for moyamoya disease: comparative study with non-moyamoya patients using N-isopropyl-p-[(123)I]iodoamphetamine single-photon emission computed tomography. Neurosurgery. 2011;68(4):957–64. discussion 964-5
Andoh T, et al. Chronic subdural hematoma following bypass surgery--report of three cases. Neurol Med Chir (Tokyo). 1992;32(9):684–9.
Fujimura M, et al. Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in moyamoya disease. Neurosurg Rev. 2009;32(2):245–9. discussion 249
Touho H. Cerebral ischemia due to compression of the brain by ossified and hypertrophied muscle used for encephalomyosynangiosis in childhood moyamoya disease. Surg Neurol. 2009;72(6):725–7.
Takanari K, et al. Operative wound-related complications after cranial revascularization surgeries. J Neurosurg. 2015;123(5):1145–50.
Mikami T, et al. Predictive factors for acute thrombogenesis occurring immediately after bypass procedure for moyamoya disease. Neurosurg Rev. 2020;43(2):609–17.
Katsuta T, et al. Reversible occlusion of donor vessel caused by mouth opening after superficial temporal artery-middle cerebral artery anastomosis in adult moyamoya patients. J Neurosurg. 2015;123(3):670–5.
Nishimoto T, et al. A ruptured middle cerebral artery aneurysm originating from the site of anastomosis 20 years after extracranial-intracranial bypass for moyamoya disease: case report. Surg Neurol. 2005;64(3):261–5. discussion 265
Hokari M, et al. Intracerebral hemorrhage from a ruptured aneurysm at the site of anastomosis 27 years after superficial temporal artery-middle cerebral artery bypass. Neurol Med Chir (Tokyo). 2010;50(11):1012–4.
Yokota H, Yokoyama K, Noguchi H. De novo aneurysm associated with superficial temporal artery to middle cerebral artery bypass: report of two cases and review of literature. World Neurosurg. 2016;92:583.e7–583.e12.
Aoki T, et al. Ruptured de novo aneurysm arising at a site remote from the anastomosis 14 years after superficial temporal artery-middle cerebral artery bypass: a case report. Neurosurgery. 2012;71(4):E905–9.
Fujimura M, et al. Development of a de novo arteriovenous malformation after bilateral revascularization surgery in a child with moyamoya disease. J Neurosurg Pediatr. 2014;13(6):647–9.
Schmit BP, et al. Acquired cerebral arteriovenous malformation in a child with moyamoya disease. Case report. J Neurosurg. 1996;84(4):677–80.
Feroze AH, et al. Development of arteriovenous fistula after revascularization bypass for Moyamoya disease: case report. Neurosurgery. 2015;11(Suppl 2):E202–6.
Peeters SM, et al. Spontaneous resolution of Dural and Pial Arteriovenous fistulae arising after superficial temporal artery to middle cerebral artery bypass for Moyamoya disease. World Neurosurg. 2020;142:404–7.
Inoue K, et al. A case of pediatric moyamoya disease with severe cerebral vasospasm and delayed cerebral infarction following an intraventricular hemorrhage. In: Childs Nerv Syst; 2020.
Karsten MB, et al. Central retinal artery occlusion occurring 30 years after successful revascularization surgery for moyamoya disease: case report. In: Acta Neurochir (Wien); 2020.
Yamada S, et al. Effects of surgery and antiplatelet therapy in ten-year follow-up from the registry study of research committee on Moyamoya disease in Japan. J Stroke Cerebrovasc Dis. 2016;25(2):340–9.
Antonucci MU, et al. Acute preoperative infarcts and poor cerebrovascular reserve are independent risk factors for severe ischemic complications following direct Extracranial-intracranial bypass for Moyamoya disease. AJNR Am J Neuroradiol. 2016;37(2):228–35.
Sim YW, et al. Unpredictable postoperative global cerebral infarction in the patient of Williams syndrome accompanying moyamoya disease. J Korean Neurosurg Soc. 2011;50(3):256–9.
Iwama T, et al. Peri-operative complications in adult moyamoya disease. Acta Neurochir. 1995;132(1–3):26–31.
Parray T, Martin TW, Siddiqui S. Moyamoya disease: a review of the disease and anesthetic management. J Neurosurg Anesthesiol. 2011;23(2):100–9.
Kikuta K, et al. Effects of intravenous anesthesia with propofol on regional cortical blood flow and intracranial pressure in surgery for moyamoya disease. Surg Neurol. 2007;68(4):421–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kazumata, K., Houkin, K. (2021). Perioperative Complications. In: Kuroda, S. (eds) Moyamoya Disease: Current Knowledge and Future Perspectives. Springer, Singapore. https://doi.org/10.1007/978-981-33-6404-2_19
Download citation
DOI: https://doi.org/10.1007/978-981-33-6404-2_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6403-5
Online ISBN: 978-981-33-6404-2
eBook Packages: MedicineMedicine (R0)